Abstract
Expression of the primary female sex behaviour, lordosis, in laboratory animals depends on oestrogen-induced expression of progesterone receptor (PgR) within a defined cell group in the ventrolateral portion of the ventromedial nucleus of the hypothalamus (VMH). The minimal latency from oestradiol administration to lordosis is 18 hours. During that time, ligand-bound oestrogen receptors (ER), members of a nuclear receptor superfamily, recruit transcriptional coregulators, which induce covalent modifications of histone proteins thus leading to transcriptional activation or repression of target genes. The aim of this study was to investigate early molecular epigenetic events underlying oestrogen-regulated transcriptional activation of the Pgr gene in the VMH of female mice. Oestradiol (E2) administration induced rapid and transient global histone modifications in the VMH of ovariectomised female mice. Histone H3 N-terminus phosphorylation (H3S10phK14Ac), acetylation (H3Ac) and methylation (H3K4me3) exhibited distinct temporal patterns facilitative to the induction of transcription; and a transcriptional repressive (H3K9me3) modification showed a different temporal pattern. Collectively these should create a permissive environment for the transcriptional activity necessary for lordosis, within 3-6 hours after E2-treatment. In the VMH, changes in the H3Ac and H3K4me3 levels of histone H3 were also detected at the promoter region of Pgr gene within the same time window, but were delayed in the preoptic area. Moreover, examination of histone modifications associated with the promoter of another ER-target gene, oxytocin receptor (Oxtr), revealed gene- and brain-region specific effects of E2 treatment. In the VMH of female mice, E2-treatment resulted in the recruitment of ERα to the oestrogen-response-elements-containing putative enhancer site of Pgr gene ~200kb upstream of the transcription start site (TSS), but failed to increase ERα association with the more proximal promoter region. Finally, E2 administration led to significant changes in the mRNA expression of several ER coregulators in a brain-region dependent manner. Taken together, these data indicate that in the hypothalamus and preoptic area of female mice, early responses to E2-treatment involve highly specific changes in chromatin structure, dependent on cell group, gene, histone modification studied, promoter/enhancer site and time following E2.
Keywords: oestrogens, oestrogen receptor, progesterone receptor, hypothalamus, transcriptional regulation, histone modification
Introduction
The ovarian steroids, oestrogens, regulate a great variety of physiological processes, from reproduction to bone formation, cardioprotection and neurotrophic effects on the central nervous system (CNS) [1]. Moreover, oestrogens have been the focus of intense study for decades for their role in endometrial and breast tumours. A primary role of oestrogens in the mammalian brain is on the hypothalamic–pituitary axis where they act as homeostatic feedback messengers between the gonads and the hypothalamus to regulate the levels of gonadotrophins and the reproductive cycle of the female [2]. In addition, oestrogens impact reproduction through facilitation of an entire chain of behaviours essential for reproduction, from courtship to maternal behaviours. The primary sex behaviour of female rodents, lordosis, is known to critically depend on the actions of oestrogens within the defined neuronal group in the hypothalamus, ventromedial nucleus of hypothalamus (VMH) [3-5].
The mechanism by which oestrogens modulate reproductive behaviour is through the transcriptional induction of target genes and the coordinated actions of these gene products promote different aspects of lordosis [6]. Oestrogens regulate transcription through binding to their cognate receptors, oestrogen receptor alpha (ERα) [7], and oestrogen receptor beta (ERβ) [8], both members of the nuclear receptor superfamily of transcription factors. Upon ligand binding ERs dimerise and translocate to the nucleus where they bind to specific DNA sequences called oestrogen-response-elements (ERE) within the regulatory regions of target genes [9-11]. Studies using genetically modified mice with disrupted ERα as well as temporally restricted knockdown of this receptor, specifically in the VMH, confirm that signalling through ERα is critical for reproduction and the expression of sexual behaviour in response to oestrogens [12-14].
One of the most important regulatory steps in the transcriptional activity of hormone receptors is the interaction with cofactors, which link them to the basal transcriptional machinery. Furthermore, these cofactors are able to regulate transcription by modifying chromatin structure through covalent histone modifications [15]. To date, a large number of cofactors involved in ER-mediated transcription have been identified. Among them are coactivators with histone acetyltransferase (HATs) activity, members of the p160 subfamily (steroid-receptor coactivators, SRCs)[16] and the integrator complex p300/CBP [17]; histone methyltransferases (HMTs) such as CARM1, PRMT1, and MLLs [18-20]; and corepressors, NCOR and SMRT, which recruit histone deacetylases to target gene promoters [21,22]. Because of the central role of coregulators in ER-mediated transcriptional regulation, they have long been regarded as the critical determining factors of specificity of oestrogen action.
Among ER target genes, the induction of the Pgr gene by oestradiol (E2)-activated ERα within the VMH, is one of the earliest and most essential steps in the sequence of events that lead to the expression of female sex behaviours and successful reproduction in rodents [23]. Pgr is considered one of the classic ERα target genes and its induction by E2 has been extensively studied in a number of E2-responsive tissues, most notably in mammary epithelial and breast cancer cells as well as in the brain. However, as the data accumulate, it has become increasingly clear that the regulation of Pgr by ligand-activated ERα is quite complex and displays tissue and age-dependent patterns [24-26]. Requirement of different SRC isoforms for the regulation of Pgr gene expression by E2-activated ER is one clear example of a tissue-specific hormonal effect. In human MCF-7 breast cancer cell line, the expression of the Pgr gene in response to E2 stimulation requires SRC3, but not SRC1 and SRC2 [27,28]. In contrast, in the brains of rats and mice, SRC1 and SRC2 are necessary for the induction of the Pgr gene as well as expression of reproductive behaviour, while SRC3 is dispensable for both [29,30].
The Pgr gene produces two protein isoforms, PgR-A (94 kDa) and PgR-B (120 kDa), through two alternate TSS. Moreover, two functional promoters that direct the transcription of each Pgr isoforms have been characterised for human and rat Pgr genes [31-33]. Although the transcription of the Pgr gene is directly regulated by ERα, there are no consensus ERE motifs near the transcription start sites. There is, however, an ERE half site within the Pgr-A promoter, that is conserved in the mouse, rat and human genome [31-33]. Recently, several ERα binding sites have been identified between 48 and 311kb upstream of the human PGR-B TSS [34,35]. Three of these sites have been shown to associate with the TSS in an E2-dependent manner suggesting that they may act as enhancers responsible for PGR gene transcription [35]. Despite these recent advances, little is known about the molecular mechanisms involved in the regulation of Pgr gene expression by ligand-activated ERα in the mouse nervous tissue.
For decades, the human breast cancer cell system has been the primary source of information about the effects of oestrogens and transcriptional activity of ERs. However, the important physiological role of oestrogens on nervous system functions creates a need to understand the molecular actions of these steroid hormones in nervous tissue. In the current study we investigated early molecular events associated with E2-mediated regulation of Pgr gene transcription in the mouse brain. Total levels of histone modifications in the VMH of female mice, as well as acetylation and K4 methylation of histone H3 at the promoter of Pgr gene were examined to determine the effects of E2 on chromatin architecture and E2-target gene expression. We also performed comparative analyses of histone modifications at the promoters of two of E2-target genes, Pgr and Oxtr, in two E2-responsive hypothalamic nuclei, VMH and POA, to investigate the molecular mechanisms of gene- and region-specific effects of E2 in the brain. Chromatin immunoprecipitation (ChIP) was performed on the VMH tissue of female mice to detect the recruitment of ERα to the putative regulatory sequences of Pgr gene and led to the identification of an E2-regulated site ~200kb upstream of TSS of the mouse Pgr gene. In addition, mRNA expression analysis in the VMH and POA of female mice revealed that E2 regulates the transcript levels of several histone-modifying factors also in a region-specific manner, providing mechanistic clues as to how the exposure to oestrogens leads to changes in the status of histone proteins.
Materials and methods
Treatment and Tissue collection
Animals were handled in accordance with the National Institutes of Health and Rockefeller University Institutional Animal Care and Use Committee guidelines. Six to eight-week-old female Swiss-Webster mice were ovariectomised (OVX). After a weeklong recovery period mice were injected intraperitonially (i.p) with 20μg 17β-oestradiol (E2)(Sigma, St. Louis, MO) diluted in 100μl vehicle solution (10% ethanol/saline). Control mice were injected only with vehicle (Veh) solution. Mice were sacrificed 1, 3, 6, and 9 hours after E2 injections, brains removed and immediately frozen. VMH and POA were dissected from each brain on a dry-ice bed and kept frozen until processed. For dissections, frozen brain was placed on dry ice bad ventral side up and slices containing areas of interest were cut using brain mold and sterile blades. For POA, optic chiasm and olfactory tubercle were used as landmarks to isolate ~0.5mm slice between 0.5 to −0.10 relative to bregma; and for VMH, pituitary stalk and mammary bodies were used as landmarks to isolate ~1mm slice between −1.22 to −2.06 relative to bregma. Subsequently areas containing POA and VMH were dissected from coronal slices according to the mouse brain atlas [36].
SDS-PAGE and Western Blot
VMH tissue was thawed in lysis buffer containing protease, phosphatase and deacetylase inhibitors. Tissue was homogenised in 2% SDS buffer to ensure total disruption of the plasma membrane as well as the nuclear envelope. For the SDS-PAGE and Western blot analysis VMH tissue from two mice were pooled. Total protein concentration was determined using BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL) and 30μg of protein was ran on 4-20% gradient Tris-glycine Ready gels (BioRad, Hercules, CA). Protein was transferred on PVDF membrane (BioRad, Hercules, CA) and blocked for 1h at room temperature (RT) in 5% TBS-milk. Primary antibodies that recognise specific modified residues on H3 and H4 histone tails were all purchased from Millipore (Bedford, MA). Primary antibody incubation was done at 4°C overnight followed by washes and incubation with a secondary horseradish peroxidase–labelled goat anti-rabbit antibody (GE Healthcare, Piscataway, NJ) for 1 h at RT. Membranes were washed and immunolabelled proteins were visualised with enhanced chemiluminescence (ECL) detection reagent (GE Healthcare). Quantitative densitometric analysis was performed using NIH Image software. All modified histone levels were normalised to that of total H3 histone (Millipore) to account for the loading variation. Statistical significance was calculated by one-way ANOVA followed by Dunnett’s Multiple Comparison Test using GraphPad Prism software. Progesterone receptor protein was detected in the same extracts using rabbit monoclonal antibody that recognises both PgR-A and PgR-B isoforms (clone SP2, Thermo Fisher Scientific). PgR levels were normalised to that of β-actin (SCBT, Santa Cruz, CA).
Chromatin immunoprecipitation and qPCR
ChIP assay was performed using EZ-Magna ChIP kit (Millipore) according to manufacturer’s instructions with some modifications. Briefly, VMH and POA tissue collected from Veh and E2-treated mice was cut into ~1mm pieces and crosslinked in 1% formaldehyde at RT for 15min. To perform ChIP with ERα antibody (Clone: TE111.5D11, Thermo Fisher Scientific) an additional cross-linking step with 2 mM Ethylene Glycol-bis (Succinimidylsuccinate) (EGS, Thermo Scientific) was introduced, followed by 30min 1% formaldehyde [37]. Tissue from 2-4 mice was pooled for each sample. After quenching and washing out the formaldehyde, tissue was homogenised in 1% SDS nuclear lysis buffer containing protease inhibitors. Chromatin was sheared using Bioruptor® sonicator (Diagenode Inc., Denville, NJ), cleared by centrifugation and the protein concentration was determined using BCA protein assay kit (Thermo Fisher Scientific). Equal amount of chromatin (300μg) were used from each sample to immunoprecipitate H3Acetyl and H3K4me3 using antibodies that recognise pan-acetylated H3 (06-599) and specific trimethylated H3K4 residue (07-473) (both Millipore). 10% of each sample amount (30μg) was removed and saved as input. For immunoprecipitating ERα 1mg of chromatin was used for each sample and 5% of sample amount was saved as input. The specificity and efficiency of ERα antibody is shown in Figure 2S. After several washes, pulled-down complexes were reverse cross-linked and genomic DNA was purified.
Quantitative PCR was used to measure the amount of immunoprecipitated DNA and Ct values obtained were normalised to those from input. Statistical significance was calculated by one-way ANOVA followed by Dunnett’s Multiple Comparison Test using GraphPad Prism software. Sequences and positions of primers used to detect binding of modified H3 histones to Pgr and Oxtr promoters are listed in Tables 1 and 2, respectively, and are based on sequences of the mouse Pgr (accession #U12644) and Oxtr gene (accession #D86631). Sequences and positions of primers used to detect binding of ERα to the upstream putative enhancer regions of the mouse Pgr gene are listed in Table 3. Primers were designed using either Primer Express software (Applied Biosystems, Carlsbad, CA) or PrimerQuest (Integrated DNA Technologies, Coralville, IA).
Table 1. Nucleotide sequences and locations of primers used to amplify mouse Pgr promoter regions in ChIP experiments.
| Name | Position | Primer sequence |
|---|---|---|
| Pro m 1 | −721/−650 | Forw 5′ -CATCC TTA ACTGC TAAG TCACATTGG -3′ Rev 5′-GCCTT CCT CTA ACACGCTCAA -3′ |
| Pro m 2 | +261/+332 | Forw 5′ -AGCGGG AGTCCTTTTTTTCAG -3′ Rev 5′-TTTTTCGT GTTT CTTC TTGA ATGG-3′ |
| Pro m 3 | +621/+683 | Forw 5′ -GGAG CTTGG GTCG TCATGA -3′ Rev 5′-GCCAGA CGTGTGGAGA AC CT-3′ |
| Pro m 4 | +841/+915 | Forw 5′ -AGCTCC CAG ACGGAAAG ACA -3′ Rev 5′-GTG GC TTCTACCC CAGAGAAAG -3′ |
Table 2. Nucleotide sequences and locations of primers used to amplify OTR 5′ UTR regions in ChIP experiments.
| Name | Position | Primer Sequence |
|---|---|---|
| Oxtr prom1 | +448/+518 | Forw 5′-CGGCGTTGGGAAGTTGTG-3′ Rev 5′-CCCAAAAGCCCATCCTTCTT-3′ |
| Oxtr prom2 | +686/+748 | Forw 5′-GGTTAATCGCAAGCTTCCTCTCT-3′ Rev 5′- GCGCTTAGGTCCCAGGAAAG-3′ |
| Oxtr prom3 | +938/+1017 | Forw 5′- CGGTTCTCCTGGTTTGTTTCA-3′ Rev 5′- GCGCTCAGTCCAGGTGTCTAT-3′ |
Table 3. Nucleotide sequences and locations of primers used to amplify putative enhancer regions of mouse Pgr gene in ChIP experiments.
| Name | Position in Mouse genome |
Position in Human genome* |
Conserved ERE* |
Primer sequence |
|---|---|---|---|---|
| Enh 1 | −81kb | −168kb | ERE1 | Forw 5′ -AGCA ACA AGT TGGG TTAGT -3′ Rev 5′-CTCT CCAGGA TTGGT ACATTT-3′ |
| ERE3 | Forw 5′ -CTGG TTGTTCTGACATT CTTAT TC-3′ Rev 5′-CACTC TGTCATGCTTGATG TA-3′ |
|||
| Enh 2 | −147kb | −205kb | ERE1 | Forw 5′ -CCTATT AGAGA ACAAGGAG ATG AC-3′ Rev 5′-GG TCACATTAACCC TTGGAA -3′ |
| ERE2 | Forw 5′ -TCAAGAT GGG CAGGT ACAA -3′ Rev 5′-TAGA GCA GTGTTCC CAGAAA -3′ |
|||
| Enh 3 | −166kb | −221kb | ERE1 | Forw 5′ -AT GCTCAA GTCACCAA GC-3′ Rev 5′-AATT GCC TCT CTGCACTTT -3′ |
| Enh 4 | −200kb | −306kb | ERE3 | Forw 5′ -TGCAAGA ATATA ATG GCAGAGA -3′ Rev 5′-CTA ACTTCGC TGC AGATAG AC-3′ |
| Enh 5 | −207kb | −311kb | ERE1 | Forw 5′ -ATGAGG GTTCTGTTT CCTTTAT -3′ Rev 5′-TAG GCTT CA CCACTTC CT-3′ |
Based on: Boney-Montoya et al., 2010.
Total RNA isolation and gene expression
OVX female mice were injected with 20μg E2 or Vehicle solution (i.p) and sacrificed 3 or 5 hours after. Brains were removed and immediately frozen. Total RNA was isolated from VMH and POA using Trizol® reagent (Life Technologies, Grand Island, NY) according to manufacturer’s instructions. Equal amount of total RNA (100ng) was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Life Technologies) and the resultant cDNA was used as a template for qPCR. Following TaqMan® Gene Expression Assays were used to amplify mRNA for genes of interest: Src1, ID:Mm00447958_m1; Src2, ID:Mm00500749_m1; Cbp, ID:Mm01342452_m1; p300, ID:Mm00625535_m1; Mll1, ID:Mm01179235; Mll3, ID:Mm01156942_m1; Ash1, ID:Mm00467322_m1; Wdr5, Mm01332446_m1; Lsd1, ID:Mm01181033_m1; Jarid1A, ID:Mm00524457. Eukaryotic 18S rRNA (4352930E) was used as internal control (All from Life Technologies). Relative ratio of the expression of each gene was calculated using the 2−ΔΔCt method [38]. Statistical significance was calculated by one-way ANOVA followed by Dunnett’s Multiple Comparison Test using GraphPad Prism software.
Results
Oestradiol induces global changes in histone modifications in the VMH
In order to determine the effects of E2-treatment on chemical modifications of histone proteins in the VMH of female mice, we first investigated the changes and the time course of total histone modifications in this brain region following E2 administration. Ovariectomised (OVX) female mice were injected with E2 (20μg) or vehicle (Veh) and the brains were analysed 1, 3, 6 and 9 hours later. The dose of E2 was chosen to correlate molecular and biochemical effects of the hormone with the expression of lordosis behaviour, which in our experience requires higher doses of E2 when used independent of progesterone priming [39]. Since sustained dose-response range for E2 is extremely wide [40], 20μg dose is believed to be within the physiological range. Total extracts of VMH tissue were isolated from E2 and Veh-treated mice and analysed by Western blot using antibodies that recognise specific chemically modified histone residues. We measured global acetylation levels of histone H3 and H4 (H3Ac and H4Ac), trimethylation levels of H3K4, H3K36 and H3K9 (H3K4me3, H3K36me3 and H3K9me3), as well as double phosphorylation/acetylation mark on histone H3 (H3S10phK14Ac) (Fig. 1). Acetylation of Lys residues on histone H3 and H4 is a well-characterised modification that leads to an open chromatin structure and promotes transcription [41]. Methylation of H3K4 and H3K36 residues is also associated with transcriptional activation, whereas methylation of H3K9 is generally regarded as a repressive histone mark [41]. Moreover, as an example of cross-regulation of histone modifications, H3S10 phosphorylation has been shown to promote H3K14 acetylation in several experimental systems and is linked to a transcriptional activation [42-44].
Fig. 1. E2 effects on the levels of histone modifications in the VMH of female mice.
Ovariectomised female mice were injected with E2 or vehicle and their brains were collected for the analyses 1, 3, 6, or 9 hours later. The levels of histone modifications in VMH tissue were measured by Western blot using antibodies that recognise specific modified residues: H3S10phK14Ac (A), H3 Acetyl (B), H3K4me3 (C), H3K9me3 (D), H3K36me3 (E) and H4 Acetyl (F). Levels of each histone modifications were normalised to that of total histone H3. Representative Western blot for each histone modification examined is shown. Data reported are the average of three to four independent experiments done in triplicate. Data are expressed as fold change ± SEM. Statistical differences were calculated using one-way ANOVA with post-hoc Dunnett’s Multiple Comparison Test. *, p<0.05, **, p<0.01.
In the VMH of female mice, levels of total histone modifications were highly regulated following E2-administration. Antibody that recognises double modification H3S10phK14Ac detected significant changes in the levels of this combinatorial histone mark. One-way ANOVA revealed a significant main effect of time of the treatment (F(4,93)=2.482, p=0.049) and post-hoc Dunnett’s multiple comparison test showed significant increase 1 hour after E2 administration. However, H3S10phK14Ac appeared to be transient and by 6 hours following E2 injection the levels returned to baseline (Fig.1A). Antibody that recognises H3 pan-acetylation detected significant changes following E2 injection (one-way ANOVA, F(4,110)=4.813, p=0.001). In contrast to H3S10phK14Ac, total H3 acetylation detected by H3 pan-acetyl antibody, increased with slower kinetics and reached significantly higher levels only 6 hours after E2 treatment according to a Dunnett’s multiple comparison test (Fig.1B). Another activating histone modification, H3K4me3 is also regulated in the VMH of female mice following E2 administration. One-way ANOVA revealed a significant main effect of time of the treatment (F(4,100)=3.944, p=0.005) and post-hoc Dunnett’s multiple comparison test showed significantly higher levels 3 and 6 hours following E2 administration (Fig.1C). Interesting pattern of changes was observed in the level of repressive H3 histone modification, H3K9me3. One-way ANOVA revealed a significant main effect of time of the treatment (F(4,49)=15.40, p<0.0001) and post-hoc Dunnett’s multiple comparison test demonstrated significant changes at all time points examined. Specifically, levels of H3K9me3 were significantly increased at the beginning (1 hour) and towards the end (9 hours) of the investigated E2 time course and significantly decreased 3 and 6 hours after injection. This decrease of H3K9me3 coincides with the significant upregulation of H3K4 trimethylation (Fig.1D). Interestingly, these data are in line with previous reports that these two methylation events are mutually exclusive [42,45]. We failed to detect any changes in the levels of H3K36me3, an active chromatin mark on histone H3 (one-way ANOVA, F(4,63)=1.699, p=0.161) (Fig.1E). Finally, analysis of total acetylation levels of histone H4 revealed a significant main effect of time of treatment (one-way ANOVA, F(4,117)=4.051, p=0.0041) and post-hoc Dunnett’s multiple comparison test showed that H4 acetylation was significantly decreased 9 hours following E2 injection (Fig.1F).
These data revealed that in the female mouse VMH E2 treatment induces rapid changes in the covalent modifications of histone H3 tail residues. Moreover, specific modifications appear to be sequential and follow distinct temporal patterns. Collectively, these covalent histone modifications establish favourable environment for the transcriptional activity in the VMH of female mice within 3-6 hours of E2-treatment.
E2-induced histone modifications are accompanied by ERα target gene upregulation
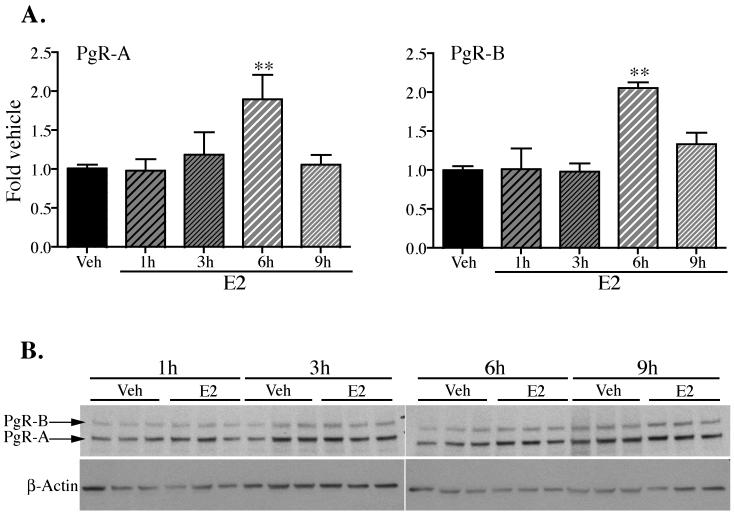
The Pgr gene is a classic ERα target gene, which is regulated by E2 in several tissues [46-48]. Thus, we tested whether E2-induced “permissive chromatin” state in VMH leads to the upregulation of PgR expression. As expected, protein levels of both PgR isoforms, PgR-A and PgR-B, were significantly increased following E2 administration (Fig. 2B and C). One-way ANOVA revealed a significant main effect of treatment (F(4,70)=5.866, p=0.0004 for PgR-A and F(4,68)=10.88, p<0.0001 for PgR-B) and post-hoc Dunnett’s multiple comparison test showed significantly higher levels of both isoforms 6 hours after hormone injection, coinciding with the E2 induced H3 acetylation and H3K4 methylation events.
Fig. 2. E2-induced upregulation of PgR-A and PgR-B in the VMH of female mice.
(A) Whole cell extracts isolated from the VMH of E2 and Veh-treated mice were subjected to Western blot using antibody that recognises both isoforms of progesterone receptor, PgR-A and PgR-B. PgR protein levels were normalised to that of β-Actin and are expressed as fold change±SEM. Data represent average of three independent experiments done in triplicate. Statistical differences were calculated using one-way ANOVA with post-hoc Dunnett’s Multiple Comparison Test. **, p<0.01. (B) Representative Western blot analysis of PgR protein expression in VMH of female mice. Bands corresponding to PgR-A and PgR-B isoforms are indicated.
To ascertain that our chosen dose of E2 was within the physiological range we performed a control experiment in which PgR protein levels were also examined in VMH tissue of OVX female mice injected with 2μg of E2. Low dose of E2, similar to the effect seen with higher dose, led to significant induction of PgR protein 6 hours after injection (Fig. 1S).
These data reveal that in the VMH of female mice, an “active” chromatin state brought about by E2 exposure coincides with the transcriptional activation of an ERα target gene, Pgr, and may contribute to its regulation.
E2 treatment induces association of activating histone marks with Pgr promoter in the VMH
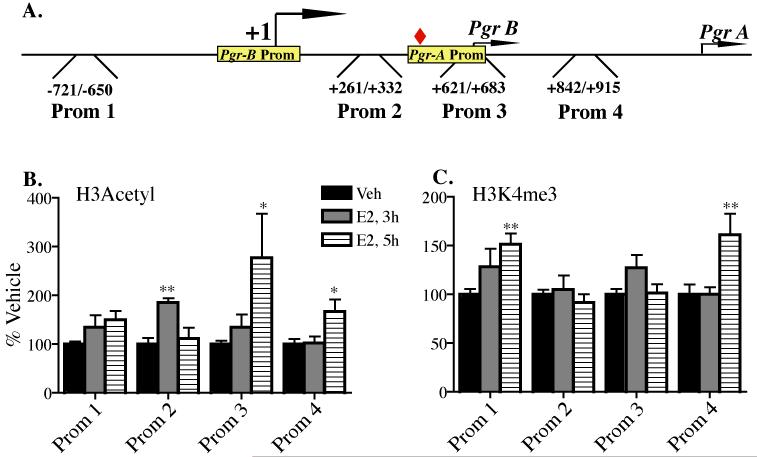
In order to investigate how E2-induced histone modifications contribute to the expression of the Pgr gene in the VMH of female mice, we examined the association of acetylated (H3Ac) and methylated (H3K4me3) histone H3 with the promoter of this gene, using ChIP analysis. OVX female mice were injected with E2 and Veh and brains collected 3 and 5 hours later. The time-points to detect “activating” histone marks at the regulatory sequences of Pgr gene was selected to precede E2-induce PgR protein upregulation. Chromatin isolated from VMH tissue of E2 and Veh-treated mice was immunoprecipitated using antibodies specific for H3Ac and H3K4me3. It has been shown that H3 histone acetylation and H3K4 methylation most often take place near the TSS of actively transcribed genes [49,50]. Although the 5′ sequence of mouse Pgr gene has been published [33], the functional analysis of mouse Pgr promoter has not been reported. On the other hand, the existence of two functional promoters for PGR-A and PGR-B isoforms has been described for rat as well as for human PGR genes [31,32]. Using sequence alignment, we mapped two regions within ~2kb sequence around the mouse Pgr TSS, which display approximately 83% sequence identity with the rat Pgr gene promoters (Fig. 3A). Thus, four primer pairs (designated Prom1-4, Table 1.) spanning the region between −720 to +915 bp relative to the TSS, were designed to analyse association of H3Ac and H3K4me3 with mouse Pgr gene promoter. Both H3Ac and H3K4me3 were enriched at the promoter sequences of Pgr gene in the VMH of E2-treated female mice compared to control mice. One-way ANOVA analysis revealed significant main effect of treatment on H3Ac at three promoter sites of Pgr gene (Prom2, F(2.32)=7.071, p=0.003; Prom3, F(2.32)=4.599, p=0.018; Prom4, F(2,29)=4.642, p=0.018). Post-hoc Dunnett’s multiple comparison test revealed significant hyperacetylation of H3 at Prom2 site, located between Pgr-B and Pgr-A promoters, 3 hours after E2 administration and at Prom3 and Prom4 sites, located upstream of Pgr-B promoter region, at 5 hour time point (Fig. 3B).
Fig. 3. E2 effects on histone modifications at the promoter of Pgr gene in the VMH.
(A) Schematic representation of mouse Pgr promoter and primer locations relative to the TSS (+1). Small arrows indicate translation start sites for Pgr-B and Pgr-A isoforms. Yellow boxes denote sequences corresponding to two functional promoters of rat Pgr gene. Red diamond indicates the location of ERE half site. Chromatin isolated from the VMH tissue of E2 and Veh-treated female mice were immunoprecipitated with antibodies specific to (B) H3Acetyl and (C) H3K4me3. Quantitative PCR was used to amplify Pgr promoter regions associated with acetylated and methylated histone H3. Data reported are the average of three independent experiments done in triplicate. Data are expressed as % Vehicle ± SEM. Statistical differences were calculated using one-way ANOVA with post-hoc Dunnett’s Multiple Comparison Test. *, p<0.05; **, p<0.01.
Another histone mark leading to an open chromatin confirmation, H3K4me3, was also significantly upregulated at Pgr gene promoter in the VMH of E2-treated female mice at two promoter sites (Prom1, F(2.31)=5.776, p=0.007; and Prom4, F(2,31)=5.611, p=0.008). Post-hoc Dunnett’s multiple comparison test revealed a significant increase in H3K4me3 associated with Prom1 and Prom4 sites, which span Pgr-B and Pgr-A promoters, respectively, 5 hours after E2 injection (Fig. 3C). These data suggest that the mechanism by which E2-activated ERα induces the transcription of Pgr gene in mouse brain involves histone modifications and changes to chromatin structure at the promoter of this target gene.
E2 affects histone modifications at Pgr promoter in the POA
It has been well documented that E2 regulates PgR expression in some brain regions but not the others [46]. One of the brain regions responsive to E2 treatment is the POA. Thus, we examined histone modification status at Pgr gene promoter in the POA of female mice using ChIP assay. One-way ANOVA analyses revealed significant effects of E2 treatment on the associations of H3Acetyl with two promoter sites of Pgr gene (Prom2, F(2,29)=3.370, p=0.048; and Prom4, F(2,32)=4.4, p=0.021). We also observed a significant increase in the H3K4me3 levels at the most distal promoter site of Pgr gene (Prom1, F(2,30)=15.24, p<0.0001) (Fig. 4A and B). Pos-hoc Dunnett’s multiple comparison test revealed that both histone modifications (H3Acetyl and H3K4me3) were upregulated in the VMH of female mice only 5 hours after E2 injection with no changes detected at 3h time point (Fig. 4A and B). These results demonstrate that the time course and the pattern of E2-induced histone modifications at the promoter of Pgr gene is brain-region specific and may reflect the differences in the expression of PgR in these nuclei.
Fig. 4. E2 effects on histone modifications at the promoter of Pgr gene in the POA.
(A) Histone H3 acetylation and (B) H3K4 trimethylation at the promoter of Pgr gene in the POA of female mice were determined using ChIP assay. Data reported are the average of three independent experiments done in triplicate. Data are expressed as % Vehicle ± SEM. Statistical differences were calculated using one-way ANOVA with post-hoc Dunnett’s Multiple Comparison Test. *, p<0.05; **, p<0.01.
E2 differentially affects histone modifications at Oxtr promoter in the POA and VMH
One of the gene products regulated by E2 that facilitate the expression of lordosis behaviour is oxytocin receptor [51,52]. Thus we examined how E2-treatment affects histone modifications at the promoter of the Oxtr gene in two brain regions, VMH and POA of female mice. Three primer pairs (Oxtr prom1-3, Table 2.) that span ~600bp sequence in the 5′UTR of the mouse Oxtr gene were designed and used to detect association of acetylated and methylated histone H3 with the regulatory region of this gene (Fig. 5A). We observed different patterns and time-course of E2-mediated regulation of H3Ac and H3K4me3 at the promoter of Oxtr gene in the two brain regions (Fig. 5B). In the mouse VMH, one-way ANOVA analysis revealed a significant effect of E2 treatment on both “activating” histone marks, H3Acetyl and H3K4me3, albeit to a different degree. Histone H3 hyperacetylation was detected at two promoter sites of Oxtr gene (Oxtr prom2, F(2,27)=3.770, p=0.036; and Oxtr prom3, F(2,31)=5.118, p=0.0120), and H3K4 trimethylation was increased at single promoter site (Oxtr prom2, F(2,32)=3.575, p=0.04) (Fig. 5B). Furthermore, Pos-hoc Dunnett’s multiple comparison test failed to detect significant changes in H3Ac or H3K4me3 at any one of the specific sites of 5′ UTR of Oxtr gene 3 hours after E2 administration but revealed significant upregulation of H3Acetyl and H3K4me3 at 5 hour time point (Fig. 5B).
Fig. 5. E2 effects on histone modifications at the promoter of Oxtr gene in the VMH and the POA.
(A) Schematic representation of mouse Oxtr gene 5′UTR and primer locations relative to the TSS (+1). Small arrow indicates translation start site. Histone H3 acetylation and H3K4 trimethylation at the promoter of mouse Oxtr gene in (B) the VMH and (C) the POA of female mice were determined using ChIP assay. Data reported are the average of three independent experiments done in triplicate. Data are expressed as % Vehicle ± SEM. Statistical differences were calculated using one-way ANOVA with post-hoc Dunnett’s Multiple Comparison Test. *, p<0.05; **, p<0.01
In the mouse POA, one-way ANOVA analysis revealed the effect of E2 treatment on H3Ac association with all three Oxtr promoter sites (Oxtr prom1, F(2,32)=8.565, p=0.001; Oxtr prom2, F(2,32)=6.471, p=0.004; and Oxtr prom3, F(2,33)=3.846, p=0.032) and Pos-hoc Dunnett’s multiple comparison test demonstrated significant hyperacetylation taking place at Oxtr prom1 and Oxtr prom2 only 5 hours after E2 administration (Fig. 5C). No changes were detected in the POA in the methylation status of H3K4 at any Oxtr promoter sites following E2 administration. These results suggest that E2-treatment induces distinct covalent modifications of histone H3 in the brains of OVX female mice in a gene-(Oxtr ≠Pgr) and region-(POA≠VMH) dependent manner.
E2 effects on the recruitment of ERα to the putative regulatory sequences on Pgr gene
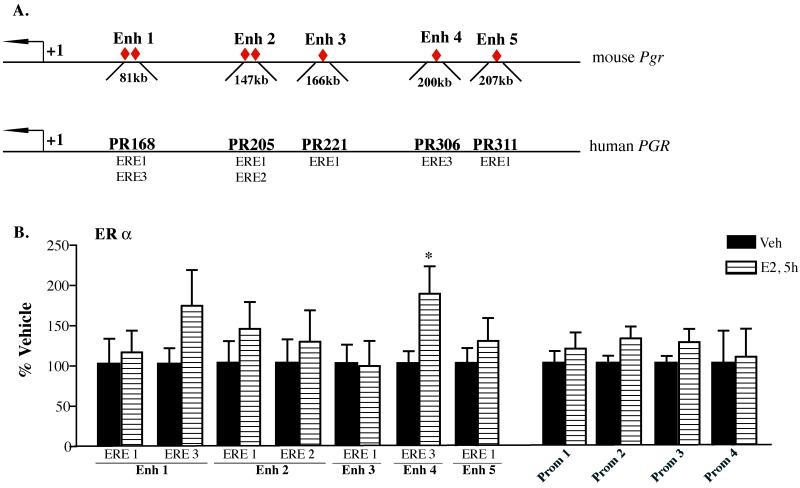
E2 regulates the transcriptional activity of target genes by binding to one of the two nuclear ERs, ERα and ERβ. Overwhelming evidence indicates that induction of Pgr gene in the VMH of female mice and subsequent expression of lordosis behaviour critically requires ERα[12,14]. Thus, we set out to examine whether E2-treatment of OVX female mice results in the direct recruitment of ERα to the promoter of Pgr gene. For this, OVX female mice were injected with Veh or E2 and chromatin isolated from the VMH 5 hours after was analysed using ChIP assay. The time-point to analyse the association of ERα with the regulatory sequences of Pgr gene was selected to precede E2-induce PgR protein upregulation.
Reportedly, there are no consensus ERE sites within the promoter region of mouse Pgr gene, however there is a half ERE site within the Pgr-A promoter (Fig. 3A). Furthermore, recent reports suggest that several sequences ~150-300kb upstream of human PGR gene contain ERE sites and act as enhancers to regulate PGR gene expression [35]. Examination of ~200kb genomic sequences upstream of mouse Pgr gene led to identification of seven ERE sites, which displayed high homology to ERE sites characterised by Boney-Montoya and colleagues in human as well as in mouse genomes (Dr. Ann Nardulli, personal communication). Thus, we examined direct association of ERα with these putative regulatory sequences of Pgr gene: four sites within the proximal promoter region (Table 1 and Fig. 3A) and seven ERE-containing sites within ~200kb upstream of the TSS (Table 3 and Fig. 6A). In concert with previous reports we did not observe E2-regulated recruitment of ERα to the promoter region of Pgr gene [28]. We did, however, detect E2-induced binding of ERα at two putative enhancer regions located ~81kb and 200kb upstream of the Pgr gene TSS (Fig. 6B). Statistical analysis using Student’s t-test revealed a significant increase in the binding of ERα 5 hours after E2 administration only at Enhancer 4 site (p=0.043). This is the first evidence that the ERE-containing site 200kb upstream of Pgr TSS may be involved in the regulation of Pgr gene by ligand-activated ERα in the mouse brain in vivo. Surprisingly, little or no E2-induced recruitment of ERα was observed at any other ERE sites following E2 administration (Fig. 6B), suggesting that, contrary to data obtained in human breast cancer cells [28,35] these ERE–containing sites are not involved in the regulation of Pgr gene expression in the mouse brain. These data suggest that different regulatory sequences contribute to Pgr gene activation in different species.
Fig. 6. Recruitment of ERα to the putative promoter and enhancer sites of mouse Pgr gene in the VMH.
(A) Location of primers used to amplify putative enhancer sequences upstream of the TSS (+1) of mouse Pgr gene and corresponding enhancer sites in the human PGR gene [35]. Red diamonds denote ERE sites which are conserved between human and mouse enhancer sequences. Locations of primers used to amplify sequences within promoter region (Prom 1-4) are as in Fig. 3A. (B) Chromatin isolated from E2 and Veh-treated mice was immunoprecipitated with antibody specific for ERα and associated genomic DNA was quantified by qPCR. Data are expressed as % Vehicle± SEM and represent the average of two independent experiments done in triplicate. Statistical differences between E2 and Veh-treated samples were calculated using Students’s t-test. *, p<0.05.
E2 effects on the expression of ERα cofactors in the VMH of female mice
Histone modifications are induced by specialised enzymes or enzyme complexes, which are recruited by transcription factors to promote or repress transcription of target genes though alteration of the chromatin structure. One-way to affect the chemical status of histone proteins is by regulating the expression and availability of histone modifying enzymes [53]. Although a large number of ERα cofactors with HAT or HMT activity has been identified in human cancer cells, little or no information exists as to which of these cofactors regulates the transcriptional activity of ERα in the nervous tissue. Thus, we examined the mRNA expression levels of some of transcriptional coregulators, which are able to bring about histone modifications we detected in the VMH following E2-treatment. For this, OVX female mice were injected with E2 and Veh and the brains were analysed 3 or 5 hours later. Total RNA was isolated from the VMH and POA tissues, reverse transcribed and resultant cDNA was used as a template for qPCR. Expression of each gene of interest was normalised to that of a housekeeping gene (ribosomal 18S gene) and the relative ratio between the Veh and E2-treated samples were calculated as described in Materials and Methods. Among the examined factors were SRC1, SRC2, CBP and p300, well-characterised ERα coactivators with HAT activity, which are involved in ER-mediated transcriptional activation in various tissues, including the brain [15,30]; MLL1 and MLL3, two important HMTs, which specifically modify H3K4 residue and are involved in the E2-regulated expression of various HOX genes in human MCF-7 cells [20]; WDR5, an essential DNA-binding subunit of MLL complexes [54,55]; ASH1L, a H3K4 methyltransferase, not yet characterised as a ERα coregulator [55]; LSD1, a coregulator with the activity to demethylase both H3K4 and H3K9, thus either repress or promote nuclear receptor-mediated transcription [56,57]; and JARID1A, which has been shown to regulate E2-induced PGR expression in human MCF-7 cells through H3K4 demethylation [58].
We found that E2 treatment induced significant changes in the levels of histone-acetylating cofactor mRNAs in the VMH of female mice (Fig.7). The expression of two Src isoforms, Src1 and Src2, were highly regulated by E2-exposure as revealed by one-way ANOVA (Src1, F(2,29)=4.255 p=0.024 and Src2, F(2,28)=11.04 p=0.0003). Pos-hoc Dunnett’s multiple comparison test revealed significant upregulation of both Src isoforms at the 3 hour time point, however, 5 hours after E2-administration Src1 stayed elevated while Src2 mRNA levels returned to baseline. The other two homologous ER cofactors with HAT activity, CBP and p300, also displayed somewhat different patterns of changes. One-way ANOVA revealed a significant main effect of the treatment on the expression of p300 (F(2,28)=22.07, p<0.0001) but not on the expression of Cbp (F(2,29)=2.247, p=0.124). Post-hoc Dunnett’s multiple comparison test demonstrated transient pattern of p300 upregulation, which reached significantly high levels at 3 hours and returned to baseline 5 hours after E2 injection (Fig. 7A).
Fig.7. E2 effects on the mRNA expression levels of histone modifying factors in the VMH.
Total RNA isolated from E2 and Veh-treated mice was reverse transcribed and the expression levels of ERα cofactors, histone acetyltransferases (A), histone methyltransferases (B), and histone demethylases (C) were analyzed by qPCR. Data are expressed as fold change± SEM and represent the average of two independent experiments each with n=5. Statistical differences were calculated using one-way ANOVA followed by post-hoc Dunnett’s Multiple Comparison Test. *, p<0.05, **, p<0.01.
The mRNA expression of two HMTs, Mll3 and Ash1, as well as the accessory protein, Wdr5, were also regulated by E2 in the VMH of OVX female mice (Fig. 7B). One-way ANOVA revealed a significant effect of the treatment on the expression of Mll3 (F(2,31)=4.204, p=0.0242), Ash1 (F(2,31)=8.141, p=0.0014) and Wdr5 (F(2,30)=8.726, p=0.001). Moreover, post-hoc Dunnett’s multiple comparison test revealed differential temporal patterns of induction: the increase in the levels of Ash1 and Wdr5 mRNA levels was transient, were detected at 3 hour time point and returned to baseline after 5 hours; Mll3 mRNA upregulation was delayed and only reached significant levels 5 hours following E2 administration (Fig. 7B). Interestingly, no changes were detected in the mRNA levels of Mll1 (Fig. 7B).
The mRNA expression of the two examined HDMs were also regulated by E2 exposure in the VMH of female mice (Fig. 7C). One-way ANOVA revealed a significant effect of the treatment on the expression of Lsd1 (F(2,28)=3.715, p=0.0371) and Jarid1A (F(2,32)=4.59, p=0.0177). However, post-hoc Dunnett’s multiple comparison test only detected significant downregulation of Lsd1 at 3 hours while Jarid1A mRNA increase detected at the same time point was not significant (Fig. 7C).
E2 effects on the expression of ERα cofactors in the POA of female mice
Since (a) we detected differential acetylation and methylation events occurring at the promoters of ERα target genes in the two brain regions, VMH and POA; and (b) we hypothesise that differential regulation and expression levels of ERα cofactors may underlie these effects; we therefore examined the mRNA expression levels of given cofactors in the POA of OVX female mice following E2-administration. Interestingly, we found that in POA mRNA levels of examined HATs, HMTs and HDMs were more stable and less dynamically regulated by E2-exposure compared to the VMH.
Specifically, we found that, in the POA of female mice Src1 and Src2 mRNA levels do not change following E2 administration (Fig. 8A). In contrast, there was a significant effect of treatment on the mRNA expression of Cbp (F(2,31)=8.139, p=0.0014) and p300 (F(2,34)=4.786, p=0.0147) as revealed by one-way ANOVA. Furthermore, post-hoc Dunnett’s multiple comparison test showed a significant downregulation of Cbp 5 hours after E2 treatment and a similar trend for p300, albeit it failed to reach statistically significant level (Fig. 8A).
Fig.8. E2 effects on the mRNA expression levels of histone modifying factors in the POA.
Total RNA isolated from E2 and Veh-treated mice was reverse transcribed and the expression levels of ERα cofactors, histone acetyl transferases (A), histone methyl transferases (B) and histone demethylases (C) were analyzed by qPCR. Data are expressed as fold change± SEM and represent the average of two independent experiments each with n=5. Statistical differences were calculated using one-way ANOVA with a post-hoc Dunnett’s Multiple Comparison Test. *, p<0.05, **, p<0.01.
Among the factors involved in H3K4 methylation, E2 effect on mRNA expression was only observed for Ash1 (F(2,31)=4.196, p=0.0244) 5 hours after E2 administration as revealed by one-way ANOVA. In contrast to the data obtained from the VMH, the expression of Jarid1A but not Lsd1 was regulated by E2-administration in the POA (F(2,32)=4.222, p=0.0236) (Fig. 8C).
Together these data demonstrate that one of the mechanisms by which E2 induces changes in the chemical makeup of histone proteins in the mouse brain includes regulation of histone modifying cofactor mRNA expression. These data also support the hypothesis that tissue-specific effects of E2-activated ERα may be mediated by availability of transcriptional cofactors.
Discussion
The present study shows that in a specific hypothalamic region, important for lordosis behaviour, exposure to oestradiol induces a specific set of histone modifications with distinct temporal characteristics. Moreover, E2-induced histone modifications are detected at the level of global chromatin as well as at the promoters of ERα target genes, and provide mechanistic insight into the early molecular events underlying hormonal effects on nervous tissue.
In the VMH of female mice E2 induces specific set of histone modifications
Acetylation of histone proteins has long been known to promote transcriptional activity and contribute to E2-induced gene expression in human MCF-7 cells [50,59]. Moreover, evidence supports the importance of histone acetylation in E2-mediated behavioural and physiological processes, such as masculinisation of sexual behaviour and formation of hippocampus-dependent memory in mice [60,61]. Our preliminary data also suggest that histone hyperacetylation induced by histone deacetylase (HDAC) inhibitor, trichostatin A (TSA) treatment, is able to facilitate E2-induced sexual behaviour in female mice (Figure 3S). Thus, it was not surprising that we observed an increase in the acetylation of histone H3 in the VMH of E2-treated mice. In contrast, we did not find an increase in the levels of histone H4Ac, but rather detected a slight albeit significant decrease at the 9-hour time point. Such differences in the patterns of H3 and H4 acetylation seem to be characteristic early response to E2 action specifically in the mouse brain. For example, in another brain region, the hippocampus, direct infusion of E2 also resulted in the acetylation of H3 but not of H4 histone, whereas E2-treatment of human MCF-7 cells does lead to H4 acetylation at the level of total chromatin as well as at ERα target gene promoters demonstrating tissue-specific epigenetic effects of E2 [60,62].
In addition, we have found that in the VMH of female mice E2 administration induces a rapid phosphorylation/acetylation event on histone H3, H3S10ph/H3K14Ac. These two cis modifications on histone H3 have been shown to occur in a cooperative fashion, with phosphorylation of H3S10 promoting subsequent acetylation of H3K14 [42,43]. Interestingly, E2-induced acetylation of H3 (on H3K14) in the mouse hippocampus has been shown to critically depend on ERK activation [60], whereas in human MCF-7 cells, E2 also induced H3S10 phosphorylation in a MEK1 kinase-dependent manner [62]. Based on this evidence we can infer that a phosphorylation event, possibly of H3S10, by MAP kinase may be a prerequisite for H3K14 acetylation and a common mechanism for E2-induced epigenetic effects.
In the VMH of female mice, E2-administration also led to a significant increase in the levels of H3K4me3, another “permissive” histone modification, which has been shown to be important for the transcriptional activity of ERα in human breast cancer cell model [28]. In addition, our data support the previous finding that H3K4 and H3K9 methylation marks are mutually exclusive [42,45]: at 3 and 6 hours after E2-administration the levels of H3K4me3 in the VMH are significantly increased whereas levels of H3K9me3 are dramatically decreased. These data, in conjunction with the marked elevation of H3 histone acetylation 6 hours following E2 injection, suggest that in this behaviourally critical hypothalamic nucleus hormone treatment induces rapid changes in the make-up of histone proteins. As a result, the combinatorial effect of these changes creates a permissive environment for the transcriptional activity within a distinct time window.
Our analyses revealed some unexpected results as well. Notably, in the VMH of female mice, we detected a significant upregulation of repressive H3K9 methylation within 1 hour of E2 administration, as well as 9 hours after E2 injection, just when hormone effects on histone H3 acetylation and H3K4me have worn off. It is well established that E2-treatment leads to the downregulation of a number of genes in various mouse tissues [63,64]. Moreover, microarray analysis of E2 regulated genes in mouse uterine tissue had revealed distinct temporal patterns of target gene activation as well as repression [64]. Specifically, ~17% of total genes identified by Watanabe and colleagues were downregulated by E2-treatment within the first hour. Among these were genes coding for signal transduction molecules and transcription factors, indicating that their rapid downregulation may lead to further amplification of E2 effects. Our data suggest that similar mechanisms may be in place in the VMH as well, however further studies are needed to examine the precise role of ERα repressed genes on the CNS function and physiological outcomes of E2-treatment. On the other hand the marked increase in the levels of H3K9me3 together with significant downregulation in the basal levels of H4 acetylation, 9 hours after E2-exposure, may be indicative of termination of E2 effects on VMH cells.
The pattern of E2-induced histone modifications at the promoters of target genes is brain-region and gene-specific
We used ChIP assay to extend our analyses and examine E2-effects on the histone modifications at the promoters of specific ERα-target genes, Pgr and Oxtr, in two hormone-responsive brain regions, VMH and POA. Our data revealed interesting brain-region and gene-specific effects of E2 treatment (Table 4). Specifically, we found differences in the pattern and the time-course of histone H3 acetylation associated with Pgr gene promoter in the two nuclei: rapid and sustained hyperacetylation (H3Ac) in the VMH and delayed and less pronounced H3Ac in the POA. These data are in agreement with the report that demonstrates anatomic specificity of PgR regulation by E2 in rodent brain [25]. In contrast, the degree and the time-course of E2-induced H3K4 methylation at Pgr promoter were found to be similar in both, the VMH and the POA, indicating the importance of this specific histone modification in the regulation of Pgr gene. Moreover, methylation of H3K4 in response to E2 seems to be a common requirement for the expression of Pgr gene across tissues, as knockdown of Mll1, the methyltransferase responsible for this modification leads to a thwarted effect of E2 in human MCF-7 cells as well [28].
Table 4. Summary of E2-induced histone modifications at the promoters of Pgr and Oxtr genes in the VMH and POA of female mice.
| Pgr | Oxtr | |||||||
|---|---|---|---|---|---|---|---|---|
| H3Ac | H3K4 me3 | H3Ac | H3K4me3 | |||||
| VMH | POA | VMH | POA | VMH | POA | VMH | POA | |
| 3h | ↑ | – | – | – | – | – | – | – |
| 5h | ↑ ↑ | ↑ | ↑ ↑ | ↑ | ↑ | ↑ ↑ | ↑ | – |
Each arrow denotes significant change at single promoter site.
p<0.05,
p<0.01.
Interesting gene-specific effects were observed when we analysed histone modifications associated with the Oxtr promoter (Table 4). Contrary to Pgr, H3 histone acetylation appeared to be more prominently regulated by E2-exposure at the promoter of this specific ERα-target gene, whereas little or no changes in the levels of H3K4me were detected. Moreover, the increase in the levels of H3Ac at the Oxtr promoter was only detected 5 hours following treatment and this effect was evident in both hypothalamic nuclei examined, POA and VMH. Such delayed kinetics of “activating” histone modifications associated with Oxtr promoter may in fact reflect the time course of E2-induced OXTR expression, which only becomes detectable in VMH after 6h and reaches significant levels at 24h [65]. These data suggest that the temporal regulation of early epigenetic events, such as covalent histone modifications induced by E2-treatment may be the determining factor of the time course of gene induction.
E2 regulates the expression of transcription cofactors in a brain-region specific manner
Transcription factors, such as nuclear receptors, recruit coregulators with intrinsic histone acetyltransferase and histone methyltransferase activity to induce specific histone modifications at the regulatory sequences of target genes [15]. It has been postulated that regulation of cofactor expression levels and availability is one of the mechanisms that impacts histone modifications and chromatin structure [52,53]. Our data, demonstrating that E2-exposure significantly upregulates mRNA levels of several ERα cofactors with HAT and HMT activity in the VMH of female mice, suggest that similar mechanisms may underlie E2-induced changes in the status of histone proteins in the brain. To date, a vast number of ERα cofactors have been identified in reproductive tissues and cell culture systems, which are important for the regulation of ERα-target genes as well as physiological and pathological effects of E2 [15]. However, only few of them have been functionally tested in the nervous system. Among them, two SRC isoforms, SRC1 and SRC2, have been shown to be co-expressed with ERα in hormone responsive brain regions, physically interact with the receptor and are required for the E2-dependent expression of Pgr gene and reproductive behaviours [29,30]. The important role of SRC1, as well as another ERα cofactor with HAT activity, CBP, has also been demonstrated in the development of sexually dimorphic behaviours during the perinatal period in female rats [66]. In the current study, we found that following E2-administration mRNA levels of two Src isoforms (1 and 2) as well as p300 are increased in the VMH of female mice. Interestingly, the observed upregulation of cofactor mRNA either coincided or preceded H3 histone acetylation events detected at the level of total chromatin and at the promoters of ERα-target genes. Although we have not explored the causal relationship between HAT mRNA induction and histone acetylation detected in the VMH of female mice, the time-courses of these two events lead us to suggest a cause-effect connection (Fig. 7). Among the examined HATs, E2-induced increase in mRNA levels of Src2 and p300 was very rapid and transient, detected only at 3 hours and gone by 5 hour time point. Src1 was the only co-activator that was persistently elevated following E2-treatment, suggesting possible functional specificity of this isoform. These results also underscore the importance of the time scale of experimental design. When studying E2 effects on the brain, molecular mechanisms and correlates are often investigated at the same time scale as behavioural outcomes, 24 or 48 hours after E2-treatment. Our study was specifically designed to investigate early molecular events, which take place on the scale of a few hours. These differences may be the bases for the discrepancies between our findings that Src1 and Src2 mRNA levels in VMH are regulated by E2, and previous reports which failed to detect such regulation 24 hours after E2 treatment [67].
Although several histone methyltransferases have been shown to contribute to ERα transcriptional activity in MCF-7 cells, the importance of H3K4me and its modifying enzymes in E2-mediated effects on the CNS have not been addressed. In our current study, we detected significant increase of mRNA levels of two HMTs, Mll3 and Ash1, as well as an essential subunit of the MLL complex, Wdr5, in the VMH, following E2-administration. The time course of the increase in HMT mRNA expression coincided with the H3K4 methylation events at the promoters of Pgr and Oxtr in the VMH of female mice, once again suggesting a causal relationship. Interestingly, MLL1 has been shown to be one of the factors required for the expression of the PGR gene in human MCF-7 cell [28], however we did not observe any changes in the levels of this specific HMT. These data suggest that the effect of MLL1 on Pgr expression may be tissue specific and that another isoform, MLL3, which is persistently upregulated in VMH by E2, is important for Pgr regulation in the brain. Alternatively, in the brain E2 may affect the recruitment of MLL1 to the promoter of Pgr gene without altering its levels, and further studies will address this possibility. Nonetheless, significant upregulation of Wdr5, a common DNA-binding subunit of both MLL1 and MLL3 complexes, in the VMH of female mice, points to the important role of these HMT complexes in the E2-induced transcriptional regulation. Interestingly, it has been reported that genetically modified female mice, which express catalytically inactive MLL3 (Mll3Δ/Δ were infertile and males with the same mutation were hypofertile [68]. Although no detailed characterization of this phenotype has been provided in the article, given the critical requirement of ERα transcriptional activity for the female reproduction, both at the level of reproductive tissues as well as at the level of the hypothalamic behavioural circuit, we can hypothesise that infertility observed in these mutant mice was due to disruptions in E2-signalling. Moreover, MLL complexes have been previously implicated in the development and various functions of the rodent nervous system, such as nerve cell differentiation, adult neurogenesis and memory formation [69-71] which underlies the importance of studying the role of these epigenetic factors in the brain.
It has been suggested that tissue- and gene-specific effects induced by ligand-activated ERs may be mediated through differential recruitment or expression of coregulator proteins. In the current study we tested whether brain-region specific histone modifications (H3 acetylation and H3K4 methylation), which we detected at the promoters of Pgr and Oxtr genes in the VMH and the POA, may be the result of differential expression of ERα cofactors. Our results, showing vast differences in the mRNA expression of several histone-modifying cofactors between these nuclei, confirmed this hypothesis. While mRNA expression of most cofactors with HAT or HMT activity was significantly upregulated in the VMH of female mice, we observed either no change or, in a few cases, a decrease in the levels of these factors in the POA (Fig. 7 and 8). These data indicate that in the POA, E2-induced histone acetylation and H3K4 methylation at the promoters of Pgr and Oxtr genes does not require the activity of given coregulators. Alternatively, E2 induced histone modifications in these brain regions may be the result of differential recruitment of these factors to the regulatory sequences of the Pgr and Oxtr genes. In conclusion, based on our results, we propose that E2-activated ERα may employ differential epigenetic mechanisms to induce brain-region specific transcription of target genes in the VMH and the POA of female mice.
E2-regulated recruitment of ERα to the putative regulatory-sequences is different between human and mouse
Although it is well established that Pgr gene expression is directly regulated by ligand-activated ERα, we failed to convincingly demonstrate the recruitment of ERα to the regulatory sequences on the Pgr gene. There is no ERE site within the promoter region of either mouse Pgr or human PGR genes. Alternatively, it was suggested that half-ERE and proximal Sp1 or AP-1 sites may be sufficient for ERα recruitment to human Pgr gene promoter [72,73]. More recent studies have shown that, in fact, ERα does not bind within the promoter region of human PGR gene but is rather recruited to the remote enhancer sequences as far as 300kb upstream of the TSS. In concert with these data, we did not observe E2-regulated binding of ERα to the proximal promoter sequences of mouse Pgr. We did, however identify two putative enhancer sites ~81kb and ~200kb upstream of the mouse Pgr gene TSS, which displayed E2-regulated recruitment of ERα (Fig. 6). These were two out of seven ERE-containing sites homologous to the enhancer regions described by Boney-Montoya and colleagues in human MCF-7 cells. Lack of E2-regulated binding at other five ERE-containing sites may be the result of sequence differences between species known to affect ERα binding [74]. Alternatively, other transcription factors may be required for the binding of ERα to the chromatin. In fact, genome-wide analysis of ERα binding sites revealed that one such factor, Forkhead (FoxA1), binds in the close proximity of ERα and is in fact required for ERα association with chromatin in human cell line [34,75]. Moreover, Fox1A has been identified as one of only four coregulators essential for the expression of Pgr gene in MCF-7 cells [28]. Thus, further analyses of the role of this pioneer factor in the transcriptional activity of ERα in the mouse brain are warranted. Such studies will provide valuable information about the molecular mechanisms of E2 regulated gene expression in the brain and lead to better understanding of hormone effects on CNS functions.
Conclusions
Although oestrogens exert considerable influence on the variety of functions of the nervous system, not only in females but also in males, the studies of such influences have mostly focused on behavioural, physiological or pathological effect. Studies on molecular mechanisms that underlie oestrogens’ actions and the analyses of gene networks regulated by hormones are most often performed using more easily accessible and manipulated cell culture systems. This is understandable, considering the challenging technical issues neuroscientists have to overcome when studying molecular events induced by ligand-activated hormone receptor in the brains of living animals: individual variability, small amount of starting material, heterogeneity of starting material (presence of glial cells, neurones that do not express ERα, and not all ERα expressing neurones are identical). As a result, molecular and biochemical changes in response to hormonal treatments are always more subtle in the brain than in the homologous cell culture systems. However, given the vastly diverse physiological, cellular and transcriptional effects that oestrogens exert on different tissues and cell types, it is of utmost importance to address the questions of molecular transcriptional mechanisms underlying oestrogens actions in a relevant system. Moreover, prevalent use of hormone replacement therapy (HRT) in postmenopausal women as well as the use of different selective oestrogen receptor modulators (SERMS) for the treatment of breast and endometrial cancers or osteoporosis, highlight immediate benefits of such studies for the understanding of hormone effects on women’s health and disease.
Supplementary Material
Acknowledgments
We would like to thank Dr. Kyung Min Noh and the Allis lab for technical assistance. We are grateful to Dr. Richard Hunter and Dr. Gen Murakami for helpful discussions. Support was provided by National Institute of Health grant HD05751-39 to DWP and Tri-institutional training fellowship 5T32DK007313-33 to KG.
References
- 1.Wend K, Wend P, Krum SA. Tissue-Specific Effects of Loss of Estrogen during Menopause and Aging. Front Endocrinol (Lausanne) 2012;3:19. doi: 10.3389/fendo.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen signaling in the hypothalamus. Vitam Horm. 2005;71:123–145. doi: 10.1016/S0083-6729(05)71005-0. [DOI] [PubMed] [Google Scholar]
- 3.Pfaff DW. Features of a hormone-driven defined neural circuit for a mammalian behavior. Principles illustrated, neuroendocrine syllogisms, and multiplicative steroid effects. Ann N Y Acad Sci. 1989;563:131–147. doi: 10.1111/j.1749-6632.1989.tb42195.x. [DOI] [PubMed] [Google Scholar]
- 4.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979 Mar;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979 Mar;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 6.Mong JA, Pfaff DW. Hormonal symphony: steroid orchestration of gene modules for sociosexual behaviors. Mol Psychiatry. 2004 Jun;9:550–556. doi: 10.1038/sj.mp.4001493. [DOI] [PubMed] [Google Scholar]
- 7.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996 Jun 11;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein-Hitpass L, Ryffel GU, Heitlinger E, Cato AC. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16:647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997 Sep;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll MD, Sathya G, Muyan M, Klinge CM, Hilf R, Bambara RA. Sequence requirements for estrogen receptor binding to estrogen response elements. J Biol Chem. 1998 Nov 6;273:29321–29330. doi: 10.1074/jbc.273.45.29321. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998 Dec;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA. 1999 Oct 26;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA. 2006 Jul 5;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai MJ, O’Malley BW. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. The Journal of Steroid Biochemistry and Molecular Biology. 1999 Mar;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- 16.Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995 Nov 24;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 17.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996 Dec 19;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 18.Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001 Jan 12;276:1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- 19.Chen D. Regulation of Transcription by a Protein Methyltransferase. Science. 1999 Jun 25;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 20.Ansari KI, Shrestha B, Hussain I, Kasiri S, Mandal SS. Histone Methylases MLL1 and MLL3 Coordinate with Estrogen Receptors in Estrogen-Mediated HOXB9 Expression. Biochemistry. 2011 May 3;50:3517–3527. doi: 10.1021/bi102037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor Dynamics and Sufficiency in Estrogen Receptor-Regulated Transcription. Cell. 2000 Dec;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 22.Shang Y. Molecular Determinants for the Tissue Specificity of SERMs. Science. 2002 Mar 29;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 23.Parsons B, Rainbow TC, Pfaff DW, McEwen BS. Oestradiol, sexual receptivity and cytosol progestin receptors in rat hypothalamus. Nature. 1981 Jul 2;292:58–59. doi: 10.1038/292058a0. [DOI] [PubMed] [Google Scholar]
- 24.Brown TJ, Clark AS, MacLusky NJ. Regional sex differences in progestin receptor induction in the rat hypothalamus: effects of various doses of estradiol benzoate. J Neurosci. 1987 Aug;7:2529–2536. [PMC free article] [PubMed] [Google Scholar]
- 25.Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology. 2008 Jun;149:3054–3061. doi: 10.1210/en.2007-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzales KL, Quadros-Mennella P, Tetel MJ, Wagner CK. Anatomically-Specific Actions of Oestrogen Receptor in the Developing Female Rat Brain: Effects of Oestradiol and Selective Oestrogen Receptor Modulators on Progestin Receptor Expression. J Neuroendocrinol. 2012 Jan 17;24:285–291. doi: 10.1111/j.1365-2826.2011.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karmakar S, Foster EA, Smith CL. Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-alpha transcriptional activity. Endocrinology. 2009 Apr;150:1588–1596. doi: 10.1210/en.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won Jeong K, Chodankar R, Purcell DJ, Bittencourt D, Stallcup MR. Gene-Specific Patterns of Coregulator Requirements by Estrogen Receptor-in Breast Cancer Cells. Molecular Endocrinology. 2012 May 18;26:955–966. doi: 10.1210/me.2012-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apostolakis EM, Ramamurphy M, Zhou D, Oñate S, O’Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Molecular Endocrinology. 2002 Jul;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 30.Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002 Feb;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 31.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus W, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Molecular Endocrinology. 1993;7:1603–1616. doi: 10.1210/mend.7.12.8145766. [DOI] [PubMed] [Google Scholar]
- 33.Hagihara K, Wupeng XS, Funabashi T, Kato J, Pfaff DW. Nucleic acid sequence and Dnase hypersensitive sites of the 5′region of the mouse progesterone receptor gene. Biochemical and Biophysical Research Communications. 1994;205:1093–1101. doi: 10.1006/bbrc.1994.2778. [DOI] [PubMed] [Google Scholar]
- 34.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006 Oct 1;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 35.Boney-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. Long-Range Transcriptional Control of Progesterone Receptor Gene Expression. Molecular Endocrinology. 2010 Jan 26;24:346–358. doi: 10.1210/me.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Gulf Professional Publishing. 2004 [Google Scholar]
- 37.Milne TA, Zhao K, Hess JL. Humana Press; Totowa, NJ: 2009. Methods in Molecular Biology™. [Google Scholar]
- 38.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008 Jun;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 39.Kow LM, Pfaff DW. Induction of lordosis in female rats: two modes of estrogen action and the effect of adrenalectomy. Horm Behav. 1975 Sep;6:259–276. doi: 10.1016/0018-506x(75)90013-6. [DOI] [PubMed] [Google Scholar]
- 40.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, Saal vom FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003 Jun;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allis CD, Jenuwein T, Reinberg D. Epigenetics. CSHL Press; 2007. [Google Scholar]
- 42.Latham JA, Dent SYR. Cross-regulation of histone modifications. Nature Structural & Molecular Biology. 2007 Nov 5;14:1017–1024. doi: 10.1038/nsmb1307. [DOI] [PubMed] [Google Scholar]
- 43.Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000 Jun;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 44.Nowak SJ. Phosphorylation of histone H3 correlates with transcriptionally active loci. Genes & Development. 2000 Dec 1;14:3003–3013. doi: 10.1101/gad.848800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Cao R, Xia L, Erdjument-Bromage H, Borchers C, Tempst P, et al. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol Cell. 2001 Dec;8:1207–1217. doi: 10.1016/s1097-2765(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 46.Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982 Oct;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May FEB, Johnson MD, Wiseman LR, Wakeling AE, Kastner P, Westley BR. Regulation of progesterone receptor mRNA by oestradiol and antioestrogens in breast cancer cell lines. Journal of Steroid Biochemistry. 1989 Dec;33:1035–1041. doi: 10.1016/0022-4731(89)90406-8. [DOI] [PubMed] [Google Scholar]
- 48.Rickard DJ, Waters KM, Ruesink TJ, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, et al. Estrogen receptor isoform-specific induction of progesterone receptors in human osteoblasts. J Bone Miner Res. 2002 Apr;17:580–592. doi: 10.1359/jbmr.2002.17.4.580. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005 Jan 28;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, et al. Genomic Analyses of Transcription Factor Binding, Histone Acetylation, and Gene Expression Reveal Mechanistically Distinct Classes of Estrogen-Regulated Promoters. Molecular and Cellular Biology. 2007 Jun 28;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schumacher M, Coirini H, Flanagan LM, Frankfurt M, Pfaff DW, McEwen BS. Ovarian steroid modulation of oxytocin receptor binding in the ventromedial hypothalamus. Annals of the New York Academy of Sciences. 1992 Jun 12;652:374–386. doi: 10.1111/j.1749-6632.1992.tb34368.x. [DOI] [PubMed] [Google Scholar]
- 52.Gao H, Dahlman-Wright K. The gene regulatory networks controlled by estrogens. Molecular and Cellular Endocrinology. 2011 Mar 1;334:83–90. doi: 10.1016/j.mce.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Jeyakumar M, Carlson KE, Gunther JR, Katzenellenbogen JA. Exploration of Dimensions of Estrogen Potency: PARSING LIGAND BINDING AND COACTIVATOR BINDING AFFINITIES. Journal of Biological Chemistry. 2011 Apr 8;286:12971–12982. doi: 10.1074/jbc.M110.205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005 Jun 17;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 55.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007 Jan 12;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 56.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004 Dec 29;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AHFM, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005 Sep 15;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 58.Stratmann A, Haendler B. The histone demethylase JARID1A regulates progesterone receptor expression. FEBS Journal. 2011 Mar 25;278:1458–1469. doi: 10.1111/j.1742-4658.2011.08058.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee DY, Hayes JJ, Pruss D, Wolffe AP. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993 Jan 15;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proceedings of the National Academy of Sciences. 2010 Mar 23;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011 Jul;152:2760–2767. doi: 10.1210/en.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Sun L, Zhang Y, Wang D, Wang F, Liang J, et al. The Histone Modifications Governing TFF1 Transcription Mediated by Estrogen Receptor. Journal of Biological Chemistry. 2011 Apr 15;286:13925–13936. doi: 10.1074/jbc.M111.223198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mong JA, Devidze N, Goodwillie A, Pfaff DW. Reduction of lipocalin-type prostaglandin D synthase in the preoptic area of female mice mimics estradiol effects on arousal and sex behavior. Proc Natl Acad Sci USA. 2003 Dec 9;100:15206–15211. doi: 10.1073/pnas.2436540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, et al. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. Journal of Molecular Endocrinology. 2003 Jun;30:347–358. doi: 10.1677/jme.0.0300347. [DOI] [PubMed] [Google Scholar]
- 65.Johnson AE, Ball GF, Coirini H, Harbaugh CR, McEwen BS, Insel TR. Time course of the estradiol-dependent induction of oxytocin receptor binding in the ventromedial hypothalamic nucleus of the rat. Endocrinology. 1989 Sep;125:1414–1419. doi: 10.1210/endo-125-3-1414. [DOI] [PubMed] [Google Scholar]
- 66.Auger AP, Perrot-Sinal TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002 Aug;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tognoni CM, Chadwick JG, Jr, Ackeifi CA, Tetel MJ. Nuclear receptor coactivators are coexpressed with steroid receptors and regulated by estradiol in mouse brain. Neuroendocrinology. 2011;94:49–57. doi: 10.1159/000323780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S, Lee D-K, Dou Y, Lee J, Lee B, Kwak E, et al. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc Natl Acad Sci USA. 2006 Oct 17;103:15392–15397. doi: 10.1073/pnas.0607313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wynder C, Hakimi M-A, Epstein JA, Shilatifard A, Shiekhattar R. Recruitment of MLL by HMG-domain protein iBRAF promotes neural differentiation. Nat Cell Biol. 2005 Oct 9;7:1113–1117. doi: 10.1038/ncb1312. [DOI] [PubMed] [Google Scholar]
- 70.Lim DA, Huang Y-C, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009 Feb 11;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, et al. Histone Methylation Regulates Memory Formation. Journal of Neuroscience. 2010 Mar 10;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petz LN, Ziegler YS, Loven MA, Nardulli AM. Estrogen receptor alpha and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology. 2002 Dec;143:4583–4591. doi: 10.1210/en.2002-220369. [DOI] [PubMed] [Google Scholar]
- 73.Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. The Journal of Steroid Biochemistry and Molecular Biology. 2004 Feb;88:113–122. doi: 10.1016/j.jsbmb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Bourque G, Leong B, Vega VB, Chen X, Lee YL, Srinivasan KG, et al. Evolution of the mammalian transcription factor binding repertoire via transposable elements. Genome Research. 2008 Nov;18:1752–1762. doi: 10.1101/gr.080663.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005 Jul 15;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.