Abstract
Purpose
With substantial variation in follow-up for patients after radical cystectomy for bladder cancer, we sought to understand the effect of urine tests, laboratory tests, physician visits, and imaging on overall survival.
Materials and Methods
We analyzed a cohort of patients treated in the fee-for-service Medicare population from 1992 through 2007 using Surveillance Epidemiology and End Results - Medicare data. Using propensity score analysis, we assessed the relationship between time- and geography-standardized expenditures on follow-up care and overall survival in three time periods after surgery: peri-operative (0–3 months), early follow-up (4–6 months), and later follow-up (7–24 months). Using instrumental variables analysis, we assessed the overall survival impact of quantity of follow up care by category (doctor visits, imaging, lab tests, urine tests).
Results
We found no improvement in survival from follow-up care in the peri-operative and early follow-up periods. Receipt of follow-up care in the later follow-up period was associated with improved survival [HR 0.23, 95% CI 0.15–0.35; 0.27, 95% CI: 0.18–0.40; 0.47, 95% CI: 0.31–0.71, low, middle and high tertile of expenditures, respectively]. Instrumental variables analysis suggested only doctor visits and urine testing [HRs: 0.96 (0.93–0.99) and 0.95 (0.91–0.99), respectively] improved survival.
Conclusion
Follow-up care after radical cystectomy in the later follow-up period was associated with improved survival. Doctor visits and urine tests were associated with this improved survival. Our results suggest aspects of follow-up care significantly improve patient outcomes, but imaging studies could be used more judiciously after cystectomy.
Keywords: Urinary bladder neoplasms, Cystectomy, Survival analysis, Follow-up studies
Introduction
For patients with definitive surgery for bladder cancer, adequate follow-up care remains undefined. While agreement exists on the need for regular physical examination and laboratory testing,1–6 and guidelines focus on finding cancer recurrence or dysfunction related to the urinary diversion,7 the frequency at which visits should occur varies substantially amongst studies. Furthermore, various recommendations have been reported for imaging studies, including CT or MRI scans,6 trans-rectal ultrasound,5 and no imaging,8 but they lack firm empirical evidence. Other recommended tests include voided cytology3, 6 and urethral wash cytology,1, 3, 4, 6 but evidence for this is also scant. Finally, effectiveness of follow-up studies in patients treated with neoadjuvant or adjuvant chemotherapy has not been fully assessed.2, 9
This lack of evidence causes wide variations in medical care provided by urologists, resulting in extensive variability in costs.10 On the one hand, if more attentive follow-up care is associated with improved survival, patients receiving less care are directly harmed. On the other hand, if the care is not improving outcomes, patients are receiving poor value for their care.
In this study we assessed the benefit of follow-up care for detection of recurrence or metabolic abnormalities among patients who have received definitive treatment for bladder cancer. We hypothesized that more follow-up care, characterized as adjusted Medicare expenditures on doctor visits, imaging, laboratory tests and urine tests, would not impact patient survival compared to less follow-up care.
Materials and Methods
Data Source
After review by the Washington University Institutional Review Board and granting of an exempt status, we assembled our study cohort from linked Surveillance Epidemiology and End Results (SEER)-Medicare data using bladder cancer cases (International Classification of Diseases for Oncology 3rd edition (ICO-3) codes 188.x,11) diagnosed between 1992 and 2005, with follow up through 2007. To have a uniform study population, we limited our cohort to only those regions which were involved in data collection through the entire study period (Seattle, Detroit, Atlanta, San Jose/Monterey, San Francisco/Oakland, Los Angeles, New Mexico, Connecticut, Utah, Iowa, and Hawaii). The rural Georgia registry was not included in the study due to a small number of eligible cases.
Study Population
Our cohort formation is illustrated in Figure 1. Similar to other studies,12, 13 we examined the inpatient (MEDPAR) and physician (NCH) claims for codes consistent with radical cystectomy (Table 1). After the restrictions as outlined in Figure 1, our study population consisted of 2010 patients. All patients were assigned to a primary urologic surgeon based on encrypted physician UPIN numbers and physician specialty coding. In the few cases where multiple surgeons were involved with surgery, the surgeon who had done more cases within the cohort was considered the primary surgeon.
Figure 1.
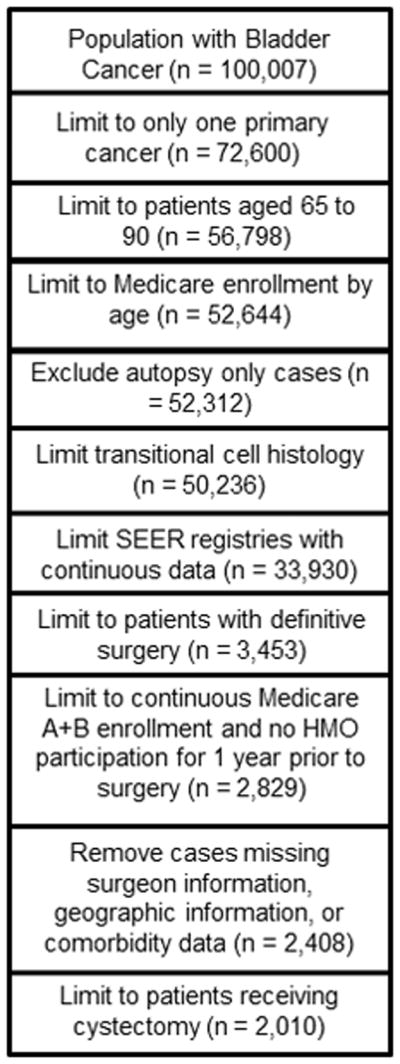
Cohort Formation
The steps taken for cohort development from the linked SEER-Medicare data are shown.
Table 1.
Codes for Partial and Radical Cystectomy
| International Classification of Disease 9th Edition | Healthcare Common Procedure Coding System |
|---|---|
| 57.6 | 51570 |
| 57.7 | 51575 |
| 57.71 | 51580 |
| 57.79 | 51585 |
| 68.8 | 51590 |
| 51595 | |
| 51596 | |
| 51597 |
Characterization of Follow-up Care
Using healthcare common procedure and coding system (HCPCS) codes, we determined outpatient care from date of surgery to 24 months of follow-up in four categories; urine testing, laboratory testing, imaging, and doctor visits (Table 2). National Comprehensive Cancer Network guidelines recommend surveillance for patients after radical cystectomy for two years with further follow-up care as clinically indicated.7 Doctor visits included all outpatient visits to any physician. We calculated the total monthly expenditures for the care of each patient, price-adjusted for time and geography using the Medicare Economic Index.14 We stopped calculation of expenditures at the date of recurrence using a previously published algorithm,15 when the patient no longer had Medicare A and B coverage, or was no longer enrolled in an HMO. We excluded from calculation of expenditures all care that took place during hospitalizations using dates from the MEDPAR file. Sensitivity analyses were performed to assess the impact of stopping assessment at these time points on the study results. Each patient’s adjusted monthly expenditure on care in two periods was calculated; from 4 to 6 months (early follow-up period), and from 7 to 24 months (later follow-up period). These periods were chosen based on the post-operative course of bladder cancer patients with the first 3 months after surgery being characterized by the development of immediate post surgery complications, the next three months characterized by resolution of any post-operative complications, and the final period in the study reflecting longer-term follow-up care. Patients were grouped based their expenditure in each time period; no expenditure, or the low, middle, or high tertile of expenditures. For each post-surgical time period, we also determined the follow-up care expenditures by category.
Table 2.
Codes for Follow-Up Care
| HCPCS | Category | |
|---|---|---|
| Urine Testing | 88104, 88106, 88107, 88108, 88112, 88160, 88161, 88162 | Urine cytology |
| 81000, 81001, 81002, 81003, 81005, 81007, 81015 | Urine analysis | |
| Laboratory Testing | 80058, 80076 | Hepatic function panel |
| 80048, 80049 | Basic metabolic panel | |
| 80051 | Electrolyte panel | |
| 80053, 80054 | Comprehensive metabolic panel | |
| 80069 | Renal function panel | |
| 80002 through 80019 | Older Panels | |
| 82040, 82247, 82310, 82374, 82435, 82565, 82947, 84075, 84132, 84155, 84295, 84460, 84450, 84520 | Other electrolytes | |
| Imaging Procedures | 71010, 71015, 71020, 71021, 71022, 71023, 71030, 71034, 71035 | Chest x-ray |
| 71250, 71260, 71270, 71275, 74150 to 74170, 72191, 72192, 72193, 72194, 76497 | Cat (CT) scan | |
| 71550, 71551, 71552, 71555, 74181, 74182, 74183, 74185, 72195, 72196, 72197, 76498 | MRI | |
| 78810, 78811, 78812, 78813, 78814, 78815, 78816 | Positron emission tomography (PET) scan | |
| 74400, 74410, 74415 | Intravenous urography | |
| 74420, 74425 | Retrograde urography | |
| 76700, 76770, 76705, 76775, 76778 | Abdominal/Renal ultrasound | |
| 78700, 78701, 78704, 78707, 78708, 78709, 78715 | Nuclear medicine renal scan | |
| 78300, 78305, 78306, 78315, 78320 | Nuclear medicine bone scan | |
| 78800, 78801, 78802, 78990 | Radiopharmaceutical localization of tumor or distribution of radiopharmaceutical agent(s) | |
| 76061, 76062 | Radiologic exam, osseous survey; (for metastases) | |
| Doctor Visits | 99201, 99202, 99203, 99204, 99205 | New patient office visits |
| 99211, 99212, 99213, 99214, 99215 | Established patient office visits | |
| 99241, 99242, 99243, 99244, 99245 | Consultation office visit |
Outcome
The primary outcome of interest was overall survival. We also assessed the contribution of each category of follow-up care (urine testing, laboratory testing, imaging, and doctor visits) to total expenditures in the tertiles of care.
Statistical Analysis
Differences in patient populations during each time period after surgery were assessed with Mantel-Haenszel chi-square testing. We then created propensity score models to balance observed covariates of the different expenditure groups.16 We assessed the impact of patient age, comorbidity (methods of Klabunde et al),17 tumor stage, tumor grade, lymph node status (positive, negative, no lymph nodes assessed), surgeon volume, hospital NCI cancer center status, hospital readmissions after surgery, receipt of chemotherapy (neoadjuvant, adjuvant, as delayed therapy, or as combinations of the therapies), ZIP code level education status, and zip code level income on the receipt of follow-up care. Statistically significant variables, including chemotherapy, stage, lymph node status, readmission, age group, comorbidity, and zip code level education status, were kept in the model. Because there is uncertainty about the most appropriate form of propensity scores, we examined the effect of continuous scores, stratified scores, and categorical scores by decile.
As unknown confounding factors may impact the results of our propensity score analysis, we performed instrumental variable (IV) analysis to control for known and unknown factors potentially biasing the impact of follow-up care on survival. We considered five types of care – doctor visits, image tests, urine tests, laboratory tests, and total services in general. Similar to other studies,12, 18, 19 we chose as the IV the patient’s distance to the nearest partial or radical cystectomy care provider. Distance was measured as the straight-line distance from the center of the patient’s residential zip code to the center of the nearest surgeon zip code. Distance was selected as an IV based on two criteria: (i) the instrument relevance condition: an IV has to be associated with the likelihood of the follow-up care; and (ii) the instrument exogeneity condition: an IV has to be uncorrelated with the mortality/survival outcome. While the second condition cannot be tested, the first condition was tested by the individual (t) and joint significance (F) tests.20–22 We used a two-stage residual inclusion approach designed for IV estimation in a nonlinear model context,23–25 including a two-part model26 to account for the right-skewed distribution of monthly frequency of care in the first stage and a Cox proportional hazard model in the second stage. The Sargan test was used to check for the lack of correlation with the error term.27 All of the Sargan test results suggested that there was no evidence to show that the error term was correlated with the covariates included in the first stage.
All analysis was performed with SAS version 9.2.
Results
With censoring for loss to follow up and death, the initial cohort of 2010 patients decreased to 1568 at the 4th month after surgery and 1396 by the 7th month after surgery (Table 3). No significant difference among follow-up care tertiles was seen in either time period by age, race, gender, or zip-code level educational achievement. Patients with more comorbidity had more follow-up care in the initial post-surgical time period, but this difference attenuated over the later time periods. Stage III and IV patients received more intense follow-up than earlier-stage patients. Higher median income was consistently associated with being in the highest tertile of follow-up intensity across the time periods.
Table 3.
Patient Characteristics by Time Period and Expenditure Groups
| Early Follow-up | Later Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expenditure Groups | Expenditure Groups | |||||||||
| 0 | low | med | high | P value | 0 | low | med | high | P value | |
| N | 377 | 586 | 586 | 605 | 264 | 557 | 557 | 575 | ||
| Age Category | ||||||||||
| 66 to 69 | 18.8 | 14.8 | 20.2 | 20.7 | 0.1423 | 18.5 | 19.1 | 19.4 | 18.9 | 0.413 |
| 70 to 74 | 26 | 31 | 26.4 | 28.8 | 24.3 | 29.6 | 28.9 | 30.7 | ||
| 75 to 79 | 29.4 | 26.9 | 29 | 28.2 | 28.6 | 26.6 | 30.9 | 26.4 | ||
| 80+ | 25.7 | 27.3 | 24.4 | 22.3 | 28.6 | 24.7 | 20.8 | 24 | ||
| Race | ||||||||||
| White | 91.8 | 95.6 | 90.7 | 90.4 | 0.003 | 88.9 | 94.2 | 92.6 | 91.6 | 0.09 |
| Non-White | 8.2 | 4.4 | 9.3 | 9.6 | 11.1 | 5.8 | 7.4 | 8.4 | ||
| Sex Male | 65.3 | 69.9 | 66.7 | 63.3 | 0.118 | 64 | 69.1 | 70.4 | 62.3 | 0.012 |
| Married | 62.9 | 66.3 | 67.3 | 67.9 | 0.452 | 66.1 | 68.2 | 67.1 | 66 | 0.991 |
| Unmarried/Unknown | 37.1 | 33.7 | 32.7 | 32.1 | 33.9 | 31.8 | 32.9 | 34 | ||
| Grade | ||||||||||
| Well differentiated | <5 | <5 | <5 | <5 | 0.0002 | <5 | <5 | <5 | <5 | 0.0001 |
| Moderately differentiated | 11.4 | 16.4 | 13.6 | 10.2 | 7.4 | 17.2 | 15.4 | 9.8 | ||
| Poorly differentiated | 54.4 | 54 | 47.9 | 49.8 | 57.7 | 49 | 51.7 | 48 | ||
| undifferentiated | 28.4 | 24.5 | 31.5 | 36.4 | 31.2 | 26.6 | 27.3 | 37.9 | ||
| Undetermined | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | ||
| Comorbidity | ||||||||||
| 0 | 56.8 | 54 | 57.4 | 53.9 | 0.6221 | 56.1 | 61 | 55.2 | 53.2 | 0.1319 |
| 1 | 27.8 | 314 | 26.7 | 28 | 25.9 | 26 | 28.1 | 30.7 | ||
| 2 | 11.7 | 10.2 | 10.8 | 12.3 | 12.2 | 9.4 | 12.5 | 10.1 | ||
| 3 | 3.7 | 4.4 | 5.1 | 5.8 | 5.8 | 3.6 | 4.2 | 6 | ||
| Nodes | ||||||||||
| All nodes neg | 33.7 | 37.7 | 35 | 32.9 | 0.0001 | 23.3 | 37.1 | 37.8 | 35.7 | 0.0001 |
| Nodes Positive | 10.3 | 3.9 | 11.1 | 18.8 | 16.4 | 4.3 | 8.4 | 16.1 | ||
| No nodes exam | 56 | 58.4 | 53.9 | 48.3 | 60.3 | 58.6 | 53.8 | 48.2 | ||
| Final Stage | ||||||||||
| Stage 1 | 23.9 | 27.8 | 24.5 | 19.5 | 0.0001 | 18 | 29.2 | 26.2 | 21.1 | 0.0001 |
| Stage 2 | 16.2 | 21.6 | 19.6 | 16 | 13.2 | 20.5 | 18.1 | 21.8 | ||
| Stage 3 | 20.1 | 18.4 | 21.6 | 25.9 | 25.9 | 19.2 | 22.6 | 22.3 | ||
| Stage 4 | 19.6 | 10.6 | 17.2 | 25.4 | 25.9 | 9.1 | 15.5 | 21.3 | ||
| Unknown, No Cancer, In Situ | 20.2 | 21.6 | 17.1 | 13.2 | 17.0 | 22.0 | 17.6 | 13.5 | ||
| Educational Achievement | ||||||||||
| <10 no HS | 32.7 | 29.4 | 30.2 | 32.6 | 0.4262 | 35.8 | 31.3 | 28.5 | 33.5 | 0.1471 |
| 10–19 | 41.8 | 47.1 | 44.5 | 43 | 36.4 | 46.8 | 46 | 42.9 | ||
| 20–29 | 13.4 | 15.6 | 14.8 | 13.4 | 13.9 | 12.6 | 15.7 | 14.6 | ||
| >=30 no HS | 12.1 | 7.9 | 10.5 | 10.9 | 13.9 | 9.3 | 9.8 | 9 | ||
| Readmission | ||||||||||
| None | 16.7 | 15.2 | 12.9 | 14.9 | 0.0414 | 16.4 | 16.5 | 14.5 | 12.7 | 0.004 |
| 1 | 23.6 | 16.3 | 20.2 | 17.2 | 22.2 | 15.6 | 17.6 | 21.3 | ||
| 2 | 15.4 | 15.2 | 15.8 | 18.9 | 18.5 | 12.4 | 14.2 | 18 | ||
| 3+ | 44.3 | 53.3 | 51.1 | 49 | 42.9 | 55.5 | 53.7 | 48 | ||
| Median Income | ||||||||||
| 1st QR | 29.7 | 24.8 | 23.8 | 22 | 0.004 | 29.4 | 24.7 | 27.2 | 19.9 | 0.004 |
| 2nd QR | 24.4 | 27.4 | 26.7 | 21.3 | 23.5 | 28.3 | 24.7 | 21.7 | ||
| 3rd QR | 26.4 | 25.3 | 24.6 | 26.1 | 23 | 24.4 | 24.8 | 28.2 | ||
| 4th QR | 29.5 | 22.5 | 24.9 | 30.6 | 24.1 | 22.6 | 23.3 | 30.2 | ||
| Chemotherapy | ||||||||||
| None | 70.8 | 76 | 64.7 | 47.8 | 0.0001 | 46.6 | 76.8 | 67.4 | 52.8 | 0.0001 |
| Neoadjuvant Only | 5 | 4.9 | 6.1 | 4.4 | <5 | 4.5 | 4 | 6.5 | ||
| Adjuvant Only | 9.5 | 2.4 | 11.6 | 25.8 | 25.4 | 4.1 | 8.8 | 17.2 | ||
| Treatment Only | 8.5 | 13.8 | 10 | 9.2 | 10 | 10.8 | 13.9 | 10.6 | ||
| Neoadjuvant and Adjuvant | <5 | <5 | 2.5 | 1.9 | <5 | 0.3 | <5 | 1.7 | ||
| Neoadjuvant or Adjuvant then Treatment | <5 | <5 | 5.1 | 8.4 | 8.5 | 2.6 | <5 | 8.8 | ||
| Continuous | <5 | <5 | <5 | 2.5 | 3.7 | 0.9 | <5 | 2.4 | ||
Cell sizes that could be derived as a cell size <11 were suppressed in accordance with the SEER-Medicare Data Use agreement. Those cells are reported as < 5%. In Situ cancer was rare at cystectomy, and for reporting in accordance with SEER-Medicare Data Use agreements has been combined with the no cancer group.
Propensity Score Analysis
Results from the survival analysis were consistent when using the continuous, stratified, and categorical methods of proportional hazards regression modeling (Table 4). Expenditures for care during the early follow up were not associated with improvements in survival. By the later follow-up period, patients with expenditures on care had improved survival compared to no expenditures on care across all expenditure categories and models [HR 0.23, 95% CI 0.15–0.35; 0.27, 95% CI: 0.18–0.40; 0.47, 95% CI: 0.31–0.71, low, middle and high tertile of expenditures respectively in the continuous propensity score adjusted model (reference group: no expenditure)].
Table 4.
Propensity Score Adjusted Analysis of Overall Survival with Hazard Ratios and 95% Confidence Intervals
| Propensity Score Adjustment | |||
|---|---|---|---|
| Continuous (HR; 95% CI) | Stratified | Categorical | |
| Early Follow-up Care | |||
| None | 1 | 1 | 1 |
| Low Tertile | 0.866 (0.679, 1.103) | 0.916 (0.712, 1.178) | 0.838 (0.657, 1.069) |
| Middle Tertile | 1.064 (0.833, 1.359) | 1.126 (0.871, 1.454) | 1.035 (0.810, 1.323) |
| High Tertile | 1.144 (0.880, 1.489) | 1.177 (0.892, 1.554) | 1.104 (0.848, 1.437) |
| Later Follow-up Care | |||
| None | 1 | 1 | 1 |
| Low Tertile | 0.228 (0.150, 0.345) | 0.198 (0.127, 0.310) | 0.222 (0.147, 0.336) |
| Middle Tertile | 0.265 (0.175, 0.402) | 0.225 (0.144, 0.351) | 0.256 (0.169, 0.388) |
| High Tertile | 0.468 (0.309, 0.709) | 0.423 (0.271, 0.660) | 0.452 (0.298, 0.685) |
Categorization of Expenditures
Figure 2 illustrates the distribution of average per patient monthly expenditures for follow-up care in the early follow-up and later follow-up periods. Expenditures for all patient groups were similar in both periods. In all both periods, imaging tests accounted for the majority of follow-up care expenditures.
Figure 2.
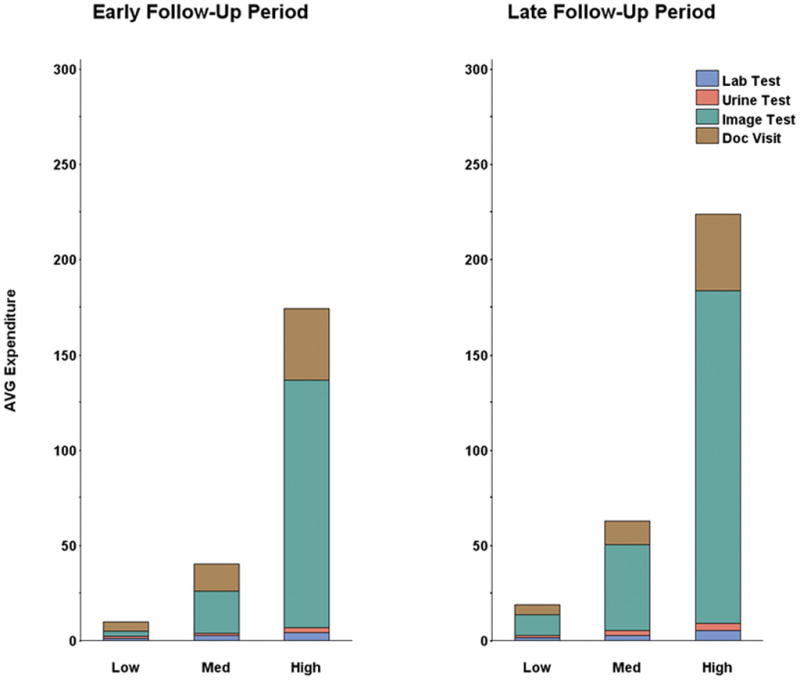
Average Expenditures on Follow-up Care by Category and Follow-up Period
Imaging tests were the single largest contributor to expenditures in all tertiles of care. Although it was possible for expenditures for any single class of care to be stable across the tertiles in each time period, all types of follow-up care had significantly higher expenditures in the high expenditure tertile compared to the low expenditure tertile.
Instrumental variable analysis
Figure 3 reports the hazard ratios and 95% confidence intervals for overall survival related to monthly follow-up care frequency, including total services, doctor visits, imaging, laboratory tests, and urinary tests for the first 24 months after surgery. The regression results suggested that doctor visits and urine testing decreased the risk of death (HR: 0.96, 95% CI: 0.93–0.99 and HR: 0.95, 95% CI: 0.91–0.99, respectively). To quantify this survival advantage; if a patient increased one additional physician visit in the first two years after surgery, then his/her risk of death would decrease by 4%. In addition, an increase of one urine test was associated with a decrease in the risk of death by 5%. Conversely, increasing use of imaging was associated with an increased risk of death (HR: 1.03, 95% CI: 1.02–1.04). Increasing laboratory testing and total services were not statistically significant (HR: 1.003, 95% CI: 0.99–1.02, HR: 0.99, 95% CI: 0.96–1.01, respectively).
Figure 3.
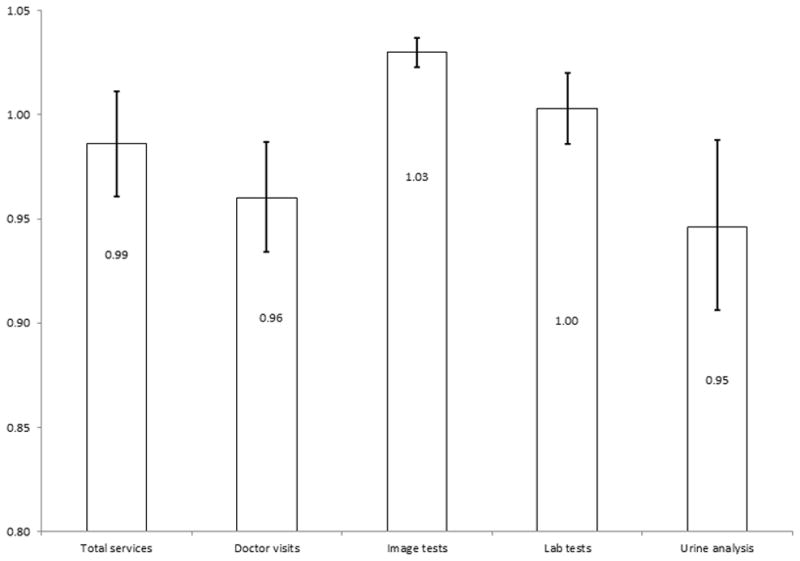
Hazard ratios and 95% Confidence Intervals for the Instrumental Variables Analysis of Monthly Intensity of Follow-up Care and Survival.
Increased use of doctor visits and urine testing was associated with improved survival. Imaging tests were associated with decreased survival.
Sensitivity Analysis
We performed sensitivity analyses to test the assumptions made during the course of assembling and analyzing our study cohort related to the ascertainment of follow-up care. None of the sensitivity analyses changed the results of the study.
Discussion
Contrary to our hypothesis, bladder cancer patients who received cystectomy and follow-up care had improved survival. However, benefits of follow-up care were restricted to the later follow-up care period (7–24 months after surgery). No difference was found between low, middle, and high tertiles of follow-up care expenditures. Further examination of follow-up care revealed that imaging studies drive expenditures, but only doctor visits and urinary tests were associated with improved survival.
Our results suggest that aspects of follow-up care are likely beneficial for patients after radical cystectomy. Empirical evidence for follow-up care affecting survival is lacking, with varying recommendations for follow-up care.1–6 While studies show that imaging studies and urine tests can detect asymptomatic recurrent disease, evidence for the survival benefit from this early detection is lacking. We find support for urine testing as a modality to diagnose recurrence at a treatable point, but we did not find a survival advantage for imaging studies. Surveillance of the remaining urinary tract, specifically the urethra for CIS, has been beneficial in single institution studies.28 Similarly, lab tests and physician visits are used to monitor patient’s general health status and complications related to the urinary diversion. Here, we found physician visits to be associated with improved survival, but lab tests did not show this same association.
Our results assist practicing urologists by defining aspects of care most likely to improve patient survival. First, this study adds to literature showing care provided during the follow-up period is important for patient’s overall survival.28, 29 Second, doctor visits and urine tests were associated with improvements in survival, suggesting these relatively low cost interventions are finding significant problems based on symptoms at a stage when intervention is still possible and helpful to the patient. Such care may be related to metabolic complications of urinary diversion and general medical conditions for which imaging does not mediate the relationship between testing and a successful intervention. Third, no difference was found between low, intermediate, and high expenditures on follow-up care. Thus, while some care is needed, our results show that the highest cost care may not be the most effective care.
Despite the fact that the highest costs in follow-up care come from imaging tests, these tests were not associated with improvements in patient survival. Imaging after definitive cancer surgery is a screening test looking for recurrent disease before it becomes symptomatic. For such screening to be effective, treatments must be available that will arrest the natural course of the disease. The lack of improvement in outcomes with the expenditures related to this imaging is not surprising given the lack of effective treatments for patients who develop metastatic transitional cell cancer of the bladder. Furthermore, imaging tests themselves may have risk, both from contrast related injury and invasive work up of positive results.
Our study needs to be interpreted acknowledging the following limitations. First, our results are limited to patients over the age of 65. Patients under 65 years of age may receive different benefits from follow-up care than older patients. However, since 70% of bladder cancers occurs in persons aged 65 or older,30 our results are applicable to the majority of bladder cancer patients. Second, the linked SEER-Medicare data does not allow identification of patient symptoms, limiting our ability to determine the reason for follow-up care received after diagnosis. Regardless of the reason for obtaining care, use of follow-up care was carefully evaluated accounting for possible confounding arising from the intention to treat through our use of the instrumental variables analysis. Third, while the instrument, distance to the provider, was significantly associated with provision of follow-up care, urine testing was marginally significant, which, to some extent, limits the strength of our IV analysis for urine testing. Finally, our results are limited by the retrospective, non-randomized, study design. Although we have used methods to attempt to control for known and unknown confounding variables, residual confounding could still influence our results. However, the greatest risk would have been that high expenditures on follow-up care would be associated with decreased survival, a finding we did not see in the study. Instead, no difference was found among the follow-up tertiles, and any follow-up care was a significant improvement over no care.
Conclusion
The results of our study support the routine follow-up of patients after definitive bladder cancer surgery, and reexamination of the likely overuse of follow up imaging in these patients. Our results suggest aspects of follow-up care significantly improve patient outcomes, but as the driver of expenditures with no clear survival advantage for patients, imaging studies could be used more judiciously after cystectomy.
Acknowledgments
Funding
Support for this publication was provided by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences, by an American Cancer Society Institutional Review Grant (IRG-58-010-53), the Barnes Jewish Hospital Foundation/ICTS Clinical and Translational Science Research award (UL1 RR024992), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ), and the Washington University KL2 Career Development Awards Program (KL2 TR000450).
References
- 1.Kuroda M, Meguro N, Maeda O, et al. Stage specific follow-up strategy after cystectomy for carcinoma of the bladder. Int J Urol. 2002;9:129. doi: 10.1046/j.1442-2042.2002.00436.x. [DOI] [PubMed] [Google Scholar]
- 2.Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 3.Raj GV, Bochner BH, Serio AM, et al. Natural history of positive urinary cytology after radical cystectomy. J Urol. 2006;176:2000. doi: 10.1016/j.juro.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 4.Solsona E, Iborra I, Rubio J, et al. Late oncological occurrences following radical cystectomy in patients with bladder cancer. Eur Urol. 2003;43:489. doi: 10.1016/s0302-2838(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 5.Volkmer BG, Kuefer R, Bartsch GC, Jr, et al. J Urol. 2009;181:1587. doi: 10.1016/j.juro.2008.11.112. [DOI] [PubMed] [Google Scholar]
- 6.Sanderson KM, Cai J, Miranda G, et al. Upper tract urothelial recurrence following radical cystectomy for transitional cell carcinoma of the bladder: an analysis of 1,069 patients with 10-year followup. J Urol. 2007;177:2088. doi: 10.1016/j.juro.2007.01.133. [DOI] [PubMed] [Google Scholar]
- 7.Clark PE, Agarwal N, Biagioli MC, et al. NCCN Clinical Practice Guidelines in Oncology: National Comprehensive Cancer Network. 2012. Bladder Cancer. [Google Scholar]
- 8.Meissner C, Giannarini G, Schumacher MC, et al. The efficiency of excretory urography to detect upper urinary tract tumors after cystectomy for urothelial cancer. J Urol. 2007;178:2287. doi: 10.1016/j.juro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 10.Dalbagni G, Bochner BH, Cronin A, et al. A plea for a uniform surveillance schedule after radical cystectomy. J Urol. 2011;185:2091. doi: 10.1016/j.juro.2011.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz A, Jack A, Parkin DM. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 12.Gore JL, Litwin MS, Lai J, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore JL, Lai J, Setodji CM, et al. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer. 2009;115:988. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown ML, Riley GF, Schussler N, et al. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 15.Earle CC, Nattinger AB, Potosky AL, et al. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40:IV. doi: 10.1097/00005650-200208001-00011. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41. [Google Scholar]
- 17.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.JMW . Introductory econometrics: a modern approach. Mason, OH: Cengage Learning; 2009. [Google Scholar]
- 21.JHS, JHW, Yogo M. A survey of weak instruments and weak identifications in generalized method of moments. Journal of Business and Economic Statistics. 2002;20 [Google Scholar]
- 22.JDA, GWI, DBR Identification of causal effects using instrumental variables. Journal of the American Statistical Association. 1996;91:444. [Google Scholar]
- 23.Newey WK. Efficient Estimation of Limited Dependent Variable Models with Endogenous Explanatory Variables. Journal of Econometrics. 1987;363:231. [Google Scholar]
- 24.Blundell RW, Smith RJ. Estimation in a Class of Simultaneous Equation Limited Dependent Variable Models. Review of Economic Studies. 1989;561:37. [Google Scholar]
- 25.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27:531. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullahy J. Specification and Testing of Some Modified Count Data Models. Journal of Econometrics. 1986;333:341. [Google Scholar]
- 27.ACC, PKT . Microeconometrics: methods and applications. New York, NY: Cambridge University Press; 2005. [Google Scholar]
- 28.Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486. doi: 10.1016/j.eururo.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Boorjian SA, Tollefson MK, Cheville JC, et al. Detection of asymptomatic recurrence during routine oncological followup after radical cystectomy is associated with improved patient survival. J Urol. 2011;186:1796. doi: 10.1016/j.juro.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]


