Abstract
We identified ventrolateral medullary nuclei in which thyrotropin-releasing hormone (TRH) regulates glucose metabolism by modulating autonomic activity. Immunolabeling revealed dense prepro-TRH-containing fibers innervating the rostroventrolateral medulla (RVLM) and nucleus ambiguus (Amb), which contain, respectively, pre-sympathetic motor neurons and vagal motor neurons. In anesthetized Wistar rats, microinjection of the stable TRH analog RX77368 (38–150 pmol) into the RVLM dose-dependently and site-specifically induced hyperglycemia and hyperinsulinemia. At 150 pmol, blood glucose reached a peak of 180 ± 18 mg% and insulin increased 4-fold. The strongest hyperglycemic effect was induced when RX77368 was microinjected into C1 area containing adrenalin cells. Spinal cord transection at cervical-7 abolished the hyperglycemia induced by RVLM RX77368, but not the hyperinsulinemic effect. Bilateral vagotomy prevented the rise in insulin, resulting in a prolonged hyperglycemic response. The hyperglycemic and hyperinsulinemic effects of the TRH analog in the RVLM was peptide specific, since angiotensin II or a substance P analog at the same dose had weak or no effects. Microinjection of RX77368 into the Amb stimulated insulin secretion without influencing glucose levels. In conscious type 2 diabetic Goto-Kakizaki (GK) rats, intracisternal injection of RX77368 induced a remarkably amplified hyperglycemic effect with suppressed insulin response compared to Wistar rats. RX77368 microinjected into the RVLM of anesthetized GK rats induced a significantly potentiated hyperglycemic response and an impaired insulin response, compared to Wistar rats. These results indicate that the RVLM is a site at which TRH induces sympathetically-mediated hyperglycemia and vagally-mediated hyperinsulinemia, whereas the Amb is mainly a vagal activating site for TRH. Hyperinsulinemia induced by TRH in the RVLM is not secondary to the hyperglycemic response. The potentiated hyperglycemic and suppressed hyperinsulinemic responses in diabetic GK rats indicate that an unbalanced “sympathetic-over-vagal” activation by TRH in brainstem RVLM contributes to the pathophysiology of impaired glucose homeostasis in type 2 diabetes.
Keywords: glucose, insulin, sympathetic, vagus, diabetes, brainstem
Increasing evidence suggests a key role of the central nervous system in the pathophysiology of type 2 diabetes (T2D), in which impaired insulin secretion is one of the major characteristics (Schwartz et al., 2005; Seeley et al., 2006). Pancreatic islets are richly innervated by vagal and sympathetic nerves, which display stimulatory and inhibitory regulation on insulin secretion, respectively (Berthoud et al., 1979; Ahren, 2000). An appropriately balanced sympathovagal efferent activation in response to metabolic demand is crucial to insure proper insulin release for maintaining glucose homeostasis (Niijima, 1975; Berthoud et al., 1983; Duttaroy et al., 2004; Gautam et al., 2006). The brainstem contains essential autonomic regulatory circuits (DiRocco et al., 1979; Harris et al., 2005). While the circuits involved in cardiovascular autonomic reflexes have been exhaustively investigated, those involved in metabolic autonomic reflexes remain much more poorly understood.
In the brainstem, thyrotropin-releasing hormone (TRH) is synthesized in the raphe nuclei, which contain vagal and sympathetic premotor neurons involved in thermal, cardiovascular, and gastrointestinal regulation (Berthoud et al., 2005). TRH-containing projections innervate the dorsal vagal complex (DVC) (Lynn et al., 1991), where dense TRH-immunoreactive nerve terminals and TRH 1 receptors are localized (Rinaman et al., 1989; Heuer et al., 2000). TRH, or its metabolically stable analog RX77368, injected into the cisterna magna (intracisternal injection, ic) or microinjected into the DVC activates vagal efferent pathways, which in turn stimulate gastric acid secretion and motility and pancreatic insulin secretion (Garrick et al., 1987; Stephens et al., 1988; Yang et al., 2002; Ao et al., 2005). Another brainstem nucleus involved in TRH regulation of vagal efferents is the nucleus ambiguus (Amb), which is part of a larger region called the ventrolateral medulla. Amb is innervated by TRH-immunoreactive fibers and its functional responses to TRH have been demonstrated in electrophysiological and in vivo studies (Ishikawa et al., 1988; Hornby et al., 1989; Johnson et al., 1992; Yang et al., 1992; Batten, 1995; Sun et al., 1995). In addition to activating brainstem vagal-regulatory circuits, TRH or RX77368 injected ic induces profound hyperglycemia by activating the sympathetic-adrenal system (Taché et al., 1980; Ao et al., 2005). This effect is remarkably potentiated in a polygenetic T2D animal model, the Goto-Kakizaki (GK) rat (Goto et al., 1976; Goto et al., 1988; Hughes et al., 1994; Galli et al., 1996; Guenifi et al., 2001; Porte et al., 2001; Proks et al., 2002), associated with a sympathetic-adrenal system-mediated suppression of insulin secretion (Ao et al., 2005). These findings suggest that brainstem TRH may play important roles in physiological and pathophysiological regulation of metabolism, in part by coordinating sympathetic and vagal efferent outflows to achieve appropriate sympathovagal balance. Despite the extensive studies on the TRH action at brainstem vagal regulatory circuits (Ishikawa et al., 1988; Yang et al., 1992; Taché et al., 1994; Yang et al., 2002), the brainstem sites at which TRH acts to influence sympathetic efferent output have not yet been determined.
A group of neurons in the rostroventrolateral medulla (RVLM) projects monosynaptically to sympathetic preganglionic motoneurons in the intermediolateral cell column of the spinal cord, and is a final common point of convergence of most brain pathways regulating sympathetic tone (Oshima et al., 2000; Allen et al., 2009). Transneuronal labeling studies showed that the RVLM is a major brain region involved in sympathetic control of the pancreas (Jansen et al., 1997). The objective of this study was to test the hypothesis that the RVLM is a brainstem TRH action site where TRH regulate glucose metabolism by modulating sympathetic efferent functions. Because approximately 60–80% of presympathetic neurons within the RVLM can be defined phenotypically as C1 adrenaline-synthesizing neurons (C1) containing the catecholamine biosynthetic enzyme tyrosine hydroxylase (TH) (Lipski et al., 1995; Phillips et al., 2001), we first investigated whether prepro-TRH immunoreactive fibers are present in the RVLM with close appositions to TH neurons. Then we studied the effects of microinjection of the TRH analog RX77368 into the RVLM and Amb on blood glucose and serum insulin levels. These effects were compared between non-diabetic Wistar rats and T2D GK rats.
EXPERIMENTAL PROCEDURES
Animals
Male non-diabetic Wistar rats (250–280 g) were purchased from Harlan (San Diego, CA) and maintained in the Animal Facilities of the Veterans Affairs Great Los Angeles Health Care System (VAGLAHS) for one week before experiments. The body weight- and sex-matched T2D GK rats were bred in VAGLAHS animal facilities with a breeding protocol approved by Institutional Animal Care and Use Committee (IACUC) of VAGLAHS. GK rats were used at the age of 3 months, when body weights were 250–280 g. The body weight of GK rats were about 10–20 g lower than the age-matched Wistar rats, as previously reported (Murakawa et al., 2002). Rats were housed under controlled conditions (21–23°C, light on from 6.00 a.m. to 6.00 p.m.) with free access to standard rat chow (Prolab Lab Diet, PMI Nutrition International, Brentwood, MO) and tap water. Food, but not water, was removed 16 h before brainstem microinjection experiments. Animal studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996. The animal protocol was approved by the IACUC and the Research & Development Committee of VAGLAHS. Efforts were made to minimize the number of animals used and their suffering.
Chemicals
Three chemicals were used for brainstem microinjection. RX77368 (p-Glu-His-(3,3′-dimethyl)-Pro-NH2, Reckitt and Colman, Kingston Upon Hull, UK) is a metabolically stable TRH analog with relatively long-lasting central effects compared to TRH in rats and mice (Stephens et al., 1988; Taché et al., 1994). DiMe-C7[pGlu5, Mephe8, MeGly9]substance P5-11 (Peninsula Laboratories, San Carlos, CA) is a metabolically protected substance P analog. This compound is equipotent with substance P in competing for 3H-substance P binding to neurokinin 1 receptors in rat brain membranes and has been characterized as a specific substance P agonist in in vitro and in vivo rat assays (Eison et al., 1982; Keeler et al., 1985). Angiotensin II was purchased from Phoenix Pharmaceuticals, Inc (Burlingame, CA).
Immunohistochemistry
Adult male rats were anesthetized with intraperitoneal injection (ip) of pentobarbital (70 mg/kg, Abbott Laboratories, North Chicago, IL) and fixed by aortic perfusion of 50 ml physiological saline followed by 500 ml of ice-cold fixative (4 % paraformaldehyde, 0.18 % picric acid in 0.1 M phosphate buffer, pH 7.4). After post-fixing in the same fixative and cryoprotecting in 20% sucrose solution, a brainstem block corresponding to interaural −2.80 to −5.08 mm (Paxinos et al., 1997) was sectioned at 30 μm in the coronal plane with a cryostat (Microtome, IEC, MA). Primary antibodies were rabbit anti-prepro-TRH160-169 (1:2000, a gift of Prof. Pekary at Department of Medicine, Endocrine Division, UCLA and VA GLAHS, the antibody specificity has been tested in previous radioimmunoassay and pre-absorption studies) (Pekary et al., 1997; Yuan et al., 1999), mouse anti-TH (1:2000, Invitrogen, Carlsbad, CA)(Mallett, 1996) and chicken anti-choline acetyltransferase (ChAT, 1:1000, Aves Labs, Inc, Tigard, OR)(Goetz et al., 2006). The antibody against prepro-TRH was combined with either the antibody against TH or the one against ChAT, and incubated with the brainstem sections at room temperature for 1 h and at 4°C then for 16 h. The secondary antibodies were donkey anti-rabbit IgG Alexa Fluor® 488 (1:1000, Invitrogen, Carlsbad, CA) and donkey anti-mouse IgG Alexa Fluor® 568 (1:1000, Invitrogen, Carlsbad, CA) or donkey anti-chicken IgG Rhodamine Red-X (1:1000, Jackson, West Grove, PA). As a control, brainstem sections were processed the same way omitting the primary antibodies, which resulted in no staining.
Confocal Microscopy
Confocal images were acquired with a Zeiss LSM-710 confocal microscope (Carl Zeiss, Inc., Thornwood, NY) with objectives of 10x (numerical aperture 0.3) and 63x oil immersion (numerical aperture 1.4). Excitation light was provided by an argon laser (488 nm) for Alexa Fluor® 488 and a diode laser (561 nm) for Alexa Fluor® 568 and Rhodamine Red-X. Emission windows were 500–550 nm for Alexa Fluor® 488 and 580–700 nm for Rhodamine Red-X and Alexa Fluor® 568. The pinhole was 1.0 Airy unit for 488 nm excitation and calculated to give the same optical section thickness for 561 nm excitation. Images were acquired as optical sections of 1024 × 1024 pixels averaged 4 times. Images were processed using Imaris 6 (Bitplane AG, Zurich, Switzerland) to observe them in three-dimensions and to generate a two-dimension projection picture. Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA) was used to compose the multi-panel figures and to add text and arrows.
Experimental protocol for brainstem microinjection
All experiments were performed between 10:00 am and 3:00 pm. Rats were anesthetized with ip pentobarbital (50 mg/kg followed by 20 mg/kg each hour until the end of the experiment) to avoid surgery- and brainstem microinjection-induced stressful influences on glycemic regulation that is typically unavoidable in conscious animals. The anesthetized rat was positioned on a stereotaxic instrument (Kopf model 900). The dorsal surface of the caudal brainstem, including the obex region and the surrounding area, were exposed by slitting the cervical musculature and removing a small piece of the occipital skull plate. The tip of a glass micropipette (50–70 μm diameter) was positioned in the RVLM or in the Amb for microinjection, which was performed by pressure ejection of 100 nl solution over 2 min using a 1 μl Hamilton syringe attached to a glass micropipette with a PE-50 polyethylene catheter filled with water. The doses of RX77368 were 38, 75 and 150 pmol (15, 30, and 60 ng), which was chosen based on previous findings that they are sufficient to induce vagally-mediated changes in gastric and pancreatic functions when microinjected into the DVC (Garrick et al., 1987; Stephens et al., 1988; Taché et al., 1994). Doses for DiMe-C7[pGlu5, Mephe8, MeGly9]substance P5-11 and angiotensin II was 150 pmol. The micropipette was left in place for another 3 min. The coordinates (in mm) used for microinjection were: RVLM, ventral from the surface of the brainstem (V) 3.3, rostral from the caudal tip of the area postrema (R) 1.5, lateral from the midline (L) 1.7; Amb, V 2.5, R 1.5, L 2.0. Blood samples (0.05 ml each time) for glucose and insulin measurements were collected before and at 15, 30, 45, 60, 75, 90, 120, 150, and 180 min after the microinjection by a needle-punch at the capillary bed of the tail tip. In some rats, sham operation, spinal cord transection at the level of the 7th cervical verterbra (C7), or bilateral cervical vagotomy was performed 30 min before the microinjection. At the end of the experiment, rats were euthanized by quick decapitation, and brains were removed and fixed in a 10% formalin/20% sucrose solution for 2 days. Frozen coronal sections of caudal brainstem were sliced at 30 μm, mounted onto glass slides, and stained with toluidine blue. Histological sections were examined microscopically. The location of the microinjection sites were identified as the termination point of the micropipette track and marked on plates reproduced from the atlas of Paxinos & Watson (Paxinos et al., 1997).
Surgeries
Spinal cord transection at C7
A dorsal midline skin incision 1.5 cm in length was made in the vicinity of C7. After blunt dissection of the muscles, the spinous process of the C7 vertebra was held in place using a wound closure clip, and dissecting scissors were inserted into the spinal canal between the C7 and the first thoracic vertebra to completely transect the spinal cord, taking care to avoid damage to adjacent vasculature. The skin flaps were then sutured closed.
Bilateral cervical vagotomy
A ventral midline incision 2 cm in length was made; its caudal terminus was at the level of the clavicle. The underlying salivary and lymphatic tissues were separated by blunt dissection to reveal the carotid artery and the cervical vagus nerves. After carefully separating the artery from each vagal trunk with a fine glass probe, the nerve was cut. The skin flaps of the ventral incision were sutured shut.
Sham operation
The ventral and dorsal midline skin incisions were made and all procedures repeated as described for spinal cord transection and bilateral cervical vagotomy, omitting the step of transecting the spinal cord or cutting the vagus nerve.
Intracisternal injection
Rats under brief (2–3 min) isoflurane-anesthesia (5% vapor concentration in oxygen; Butler Animal Health Supply, Dublin, OH) were positioned on the stereotaxic instrument. A 50 μl Hamilton syringe was inserted through the skin, posterior altantooccipital membrane, dura mater, and underlying arachnoid membrane into the cisterna magna. The successful insertion of the needle into the cisterna magma was verified by aspirating clear cerebrospinal fluid into the syringe before and after the injection. Ten microliters of physiological saline or RX77368 solution (25 or 50 ng/10 μl) were injected. The animal was returned to its cage where recovery from the anesthesia occurred within 1–2 min.
Measurement of blood glucose and serum insulin levels
Blood glucose levels were measured with the One Touch Ultra Blood Glucose Monitoring System (Lifescan, Milpitas, CA) using 5 μl of whole blood, and serum insulin levels by a rat insulin enzyme-linked immunosorbent assay kit (LINCO, St. Charles, MO) using 10 μl of serum.
Statistical analysis
Data are expressed as mean ± SEM for each value. Statistical comparisons between Wistar and GK rats receiving the same treatment or between RX77368-treated and vehicle-treated rats of the same strain were performed using an unpaired Student’s t-test. Comparisons of values before and after microinjection in the same group were performed using a paired Student’s t-test. Comparisons among multiple groups were performed using a one-way ANOVA. All the statistical tests were performed using SigmaStat 2.0 software. P values less than 0.05 were considered statistically significant.
RESULTS
Prepro-TRH immunoreactive nerve fibers innervate the RVLM and Amb
Fluorescence double-labeling revealed that in the rat brainstem (interaural levels −3.30 to −4.68 mm (Paxinos et al., 1997)) nerve fibers brightly stained for prepro-TRH160-169 were present in the RVLM, forming close apposition to TH-positive cell bodies and axons (Fig. 1A, Fig. 1C), suggesting the existence of synapses with axodendritic and axosomatic contacts. Less bright, punctate prepro-TRH160-169 immunoreactivity was found inside the TH and non-TH neurons (Fig. 1A, Fig. 1C). As a positive control, we confirmed the presence of staining for prepro-TRH160-169 and TH in the nucleus tractus solitarius (NTS) (Fig. 1B, Fig. 1D), which is known to receive TRH innervation and to contain TH-immunoreactive neurons (Rinaman et al., 1989; Kachidian et al., 1993; Yuan et al., 2002). Similarly, double staining for prepro-TRH160-169 and ChAT showed prepro-TRH160-169 positive nerve fibers innervate ChAT-positive and ChAT-negative neurons in the Amb (Fig. 1G). Most ChAT-immunoreactive neurons in the Amb appear also prepro-TRH160-169 positive (Fig. 1E, Fig. 1G). The prepro-TRH160-169 and ChAT double-stained neurons were distributed ventrally into the RVLM, indicating that most of the prepro-TRH160-169 positive non-TH neurons seen in Figs. 1A and 1C express ChAT. As a positive control, double staining of prepro-TRH160-169 and ChAT was observed in the dorsal motor nucleus of the vagus (DMV) (Fig. 1F, Fig. 1H), which is known to receive TRH innervation and containing ChAT-synthesizing vagal motor neurons (Rinaman et al., 1989; Lynn et al., 1991; Yuan et al., 2002).
Fig. 1.
Confocal images of brainstem sections double-labeled for prepro-TRH160-169 (ppTRH) and tyrosine hydroxylase (TH) or ppTRH and choline acetyltransferase (ChAT). Transverse sections of the brainstem at interaural levels of −4.24 mm (left panels) or −4.68 mm (right panels) were labeled with antibodies against ppTRH (green, all panels), TH (red, panels A–D) and ChAT (red, panels E–H). The location of the illustrated fields is indicated by the rectangles in the diagrams in the middle panels, which were reproduced from the Paxinos & Watson Atlas of the Rat Brain (Paxinos et al., 1997): RVLM, rostroventrolateral medulla (A, C); NTS, nucleus tractus solitarius (B, C); Amb, nucleus ambiguus (E, G); DMV, dorsal motor nucleus of the vagus (F, H). Images in panels C, D, E and F are 3 optical sections acquired with a 10x objective (scale bars are 100 μm). Images in panels A, B, G and H are 13–29 optical sections acquired with a 63x objective (scale bars are 10 μm). In the RVLM (A, C) and the NTS (B, D), strong ppTRH immunoreactivity was found in fibers and puncta, which represent axons and presynaptic terminals containing ppTRH. The ppTRH staining was also detected inside neurons, both TH-positive and TH-negative (yellow arrows in A), indicating that the RVLM contains neurons synthesizing TRH. In the Amb-RVLM (E, G) and the DVM (F, H), ppTRH-immunoreactivity was found in fibers surrounding ChAT-positive and ChAT-negative neurons, and also inside the ChAT-immunoreactive neurons. The white arrowheads in panels A and G indicate examples of putative axodendritic and axosomatic interactions between TRH axons/dendrites and TH (A) or ChAT (G) neurons/axons. The nature of these structures was inferred by close examination and rotation of the whole confocal stack in three dimensions. cc: central canal.
TRH analog RX77368 microinjected into the RVLM induced hyperglycemia and hyperinsulinemia in Wistar rats
In pentobarbital-anesthetized Wistar rats, microinjection of the stable TRH analog RX77368 into the RVLM at doses of 38, 75, or 150 pmol (15, 30, or 60 ng)/100 nl induced a prompt, dose-dependent, and sustained elevation in blood glucose levels. At 150 pmol, blood glucose increased from a basal level of 103 ± 5 mg% to a peak of 180 ± 18 mg% at 90 min (Fig. 2). Thereafter, glucose levels gradually subsided, but still remained significantly above (139 ± 9 mg%) basal levels at 180 min (Fig. 2). The most potent hyperglycemic effect was induced when RX77368 was microinjected into the ventral part of the RVLM, the C1 area containing adrenalin cells (Fig. 2, Fig. 3). By contrast, when RX77368 was microinjected into ventral brainstem sites adjacent to, but outside of the RVLM, no significant increase in blood glucose levels were detected (Fig. 2, Fig. 4C). Glucose levels in this condition were significantly lower than those evoked by RX77368-microinjection inside the RVLM (Fig. 2, Fig. 3, Fig. 4B). To exclude the possibility that the hyperglycemia was a non-specific effect of the injection, we injected 100 nl of physiological saline into the RVLM of Wistar rats. This did not induce any significant change in blood glucose levels (Fig. 3, Fig. 4A).
Fig. 2.
Left panel: Dose-related hyperglycemic effect of the stable TRH analog RX77368 microinjected into the RVLM of overnight-fasted Wistar rats. Each point represents the mean ± SEM of the number of rats indicated in the parenthesis. * P < 0.05 compared with the effect of microinjection into sites outside of the RVLM. # P< 0.05 compared with the basal levels of the same group. Right panel: Effective and ineffective microinjection sites of the TRH analog RX77368 to induce hyperglycemia, marked on plates reproduced from the atlas of Paxinos & Watson (Paxinos et al., 1997).
Fig. 3.
Examples of glucose and insulin responses to microinjection of normal saline (100 nl) or TRH analog RX77368 (150 pmol/100 nl) into the RVLM in individual Wistar rats.
Fig. 4.
The hyperglycemic and hyperinsulinemic effects of TRH analog RX77368 (150 pmol) microinjected into the RVLM in sham-operated, spinal cord-transected, and bilateral cervical-vagotomized Wistar rats. Each point represents mean ± SEM of the number of rats indicated at the top of each graph in the lower panel. * P < 0.05 compared with the value of the time point in sham operated rats with saline microinjection into the RVLM (group A). # P< 0.05 compared with the value of the time point in sham operated rats with TRH analog RX77368 microinjection into the RVLM (group B).
The hyperglycemia induced by RX77368-microinjection (150 pmol) into the RVLM occurred concomitantly with an increase in serum insulin levels (Fig. 3, Fig. 4B), from a basal level of 0.48 ± 0.16 ng/ml to a peak of 1.98 ± 0.39 ng/ml at 90 min (Fig. 4B). The most potent hyperinsulinemic effect was induced when RX77368 was microinjected into the dorsal part of the RVLM, near the Amb (Fig. 3). Physiological saline (100 nl) microinjected into the RVLM, or RX77368 microinjected into sites adjacent to, but outside of the RVLM, did not change serum insulin levels significantly (Fig. 3, Fig. 4A, Fig. 4C).
The hyperglycemia induced by RVLM microinjection of TRH analog was abolished by spinal cord transection and the hyperinsulinemia by vagotomy
The hyperglycemic effect of RVLM RX77368-microinjection (150 pmol) was completely abolished in rats with spinal cords transected at C7 to block sympathetic descending pathways. At all post-injection time points, blood glucose levels did not differ significantly from basal levels (Fig. 4D). By contrast, the hyperinsulinemic effect was retained, with the peak (2.44 ± 0.69 ng/ml from a basal level of 0.54 ± 0.18 ng/ml) occurring more promptly at 45 min post-microinjection (Fig. 4D). The elevated insulin in spinal cord-transected rats quickly declined from the peak values, and returned to basal levels at 90 min (Fig. 4D), at which time point the insulin levels of this group was significantly different than that in sham-operated group (Fig. 4B, Fig. 4D).
The hyperinsulinemic effect of RVLM RX77368-microinjection (150 pmol) was largely prevented, especially in the first 60 min, in rats received bilateral cervical vagotomy to block vagal descending pathways. Insulin levels in vagotomized rats were not significantly different compared to the pre-injection levels at any time point (Fig. 4E). By contrast, the hyperglycemic effect was retained and lasted longer compared to that in sham-operated rats. The peak response (249 ± 36 mg%) occurred at 120 min after the microinjection, and glucose levels remained elevated at 180 min (Fig. 4E).
RVLM microinjection of substance P analog or angiotensin II produced weaker or no hyperglycemic and hyperinsulinemic effects compared to TRH analog
In pentobarbital-anesthetized Wistar rats, microinjection of the metabolically stable substance P analog DiMe-C7[pGlu5, Mephe8, MeGly9]substance P5-11 (150 pmol) into the RVLM did not significantly influence blood glucose levels, but elevated serum insulin when compared to the basal levels (Fig. 5). In contrast, angiotensin II (150 pmol) induced significant hyperglycemic and hyperinsulinemic responses. Blood glucose levels increased from a basal level of 95 ± 5 to a peak of 131 ± 11 mg% at 75 min post injection (Fig. 5). However, the hyperglycemic effect of angiotensin II was smaller than that of the TRH analog RX77368 injected at the same dose (line with filled circles in Fig. 5, data adopted from Fig. 2).
Fig. 5.
The hyperglycemic and hyperinsulinemic effects of RVLM microinjection of the TRH analog RX77368, the substance P analog DiMe-C7 [pGlu5, Mephe8, MeGly9]substance P5-11, and angiotensin II (all at 150 pmol, data of the TRH analog are adapted from Fig. 2) in Wistar rats. Each point represents mean ± SEM of the number of rats indicated in the parenthesis. * P < 0.05 compared with the value of the same time point in TRH analog RX77368 injected rats. # P< 0.05 compared with the basal levels of the same group.
TRH analog RX77368 microinjected into the Amb induced hyperinsulinemia without hyperglycemia
Microinjection of the stable TRH analog RX77368 (150 pmol) into the Amb, a nucleus dorsal to the RVLM which contains vagal preganglionic neurons that innervate the thoracic and abdominal organs (Bieger et al., 1987), did not influence blood glucose levels but significantly elevated serum insulin, which increased from a basal level of 0.49 ± 0.20 ng/ml to a peak of 1.62 ± 0.61 ng/ml at 45 min after microinjection (Fig. 7B, Fig. 8B, lines with open circle).
Fig. 7.
Top panel: A. Potentiated hyperglycemic response to TRH analog RX77368 (150 pmol) microinjected into the RVLM in GK rats compared to Wistar rats (data of the Wistar rats are adapted from Fig. 2). B, Unchanged blood glucose levels after RX77368 (150 pmol) microinjected into the nucleus ambiguus (Amb) in Wistar and GK rats. Each point represents mean ± SEM of the number of rats indicated near the line. * P < 0.05 compared with the value of the same time point in Wistar rats. # P< 0.05 compared with the basal levels of the same group. Lower panel: Coronal sections showing the microinjection sites in the RVLM (A) and Amb (B). Black circles are the microinjection sites in Wistar rats and the gray circles are those in GK rats.
Fig. 8.
Top panel: A, Impaired first-hour insulin response to TRH analog RX77368 (150 pmol) microinjection into the RVLM in GK rats compared to Wistar rats. B. Similar insulin response to RX77368 (150 pmol) microinjected into the Amb in Wistar and GK rats. Lower panel: The corresponding changes in blood glucose levels in the same rats. Each point represents mean ± SEM of the number of rats indicated near the line. * P < 0.05 compared with the value of the same time point in Wistar rats. # P< 0.05 compared with the basal levels of the same group.
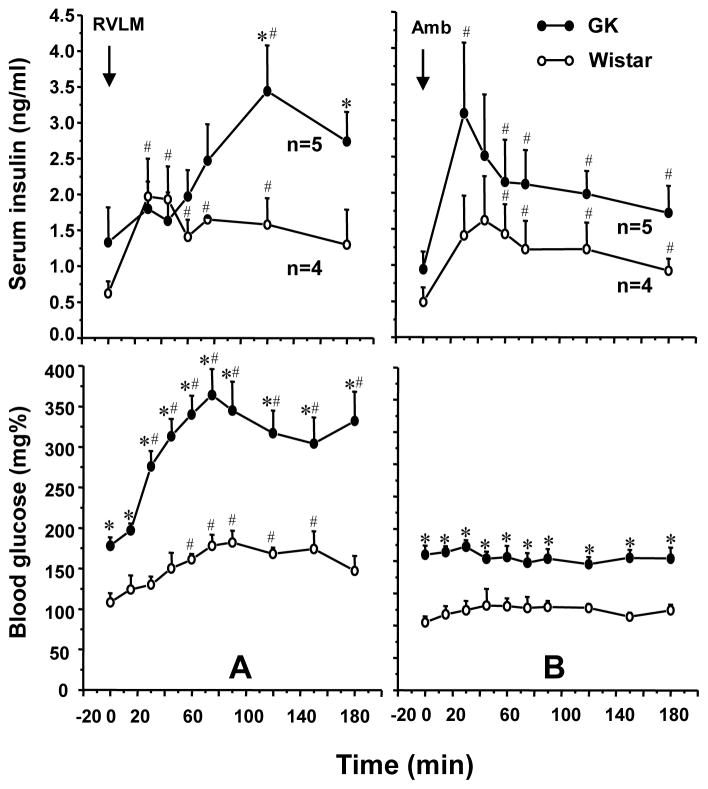
TRH analog RX77368 ic induced markedly potentiated hyperglycemic and insulin-suppressive responses in non-anesthetized T2D GK rats compared to Wistar rats
To assess whether results obtained in anesthetized conditions (Ao et al., 2005) were relevant to that in non-anesthetized conditions, we studied the effects of ic saline or RX77368 (10 μl), performed under brief (1–2 min) isoflurane-anesthesia, on glucose and insulin levels in awake rats. Saline did not influence blood glucose or serum insulin levels in Wistar rats, whereas TRH analog RX77368 (25 ng) significantly increased blood glucose and induced a weak, insignificant elevation in serum insulin levels. When the dose of RX77368 was increased to 50 ng, the glucose response was the same as that induced by 25 ng, but the insulin response was 2.5-fold higher (Fig. 6). In contrast to Wistar rats, T2D GK rats showed a remarkably potentiated hyperglycemic response to ic RX77368 (25 ng). Glucose levels in all GK rats reached above 600 mg% (the highest glucose level measurable by the monitoring system) at 60–120 min after ic injection. Serum insulin levels were significantly suppressed for 120 min in GK rats, despite the simultaneous hyperglycemia (Fig. 6). These results indicate that the “sympathetic-over-vagal” response to ic TRH analog, i.e. an elevation of both sympathetic and vagal drive with the sympathetic effect dominating the vagal effect, in T2D GK rats is more pronounced in the awake state than in the anesthetized state.
Fig. 6.
The potentiated hyperglycemic and insulin-suppressive responses to intracisternal injection (ic) of the stable TRH analog RX77368 in type 2 diabetic (T2D) Goto-Kakizaki (GK) rats, compared to Wistar rats. Each point represents mean ± SEM of the number of rats indicated in the parenthesis. * P < 0.05 compared with the response of Wistar rats receiving ic saline. # P< 0.05 compared with the response of Wistar rats receiving ic RX77368 (25 ng).
TRH analog RX77368 microinjected into the RVLM induced potentiated hyperglycemic response in T2D GK rats compared to Wistar rats
Basal blood glucose levels in anesthetized T2D GK rats were 175.6 ± 5.7 mg% (n=25). After receiving TRH analog RX77368 (150 pmol/100 nl) microinjection into the RVLM, GK rats showed a markedly potentiated hyperglycemic response compared to Wistar rats. The peak response (328 ± 25 mg%) in GK rats occurred at 75–90 min post-injection, showing a 140 mg% increase from the basal levels, which was almost twice the 77 mg% net increase from the basal levels in Wistar rats (Fig. 7A, line with open circle, adopted from Fig. 2). In addition, unlike the quick decline following the peak at 90 min in Wistar rats, the hyperglycemic response in GK rats persisted until the end of the experiment (180 min), at which point it remained 134 mg% higher than its basal levels, compared to a 36 mg% net difference from the basal in Wistar rats (Fig. 7A, Fig. 8A). Microinjection of RX77368 into the Amb of GK rats, like that in Wistar rats, did not significantly affect blood glucose levels (Fig. 7B, Fig. 8B).
Impaired insulin response to TRH analog RX77368 microinjected into the RVLM in T2D GK rats
Basal insulin levels in anesthetized GK rats (1.08 ± 0.19 ng/ml, n=14) were significantly higher than that in Wistar rats (0.51 ± 0.09 ng/ml, n=11). Unlike the similar insulin response patterns to microinjection of RX77368 between the RVLM and Amb injection sites in Wistar rats, responses in GK rats were nucleus-specific (Fig. 8). The significant elevation of insulin levels during the first hour after RVLM microinjection of TRH analog that was observed in Wistar rats did not occur in GK rats. Insulin levels in GK rats at 30, 45, and 60 min post RVLM microinjection of RX77368 were not significantly different compared to the basal levels (Fig. 8A). Thereafter, insulin levels increased during the second hour from a basal level of 1.33 ± 0.49 to a peak of 3.44 ± 0.64 ng/ml at 120 min. Insulin levels remained as high as 2.74 ± 0.41 ng/ml at the end of the experiment (Fig. 8A). By contrast, microinjection of RX77368 into the Amb in GK rats induced a prompt insulin response, increasing from a basal level of 0.94 ± 0.25 ng/ml to a peak of 3.10 ± 0.98 ng/ml at 30 min and then quickly declining, a pattern similar to that seen in Wistar rats (Fig. 8B).
DISCUSSION
The present results indicate that the RVLM is an important site of action for TRH and is involved in sympathovagal regulation of glucose metabolism.
Distribution of prepro-TRH immunoreactive fibers in the RVLM and Amb
Immunohistochemistry data show that prepro-TRH160-169-immunoreactive nerve terminals are abundant in the RVLM and were in close apposition with TH neurons, the typical component of the C1 area, of which 50% project to the spinal cord sympathetic motor neurons (Phillips et al., 2001; Farnham et al., 2008). In addition, prepro-TRH160-169-immunoreactivity was found in the cytoplasm of the TH neurons in the RVLM and the non-TH neurons in the RVLM and Amb, most of which appear with ChAT-immunoreactivity. The close appositions of prepro-TRH and TH or prepro-TRH and ChAT immunostaining suggest axodendritic and axosomatic interactions between TRH axons/dendrites and the TH or ChAT neurons/axons. To further prove the specificity of the immunostaining, we show that prepro-TRH160-169, TH and ChAT immunoreactivities were also observed in the NTS and the DMV, nuclei well known to contain TRH fibers and TH or ChAT neurons (Rinaman et al., 1989; Lynn et al., 1991; Yuan et al., 2002). Prepro-TRH160-169 corresponds to an amino acid region linking, but not including, two pro-TRH sequences in prepro-TRH (Bulant et al., 1988). Prepro-TRH160-169 has a similar pattern of distribution in rat brain as TRH and is co-released with TRH to modulate the TRH action in some brain regions (Bulant et al., 1988; Valentijn et al., 1991; Ladram et al., 1994; Yang et al., 1994a). The present finding is consistent with previous reports that TRH immunoreactive fibers are present in the ventrolateral medulla and TRH immunoreactive boutons form close appositions with expiratory neurons in the RVLM (Merchenthaler et al., 1988; Sun et al., 1996). The morphological data illustrated here provides anatomic support for a possible TRH action on the C1 catecholaminergic neurons. The presence of local interneurons producing prepro-TRH in the RVLM and Amb has not previously been emphasized, and is of substantial interest. Further studies focused on tracing the sources of TRH innervation in the RVLM and Amb are needed to establish the relative contributions of extra-nuclear vs. intranuclear sources to the TRH inputs in each of these nuclei.
Hyperglycemic effect of TRH analog microinjected into the RVLM
Microinjection of the TRH analog RX77368 into the RVLM potently induced dose-related, nucleus-specific, and peptide-specific hyperglycemic response. The nucleus-specificity was evidenced by the fact that microinjection of RX77368 into sites close to, but outside of the RVLM had no significant effect. It was further supported by the absence of hyperglycemic response when RX77368 was microinjected into the Amb, a nucleus located near the RVLM. That spinal cord transection completely abolished the hyperglycemic response indicates a sympathetic-adrenal mediation of the TRH analog action. The peptide specificity was shown by the variation in glucose/insulin responses to microinjection into the RVLM of the TRH analog, the substance P analog, and angiotensin II. Substance P is co-localized with TRH in raphe neurons and raphe projections innervating brainstem vagal and spinal sympathetic preganglionic motor neurons (Sasek et al., 1990; Baude et al., 1998). The RVLM area also contains angiotensin II-immunoreactive nerve terminals and a moderately high density of angiotensin II type 1 receptor (Allen et al., 1999; Hu et al., 2002). Electrophysiological studies revealed that the substance P analog DiMe-C7 [pGlu5, Mephe8, MeGly9]substance P5-11 and angiotensin II excite RVLM neurons (Li et al., 1995; Li et al., 1997). When microinjected into the RVLM, angiotensin II (20–100 pmol) or the substance P analog (600 pmol) increased sympathetic nerve activity, arterial blood pressure, and heart rate (Muratani et al., 1991; Makeham et al., 2005). In this study, we found a significant hyperglycemic effect when angiotensin II was microinjected into the RVLM, whereas the substance P analog had no effect at the dose of 150 pmol. Sympathetic activation by the substance P analog could not be excluded. However, the hyperglycemic effect mediated by sympathetic activation could have been countered by increased insulin release following substance P-induced vagal activation, since it has been reported that adrenaline-mediated hyperglycemia can be normalized by insulin (Jensen et al., 2010). In contrast to the effects of these two neuropeptides, the TRH analog RX77368 in the RVLM induced a stronger hyperglycemic response at the same molar dose, demonstrating its potent, peptide-specific action in activating sympathetic efferent outflow. Similarly, in vitro studies have shown that TRH applied to brain slices within the confines of the RVLM activated neurons with intrinsic pacemaker activity and presumed sympathoexcitatory function (Sun et al., 1989). These findings contribute to the delineation of TRH-containing brainstem circuits involved in activating the sympathetic-adrenal system, which are in addition to the well-established vagal-activating raphe-DVC pathways (Yang et al., 1993; Taché et al., 1994; Yang et al., 2002). The results also suggest that TRH action in the RVLM-C1 area may participate in the sympathetic-adrenal response to homeostatic challenges of glucoprivation and energy demand. Indeed, brainstem TRH gene expression is up-regulated during food restriction and by acute cold exposure (Yang et al., 1994b; Ao et al., 2006). Future studies using pharmacologic blockade of TRH or prepro-TRH peptides action in the RVLM-C1 area are needed to investigate the physiological functions of endogenous TRH in sympathetic activation.
Hyperinsulinemic effect of TRH analog microinjected into the RVLM
TRH analog microinjected into the RVLM induced hyperinsulinemia, which is not likely a sole secondary effect following the sympathetically-driven hyperglycemia. In spinal cord-transected rats, in which the descending pathway driving sympathetic outflow is interrupted, RVLM microinjection of the TRH analog induced a prompt hyperinsulinemic effect without increasing blood glucose levels. The short-lasting insulin elevation in these rats and the absence of hyperinsulinemic response in vagotomized rats indicate that the first-hour insulin response to RVLM TRH analog was solely mediated by vagal efferent activation, whereas the second- and third-hour insulin elevation observed in sham operated rats is most likely mediated by mechanisms secondary to hyperglycemia, such as the glucose-dependent secretion of incretins. However, the lack of insulin response to the extremely high glucose levels in vagotomized rats indicates that the β-cell-exciting mechanism triggered by hyperglycemia requires an intact vagal nerve.
Overlapped sympathovagal regulatory function of TRH in the RVLM-Amb area
The simultaneous hyperglycemic and hyperinsulinemic responses to TRH analog microinjected into the RVLM, combined with the relationship between the location of individual microinjection sites and the corresponding effects, suggest that the RVLM contains vagal-activating elements responding to TRH, which are more abundant in the dorsal portion of the RVLM, near the Amb. The Amb contains vagal preganglionic motoneurons innervated by TRH-containing nerve terminals (Sun et al., 1995). TRH or TRH analog microinjected into the Amb excites Amb neurons and evokes a vagally-mediated stimulation of gastric/pancreatic functions (Bieger et al., 1987; Ishikawa et al., 1988; Batten, 1995; Sun et al., 1995). Electrical stimulation of the Amb in anesthetized rats produced a rapid rise (in 1 min) in plasma insulin levels (Bereiter et al., 1981). Not unexpectedly, microinjection of RX77368 into the Amb induced a prompt hyperinsulinemic response peaked at 30 min. The unchanged glucose levels indicate a predominant vagal activation by TRH in this nucleus. It has been pointed out that the Amb lacks the structural and functional homogeneity implicit in the classical neuroanatomical concept of a nucleus (Bereiter et al., 1981). After injection of horseradish peroxidase into the cervical vagus nerve, retrograde labeled neurons in the ventrolateral brainstem were found compactly localized in the Amb and scattered in the RVLM. Cardiovascular studies showed that the RVLM region contains cholinergic parasympathetic neurons controlling heart function (Guyenet, 2006). The present double immunostaining data are consistent with these findings. Although it still need to clarify whether the ChAT/prepro-TRH colocalized cells in the RVLM are vagal neurons involved in stimulating insulin secretion, the current morphological and functional results support the view that the sympathetic- and vagal-regulatory brainstem circuits participating in controlling glucose metabolism overlap at the RVLM-Amb area. Given the typically reciprocal influences of sympathetic and vagal drive on the visceral organs they innervate, coordinated control of the two is needed to effect stable physiological function. That TRH acts at these structures to activate vagal and sympathetic efferent pathways indicates its cardinal role in maintaining metabolic homeostasis. The detailed mechanisms of the integrated actions of TRH, substance P, angiotensin II, and other neurotransmitter/neuropeptides in this area in balancing sympathovagal efferent drive to various viscera remain to be investigated.
“Sympathetic-over-vagal” response to RVLM microinjection of TRH analog in T2D GK rats
Relative predominance of sympathetic activity and attenuation of vagal motor activity are common in T2D (Oida et al., 1999). These alterations are associated with insulin resistance and contribute to the pathogeneses of hypertension, lipid dysregulation, and cardiovascular mortality (Oida et al., 1999; Perin et al., 2001; Huggett et al., 2003). We have observed a “sympathetic-over-vagal” excitation following TRH analog ic that was responsible for the suppressed insulin response to hyperglycemia in anesthetized GK rats (Ao et al., 2005). Here we found that the ic TRH analog-induced sympathetically-driven hyperglycemia and suppression of insulin secretion are more pronounced in awake than in anesthetized T2D GK rats. This indicates that the unbalanced sympathovagal response to brainstem TRH observed in anesthetized GK rats is relevant to that in awake conditions. Furthermore, we identified the RVLM as one of the brainstem sites where TRH induces elevated sympathetic activation in GK rats. The failed first hour insulin response to RVLM TRH analog in GK rats most likely resulted from suppression of vagally-stimulated insulin secretion by over-activated sympathetic outflow (Ao et al., 2005). The ample insulin secretion in the second and third hours in GK rats indicates a compensatory peripheral mechanism for stimulating insulin release during hyperglycemia, possibly involving increased glucose-dependent secretion of incretins. The detailed mechanisms responsible for the sympathetic overactivation by TRH in the RVLM and the enhanced insulin secretion in the second and third hours in T2D GK rats remain to be studied.
CONCLUSION
The RVLM is innervated with prepro-TRH nerve terminals and is a site at which TRH induces sympathetically-mediated hyperglycemia and vagally-mediated hyperinsulinemia. In T2D GK rats, a “sympathetic-over-vagal” activation by TRH in the RVLM results in impaired vagal stimulation of insulin secretion. TRH action in the RVLM may play a role in the physiological “metabolic autonomic reflexes” and the pathophysiology of sympathovagal imbalance in metabolic diseases.
Acknowledgments
This work was funded by VA Merit Award (H. Yang) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41301 (CURE Center grant Animal Core). The authors thank Prof. Pekary AE of UCLA and VA GLAHS for providing the prepro-TRH160-169 antibody.
Abbreviations
- Amb
nucleus ambiguus
- C1
a rostroventrolateral medullary area containing adrenaline cells
- C7
7th cervical verterbra
- cc
central canal
- ChAT
choline acetyltransferase
- DMV
dorsal motor nucleus of the vagus
- DVC
dorsal vagal complex
- GK rats
Goto-Kakizaki rats
- IACUC
Institutional Animal Care and Use Committee
- ic
intracisternal injection
- ip
intraperitoneal injection
- NTS
nucleus tractus solitarius
- ppTRH
prepro-TRH
- RVLM
rostroventrolateral medulla
- T2D
type 2 diabetes
- TH
tyrosine hydroxylase
- TRH
thyrotropin-releasing hormone
- VAGLAHS
Veterans Affairs Great Los Angeles Health Care System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Allen AM, MacGregor DP, McKinley MJ, Mendelsohn FA. Angiotensin II receptors in the human brain. Regul Pept. 1999;79:1–7. doi: 10.1016/s0167-0115(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Allen AM, O’Callaghan EL, Chen D, Bassi JK. Central Neural Regulation of Cardiovascular Function by Angiotensin: A Focus on the Rostral Ventrolateral Medulla. Neuroendocrinology. 2009;89:361–369. doi: 10.1159/000197863. [DOI] [PubMed] [Google Scholar]
- Ao Y, Go VL, Toy N, Li T, Wang Y, Song MK, Reeve JRJ, Liu Y, Yang H. Brainstem Thyrotropin-Releasing Hormone Regulates Food Intake through Vagal-Dependent Cholinergic Stimulation of Ghrelin Secretion. Endocrinology. 2006;147:6004–6010. doi: 10.1210/en.2006-0820. [DOI] [PubMed] [Google Scholar]
- Ao Y, Toy N, Song MK, Go VL, Yang H. Altered glucose and insulin responses to brain medullary thyrotropin-releasing hormone (TRH)-induced autonomic activation in type 2 diabetic Goto-Kakizaki rats. Endocrinology. 2005;146:5425–5432. doi: 10.1210/en.2005-0553. [DOI] [PubMed] [Google Scholar]
- Batten TF. Immunolocalization of putative neurotransmitters innervating autonomic regulating neurons (correction of neurones) of cat ventral medulla. Brain Res Bull. 1995;37:487–506. doi: 10.1016/0361-9230(95)00029-e. [DOI] [PubMed] [Google Scholar]
- Baude A, Shigemoto R. Cellular and subcellular distribution of substance P receptor immunoreactivity in the dorsal vagal complex of the rat and cat: a light and electron microscope study. J Comp Neurol. 1998;402:181–196. [PubMed] [Google Scholar]
- Bereiter DA, Berthoud HR, Brunsmann M, Jeanrenaud B. Nucleus ambiguus stimulation increases plasma insulin levels in the rat. Am J Physiol. 1981;241:E22–E27. doi: 10.1152/ajpendo.1981.241.1.E22. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Jeanrenaud B. Acute hyperinsulinemia and its reversal by vagotomy after lesions of the ventromedial hypothalamus in anesthetized rats. Endocrinology. 1979;105:146–151. doi: 10.1210/endo-105-1-146. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Niijima A, Sauter JF, Jeanrenaud B. Evidence for a role of the gastric, coeliac and hepatic branches in vagally stimulated insulin secretion in the rat. J Auton Nerv Syst. 1983;7:97–110. doi: 10.1016/0165-1838(83)90039-5. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Bulant M, Delfour A, Vaudry H, Nicolas P. Processing of thyrotropin-releasing hormone prohormone (pro-TRH) generates pro-TRH-connecting peptides. Identification and characterization of prepro-TRH-(160-169) and prepro-TRH-(178-199) in the rat nervous system. J Biol Chem. 1988;263:17189–17196. [PubMed] [Google Scholar]
- DiRocco RJ, Grill HJ. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science. 1979;204:1112–1114. doi: 10.1126/science.451558. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720. doi: 10.2337/diabetes.53.7.1714. [DOI] [PubMed] [Google Scholar]
- Eison AS, Iversen SD, Sandberg BE, Watson SP, Hanley MR, Iversen LL. Substance P analog, DiMe-C7: evidence for stability in rat brain and prolonged central actions. Science. 1982;215:188–190. doi: 10.1126/science.6171884. [DOI] [PubMed] [Google Scholar]
- Farnham MM, Li Q, Goodchild AK, Pilowsky PM. PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am J Physiol. 2008;294:R1304–R1311. doi: 10.1152/ajpregu.00753.2007. [DOI] [PubMed] [Google Scholar]
- Galli J, Li LS, Glaser A, Ostenson CG, Jiao H, Fakhrai-Rad H, Jacob HJ, Lander ES, Luthman H. Genetic analysis of non-insulin dependent diabetes mellitus in the GK rat. Nat Genet. 1996;12:31–37. doi: 10.1038/ng0196-31. [DOI] [PubMed] [Google Scholar]
- Garrick T, Buack S, Veiseh A, Tache Y. Thyrotropin-releasing hormone (TRH) acts centrally to stimulate gastric contractility in rats. Life Sci. 1987;40:649–657. doi: 10.1016/0024-3205(87)90266-9. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, Heard T, Wess J. A critical role for beta cell M(3) muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Goetz AK, Scheffler B, Chen HX, Wang S, Suslov O, Xiang H, Brustle O, Roper SN, Steindler DA. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:11063–11068. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- Goto Y, Suzuki K, Sasaki M, Ono T, Abe S. GK rat as a model of nonobese, noninsulin-dependent diabetes. Selective breeding over 35 generations. In: Renold AE, Renold, editors. Frontiers in Diabetes Research. Lessons from Animal Diabetes II. London, UK: John Libbey; 1988. pp. 301–303. [Google Scholar]
- Guenifi A, Simonsson E, Karlsson S, Ahren B, Abdel-Halim SM. Carbachol restores insulin release in diabetic GK rat islets by mechanisms largely involving hydrolysis of diacylglycerol and direct interaction with the exocytotic machinery. Pancreas. 2001;22:164–171. doi: 10.1097/00006676-200103000-00009. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy Expenditure and Body Composition of Chronically Maintained Decerebrate Rats in the Fed and Fasted Condition. Endocrinology. 2005;147:1365–1376. doi: 10.1210/en.2005-1156. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schafer MK, O’Donnell D, Walker P, Bauer K. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J Comp Neurol. 2000;428:319–336. [PubMed] [Google Scholar]
- Hornby PJ, Rossiter CD, Pineo SV, Norman WP, Friedman EK, Benjamin S, Gillis RA. TRH: immunocytochemical distribution in vagal nuclei of the cat and physiological effects of microinjection. Am J Physiol. 1989;257:G454–G462. doi: 10.1152/ajpgi.1989.257.3.G454. [DOI] [PubMed] [Google Scholar]
- Hu L, Zhu DN, Yu Z, Wang JQ, Sun ZJ, Yao T. Expression of angiotensin II type 1 (AT(1)) receptor in the rostral ventrolateral medulla in rats. J Appl Physiol. 2002;92:2153–2161. doi: 10.1152/japplphysiol.00261.2001. [DOI] [PubMed] [Google Scholar]
- Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- Hughes SJ, Suzuki K, Goto Y. The role of islet secretory function in the development of diabetes in the GK Wistar rat. Diabetologia. 1994;37:863–870. doi: 10.1007/BF00400940. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yang H, Taché Y. Medullary sites of action of the TRH analogue, RX 77368, for stimulation of gastric acid secretion in the rat. Gastroenterology. 1988;95:1470–1476. doi: 10.1016/s0016-5085(88)80065-9. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Hoffman JL, Loewy AD. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res. 1997;766:29–38. doi: 10.1016/s0006-8993(97)00532-5. [DOI] [PubMed] [Google Scholar]
- Jensen J, Ruge T, Lai YC, Svensson MK, Eriksson JW. Effects of adrenaline on whole-body glucose metabolism and insulin-mediated regulation of glycogen synthase and PKB phosphorylation in human skeletal muscle. Metabolism. 2010 Feb 11; doi: 10.1016/j.metabol.2009.12.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Getting PA. Excitatory effects of thyrotropin-releasing hormone on neurons within the nucleus ambiguus of adult guinea pigs. Brain Res. 1992;590:1–5. doi: 10.1016/0006-8993(92)91074-o. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Pickel VM. Localization of tyrosine hydroxylase in neuronal targets and efferents of the area postrema in the nucleus tractus solitarii of the rat. J Comp Neurol. 1993;329:337–353. doi: 10.1002/cne.903290305. [DOI] [PubMed] [Google Scholar]
- Keeler JR, Charlton CG, Helke CJ. Cardiovascular effects of spinal cord substance P: studies with a stable receptor agonist. J Pharmacol Exp Ther. 1985;233:755–760. [PubMed] [Google Scholar]
- Ladram A, Bulant M, Montagne JJ, Nicolas P. Distribution of TRH-potentiating peptide (Ps4) and its receptors in rat brain and peripheral tissues. Biochem Biophys Res Commun. 1994;200:958–965. doi: 10.1006/bbrc.1994.1543. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Neuronal excitation by angiotensin II in the rostral ventrolateral medulla of the rat in vitro. Am J Physiol. 1995;268:R272–R277. doi: 10.1152/ajpregu.1995.268.1.R272. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Effect of substance P on C1 and other bulbospinal cells of the RVLM in neonatal rats. Am J Physiol. 1997;273:R805–R813. doi: 10.1152/ajpregu.1997.273.2.R805. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: morphological properties and relationship to C1 adrenergic neurons. Neuroscience. 1995;69:601–618. doi: 10.1016/0306-4522(95)92652-z. [DOI] [PubMed] [Google Scholar]
- Lynn RB, Kreider MS, Miselis RR. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. J Comp Neurol. 1991;311:271–288. doi: 10.1002/cne.903110208. [DOI] [PubMed] [Google Scholar]
- Makeham JM, Goodchild AK, Pilowsky PM. NK1 receptor activation in rat rostral ventrolateral medulla selectively attenuates somato-sympathetic reflex while antagonism attenuates sympathetic chemoreflex. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1707–R1715. doi: 10.1152/ajpregu.00537.2004. [DOI] [PubMed] [Google Scholar]
- Mallett J. The TiPs/TINS lecture. Catecholamines from Gene Regulation to Neuropsychiatric Disorders. Trends Pharmacol Sci. 1996;17:129–135. doi: 10.1016/0165-6147(96)81587-2. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Csernus V, Csontos C, Petrusz P, Mess B. New data on the immunocytochemical localization of thyrotropin-releasing hormone in the rat central nervous system. Am J Anat. 1988;181:359–376. doi: 10.1002/aja.1001810404. [DOI] [PubMed] [Google Scholar]
- Murakawa Y, Zhang W, Pierson CR, Brismar T, Ostenson CG, Efendic S, Sima AA. Impaired glucose tolerance and insulinopenia in the GK-rat causes peripheral neuropathy. Diabetes Metab Res Rev. 2002;18:473–483. doi: 10.1002/dmrr.326. [DOI] [PubMed] [Google Scholar]
- Muratani H, Averill DB, Ferrario CM. Effect of angiotensin II in ventrolateral medulla of spontaneously hypertensive rats. Am J Physiol. 1991;260:R977–R984. doi: 10.1152/ajpregu.1991.260.5.R977. [DOI] [PubMed] [Google Scholar]
- Niijima A. Studies on the nervous regulatory mechanism of blood sugar levels. Pharmacol Biochem Behav. 1975;3:139–143. [PubMed] [Google Scholar]
- Oida E, Kannagi T, Moritani T, Yamori Y. Diabetic alteration of cardiac vago-sympathetic modulation assessed with tone-entropy analysis. Acta Physiol Scand. 1999;165:129–134. doi: 10.1046/j.1365-201x.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- Oshima N, Kumagai H, Kawai A, Sakata K, Matsuura T, Saruta T. Three types of putative presympathetic neurons in the rostral ventrolateral medulla studied with rat brainstem-spinal cord preparation. Auton Neurosci. 2000;84:40–49. doi: 10.1016/S1566-0702(00)00179-X. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pekary AE, Sattin A, Lloyd RL. Electroconvulsive seizures increase levels of PS4, the TRH-enhancing peptide [prepro-TRH(160-169)], in rat brain. Neuroendocrinology. 1997;65:377–384. doi: 10.1159/000127199. [DOI] [PubMed] [Google Scholar]
- Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23:45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J Comp Neurol. 2001;432:20–34. doi: 10.1002/cne.1086. [DOI] [PubMed] [Google Scholar]
- Porte DJ, Kahn SE. beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50(Suppl 1):S160–S163. doi: 10.2337/diabetes.50.2007.s160. [DOI] [PubMed] [Google Scholar]
- Proks P, Treinies I, Mest HJ, Trapp S. Inhibition of recombinant K(ATP) channels by the antidiabetic agents midaglizole, LY397364 and LY389382. Eur J Pharmacol. 2002;452:11–19. doi: 10.1016/s0014-2999(02)02234-3. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR, Kreider MS. Ultrastructural localization of thyrotropin-releasing hormone immunoreactivity in the dorsal vagal complex in rat. Neurosci Lett. 1989;104:7–12. doi: 10.1016/0304-3940(89)90320-0. [DOI] [PubMed] [Google Scholar]
- Sasek CA, Wessendorf MW, Helke CJ. Evidence for co-existence of thyrotropin-releasing hormone, substance P and serotonin in ventral medullary neurons that project to the intermediolateral cell column in the rat. Neuroscience. 1990;35:105–119. doi: 10.1016/0306-4522(90)90125-n. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte DJ. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Tschop M. How diabetes went to our heads. Nat Med. 2006;12:47–49. doi: 10.1038/nm0106-47. [DOI] [PubMed] [Google Scholar]
- Stephens RL, Ishikawa T, Weiner H, Novin D, Taché Y. TRH analogue, RX 77368, injected into dorsal vagal complex stimulates gastric secretion in rats. Am J Physiol. 1988;254:G639–G643. doi: 10.1152/ajpgi.1988.254.5.G639. [DOI] [PubMed] [Google Scholar]
- Sun MK, Guyenet PG. Effects of vasopressin and other neuropeptides on rostral medullary sympathoexcitatory neurons ‘in vitro’. Brain Res. 1989;492:261–270. doi: 10.1016/0006-8993(89)90909-8. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Llewellyn-Smith I, Minson J, Arnolda L, Chalmers J, Pilowsky P. Thyrotropin-releasing hormone immunoreactive boutons form close appositions with medullary expiratory neurons in the rat. Brain Res. 1996;715:136–144. doi: 10.1016/0006-8993(95)01569-8. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Pilowsky P, Llewellyn-Smith IJ. Thyrotropin-releasing hormone inputs are preferentially directed towards respiratory motoneurons in rat nucleus ambiguus. J Comp Neurol. 1995;362:320–330. doi: 10.1002/cne.903620303. [DOI] [PubMed] [Google Scholar]
- Taché Y, Vale W, Brown M. Thyrotropin-releasing hormone--CNS action to stimulate gastric acid secretion. Nature. 1980;287:149–151. doi: 10.1038/287149a0. [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H. Role of medullary TRH in the vagal regulation of gastric function. In: Wingate DL, Butkd TF, editors. Innervation of the Gut: Pathophysiological Implications. Boca Raton: CRC; 1994. pp. 67–80. [Google Scholar]
- Valentijn K, Bunel DT, Liao N, Pelletier G, Vaudry H. Release of pro-thyrotropin-releasing hormone connecting peptides PS4 and PS5 from perifused rat hypothalamic slices. Neuroscience. 1991;44:223–233. doi: 10.1016/0306-4522(91)90263-n. [DOI] [PubMed] [Google Scholar]
- Yang H, Ohning GV, Taché Y. TRH in dorsal vagal complex mediates acid response to excitation of raphe pallidus neurons in rats. Am J Physiol. 1993;265:G880–G886. doi: 10.1152/ajpgi.1993.265.5.G880. [DOI] [PubMed] [Google Scholar]
- Yang H, Stephens RL, Taché Y. TRH analogue microinjected into specific medullary nuclei stimulates gastric serotonin secretion in rats. Am J Physiol. 1992;262:G216–G222. doi: 10.1152/ajpgi.1992.262.2.G216. [DOI] [PubMed] [Google Scholar]
- Yang H, Tache Y, Ohning G, Go VL. Activation of raphe pallidus neurons increases insulin through medullary thyrotropin-releasing hormone (TRH)-vagal pathways. Pancreas. 2002 Oct;25 (3):301–7. doi: 10.1097/00006676-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Yang H, Taché Y. Prepro-TRH-(160-169) potentiates gastric acid secretion stimulated by TRH microinjected into the dorsal motor nucleus of the vagus. Neurosci Lett. 1994a;174:43–46. doi: 10.1016/0304-3940(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu SV, Ishikawa T, Taché Y. Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience. 1994b;61:655–663. doi: 10.1016/0306-4522(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Yuan PQ, Yang H. Hypothyroidism induces Fos-like immunoreactivity in ventral medullary neurons that synthesize TRH. Am J Physiol. 1999;277:E927–E936. doi: 10.1152/ajpendo.1999.277.5.E927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan PQ, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am J Physiol. 2002;283:E436–E448. doi: 10.1152/ajpendo.00538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]