Abstract
MicroRNAs regulate post-transcriptomic landscape in many tumors including renal cell carcinoma. We have recently shown significantly increased expression of miR-21 in renal tumors and that this miRNA contributes to the proliferation of renal cancer cells in culture. However, the mechanism by which miR-21 regulates renal cancer cells proliferation is poorly understood. Addiction to constitutive NFκB activity is hallmark of many cancers including renal cancer. Using miR-21 Sponge in renal cancer cells to block endogenous function of miR-21, we show inhibition of phosphorylation of p65 subunit of NFκB, IKKβ and IκB, which results in attenuation of NFκB transcriptional activity. Subtle reduction in the tumor suppressor PTEN has been linked to various malignancies. We showed previously that miR-21 targeted PTEN in renal cancer cells. Inhibition of PTEN by siRNAs restored miR-21 Sponge-induced suppression of phosphorylation of p65, IKKβ, IκB and NFκB transcriptional activity along with reversal of miR-21 Sponge-reduced phosphorylation of Akt. Expression of constitutively active Akt protected against miR-21 Sponge- and PTEN-mediated decrease in p65/IKKβ/IκB phosphorylation and NFκB transcriptional activity. Furthermore, IKKβ and p65 were required for miR-21-induced renal cancer cell proliferation. Interestingly, miR-21 controlled the expression of cyclin D1 through NFκB-dependent transcription. Finally, we demonstrate that miR-21-regulated renal cancer cell proliferation is mediated by cyclin D1 and CDK4. Together, our results establish a molecular order of a phosphatase-kinase couple involving PTEN/Akt/IKKβ and NFκB-dependent cyclin D1 expression for renal carcinoma cell proliferation by increased miR-21 levels.
Keywords: microRNA, Renal cancer, PTEN, NFκB
1. INTRODUCTION
Renal cell carcinoma represents about 5% of all epithelial cancers and one of the 10 most common cancers. Patients with advanced disease have poor prognosis and overall survival. Morphologically renal tumors are classified into different subtypes; however, clear cell renal carcinoma is the most common and represents nearly 75% of all renal tumors [1]. Genetic analyses of the families with hereditary benign and malignant kidney cancer led to the identification of mutation in VHL gene located on chromosome 3p25. In fact, about 50% of sporadic renal tumors produce mutated VHL tumor suppressor protein due to intragenic mutation in one copy of the gene and loss of remaining copy in the other 3p arm [2]. Also, hypermethylation of the both parental and maternal copies of VHL allele can cause transcriptional silencing of this gene [3]. Examination of kidneys from VHL patients, who display pre-neoplastic lesions with sustained loss of VHL allele, shows that they often have few carcinomas [4]. These results suggest that VHL mutation may not be the sole cause of the disease. Indeed, deletion of VHL in mouse kidney does not cause renal cell carcinoma [5]. Oncogene amplification, constitutive activation of growth regulatory genes and loss/deregulation of tumor suppressor genes play significant role in the pathogenesis of renal cell carcinoma [6].
MicroRNAs act as rheostats to tune expression of genes by post-transcriptional mechanism. These short oligoribonucleotides are 19–22 nucleotides long and bind predominantly to the 3' untranslated region of target mRNAs by imperfect complementarity [7]. miRNAs block protein abundance essentially by inhibiting mRNA translation although mRNA cleavage may also contribute to the downregulation of proteins. They are synthesized as RNA polymerase II products in the nucleus. Drosha, an RNase III like enzyme, processes this primary transcript to produce pre-miRNA, which contains a stem-loop structure. Pre-miRNAs are then transported to the cytoplasm, where they are further processed by dicer to produce the ~ 22 nucleotide long double stranded RNAs. Finally, the guide strand, which represents the mature miRNA, associates with the Argonaute 2 in the RISC (RNA-induced silencing complex) and binds to the miRNA recognition element present in the 3' untranslated region of the target mRNA to induce translational repression [8].
Aberrant expression of miRNAs have been linked to the development of many diseases including different cancers. Profiling studies have demonstrated both enhanced and decreased expression of select miRNAs in urological cancers including renal tumors [9–11]. Interestingly, expression of the miRNA miR-34a is downregulated in both bladder and prostate cancers while its levels are increased in renal tumors [12], These results suggest tissue-specific roles of specific miRNA in cancer. Recently, a significant increase in the expression of miR-21 has been found in 31 different solid tumors suggesting a strong association of this miRNA with oncogenesis [13]. More recently, we have demonstrated enhanced expression of this miRNA in clear cell renal carcinoma [14], We showed that miR-21 contributes to the signal transduction pathways necessary for renal cancer cell proliferation and invasion. Cancer progression impacts key biological control points, including cell cycle entry. In the present study, we demonstrate a mechanism by which miR-21 regulates expression of an NFκB target gene, cyclin D1, to control renal cancer cell proliferation.
2. MATERIALS AND METHODS
2.1. Reagents
NP-40, Na3VO4, phenylmethylsulfonylfluoride and actin antibody were purchased from Sigma, St Louis, MO. Fugene HD was purchased from Roche Applied Science, Indianapolis, IN. Phospho-p65 (Ser-536), p65, phospho-IKKβ (Ser-180/181), IKKβ, phospho-IκB (Ser-32), IκB, phospho-Akt (Ser-473), phospho-Akt (Thr-308) and Akt antibodies were obtained from Cell Signaling, Boston, MA. PTEN and cyclin D1 antibodies and pool of siRNAs targeting PTEN mRNA were from Santa Cruz, Delaware, CA. Anti-HA antibody was obtained from Covance, Princeton, NJ. TRIzol reagent for preparation of total RNA was purchased from Invitrogen, Carlsbad, CA. RT2 real-time SYBR green/ROX PCR master mix, mature miR-21 detection primers and cyclin D1 primers were obtained from SA Biosciences, Frederick, MD. U6 primers were purchased from Ambion, Austin, TX. Luciferase assay kit was obtained from Promega, Madison, WI. Forward and Reverse primers for chromatin immunoprecipitation assay were made at The University of Texas Health Science Center at San Antonio Molecular Biology Core facility. pCDNA HA p65 and pCDNA HA p65 S536A plasmids were provided by Dr. H. Sakurai, University of Toyama, Japan. pCMV HA CDK4 expression vector was a kind gift from Dr. R. H. Medema, Netherlands Cancer Institute, Netherlands. Cyclin D1 HA pBabe-Hygro vector was purchased from Addgene and was constructed in Dr. P. Sicinski's laboratory. pCMV miR-21, pSGL HA PTEN, HA Myr Akt and miR-21 Sponge plasmids have been described previously [14, 15].
2.2. Cell culture
Human ACHN renal carcinoma cells were purchased from American Type Culture Collection, Manassas, VA. These cells were chosen as they are PTEN positive. The cells were grown in RPMI 1640 medium containing 10% fetal bovine serum as described previously [14].
2.3. Immunoblotting
The cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, 50 mM NaF, 0.1% protease inhibitor cocktail and 1% NP-40) for half an hour at 4°C. The cells were scraped and centrifuged at 10,000×g for 30 minutes at 4°C. The supernatant was collected and protein estimated using BioRad reagent. Equal amounts of cell lysates were separated by SDS polyacrylamide gel electrophoresis. The separated proteins were transferred to PVDF membrane and immunoblotted with the indicated antibodies as described previously [15, 16].
2.4. DNA synthesis and proliferation assay
Transfected cells were serum-starved for 24 hours and incubated with 3H-thymidine for 18 hours. 3H-thymidine incorporation was determined as a measure of DNA synthesis as described previously [14]. For proliferation assay, the transfected cells in serum-free medium were maintained for the indicated times. The time at which the cells were placed in serum-free medium was considered as zero time point. The cells were trypsinized and counted using a hemocytometer as described [14].
2.5. Real-time quantitative RT-PCR
Total RNAs were prepared using the TRIzol kit as described previously [16]. 1 μg of RNA was used to synthesize cDNA using the RT2 miRNA first strand kit according to manufacturer's protocol. For mature miR-21, qRT-PCR was performed using a real-time PCR machine (7500, Applied Biosystems). Each sample was analyzed in duplicate. The PCR conditions for cyclin D1 were: 94°C for 10 minutes followed by 40 cycles of 94°C for 30 seconds, 58°C for 30 seconds and 72°C for 30 seconds, respectively.
2.6. Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed using a kit. Transfected ACHN cells were used to prepare sheared chromatin as per vendor's protocol. Sheared chromatin was incubated with protein G-agarose beads to precipitate. This was used as input control. Similarly, sheared chromatins were incubated with Erk1 antibody (used as control) or p65 NFκB antibody to immunoprecipitate the p65-bound DNA fragment. DNA was eluted from the immunoprecipitates and PCR amplified with cyclin D1 promoter specific primers (Forward: CCGGGCTTTGATCTTTGCTTAAC; Reverse: TCGCTGCTACTGCGCCGAC) and analyzed by agarose gel electrophoresis. Also, we amplified the product in a real-time PCR machine. PCR condition was: 94°C for 10 minutes followed by 40 cycles of 94°C for 30 seconds, 58°C for 30 seconds and 72°C for 30 seconds respectively.
2.7. Transfection
The cells were split at a density of 80% confluency. After 18–24 hours of cell seeding, they were transfected with indicated plasmids or siRNAs using Fugene HD as described previously [15, 16].
2.8. Luciferase assay
Reporter-transfected cells were serum starved for 24 hours. The cell lysates were assayed for luciferase activity using the kit as described previously [15, 16]. The activity was corrected by total amounts of protein assayed. Mean ± SE for indicated measurements is shown.
2.9. Statistics
The significance of the data was analyzed by paired t-test or ANOVA followed by Student-Newman-Keuls analysis where necessary [15, 16]. A p value of less than 0.05 was considered as significant.
3. RESULTS
3.1. miR-21 regulates NFκB activation via PTEN in renal cancer cells
Enhanced expression of NFκB p65 subunit and its upstream regulator IKKβ has been linked to increased tumor grade, proliferation of tumor cells, invasion and metastasis of clear cell renal carcinoma [17]. In many cells phosphorylation of p65 subunit at Ser-536 often correlates with transcriptional activity of NFκB [18, 19]. To examine this in ACHN renal cancer cells, we used a plasmid containing the firefly luciferase gene driven by a basal promoter containing three copies of NFκB binding element, which reports activation of this transcription factor. Expression of a phosphorylation deficient mutant of p65 (p65 S536A) significantly inhibited the reporter activity (Supplementary Fig. S1). These results suggest that phosphorylation of NFκB is required for induction of its activation in renal cancer cells. We investigated the role of miR-21 in activation of NFκB in these cells. To block the function of endogenous miR-21, we used a plasmid vector (miR-21 Sponge) in which imperfect complementary sequences of mature miR-21 were cloned downstream of GFP cDNA (Supplementary Fig. S2A). Transfection of miR-21 Sponge did not have any gross effect on the morphology of ACHN renal cancer cells (Supplementary Figs. S2B, S2C and S2D). Expression of miR-21 Sponge significantly inhibited phosphorylation of p65 (Fig. 1A). GFP expression demonstrates successful expression of miR-21 in these cells (Supplementary Fig. S3A). Phosphorylation of IKKβ induces its activation and results in phosphorylation of IκB leading to activation of NFκB. Also, IKKβ has been shown to phosphorylate p65 [19]. Therefore, we tested the role of miR-21 in phosphorylation of IKKβ. Expression of miR-21 Sponge inhibited phosphorylation of IKKβ (Fig. 1B and Supplementary Fig. S3B). Consequently, phosphorylation of IκB was significantly suppressed (Fig. 1C and Supplementary Fig. S3C). Furthermore, miR-21 Sponge significantly inhibited NFκB-dependent reporter activity (Fig. 1D and Supplementary Fig. S3D). In contrast to these results, overexpression of miR-21 significantly increased the reporter activity (Fig. 1E and Supplementary Fig. S3E). These results indicate that miR-21 regulates activation of NFκB in renal cancer cells.
Figure 1.
miR-21 regulates activation of NFκB in renal cancer cells. (A –C) Expression of miR-21 Sponge inhibits phosphorylation of p65, IKKβ and IκB. ACHN renal carcinoma cells were transfected with miR-21 Sponge or vector plasmids as indicated. Equal amounts of cell lysates were immunoblotted with phospho-p65 (Ser-536), p65 (panel A), phospho-IKKβ (Ser-180/181), IKKβ (panel B), phospho-IκB (Ser-32) and IκB (panel C) and actin antibodies (panels A –C). (D and E) miR-21 regulates NFκB-dependent transcription. ACHN renal cancer cells were transfected with NFκB-Luc reporter plasmid along with miR-21 Sponge (panels D) or CMV miR-21 (panel E). The cell lysates were used to assay luciferase activity as described in the Experimental Procedures. Mean ± SE of triplicate (panel D) and quadruplicate (panel E) measurements is shown. *p = 0.001 for panel D and 0.006 for panel E. Expression of miR-21 Sponge for panels A,B, C and D is shown in Supplementary Fig. S3A – S3D. Expression of mature miR-21 for panel E is shown in Supplementary Fig. S3E. Quantification of panels A, B and C are shown at the bottom of each panel. Mean ± SE of triplicate measurements is shown. *p = 0.003, 0. 0005 and 0.0003 vs control for panel A, B and C respectively.
3.2. miR-21 targets PTEN to activate NFκB
The 3' UTR of PTEN mRNA has miR-21 recognition element [20]. We have recently shown that miR-21 targets PTEN mRNA to reduce its protein levels in renal cancer cells [14]. In order to investigate the mechanism of miR-21-induced activation of NFκB, we examined the role of PTEN. We transfected miR-21 Sponge along with a pool of siRNAs (siPTEN), which target PTEN mRNA, into renal cancer cells. Note that expression of miR-21 Sponge significantly increased levels of PTEN protein and siPTEN reduced this increase to basal level (Fig. 2A, quantification is shown in bottom right panel). Downregulation of PTEN prevented decrease in phosphorylation of p65 induced by miR-21 Sponge (Fig. 2A and Supplementary Fig. S4A). Similarly, inhibition of miR-21 Sponge-induced increase in PTEN by siPTEN significantly abrogated the miR-21 Sponge-induced reduction in phosphorylation of IKKβ (Fig. 2B and Supplementary Fig. S4B). Consequently, siTPEN reversed the decrease in phosphorylation of IκB induced by miR-21 Sponge (Fig. 2C and Supplementary Fig. S4C). Next, we determined the effect of siPTEN on activation of NFκB. As expected miR-21 Sponge inhibited the NFκB-dependent reporter activity (Fig. 2D). Expression of siPTEN significantly suppressed the inhibitory effect of miR-21 Sponge on the reporter activity (Fig. 2D and Supplementary Fig. S4D).
Figure 2.
PTEN regulates miR-21-induced activation of NFκB. (A – C) ACHN renal cancer cells were transfected with miR-21 Sponge, siRNAs against PTEN, vector or scrambled RNA as indicated. The cell lysates were immunoblotted with phospho-p65 (Ser-536), p65, phospho-IKKβ (Ser-180/181), IKKβ, phospho-IκB (Ser-32), IκB, PTEN and actin antibodies. (D) ACHN renal cancer cells were transfected with NFκB-Luc reporter along with miR-21 Sponge and siRNAs against PTEN or vector or scrambled RNA. Luciferase activity was determined as described previously. Mean ± SE of 6 measurements is shown. *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. Expression of miR-21 Sponge for panels A,B, C and D is shown in Supplementary Fig. S4A – S4D. Also, expression of PTEN for Fig. 2D is shown in Supplementary Fig. S4D. Quantification of results in panels A, B and C is shown at the bottom of each panel. Each bar represents the condition illustrated on the top of corresponding panels. Mean ± SE of 3 experiments is shown. In panel A left part, *p < 0.01 vs control; **p < 0.01 vs miR-21 Sponge. In panel A right part, *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. In panels B and C, for both left and right parts, *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge.
3.3. miR-21 regulates NFκB activation via PTEN-Akt axis
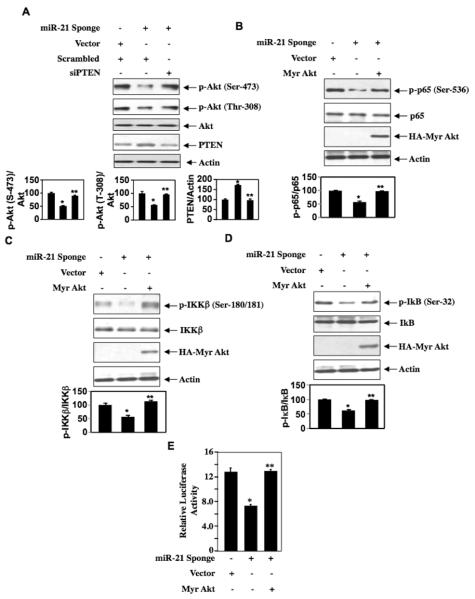
Our results demonstrate that miR-21 regulates activation of NFκB by downregulating PTEN. The lipid phosphatase activity of PTEN dephosphorylates the phosphatidylinositol 3,4,5-tris-phosphate, which increases the Akt kinase activity [21]. Therefore, we tested involvement of PTEN in Akt activation. Quenching of miR-21 levels by miR-21 Sponge in the renal cancer cells inhibited phosphorylation of Akt at both activating sites (Ser-473 and Thr-308; Fig. 3A). Downregulation of PTEN by siRNAs prevented miR-21 Sponge-induced suppression of phosphorylation of Akt (Fig. 3A and Supplementary Fig. S5A). Next, we determined the role of Akt in NFκB activation. Since miR-21 Sponge inhibited Akt phosphorylation, we used a plasmid expressing constitutively active Akt (Myr Akt). As expected, miR-21 Sponge inhibited the phosphorylation of p65 subunit of NFκB. This inhibition was reversed by expression of constitutively active Akt (Fig. 3B and Supplementary Fig. S5B). Similarly, expression of Myr Akt rescued inhibition of phosphorylation of IKKβ induced by miR-21 Sponge (Fig. 3C and Supplementary Fig. S5C). Identical results were obtained when we examined the phosphorylation of IKKβ substrate IκB (Fig. 3D and Supplementary Fig. S5D). To assess the functional consequences of these results, we examined the NFκB-dependent reporter activity. miR-21 Sponge-induced inhibition of reporter activity was significantly prevented by expression of Myr Akt (Fig. 3E and Supplementary Fig. S5E).
Figure 3.
miR-21 regulates NFκB activation through Akt kinase. (A) ACHN cells were transfected with miR-21 Sponge and siRNAs against PTEN or vector or scrambled RNA. The cell lysates were immunoblotted with phospho-Akt (Ser-473), phospho-Akt (Thr-308), Akt, PTEN and actin antibodies. (B –D) ACHN cells were transfected with miR-21 Sponge and Myr Akt or vector. The cell lysates were immunoblotted with phospho-p65 (Ser-536), p65, phospho-IKKβ (Ser-180/181), IKKβ, phospho-IκB (Ser-32), IκB, HA (to detect Myr Akt) and actin antibodies. For panels A – D, the quantification of the mean ± SE of 3 experiments is shown at the bottom of each panel. In panel A, *p < 0.01 vs control; **p < 0.001 vs miR-21 Sponge for all parts. For panel B and D, *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. For panel c, *p < 0.01 vs control; **p < 0.01 vs miR-21 Sponge. (E) ACHN cells were transfected with NFκB-Luc reporter along with miR-21 Sponge and Myr Akt or vector. Luciferase activity was determined as described previously. Mean ± SE of 6 measurements is shown. *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. Expression of Myr-Akt is shown in Supplementary Fig. S5E. Expression of miR-21 Sponge for all panels are shown in Supplementary Fig. S5A – S5E.
We have shown above that phosphorylation of Akt is enhanced in renal cancer cells possibly due to miR-21-forced reduced levels of PTEN. To confirm the role of PTEN in NFκB activation, we transfected wild type PTEN into renal cancer cells. Expression of PTEN inhibited phosphorylation of p65 (Fig. 4A). Co-expression of Myr Akt reversed this phosphorylation (Fig. 4A). Similarly, expression of Myr Akt rescued reduced phosphorylation of IKKβ and IκB by PTEN (Figs. 4B and 4C). Consequently, PTEN inhibited activation of NFκB as determined by the reporter assay, which was rescued by expression of Myr Akt (Fig. 4D and Supplementary Fig. S6). Collectively, these results demonstrate that miR-21 regulates activation of NFκB using PTEN-Akt axis.
Figure 4.
PTEN regulates activation of NFκB via Akt. (A – C) ACHN renal cancer cells were transfected with HA tagged PTEN and Myr Akt or vector as indicated. The cell lysates were immunoblotted with phospho-p65 (Ser-536), p65, phospho-IKKβ (Ser-177/181). IKKβ, phospho-IκB (Ser-), IκB, HA (to detect PTEN and Myr Akt) and actin antibodies. For panels A – C, the quantification of the mean ± SE of 3 experiments is shown at the bottom of each panel. In panel A, *p < 0.001 vs control; **p < 0.01 vs PTEN. For panel B and C, *p < 0.001 vs control; **p < 0.01 vs PTEN. (D) ACHN cells were transfected with NFκB-Luc reporter along with PTEN and Myr Akt or vector. Luciferase activity was determined as described previously. Mean ± SE of 4 measurements is shown. *p < 0.01 vs control; **p < 0.01 vs PTEN-transfected. Expression of HA-PTEN and HA-Myr Akt for Fig. 4D is shown in Supplementary Fig. S6.
3.4. miR-21 regulates proliferation of renal cancer cells via NFκB
Activation of NFκB has been correlated with development of renal tumors. We tested the role of NFκB in proliferation of renal cancer cells in culture. First, we examined the role of IKKβ, which activates NFκB. Expression of dominant negative IKKβ significantly decreased DNA synthesis in renal cancer cells (Fig. 5A and Supplementary Fig. S7A). Dominant negative IKKβ also inhibited proliferation of these cells (Fig. 5B and Supplementary Fig. S7B). Similarly, phospho-deficient mutant of p65 subunit of NFκB markedly inhibited DNA synthesis and proliferation (Figs. 5C, 5D and Supplementary Figs. S7C, S7D).
Figure 5.
NFκB controls miR-21-induced renal cancer cell proliferation. (A – D) ACHN cells were transfected with dominant negative IKKβ (panels A, B) and p65 S536A (C, D) or vector plasmids. 3H-Thymidine incorporation was determined as described in the Experimental Procedures (A and C). Mean ± SE of quadruplicate measurements is shown, p = 0. 0001 vs control. (B and D) Number of cells were counted at indicated time points as described in the Experimental Procedures. Mean ± SE of quadruplicate measurements is shown. In panel B, *p = 0.01 vs vector for 24 and 48 hours; *p = 0.001 vs vector for 72 hours. In panel D, *p = 0.003 vs vector for 24 hours; 0.002 vs vector for 48 and 72 hours. (E – H) ACHN cells were transfected with miR-21 Sponge and IKKβ (E and F) or miR-21 Sponge and p65 (G and H) or vector. 3H-Thymidine incorporation was determined as described in the Experimental Procedures (E and G). Mean ± SE of quadruplicate measurements is shown. *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. (F and H). Cells were counted at indicated time points as described in the Experimental Procedures. Mean ± SE of quadruplicate measurements is shown. *p < 0.01 vs vector; **p < 0.001 vs miR-21 Sponge. Expressions of dominant negative IKKβ, p65 S536A, wild type IKKβ, p65 and miR-21 Sponge are shown in Supplementary Fig. S7A – S7H.
We have shown previously that miR-21 contributes to proliferation of renal cancer cells [14]. We examined the involvement of NFκB in miR-21-induced renal cancer cell proliferation. As expected, miR-21 Sponge significantly inhibited DNA synthesis. Expression of wild type IKKβ markedly rescued miR-21 Sponge-induced suppression of DNA synthesis (Fig. 5E and Supplementary Fig. S7E). Similarly, inhibition of proliferation of renal cancer cells by miR-21 Sponge was reversed by expression of IKKβ (Fig. 5F and Supplementary Fig. S7F). Next, we directly tested the effect of NFκB. The results showed that expression of the p65 subunit of NFκB significantly prevented the suppression of DNA synthesis and renal cancer cell proliferation induced by miR-21 Sponge (Figs. 5G, 5H and Supplementary Figs. S7G, S7H).
3.5. miR-21 regulates cyclin D1 expression
G1 cyclins including cyclin D1 regulate progression of cells from G1 to S phase to initiate DNA synthesis. Since our results described above show that miR-21 controls DNA synthesis in renal cancer cells, we tested the role of this miRNA in cyclin D1 expression. Expression of miR-21 Sponge inhibited cyclin D1 protein levels in renal tumor cells (Fig. 6A and Supplementary Fig. S8A). Next, we examined the involvement of PTEN and Akt in this process. Downregulation of miR-21 Sponge-mediated increase in PTEN by siRNAs prevented miR-21 Sponge-induced inhibition of cyclin D1 expression (Fig. 6B and Supplementary Fig. S8B). Similarly, expression of constitutively active Myr Akt reversed the suppression of cyclin D1 induced by miR-21 Sponge (Fig. 6C and Supplementary Fig. S8C). Since miR-21 regulates IKKβ through PTEN-Akt, we investigated the role of this kinase in cyclin D1 expression. Expression of wild type IKKβ rescued the inhibition of cyclin D1 levels in response to miR-21 Sponge (Fig. 6D and Supplementary Fig. S8D). These results indicate that miR-21 may transcriptionally regulate cyclin D1 expression via NFκB pathway.
Figure 6.
miR-21 regulates cyclin D1 protein expression via PTEN-Akt-IKKβ axis. ACHN renal cancer cells were transfected with miR-21 Sponge (panel A) or miR-21 Sponge and siRNAs against PTEN (panel B) or miR-21 Sponge and Myr Akt (panel C) or miR-21 Sponge and wild type IKKβ (panel D) or vector as indicated. The cell lysates were immunoblotted with indicated antibodies. For panels A – D, the quantification of the mean ± SE of 3 experiments is shown at the bottom of each panel. In panel A, *p = 0.004 vs control. In panel B, *p < 0.01 vs control; **p < 0.001 vs miR-21 Sponge for let and right parts. In panel C, *p < 0.01 vs control; **p < 0.001 vs miR-21 Sponge. In panel D, *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. Expression of miR-21 Sponge for all panels is shown in Supplementary Fig. S8A – S8D.
To examine the role of miR-21 in transcription of cyclin D1, we first tested its role in expression of cyclin D1 mRNA. miR-21 Sponge significantly inhibited cyclin D1 mRNA expression (Fig. 7A and Supplementary Fig. S9A). Downregulation of PTEN as well as expression of constitutive active Akt reversed the inhibitory effect of miR-21 Sponge on expression of cyclin D1 mRNA (Figs. 7B, 7C and Supplementary Figs. S9B, S9C). Similarly, expression of wild type IKKβ significantly reversed miR-21 Sponge-induced inhibition of cyclin D1 mRNA expression in renal cancer cells (Fig. 7D and Supplementary Fig. S9D).
Figure 7.
miR-21 regulates expression of cyclin D1 mRNA via PTEN-Akt-IKKβ axis. ACHN renal cancer cells were transfected with miR-21 Sponge (panel A) or miR-21 Sponge and siRNAs against PTEN (panel B) or miR-21 Sponge and Myr Akt (panel C) or miR-21 Sponge and wild type IKKβ (panel D) or vector as indicated. Total RNAs were used to detect cyclin D1 mRNA as described in the Materials and Methods. The results were corrected by GAPDH mRNA. Mean ± SE of triplicate measurements is shown. For panel A, *p = 0.003 vs vector. For panel B, *p < 0.001 vs vector alone; **p < 0.001 vs miR-21 Sponge. For panel C, *p < 0.01 vs vector alone; **p < 0.01 vs miR-21 Sponge. For panel D, *p < 0.01 vs vector alone; **p < 0.01 vs miR-21 Sponge. Expression of miR-21 Sponge, PTEN, Myr Akt and IKKβ for all panels is shown in Supplementary Fig. S9A – S9D.
Previously it was shown that activation of NFκB results in cyclin D1 expression. In fact NFκB binding DNA element was identified in cyclin D1 promoter [22], In ACHN renal cancer cells, we tested whether endogenous NFκB occupies this site in the cyclin D1 promoter by performing ChIP assay. As shown in Fig. 8A, we detected physical association of p65 subunit of NFκB with the cyclin D1 promoter sequence in renal cancer cells. Next, we examined the role of miR-21 in binding of NFκB to the cyclin D1 promoter. The results show significant inhibition of endogenous p65 occupancy in the cyclin D1 promoter by miR-21 Sponge (Fig. 8B and Supplementary Fig. S10). These results conclusively demonstrate that NFκB controls miR-21-induced transcription of cyclin D1 in renal cancer cells.
Figure 8.
Detection of p65 NFκB in the cyclin D1 promoter. (A) Sheared chromatin from ACHN renal cancer cells were incubated with Erkl antibody (as control) or p65 antibody as described in the Materials and Methods. The bound DNA was eluted and amplified using cyclin D1 promoter-specific primers flanking NFκB binding element as described in the Materials and Methods. The amplified product was separated by agarose gel electrophoresis. Empty indicates an empty lane in agarose gel. (B) ACHN cells were transfected with miR-21 Sponge or Vector. Sheared chromatins were prepared and used in immunoprecipitation as described above in panel A except the amplification was performed using SYBR Green real time PCR as described in the Materials and Methods. Relative amount of bound p65 was calculated by the ratio of ChIPed DNA to input control DNA. Mean ± SE of triplicate measurements is shown. *p < 0.001 vs anti-p65 alone in vector-transfected cells. Expression of miR-21 Sponge for Fig. 8B is shown in Supplementary Fig. S10.
3.5. Cyclin D1 regulates proliferation of renal cancer cells
For cell cycle progression, cyclin D1 activates CDK4. Sequential phosphorylation of pRb by cyclin-dependent kinases including CDK4 regulates S-phase of cells [23], Cyclin D1/CDK4 complex phosphorylates pRb at Ser-795 residue to induce DNA synthesis [24], We directly determined the role of miR-21-regulated cyclin D1 in renal cancer cell proliferation. Expression of wild type cyclin D1 significantly recovered the miR-21 Sponge-induced inhibition of DNA synthesis and proliferation (Figs. 9A, 9B and Supplementary Figs. S11A, S11B). We showed above that inhibition of cyclin D1 by miR-21 Sponge results in attenuation of renal cancer cell proliferation. Since cyclin D1 activates CDK4, we tested the effect of this kinase on miR-21 Sponge-induced inhibition of proliferation of renal cancer cells. Expression of wild type CDK4 markedly prevented the inhibition of DNA synthesis and proliferation induced by miR-21 Sponge (Figs. 9C, 9D and Supplementary Figs. S11C, S11D). Collectively, these results suggest that miR-21 regulates cyclin D1/CDK4 activity to control renal cancer cell proliferation.
Figure 9.
Cyclin D1 and CDK4 regulate miR-21-induced renal cancer cell proliferation. ACHN cells were transfected with miR-21 Sponge and cyclin D1 or CDK4 as indicated. (A and C) 3H-thymidine incorporation was determined as a measure of DNA synthesis as described in the Materials and Methods. Mean ± SE of quadruplicate measurements is shown. *p < 0.001 vs control; **p < 0.001 vs miR-21 Sponge. (B and D) Cells were counted at indicated time points. Mean ± SE of quadruplicate measurements is shown. *p < 0.05 vs vector at 48 and 72 hours' **p < 0.001 vs miR-21 Sponge at 48 and 72 hours. For all panels expression of miR-21 Sponge, cyclin D1 and CDK4 are shown in Supplementary Fig. S11A – S11D.
4. DISCUSSION
miRNAs play an important role in regulating expression of genes via post-transcriptional mechanisms, which contribute to the development, progression and metastasis of cancer. miR-21 is ubiquitously expressed and has been shown to be upregulated in multiple cancers. It regulates proliferation and apoptosis of cells by controlling cell cycle regulators, tumor suppressor proteins and growth factors [25]. Abundant expression of miR-21 has been detected in kidney proximal tubules [26]. In rodent models of renal fibrosis including ureteral obstruction, ischemia reperfusion injury, glomerulonephritis, aging and diabetic nephropathy, significant upregulation of miR-21 has been reported [27]. We have recently shown increment of miR-21 in renal cells in response to hyperglycemia and pro-fibrotic growth factor TGFβ [14, 15]. Enhanced expression of miR-21 was found in clear cell renal carcinoma. In fact a weak reciprocal association was observed between miR-21 expression and survival of patients with renal cancer [28]. In the present study, we show a linear signaling pathway, which involves miR-21 regulation of PTEN-Akt-IKKβ axis for activation of NFκB, resulting in augmented cyclin D1 expression for sustained renal cancer cell proliferation (Fig. 10). Our current observations, coupled with our previous report that expression of miR-21 was mediated by NFκB [14], suggest that a positive feedback loop maintains a sustained level of miR-21 to perpetuate proliferation of renal cancer cells (Fig. 10).
Figure 10.
Schematic representing the results presented in the paper.
Activation of NFκB plays a significant role in inducing transcription of genes, which act in all steps of tumorigenesis [29]. Five family members of NFκB all of which contain a single Rel homology domain can form various hetero or homodimer combinations [30]. The canonical pathway involving TAK-1-mediated phosphorylation and activation of IKKβ present in the IKKα/IKKβ/IKKγ complex phosphorylates IκB complexed with the p65/p50 heterodimer, the predominant NFκB isoforms found in renal cancer. Phosphorylated IκB undergoes polyubiquitination at specific lysine residues by SCF-βTrCP E3 ubiquitine ligase and subsequently degraded by the 26S proteasome [31]. Free p65/p50 dimer then translocates to the nucleus to induce gene expression necessary for tumorigenesis. Also a role of IKKβ-mediated phosphorylation of p65 at Ser-536 has been shown to increase its binding to the transcriptional coactivators CBP/p300 [18, 19, 32]. In the present study, we show that neutralization of endogenous miR-21 in renal cancer cells blocks this phosphorylation indicating suppression of its transcriptional activity (Figs. 1A and 1D). Our results demonstrate that miR-21 regulates activating phosphorylation of IKKβ, which phosphorylates IκB (Figs. 1B and 1C). These results indicate that miR-21 controls events upstream of IKKβ.
In many cancers, mutation of PTEN gene has been identified. However, a previous study using 15 renal cell tumors showed no somatic mutation or homozygous deletion of PTEN in renal cancer [33]. In a recent study using primary and metastatic renal tumors mutations upto 11% has been identified in PTEN gene [34, 35]. However, inactivation of PTEN in cancer cells with wild type allele has been reported in many cancers. For example, reduced levels of immunoreactive PTEN were found in breast cancer samples where the oncogene DJ-1 was increased [36]. The E3 ubiquitin ligase NEDD4-1 inhibits PTEN in many cancer cells [37]. Also, the PTEN interacting protein P-REX2a inhibits its enzymatic activity [38]. Along with these mechanisms PTEN activity in cancer is often associated with epigenetic silencing, transcriptional repression, post-translational modification and aberrant localization [39]. This mode of reduced PTEN activity in cancer is of pivotal importance. In fact, 50% reduction in PTEN levels in a heterozygous mouse resulted in cancers of multiple organs suggesting that PTEN is a haploinsufficient gene [40]. However, a unique `hyper' mouse model containing a PTEN allele, which expresses 80% of normal PTEN mRNAs and protein showed many phenotypes of PTEN heterozygous mice including signs of cancer susceptibility suggestive of quasi-insufficiency of this tumor suppressor [41]. In fact, in a cohort of breast tumor patients, 20% of the tumors displayed 65% of PTEN levels compared to that found in normal tissues [41]. Interestingly, a geneset expression, which was found to be increased in the `hyper' mouse model of breast tumorigenesis, was also increased in the patients' tumor samples with reduced PTEN levels [42]. These results suggest that a subtle change in PTEN expression may be sufficient to contribute to changes in gene expression profile leading to tumorigenesis. In fact, a reduction in PTEN levels have been reported in 31% of renal tumors [43]. In this study, a strong correlation between grade/stage of renal cancer and reduced PTEN expression was observed. Also, the proliferative index, expression of Ki67, was significantly increased in the tumors with reduced PTEN expression [43]. These results indicate that aberrant PTEN levels may contribute to renal tumorigenesis.
Most recent addition to the spectrum of mechanisms of PTEN repression in many diseases including cancer is the post-transcriptional regulation by miRNAs [44]. Many miRNAs including miR-221-222, miR-205, miR-19a, miR-19b, miR-22, miR-25, miR-93 and miR-214 regulate expression of PTEN in multiple cancers [44]. Increased miR-21 in cancer directly targets mRNAs to decrease expression of many proteins, which contribute to tumorigenesis. We and others have validated PTEN as a target of miR-21, which has been shown to be upregulated in 31 different solid tumors and also in models of renal fibrosis [14, 15, 27]. Recently we have shown upregulation of miR-21 in renal tumors and in cultured renal cancer cells [14]. More recently, gene expression analysis of renal cancer showed a correlation between increased miR-21 expression and worst outcome [35]. Now we show that miR-21 regulates phosphorylation of IKKβ to increase NFκB activity by targeting PTEN in renal cancer cells (Fig. 2).
Increased level of PI 3 kinase product, phosphatidylinositol 3,4,5-tris-phosphate, results in activation of Akt kinase which contributes to cell proliferation and survival necessary for tumorigenesis. Although 3000 somatic mutations have been identified in the PI 3 kinase catalytic subunit (p110α), its mutation is rare in renal cancer [45]. A recent study demonstrated activating mutation in 3% of renal tumor samples [35]. Similarly, activating mutation of Akt has been found in various cancers [46]: however, it is also rare in renal cancer (2%) [35]. An alternative way of maintaining sustained PIP3 level in cells is decreased expression of PTEN, which dephosphorylates PIP3 resulting in reduced Akt activation [21]. In fact genetic experiment in Drosophila showed that loss of PTEN promotes translocation of Akt to the cell membrane to increase its kinase activity [47]. In the present study, we show that miR-21-targeted PTEN regulates Akt activity (Fig. 3A). Furthermore, we demonstrate a contribution of miR-21 in activating NFκB through Akt activation via PTEN (Figs. 3 and 4).
Initially a correlation was identified between activation of NFκB and, tumor grade and metastasis of renal cancer [48]. Recently Sourbier et al reported significant increase in levels of p65 and phosphorylated p65 in a cohort of patients with renal cell carcinoma [49]. Using renal tumor tissue, two more studies also showed increased expression of NFκB subunits that was associated with increased growth factors and anti-apoptotic proteins [50, 51]. Furthermore, a more recent study demonstrated that 70% of renal tumors possess activated NFκB [52]. Many renal cancer cells including the one used in this study are known to express increased levels of canonical NFκB subunits [48, 49, 53]. Additionally, nuclear localization of NFκB was observed in majority of the renal tumors, which was associated with rate of proliferation of tumor cells [51]. We show a correlation between increased miR-21 and phosphorylated active NFκB due to upregulated active IKKβ. We also show that that miR-21 Sponge-mediated inhibition of DNA synthesis and proliferation of renal cancer cells is due to activation of IKKβ and NFκB (Fig. 5).
Proliferation of cells is driven by G1 cyclin family of proteins. During G1/S transition, cyclin D1 promotes DNA synthesis [23]. Increased expression of cyclin D1 is found in many tumors in the absence of any detectable genetic alteration [54]. It occurs due to either amplification of its gene or impaired degradation. Overexpression of cyclin D1 is detected as the earliest change in human breast cancer [55]. This indicates that cyclin D1 might act as the driver for breast cancer progression. In fact, overexpression of cyclin D1 in mammary glands promotes mammary tumorigenesis [56]. Also, B-cell specific overexpression of cyclin D1 sensitized mice to develop B cell lymphomas [57]. Cyclin D1 deficient mice were resistant to ErbB2-induced breast adenocarcinomas and showed reduced skin cancer suggesting its role in tumorigenesis [58, 59]. Furthermore, cyclin D1 expression is necessary for maintenance of Erb2-driven tumors as cyclin D1 deleted mouse showed significantly reduced tumor cell proliferation [60]. Majority of tumors in renal cancer patients express significantly high levels of cyclin D1 [61–63]. However, gene amplification of cyclin D1 in renal cancer was not detected [63]. In the present study, in renal cancer cells we found appreciable expression of cyclin D1 protein, which was significantly inhibited by miR-21 sponge (Fig. 6A). We also found inhibition of cyclin D1 mRNA expression by miR-21 sponge, suggesting a transcriptional regulation. Furthermore, our results demonstrate that a PTEN-Akt-IKKβ axis contributes to the expression of cyclin D1 mRNA and protein in renal cancer cells (Figs. 6 and 7). Cyclin D1 is an NFκB-regulated gene and its promoter contains NFκB DNA binding element [22]. In renal cancer cells, we show direct binding of NFκB to the cyclin D1 promoter. Also our results for the first time show that quenching of endogenous miR-21 significantly blocked NFκB binding to the cyclin D1 promoter (Fig. 8). These results conclusively demonstrate that miR-21 contributes to transcriptional activation of cyclin D1 gene to increase its protein levels in renal cancer cells.
Mutation of retinoblastoma protein (pRb) or its inactivation has been detected in many cancers in which multiple mitotic genes are upregulated [64], However, both in primary renal tumors and in metastatic renal cell carcinomas, alterations in the pRb gene are extremely rare [65, 66], pRb is detected in the nucleus of renal tumors similar to that observed in normal kidneys [66], Functional inactivation of pRb occurs via phosphorylation by CDK4/6 complex, which initiates cell cycle progression [23], Increased cyclin D1 levels enhance the activity of CDK4/6 to stimulate cell cycle progression and DNA synthesis. In fact, micro injection of cells with CDK4-phosphorylated pRb induced cell cycle entry [67], Also, mice bearing ErbB2-driven breast tumors when treated with CDK4 inhibitor showed reduced tumor progression similar to that found with cyclin D1 deletion [60], As inactivation of pRb by phosphorylation is necessary for cell proliferation, our results further establish a significant role of miR-21-controlled contribution of cyclin D1 and CDK4 to DNA synthesis and proliferation of renal cancer cells (Fig. 9).
Kidney cancer represents 2% of all malignancies. Understanding of underlying genetic mechanisms and resulting signal transduction pathways have led to recent progress in targeted therapies, which include antibodies to growth factors, growth factor receptor tyrosine kinase inhibitors and mechanistic target of rapamycin inhibitors [68], Our results demonstrate a mechanism for renal cancer cell proliferation involving miR-21 and NFκB. These data provide therapeutic options, which may include combinatorial use of inhibitors of miR-21 and NFκB pathway for this devastating cancer.
Supplementary Material
Highlights
Our data for the first time document that miR-21-dependent activation of NFκB regulate renal cancer cell proliferation.
Interestingly, we find that miR-21 utilize PTEN tumor suppressor protein to activate NFκB and its activating kinase.
We demonstrate that miR-21 regulates transcription of cyclin D1.
We show that miR-21-regulated cyclin D1 and its associated CDK4 contribute to renal cancer cell proliferation.
Our results provide evidence for a novel mechanism where a signal transduction pathway involving miR-21/PTEN/Akt/NFκB/Cyclin D1/CDK4 regulates renal cancer cell proliferation.
ACKNOWLEDGEMENT
This work was supported partly by the VA Merit Review and NIH RO1 DK50190 grants to GGC. GGC is a recipient of VA Senior Research Career Scientist Award. NGC is supported by VA Merit Review grant and Cancer Therapy and Research Center at San Antonio Pilot Project grant. BSK is supported by grants from NIH RC2A 036613 and VA Research Service. HEA is supported by NIH RO1 DK 78971, VA Merit Review and Juvenile Diabetes Research Foundation grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Diaz JI, Mora LB, Hakam A. Cancer Control. 1999;6:571–579. doi: 10.1177/107327489900600603. [DOI] [PubMed] [Google Scholar]
- [2].Kim WY, Kaelin WG. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- [3].Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, Linhart D, Worrell RA, Moch H, Rubin MA, Sellers WR, Meyerson M, Linehan WM, Kaelin WG, Jr., Signoretti S. Cancer Res. 2009;69:4674–4681. doi: 10.1158/0008-5472.CAN-09-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Montani M, Heinimann K, von Teichman A, Rudolph T, Perren A, Moch H. Am J Surg Pathol. 2010;34:806–815. doi: 10.1097/PAS.0b013e3181ddf54d. [DOI] [PubMed] [Google Scholar]
- [5].Rankin EB, Tomaszewski JE, Haase VH. Cancer Res. 2006;66:2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li L, Kaelin WG., Jr. Hematol Oncol Clin North Am. 2011;25:667–686. doi: 10.1016/j.hoc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Couzin J. Science. 2008;319:1782–1784. doi: 10.1126/science.319.5871.1782. [DOI] [PubMed] [Google Scholar]
- [8].Fabian MR, Sonenberg N, Filipowicz W. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- [9].Huang Y, Dai Y, Yang J, Chen T, Yin Y, Tang M, Hu C, Zhang L. Eur J Surg Oncol. 2009;35:1119–1123. doi: 10.1016/j.ejso.2009.04.010. [DOI] [PubMed] [Google Scholar]
- [10].Juan D, Alexe G, Antes T, Liu H, Madabhushi A, Delisi C, Ganesan S, Bhanot G, Liou LS. Urology. 2010;75:835–841. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- [11].Petillo D, Kort EJ, Anema J, Furge KA, Yang XJ, Teh BT. Int J Oncol. 2009;35:109–114. doi: 10.3892/ijo_00000318. [DOI] [PubMed] [Google Scholar]
- [12].Fendler A, Stephan C, Yousef GM, Jung K. Clin Chem. 2011;57:954–968. doi: 10.1373/clinchem.2010.157727. [DOI] [PubMed] [Google Scholar]
- [13].Volinia S, Galasso M, Costinean S, Tagliavini L, Gamberoni G, Drusco A, Marchesini J, Mascellani N, Sana ME, Abu Jarour R, Desponts C, Teitell M, Baffa R, Aqeilan R, Iorio MV, Taccioli C, Garzon R, Di Leva G, Fabbri M, Catozzi M, Previati M, Ambs S, Palumbo T, Garofalo M, Veronese A, Bottom A, Gasparini P, Harris CC, Visone R, Pekarsky Y, de la Chapelle A, Bloomston M, Dillhoff M, Rassenti LZ, Kipps TJ, Huebner K, Pichiorri F, Lenze D, Cairo S, Buendia MA, Pineau P, Dejean A, Zanesi N, Rossi S, Calin GA, Liu CG, Palatini J, Negrini M, Vecchione A, Rosenberg A, Croce CM. Genome Res. 2010;20:589–599. doi: 10.1101/gr.098046.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dey N, Das F, Ghosh-Choudhury N, Mandal CC, Parekh DJ, Block K, Kasinath BS, Abboud HE, Choudhury GG. PLoS One. 2012;7:e37366. doi: 10.1371/journal.pone.0037366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dey N, Das F, Mariappan MM, Mandal CC, Ghosh-Choudhury N, Kasinath BS, Choudhury GG. J Biol Chem. 2011;286:25586–25603. doi: 10.1074/jbc.M110.208066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Das F, Ghosh-Choudhury N, Dey N, Mandal CC, Mahimainathan L, Kasinath BS, Abboud HE, Choudhury GG. J Biol Chem. 2012;287:3808–3822. doi: 10.1074/jbc.M111.246397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ozbek E, Aliskan T, Otunctemur A, Calik G, Cakir S, Dursun M, Somay A. Arch Ital Urol Androl. 2012;84:53–60. [PubMed] [Google Scholar]
- [18].Chen LF, Greene WC. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- [19].Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- [20].Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cully M, You H, Levine AJ, Mak TW. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- [22].Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sherr CJ. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- [24].Kim H, Jo C, Jang BG, Oh U, Jo SA. Cell Signal. 2008;20:120–129. doi: 10.1016/j.cellsig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [25].Krichevsky AM, Gabriely G. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saal S, Harvey SJ. Curr Opin Nephrol Hypertens. 2009;18:317–323. doi: 10.1097/MNH.0b013e32832c9da2. [DOI] [PubMed] [Google Scholar]
- [27].Kato M, Natarajan R. Semin Nephrol. 32:253–260. doi: 10.1016/j.semnephrol.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Neal CS, Michael MZ, Rawlings LH, Van der Hoek MB, Gleadle JM. BMC Med. 2010;8:64. doi: 10.1186/1741-7015-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Karin M. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- [30].Ghosh S, Hayden MS. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- [31].Skaug B, Jiang X, Chen ZJ. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- [32].Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- [33].Cairns P, Evron E, Okami K, Halachmi N, Esteller M, Herman JG, Bose S, Wang SI, Parsons R, Sidransky D. Oncogene. 1998;16:3215–3218. doi: 10.1038/sj.onc.1201855. [DOI] [PubMed] [Google Scholar]
- [34].Abou Youssif T, Fahmy MA, Koumakpayi IH, Ayala F, Al Marzooqi S, Chen G, Tamboli P, Squire J, Tanguay S, Sircar K. Cancer. 2011;117:290–300. doi: 10.1002/cncr.25402. [DOI] [PubMed] [Google Scholar]
- [35].T.C.G.A.R. Network Nature. 2013;499:43–49. [Google Scholar]
- [36].Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW. Cancer Cell. 2005;7:263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [37].Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fine B, Hodakoski C, Koujak S, Su T, Saal LH, Maurer M, Hopkins B, Keniry M, Sulis ML, Mense S, Hibshoosh H, Parsons R. Science. 2009;325:1261–1265. doi: 10.1126/science.1173569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Salmena L, Carracedo A, Pandolfi PP. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- [40].Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R. Proc Natl Acad Sci USA. 1999;96:1563–1568. doi: 10.1073/pnas.96.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP. Nat Genet. 2010;42:454–458. doi: 10.1038/ng.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carracedo A, Alimonti A, Pandolfi PP. Cancer Res. 2011;71:629–633. doi: 10.1158/0008-5472.CAN-10-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shin Lee J, Seok Kim H, Bok Kim Y, Cheol Lee M, Soo Park C. J Surg Oncol. 2003;84:166–172. doi: 10.1002/jso.10302. [DOI] [PubMed] [Google Scholar]
- [44].He L. Sci Signal. 2010;3:pe39. doi: 10.1126/scisignal.3146pe39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vadas O, Burke JE, Zhang X, Berndt A, Williams RL. Sci Signal. 2011;4 doi: 10.1126/scisignal.2002165. [DOI] [PubMed] [Google Scholar]
- [46].Castaneda CA, Cortes-Funes H, Gomez HL, Ciruelos EM. Cancer Metastasis Rev. 2010;29:751–759. doi: 10.1007/s10555-010-9261-0. [DOI] [PubMed] [Google Scholar]
- [47].Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Science. 2002;295:2088–2091. doi: 10.1126/science.1068094. [DOI] [PubMed] [Google Scholar]
- [48].Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Oncogene. 2001;20:3888–3896. doi: 10.1038/sj.onc.1204525. [DOI] [PubMed] [Google Scholar]
- [49].Sourbier C, Danilin S, Lindner V, Steger J, Rothhut S, Meyer N, Jacqmin D, Helwig JJ, Lang H, Massfelder T. Cancer Res. 2007;67:11668–11676. doi: 10.1158/0008-5472.CAN-07-0632. [DOI] [PubMed] [Google Scholar]
- [50].Meteoglu I, Erdogdu IH, Meydan N, Erkus M, Barutca S. J Exp Clin Cancer Res. 2008;27:53. doi: 10.1186/1756-9966-27-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Djordjevic G, Matusan-Ilijas K, Sinozic E, Damante G, Fabbro D, Grahovac B, Lucin K, Jonjic N. Croat Med J. 2008;49:608–617. doi: 10.3325/cmj.2008.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Matusan-Ilijas K, Damante G, Fabbro D, Dordevic G, Hadzisejdic I, Grahovac M, Marie I, Spanjol J, Grahovac B, Jonjic N, Lucin K. Pathol Res Pract. 2011;207:104–110. doi: 10.1016/j.prp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- [53].Steiner T, Junker U, Henzgen B, Nuske K, Durum SK, Schubert J. Eur Urol. 2001;39:478–483. doi: 10.1159/000052489. [DOI] [PubMed] [Google Scholar]
- [54].Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- [55].Weinstat-Saslow D, Merino MJ, Manrow RE, Lawrence JA, Bluth RF, Wittenbel KD, Simpson JF, Page DL, Steeg PS. Nat Med. 1995;1:1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- [56].Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- [57].Lovec H, Grzeschiczek A, Kowalski MB, Moroy T. EMBO J. 1994;13:3487–3495. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yu Q, Geng Y, Sicinski P. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- [59].Robles AI, Rodriguez-Puebla ML, Glick AB, Trempus C, Hansen L, Sicinski P, Tennant RW, Weinberg RA, Yuspa SH, Conti CJ. Genes Dev. 1998;12:2469–2474. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer H, Sicinski P. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Aaltomaa S, Lipponen P, Ala-Opas M, Eskelinen M, Syrjanen K, Kosma VM. Br J Cancer. 1999;80:2001–2007. doi: 10.1038/sj.bjc.6690634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hedberg Y, Davoodi E, Roos G, Ljungberg B, Landberg G. Int J Cancer. 1999;84:268–272. doi: 10.1002/(sici)1097-0215(19990621)84:3<268::aid-ijc12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- [63].Lin BT, Brynes RK, Gelb AB, McCourty A, Amin MB, Medeiros LJ. Mod Pathol. 1998;11:1075–1081. [PubMed] [Google Scholar]
- [64].Manning AL, Dyson NJ. Nat Rev Cancer. 2012;12:220–226. doi: 10.1038/nrc3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ishikawa J, Xu HJ, Hu SX, Yandell DW, Maeda S, Kamidono S, Benedict WF, Takahashi R. Cancer Res. 1991;51:5736–5743. [PubMed] [Google Scholar]
- [66].Hedberg Y, Ljungberg B, Roos G, Landberg G. Int J Cancer. 2004;109:189–193. doi: 10.1002/ijc.11665. [DOI] [PubMed] [Google Scholar]
- [67].Connell-Crowley L, Harper JW, Goodrich DW. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Garcia JA, Danielpour D. Mol Cancer Ther. 2008;7:1347–1354. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.