Abstract
Purpose of review
HIV-infected individuals are living longer due to effective antiretroviral therapy and may therefore have a greater opportunity to develop HPV-associated malignancies. This review describes the risk factors and burden of oral HPV infection and HPV-associated Head and Neck Cancer (HNC) among HIV-infected individuals.
Recent findings
Oral HPV infection is commonly detected in HIV-infected individuals and is elevated among those with a higher number of lifetime oral sexual partners, current tobacco use, and immunosuppression. There is limited data on the natural history of oral HPV, but initial studies suggest the majority of infections clear within two years. While HIV-infected individuals are at much higher risk of most HPV-associated cancers than the general population, studies suggest HIV-infected individuals have a more modest 1.5-4 fold greater risk for HPV-associated HNC.
Summary
HIV-infected individuals are living longer, have a high prevalence of oral HPV infection and have many of the currently determined risk factors for HPV-associated HNC.
Keywords: Head and Neck Cancer, HIV, oral HPV, risk factors
Introduction
Human Papillomavirus (HPV) infection, a commonly detected DNA virus widely known as the necessary cause of cervical cancer,[1] has been established as a major etiologic factor for head and neck cancer (HNC).[2] Research suggests that HIV-infected individuals are at higher risk for oral HPV infection and HPV-associated HNC.[3,4] In this article, we review the literature on the risk factors and burden of oral HPV and HPV-associated HNC among HIV-infected individuals, discuss cancer prevention possibilities, and suggest future research directions.
HPV and Head and Neck Cancer Overview
HNC is a heterogeneous group of cancers which includes cancer of the oral cavity, pharynx, and larynx, and is the sixth most common cancer worldwide with an annual incidence of over 400,000.[5] HPV is known to cause a subset of HNCs, with HPV-associated HNCs having distinct genetic, clinical, and epidemiological characteristics from HPV-unassociated HNCs.[6] HPV-associated HNCs represent approximately 25% of all HNCs in the general population,[7,8] and usually arise in the oropharynx, which includes the base of the tongue and the lingual and palatine tonsils.[9] These HPV-associated cancers are independently associated with sexual behavior including recent and lifetime number of oral sex partners.[6] In contrast, the majority of HPV-unassociated HNC occurs in the oral cavity and larynx and is primarily associated with tobacco and alcohol use.[6]
Control of the cell cycle is impacted in both HPV-associated and HPV-unassociated cancers. HPV-associated HNCs involves E6 and E7 oncogene expression which functionally inactivate tumor suppressor genes p53 and pRB, while HPV-unassociated HNCs mutationally inactivate p53 and p16 which can lead to unregulated cell growth.[10,11] HPV-associated HNCs, which now account for many of the HNCs diagnosed before age 60, have shown better response to chemotherapy and radiotherapy, and improved survival compared to HPV-unassociated HNCs. [12,13]
The incidence of HPV-associated HNC has increased in the general population of many developed countries over the past several decades.[14*,15] This increase could be explained by generational differences in sexual behavior or increased persistence or progression of oral HPV due to changes in co-factors. Despite this increasing trend of HPV-associated HNC, the natural history of oral HPV has been largely unexplored.
Oral HPV infection among HIV-infected individuals
Several cross-sectional studies have observed that HIV-infected individuals have a 2-3 fold higher odds of prevalent oral HPV infection compared to HIV-uninfected individuals, even after adjustment for sexual behavior and other relevant factors.[3,16**] Recent studies suggest HIV-infected individuals have an overall oral HPV DNA prevalence between 20% and 45% (in the alpha genus), and an oncogenic oral HPV DNA prevalence between 12% and 26%,(Table 1).[3,16**,17] HPV16, which causes more than 80% of HPV-associated oropharyngeal cancers,[7,18] is the most commonly detected oral HPV type in HIV-infected individuals with a prevalence around 2-6%.[16,19-21*] In contrast, a study utilizing a representative population of the United States (US) found that 7% of healthy adults have a detectable oral HPV infection, while about 1%have detectable oral HPV16.[22*] Estimates of oral HPV prevalence among HIV-infected individuals likely vary because of multiple factors including differences in sample collection, processing, number of HPV types tested, DNA detection methods, and the study participant characteristics (Table 1).
Table 1. Summary of results from studies reporting prevalence of oral HPV DNA among HIV-infected adults, contrasted with US general population results.
| Study | Description | Sample Size | Sample Method | Any HPV* | Oncogenic HPV& | HPV16 | Multiple types |
|---|---|---|---|---|---|---|---|
| Coutlee STD 1997[19] | US men and women | 201 | oral brush | 14% | 12% | 3.0% | 0.5% |
|
| |||||||
| Kreimer et al JID 2004[3] | US men and women | 190 | oral rinse/brush biopsy | 25% | 14% | --- | 5.8% |
|
| |||||||
| Cameron STD 2005[17] | US men and women | 98 | Saliva | 37% | 26% | 6.1% | 7.0% |
|
| |||||||
| Marais JMV 2008[23] | South African women | 33 | oral brush | 33% | 12% | 3.0% | 9.1% |
|
| |||||||
| Richter JOPM 2008[24] | South African women | 30 | oral brush | 20% | 7% | 0.0% | 6.7% |
|
| |||||||
| Fakhry Plos One 2010∼[25] | US men and women | 112 | oral rinse | 45% | 26% | 5.9% | --- |
|
| |||||||
| Parisi BMCID 2011[26] | Italian MSM | 166 | oral swab | 20% | 1.5% | 0.8% | --- |
|
| |||||||
| Beachler et al CEBP 2012[16**] | US MSM and women | 379 | oral rinse | 40% | 21% | 6.1% | 19% |
|
| |||||||
| Read et al Plos One 2012[20*] | Australian MSM | 249 | oral rinse/brush | 19% | 8% | 4.4% | 7.2% |
|
| |||||||
| Del Mistro STD 2012[27*] | Italian men and women | 100 | Saliva | 37% | 13% | 3.0% | 6.0% |
|
| |||||||
| Steinau et al JOPM 2012[28**] | US men and women | 100 | oral rinse | 39% | 24% | 3.0% | 17% |
|
| |||||||
| Fatahzadeh et al OOOOE 2013 [29*] | US men and women | 52 | oral rinse | 38% | 23% | 6.0% | --- |
|
| |||||||
| Videla et al STD 2013[21**] | Spanish men | 650 | oral brush/rinse | 16% | 15% | 5.2% | 3.8% |
|
| |||||||
| Beachler et al JID 2013[30*] | US men and women | 404 | oral rinse | 28% | 13% | 2.3% | 11% |
|
| |||||||
| ------ | HIV+ Summaryˆ | 2764 | ----- | 26%ˆ | 15%ˆ | 4.2%ˆ | 8.5%ˆ |
| Gillison et al JAMA 2012[22*] | US General Population+ | 5501 | oral rinse | 7% | 3.7% | 1.0% | --- |
Number of total alpha HPV types tested varied from 9 (Coutlee 1997) to 47 (Parisi 2011) although untyped genotypes were included in the prevalence estimates for several studies (Coutlee 1997, Cameron 2005, Del Mistro 2012). Cutaneous HPV types were not included in this summary.
Number of oncogenic types varied from 7 (Coutlee 1997) to 22 (Kreimer 2004, Marais 2008) while most considered 13-14 types to be oncogenic
Average point prevalences reported
Pooled oral HPV prevalence summary includes studies with differences in sample collection, processing, number of HPV types tested, DNA detection methods, and the study participant characteristics
Data from a representative sample of the US population, from the National Health and Nutrition Examination Survey (NHANES)
The natural history of oral HPV has been largely uninvestigated. Preliminary studies suggest that similar to anogenitial HPV, a substantial proportion of oral HPV infections clear within one or two years.[21*,23*,24] In fact, oral HPV has been observed to clear modestly faster than anal HPV,[21*,23*] potentially due to local mucosal immunity differences in the anatomic sites. Oral HPV infection has also been observed to be intermittently detected among HIV-infected individuals who are recently sexually abstinent[23*] suggesting that oral HPV may be variably expressed or re-expressed from a prior latent state similar to anogenital HPV.[25]
Head and Neck Cancer in HIV-infected individuals
The risks of several different cancer types are elevated in HIV-infected individuals due to behavioral and biological characteristics, immunodeficiency, and potentially chronic inflammation and immune dysfunction/senescence.[26*] Indeed, HIV-infected individuals in developed countries are at modestly increased risk of both HPV-associated and HPV-unassociated HNCs compared to the general population. AIDS-cancer registry match studies have found that the standardized incidence ratios (SIRs) for HNC is between 1.5 and 4 fold higher among HIV-infected individuals compared with the general population (Table 2).[4,27-29] HIV-infected individuals are also at increased risk of laryngeal, oral cavity and other HPV-unassociated HNCs,[4] which is likely due to their high prevalence of tobacco use,[30*] the strongest risk factor for these cancers.[31] While these registry match studies are not adjusted for various potential confounders other than age, one study which did control for risk factors including tobacco and alcohol use recently found a non-significant risk of oral cavity/pharynx cancer overall comparing HIV-infected and HIV-uninfected individuals in California (aRR=1.4, 95%CI=0.9-2.1).[32**]
Table 2. Increased risk of Head and Neck Cancer comparing HIV-infectedindividuals with the general population.
| Study | Study Population | Type of Cancer | Standardized Incidence Ratios (SIRs) and (95%CIs) | |
|---|---|---|---|---|
| Overall | HIV-Transmission Subgroup# | |||
| Shiels et al. JAIDS 2009[4]& | Meta-analysis of developed countries (1980-2007) | Head and Neck | 2.0 (1.1-3.6) | --- |
| Simard et al. AIM 2010[34]ˆ | United States (1996-2006) | Oral Cavity and Pharynx | 1.8 (1.5-2.0) | --- |
| Silverberg et al. CEBP 2011[35**] | United States (1996-2008) | Oral Cavity and Pharynx | aRR*=1.4 (0.9-2.1) | --- |
| Shiels et al. JAIDS 2009[4]& | Meta-analysis of developed countries (1980-2007) | Oropharyngeal | 1.9 (1.2-2.5) | --- |
| Chatervedi et al. JNCI 2009[36] | United States (1980-2004) | Oropharyngeal | 1.6 (1.2-2.1) | MSM: 1.1 (0.7-1.8), IDU: 2.1 (1.3-3.2), Hetero:3.2 (1.6-5.7) |
| Clifford et al. JNCI 2005[37] | Switzerland (1985-2002) | Lip, Oral Cavity and Pharynx | 4.1 (2.1-7.4) | MSM: 2.0 (0.4-5.8), IDU: 13.7 (4.9-30.1), Hetero: 2.9 (0.3-10.5) |
| Frisch et al. JNCI 2000[38]ˆ | United States (1987-1996) | Tonsillar | 2.6 (1.8-3.8) | Hetero Men: 5.3 (1.1-15.4) |
Meta-analysis included three studies considering oropharynx cancers and four studies exploring head and neck cancer.
Both studies used data from the US HIV/AIDS Cancer match study
Relative Risk based on an observational study controlling for potential risk factors such as tobacco and alcohol use
MSM= Men-who-have-sex-with-men, IDU=Injection drug user, Hetero=Heterosexual
While most studies have considered all HNC sites together, several registry-match studies attempting to approximate the risk of HPV-associated HNC have explored cancer risk in specific anatomical subsites and found that HIV-infected individuals have a 1.5-4 fold higher risk of oropharyngeal or tonsillar cancer compared to the general population (Table 2).[4,27,33,34] While estimates suggest that half or more of oropharyngeal cancers are HPV-positive in the general population,[14**,15] the proportion of HPV-positive oropharyngeal tumors in HIV-infected individuals is unknown. While their exact level of risk is unclear, HIV-infected individuals appear to be at moderately increased risk of HPV-associated HNC compared to the general population based on the modestly higher oropharyngeal cancer incidence. Interestingly, the magnitude of this increase (SIR∼1.5-4) is lower than other HPV-associated cancers such as anal, cervical, vulvar, and penile cancer which each have SIRs≥5.[4,27]
Impact of Sexual Behavior
An increased number of oral sexual partners is a risk factor for both oral HPV infection[16,35] and HPV-associated HNC.[6] Given the high number of lifetime sexual partners among many HIV-infected individuals,[16**,36] one might expect the incidence of HPV-associated HNC among HIV-infected individuals to be higher than what is currently observed; particularly among the most sexual active groups such as men who have sex with men (MSM).[16**,37] However, several registry based studies have found a non-significantly lower incidence of oropharyngeal cancer in HIV-infected MSM compared to HIV-infected injection drug users and heterosexual men (Table 2).[27,28,34] One potential explanation for this relatively modest HNC incidence among HIV-infected MSM is that the probability of acquiring oral HPV from performing oral sex on a man (fellatio) may be lower than when performing oral sex on a woman (cunnilingus). Indeed, a recent study we performed involving HIV-infected individuals suggests that heterosexual males have a higher incidence of oral HPV infection compared to MSM and heterosexual females after adjusting for relevant factors such as number of sexual partners.[23*] Two large studies also recently suggested that oral HPV DNA prevalence[22*] and HPV-associated oropharyngeal cancer[14*] are considerable higher in males compared to females in the US general population.
There are at least two hypotheses on why oral HPV may be more transmissible when performing oral sex on women. One hypothesis is that the female genital region may have a greater HPV viral load than the male genitals [38-41] despite having similar genital HPV DNA prevalences,[42-44] and this higher viral load in the female genitals could increase the likelihood of oral HPV acquisition. In contrast, a second hypothesis suggests that the keratinized epithelium from male genitals may be less likely to induce an immune (antibody) response than mucosal surfaces such as the cervix or the anal canal.[45,46*] Thus, the high level of natural antibodies developed after a cervical or anal HPV infection in women and MSM might conceivably be more likely to protect them from acquiring subsequent oral HPV infections.[45,47-49*] Further investigation is necessary to explore these hypotheses.
These hypotheses coupled with the demographics of the HIV-epidemic in developed countries may in part explain the moderate risk of HPV-associated HNC seen in the HIV-infected population. In the US, approximately half of HIV-infected individuals are MSM, while a little over a quarter are women (who are almost all heterosexual).[50] This suggests that the proportion of heterosexual males (who perform oral sex on women) is lower among the HIV-infected population than the general population. If the risk of acquiring oral HPV infection is truly highest among heterosexual males, then this could help explain the relatively moderate risk of HPV-associated HNC (compared to other HPV-associated cancers) seen in HIV-infected individuals in developed countries.
Impact of Immunosuppression and Antiretroviral Therapy (ART) Use
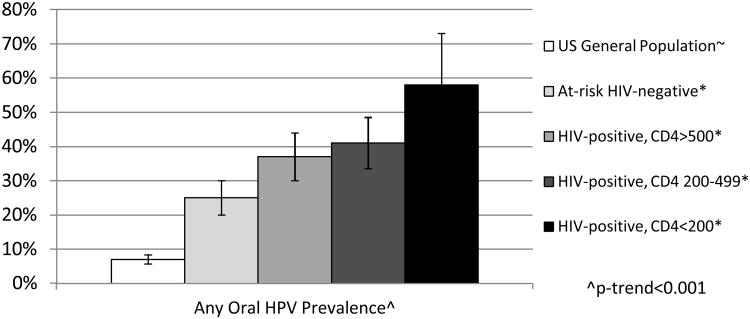
HIV-related immunosuppression may be a strong risk factor for oral HPV incidence or persistence given the 2-3 times higher adjusted odds of oral HPV prevalence in HIV-infected individuals compared to HIV-uninfected individuals.[3,16**] Advanced stage of HIV disease, characterized by low CD4 T cell count and high HIV viral load, has also been associated with increased oral HPV prevalence which may reflect a loss of viral control in those with compromised immune systems (Figure 1).[3,16**]
Figure 1. Impact of immunosuppression on oral HPV prevalence.
∼Data from a representative sample of the US population, from the National Health and Nutrition Examination Survey (NHANES)[22*]
*Data from the Multicenter AIDS Cohort Study (MACS) and Women's Interagency HIV Study(WIHS),[16**] HIV-uninfected individuals in this study were are at-risk for oral HPV due to their higher number of sexual partners and higher use of tobacco
The direct effect of immunosuppression on oral HPV persistence and HPV-associated HNC is currently less understood, but research on other HPV-associated cancers suggest immunosuppression may act more on the earlier stages of the HPV carcinogenesis process.[34,51] Oral cavity/pharynx cancer is elevated among both HIV-infected individuals and solid organ transplant recipients (another immunosuppressed population) suggesting a potential link between immunosuppression and HPV-associated HNC.[52] In addition, three studies have found that the incidence of oral cavity/pharynx cancer was non-significantly higher among those with a reduced CD4 T cell count,[28,32**,53] with Engels et al also finding a higher risk of oral cavity/pharynx cancer in individuals with AIDS relative to HIV-infected individuals who have not developed AIDS.[53] However, another recent study suggested reduced CD4 at AIDS diagnosis was associated with a reduced risk of oropharyngeal cancer among patients 28-60 months after AIDS offset.[27] One explanation for this heterogeneity in results could be from a higher proportion of HPV-unassociated HNCs in certain populations, as HPV tumor status has not been explored and HPV-associated and unassociated HNC might be differentially related to immunosuppression. These registry based studies also lack detailed covariate information such as sexual behavior and smoking status and cannot comprehensively evaluate the effect of cumulative and recent immunosuppression.
Effective antiretroviral therapy (ART, also known as HAART) has greatly improved the life expectancy of HIV-infected individuals while reducing viral-related malignancies such as Kaposi Sarcoma and Non-Hodgkin's lymphoma.[54] However, the incidence rates of HPV-associated malignancies have remained stable in the ART era, or have increased in the case of anal cancer. A preliminary study suggested ART use was associated with increased six month oral HPV persistence,[55] and other studies have suggested ART use is associated with an increase in oral lesions/warts.[56,57*] However, these studies may be prone to confounding by indication, as ART is more likely to be indicated for sicker individuals.
The role of ART on cervical HPV and related squamous epithelial lesions (SILs) has been more extensively explored with the majority of well-designed studies suggesting a benefit.[58-60] While some of the initial studies suggested a similar cervical HPV persistence and progression of SILs comparing ART users and non-users,[61,62] more recent reports suggest ART reduces the incidence of cervical HPV,[59] decreases the incidence of squamous epithelial lesions[58,60] and increases the regression of these lesions.[58,59] However, if ART use does not fully recover oral HPV-specific immunity it may not be able to substantially modify the elevated oral HPV incidence or persistence seen in HIV-infected individuals. Therefore, HPV-associated HNC could pose a further increasing threat for immune-competent HIV-infected individuals, if ART improves survival but did not improve control of oral HPV infections.
Tobacco use and other co-factors for HPV-associated HNC
Although HPV-associated HNC has often been described as a cancer among non-smokers and non-drinkers, there is growing evidence that tobacco may play a substantial role in the development of some of these cancers.[63] Tobacco use is an established risk factor for cervical cancer,[64] and is associated with oral HPV prevalence [22*,35,65,66] and six month oral HPV persistence[67] in HIV-infected and HIV-uninfected individuals. Other studies have shown tobacco use can reduce the innate and cell-mediated immunity at the systemic level and in the local oral region[68,69], suggesting an immunosuppressive effect of tobacco.
The direct role of tobacco on HPV-associated HNCs is less clear as several studies have found an association,[18,70-72] while others have not.[6,73] This question is particularly important for HIV-infected individuals, as the prevalence of tobacco use in this population is considerable higher than the general population with estimates suggesting 40-70% of HIV-infected individuals in developed countries may be current smokers.[30,74]
There are several other factors being investigated that may increase the risk of HPV-associated HNC including marijuana and alcohol use. While there is a lack of data on these factors in HIV-infected individuals, epidemiologic studies among HIV-uninfected individuals have found inconsistent results between risk of HPV-associated HNC and both marijuana [6,75-77] and alcohol use [6,70] as some have observed an association and others have not.
Future steps and challenges
While prevention of HPV-associated HNC is a developing research area, screening for HNC remains a challenge. A recent study found that feasibility of an oral Pap smear equivalent remains poor in HIV-infected individuals, as the limited number of cytopathologic abnormalities observed were not associated with HPV16.[78**] Fakhry et al suggest that this potential screening tool may not be feasible due to anatomic sampling limitations and the relatively low incidence of HNC.[78**]
Although prophylactic vaccination is an effective tool to prevent other HPV-associated cancers, its efficacy in preventing HPV-associated HNC among HIV-infected individuals has not yet been evaluated. The recently developed HPV vaccines likely have the potential to protect against HPV-associated HNC, considering they include prevention of HPV16 which accounts for over 80% of HPV-associated HNCs.[7,8,18] While rigorous efficacy studies have not been performed, initial observational data suggest the vaccine may provide protection against prevalent oral HPV16 and HPV18 in young HIV-uninfected women.[79] The quadrivalent vaccine has been shown to be safe and immunogenic among HIV-infected individuals,[80,81] and is recommend by the American Council on Immunization Practices for HIV-infected individuals aged 11-26.[82] Efficacy studies for anogenital HPV are currently being performed in older HIV-infected individuals.[83*]
There are still several other unknowns regarding oral HPV infection and HPV-associated HNC among HIV-infected individuals. First, the proportion of HNCs cancers caused by HPV among HIV-infected individuals is currently unknown and would help to understand its etiology and identify who is at the greatest risk for this disease. In addition, it is not clear what factors increase the risk of oral HPV incidence, persistence and progression to subsequent HNC. The relative effects of HIV, reduced immunity, tobacco use, sexual behavior and other factors on the natural history of oral HPV are still not well understood. Long term longitudinal studies are needed to further explore these factors and how they differ between HIV-infected and HIV-uninfected individuals.
Conclusion
HIV-infected individuals are living longer and therefore may have the opportunity to acquire more slowly developing HPV-associated malignancies. Indeed, HIV-infected individuals currently appear to have a moderately increased risk of HPV-associated HNC, which might potentially increase as this population ages. In addition, HIV-infected individuals have many of the potential risk factors for this disease (immunosuppression, increased sexual partners, tobacco use) and thus should be further studied and considered for future potential preventative measures.
Key points.
HIV-infected individuals are at increased risk for all HPV-associated cancers, including HPV-associated head and neck cancercompared to the general population.
Oral HPV infection is commonly detected in HIV-infected individuals, but the vast majority of infections appear to clear or be controlled within two years.
Risk factors for oral HPV infection include immunosuppression, increased number of oral sexual partners and tobacco use.
HIV-infected heterosexual males may be at higher risk of oral HPV and subsequent HPV-associated HNCcompared to heterosexual women and men who have sex with men with similar number of sexual partners.
Acknowledgments
Disclosure of funding: The NIH related research grant related to this article is R01DE021395. Daniel Beachler is supported on the T32 Pre-doctoral Cancer Epidemiology Training Grant (T32 CA CA009314).
GD has research funding from Merck Inc.
Footnotes
Conflicts of Interest: DCB has no conflicts.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Human Papillomaviruses. IARC Monographs on the Evolution of Carcinogenic Risks to Humans. 2007:1–670. [PMC free article] [PubMed] [Google Scholar]
- 3.Kreimer AR, Alberg AJ, Daniel R, et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J Infect Dis. 2004;189:686–98. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 4.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 7.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–54. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Gillison M, Lowy D. A causal role for human papillomavirus in head and neck cancer. The Lancet. 2004;363:1488–1489. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 10.Munger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Psyrri A, Dimaio D. Human papillomavirus in cervical and head-and-neck cancer. Nat Clin Pract Oncol. 2008;5:24–31. doi: 10.1038/ncponc0984. [DOI] [PubMed] [Google Scholar]
- 12.Fakhry C, Westra WH, Li S, et al. Improved Survival of Patients With Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J Natl Cancer Inst. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. This study estimated the incidence of HPV-associated cancer by testing banked oropharyngeal cancers collected from HIV-uninfected individuals in the United States. They found the incidence of HPV-positive oropharyngeal cancer increased by 225% between 1998 and 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 16**.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122–133. doi: 10.1158/1055-9965.EPI-11-0734. This cross-sectional study found the adjusted odds of prevalant oral HPV infection is two fold higher among HIV-infected individuals compared to HIV-uninfected individuals. Risk factors for prevalent oral HPV infection included higher number of lifetime oral sex partners and reduced CD4 T cell count. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cameron JE, Mercante D, O'Brien M, et al. The impact of highly active antiretroviral therapy and immunodeficiency on human papillomavirus infection of the oral cavity of human immunodeficiency virus-seropositive adults. Sex Transm Dis. 2005;32:703–709. doi: 10.1097/01.olq.0000175398.34610.2e. [DOI] [PubMed] [Google Scholar]
- 18.Herrero R. Human Papillomavirus and Oral Cancer: The International Agency for Research on Cancer Multicenter Study. Cancer Spectrum Knowledge Environment. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 19.Coutlee F, Trottier AM, Ghattas G, et al. Risk factors for oral human papillomavirus in adults infected and not infected with human immunodeficiency virus. Sex Transm Dis. 1997;24:23–31. doi: 10.1097/00007435-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 20*.Read TR, Hocking JS, Vodstrcil LA, et al. Oral human papillomavirus in men having sex with men: risk-factors and sampling. PLoS One. 2012;7:e49324. doi: 10.1371/journal.pone.0049324. This cross-sectional study from Australia found that HIV-infected MSM have a higher prevalence of oral HPV than HIV-uninfected MSM. They found the oral rinse was the most sensitive sampling technique and risk factors for prevalent oral HPV included higher number of sex patners, current smoking, and recent tooth brushing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Videla S, Darwich L, Canadas MP, et al. Natural History of Human Papillomavirus Infections Involving Anal, Penile, and Oral Sites Among HIV-Positive Men. Sex Transm Dis. 2013;40:3–10. doi: 10.1097/OLQ.0b013e31827e87bd. This longitudinal study of HIV-infected men from Spain compared the natural history of anal, oral, penile HPV infection. They found the incidence of oral HPV was lower than anal and penile infections, and 44% of oral HPV infections cleared during their study compared to 30% and 56% of anal HPV infection and penile infections, respectively. [DOI] [PubMed] [Google Scholar]
- 22*.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. This cross sectional study from the National Health and Nutrition Examination Survey (NHANES), a representative sample of the US population, found an oral HPV prevalence of 7% with a oral HPV16 prevalence of 1%. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Beachler DC, D'Souza G, Sugar EA, et al. Differences in Oral and Anal HPV Natural History among HIV-infected Individuals abstract; Proceedings of the 28th International Papillomavirus Conference; Epidemiology/Public Health; 2012. p. 213. This longitudinal study described the natural history of oral HPV infection in HIV-infected individuals. They found that the majority of oral HPV infections do not persist for longer than two years, and that the incidence and persistance of oral HPV was lower than anal HPV. [Google Scholar]
- 24.Mooij S, Speksnijder A, King A, et al. Six-month incidence, clearance and persistence of oral high risk HPV infection in HIV-negative and HIV-infected men who have sex with men abstract; Proceedings of the 28th International Papillomavirus Conference; Epidemiology/Public Health; 2012. p. 198. [Google Scholar]
- 25.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–4599. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Dubrow R, Silverberg MJ, Park LS, et al. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24:000. doi: 10.1097/CCO.0b013e328355e131. This review article summarizes cancer risk among HIV-infected individuals, and provides biological hypotheses for the increased risk of several cancers seen in this population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 29.Simard EP, Pfeiffer RM, Engels EA. Spectrum of Cancer Risk Late After AIDS Onset in the United States Original Investigation. Arch Intern Med. 2010;170:1337–1345. doi: 10.1001/archinternmed.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirk GD, Merlo CA Lung HIV Study. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc. 2011;8:326–332. doi: 10.1513/pats.201009-061WR. This review article provides a summary of tobacco usage among HIV-infected individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubin JH, Purdue M, Kelsey K, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170:937–947. doi: 10.1093/aje/kwp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2551–2559. doi: 10.1158/1055-9965.EPI-11-0777. This California based study found a relative risk of 1.4 (95%=0.9-2.1) of oral cavity/pharynx cancer when comparing HIV-infected to HIV-uninfected individuals and controlling for various risk factors including tobacco and alcohol use. They also found a non-significantly higher incidence of oral cavity/pharynx cancer among immunosuppressed individuals (p=0.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 34.Frisch M. Human Papillomavirus-Associated Cancers in Patients With Human Immunodeficiency Virus Infection and Acquired Immunodeficiency Syndrome. J Natl Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 35.D'Souza G, Agrawal Y, H J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus (HPV) infection. Journal of Infectious Disease. 2009;199:1–7. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aidala AA, Lee G, Garbers S, Chiasson MA. Sexual behaviors and sexual risk in a prospective cohort of HIV-positive men and women in New York City, 1994-2002: implications for prevention. AIDS Educ Prev. 2006;18:12–32. doi: 10.1521/aeap.2006.18.1.12. [DOI] [PubMed] [Google Scholar]
- 37.Elford J. Changing patterns of sexual behaviour in the era of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19:26–32. doi: 10.1097/01.qco.0000199018.50451.e1. [DOI] [PubMed] [Google Scholar]
- 38.Flores R, Papenfuss M, Klimecki WT, Giuliano AR. Cross-sectional analysis of oncogenic HPV viral load and cervical intraepithelial neoplasia. Int J Cancer. 2006;118:1187–1193. doi: 10.1002/ijc.21477. [DOI] [PubMed] [Google Scholar]
- 39.Flores R, Lu B, Nielson C, et al. Correlates of human papillomavirus viral load with infection site in asymptomatic men. Cancer Epidemiol Biomarkers Prev. 2008;17:3573–3576. doi: 10.1158/1055-9965.EPI-08-0467. [DOI] [PubMed] [Google Scholar]
- 40.Bleeker MC, Hogewoning CJ, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis. 2005;41:612–620. doi: 10.1086/431978. [DOI] [PubMed] [Google Scholar]
- 41.Tamalet C, Richet H, Carcopino X, et al. Testing for human papillomavirus and measurement of viral load of HPV 16 and 18 in self-collected vaginal swabs of women who do not undergo cervical cytological screening in Southern France. J Med Virol. 2010;82:1431–1437. doi: 10.1002/jmv.21835. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giuliano AR, Anic G, Nyitray AG. Epidemiology and pathology of HPV disease in males. Gynecol Oncol. 2010;117:S15–9. doi: 10.1016/j.ygyno.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunne EF, Nielson CM, Stone KM, et al. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 45.Safaeian M, Porras C, Schiffman M, et al. Epidemiological Study of Anti-HPV16/18 Seropositivity and Subsequent Risk of HPV16 and -18 Infections. J Natl Cancer Inst. 2010 doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Lu B, Viscidi RP, Wu Y, et al. Seroprevalence of Human Papillomavirus (HPV) Type 6 and 16 Vary by Anatomic Site of HPV Infection in Men. Cancer Epidemiol Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0483. This cross-sectional study found that HPV seroprevalence is higher with a corresponding anal HPV infection than with a corresponding genital HPV infection among heterosexual men and MSM. This suggests that differences in the epithelial types may affect the HPV-antibody response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markowitz LE, Sternberg M, Dunne EF, et al. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis. 2009;200:1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 48.Kreimer AR, Alberg AJ, Viscidi R, Gillison ML. Gender differences in sexual biomarkers and behaviors associated with human papillomavirus-16, -18, and -33 seroprevalence. Sex Transm Dis. 2004;31:247–256. doi: 10.1097/01.olq.0000118425.49522.2c. [DOI] [PubMed] [Google Scholar]
- 49*.Tiggelaar SM, Lin MJ, Viscidi RP, et al. Age-specific human papillomavirus antibody and deoxyribonucleic Acid prevalence: a global review. J Adolesc Health. 2012;50:110–131. doi: 10.1016/j.jadohealth.2011.10.010. This review article describes how research has found a higher natural HPV seroprevalence in females than males, despite the prevalence of HPV DNA being similar between females and males. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (CDC) HIV surveillance--United States, 1981-2008. MMWR Morb Mortal Wkly Rep. 2011;60:689–693. [PubMed] [Google Scholar]
- 51.Palefsky J. Biology of HPV in HIV infection. Adv Dent Res. 2006;19:99–105. doi: 10.1177/154407370601900120. [DOI] [PubMed] [Google Scholar]
- 52.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 53.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 54.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103:753–762. doi: 10.1093/jnci/djr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 56.Greenspan D, Canchola AJ, MacPhail LA, et al. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet. 2001;357:1411–1412. doi: 10.1016/S0140-6736(00)04578-5. [DOI] [PubMed] [Google Scholar]
- 57*.Gabriela AS, Bertha FM, Alejandro GC, et al. HPV oral lesions in HIV-infected patients: the impact of long-term HAART. J Oral Pathol Med. 2012 doi: 10.1111/jop.12032. This cross-sectional study from Mexico found that longer time on ART was associated with oral lesions/warts. [DOI] [PubMed] [Google Scholar]
- 58.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of human papillomavirus-related cervical lesions among HIV-infected women on HAART. AIDS. 2012;26:1645–1652. doi: 10.1097/QAD.0b013e32835536a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201:681–690. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soncini E, Zoncada A, Condemi V, et al. Reduction of the risk of cervical intraepithelial neoplasia in HIV-infected women treated with highly active antiretroviral therapy. Acta Biomed. 2007;78:36–40. [PubMed] [Google Scholar]
- 61.Lillo FB, Ferrari D, Veglia F, et al. Human papillomavirus infection and associated cervical disease in human immunodeficiency virus-infected women: effect of highly active antiretroviral therapy. J Infect Dis. 2001;184:547–551. doi: 10.1086/322856. [DOI] [PubMed] [Google Scholar]
- 62.Schuman P, Ohmit SE, Klein RS, et al. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188:128–136. doi: 10.1086/375783. [DOI] [PubMed] [Google Scholar]
- 63.Beachler DC, D'Souza G. Nuances in the changing epidemiology of head and neck cancer. Oncology (Williston Park) 2010;24:924–926. [PMC free article] [PubMed] [Google Scholar]
- 64.Office of the Surgeon General (US), Office on Smoking and Health (US) The Health Consequences of Smoking: A Report of the Surgeon General. 2004 [Google Scholar]
- 65.Smith EM, Swarnavel S, Ritchie JM, et al. Prevalence of human papillomavirus in the oral cavity/oropharynx in a large population of children and adolescents. Pediatr Infect Dis J. 2007;26:836–40. doi: 10.1097/INF.0b013e318124a4ae. [DOI] [PubMed] [Google Scholar]
- 66.Kreimer AR, Villa A, Nyitray AG, et al. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev. 2011;20:172–182. doi: 10.1158/1055-9965.EPI-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'Souza G, Fakhry C, Sugar EA, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121:143–50. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 68.Zeidel A, Beilin B, Yardeni I, et al. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46:959–964. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- 69.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res. 2012;91:142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith EM, Rubenstein LM, Haugen TH, et al. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010;9:1369–1378. doi: 10.1007/s10552-010-9564-z. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz SM, Daling JR, Doody DR, et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J Natl Cancer Inst. 1998;90:1626–36. doi: 10.1093/jnci/90.21.1626. [DOI] [PubMed] [Google Scholar]
- 72.Smith EM, Ritchie JM, Summersgill KF, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 73.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 74.Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang ZF, Morgenstern H, Spitz MR, et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999;8:1071–1078. [PubMed] [Google Scholar]
- 76.Berthiller J, Lee YC, Boffetta P, et al. Marijuana smoking and the risk of head and neck cancer: pooled analysis in the INHANCE consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:1544–1551. doi: 10.1158/1055-9965.EPI-08-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aldington S, Harwood M, Cox B, et al. Cannabis use and cancer of the head and neck: case-control study. Otolaryngol Head Neck Surg. 2008;138:374–380. doi: 10.1016/j.otohns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Fakhry C, Rosenthal B, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “Pap-test equivalent” in high-risk populations. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-11-0284. This study of HIV-infected individuals found the number of cytopathologic abnormalities among HIV-infected individuals was low and not associated with HPV16. They suggest that this potential pap based screening tool may not be feasible for the oropharynx due to anatomic sampling limitations and the relatively low incidence of HNC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herrero R, Quint W, Hildesheim A, et al. Efficacy of an HPV16/18 vaccine against oral HPV infections: a randomized clinical trial [abstract]; Proceedings of the 28th International Papillomavirus Conference; Clincal Science; 2012. p. 196. [Google Scholar]
- 80.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202:1246–1253. doi: 10.1086/656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune Defic Syndr. 2010;55:197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–1708. [PubMed] [Google Scholar]
- 83*.Firnhaber C, Wilkin T. Human papillomavirus vaccines: where do they fit in HIV-infected individuals? Curr HIV/AIDS Rep. 2012;9:278–286. doi: 10.1007/s11904-012-0128-6. This review article summarizes the research on the HPV vaccine in HIV-infected individuals. It highlights support for the vaccine's safety and immunogenicity in HIV-infected individuals and suggests efficacy studies are currently ongoing. [DOI] [PubMed] [Google Scholar]
- 84.Marais DJ, Passmore JA, Denny L, et al. Cervical and oral human papillomavirus types in HIV-1 positive and negative women with cervical disease in South Africa. J Med Virol. 2008;80:953–959. doi: 10.1002/jmv.21166. [DOI] [PubMed] [Google Scholar]
- 85.Richter KL, van Rensburg EJ, van Heerden WF, Boy SC. Human papilloma virus types in the oral and cervical mucosa of HIV-positive South African women prior to antiretroviral therapy. J Oral Pathol Med. 2008;37:555–559. doi: 10.1111/j.1600-0714.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 86.Fakhry C, Sugar E, D'Souza G, Gillison M. Two-week versus six-month sampling interval in a short-term natural history study of oral HPV infection in an HIV-positive cohort. PLoS One. 2010;5:e11918. doi: 10.1371/journal.pone.0011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87*.Parisi SG, Cruciani M, Scaggiante R, et al. Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: a longitudinal cohort study among men who have sex with men. BMC Infect Dis. 2011;11:150. doi: 10.1186/1471-2334-11-150. This Italian study compared oral and anal HPV infection over a six-month period, finding a higher prevalence anal HPV and low concordance between anal and oral HPV infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88*.Del Mistro A, Baboci L, Frayle-Salamanca H, et al. Oral human papillomavirus and human herpesvirus-8 infections among human immunodeficiency virus type 1-infected men and women in Italy. Sex Transm Dis. 2012;39:894–898. doi: 10.1097/OLQ.0b013e31826ef2da. This cross-sectional study from Italy found a high prevalence (37%) of oral HPV in saliva in HIV-infected MSM, heterosexual men, and women. [DOI] [PubMed] [Google Scholar]
- 89.Steinau M, Reddy D, Sumbry A, et al. Oral sampling and human papillomavirus genotyping in HIV-infected patients. Journal of Oral Pathology & Medicine. 2012;41:288–291. doi: 10.1111/j.1600-0714.2011.01093.x. This cross-sectional study found that oral rinses have the highest sensitivity to detect oral HPV DNA, and receptive oral sex was associated with oral HPV. [DOI] [PubMed] [Google Scholar]
- 90.Fatahzadeh M, Schlecht NF, Chen Z, et al. Oral human papillomavirus detection in older adults who have human immunodeficiency virus infection; Oral Surg Oral Med Oral Pathol Oral Radiol; 2013. This cross-sectional from the US found a high prevalence of alpha, beta, and gamma oral HPV types in HIV-infected individuals. They found increased HIV-viral load and concorruent sexually transmitted diseases were associated with oral HPV prevalence. [DOI] [PMC free article] [PubMed] [Google Scholar]