Abstract
Background
Most heart failure (HF) risk stratification models were developed for inpatient use, and available outpatient models use a complex set of variables. We hypothesized that routinely collected clinical data could predict the 6-month risk of death and all-cause medical hospitalization in HF clinic outpatients.
Methods and Results
Using a quality improvement database and multivariable Cox modeling, we derived the Heart Failure Patient Severity Index (HFPSI) in the University of Michigan HF clinic (UM cohort, n = 1,536; 314 reached primary outcome). We externally validated the HFPSI in the Ann Arbor Veterans’ Affairs HF clinic (VA cohort, n = 445; 106 outcomes) and explored “real-time” HFPSI use (VA-RT cohort, n = 486; 141 outcomes) by tracking VA patients for 6 months from their most recently calculated HFPSI, rather than using an arbitrary start date for the cohort. The HFPSI model included blood urea nitrogen, B-type natriuretic peptide, New York Heart Association class, diabetes status, history of atrial fibrillation/ flutter, and all-cause hospitalization within the prior 1 and 2 to 6 months. The concordance c statistics in the UM/VA/VA-RT cohorts were 0.71/0.68/0.74. Kaplan-Meier curves and log-rank testing demonstrated excellent risk stratification, particularly between a large, low-risk group (40% of patients, 6-month event rates in the UM/VA/VA-RT cohorts 8%/12%/12%) and a small, high-risk group (10% of patients, 6-month event rates in the UM/VA/VA-RT cohorts 57%/58%/79%).
Conclusions
The HFPSI uses readily available data to predict the 6-month risk of death and/or all-cause medical hospitalization in HF clinic outpatients and could potentially help allocate specialized HF resources within health systems.
Nearly 6 million Americans have heart failure (HF), and in 2010, an estimated $39 billion1 was spent on HF care in the United States. Hospitalization costs are a large majority of this expenditure. Recurrent admissions significantly impair quality of life in patients with HF and now affect hospital Medicare reimbursement. Mortality and HF hospitalization rates have improved slightly in recent years but remain unacceptably high,2 and the prevalence of HF is growing as the population ages.
Specialty HF clinics and disease management programs were developed to address the root causes of hospitalizations and expand use of mortality-reducing therapies. Such programs can reduce adverse events in participating patients with HF.3,4 However, the benefits are not universal,5,6 and although there have been notable recent exceptions,7 similar interventions are typically applied across all patients with HF in a given cohort without regard for individual patient risk. Methods to risk stratify ambulatory patients with HF are urgently needed to target outpatient resources to the patients with HF who need them the most.
Several models predict mortality in ambulatory patients with HF,8-10 but hospitalization prediction models were developed for inpatient use,11,12 have low discrimination for individual patients,11,13 or include a complex set of variables that are not always routinely available.14 An ideal risk model would discriminate between a relatively small group of “high-risk” patients who could benefit from intensive management and a large cohort of “low-risk” patients who could be followed up in a less resource-intensive manner. In addition, the model would need to adapt to ongoing variations in risk predictors because the clinical status of patients with HF changes frequently.15 We hypothesized that routinely obtained data could adequately risk stratify HF clinic outpatients for death and/or all-cause hospitalization.
Methods
The study was originally conceived as part of an HF quality improvement initiative at the University of Michigan and the Ann Arbor Veterans Affairs Health System and was approved by the institutional review boards at both facilities. Dr Hummel is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute Mentored Patient-Oriented Career Development Award (K23HL109176); no other funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
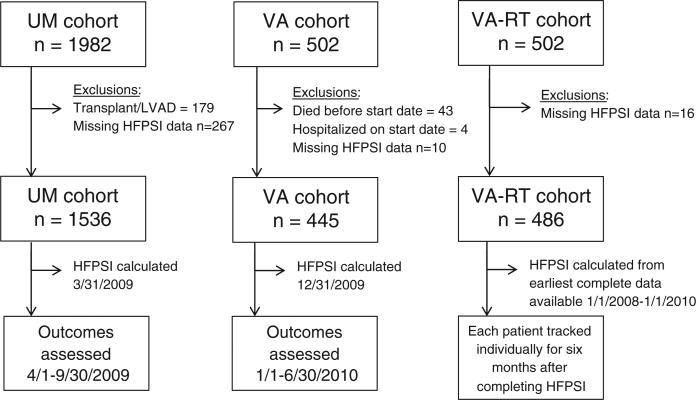
The primary study outcome was the combination of all-cause death and medical hospitalization (defined as any admission to a nonsurgical service) over a 6-month period. We used STATA version 10.0 (STATACorp, College Station, TX) for statistical analyses. We used unpaired t testing and χ2 testing for between-group differences and Cox proportional hazard modeling to investigate the primary outcome. All subjects were followed up for 6 months or until the time of death or first all-cause medical hospitalization. We report model discrimination with concordance c statistics and graphically display survival free from death or all-cause medical hospitalization with Kaplan-Meier curves. The composition of the 3 HF clinic cohorts used in the study (see below) and the timeline for outcome assessment in each are shown in Figure 1.
Figure 1.
Cohort composition and time frame for outcome assessment. Abbreviations: LVAD, left ventricular assist device; UM, University of Michigan; VA, Veterans Affairs; VA-RT, Veterans Affairs Real-Time.
Derivation of risk model
We evaluated predictors of the primary outcome in the cohort of patients managed by the University of Michigan outpatient HF clinic (UM cohort), excluding patients with prior left ventricular assist device or cardiac transplantation from the sample. As part of local quality improvement initiatives, clinical data for HF clinic patients are routinely extracted from the University of Michigan electronic medical record and stored in a searchable database. Clinical variables in this database included age, self-reported race, gender, serum sodium, blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR; calculated with the Modification of Diet in Renal Disease equation), B-type natriuretic peptide (BNP), left ventricular ejection fraction, the most recently assessed New York Heart Association (NYHA) functional class, and the dates of previous all-cause medical hospitalizations. All of these variables have previously been shown to predict mortality and/or morbidity in patients with HF.8,16,17 Based on prior studies indicating prognostic importance in HF, we obtained data from the electronic medical record on history of coronary artery disease (CAD; defined as problem summary list diagnosis or previous percutaneous/surgical coronary revascularization),8 atrial fibrillation/flutter (problem summary list diagnosis),14,18 and diabetes mellitus (problem summary list diagnosis).14
All-cause medical hospitalizations at the University of Michigan are tracked automatically, with dates of admission at other facilities added by HF clinic telemanagement nurses. Dates of death are obtained from the Social Security Death Index. We considered left ventricular assist device or cardiac transplantation during the follow-up period as mortality surrogates. Accordingly, the dates of these procedures were obtained from local University of Michigan databases and were treated as deaths for this analysis.
Using Cox proportional hazards modeling, we created a risk model (Heart Failure Patient Severity Index [HFPSI]) for the primary outcome of death and all-cause medical hospitalization in the UM cohort. Because the distribution of BNP was highly skewed, this variable was log transformed for analysis. Previous all-cause hospitalizations were dichotomized into within the past 1 month and within the prior 2 to 6 months. Self-reported race was dichotomized as white and nonwhite.
The significant predictors were identical both when including all variables simultaneously and when using backward stepwise regression (see the Results section). We performed the primary analysis excluding race and gender information because within the context of our quality improvement initiative, we felt that it would be inappropriate to allocate HF resources based on these characteristics. We performed a sensitivity analysis including these demographic variables and also evaluated model performance within dichotomized age, race, gender, CAD history, and ejection fraction subgroups.
We calculated the HFPSI on April 1, 2009, and obtained patient outcomes in the UM cohort through September 30, 2009. We graphically confirmed the proportional hazards assumption for the HFPSI and then defined 4 risk categories using linear prediction deciles derived from the variable coefficients (group 4, highest HFPSI decile [10% of patients]; group 3, deciles 8-9 [20%]; group 2, deciles 5-7 [30%]; and group 1, deciles 1-4 [40%]). Although the primary outcome was all-cause admission or death at 6 months, for comparison purposes, we also evaluated the performance of the HFPSI model in the UM cohort at 12 months of follow-up.
A subgroup of UM cohort patients had complete Medicare claims data owing to institutional participation in the Medicare Pay-for-Performance Demonstration Project. In this subset, we examined the performance of the HFPSI and how well the UM cohort database captured previous hospitalizations.
External validation
Next, we externally validated the HFPSI in patients followed up by the HF clinic at the Ann Arbor Veterans Affairs Medical Center (VA cohort). The VA cohort was identified via an electronic medical record search (Computerized Patient Record System [CPRS]) for patients with HF clinic visits in 2009, with HF diagnosis confirmed by International Classification of Diseases, Ninth Revision codes in their chart or utilization data. Patients with prior cardiac transplantation were excluded; the clinic does not follow up patients with left ventricular assist devices. We created an algorithm to electronically abstract all HFPSI variable data from local CPRS data servers. As in other recent studies,19 dates of death, medical hospitalization at Veterans Affairs medical centers, and fee-basis hospitalization at private facilities were obtained from Veterans Affairs national databases. No VA cohort patients underwent transplantation or left ventricular assist device placement during the follow-up interval, so these outcomes were not used. We calculated the HFPSI in the VA cohort on January 1, 2010, and obtained patient outcomes through June 30, 2010. We used the same variables and Cox model in the VA cohort and divided patients into 4 groups based on HFPSI deciles as in the UM cohort above.
“Real-time” implementation
We then explored the potential for real-time implementation of the HFPSI in the VA cohort (VA-RT cohort). For this analysis, we obtained data for all HFPSI variables between January 1, 2008, and January 1, 2010, from CPRS. We individually tracked each patient for 6 months from the date of “completing” their HFPSI (ie, the earliest date within this time frame when they had no missing data for laboratory and NYHA class variables). In patients who completed their HFPSI during a hospitalization, the date of hospital discharge was used as the first day for 6-month follow-up. Patients who died during a hospitalization where they completed their initial HFPSI were excluded from this analysis. To evaluate a completely outpatient VA-RT cohort, we performed a sensitivity analysis by excluding patients who completed their HFPSI during a hospital admission, Again, we also evaluated the HFPSI model at 12 months of follow-up in the VA and VA-RT cohorts.
Results
The UM cohort initially included 1,982 patients; after the exclusion of subjects for prior transplant or left ventricular assist device and for missing HFPSI variable data (Figure 1), we analyzed 1,536 patients. The baseline characteristics and 6-month outcomes of the UM cohort are detailed in Table I.
Table I.
Baseline characteristics
| Variables | UM cohort (n = 1536) | VA cohort (n = 445) | VA-RT cohort (n = 486) |
|---|---|---|---|
| Age (y) | 65 (57-72) | 67 (62-78) | 68 (62-78) |
| Gender (female)* | 36% | <1% | <1% |
| Race (white) | 78% | Data not available | |
| Ejection fraction (%) | 40 ± 19 | Data not available | |
| NYHA class* | |||
| I | 10% | 7% | 7% |
| II | 72% | 46% | 45% |
| III | 16% | 36% | 37% |
| IV | 2% | 11% | 12% |
| BUN (mg/dL) | 22 (16-31) | 22 (14-28) | 21 (15-29) |
| BNP (pg/mL)* | 138 (53-341) | 148 (46-439) | 174 (51-512) |
| Diabetes mellitus (any type) | 34% | 52% | 52% |
| CAD | 40% | 80% | 79% |
| Atrial fibrillation/flutter | 38% | 33% | 33% |
| Prior hospitalizations* | |||
| Within 1 mo | 6% | 4% | 10% |
| Within 2-6 mo | 21% | 19% | 24% |
| Outcomes during study† | 314 (20%) | 106 (24%) | 141 (29%) |
| All-cause hospitalizations | 278 (18%) | 92 (21%) | 97 (20%) |
| Mortality | 68 (4%) | 14 (3%) | 44 (9%) |
Quantitative values are expressed as mean (interquartile range).
P < .05 for differences between cohorts by analysis of variance or χ2 testing.
Event totals reflect the first event of each type during the study; totals may not match because some patients had both events during the 6-month study period.
In the primary analysis, the variables independently associated with the primary outcome were as follows: age, BUN, eGFR, log BNP, history of atrial fibrillation/ flutter, diabetes status, NYHA class, and all-cause medical hospitalization within the prior 1 and the prior 2 to 6 months. Ejection fraction and history of CAD were not associated with the primary outcome. Given the necessity of calculating eGFR based on multiple additional variables, a stronger relationship between BUN and the primary outcome, and collinearity of these 2 factors, BUN was retained and eGFR dropped from the model. Serum sodium and age were not independent predictors in the UM cohort and were also removed from the model. Therefore, the final HFPSI model included BUN, log BNP, NYHA class, hospitalization within the past 1 month, hospitalization within the prior 2 to 6 months, history of atrial fibrillation/flutter, and diabetes status.
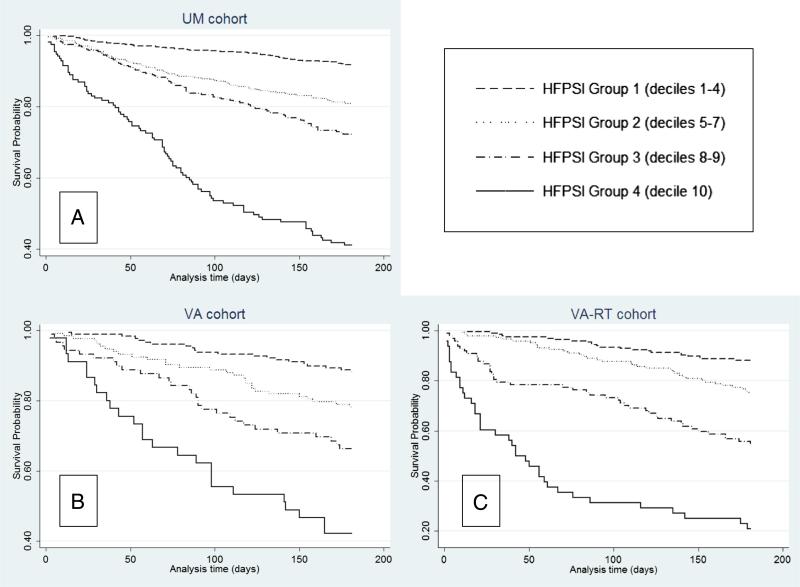
The coefficients, hazard ratios, and CIs for the HFPSI variables in the UM cohort are shown in Table II. The c statistic for the HFPSI was 0.72 for the primary outcome, 0.71 for all-cause medical hospitalization, and 0.77 for mortality. Kaplan-Meier curves for death and/or all-cause hospitalization in HFPSI groups 1 to 4 are depicted in Figure 2A; test for trend across groups was highly significant (log-rank χ2 = 206.00, P < .001). The HFPSI model performed similarly in the presence or absence of CAD (c statistics = 0.71 and 0.72 respectively) and left ventricular ejection fraction <40% and ≥40% (c statistics = 0.71 and 0.72 respectively).
Table II.
Univariable and multivariable predictors of 6 month all-cause mortality and/or medicalhospitalization (n = 1536, 314 outcomes)
| UM cohort, univariable predictors |
UM cohort, multivariable predictors |
UM cohort, final HFPSI model |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient | Hazard ratio (95% CI) | P | Coefficient | Hazard ratio (95% CI) | P | Coefficient | Hazard ratio (95% CI) | P |
| Age | 0.012 | 1.01 (1.00-1.02) | .02 | 0.006 | 1.01 (0.99-1.02) | .29 | |||
| Female gender | –0.273 | 0.76 (0.61-.95) | .02 | –0.302 | 0.74 (0.59-0.93) | .01 | |||
| Nonwhite race | 0.463 | 1.59 (1.25-2.02) | <.001 | 0.415 | 1.51 (1.18-1.94) | .001 | |||
| Ejection fraction (per %) | –0.003 | 1.00 (0.99-1.00) | .37 | ||||||
| Serum sodium (per mg/dL) | –0.050 | 0.95 (0.92-0.98) | .002 | –0.008 | 0.99 (0.96-1.02) | .62 | |||
| BUN (per mg/dL) | 0.024 | 1.02 (1.02-1.03) | <.001 | 0.013 | 1.01 (1.01-1.02) | <.001 | 0.012 | 1.01 (1.00-1.02) | <.001 |
| BNP (pg/mL; per log) | 0.297 | 1.35 (1.24-1.46) | <.001 | 0.177 | 1.26 (1.04-1.24) | .006 | 0.135 | 1.15 (1.05-1.25) | .003 |
| NYHA class | |||||||||
| I | Reference | Reference | Reference | ||||||
| II | 0.734 | 2.08 (1.25-3.46) | .005 | 0.489 | 1.63 (0.98-2.72) | .06 | 0.496 | 1.64 (0.99-2.74) | .06 |
| III | 0.985 | 2.68 (1.54-4.64) | <.001 | 0.546 | 1.73 (0.99-3.06) | .06 | 0.576 | 1.78 (1.02-3.11) | .04 |
| IV | 1.757 | 5.80 (2.90-11.59) | <.001 | 0.704 | 2.02 (0.99-4.14) | .053 | 0.835 | 2.31 (1.13-4.70) | .02 |
| Diabetes mellitus | 0.557 | 1.74 (1.40-2.18) | <.001 | 0.298 | 1.35 (1.07-1.70) | .01 | 0.317 | 1.38 (1.09-1.74) | .008 |
| Atrial fibrillation/flutter | 0.569 | 1.76 (1.42-2.21) | <.001 | 0.328 | 1.39 (1.09-1.76) | .007 | 0.288 | 1.33 (1.06-1.68) | .01 |
| CAD | –0.113 | 0.89 (0.71-1.12) | .33 | ||||||
| Hospitalization within: | |||||||||
| 1 mo | 1.409 | 4.09 (3.04-5.52) | <.001 | 1.017 | 2.76 (2.02-3.79) | <.001 | 1.051 | 2.86 (2.10-3.89) | <.001 |
| 2-6 mo | 0.897 | 2.45 (1.95-3.09) | <.001 | 0.670 | 1.95 (1.54-2.48) | <.001 | 0.689 | 1.99 (1.57-2.52) | <.001 |
| c Statistic | 0.722 | 0.716 | |||||||
Figure 2.
Survival free of death and all-cause medical hospitalization, HFPSI score decile groups.
Upon adding demographic variables, gender and race were also individual and independent predictors of the primary outcome. However, the HFPSI c statistic improved only modestly (from 0.716 to 0.722) after including this demographic information. The overall performance of the HFPSI in the UM cohort was similar in patients younger than 65 years and those 65 years or older, men and women, and white and nonwhite patients (c statistic range 0.70-0.74 for all). We therefore chose to maintain the original HFPSI variables (without race and gender information) for external validation.
Full Medicare claims data for the study interval were available for 136 UM cohort patients, 55 of which reached the primary outcome. In this group, all hospitalizations within the prior 1 and 2 to 6 months as well as all deaths and all-cause hospitalizations over the follow-up period were appropriately captured in the UM cohort database. The HFPSI c statistic in this Medicare claims-verified subgroup was 0.72.
The VA cohort initially included 502 patients. Excluding subjects who died before the start date for follow-up were hospitalized on the start date, and for missing data, we analyzed 445 patients (Figure 1). The VA-RT cohort contained 486 patients; fewer were excluded because none died before or were hospitalized on an arbitrary start date. Baseline characteristics are shown in Table I. The c statistic for the HFPSI coefficients was 0.68 in the VA cohort and 0.74 in the VA-RT cohort. When the 31 VA-RT patients who completed their HFPSI during a hospitalization were excluded, the c statistic decreased to 0.71. Kaplan-Meier curves for the VA and VA-RT cohorts are shown in Figures 2B and C; both had significant log-rank test for trend across HFPSI groups (χ2 = 52.31 [P < .001] and χ2 = 127.57 [P < .001], respectively).
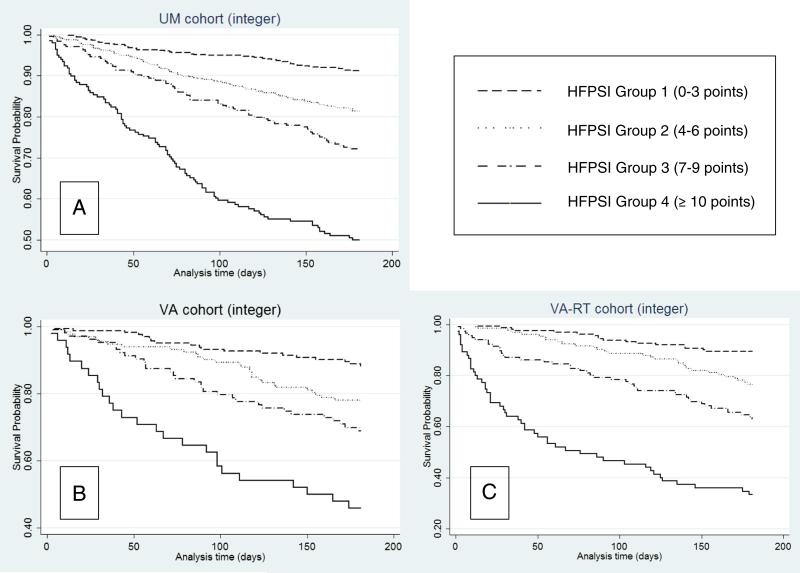
To simplify risk assessment, we created an integer HFPSI score (Table III) based on the relative importance of each variable to the final HFPSI model. The c statistic for this integer HFPSI score in the UM, VA, and VA-RT cohorts was 0.69, 0.67, and 0.71, respectively, with similar separation of risk groups to the full HFPSI model (Figures 3A-C).
Table III.
Integer HFPSIscore
| Variable | Point score |
|---|---|
| Starting score | 0 |
| BUN (mg/dL) | |
| 21-34 | +1 |
| 35-50 | +2 |
| >50 | +3 |
| BNP (pg/mL) | |
| >55 (log BNP >4) | +2 |
| >148 (log BNP >5) | +3 |
| >403 (log BNP >6) | +5 |
| Diabetes (any type) | +1 |
| Atrial fibrillation/flutter | +1 |
| NYHA class III | +1 |
| NYHA class IV | +2 |
| Prior hospitalizations | |
| Within 1 mo | +5 |
| Within 2-6 mo | +2 |
| Risk group | Total points |
|---|---|
| Group 1 | 0-3 |
| Group 2 | 4-6 |
| Group 3 | 7-9 |
| Group 4 | ≥10 |
Figure 3.
Survival free of death and all-cause medical hospitalization, HFPSI integer score group.
The HFPSI model performed similarly at 12 months of follow-up, with c statistics in the UM, VA, and VA-RT cohorts of 0.70, 0.68, and 0.72 respectively (see online Appendix Supplementary Table). Again, the HFPSI stratified effectively between groups 1 and 4 (log-rank test for trend: UM cohort χ2 = 223.80, P < .001; VA cohort χ2 = 62.83, P < .001; VA-RT cohort χ2 = 143.80, P < .001; see online Appendix Supplementary Figure).
Discussion
The HFPSI uses routinely available data to predict the 6-month risk of death and all-cause hospitalization in HF clinic outpatients. The model stratifies very well between a large, low-risk group and a small high-risk group. The HFPSI could potentially be used to target specialized HF resources within a health system and shows promise for automated implementation.
Previous studies
Most of previous HF outcome prediction models compiled data from inpatients who were then tracked after hospital discharge. The goal of these tools was to assess the risk of early adverse events in a high-risk population. In general, discrimination, particularly for readmission, is not high and/or the models are intended for hospital-to-hospital comparison rather than risk assessment per se.16,20-22 Moreover, because HF hospitalization is a well-accepted poor prognostic marker,17 these prediction models may be more robust in “sicker” patients with HF. The validity and use of these tools in a more heterogeneous ambulatory HF cohort have not been established.
Several excellent models exist to risk stratify ambulatory patients with HF for long-term mortality.8-10 However, these models either use a large number of variables9,10 and/or require specialized testing such as oxygen consumption treadmill testing or echocardiography.8,10 Moreover, these models predict events over a longer interval (12-44 months) than the HFPSI. This time frame is appropriate when discussing long-term prognosis with patients with HF or making decisions regarding the risk-benefit ratio of advanced HF therapies, but may be of less use when trying to assess overall risk across an HF clinic population. Most importantly, these risk scores do not address the expensive and frequent problem of hospitalization in patients with HF.
The Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity (CHARM) investigators evaluated all-cause mortality and the combined outcome of cardiovascular death and HF hospitalization over a 2-year period.14 The discrimination of the resulting model was good (c statistic = 0.75), but the performance of this model in nonclinical trial populations or in predicting all-cause medical admissions is unknown. The CHARM model included 24 variables, several based on physical examination findings or other parameters that would be challenging to extract electronically. The authors acknowledged that their purpose was to define associations of specific variables with adverse outcomes in a robust database rather than to create a clinical prediction tool.
Specific comments on the HFPSI
The HFPSI includes a broad range of information from clinical assessment (NYHA class), comorbidities (diabetes mellitus, atrial dysrhythmias), laboratory studies (BUN, log BNP), and illness course (recent all-cause hospitalizations). Nonetheless, these data are customarily collected during the course of outpatient HF management and do not require any specialized testing. Despite its simplicity, the HFPSI model performance compares favorably to the much more complex CHARM model,14 the only other published risk model for combined death and all-cause hospitalization in ambulatory patients with HF. Most importantly for resource allocation purposes, the HFPSI stratifies very well between a large, low-risk group (40% of patients, 6-month event rates in UM/VA/VA-RT cohorts 8%/12%/12%) and a small, high-risk group (10% of patients, 6-month event rates in UM/VA/VA-RT cohorts 57%/58%/79%).
The HFPSI had adequate discrimination in the UM cohort, a large academic hospital HF clinic population. As expected, the VA cohort comprised patients who were older and almost-exclusively male (Table I). We could not obtain complete comorbidity data for the VA cohort, but the prevalence and severity of non-HF medical conditions were likely higher in this group when compared with the general population.23 Although the stratification between low- and high-risk patients remained strong, the overall model discrimination was lower in the VA cohort. We suspect that this relates to unmeasured predictors of adverse outcomes that may be more prevalent in this population, for example, substance abuse, psychiatric illness, and lower health literacy.24,25
The clinical status of patients with HF changes frequently, particularly as disease severity worsens.15 Ideally, HF risk stratification models could be periodically or continuously updated, enabling recalculation of risk as new information becomes available. The HFPSI c statistic significantly improved in the VA-RT cohort, which tracked VA cohort patients individually for 6 months beginning on the date they “completed” their HFPSI data, as opposed to using an arbitrary start date for the entire cohort. We view this finding as encouraging evidence that the HFPSI could be implemented in real-time, that is, recalculated when new prognostic information becomes available for an individual patient. Electronic medical record systems, encouraged by recent legislation and financial incentives, would provide an ideal framework for automated HFPSI calculation and provider notification if a patient's risk profile changes.
Limitations
The HFPSI model is not comprehensive and does not include many known predictors of adverse outcomes in ambulatory patients with HF. However, the discrimination of the HFPSI model for mortality and all-cause admission compares favorably with other HF risk models.8-10,14,16,20-22 Recent data indicate that more than half of hospitalizations after the diagnosis of HF are due to noncardiac causes,26 and current hospital Medicare penalties are based on all-cause readmission. However, we did not have full information on causes of death or reasons for hospital admission and were unable to specifically assess the capability of the HFPSI to predict cardiovascular or HF-related mortality or hospitalization.
Our goal was to create a risk model that incorporated a small number of common factors that could be easily tracked and updated. The main use of the HFPSI lies in assigning risk categories to outpatients across a large HF clinic cohort, although the quickly calculated integer HFPSI could aid in general discussions of risk with specific patients. Beyond illness severity, factors such as health literacy, psychiatric comorbidities, and social support networks greatly affect HF outcomes.27-29 Supplementing the HFPSI with clinical judgment and consideration of psychosocial and other noncardiac factors would likely improve risk assessment and guide appropriate HF-, comorbidity-, and environment-focused interventions for high-risk individuals.
Both the derivation and validation cohorts comprise relatively young patients followed up in HF clinics at tertiary care centers in southeast Michigan. Our results may not be generalizable, and further validation is needed in patients with HF followed up in the general community and in other geographic areas. We specifically sought to validate the HFPSI in an HF clinic population with very different characteristics from those of the UM cohort. However, the high prevalence of CAD and predominantly male gender in the VA cohorts may also limit the generalizability of our study. Data for 1 or more HFPSI variables were missing in 13% of UM cohort patients, and it is possible that systematic differences in these patients could have biased our results; missing data were uncommon in the VA cohorts. Some hospitalization outcomes may be missing in the UM cohort, given that some occurred at outside facilities and insurance claims information was not available from all payers. However, the subset of UM cohort patients with complete Medicare claims data suggests that the derivation database captures most hospitalizations. Our strategy for obtaining outcomes in the VA cohorts was identical to that used in other recent articles.19
Conclusions
The HFPSI predicts the 6-month risk of death and/or all-cause medical hospitalization in HF clinic outpatients. The HFPSI is based on proven prognostic variables that are routinely collected in HF outpatient management and appears particularly effective at dividing patients into discrete risk groups. Preliminary work suggests that it could be used as an electronically tracked and continually updated prognostic index within large ambulatory HF cohorts.
Supplementary Material
Acknowledgments
Dr. Hummel is supported by an NIH/NHLBI Mentored Patient-Oriented Career Development Award, K23HL109176. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. on behalf of the American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Normand S-LT, Wang Y, et al. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–78. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klersy C, De Silvestri A, Gabutti G, et al. A meta-analysis of remote monitoring of heart failure patients. J Am Coll Cardiol. 2009;54:1683–94. doi: 10.1016/j.jacc.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Gohler A, Januzzi JL, Worrell SS, et al. A systematic meta-analysis of the efficacy and heterogeneity of disease management programs in congestive heart failure. J Card Fail. 2006;12:554–67. doi: 10.1016/j.cardfail.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Galbreath AD, Krasuski RA, Smith B, et al. Long-term healthcare and cost outcomes of disease management in a large, randomized, community-based population with heart failure. Circulation. 2004;110:3518–26. doi: 10.1161/01.CIR.0000148957.62328.89. [see comment]. [erratum appears in Circulation. 2004 dec 7;110(23):3615]. [DOI] [PubMed] [Google Scholar]
- 6.Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating study evaluating Outcomes of Advising and Counseling in Heart failure (COACH). Arch Intern Med. 2008;168:316–24. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 7.Berger R, Moertl D, Peter S, et al. N-terminal pro–B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure: a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55:645–53. doi: 10.1016/j.jacc.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 8.Koelling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving beta-blockers. J Heart Lung Transpl. 2004;23:1414–22. doi: 10.1016/j.healun.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez R, Bayes-Genis A, Cygankiewicz I, et al. The MUSIC risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J. 2009;30:1088–96. doi: 10.1093/eurheartj/ehp032. [DOI] [PubMed] [Google Scholar]
- 11.Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;1:245–51. doi: 10.1016/j.jchf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Howell S, Coory M, Martin J, et al. Using routine inpatient data to identify patients at risk of hospital readmission. BMC Health Serv Res. 2009;9:96. doi: 10.1186/1472-6963-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krumholz HM, Chen YT, Wang Y, et al. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–7. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 14.Pocock S, Wang D, Pfeffer M, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 15.Hauptman PJ, Masoudi FA, Weintraub WS, et al. Variability in the clinical status of patients with advanced heart failure. J Card Fail. 2004;10:397–402. doi: 10.1016/j.cardfail.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168:1371–86. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Austin PC, Stukel TA, et al. “Dose-dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med. 2009;122:162–9. e161. doi: 10.1016/j.amjmed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 19.Heidenreich PA, Sahay A, Kapoor JR, et al. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56:362–8. doi: 10.1016/j.jacc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 20.Amarasingham R, Moore B, Tabak Y, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48:981–8. doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow GC, Adams KF, Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 22.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 23.Petersen LA, Normand S-LT, Daley J, et al. Outcome of myocardial infarction in Veterans Health Administration patients as compared with Medicare patients. N Engl J Med. 2000;343:1934–41. doi: 10.1056/NEJM200012283432606. [DOI] [PubMed] [Google Scholar]
- 24.Peterson PN, Shetterly SM, Clarke CL, et al. Health literacy and outcomes among patients with heart failure. JAMA. 2011;305:1695–701. doi: 10.1001/jama.2011.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins KE, Pincus HA, Paddock S, et al. Care for veterans with mental and substance use disorders: good performance, but room to improve on many measures. Health Aff (Millwood) 2011;30:2194–203. doi: 10.1377/hlthaff.2011.0509. [DOI] [PubMed] [Google Scholar]
- 26.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol. 2009;54:1695–702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert NM, Fonarow GC, Abraham WT, et al. Depression and clinical outcomes in heart failure: an OPTIMIZE-HF analysis. Am J Med. 2009;122:366–73. doi: 10.1016/j.amjmed.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 28.McNaughton C, Collins S, Kripalani S. Health literacy and patients with heart failure. JAMA. 2011;306:489–90. doi: 10.1001/jama.2011.1064. [author reply 490–481] [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Artalejo F, Guallar-Castillon P, Herrera MC, et al. Social network as a predictor of hospital readmission and mortality among older patients with heart failure. J Card Fail. 2006;12:621–7. doi: 10.1016/j.cardfail.2006.06.471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.