Abstract
Objectives
In pancreatic cancer (PaC) the prevalence of diabetes mellitus (DM), especially new-onset DM (≤ 36 months of PaC diagnosis), is high. To determine if this observation is unique to PaC, we compared the prevalence and characteristics of DM in lung, breast, prostate and colorectal cancer with PaC and non-cancer controls.
Methods
We retrospectively reviewed medical records of 500 consecutive cancer patients (100 each with lung, breast, prostate, colorectal and PaC) and 100 non-cancer controls.
Results
Patients with PaC (mean age: 71.6±9.4 years; 53% male) had a significantly (p<0.0001) higher prevalence of DM (68%) compared to age-matched patients with lung (71.6±9.4 years, 59% male, 19.6% DM), breast (71.6±9.6 years, 100% female, 19.4% DM), prostate (71.3±9.4 years, 100% male, 14.8% DM), and colorectal cancer (71.6±9.5 years, 56% male, 20.7% DM), and non-cancer controls (70.7±9.2 years, 57% male, 23.5% DM). Among PaC patients, 40% developed DM in the 36 months preceding the diagnosis of PaC, as compared to 3.3-5.7% in the other groups (p<0.0001).
Conclusions
While the prevalence of DM in PaC is very high, DM prevalence in other common cancers is no different from that in non-cancer controls. In particular, new-onset DM is a phenomenon that is unique to PaC.
Keywords: Pancreatic cancer, diabetes mellitus, screening
Background
Diabetes mellitus (DM) is a group of heterogeneous disorders that is characterized by hyperglycemia. In 2010, 10.9 million or 26.9 percent, US residents ≥ 65 years carried a diagnosis of DM.1 The burden of DM is increasing worldwide and is associated with acute and chronic complications, leading to significant morbidity and mortality.2 Similar to DM, the prevalence of cancer has also been increasing worldwide. According to the National Cancer Institute, there were an estimated 11.7 million cases of cancer in the United States in 2007.3
The association between DM and cancer has long been debated.4 Epidemiologic studies have demonstrated an increased risk (relative risk 1.18-2.51) of certain cancers (breast, colorectal, pancreatic, liver, endometrial, and bladder) in patients with DM.5 The relevance of this finding is complicated by the fact that both DM and cancer have similar risk factors (e.g. age, obesity, family history, etc), which might confound the results of these studies.4,5 Hyperinsulinemia also likely favors the development of cancer in patients with DM since insulin is a growth factor with not only metabolic but also mitogenic effects.5 Further, some medications used in the treatment of DM have been postulated to stimulate insulin-mediated mitogenesis leading to a heightened risk of cancer in treated patients.4-6
More specifically, long standing DM (onset > 36 months before the diagnosis of PaC) is considered a risk factor (RR 1.73-1.94) for the development of pancreatic cancer (PaC).5,7 In addition, abnormalities of glucose metabolism become increasingly evident in the time period leading to the diagnosis of PaC.8 In our own studies, we have shown that ~85% of patients with PaC have an abnormal fasting glucose and nearly half have DM which is frequently new-onset, i.e. develops in the 36 months preceding the diagnosis of cancer.8-10 In a population-based study we found that subjects with new-onset DM had an 8-fold higher risk of being diagnosed with PaC within 3-years of meeting criteria for DM as compared to the general population.11 Based on these findings, new-onset DM in PaC has been postulated to be a paraneoplastic phenomenon caused by the cancer itself.10,12 However, whether this phenomenon is specific to PaC or to cancer in general has not been studied.
The aim of the present study was to retrospectively compare the prevalence and temporal correlation of DM in patients with the 4 most common cancers in the United States (lung, breast, prostate and colorectal) and non-cancer controls to PaC patients.
Methods
This study was approved by the Mayo Foundation Institutional Review Board.
Patient selection
From our database of patients, we identified consecutive patients who were diagnosed with lung cancer, breast cancer, prostate cancer, and colorectal cancer, at Mayo Clinic, Rochester in 2007. Cases were age and gender matched to the first 100 consecutive PaC patients seen at Mayo Clinic, Rochester in 2007. Gender matching was not performed for sex-specific cancers i.e. breast and prostate cancer. Controls were identified from patients followed at the Mayo Clinic’s primary care clinics who had atleast one physician visit in 2007. For non-cancer controls with multiple visits in 2007, the clinic visit closest to July 1, 2007 was used as the index date.
Data Collection
The medical records of these 600 patients were reviewed to abstract demographic information and clinical data, including age, gender, family history of DM (grandparents, parents, siblings, children), height, serial weights, serial blood glucose, DM status, duration of DM, onset and date of cancer diagnosis. Height and weight were used to calculate body mass index (BMI, calculated by weight (kg)/height2 (m2)) at the index date. Note was also made for the proportion of patients in each group who did not have a fasting blood glucose measurement prior to the index date.
Definitions
Subjects were defined as having DM using the 1997 American Diabetes Association (ADA) criteria (i.e., a fasting blood glucose (FBG) ≥126 mg/dL, random blood glucose (RBG) ≥200 mg/dL, or Hemoglobin A1c ≥ 6.5%).13 In keeping with the ADA guidelines, a repeat test was necessary to confirm the diagnosis of DM unless two different tests (FBG, RBG or A1c) were concordant for the diagnosis of DM.13 Patients who were on anti-diabetic medications were classified as having DM as well. Onset of DM was defined by the date of the first of 2 consecutive tests that met the ADA criteria for DM diagnosis.
Duration of DM was defined as the time period between the onset of DM and the diagnosis of cancer. For non-cancer controls, duration of DM was defined as the time period between the onset of DM and the index date. DM was considered to be new-onset if the onset preceded the cancer diagnosis by ≤ 36 months.
Statistical analysis
While calculating the proportion of patients with DM in each group, the number of patients without a previous fasting blood glucose measurement was subtracted from the denominator. The duration of DM was also calculated.
All statistical analyses were carried out using the SAS JMP 8 statistical software (Cary, NC). Statistical comparison of continuous variables was performed using the ANOVA and t-tests. Comparison of categorical variables was done using the Pearson Chi-square test. A p-value <0.05 was considered statistically significant.
Results
Patient Demographics (Table 1)
Table 1. Case Demographics.
| Characteristic | Controls | Lung Cancer |
Breast Cancer |
Prostate Cancer |
Colon Cancer |
PaC | p-value |
|---|---|---|---|---|---|---|---|
| N | 100 | 100 | 100 | 100 | 100 | 100 | n/a |
| Age at index date (yrs) (mean±SD) |
70.7±9.2 | 71.6±9.4 | 71.6±9.6 | 71.3±9. | 71.6±9.5 | 71.6±9.4 | 0.97 |
| Males (%) | 57 | 59 | 0 | 100 | 56 | 53 | 0.86* |
| Positive family history of DM (%) |
37 | 33 | 37 | 33 | 37 | 35 | 0.84 |
| BMI at index date (kg/m2)(mean±SD) |
28.5±5.6 | 26.8±4.7 | 28.8±5.6 | 28.6±3.8 | 28.4±6.1 | 27.3±6 | 0.06** |
Breast and prostate cancer excluded in analysis of gender distribution
p-value=0.40, if lung cancer excluded from analysis of BMI
The cohort of 600 patients had 100 patients each with lung cancer (mean age 71.6±9.4 years, 59 male), breast cancer (mean age 71.6±9.6 years), prostate cancer (mean age 71.3±9.4 years), colorectal cancer (mean age 71.6±9.5 years, 56 male), controls (mean age 70.7±9.2 years, 57 male) and PaC (mean age 71.6±9.4 years; 53 male) with no differences in the age distribution (p=0.97). When gender predominant malignancies (breast and prostate cancer) were excluded, the gender distribution among the remaining groups was also similar (p=0.86). A family history of DM was noted in 33-37% patients in each of the 5 groups (p=0.84). The mean BMI was comparable (p=0.40) among breast cancer (mean 28.8±5.6 kg/m2), prostate cancer (mean 28.6±3.8 kg/m2), colorectal cancer (mean 28.4±6.1 kg/m2), controls (mean 28.5±5.6 kg/m2) and PaC patients (mean 27.3±6 kg/m2). Lung cancer patients had a lower BMI (mean 26.8±4.7 kg/m2) as compared to breast cancer, colorectal cancer, prostate cancer and controls (p<0.05) but were comparable to PaC patients (p=0.60).
Blood glucose values
The mean FBG in patients without DM was significantly higher in patients with PaC (109.3±10.6 mg/dl) compared to colorectal cancer (103.1±10.2 mg/dl, p=0.003), prostate cancer (102.6±9 mg/dl, p=0.001), lung (102±10.3 mg/dl, p=0.0006), controls (100.3±9.2 mg/dl, p<0.0001) and breast cancer (99.7±10 mg/dl, p<0.0001). When non-PaC patients and controls were compared, there was no difference in the FBG across the different groups with the exception of breast cancer patients (99.7±10 mg/dl) who had a lower mean FBG compared to colorectal cancer (103.1±10.2 mg/dl). (p=0.04)
Out of 600 patients, there were 35 patients (8 lung cancer, 2 breast cancer, 2 colorectal cancer, 12 prostate cancer and 3 pancreatic cancer) for whom a previous fasting blood glucose measurement was not available. While these patients did not carry a diagnosis of DM in their medical record and were not on anti-diabetic medications, accurate glycemic status could not be ascertained in the absence of a previous blood glucose measurement. Therefore, these patients were not used in calculating the prevalence of DM.
Prevalence of Diabetes Mellitus
Out of the cohort of 600 patients, 158 patients (66 PaC, 18 lung cancer, 19 breast cancer, 13 prostate cancer, 19 colorectal cancer and 23 controls) met criteria for a diagnosis of DM by the index date.
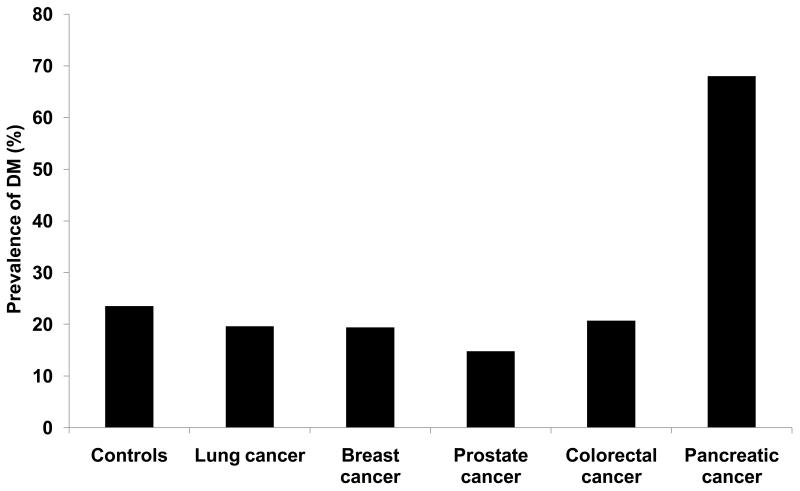
The prevalence of DM was significantly higher (p<0.0001) in patients with PaC 68% (66/97) as compared to lung cancer 19.6% (18/92), breast cancer 19.4% (19/98), prostate cancer 14.8% (13/88), colorectal cancer 20.7% (19/92) and controls 23.5% (23/98). (Figure 1) There was no difference in the prevalence of DM among non-PaC cancer patients and controls (p=0.49).
Figure 1.
Prevalence of DM in various cancers and non-cancer controls
Duration of Diabetes Mellitus
Data on duration of DM was available in 136 (59/66 PaC, 14/18 lung cancer, 17/19 breast cancer, 7/13 prostate cancer, 16/19 colorectal cancer, and 23/23 controls) out of the 158 patients who met criteria for DM. The median duration of DM was 6 months (range 0-276 months) in patients with PaC followed by 26 (3-91) months in prostate cancer, 52.5 months (range 7-249 months) in lung cancer, 120 months (range 0-230 months) in breast cancer, 110 months (0-324 months) in colorectal cancer, and 117 (0-569) months in controls.
In patients with PaC and DM, 59% (39/66) developed DM in the 36 months preceding the diagnosis of PaC. In contrast when patients with DM in the non-PaC group were compared, only 16% (3/18) patients with lung cancer, 21% (4/19) with breast cancer, 38% (5/13) with prostate cancer, 16% (3/19) with colorectal cancer and 17% (4/23) controls developed DM in the 36 months preceding the diagnosis of cancer/index date. The prevalence of new-onset DM (developing in the 36 months preceding the diagnosis of cancer/index date) was markedly higher (p<0.0001) in patients with PaC (40.2%) as compared to lung cancer (3.3%), breast cancer (4.1%), prostate cancer (5.7%), colorectal cancer (3.3%) and controls (4.1%). (Figure 2)
Figure 2.
Prevalence of new-onset DM in various cancers and non-cancer controls
Discussion
In this retrospective study of 600 patients, we show that the prevalence of DM in PaC is high (68%) compared to that found in lung cancer, breast cancer, prostate cancer, colorectal cancer, prostate cancer and non-cancer control patients (14.8-23.5%). This high prevalence of DM in PaC could not be explained based on classical risk factors for DM including age, gender, family history of DM or BMI, which were similar between PaC patients and the other groups. Even among patients who did not meet criteria for DM, patients with PaC had a significantly higher mean FBG value (109.3 mg/dl) compared to other cancers and non cancer control patients (99.7-103.1 mg/dl). Further, a majority (59%) of DM in PaC patients was new-onset (<36 months in duration), as compared to only a minority (16-38%) in patients with other cancers and non-cancer controls.
Diabetes mellitus is a complex disorder that is characterized by hyperglycemia and is typically a result of an imbalance between insulin resistance and beta-cell function.14,15 Type 2 DM commonly occurs as a result of increasing insulin resistance, typically in the context of weight gain, with a failure of the beta-cells to maintain a compensatory increase in insulin secretion.15 In addition to the more commonly described type 2 DM, the American Diabetes Association classifies DM into another sub-type that is associated with diseases of the pancreas, processes that diffusely injure the pancreas resulting in development of DM.16 As would be expected, the insult to the pancreas needs to be extensive before patients burn their endocrine pancreatic reserves and develop DM.16 While this is true of most patho-physiologic entities (e.g. chronic pancreatitis) that result in the development of this type of DM, patients with pancreatic cancer often develop DM at a time when the cancer might be small enough (typically in the 36 months preceding cancer diagnosis) to not be visualized on routine imaging, suggesting a different mechanism in the development of DM in this sub-group of patients.17 This, together with the temporal correlation of the DM with the PaC (development in the 36 months preceding the cancer diagnosis) and resolution of the DM following cancer resection has lead to the proposal that DM in PaC is a paraneoplastic phenomenon.10,12, 18,19
Our results lend further credence to the hypothesis that new-onset DM is indeed unique to PaC and is relatively uncommon in other cancers and non-cancer patients, suggesting that it is not merely a reflection of an increased risk of PaC in patients with DM but rather an early manifestation of the cancer. Further, the finding that this high prevalence in PaC was noted despite no difference in canonical risk factors for DM including age, gender, family history and BMI, supports an alternative pathophysiologic basis for this type of DM. Previous studies have postulated the presence of a tumor secreted factor that might mediate DM by inducing insulin resistance and/or causing beta-cell function.20-22 This is reiterated by our observation that even non-DM patients with PaC had higher mean fasting blood glucose levels as compared to their non-PaC counterparts, suggesting significant alterations in glucose metabolism in patients with PaC.
New-onset DM in PaC is likely a paraneoplastic phenomenon, mediated by tumor secreted product(s). Since DM has a high prevalence in PaC patients and develops at a time when the cancer may not even be visualized on imaging, patients with new-onset DM offer a group of high risk patients who could be screened for sporadic pancreatic cancer. Further studies to discover the mediators of PaC associated DM will not only improve our understanding the pathophysiology of this phenomenon but could also offer potential biomarkers to screen patients for PaC.
Acknowledgments
Grant Support: Dr. Chari’s research was funded by grants from NIH (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 102701)
Abbreviations
- DM
Diabetes Mellitus
- PaC
Pancreatic cancer
- BMI
Body mass index
- FBG
Fasting blood glucose
- RR
Relative risk
- RBG
Random blood glucose
Footnotes
Author contributions: GA: acquisition, analysis and interpretation of data, statistical analysis and drafting of the manuscript; PK: acquisition and analysis; STC: study concept and design, drafting and critical revision of the manuscript.
Conflicts of interest/disclosures: None
References
- 1.Centers for Disease Control and Prevention . National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1976-2007. National Cancer Institute; Bethesda, MD: [Accessed on June 1, 2011]. 2010. based on November 2009 SEER data submission, posted to the SEER Web site. Available at: http://www.cancer.org/cancer/cancerbasics/cancer-prevalence. [Google Scholar]
- 4.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigneri P, Frasca F, Sciacca L, et al. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 6.Smith U, Gale EA. Does diabetes therapy influence the risk of cancer? Diabetologia. 2009;52(9):1699–708. doi: 10.1007/s00125-009-1441-5. [DOI] [PubMed] [Google Scholar]
- 7.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British Journal of Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chari ST, Klee GG, Miller LJ, et al. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–5. doi: 10.1053/gast.2001.27210. [DOI] [PubMed] [Google Scholar]
- 10.Pannala R, Leirness JB, Bamlet WR, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):981–7. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chari ST, Leibson CL, de Andrade M, et al. Probability of pancreatic cancer following diabetes: A population-based study. Gastroenterology. 2005;129:504–511. doi: 10.1053/j.gastro.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Permert J, Adrian TE, Jacobsson P, et al. Is profound peripheral insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg. 1993;165:61–6. doi: 10.1016/s0002-9610(05)80405-2. [DOI] [PubMed] [Google Scholar]
- 13.Engelgau MM. Diabetes Diagnostic Criteria and Impaired Glycemic States: Evolving Evidence Base. Clinical Diabetes. 2004;22:69–70. [Google Scholar]
- 14.Vella A, Camilleri M, Rizza RA. The gastrointestinal tract and glucose tolerance. Curr Opin Clin Nutr Metab Care. 2004;7:479–84. doi: 10.1097/01.mco.0000134375.01310.97. [DOI] [PubMed] [Google Scholar]
- 15.Dinneen S, Gerich J, Rizza R. Carbohydrate metabolism in non-insulin-dependent diabetes mellitus. N Engl J Med. 1992;327:707–13. doi: 10.1056/NEJM199209033271007. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 17.Pelaez-Luna M, Takahashi N, Fletcher JG, et al. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102(10):2157–63. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 18.Permert J, Ihse I, Jorfeldt L, et al. Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg. 1993;80:1047–50. doi: 10.1002/bjs.1800800841. [DOI] [PubMed] [Google Scholar]
- 19.Fogar P, Pasquali C, Basso D, et al. Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res. 1994;14:2827–30. [PubMed] [Google Scholar]
- 20.Permert J, Larsson J, Fruin AB, et al. Islet hormone secretion in pancreatic cancer patients with diabetes. Pancreas. 1997;15:60–8. doi: 10.1097/00006676-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Basso D, Plebani M, Fogar P, et al. Beta-cell function in pancreatic adenocarcinoma. Pancreas. 1994;9:332–5. doi: 10.1097/00006676-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Isaksson B, Strommer L, Friess H, et al. Impaired insulin action on phosphatidylinositol 3-kinase activity and glucose transport in skeletal muscle of pancreatic cancer patients. Pancreas. 2003;26:173–7. doi: 10.1097/00006676-200303000-00014. [DOI] [PubMed] [Google Scholar]