Though psychotherapy, medications and brain stimulation are used to treat major depressive disorder (MDD), treatment response depends on several individualized clinical factors and frequently requires multiple treatment trials. To guide more effective personalized interventions, there is thus a need to identify biological markers to elucidate the mechanisms of response to predict treatment outcomes in MDD. Prior evidence suggests neurochemical disturbances in mood regulating networks, including the anterior cingulate cortex (ACC) and connected prefrontal and subcortical centers, contribute to MDD and play a role in treatment response1–4. To identify treatment-related changes in neurochemical markers implicated in MDD2, 4, 5, we applied single-voxel Proton Magnetic Resonance Spectroscopy (1HMRS) for cross sectional and longitudinal analysis of ACC glutamate (Glu), choline (Cho) and N-acetyl aspartate (NAA) in 10 patients with DSM-IVR diagnoses of MDD (mean age: 44.0 years, 7.93 SD; 6 females) followed prospectively during Index treatment and 10 demographically similar controls (mean age: 39.03 years, 9.55 SD; 6 females). Electroconvulsive therapy (ECT) was used as the therapeutic modality since it is a highly effective procedure for treating severe MDD that elicits rapid response in eligible individuals6.
Patients were assessed at three time points: baseline, and after the 2nd and 6th ECT sessions, corresponding to ~48 hrs and 2 weeks post treatment initiation (see Supplementary Information (SI) for clinical details). The Hamilton (HAMD) and Montgomery-Asberg (MADRS) Depression Rating Scales recorded therapeutic response. Controls were assessed twice, ~2-weeks apart, to determine disease effects, normative values and to estimate the variance of serial scanning. All subjects, without neurological disorders, alcohol/substance abuse or dementia, provided UCLA Institutional Review Board-approved informed consent. High-resolution T1-weighted MPRAGE structural (FOV: 256; voxel size: 1 mm3; TR/TE: 2200/5.16 ms; flip angle: 12°) and single-voxel Point Resolved Spectroscopy (PRESS) acquisitions (TR/TE: 2000/30 ms; spectral width 2000 Hz; 1024 points) with (128 averages) and without water suppression (8 averages) were collected on a Siemens 3T Allegra system. Automatic and manual 3D shimming was applied to reduce B0 inhomogeneity for voxels (20×18×12 mm3) placed in midline anterior ACC gray matter [Fig 1A; see SI]. LCModel computed the levels of Glu, Cho and NAA relative to the unsuppressed water signal. Derived metabolites levels were corrected for voxel CSF content using corresponding tissue classified T1-weighted images7.
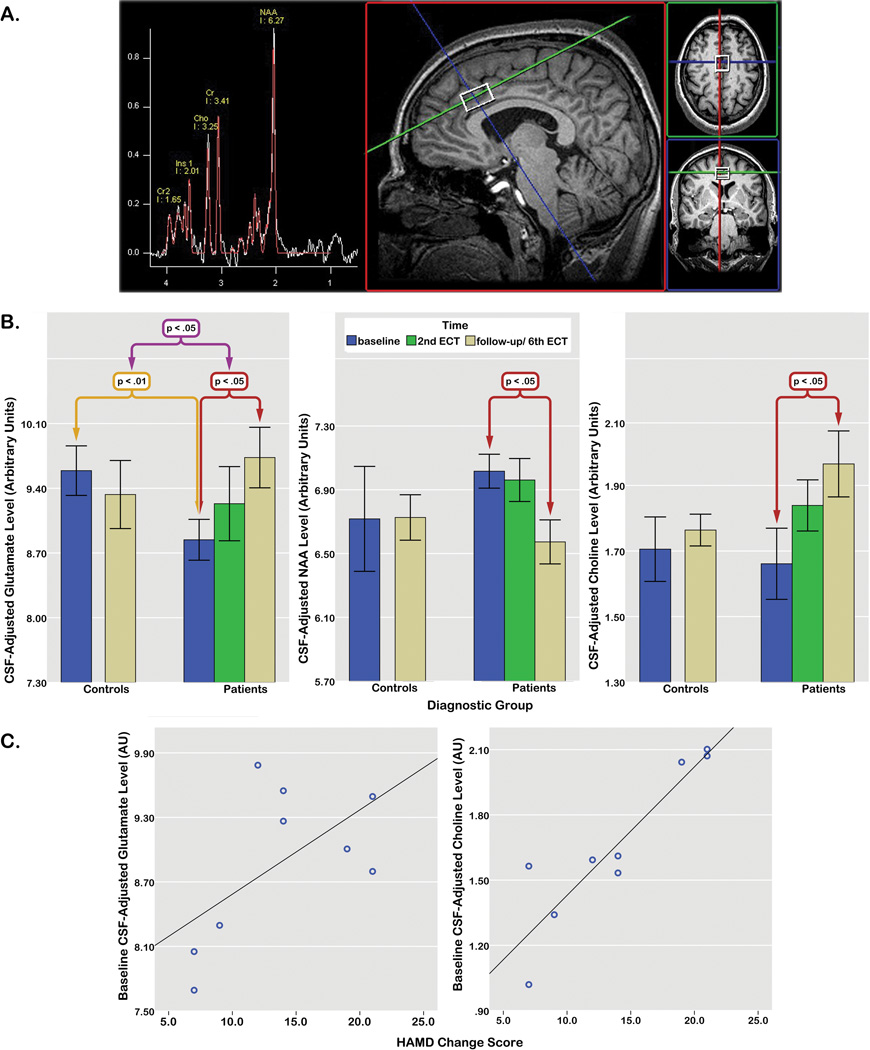
Figure 1.
A) Voxel placement and 1HMRS spectra in a single subject, B) Mean CSF-adjusted glutamate (left), NAA (middle) and choline (right) levels at each time point in patients and controls indicating significant disease effects (purple), group by time interactions (yellow), and differences between metabolite levels at baseline compared to the 6th ECT session in patients (red). The linear effect of time for glutamate within the patient group is not marked, C) relationships between HAMD change scores (subtracted between baseline and the 6th ECT session) and baseline glutamate (left) and choline (right) levels in patients. One data point is missing for a single patient (from the 6th ECT session) for B and C above.
Metabolites (with variances <20%) did not deviate from normality (Kolmogorov–Smirnov test, all p>0.50). For baseline comparisons, including age and gender as covariates, main effects of diagnosis were observed for Glu only, F(1,19)=8.61, p<.01 (lower in patients). General Linear Mixed Models (GLMMs) including metabolites from each time point and a subject variable to control for within-subject correlations, also showed a significant diagnosis by time interaction for Glu, F(1, 21.98)=4.94, p<.037. Glu levels increased significantly over time in patients approximating normal levels, F(2, 16.56)=4.05, p<.037, but remained stable in controls (p=.47). In patients, subsequent pairwise comparisons showed significant Glu elevations between baseline and the 6th ECT, p<.018. In the absence of overall effects of time, pairwise comparisons in patients also showed significant decreases in NAA, p<.048, and increases in Cho, p<.047 between baseline and the 6th ECT [Fig 1B]. HAMD and MADRS scores improved significantly with treatment, F(2, 16.67)=29.48 and F(2, 13.12)=19.79, both p<.0001. Variations in Glu correlated with MADRS scores across time, F(1, 15.78)=4.49, p<.05. Baseline Cho levels correlated significantly with overall change in HAMD ratings, r=.85, p<.0001; baseline Glu levels also showed a trend for predicting HAMD change, r=.59, p<.08 [Fig 1C].
MDD-related reductions in ACC glutamate normalize with ECT treatment and associate with clinically determined therapeutic response. Results are in line with prior reports indicating glutamate deficits in MDD2, 4 and extend earlier observations of increased glutamate/glutamine (Glx) in the left ACC in patients treated with ECT8, shown here to also mirror clinical response. NAA and Choline, though not discriminating diagnostic groups at baseline, also showed treatment effects in patients. Together, these neurochemical changes suggest treatment-induced neuroplasticity in the structure, density and integrity of mature neurons9 and/or may reflect changes relating to adult neurogenesis in connected hippocampal and subventricular regions10. Results also support that neurochemical signatures in the ACC may predict future therapeutic response through mechanisms potentially overlapping with other forms of treatment. Studies including additional treatment modalities, larger samples, and longer follow-ups with relapse rates may confirm these observations.
Supplementary Material
Footnotes
Conflict of interest:
The authors report no conflicts of interest
References
- 1.Drevets WC, Price JL, Furey ML. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capizzano AA, Jorge RE, Acion LC, Robinson RG. J Magn Reson Imaging. 2007;26:1378–1389. doi: 10.1002/jmri.21144. [DOI] [PubMed] [Google Scholar]
- 3.Castren E. Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- 4.Yuksel C, Ongur D. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yildiz-Yesiloglu A, Ankerst DP. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Kho KH, van Vreeswijk MF, Simpson S, Zwinderman AH. J ECT. 2003;19:139–147. doi: 10.1097/00124509-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 8.Pfleiderer B, Michael N, Erfurth A, Ohrmann P, Hohmann U, Wolgast M, et al. Psychiatry Res. 2003;122:185–192. doi: 10.1016/s0925-4927(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 9.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger C, Duman RS. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.