Abstract
In nucleophilic phosphine catalysis, tertiary phosphines undergo conjugate additions to activated carbon–carbon multiple bonds to form β-phosphonium enolates, β-phosphonium dienolates, β-phosphonium enoates, and vinyl phosphonium ylides as intermediates. When these reactive zwitterionic species react with nucleophiles and electrophiles, they may generate carbo- and heterocycles with multifarious molecular architectures. This Article describes the reactivities of these phosphonium zwitterions, the applications of phosphine catalysis in the syntheses of biologically active compounds and natural products, and recent developments in the enantioselective phosphine catalysis.
1. Introduction
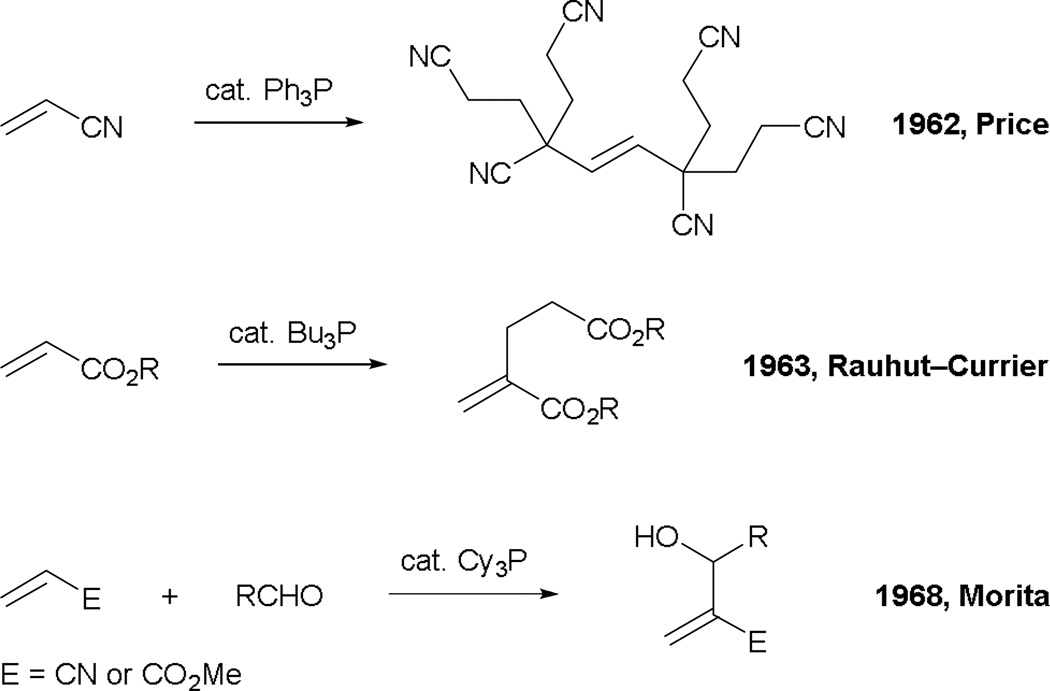
Carbon–carbon bond formation via phosphine catalysis has been known since Price reported the hexamerization of acrylonitrile using triphenylphosphine in 1962 (Scheme 1).1 A more widely known example was disclosed by Rauhut and Currier in 1963 in a patent concerning the synthesis of dialkyl-2-methylenepentadioates.2 Several years after, Mortia and co-workers reported the formation of α-hydroxymethyl acrylates and acrylonitriles, catalyzed by tricyclohexylphosphine.3a That report described the first phosphine-catalyzed reactions of activated alkenes with aldehydes, forming what are now recognized as Morita–Baylis–Hillman (MBH) adducts. Similar transformations can also be achieved using tertiary amines (such as DABCO, quinuclidine, indolizine) as catalysts, as reported later by Baylis and Hillman.3b
Scheme 1.
Phosphine-catalyzed reactions at an early stage.
After those three seminal reports in the 1960s of carbon–carbon bond formation through nucleophilic phosphine catalysis, only sporadic communications appeared thereafter in the field of phosphine catalysis. It was not until the beginning of the 21st century that phosphine catalysis attracted interest from a large number of research groups, leading to an explosion in the reporting of new reaction modes. One of the advantages of organocatalysis, especially phosphine catalysis, is that highly efficient reaction processes, involving the attachment of two or more readily available starting materials, can yield complex molecular architectures with simple post-reaction workup. To provide a focused perspective on the nucleophilic phosphine catalysis, this Article highlights only selected examples of a number of phosphine-catalyzed reactions. The Sections are organized based on the type of activated carbon–carbon multiple bond-containing starting material (alkene, allene, alkyne, or MBHAD), further divided by the nature of the second reaction partner (nucleophile, electrophile, or electrophile–nucleophile).
2. Phosphine Catalysis of Alkenes
2.1. Phosphine-Initiated Michael Addition
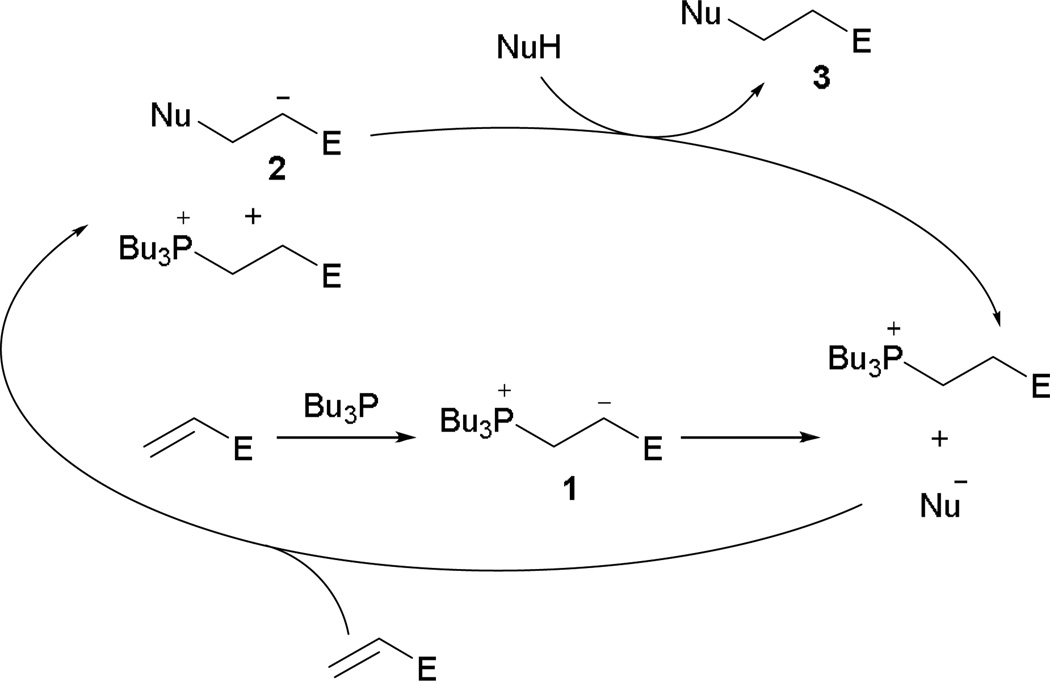
One of the oldest phosphine-catalyzed reactions with activated olefins and nucleophiles is the Michael addition. In 1973, White and Baizer demonstrated, in a short Communication, the first Michael additions of 2-nitropropane onto activated alkenes, mediated by tertiary phosphines (e.g., triphenylphosphine, methyldiphenylphosphine, dimethylphenylphosphine, tributylphosphine).4 In contrast to the traditional strong base-mediated Michael additions, the zwitterion 1 generated in situ upon addition of tributylphosphine to the activated alkene served as the base to activate the pronucleophile (Scheme 2).
Scheme 2.
Phosphine-initiated general base catalysis.
In the proposed mechanism, tributylphosphine adds conjugatively to the activated alkene to provide the phosphonium enolate 1, which deprotonates the pronucleophile for Michael addition. Post-addition, the enolate 2 is formed and can further catalyze the reaction, providing the Michael adduct 3.
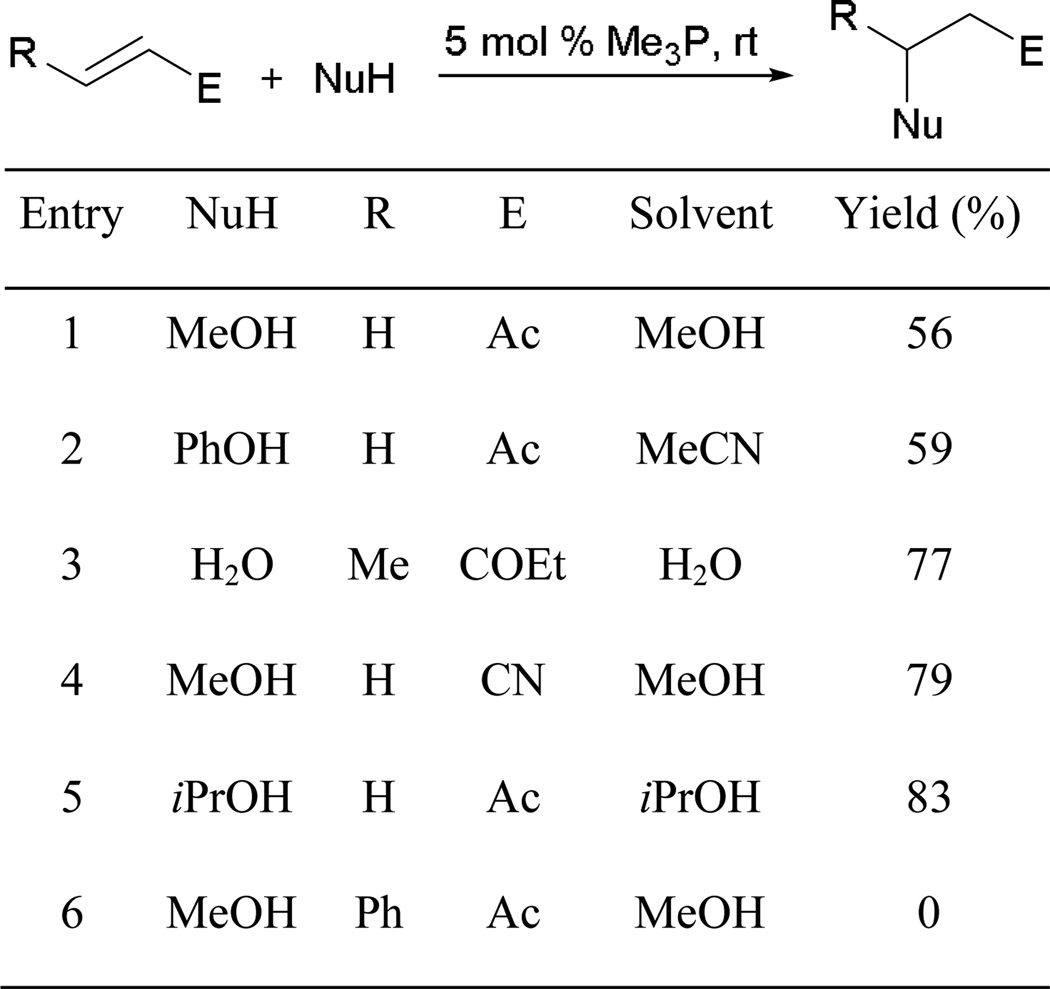
Among various pronucleophiles, alcohols appear to undergo Michael additions particularly efficiently in the presence of organophosphine catalysts. In 2003, Toste and Bergman reported an efficient transformation involving conjugate addition of alcohols to methyl vinyl ketone (MVK), ethyl vinyl ketone, and acrylonitrile in aqueous medium.5 Using 5 mol % trimethylphosphine as the catalyst, alcohols served both as pronucleophiles and solvent to furnish functionalized Michael products (Scheme 3). It is noteworthy that water, in a rare example, could also be used as a pronucleophile (entry 3). Although most alcohols/water underwent smooth addition, no Michael adduct was isolated when 4-phenylbut-3-en-2-one was employed, presumably due to steric hindrance at the β-position (entry 6).
Scheme 3.
Phosphine-mediated Michael additions of alcohols and water to activated alkenes.
2.2. Reactions of Alkenes with Electrophiles
Although the Rauhut–Currier (RC) reaction and the Morita (or Morita–Baylis–Hillman) reaction belong to this category, they are not covered in this Perspective. For detailed account of the Morita reaction, the reader should consult several reviews.6 For the RC reaction, the reader should refer to Miller’s review.7
2.2.1. Phosphine-Catalyzed Annulation through Intramolecular RC–Aldol Reaction
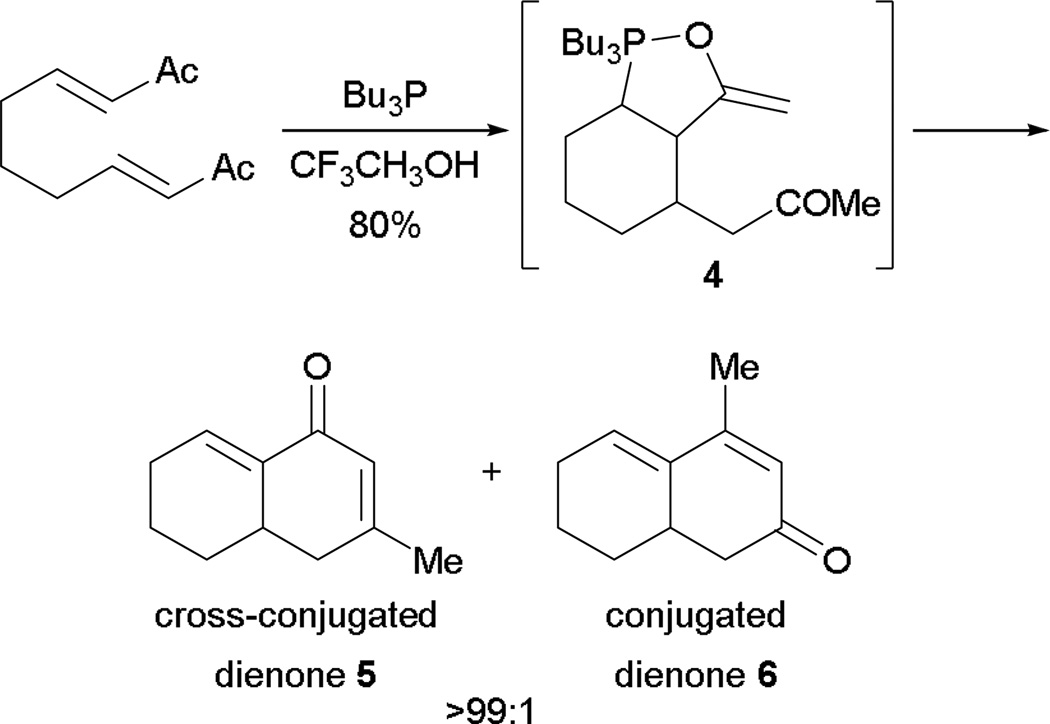
In 2005, Roush and co-workers demonstrated an intramolecular RC reaction followed by an aldol reaction, leading to several 1-decala-2,8-dienones.8 Through phosphine catalysis, regioselective aldol addition formed the cross-conjugated dienones 5 exclusively over conjugated dienone systems 6 (Scheme 4). This selectivity contrasts that of intramolecular aldol reaction performed using Brønsted bases, where conjugated dienones 6 are formed preferentially. The highly selective formation of the cross-conjugated dienone system 5 was due to the large oxophilicity of phosphine, to form the oxaphospholidine enol ether 4, which in turn dictated the regiochemical outcome of the reaction.
Scheme 4.
Aldol reaction through oxaphospholidine-stabilized enol ether.
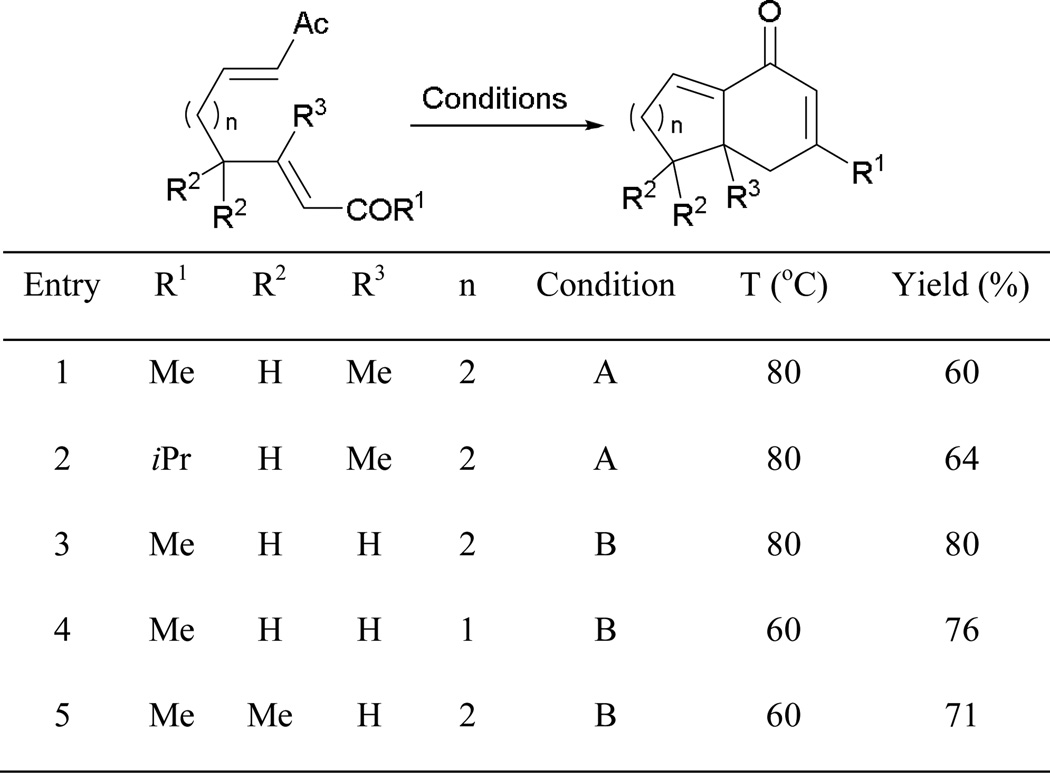
The highly selective intramolecular RC–aldol reaction allowed the synthesis of functionalized 1-decala-2,8-dienones in good yields (Scheme 5). Aside from 6,6-fused bicyclic systems, a shorter tether also allows the formation of 5,6-fused ring constructs in comparable efficiency (entry 4). Remarkably, only one regioisomer is formed in a non-symmetric bis-enone system where four different regioisomers are possible (entry 5).
Scheme 5.
Preparation of bicyclic cross-conjugated bis-enones.
Condition A: Me3P (5 equiv) in 2-methylbutan-2-ol. Condition B: Bu3P (1 equiv) in 2,2,2-trifluoroethanol.
2.2.2. Phosphine-catalyzed Annulation through Michael-Interfered aza-MBH Reaction
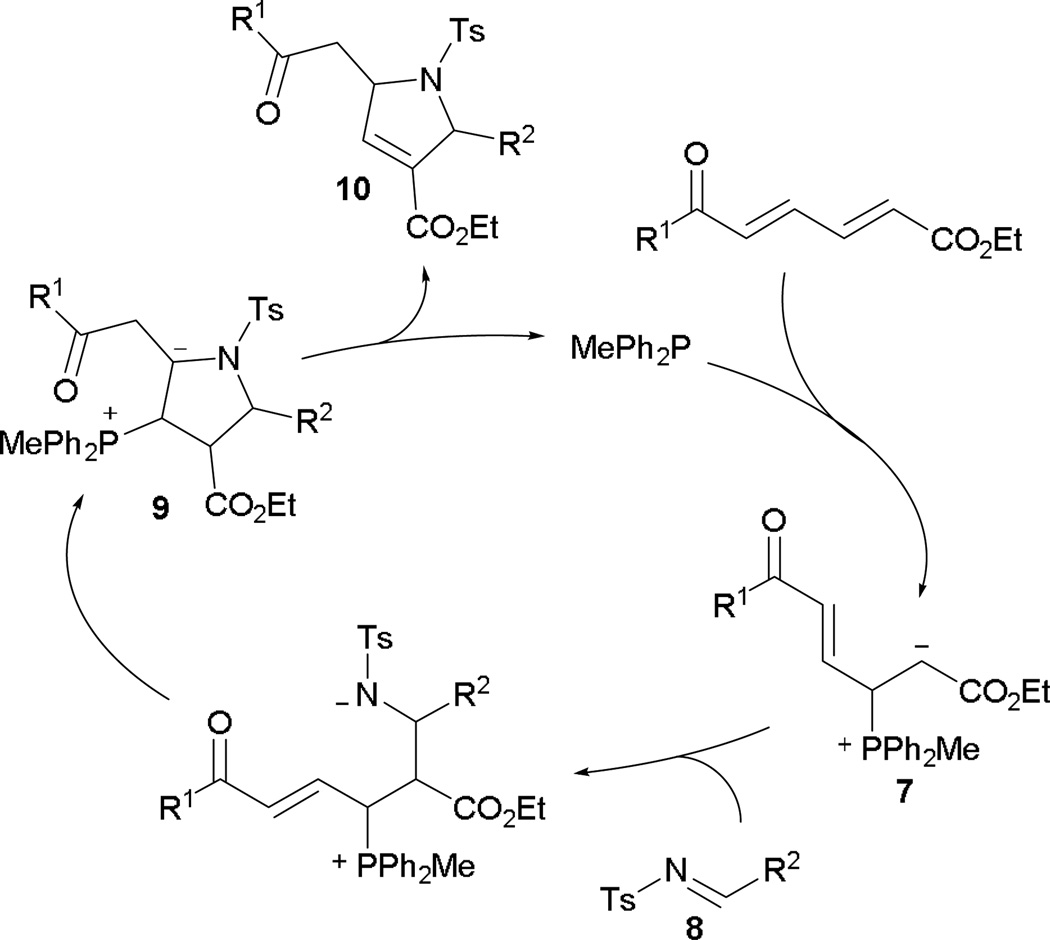
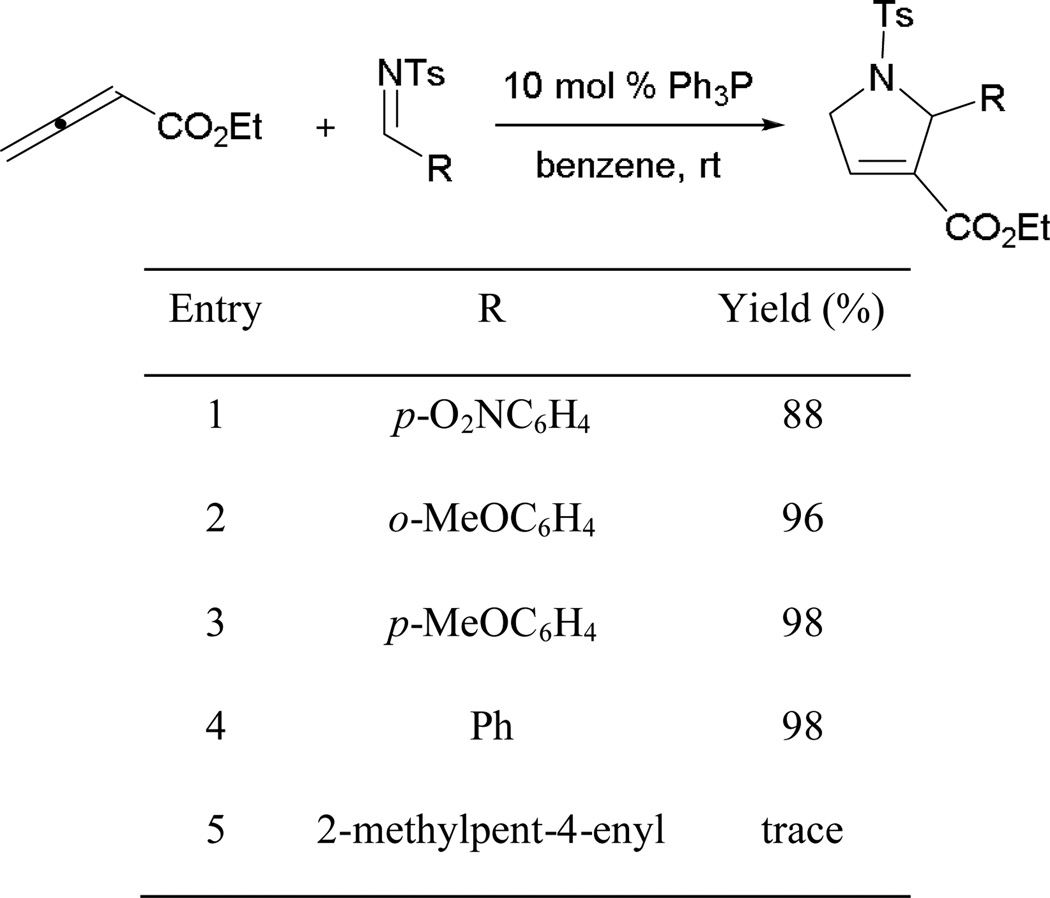
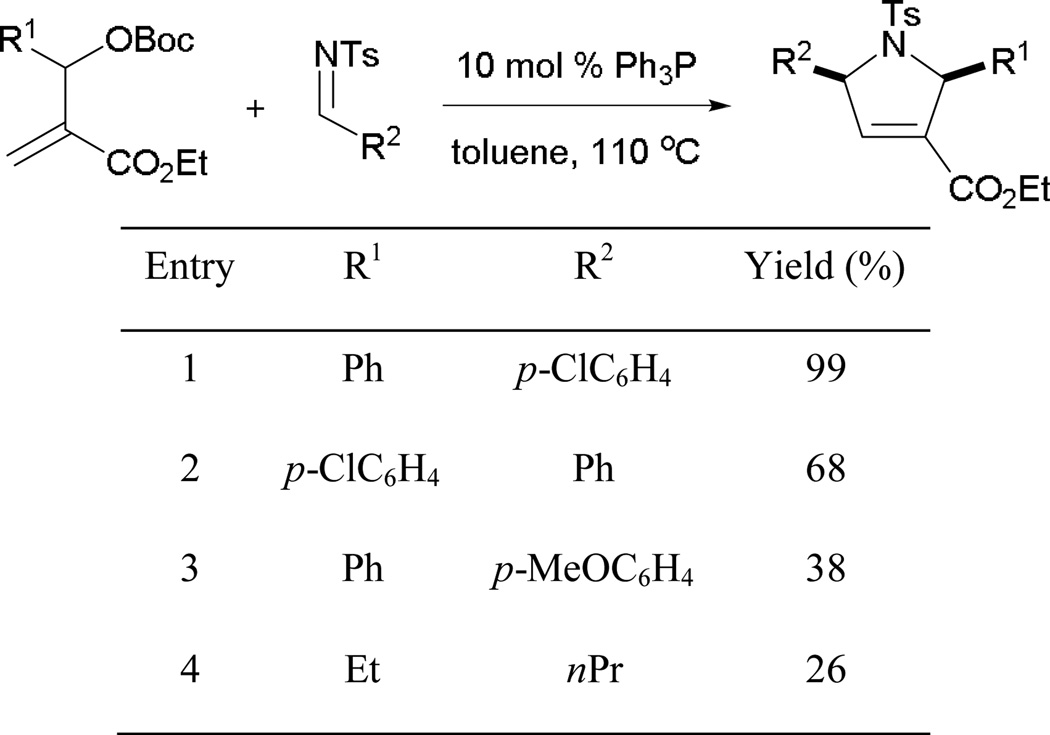
Employing ethyl 6-oxohexa-2,4-dienoates and imines, Marinetti and co-workers developed an effective annulation pathway leading to pyrrole derivatives.9 The proposed mechanism begins as an aza-MBH reaction, with the phosphine adding across the activated alkene to form the phosphonium enoate 7 (Scheme 6). In the presence of the tosylimine 8, aza-MBH occurs, followed by Michael addition, giving the intermediate 9. Upon proton transfer and regeneration of the catalyst, the elaborated pyrrole 10 is afforded.
Scheme 6.
Mechanism of Michael-interfered aza-MBH reaction.
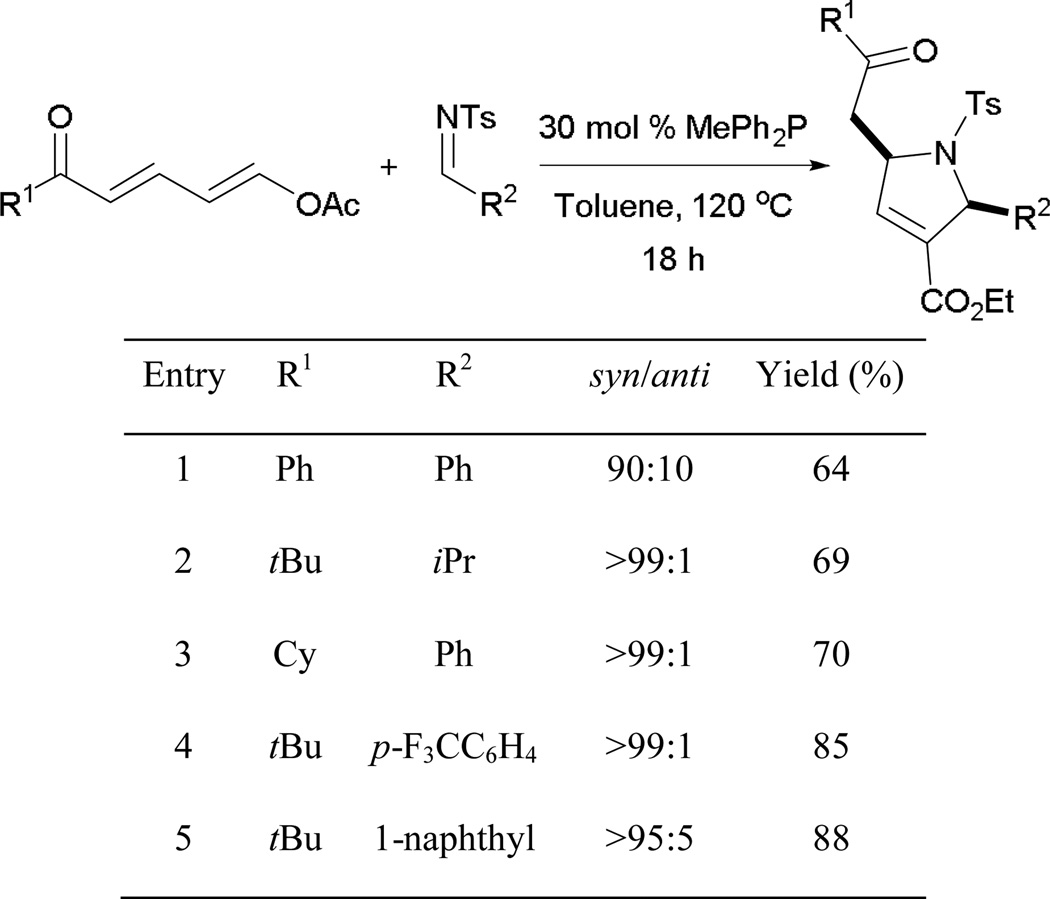
Aryl tosylimines are commonly used electrophiles in phosphine catalysis. Varyious aryl tosylimines are applicable in this transformation, yielding functionalized pyrroles with high diastereoselectivities (Scheme 7). Among the tested imines, a rare example involves the use of tosyl isopropylaldimine (entry 2). Generally, alkylaldimines are not employed broadly in phosphine catalysis, whereas the use of arylaldimines is common.
Scheme 7.
Construction of 2,5-dihydropyrrole derivatives.
2.3. Reactions of Alkenes with Electrophile–Nucleophiles
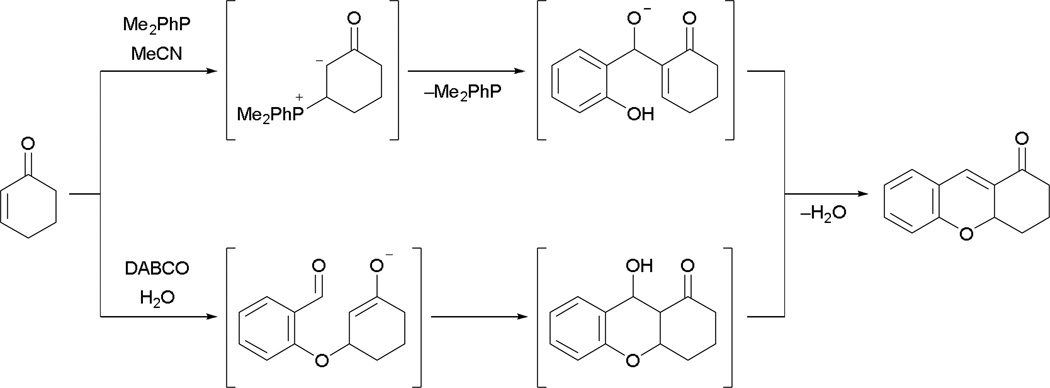
In addition to simple external sources of nucleophiles or electrophiles, annulation events also occur in more-complex systems comprising both electrophiles and nucleophiles. Shi demonstrated this possibility, employing readily available salicylaldehyde as the source of the electrophile–nucleophile in the MBH–Michael cyclization event.10 The reaction sequence is speculated to involve an initial MBH reaction followed by intramolecular Michael addition (Scheme 8). The same product was formed when using 1,4-diazabicyclo[2.2.2]octane (DABCO) in water. In the DABCO-catalyzed system, basicity plays an important role in activating salicylaldehyde through deprotonation for the initial Michael addition, followed by the aldol reaction. In contrast, a tertiary phosphine favors nucleophilic addition across activated alkenes over deprotonation. This observation hints at the possibility of an initial MBH reaction followed by a Michael reaction.
Scheme 8.
Proposed MBH–Michael pathway.
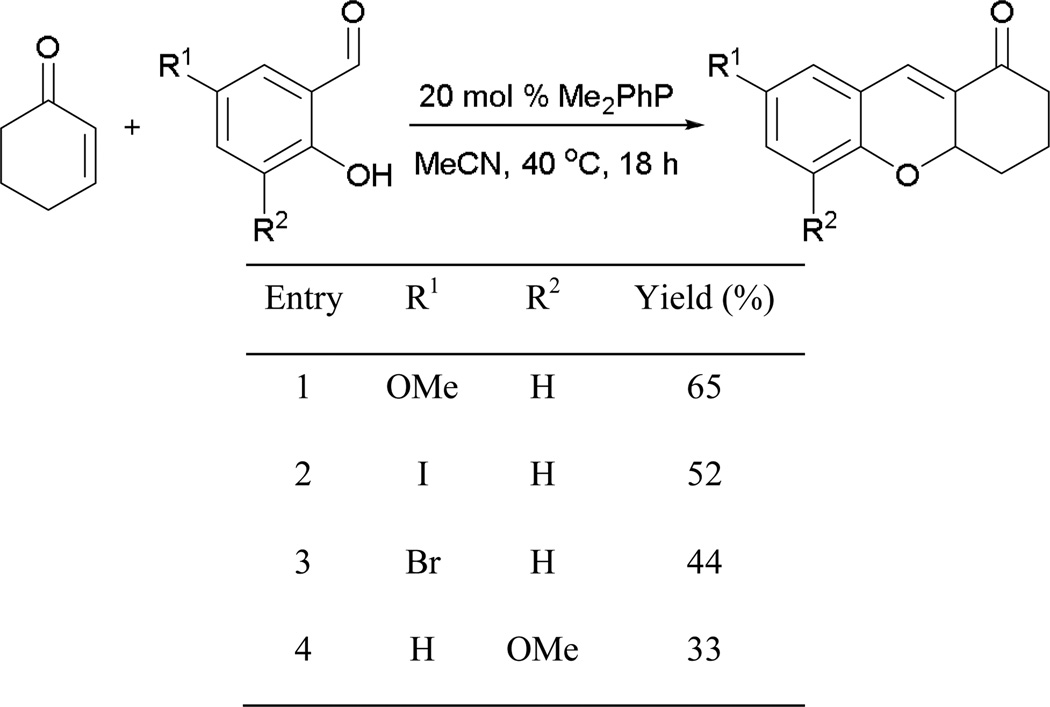
Although the concept of the MBH–Michael reaction has been demonstrated, the scope of the reaction remains limited to cyclohexenone and simply substituted aryl aldehydes (Scheme 9). In general, the reaction can provide several xanthenones, but in mediocre yields.
Scheme 9.
Preparation of xanthenone derivatives.
3. Phosphine Catalysis of Allenes
3.1. Reactions of Allenes with Nucleophiles
When treating activated alkenes with phosphine catalysts, the formation of the phosphonium enolate prompts activation of the pronucleophiles to undergo general base-catalyzed, Michael addition. Unlike phosphine-catalyzed reactions with alkenes, the phosphonium dienolate generated in situ deprotonates the pronucleophile, triggering what is known as γ-umpolung addition.
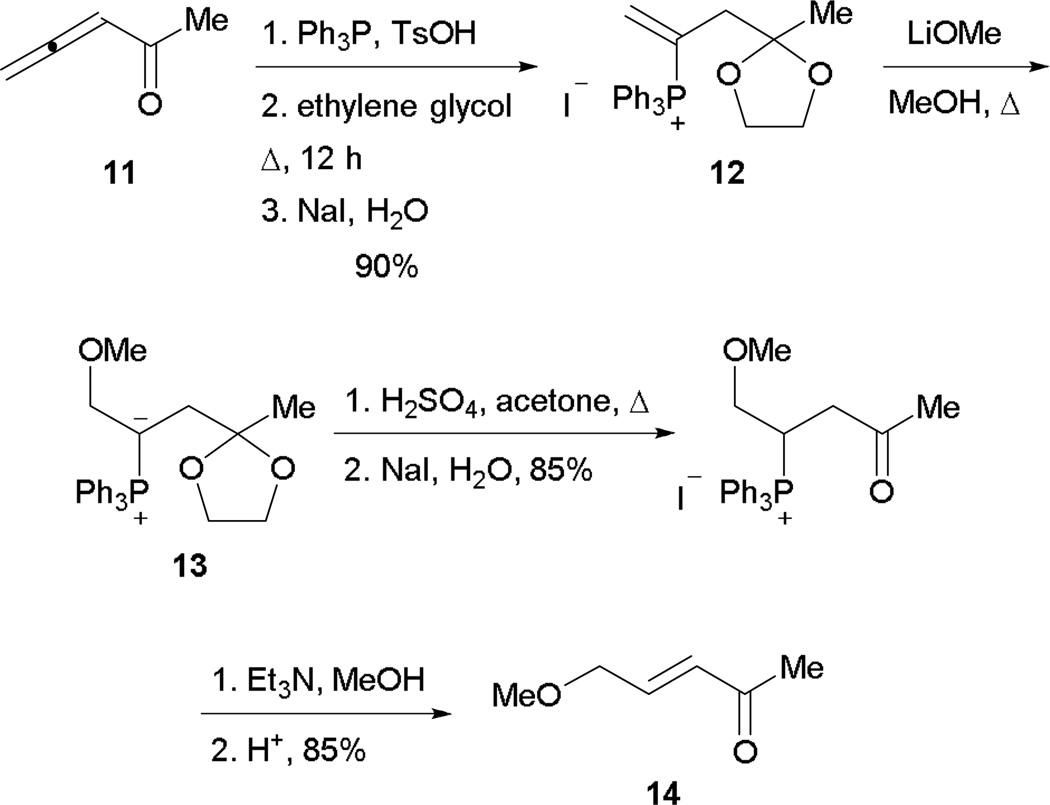
The concept of γ-umpolung addition was introduced and manifested by Cristau and co-workers in 1982.11 Although the reaction is not catalytic in nature, the concept of γ-umpolung addition was realized through a stepwise sequence (Scheme 10). To test the feasibility of nucleophilic addition into a vinyl phosphonium ion, triphenylphosphine was added into 3,4-pentadien-2-one 11, followed by protection and counter ion exchange. After isolating the vinyl phosphonium iodide 12, external methoxide was used as the nucleophile to verify the possibility of γ-umpolung addition, yielding the ylide 13. After removal of the acetal group and elimination of phosphine, the γ-umpolung product 14 was obtained.
Scheme 10.
Stepwise γ-umpolung addition.
3.1.1. Phosphine-Catalyzed γ-Umpolung Addition
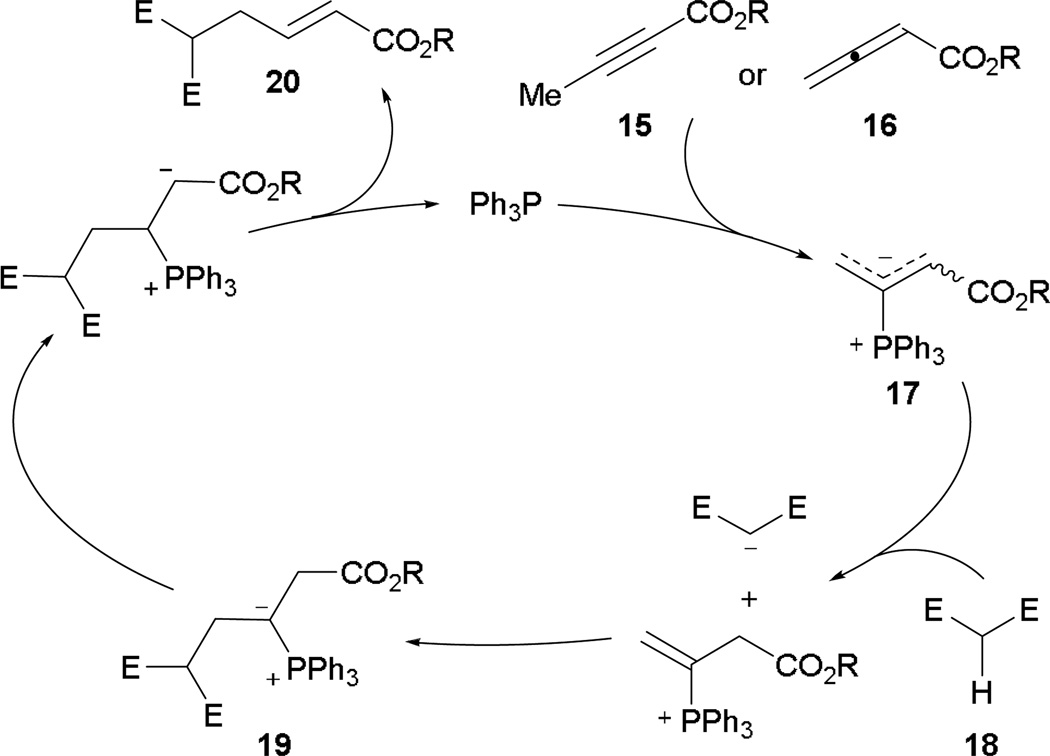
It was not until 1994 that the first catalytic variant of γ-umpolung addition appeared in the phosphine literature. Trost and Li demonstrated the first phosphine-catalyzed γ-umpolung addition of various β-carbonyl esters and activated sulfones onto 2-butynoate.12 Under phosphine catalysis, both 2-butynoate and 2,3-butadienoate lead to the common phosphonium dienolate 17 (Scheme 11). In the presence of dienolate 17, the pronucleophile 18 becomes activated through deprotonation; immediate γ-umpolung addition across the electrophilic vinyl phosphonium carbon–carbon double bond gives the ylide 19. After proton transfer and elimination of the catalyst, the γ-umpolung product 20 is afforded.
Scheme 11.
Proposed mechanism for phosphine-catalyzed γ-umpolung addition.
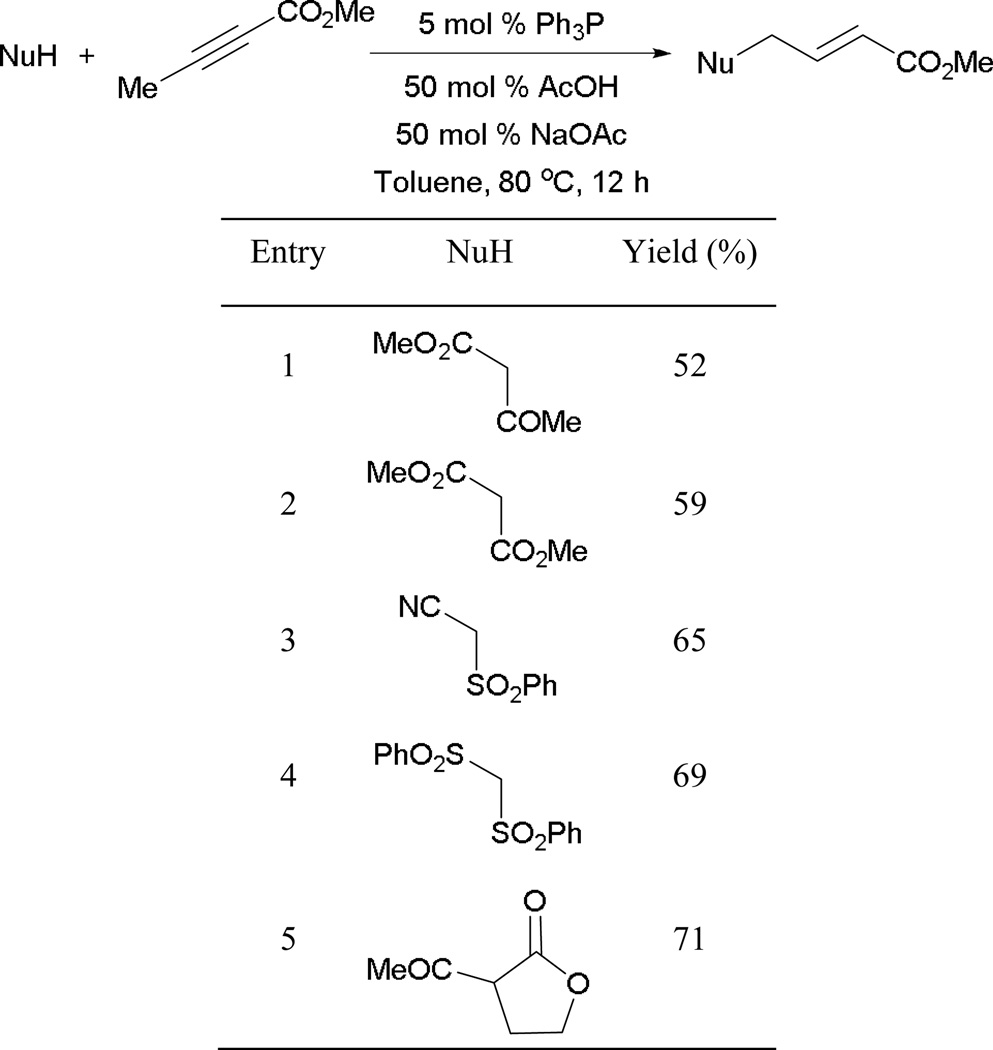
To further enhance the efficiency of γ-umpolung addition, a buffer system containing an equal ratio of AcOH and NaOAc was found to be necessary (Scheme 12). Immediate protonation of the dienolate 17 is pivotal for the reaction to provide good yields, preventing undesired self-oligomerization of 2-butynoate/2,3-butadienoate. The reaction typically favors pronucleophiles having a pKa of less than 16, such as β-keto esters, β-diesters, β-cyano sulfones, and bis(phenylsulfonyl)methane.
Scheme 12.
Preparation of γ-substituted 2-butenoates.
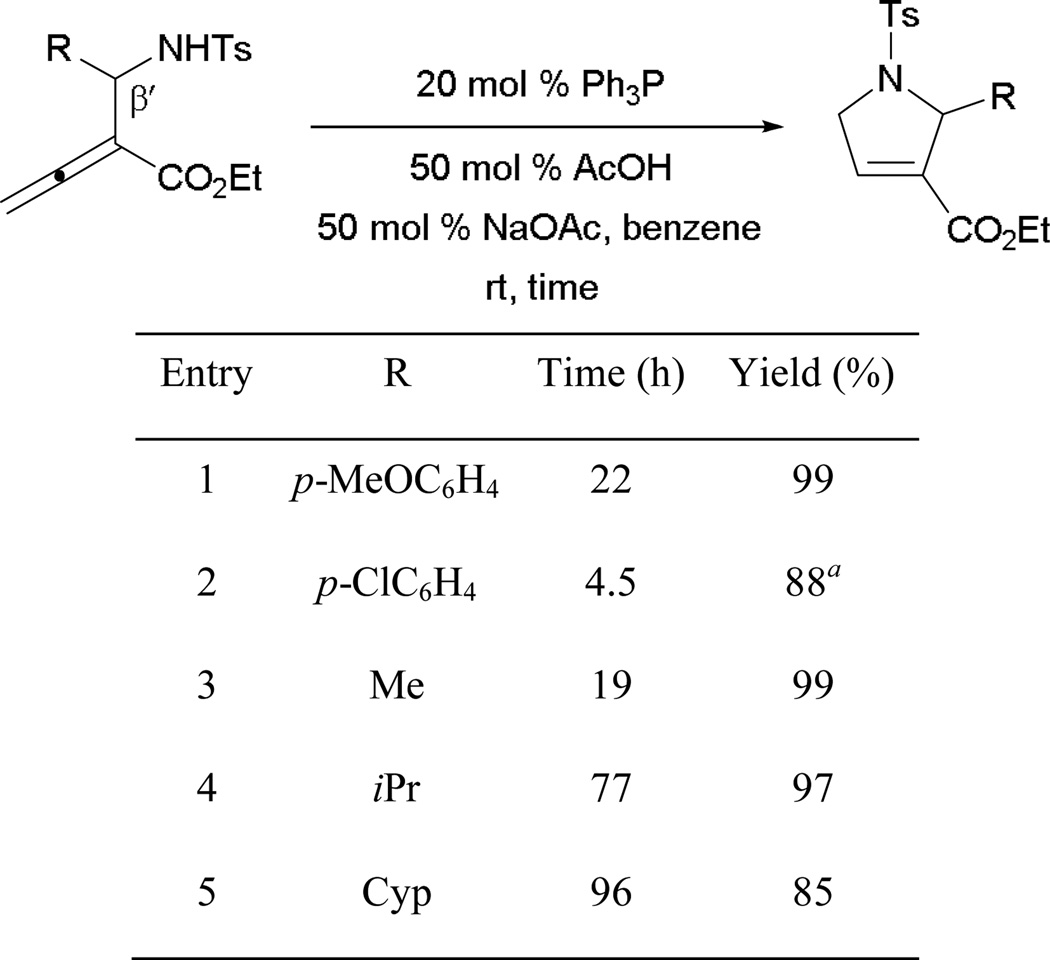
Recently, Andrews and Kwon disclosed the formation of functionalized 2-alkyl dihydropyrroles, highlighting the first example of 5-endo cyclization via intramolecular γ-umpolung addition (Scheme 13).13 High isolated product yields were observed from allenoates bearing β'-aryl substituents of various electronic properties (entries 1 and 2). Remarkably, no additives were necessary when the p-chlorophenyl group was present (entry 2). Aside from aryl functionalities, excellent yields were also achieved from several β'-alkyl substituted allenoates (entries 3–5).
Scheme 13.
Formation of dihydropyrroles via intramolecular γ-umpolung addition.
a Reaction performed without additives.
3.1.2. Phosphine-Catalyzed β'-Umpolung Addition
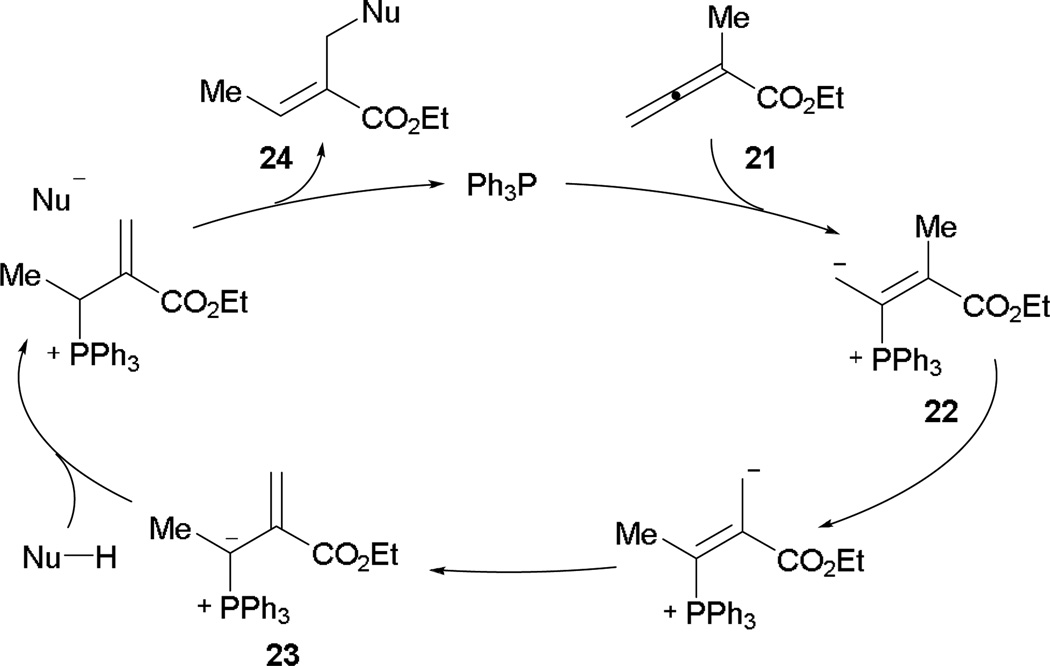
Recently, Kwon and co-workers reported a novel β'-umpolung addition en route to functionalized acrylates.14 The postulated mechanism proceeds with initial phosphine addition to 2-methyl-2,3-butadienoate 21, generating the phosphonium dienolate 22 (Scheme 14). After proton transfer, the vinyl phosphonium ylide 23 is formed and activates the pronucleophile for β'-umpolung addition. After eliminating the catalyst, the acrylate 24 is obtained readily.
Scheme 14.
Proposed β'-umpolung addition mechanism.
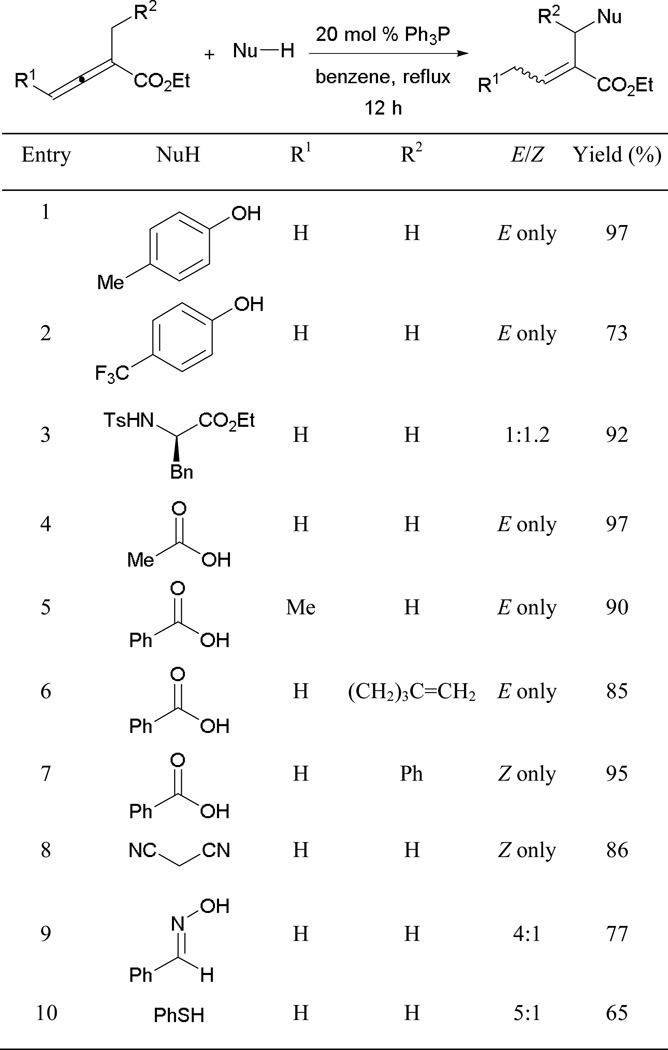
Oxygen-, nitrogen-, sulphur-, and carbon-centered nucleophiles are well suited for the β'-umpolung reaction (Scheme 15). It is noteworthy that no additives are required to facilitate the proton transfer events, unlike the case for γ-umpolung additions. Phenols having different substitution patterns undergo additions smoothly to provide high product yields (entries 1 and 2). Tosylated phenylalanine is also compatible, giving a great yield of the acrylate product, albeit as an almost equal mixture of E- and Z-isomers (entry 3). Various carboxylic acids are tolerated well, with high product conversions (entries 4–6). For more sterically demanding substrates, the stereoselectivity can be reversed to obtain Z-acrylates exclusively (entries 7 and 8). Aside from the aforementioned pronucleophiles, malononitrile, oxime, and benzenethiol can also be used to afford desired products (entries 8–10).
Scheme 15.
Formation of functionalized acrylates.
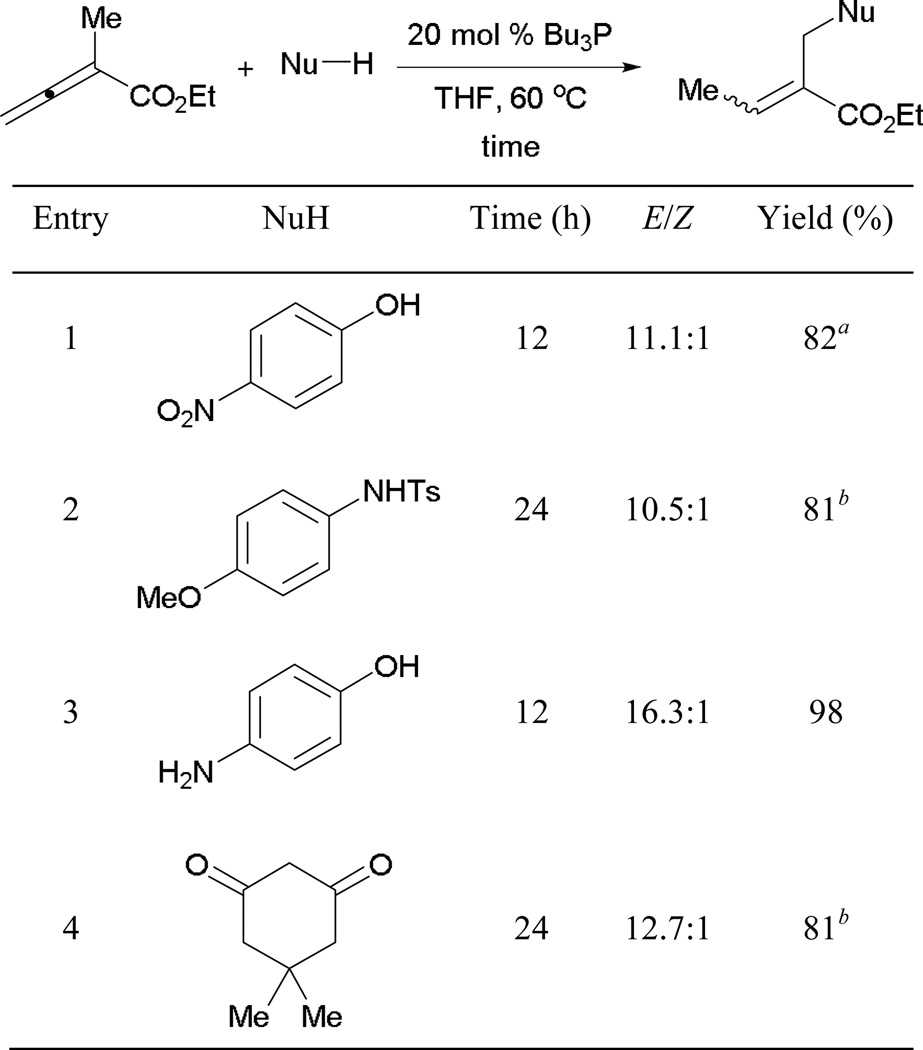
Contemporaneously, Shi and co-workers reported the formation of acrylate derivatives through β'-umpolung addition.15 Oxygen-, nitrogen-, and carbon-based pronucleophiles are well suited for this reaction (Scheme 16). Unlike Kwon’s approach, this reaction is mediated by the more-nucleophilic tributylphosphine, with THF as the solvent. Interestingly, addition occurs exclusively at the oxygen-atom when p-amino phenol is the pronucleophile (entry 3).
Scheme 16.
Synthesis of acrylate derivatives.
a Reaction conducted using Ph3P. b Reaction conducted at room temperature.
3.2. Reactions of Allenes with Di-nucleophiles
When treating allenoates with mono-pronucleophiles, the two common reaction pathways are γ-umpolung and β'-umpolung additions. Unless the pronucleophile is tethered onto the allenoate, no annulation event could occur. On the other hand, different modes of annulation have been discovered, yielding various heterocycles, when di-pronucleophiles have been employed in the reaction.
3.2.1. Phosphine-Catalyzed γ-Umpolung–Michael Reaction
When more than one nucleophile is present in a tethered system, a novel annulation pathway can occur, allowing the formation of piperazines. In 2002, Lu and co-workers disclosed the formation of piperazines from 2-alkynone via γ-umpolung addition with subsequent ring closure through intramolecular Michael addition (Scheme 17).16 In general, 2-alkynones provided higher reaction yields than 2-alkynoates.
Scheme 17.
Synthesis of piperazines via γ-umpolung–Michael addition.
3.2.2. Phosphine-Catalyzed Mixed Double-Michael [4 + 1] Annulation
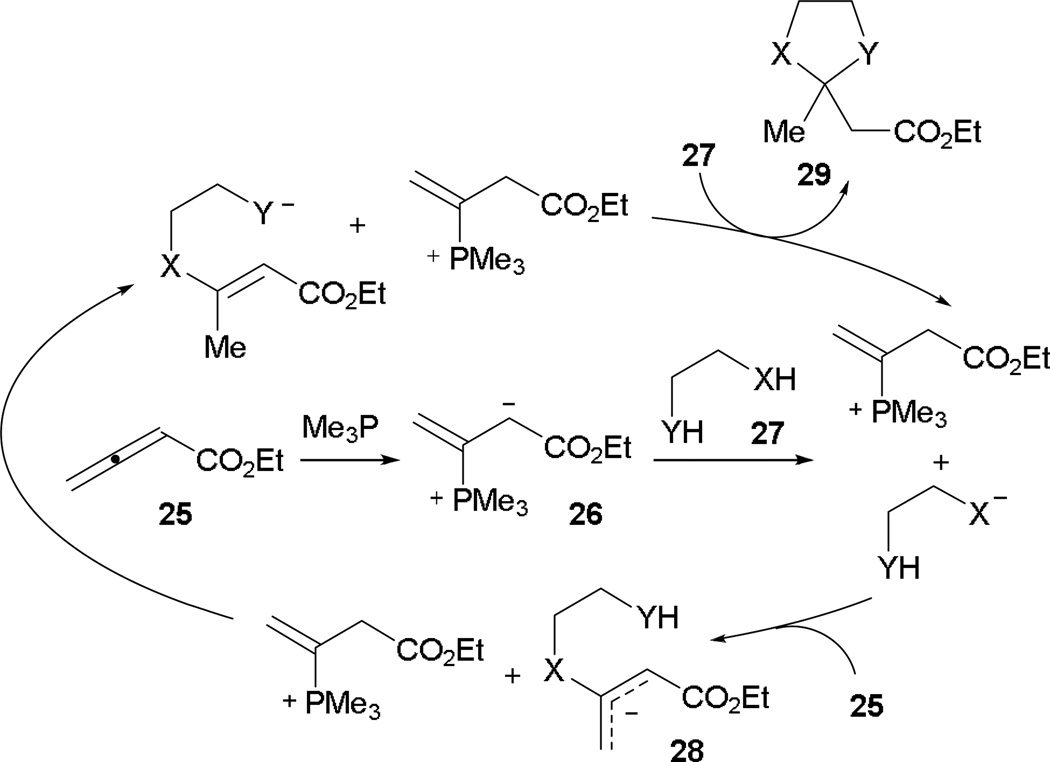
Recently, Szeto and Kwon disclosed the first examples of mixed double-Michael reactions between dinucleophiles and allenoates.17 The mechanism of this powerful reaction is proposed to be a general base catalysis triggered by the phosphine (Scheme 18). Facile formation of the phosphonium dienolate 26 prompts the activation of the tethered di-pronucleophile 27. In the presence of an allenoate 25, initial Michael addition occurs to afford the intermediate 28. Another proton transfer enables intramolecular Michael reaction, leading to the annulation product 29.
Scheme 18.
Proposed mechanism for mixed double-Michael [4+1] annulation.
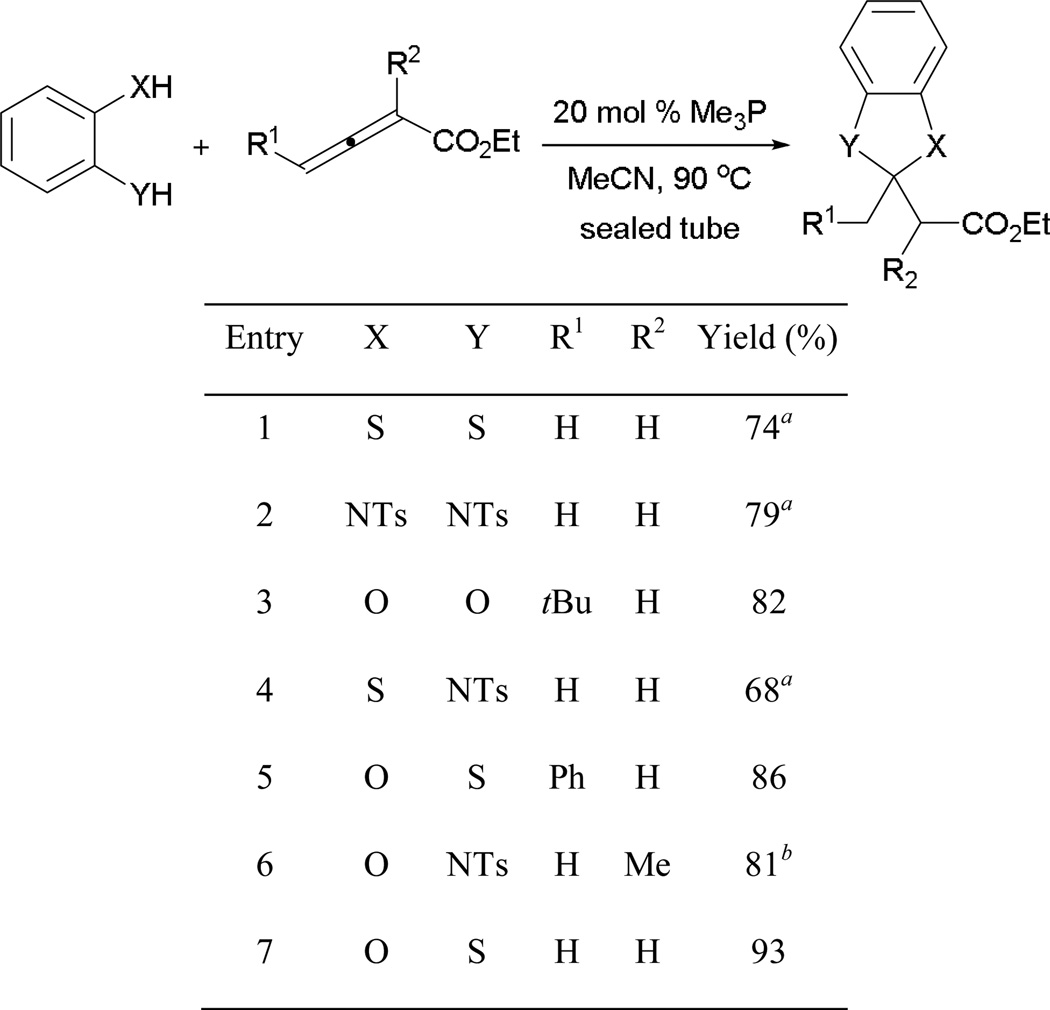
The reaction operates smoothly for di-pronucleophiles bearing various substitution patterns (Scheme 19). This method allows access to several different heterocyclic systems—benzimidazolines, benzoxazolines, benzothiazolines, 1,3-benzodioxoles, 1,3-benzoxathioles, and 1,3-benzodithioles—in excellent yields.
Scheme 19.
Mixed double-Michael addition with allenoates.
a 10 mol % catalyst was used. b dr = 1:1
3.1.3. Phosphine-Catalyzed [4+n] Annulation
In reactions of 2,3-butadienoate 16 and carbon-pronucleophiles, simple γ-umpolung additions occur to yield functionalized 2-butenoates. When employing 2-(acetoxymethyl)-2,3-butadienoate 30 and carbon-pronucleophiles, Tong reported a novel annulation sequence that can provide various cyclopentenes.18
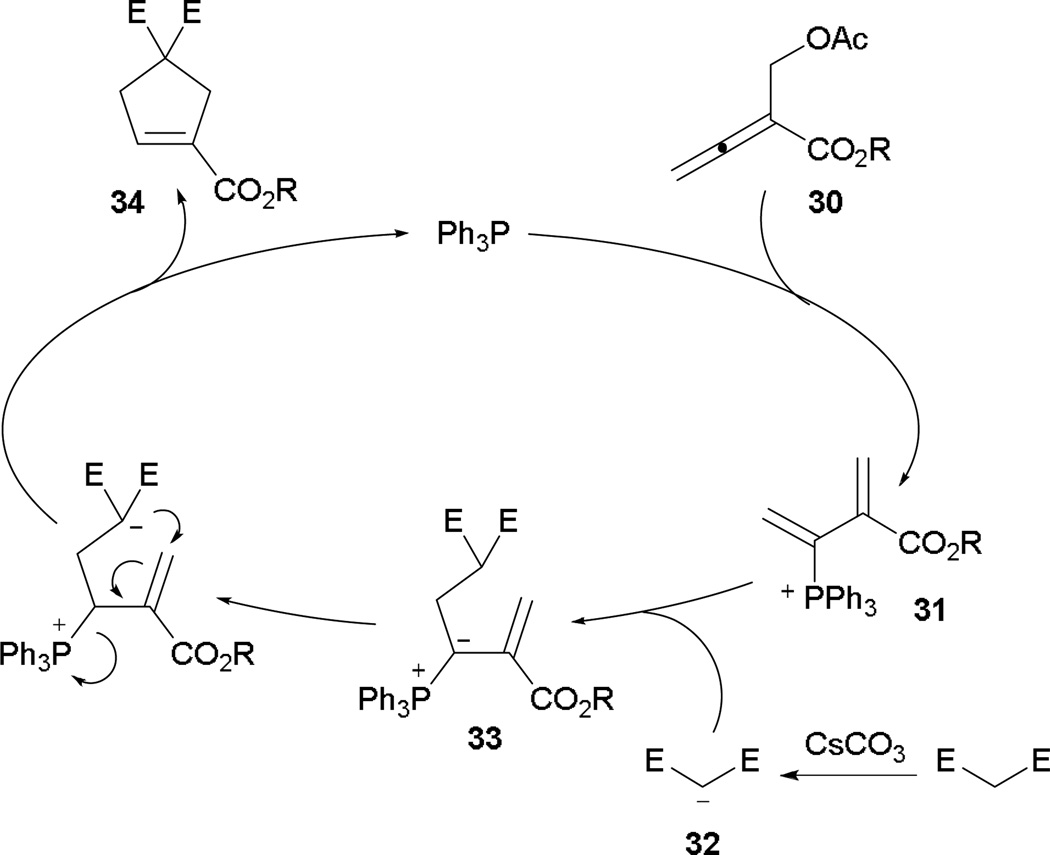
Instead of generating the phosphonium dienolate 17 (Scheme 11), the acetate group at the β'-position undergoes SN2′ displacement, creating the phosphonium diene 31 (Scheme 20). After the formation of the active nucleophile 32, the annulation event proceeds with initial γ-umpolung addition to provide the ylide 33. After proton transfer, Michael addition ensues, followed by elimination of the phosphine, to furnish the cyclopentene 34.
Scheme 20.
Proposed mechanism for γ-umpolung–Michael reaction.
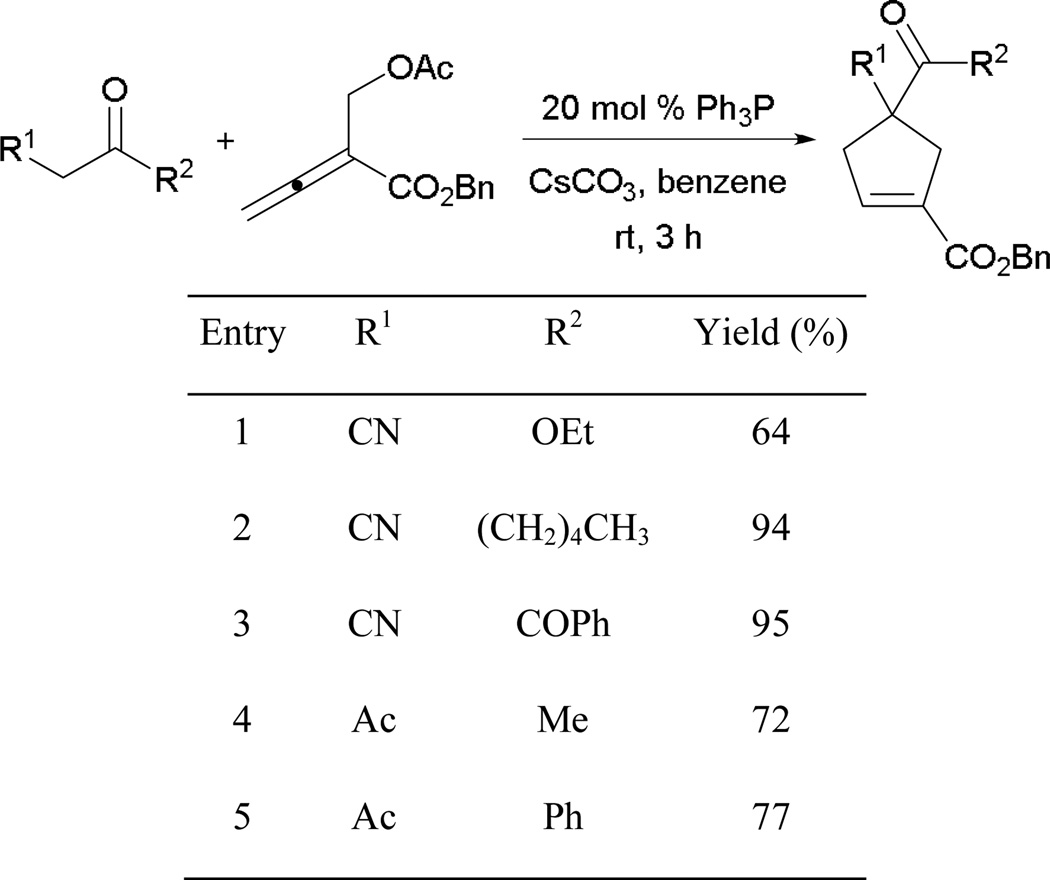
Several carbon-based pronucleophiles are suitable for this transformation, including α-cyano esters, α-cyano ketones, and acetoacetates (Scheme 21). To avoid significant loss of product, a stoichiometric amount of cesium carbonate and 20 mol % of triphenylphosphine are used.
Scheme 21.
Synthesis of cyclopentenes derivatives.
3.2. Reactions of Allenes with Electrophiles
3.2.1. Phosphine-Catalyzed Allene–Alkene [3 + 2] Annulation
In 1995, Zhang and Lu published the first example of phosphine-catalyzed [3 + 2] annulation between allenes and alkenes to yield functionalized cyclopentenes.19 Lu’s [3 + 2] annulation is highly versatile and powerful in forming functionalized cyclopentenes and dihydropyrroles. Currently, there are more than 20 research groups that are studying and applying Lu’s [3 + 2] annulation to the syntheses of natural products and biologically active molecules. There are also many reports of asymmetric variant of Lu’s [3 + 2] annulation, performed using chiral phosphines.
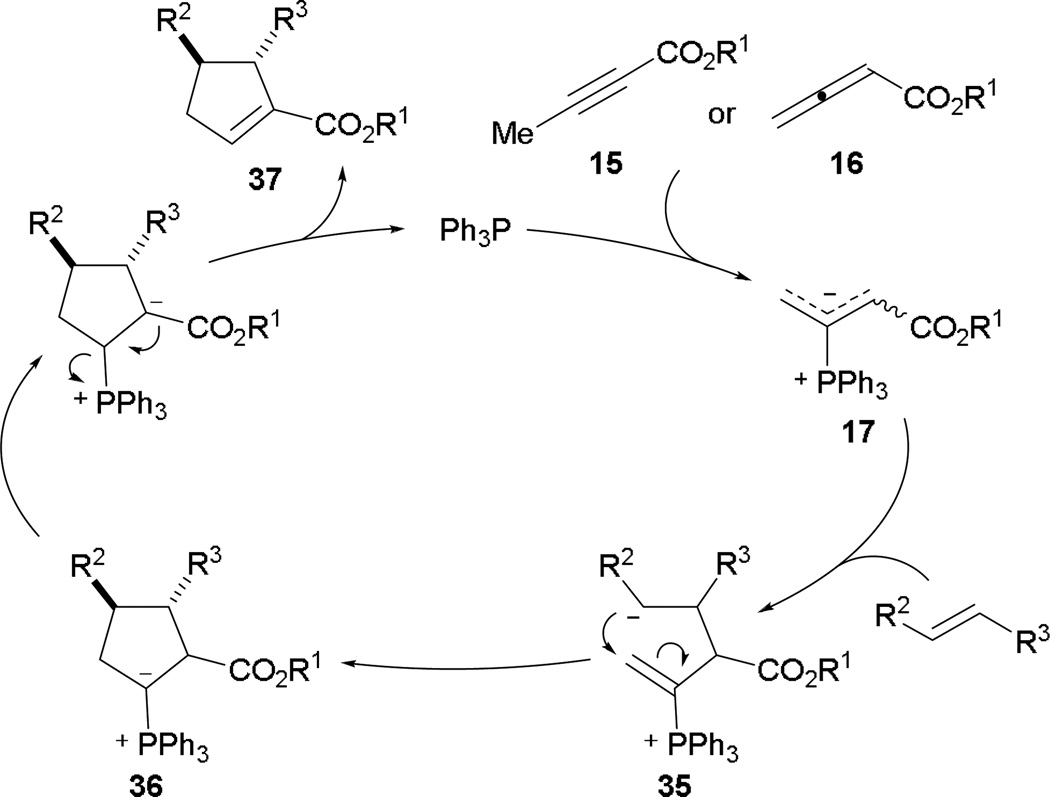
Lu’s annulation can be achieved by treating either 2-butynoate or 2,3-butadienoate with a phosphine catalyst in the presence of an activated alkene. In the mechanism, the phosphonium dienolate 17 is generated readily through phosphine conjugative addition into the 2-butynoate 15 or 2,3-butadienoate 16 (Scheme 22). Initial carbon–carbon bond formation occurs at the α-position of the phosphonium dienolate 17 to provide the intermediate 35. After the first bond formation, the γ-carbon atom becomes electrophilic, allowing the second bond formation to occur, giving the ylide 36. With facile proton transfer and regeneration of the phosphine, the functionalized cyclopentene 37 is obtained. Although α-addition is greatly favored, the minor γ-regioisomer is also observed. Through computational analysis using density functional theory (DFT) with the B3LYP/6-31G(d) basis set Yu and Kwon independently verified that Lu’s [3 + 2] annulation proceeds in a stepwise manner with distinct intermediates.20
Scheme 22.
Proposed reaction mechanism for Lu’s [3 + 2] annulation.
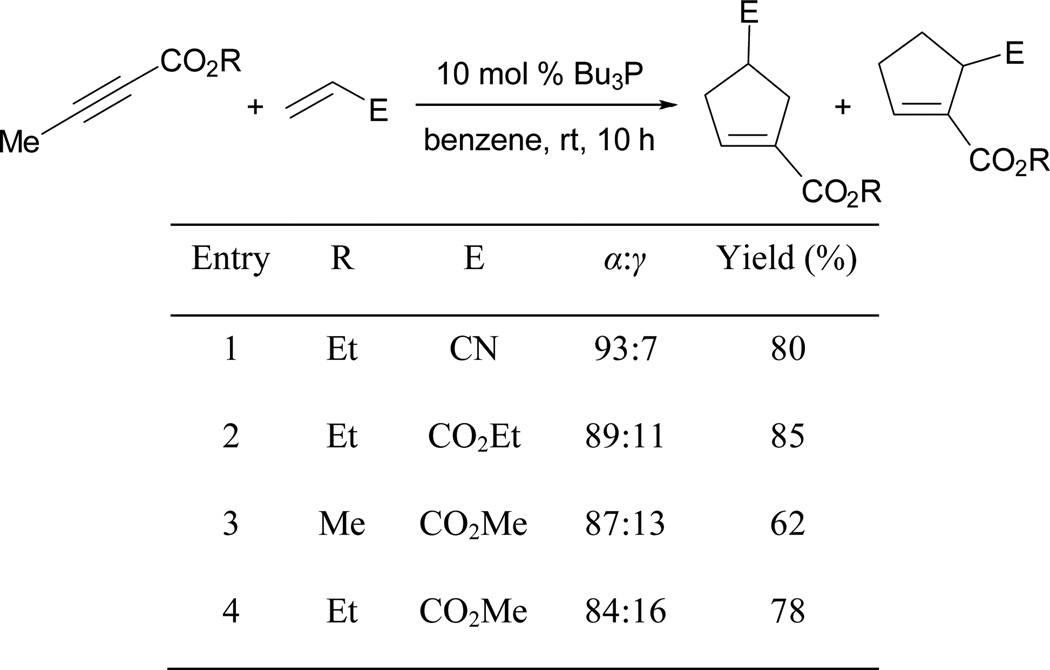
The reaction is faster when tributylphosphine is used as the catalyst. Furthermore, the formation of the γ-regioisomer can be suppressed when substituting 2-butynoate for 2,3-butadienoate. Good yields of cyclopentene derivatives can be obtained when using alkyl acrylates and acrylonitrile as electrophiles (Scheme 23).
Scheme 23.
Lu’s [3+2] annulation with alkenes.
3.2.2. Phosphine-Catalyzed Intramolecular Allene–Alkene [3 + 2] Annulation
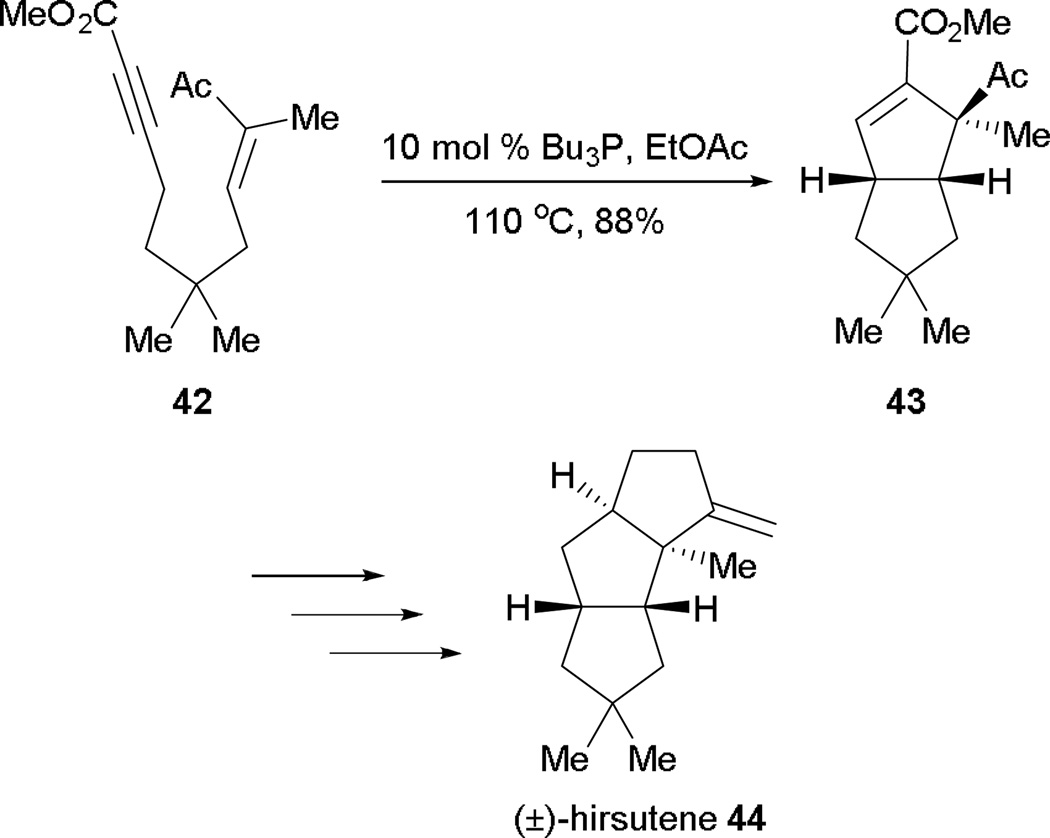
In 2003, Krische and co-workers applied Lu’s [3 + 2] annulation in an intramolecular setting to create several highly functionalized diquinanes.21 This intramolecular [3 + 2] strategy was also adopted in the total synthesis of (–)-hirsutene, generating two of the three rings in the natural product.22 Tributylphosphine can be used for systems with large steric bulk to yield annulation products efficiently (Scheme 24). Several 1,7-enynes have been subjected to the reaction conditions to render good yields of diquinanes (entries 1–3). Interestingly, a trace of the cyclization product was obtained from a tethered enoate motif (entry 4), presumably because of its lower electrophilicity.
Scheme 24.
Synthesis of diquinanes through intramolecular [3 + 2] annulation.
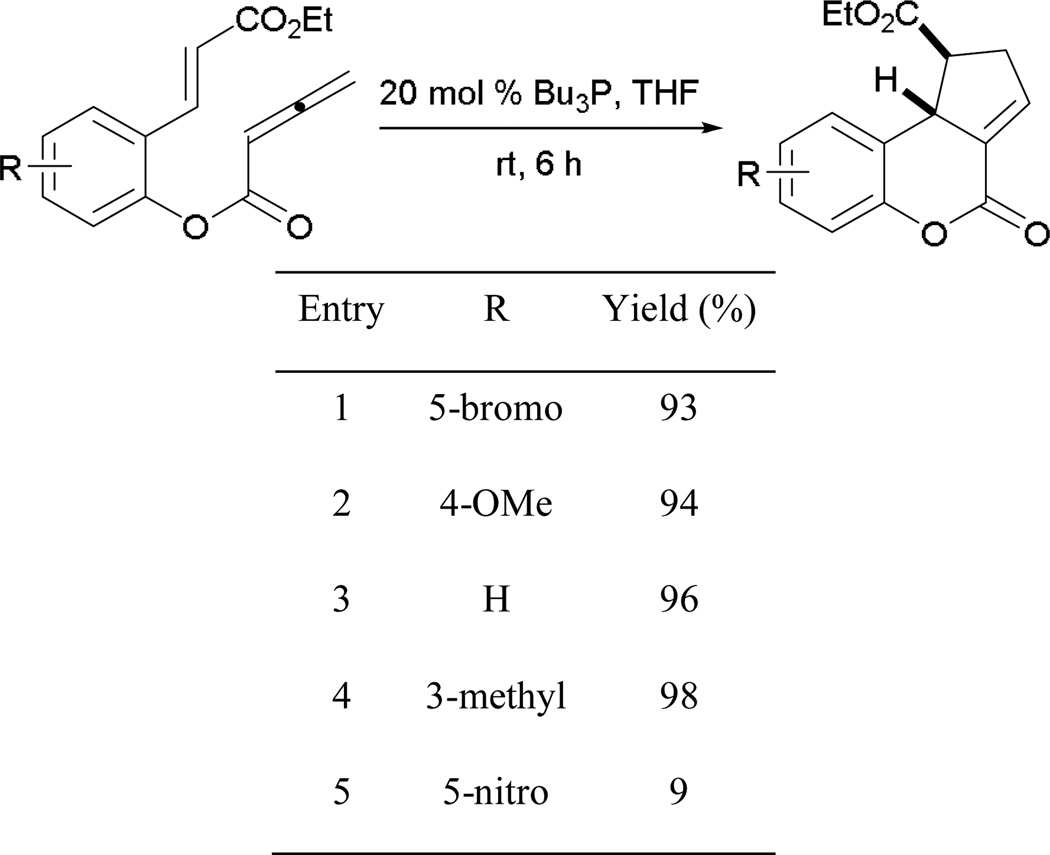
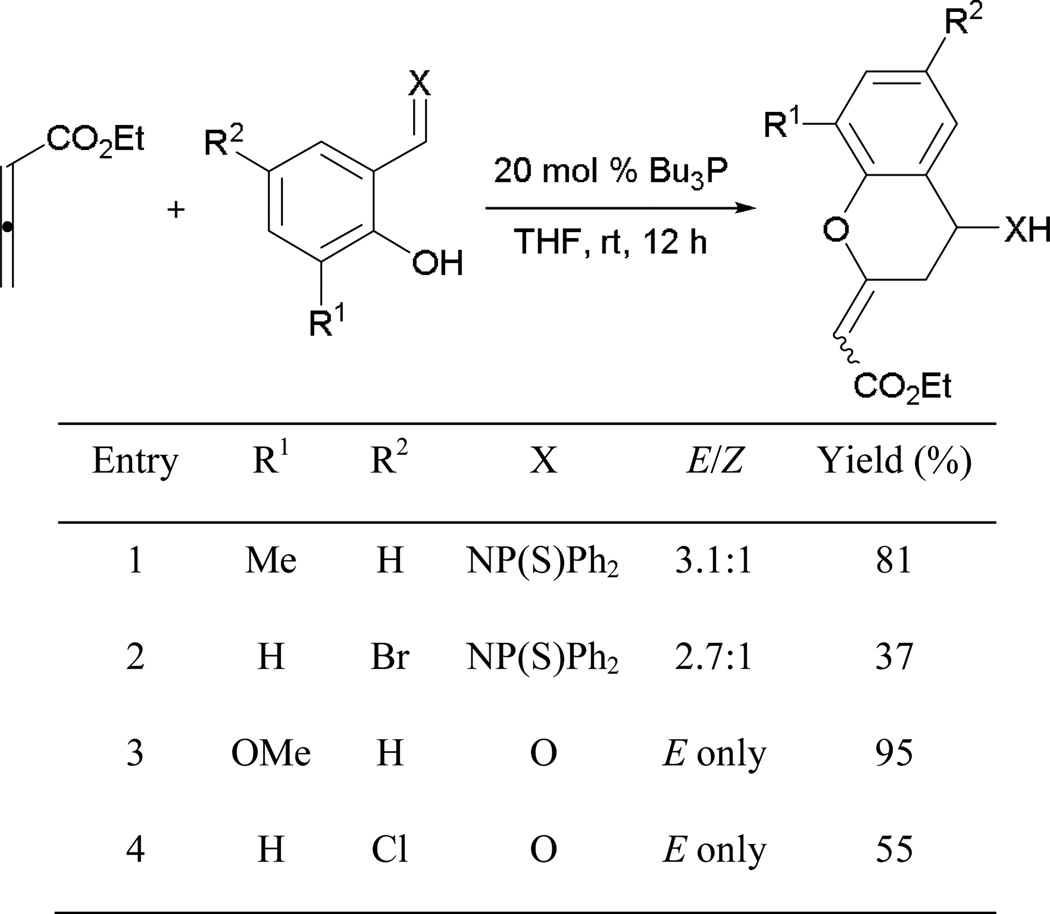
Henry and Kwon reported another intramolecular [3 + 2] process in which tethering of the carboxylic acid moiety of the allene with functionalized 2-hydroxycinnamates afforded functionalized dihydrocoumarins,23 which are important synthetic intermediates for the preparation of coumarin-containing natural products (e.g. warfarin). One of the attractive features of this method is the ease of substrate preparation through coupling at the ester linkage.
With different types of functionalization of the 2-hydroxycinnamate ring portion, numerous dihydrocoumarins can be synthesized in high efficiency (Scheme 25). Electron-rich methyl and methoxy and electron-poor bromo substituents produce their cyclization products in comparable yields (entries 1–4). Although high yields of annulation products can be obtained in the presence of various substituents with different electronic properties, a trace of product was observed when using 2-hydroxy-5-nitrocinnamate, due to ready hydrolysis of its allenoate ester moiety (entry 5).
Scheme 25.
Preparation of functionalized dihydrocoumarins.
3.2.3. Phosphine-Catalyzed [3 + 2] Annulations of 2,3,4-Pentatrienoate
In 2009, Shi reported an interesting use of 2,3,4-pentatrienoates in the [3 + 2] annulation.24 With an extra unit of unsaturation installed in the system, an unforeseen annulation pathway might have been followed. The regular [3 + 2] annulation occurred, however, with the 2,3,4-pentatrienoate serving as a three-carbon synthon in the presence of alkenes or imines.
A high loading of tributylphosphine is needed to maintain good reaction efficiency (Scheme 26). In the case of cyclopentene formation, both electron-donating and -withdrawing functionalities on arylidenemalononitrile are well accommodated, giving high yields of products (entries 1 and 2). When a less-reactive alkylidenemalononitrile is employed, significant product loss occurs (entry 3). From corresponding reactions with imines, dihydropyrroles are produced in diminished yields (entries 4 and 5).
Scheme 26.
Formation of polysubstituted cyclopentenes and dihydropyrroles.
3.2.4. Phosphine-Catalyzed [3 + 2] Annulations of Phenyl Allenone
In phosphine catalysis, alkoxycarbonyls are commonly used as activating groups on allenes or alkynes. In an attempt to use an acyl moiety as the activating group, Wallace observed allenone dimerization, even in the presence of activated alkenes.25 Such an event was first noted in Lu’s 1995 seminal report, where 2,3-butadienoate underwent [3 + 2] dimerization.19 To circumvent this undesired pathway, Loh introduced a trimethylsilyl (TMS) group at the α-position of allenone, thereby prohibiting the dimerization.26
With the α-TMS unit in place, highly reactive phenyl allenone undergoes the desired [3 + 2] annulation in the presence of several activated alkenes (Scheme 27). The appendant TMS group is hydrolyzed during the reaction. The steric congestion at the α-position causes the reaction to give exclusively the γ-addition product, with excellent diastereoselectivity favoring the trans isomer.
Scheme 27.
Synthesis of cyclopentenes using phenyl allenone.
3.2.5. Synthetic Applications of Allene–Alkene [3 + 2] Annulation
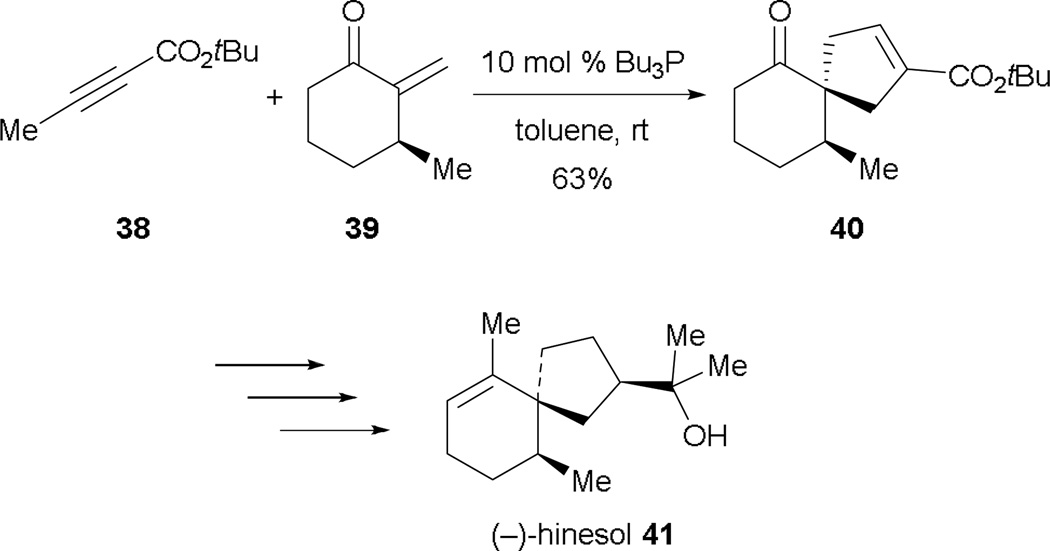
In 2003, Lu completed the total synthesis of (–)-hinesol (41), with allene–alkene [3 + 2] annulation as the key transformation for constructing the spirocyclic ring skeleton (Scheme 28).27 By treating the 2-alkynoate 38 with 2-methylene cyclohexenone 39 in the presence of tributylphosphine, the spirocyclic intermediate 40 is formed in good yield. Further functional transformations afforded (–)-hinesol (41) in good yield.
Scheme 28.
Total synthesis of (–)-hinesol.
In the total synthesis of (±)-hirsutene 44, Krische and Yang showcased the intramolecular allene–alkene [3 + 2] annulation to construct the 5,5-fused ring system rapidly, providing two of the three rings in hirsutene (Scheme 29).22 The intramolecular [3 + 2] annulation of the advanced intermediate 42 proceeded smoothly to furnish the 5,5-fused bicyclic intermediate 43 in high yield. Late-stage reductions and oxidations completed the synthesis of (±)-hirsutene 44.
Scheme 29.
Total synthesis of (±)-hirsutene.
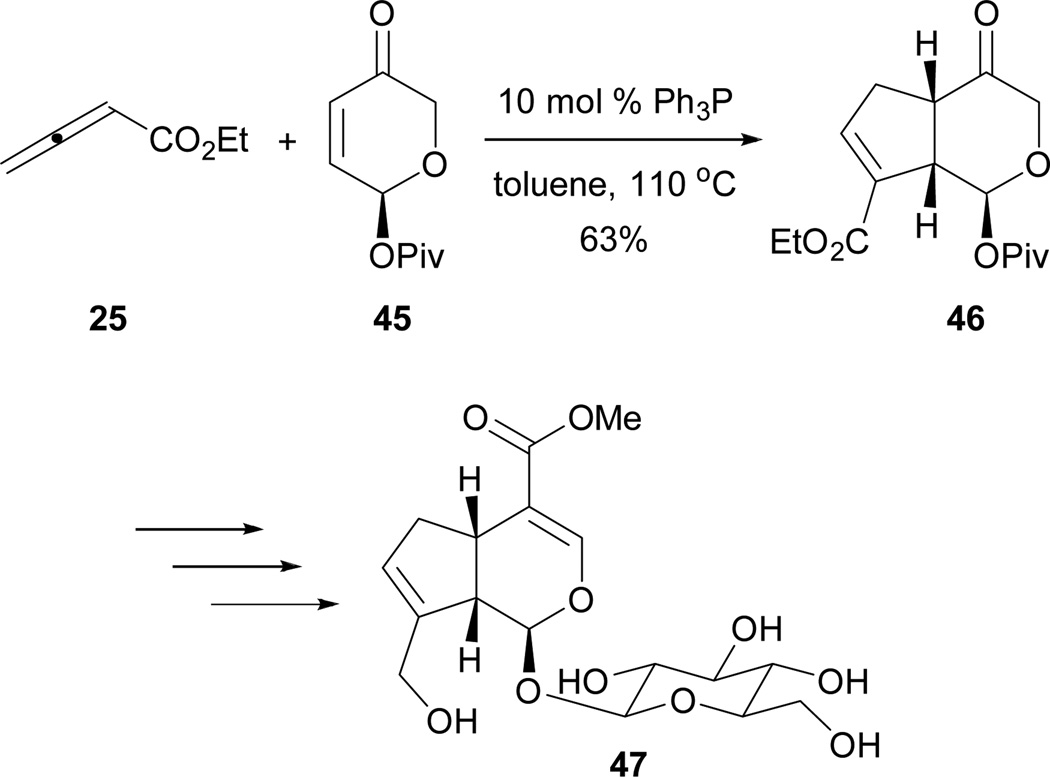
Krische’s group also reported the synthesis of (+)-geniposide 47 utilizing Lu’s [3 + 2] annulation (Scheme 30).28 They rapidly constructed the core from the reaction of the 2,3-butadienoate 25 and enantiomerically enriched enone 45, providing a good yield of the cycloadduct 46. Functional group manipulations and late-stage glycosidation yielded (+)-geniposide 47.
Scheme 30.
Total synthesis of (+)-geniposide.
3.2.6. Phosphine-Catalyzed Allene–Imine [3 + 2] Annulation
Shortly after his seminal 1995 report, Lu expanded the allene–alkene [3 + 2] annulation to an allene–imine variant, providing functionalized dihydropyrroles.29 Although it shares the same mechanism as the allene–alkene [3 + 2] annulation, this reaction provides only one regioisomer that formed through α-addition.
In general, the product yields from allene–imine [3 + 2] annulation are higher than those from allene–alkene annulation (Scheme 31). No dihydropyrrole is generated when employing an alkyl aldimine (entry 5). This common observation arises possibly because of ready hydrolysis of alkyl aldimines.
Scheme 31.
Preparation of functionalized dihydropyrroles.
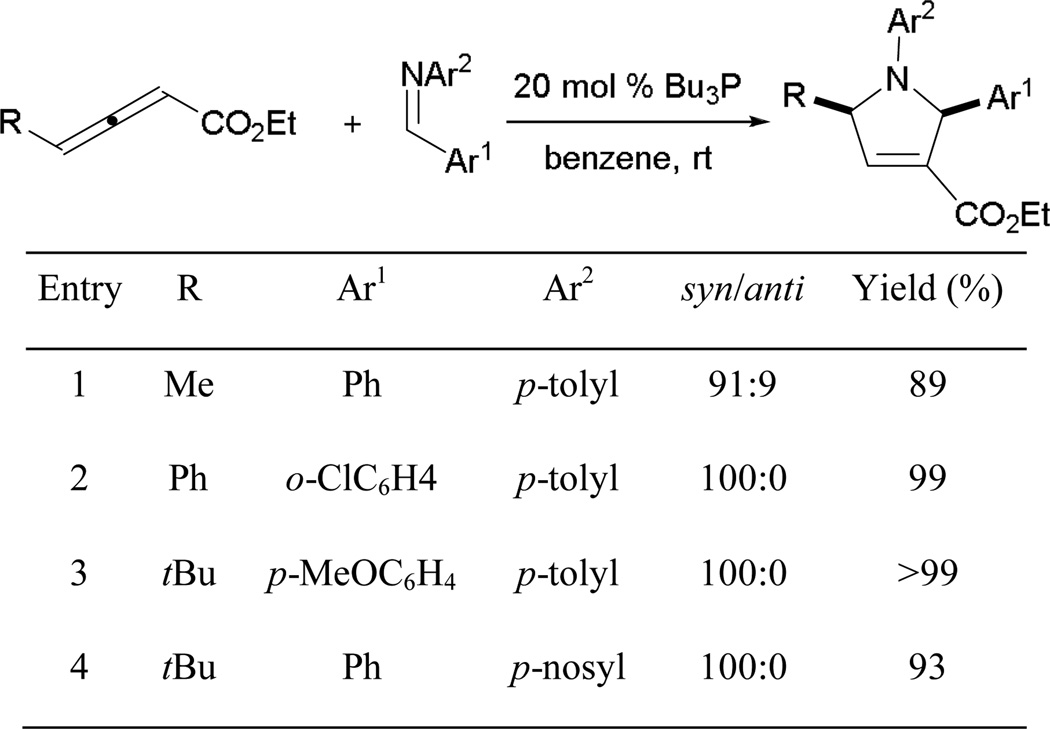
3.2.7. Phosphine-Catalyzed γ-Substituted Allene–Imine [3 + 2] Annulation
To further expand the utility of the Lu’s reaction, Kwon reported the use of either γ-substituted allenoates to access 1,2,3,5-tetrafunctionalized dihydropyrroles in high efficiency.30 Kwon and co-workers applied these reactions to solid-phase synthesis, generating libraries of dihydropyrroles and pyrrolidines and leading to the identification of protein geranylgeranyltransferase type-I inhibitors.31
When dealing with more sterically demanding 2,3-pentadienoates, tributylphosphine can be used as the catalyst because its nucleophilicity is higher than that of triphenylphosphine (Scheme 32).30a Two diastereoisomers are obtained when ethyl 2,3-pentadienoate is employed, with good cis-selectivity (entry 1). When the substituent at the γ-position of the allenoate is larger than a methyl group, cis-tetrasubstituted dihydropyrroles are isolated exclusively (entries 2–4). The reaction affords the dihydropyrrole smoothly even when a p-nosyl benzaldimine is used instead of its usual tosyl-protected counterpart (entry 4).
Scheme 32.
Kwon’s synthesis of tetrasubstituted dihydropyrroles.
3.2.8. Phosphine-Catalyzed Allene–Alkylimine [3 + 2] Annulation
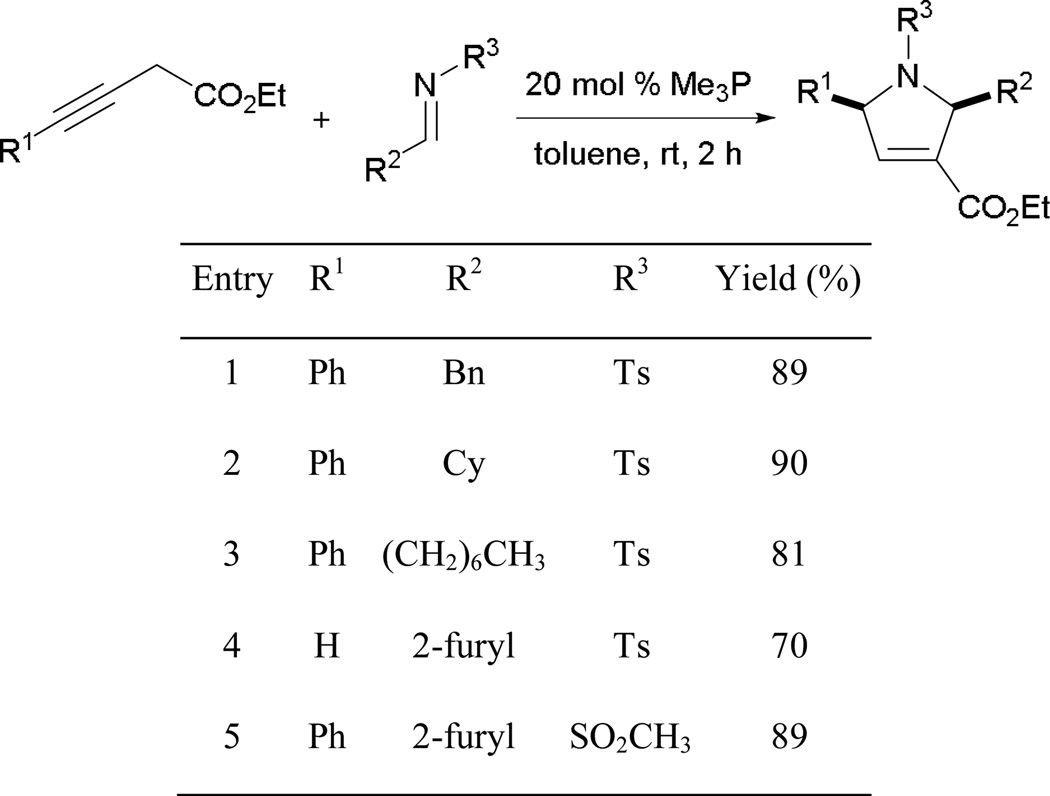
Alkylimines have always been challenging substrates to incorporate in allene–imine annulations because of their decomposition through rapid hydrolysis. Recently, Loh reported the first examples of highly efficient dihydropyrrole formation from various tosylimines derived from alkyl aldehydes (Scheme 33).32 Unlike previous attempts, Loh’s approach employs 3-alkynoates as substrates and highly nucleophilic trimethylphosphine as the catalyst. Loh suggested that in situ isomerization of the 3-alkynoates formed allenoates that underwent subsequent [3 + 2] annulation. The reaction tolerates both aryl and alkylimines with various substitution patterns.
Scheme 33.
Synthesis of functionalized 2-aryl and 2-alkyl dihydropyrroles.
3.2.9. Synthetic Applications of Allene–Imine [3 + 2] Annulation
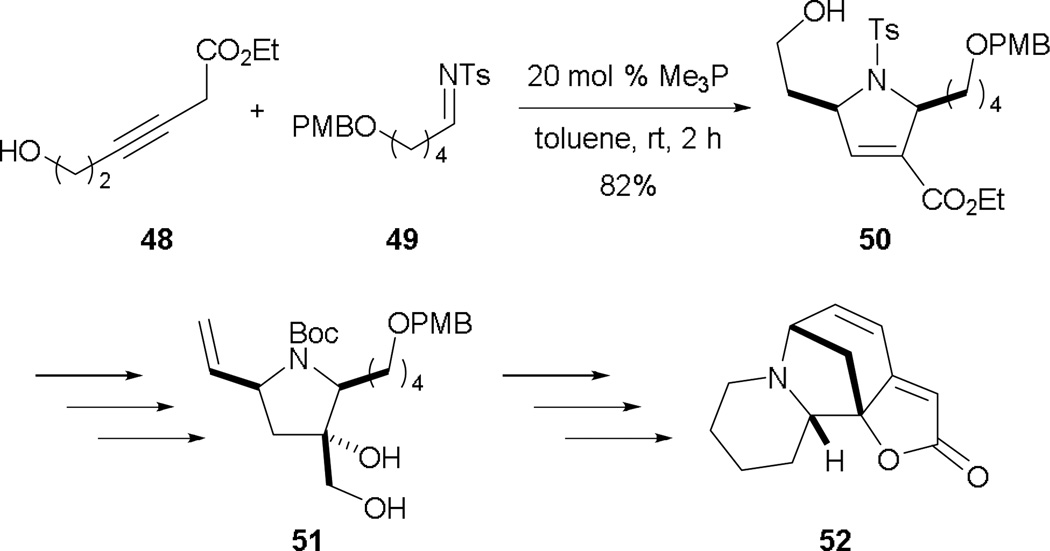
To demonstrate the utility of the allene–alkylimine annulation, Loh and co-workers completed the formal synthesis of (±)-allosecurinine 52 (Scheme 34).32 Remarkably, the unmasked 6-hydroxy-3-hexynoate 48 underwent annulation with the alkylimine 49 to produce a high yield of the desired dihydropyrroline 50 (Scheme 34). After several functional group manipulations, they obtained 51, a known synthetic intermediate of (±)-allosecurinine 52.
Scheme 34.
Formal synthesis of (±)-allosecurinine.
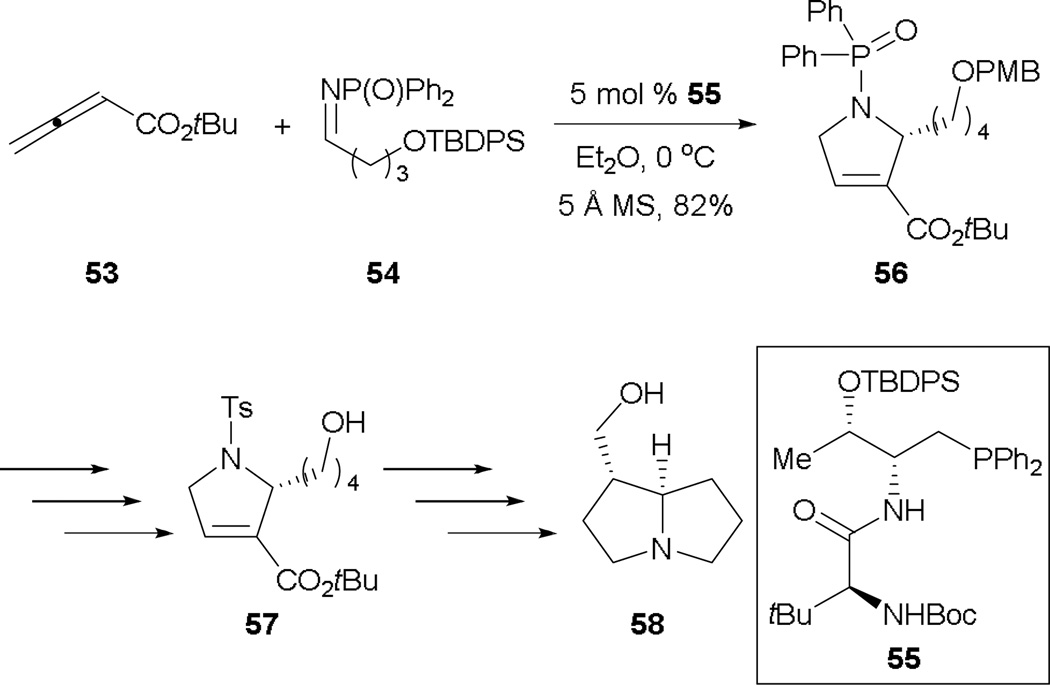
An asymmetric allene–alkylimine [3 + 2] has been developed by Lu (vide infra),33 who reported a concise formal asymmetric synthesis of (+)-trachelanthamidine 58 (Scheme 35). From the reaction of the allenoate 53 and the alkylimine 54, they isolated 2-alkyl dihydropyrroline 56 in good yield and enantioselectivity. Subsequent removal of protecting groups completed the synthesis 57, a known intermediate of (+)-trachelanthamidine 58.
Scheme 35.
Formal synthesis of (+)-trachelanthamidine.
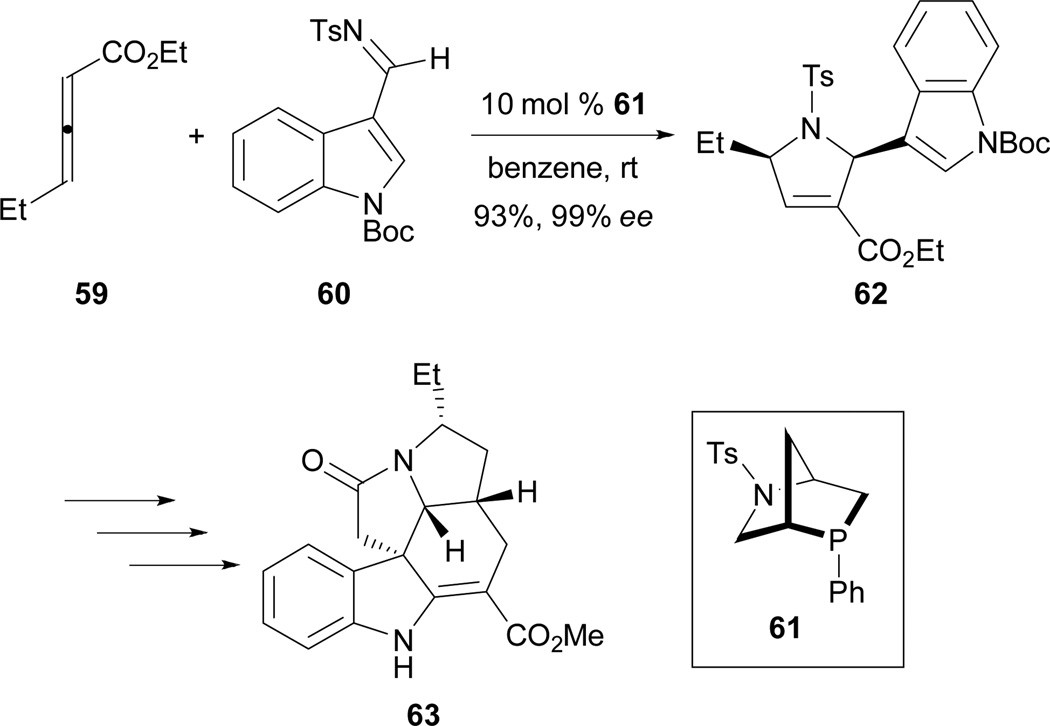
Recently, Andrews and Kwon reported the first example of asymmetric allene–imine [3 + 2] annulation in the total synthesis of (+)-ibophyllidine 63 (Scheme 36).34 Employing the readily accessible 2,3-hexadienoate 59 and the indole-3-carboxaldimine 60 as substrates and the chiral phosphine 61 as the catalyst, they synthesized the 2-indolyl-dihydropyrrole 62 in excellent yield and with excellent enantiocontrol. Using this strategy, three of the five rings of (+)-ibophyllidine 63 were accessed rapidly in high efficiency. After formation of the remaining two rings and functional group installation, the concise enantioselective synthesis of (+)-ibophyllidine 63 was achieved.
Scheme 36.
Enantioselective total synthesis of (+)-ibophyllidine.
3.2.10. Phosphine-Catalyzed Azomethine Imine–Allene [3 + 2] Annulation
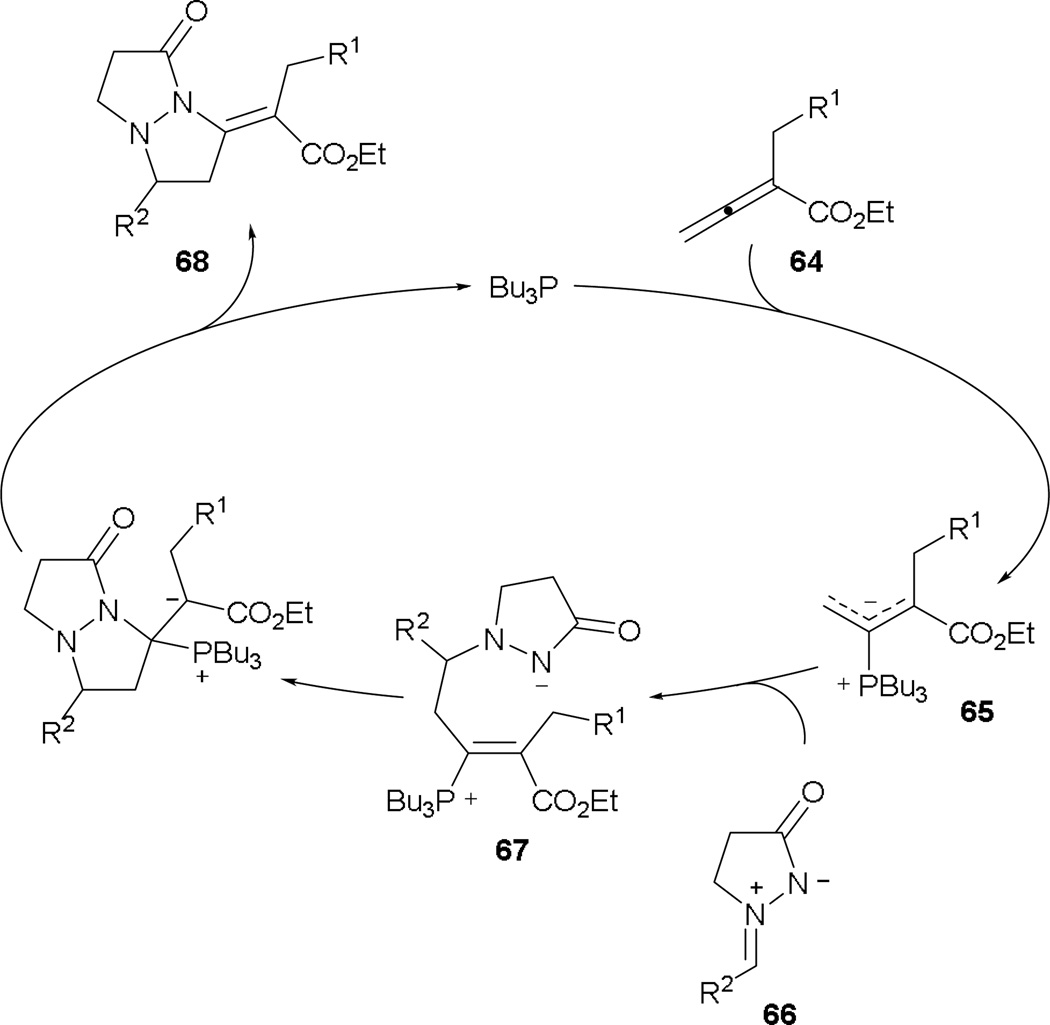
In Lu’s [3 + 2] annulation, the phosphonium dienolate behaves as a 1,3-dipole, providing three carbon atoms in the annulation product. Interestingly, Guo and Kwon used azomethine imines to provide three-atom unit in the formation of five-membered ring systems, obtaining functionalized tetrahydropyrazolopyrazolones in high yields.35 The postulated mechanism proceeds with the initial generation of the phosphonium dienolate 65 from the allenoate 64 (Scheme 37). In the presence of the azomethine imine 66, addition occurs at the γ-position to yield the intermediate 67. Immediate intramolecular Michael addition ensues with subsequent ejection of the phosphine catalyst, providing the tetrahydropyrazolopyrazolone 68.
Scheme 37.
Proposed mechanism for azomethine imine–allene [3 + 2] annulation.
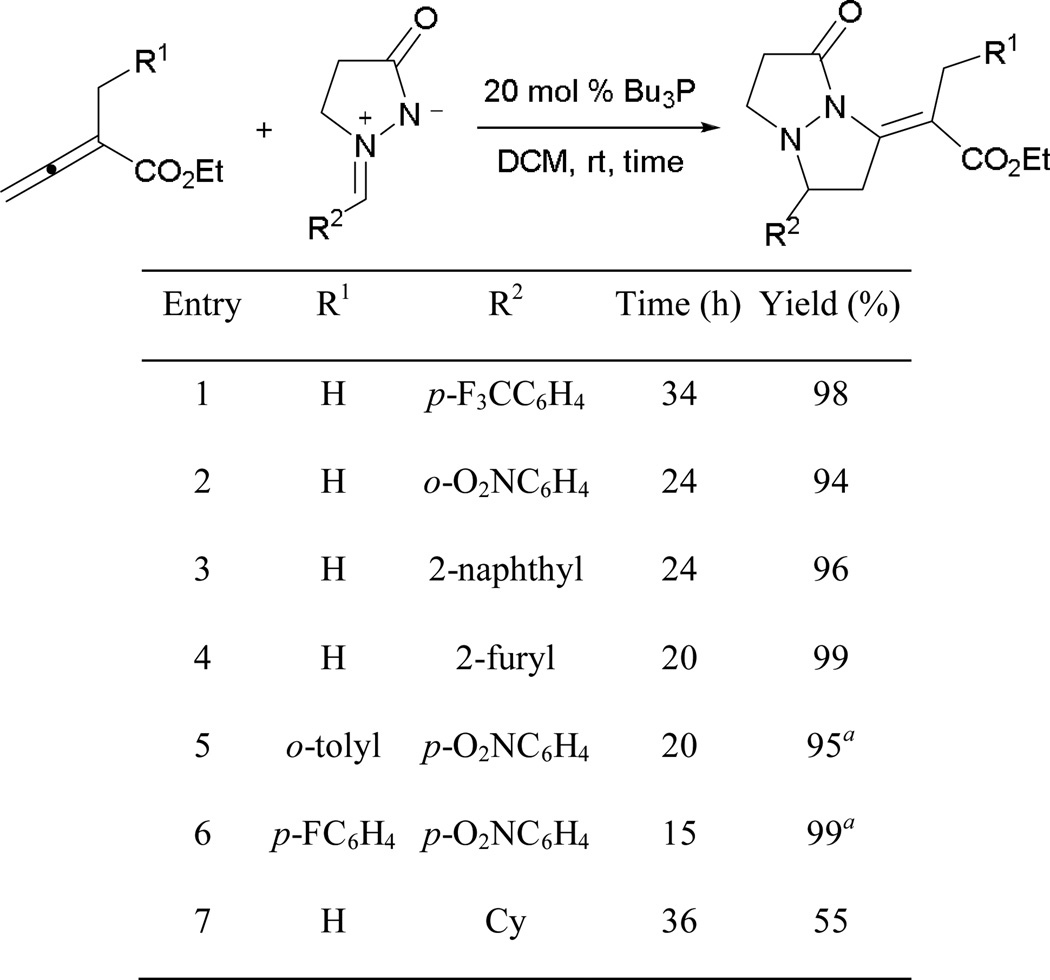
Annulation products are formed in high yields from various azomethine arylimines presenting different substitution patterns (Scheme 38). Azomethine imines bearing electron-withdrawing functionalities are favored, providing higher yields. A lower yield was obtained when employing an azomethine alkylimine (entry 7).
Scheme 38.
Formation of tetrahydropyrazolopyrazolone derivatives.
a Me3P was used as catalyst.
3.2.11. Phosphine-Catalyzed Allene–Imine [4 + 2] Annulation
In 2003 Kwon and co-workers reported a novel annulation pathway in which α-alkyl-2,3-butadienoates serve as four-carbon synthons in the presence of imines to produce densely functionalized tetrahydropyridines.36 This allene–imine [4 + 2] annulation is amenable to large-scale preparation, making it amenable to natural product syntheses.37 It is also very robust and can be applied in solid-phase synthesis, generating diverse chemical libraries for biological screening, similar to Lu’s [3 + 2] annuation.31,38
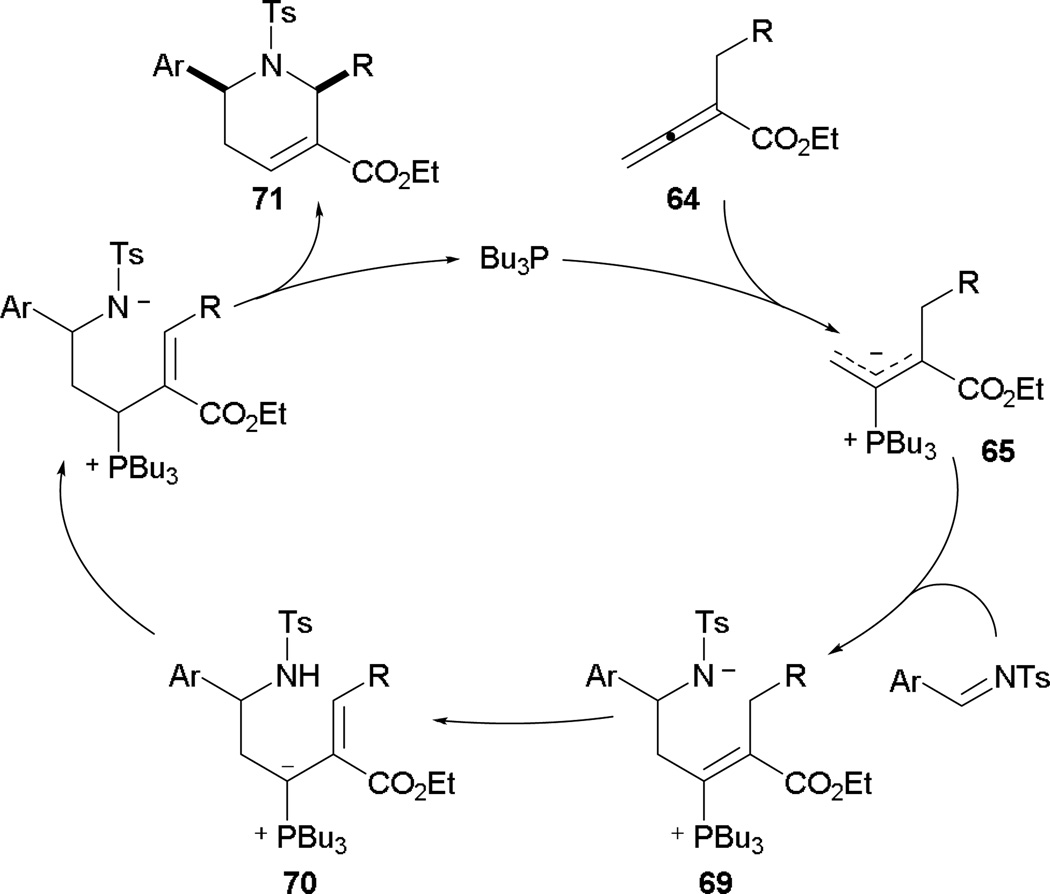
The [4 + 2] annulation begins with initial addition of phosphine into the α-alkyl-2,3-butadienoate 64 to give the phosphonium dienolate 65 (Scheme 39). Unlike Lu’s [3 + 2] annulation, addition at the α-position is prohibited by the steric bulk; therefore initial addition occurs only at the γ-position. In the presence of an imine, the zwitterion 69 is subsequently generated. Proton transfer provides the vinyl phosphonium ylide 70, which is converted to the more stable phosphonium amide zwitterion. The final nitrogen–carbon bond is formed upon the Michael addition of the amide anion, followed by extrusion of the phosphine catalyst to provide the tetrahydropyridine 71.
Scheme 39.
Proposed mechanism for Kwon’s [4 + 2] annulation.
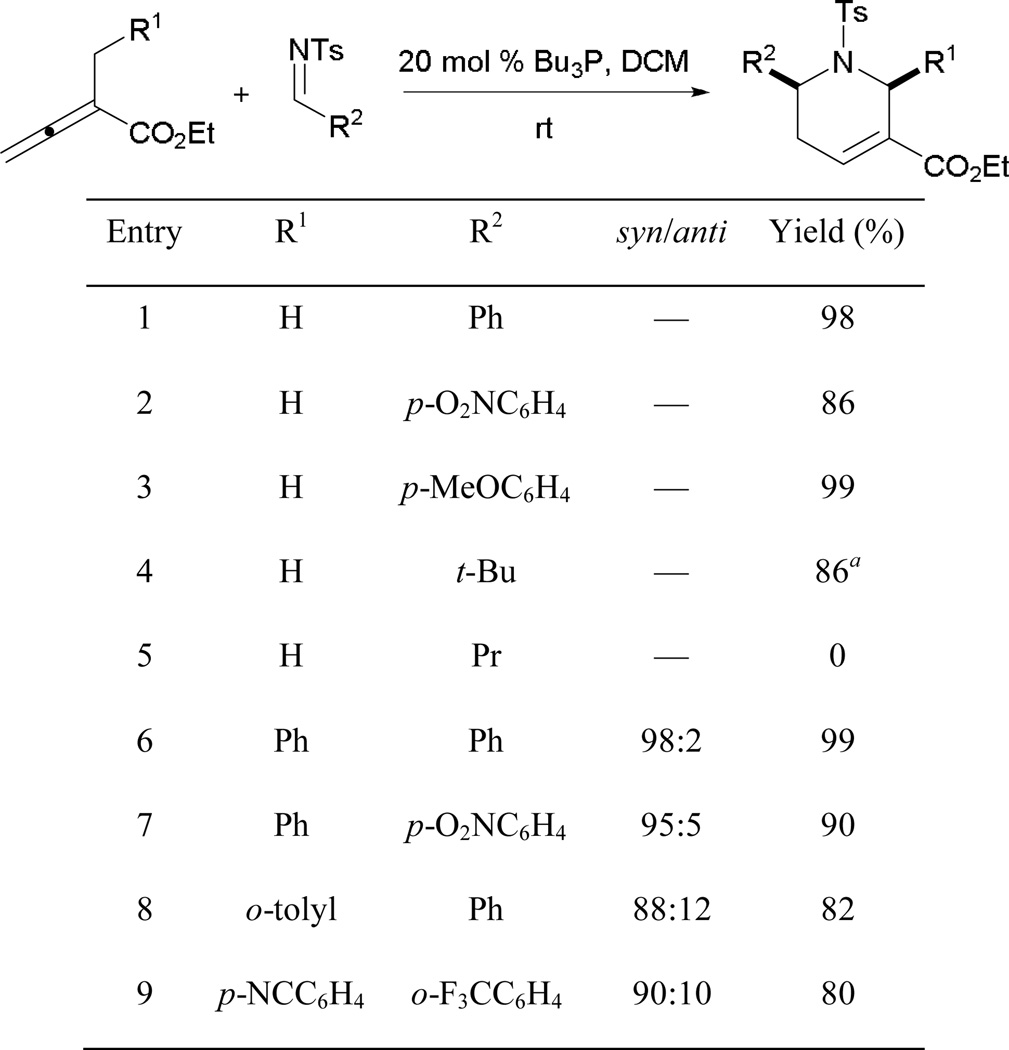
A range of electron-rich and -poor substituents on the aryl aldimines consistently provides tetrahydropyridines as [4 + 2] annulation products in excellent yields (Scheme 40). Reminiscent to Lu’s [3 + 2] annulation, no tetrahydropyridine was obtained when alkylimines were tested with the rare exception of tosyl tert-butylaldimine, which provided the annulation product when using sodium carbonate as an additive (entry 4). When different α-benzyl-2,3-butadienaotes are employed as substrates, many tetrahydropyridines can be acquired, with excellent diastereoselectivities favoring the cis-diastereoisomer (entries 6–9). The methodology can also be extended to allenoates immobilized on solid supports, generating libraries of compounds. Such a collection of compounds has led to discoveries of inhibitors of protein geranylgeranyltransferase-I and Rab geranylgeranyltransferase.31,39 Compounds with antimigratory activity against MDA-MB-231 breast cancer cells have also been identified.40 Furthermore, the allene–imine [4 + 2] annulation when combined with subsequent Tebbe/Diels–Alder reactions leads to the formation of octahydro-1,6-naphthyridin-4-ones that serve as activators for endothelium.41
Scheme 40.
Formation of densely functionalized tetrahydropyridines using Kwon’s [4+2] annulation.
a 3 equivalents of Na2CO3 were added.
3.2.12. Synthetic Applications of the Allene–Imine [4 + 2] Annulation
The versatile allene–imine [4 + 2] annulation has been applied to the formal synthesis of (±)-alstonerine 76.42 Employing 2-vinylidenesuccinate 72 and indole-2-carboxaldimine 73 as substrates, the tetrahydropyridine 74 was obtained in good yield and good diastereoselectivity (Scheme 41). Through this route, three of the five rings of (±)-alstonerine 76 were formed in a facile manner. Further functionalization led to 75, a known synthetic intermediate of (±)-alstonerine 76.
Scheme 41.
Formal synthesis of (±)-alstonerine.
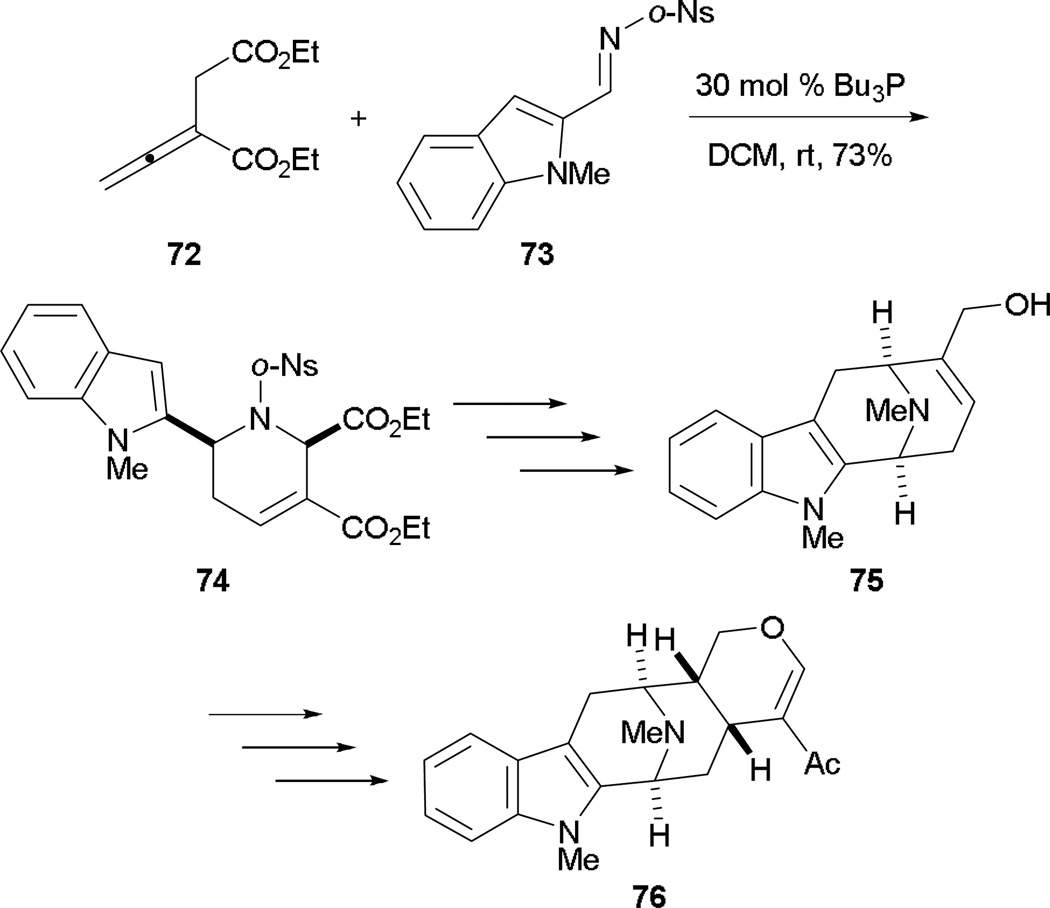
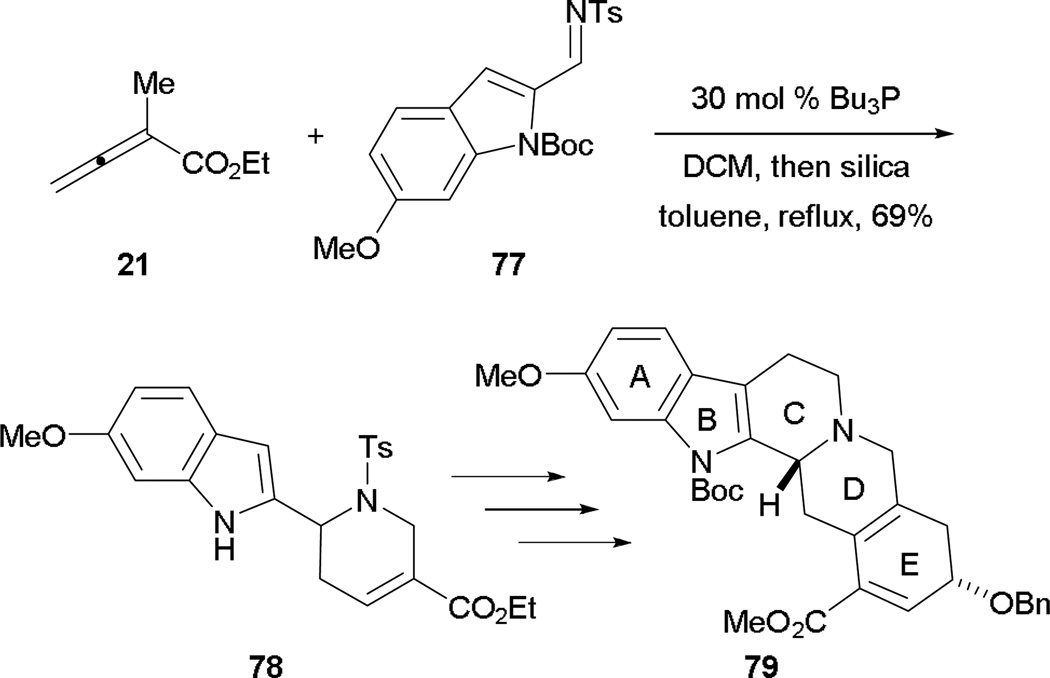
The allene–imine [4 + 2] annulation can also be applied to the synthesis of the skeletal framework of reserpine 79.43 Barcan and Kwon used this [4 + 2] annulation to construct the D-ring of reserpine 79. From the allenoate 21 and the indole-2-carboxaldimine 77, they synthesized the tricycle 78 in good yield with deprotection of the indole nitrogen atom (Scheme 42). After formation of the C-ring through intramolecular alkylation and 6π electrocyclization providing the E-ring, the skeletal framework of reserpine 79 was obtained in a concise manner.
Scheme 42.
Synthesis of the skeletal framework of reserpine.
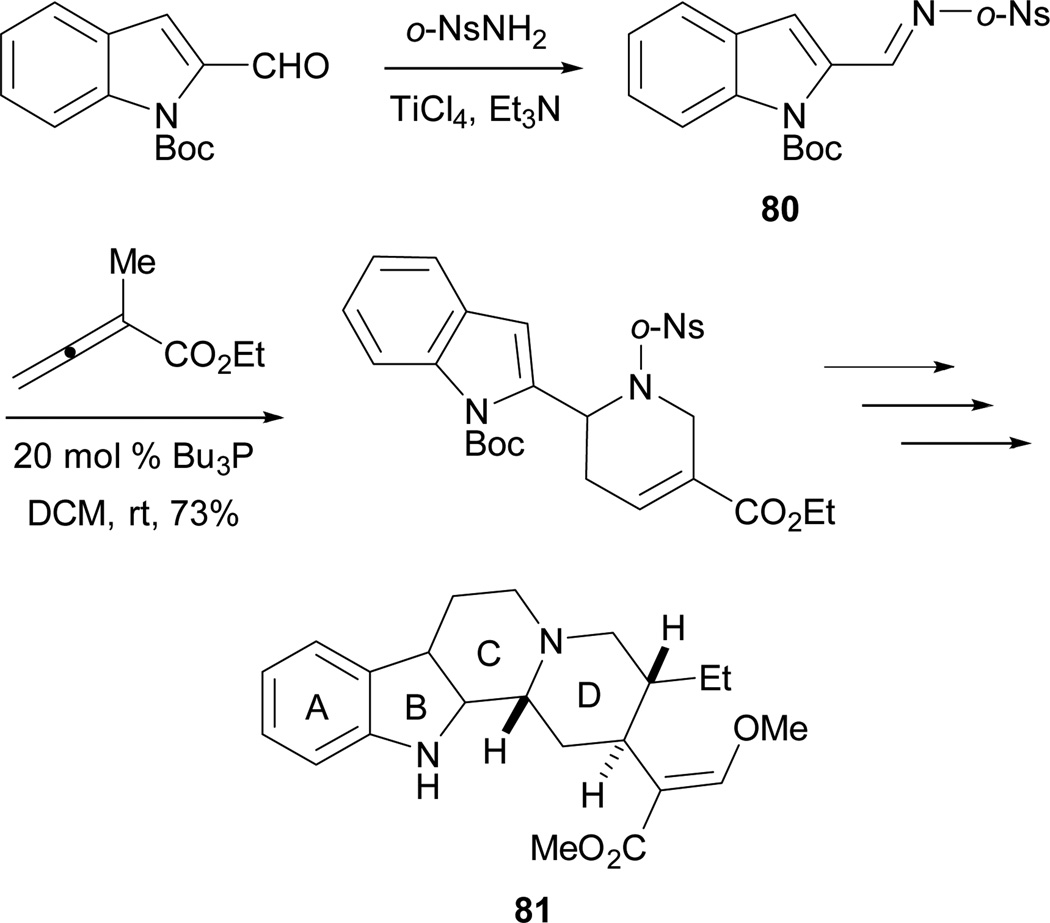
Recently, Kwon and co-workers completed the total synthesis of (±)-hirsutine 81 with allene–imine [4 + 2] annulation preparing the D-ring in good efficiency (Scheme 43).44 Remarkably, the annulation reaction could be achieved from the crude imine 80 while maintaining good yield. After formation of the C-ring and functional group installation, they achieved the total synthesis of (±)-hirsutine 81.
Scheme 43.
Total synthesis of (±)-hirsutine.
3.2.13. Phosphine-Catalyzed Allene–Alkene [4 + 2] Annulation
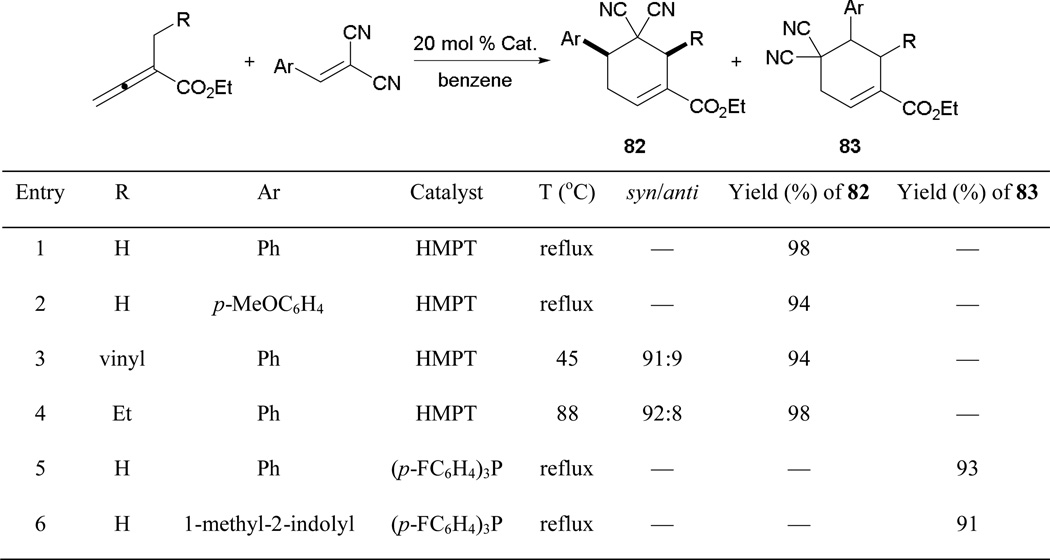
After demonstrating the feasibility of allene–alkene [4 + 2] annulation, Kwon and Tran further expanded it to the formation of cyclohexene derivatives by reacting α-alkyl-2,3-butadienoates with activated alkenes.45 Similar to Lu’s approach, Kwon’s [4 + 2] annulation is also well-suited to the generation of carbocycles, with various possible regioisomers. With fine-tuning of the catalyst’s electronic properties, each regioisomer can be obtained exclusively (Scheme 44). In the presence of hexamethylphosphorous triamide (HMPT), the reaction favors γ-addition with high diastereoselectivities (entries 3 and 4). Furthermore, consistently high yields of the cyclohexenes 82 are obtained from arylidenemalononitriles bearing either electron-rich or -poor substituents. Switching to the electron-poor catalyst tris-(p-chlorophenyl)phosphine initiates an alternative addition pathway—through initial addition at the β'-position via the vinylogous phosphonium ylide—to provide high yields of the cyclohexenes 83 (entries 5 and 6).
Scheme 44.
Synthesis of functionalized cyclohexenes through allene–alkene [4 + 2] annulation.
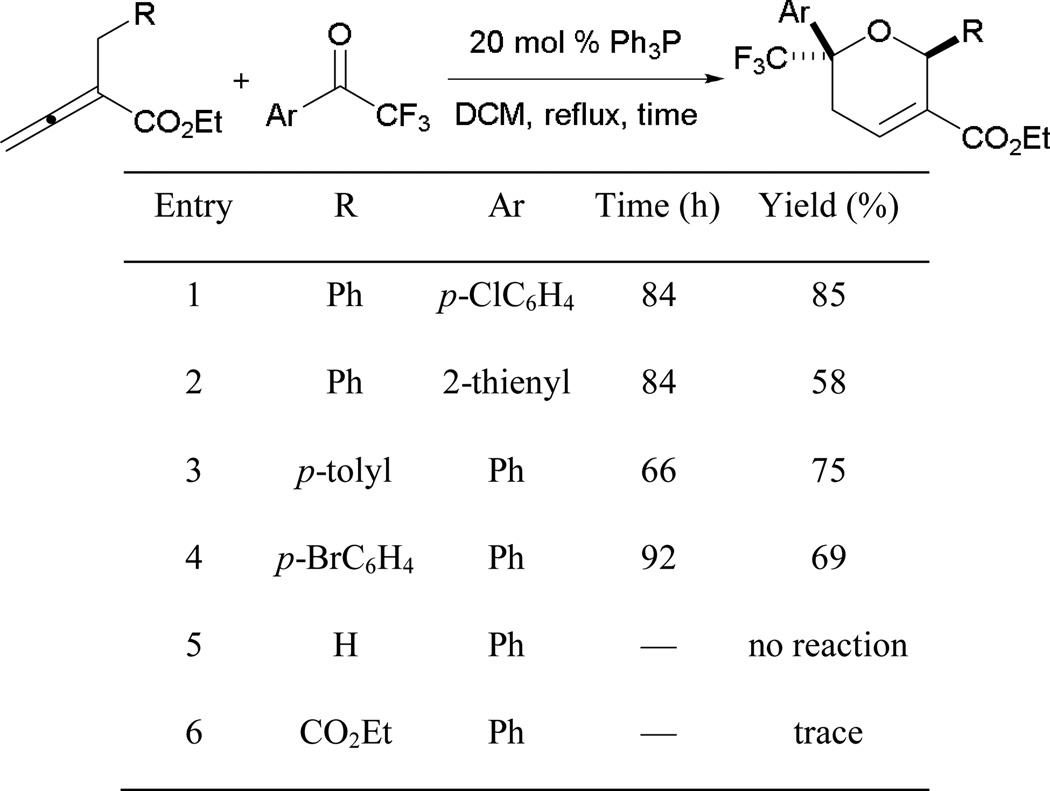
3.2.14. Phosphine-Catalyzed Allene–Ketone [4 + 2] Annulation
In addition to the formation of tetrahydropyridines and cyclohexenes through Kwon’s [4 + 2] annulation, Ye has shown that this annulation can also generate dihydropyrans.46 The reaction utilizes highly activated aryl trifluoromethyl ketones as coupling partners for the allenoates (Scheme 45). Functionalized dihydropyrans are prepared in good yields from α-benzyl allenoates and aryl trifluoromethyl ketones bearing substituents of various electronic properties. Unlike the allene–imine and allene–alkene combinations, the allene–ketone annulation does not tolerate α-methyl allenoate and 2-vinylidenesuccinate as substrates (entries 5 and 6).
Scheme 45.
Synthesis of functionalized dihydropyrans.
3.2.15. Phosphine-Catalyzed Allene–Aldehyde Annulation
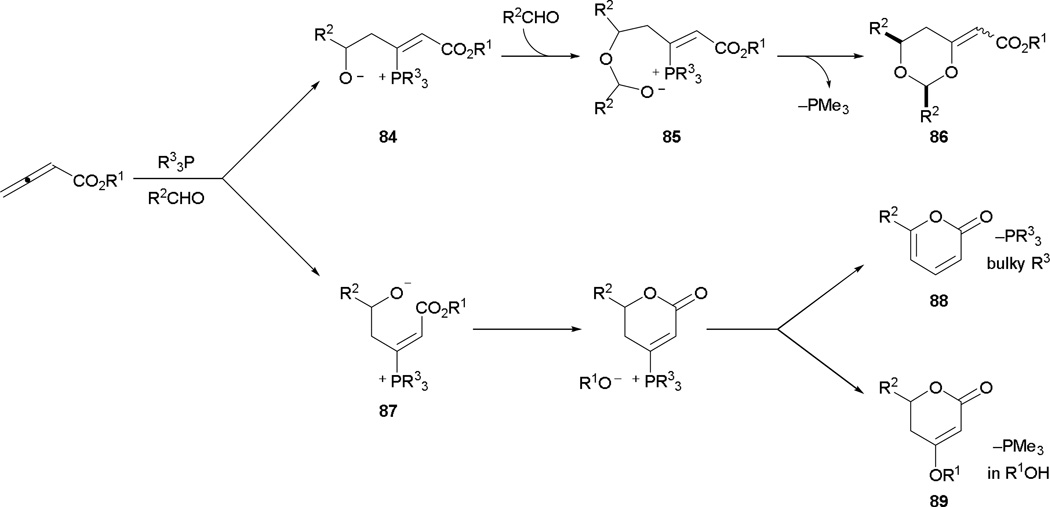
In the realm of phosphine catalysis, activated alkenes and aldimines are commonly used as electrophiles as shown in the studies of Lu and Kwon. On the other hand, reactions employing aldehydes are rare and they operate under different mechanisms. In a series of publications, Kwon and co-workers demonstrated the formation of several heterocycles including dioxanes, 2-pyranones, and dihydro-2-pyranones when using aldehydes as substrates.47
The type of annulation product obtained is dictated by the nature of the phosphine catalyst and the reaction medium (Scheme 46). When a sterically non-demanding phosphine is used, the Z-zwitterion 84 is formed, whose reaction with two equivalents of the aldehyde gives the zwitterion 85. Upon subsequent Michael addition and elimination of the catalyst, the functionalized dioxane 86 is afforded. Conversely, the E-zwitterion 87 is generated when using a bulky phosphine. The close proximity of the alkoxide and the carboxylic ester in 87 results in ready lactonization to form the 2-pyranone 88. Even with sterically unhindered trimethylphosphine as the catalyst, the E-zwitterion 87 is formed in the presence of hydrogen bond donors (e.g., methanol). With added external alkoxide, the dihydro-2-pyranones 89 can also be synthesized.
Scheme 46.
Synthesis of dioxanes, 2-pyranones, and dihydro-2-pyranones via phosphine catalysis.
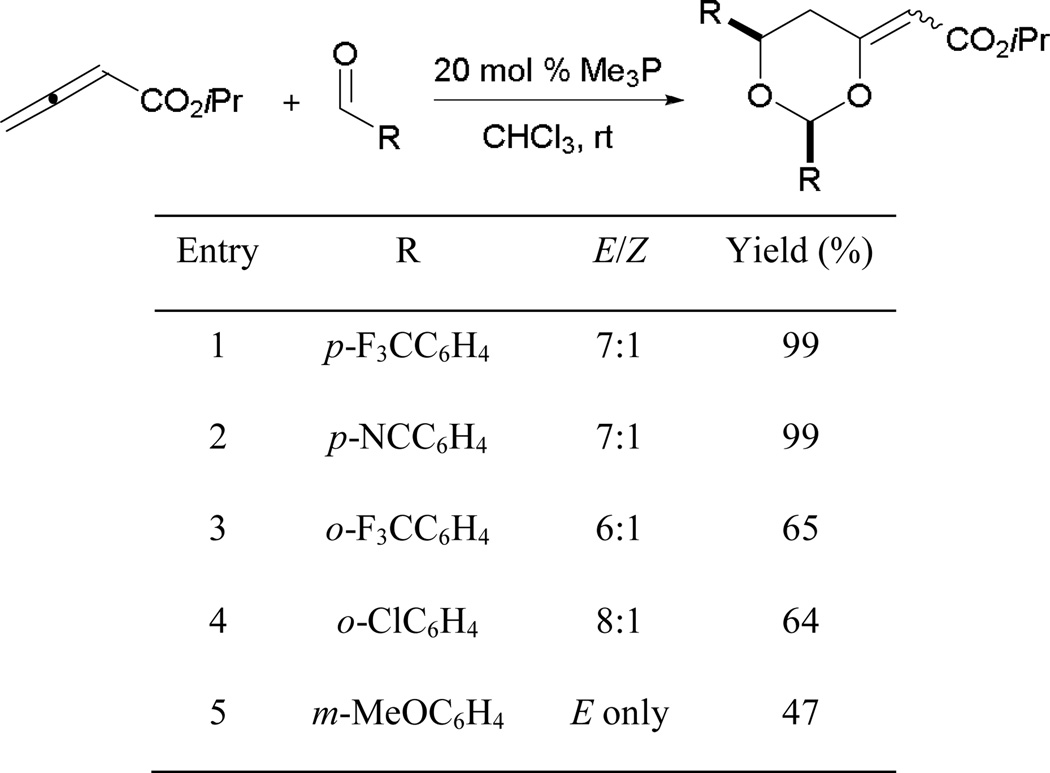
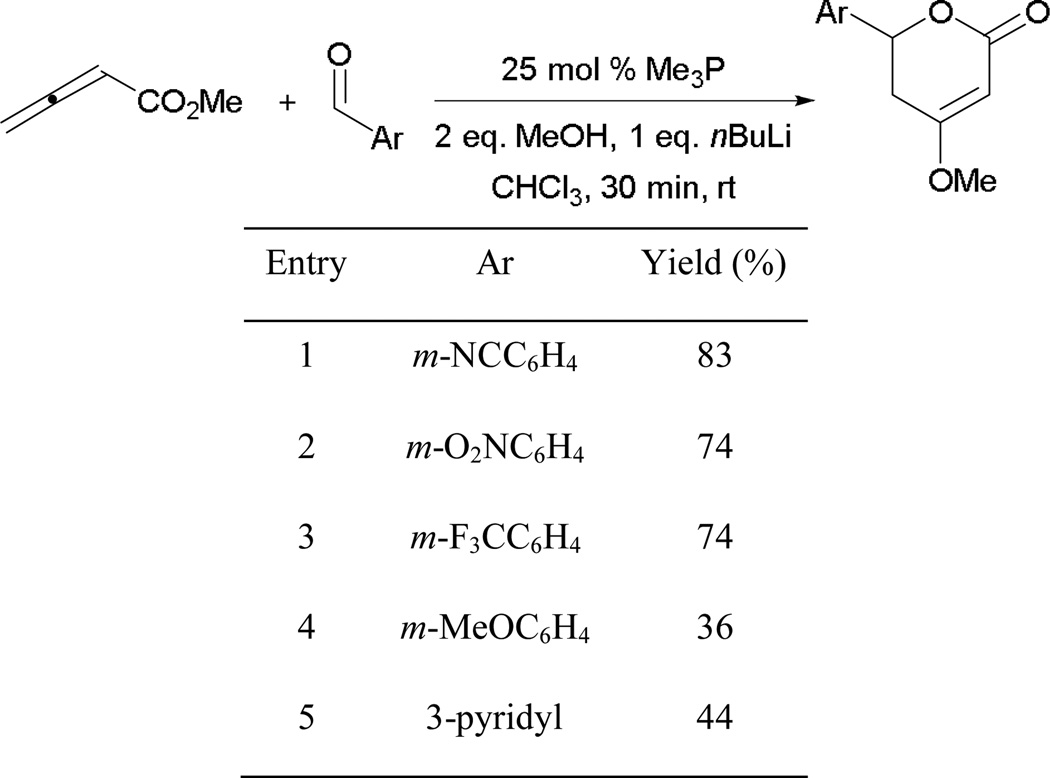
In the formation of functionalized dioxanes, the small catalyst trimethylphosphine is employed to facilitate successful generation of the Z-zwitterion 84 (Scheme 47). Generally, the reaction proceeds in higher efficiency when electron-withdrawing aryl aldehydes are used (entries 1 and 2). Lower yields are observed with aryl aldehydes bearing ortho-substituent (entries 3 and 4). Although a moderate yield is obtained when switching to less-activated m-methoxybenzaldehyde, the reaction proceeds to give the dioxane as the E-stereoisomer exclusively (entry 5).
Scheme 47.
Synthesis of dioxane derivatives through phosphine catalysis.
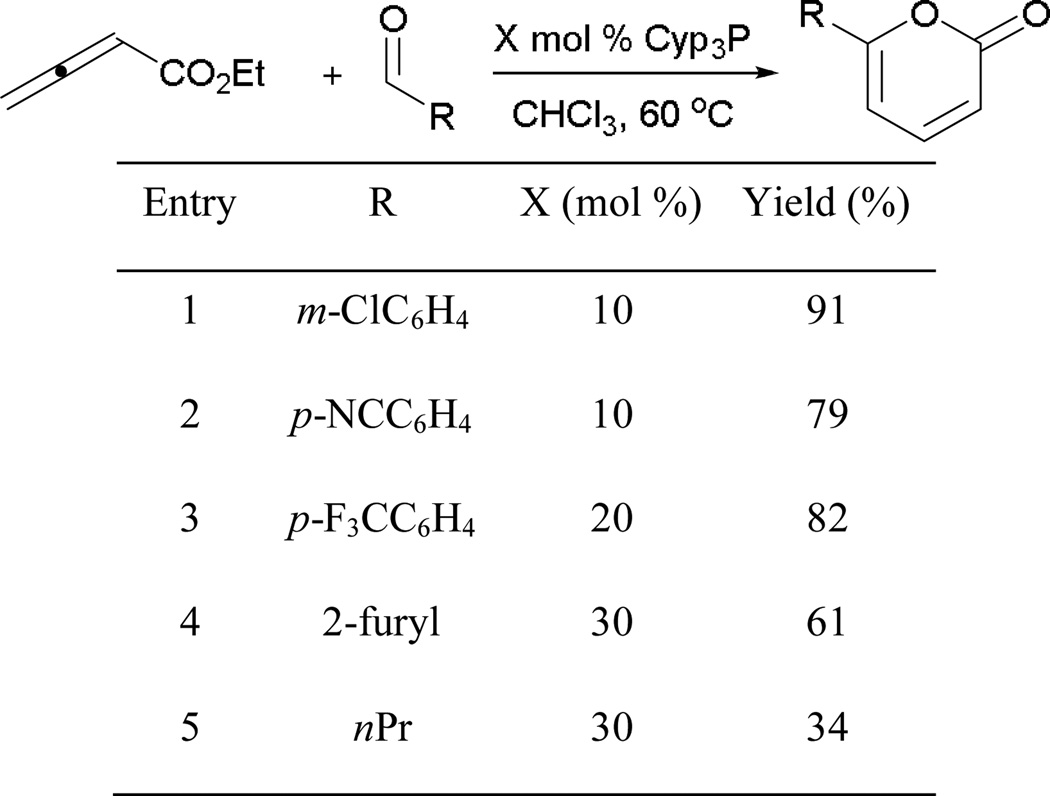
Similar to the synthesis of dioxanes, the reaction is well suited to aryl aldehydes bearing strongly electron-withdrawing functionalities (Scheme 48). Aryl aldehydes with m-chloro, p-cyano, and p-trifluoromethyl groups provide their target 2-pyranones in good yields (entries 1–3). With a heteroaryl aldehyde, a moderate yield of the cyclization product was isolated (entry 4). The effectiveness of the reaction drops when alkyl aldehydes are employed (entry 5).
Scheme 48.
Phosphine-catalyzed formation of 2-pyranones.
When external alcohol and alkoxide are introduced in the reaction mixture, dihydro-2-pyranones are generated with good efficacy (Scheme 49). Consistent with the formation of dioxanes and 2-pyranones, aryl aldehydes presenting electron-withdrawing substituents are well suited to the reaction (entries 1–3). In contrast, heteroaryl and less-activated aryl aldehydes form their products in diminished yields (entries 4 and 5).
Scheme 49.
Formation of various dihydro-2-pyranones.
3.3. Reaction of Allenes with Electrophile–Nucleophiles
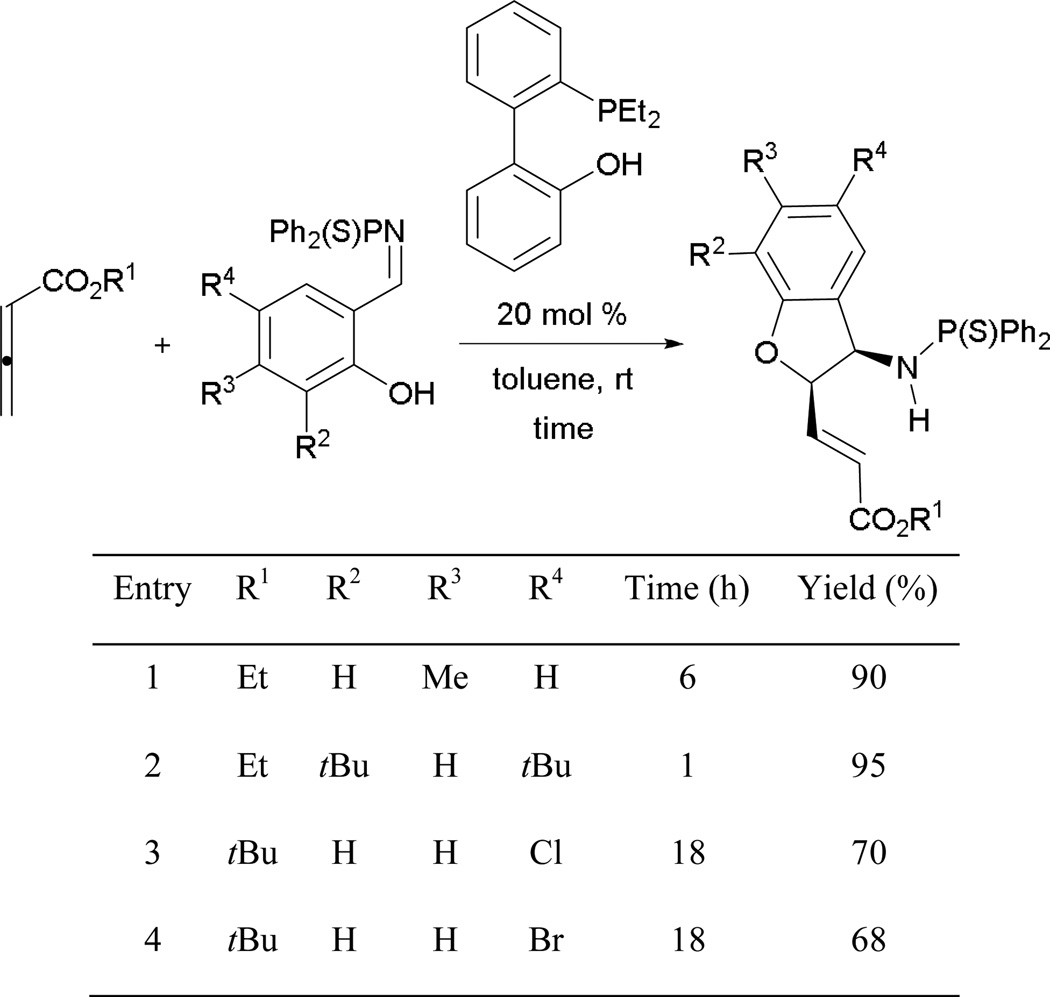
Typically, annulations with allenes employ electrophiles and starting materials possessing both nucleophilic and electrophilic functionalities had not been utilized until recently. In Huang and Chen’s studies, allenoates were reacted with salicyl aldimines to afford functionalized 2,3-dihydrobenzofurans and aminochromans.48 They found that the reaction produced only the oxo-Michael adduct when using amines as catalysts.48a Such adduct was not observed when the reaction was catalyzed by a phosphine. This observation further supports Shi’s finding in the formation of xanthenones that initial bond formation occurs at the aldehyde/aldimine center.10
With alkyl 2,3-butadienoates and salicyl aldimines, as substrates, dihydrobenzofurans are prepared in good yields (Scheme 50). The reactions of salicyl aldimines bearing electron-donating functionalities reach completion within a few hours, giving high yields (entries 1 and 2). In contrast, prolonged reaction times are required and diminished yields are observed when electron-poor substituents are present on the salicyl aldimines (entries 3 and 4).
Scheme 50.
Preparation of functionalized dihydrobenzofuran.
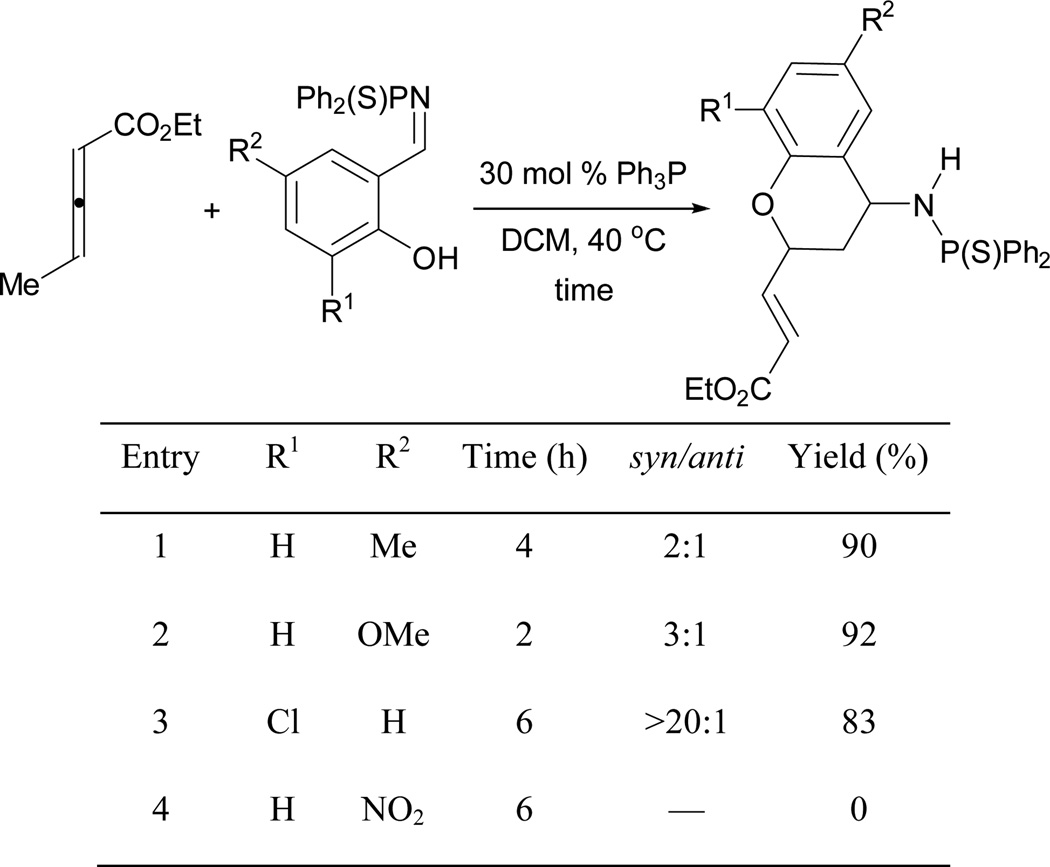
When ethyl 2,3-pentadienoate is used as a substrate instead of 2,3-butadienoate, aminochromans are produced by incorporating the allenoate as two-carbon unit (Scheme 51).48b Similar to the formation of dihydrobenzofurans, substrates with electron-donating functionalities react faster (entries 1 and 2). Although the reaction of an electron-deficient salicyl aldimine required a longer time, the final product exhibited better diastereoselectivity (entry 3). Interestingly, a nitro substituent stopped the reaction completely (entry 4).
Scheme 51.
Synthesis of aminochromans.
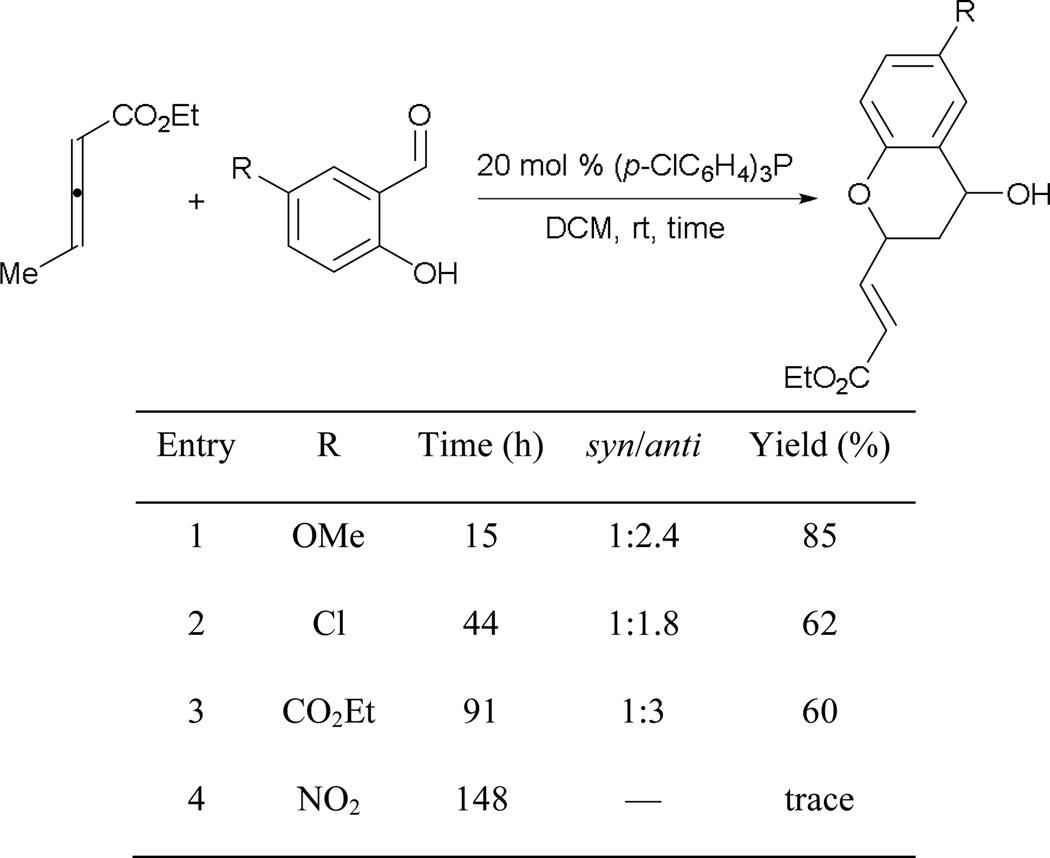
Chromans can also be prepared from derivatives of salicylaldehyde, producing hydroxychromans (Scheme 52) as reported by He and co-workers.49 Similar to Huang and Chen’s transformation, this reaction also proceeds faster for electron-rich substrates (entry 1), slower for salicylaldehydes bearing electron-withdrawing substituents, and not at all in the presence of a nitro group (entries 2–4). Unlike Huang and Chen’s approach, however, a less-nucleophilic catalyst, tris-(p-chlorophenyl)phosphine is required and the hydroxychromans are formed with poor diastereoselectivities.
Scheme 52.
Synthesis of hydroxychromans.
Around the same time, Shi and co-workers reported the formation of both aminochromans and hydroxychromans when using 2,3-butadienoate instead of 2,3-pentadienoate (Scheme 53).50 Interestingly, the reaction employs the more-nucleophilic catalyst tributylphosphine when ethyl-2,3-butadienoate was a substrate. In terms of reactivity, this transformation has many similarities with that reported by Huang and He, but with a few differences. In the synthesis of aminochromans, the products are formed mixtures of E- and Z-isomers (entries 1 and 2). For hydroxychromans, only the E-olefin geometry is obtained in the products when using tributylphosphine as the catalyst (entries 3 and 4).
Scheme 53.
Preparing functionalized aminochromans and hydroxychromans.
4. Phosphine Catalysis of Alkynes
4.1. Phosphine-Catalyzed Isomerization of Alkynes
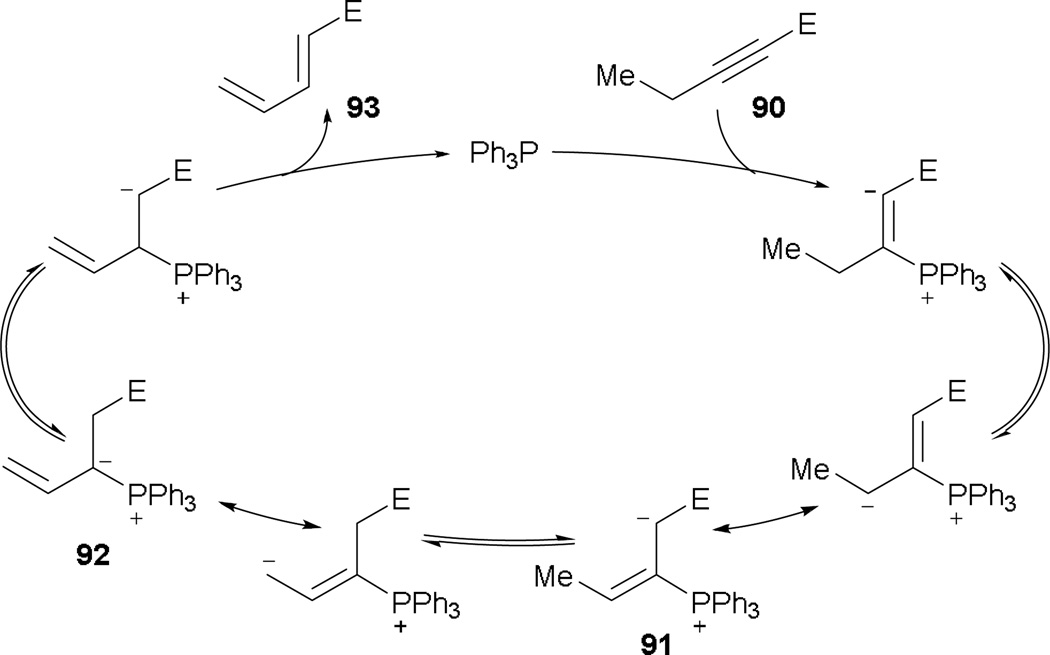
The isomerization of carbon–carbon multiple bonds in activated alkynes was first observed by Trost’s group in 1992.51 It was thought that the nucleophilic addition of the phosphine into the 2-alkynone 90 occurs spontaneously to arrive at the familiar phosphonium dienolate 91 after proton transfer (Scheme 54). After another proton transfer and equilibration, the vinyl phosphonium ylide 92 is produced. Setting the stage for elimination of the phosphine, one more proton transfer is needed to yield the diene 93.
Scheme 54.
Isomerization of alkynes through nucleophilic addition.
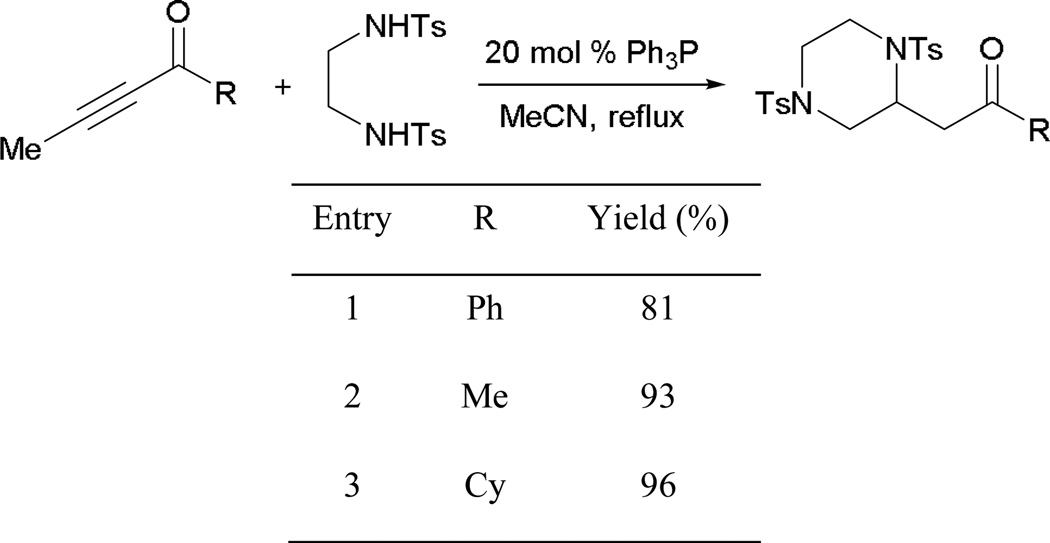
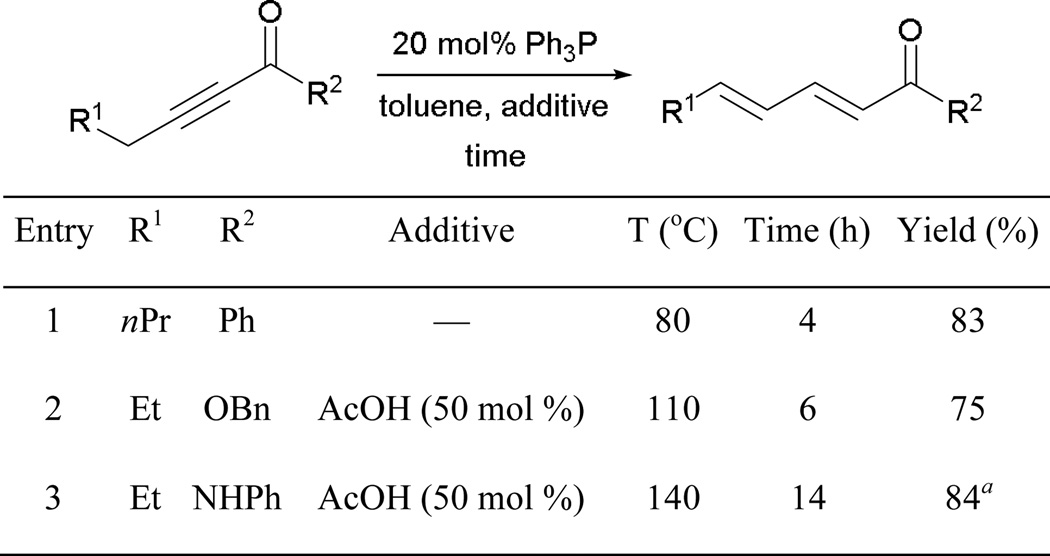
Trost reported that both alkyl and aryl alkynones are well suited for the isomerization (Scheme 55). Although a phosphine possessing strong nucleophilicity can be employed, less oligomerization side products are seen when using triphenylphosphine as the catalyst. When a 2-alkynoate or a 2-alkynamide is subjected to the reaction, higher temperature and the addition of acetic acid are required to facilitate the reaction (entries 2 and 3). These observations suggest the following general reactivity trend: 2-alkynone > 2-alkynoate > 2-alkynamide.
Scheme 55.
Isomerization of activated alkynes.
a Reaction performed in xylene as the solvent.
4.2. Reaction of Alkynes with Nucleophiles
4.2.1. Phosphine-Catalyzed Michael Addition
The first example of phosphine-catalyzed Michael addition between alcohols and activated alkynes was documented by Inanaga in 1993, two decades after White and Baizer.52 Unlike the Michael addition of alkenes, the reaction proceeds through a phosphine catalysis pathway, providing E-Michael adducts (Scheme 56).
Scheme 56.
Proposed phosphine-catalyzed Michael addition of alkynes.
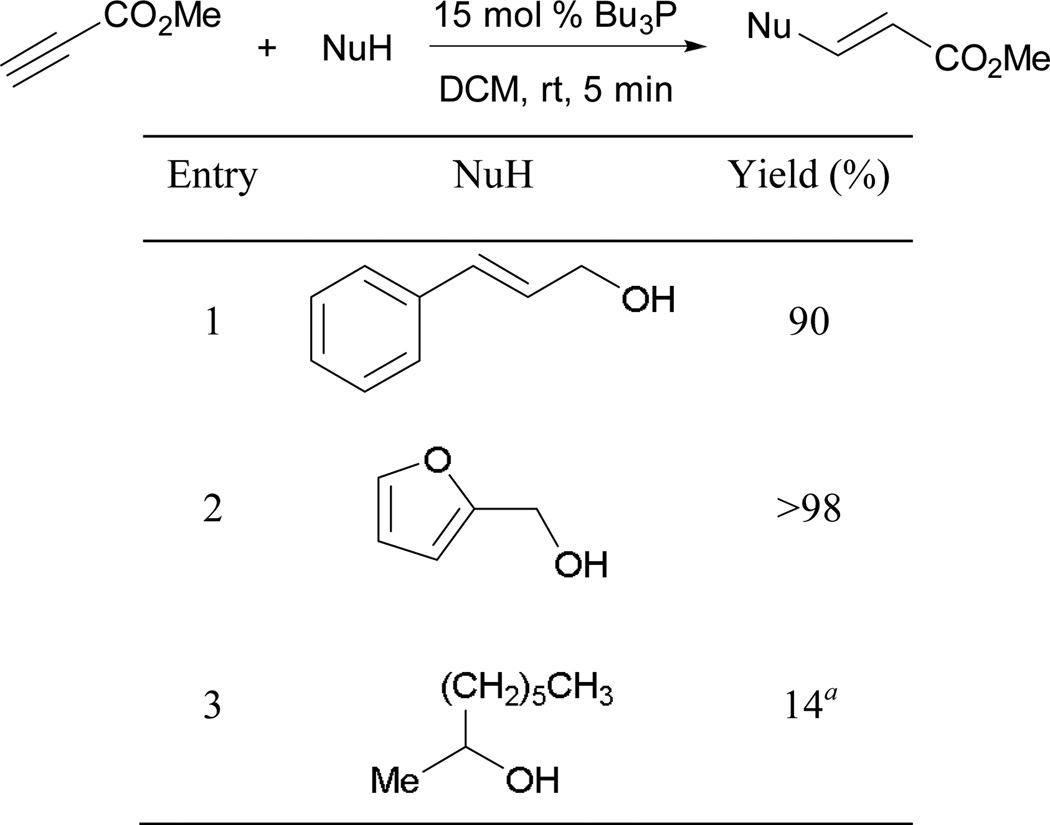
The reactions were complete within a few minutes in the presence of strongly nucleophilic tributylphosphine (Scheme 57). Primary alcohols (entries 1 and 2) underwent Michael additions smoothly to give their desired adducts, whereas a secondary alcohol (entry 3) required a longer reaction time to provide a minimal yield.
Scheme 57.
Phosphine-initiated Michael addition of alcohols.
a Reaction was completed within 30 min.
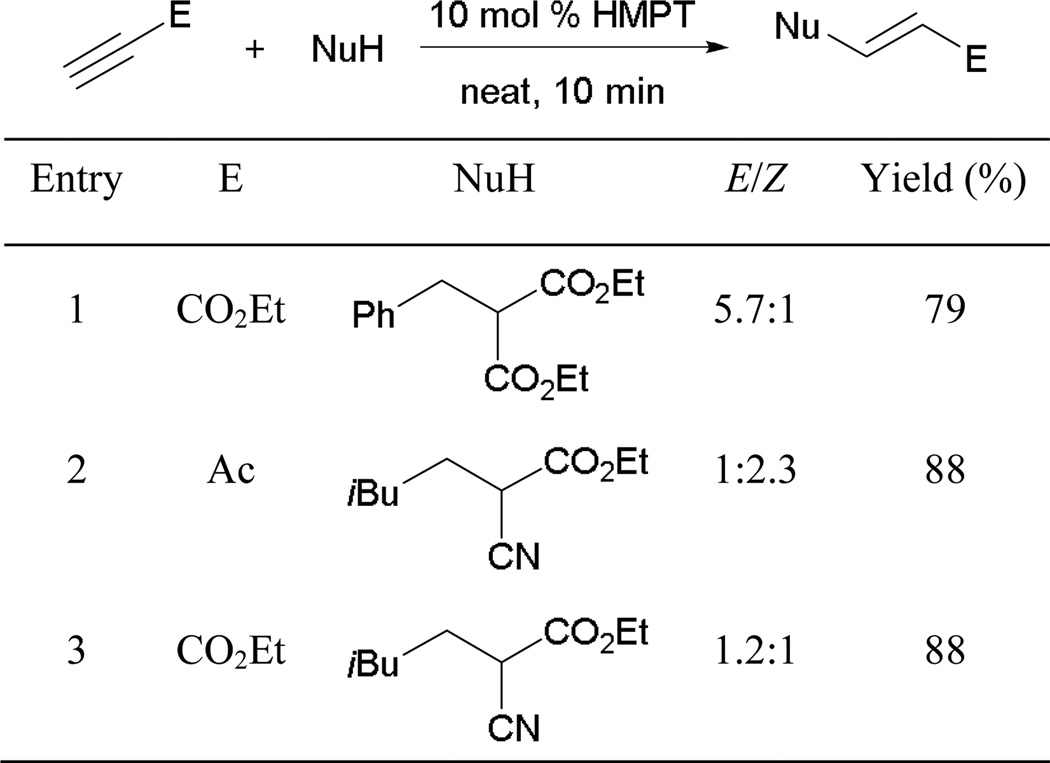
In addition to alcohols serving as pronucleophiles, Grossman’s group reported the application of carbon-centered pronucleophiles as excellent Michael donors when using HMPT as the catalyst (Scheme 58).53 In contrast to Inanaga’s proposal, Grossman suggested phosphine-initiated general base catalysis as a possible reaction mechanism occurring along with the phosphine-catalyzed pathway. Using his method, several functionalized acrylates and α,β-unsaturated ketones can be generated within minutes from β-diesters or α-cyano esters under solvent-free conditions.
Scheme 58.
Preparation of functionalized acrylates.
4.2.2. Phosphine-Catalyzed α-Umpolung Addition
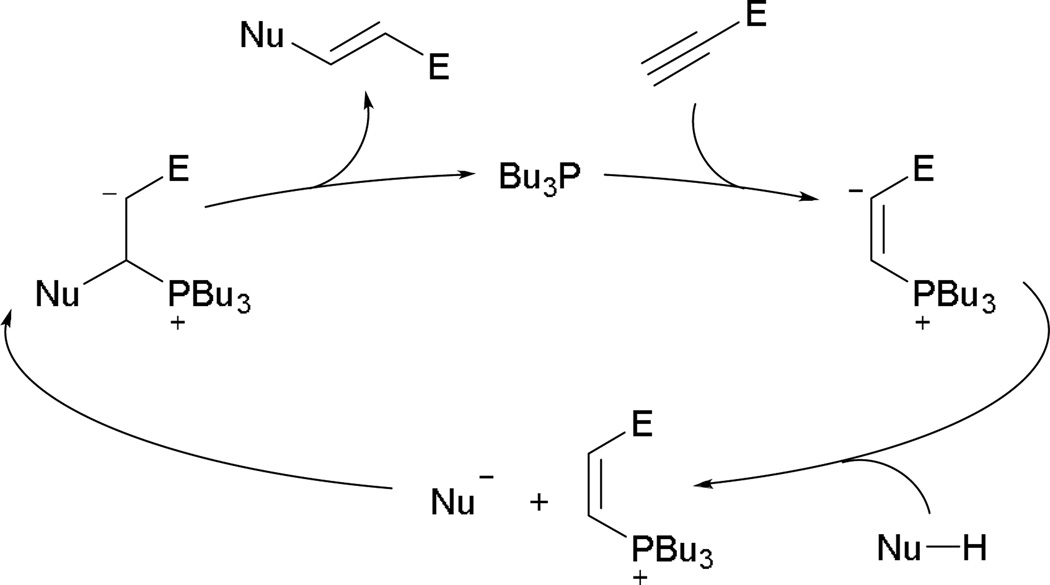
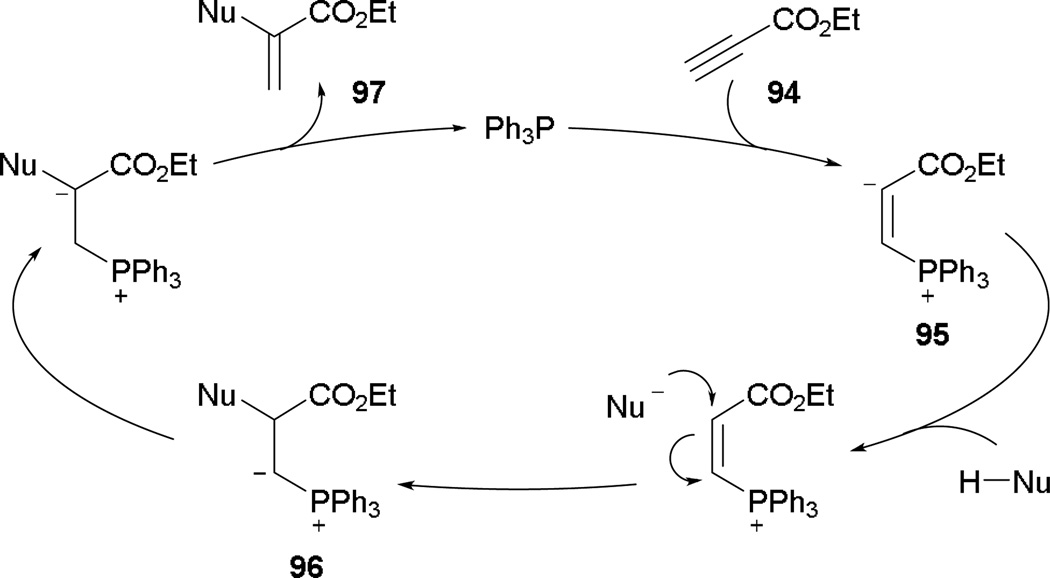
An alternative reaction can occur from activated alkynes in the presence of pronucleophiles and a phosphine. In the presence of nucleophiles, both 2,3-butadienoates and 2-butynoates undergo γ-umpolung addition readily. If propiolates lacking a γ-proton are employed, however, nucleophilic addition ensues through α-umpolung addition. Trost and co-workers made such an observation in the formation of α-aminoacrylates in 1997.54 Their study suggests nucleophilic addition of the phosphine onto alkyl propiolate 94 to give the vinyl phosphonium enoate 95 (Scheme 59). Activation of the pronucleophile and α-umpolung addition gives rise to the ylide 96. With subsequent proton transfer and elimination of the phosphine, the acrylate 97 is afforded.
Scheme 59.
Proposed mechanism of α-umpolung addition.
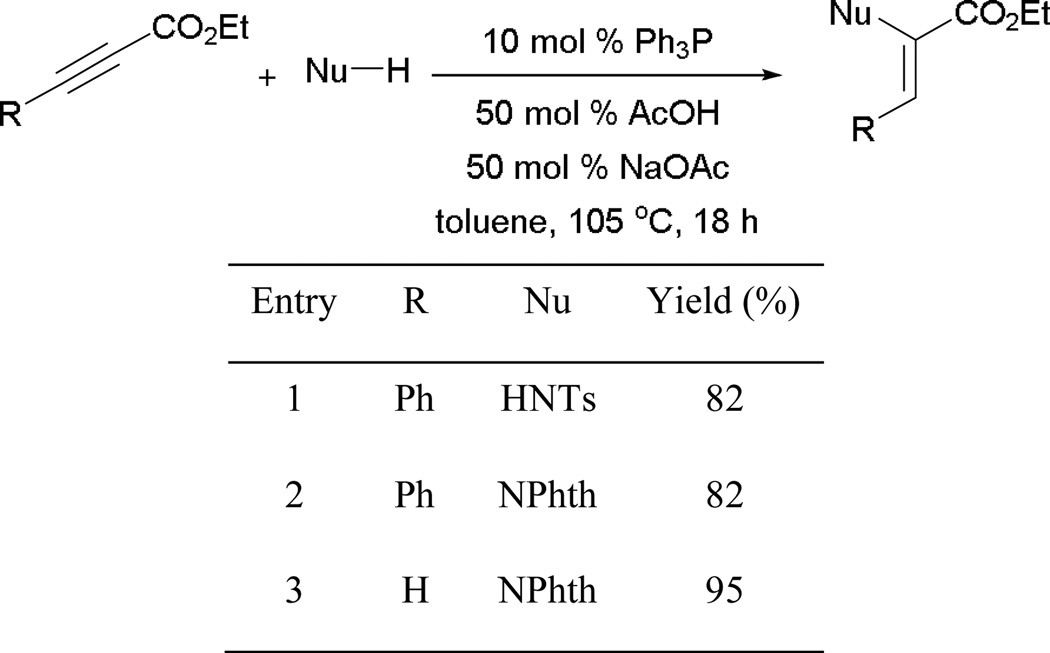
Several functionalized α-aminoacrylates can be synthesized by treating ethyl propiolates with tosylamide or phthalimide in the presence of triphenylphosphine (Scheme 60). With the assistance of acetic acid/sodium acetate as additives for efficient proton transfer, good yields of α-aminoacrylates can be obtained (entries 1–3).
Scheme 60.
Formation of α-aminoacrylates.
4.2.3. Phosphine-Catalyzed Michael–Heck Annulation
Traditionally, phosphines serve as good ligands for transition metals, such as rhodium, ruthenium, and palladium. Capitalizing on this favorable compatibility, a novel tandem phosphine–palladium annulation pathway can be envisioned. Recently, Fan and Kwon reported the formation of functionalized alkylidene phthalans through tandem Michael–Heck reactions.55 Unlike the traditional one-step, one-transformation reactions, the Michael–Heck strategy is a two-step, single-flask operation, which eliminates intermediate work-up and purification. It is also notable that the phosphine possesses dual-reactivity in this tandem transformation; first serving as a nucleophilic catalyst and then as a ligand for palladium catalyst. The proposed mechanism of this transformation begins with Michael addition. Subsequent introduction of the palladium source upon complete generation of the Michael adduct gives the alkylidene phthalan derivatives (Scheme 61).
Scheme 61.
Synthesis of functionalized alkylidene phthalans via Michael–Heck reaction.
a Major Z-phthalan isolated.
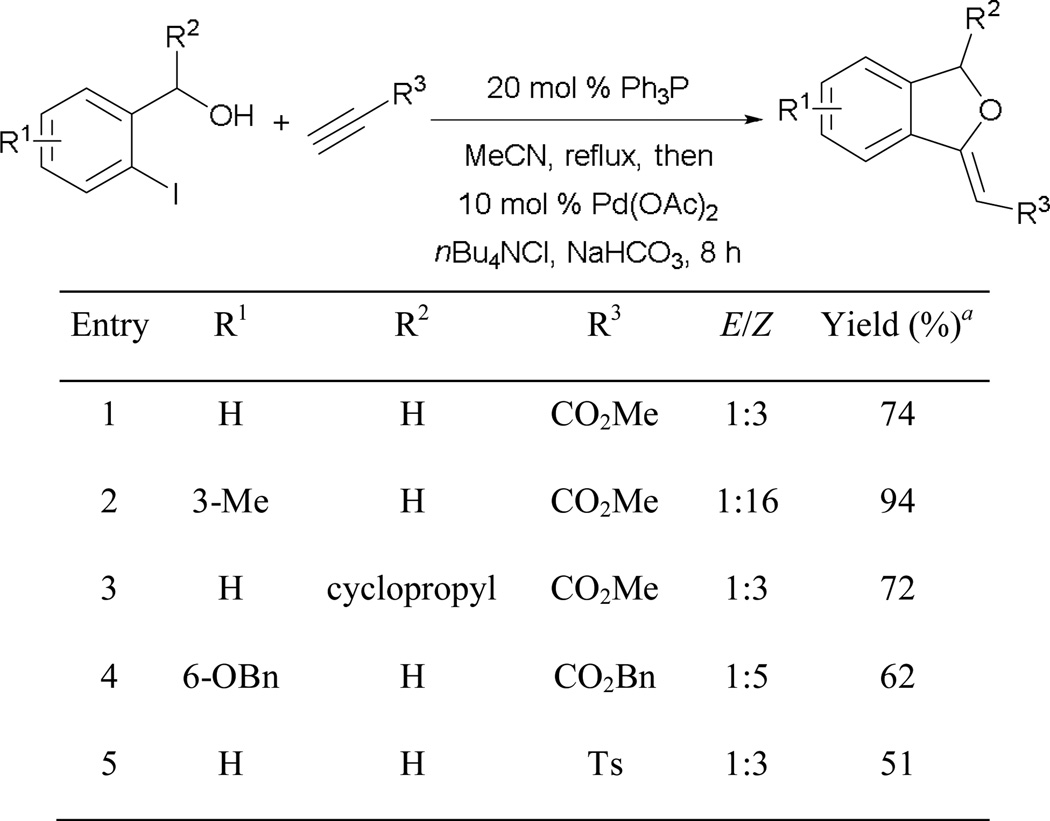
An excellent yield of the alkylidene phthalan, with stereoselectivity, is obtained when using 2-iodo-3-methylbenzyl alcohol as the pronucleophile (entry 2). A secondary alcohol with a cyclopropyl group at the benzylic position can undergo annulation smoothly, giving a good yield of the product (entry 3). In addition to alkyl propiolate, tosyl acetylene is also well suited as a Michael acceptor in this reaction, providing the desired phthalan in moderate yield (entry 5).
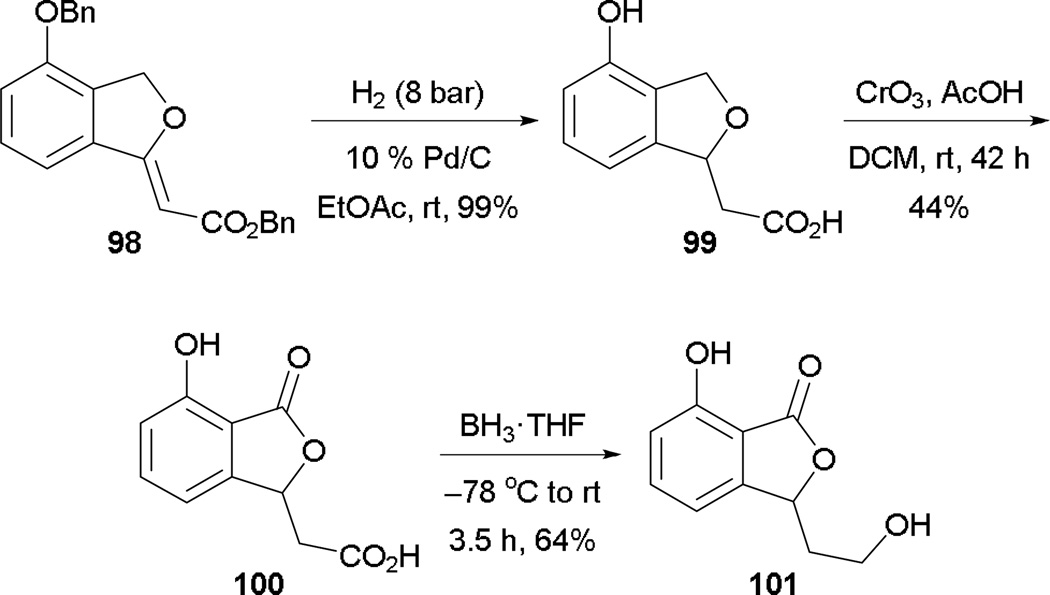
To further demonstrate the utility of Michael–Heck annulation, the transformation has been applied in the syntheses of a group of rare fungal metabolites isolated from Cladosporium sp.: 3-deoxyisoochracinic acid (99), isoochracinic acid (100), and isoochracinol (101) (Scheme 62). The synthesis begins with global debenzylation and hydrogenation of the phthalan 98, affording 3-deoxyisoochracinic acid (99). Further oxidation at the benzylic position with CrO3 provides isoochracinic acid (100). Completing the synthesis, the carboxylic acid moiety of isoochracinic acid (100) is selectively reduced to give isoohracinol (101).
Scheme 62.
Total syntheses of 3-deoxyisoochracinic acid, isoochracinic acid, and isoochracinol.
4.3. Reactions of Alkynes with Di-nucleophiles
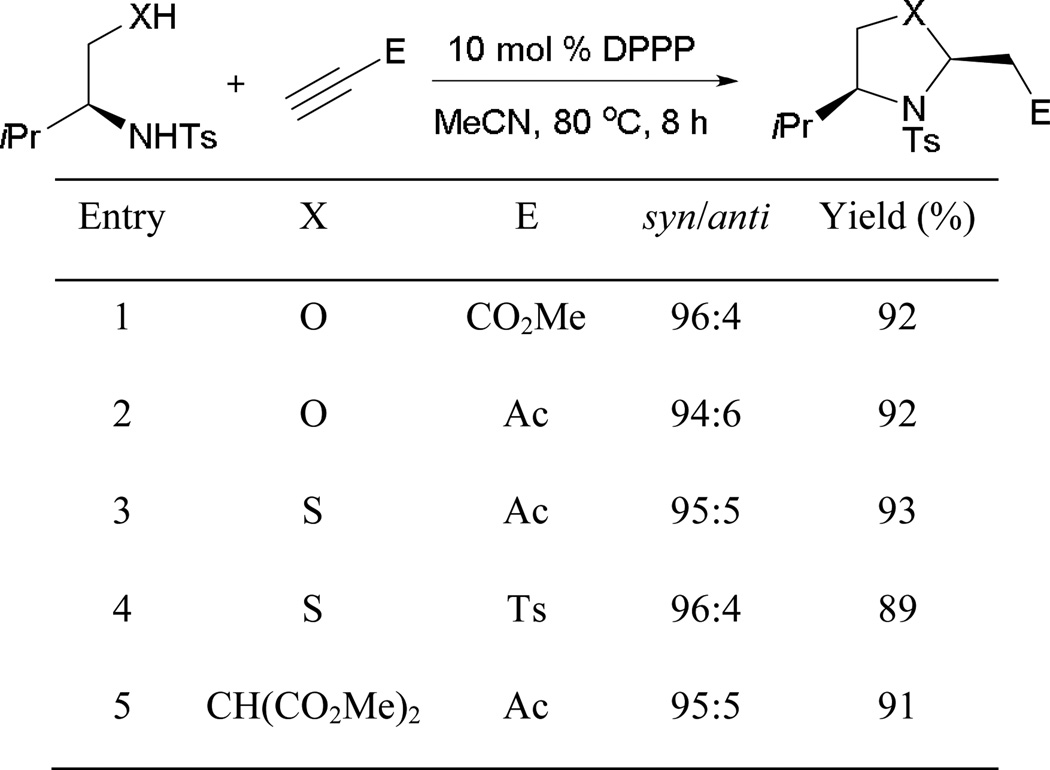
Similar to reactions with allenes, annulation also occurs from activated alkynes and tethered dinucleophiles. Kwon and Sriramurthy reported the first examples of mixed double-Michael reactions, allowing access to highly functionalized oxazolidines, thiazolidines, and pyrrolidines with excellent efficiencies and diastereoselectivities.56 Using di-nucleophiles derived from L-amino acids, the annulation products were obtained in enantiomerically pure form (Scheme 63). The use of 1,3-bis(diphenylphosphino)propane is critical, providing anchimeric assistance in stabilizing the reaction intermediates.
Scheme 63.
Syntheses of oxazolidines, thiazolidines, and pyrrolidines via mixed double-Michael additions.
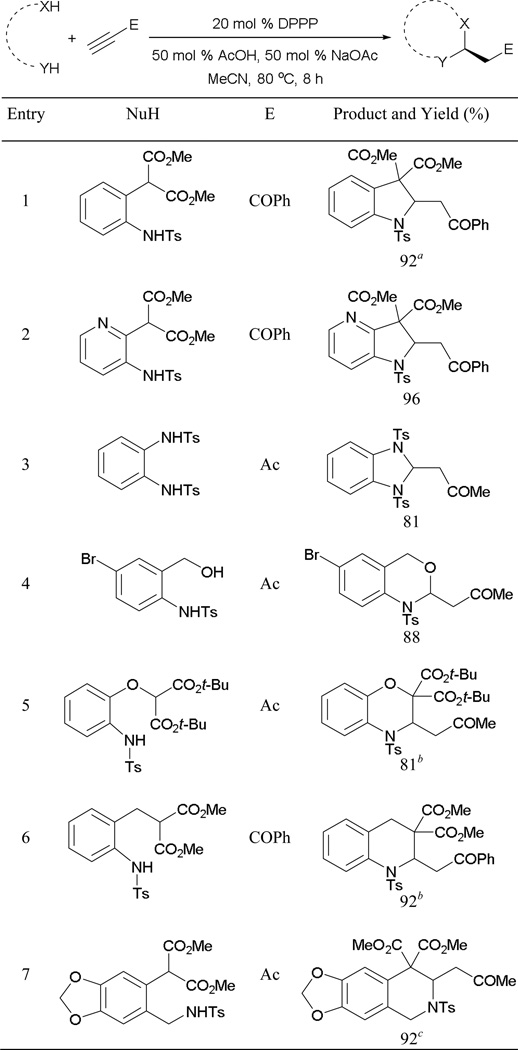
Several other heterocycles including 2,3-dihydroindoles, 2,3-dihydropyrrolopyridines, 2,3-dihydrobenzoimidazoles, tetra-hydroquinolines, tetrahydroisoquinolines, 3,4-dihydrobenzo-oxazines, and 2,4-dihydrobenzooxazines can also be generated through the mixed double-Michael reaction (Scheme 64).57 When employing several aromatic dinucleophiles as reaction partners, a buffer system of acetic acid and sodium acetate can be used to ensure efficient proton transfer.
Scheme 64.
Phosphine-catalyzed mixed double-Michael addition.
a Reaction performed at rt. b Reaction performed in the absence of AcOH/NaOAc. c Reaction performed in the absence of AcOH/NaOAc at rt.
4.4. Reactions of Alkynes with Electrophiles
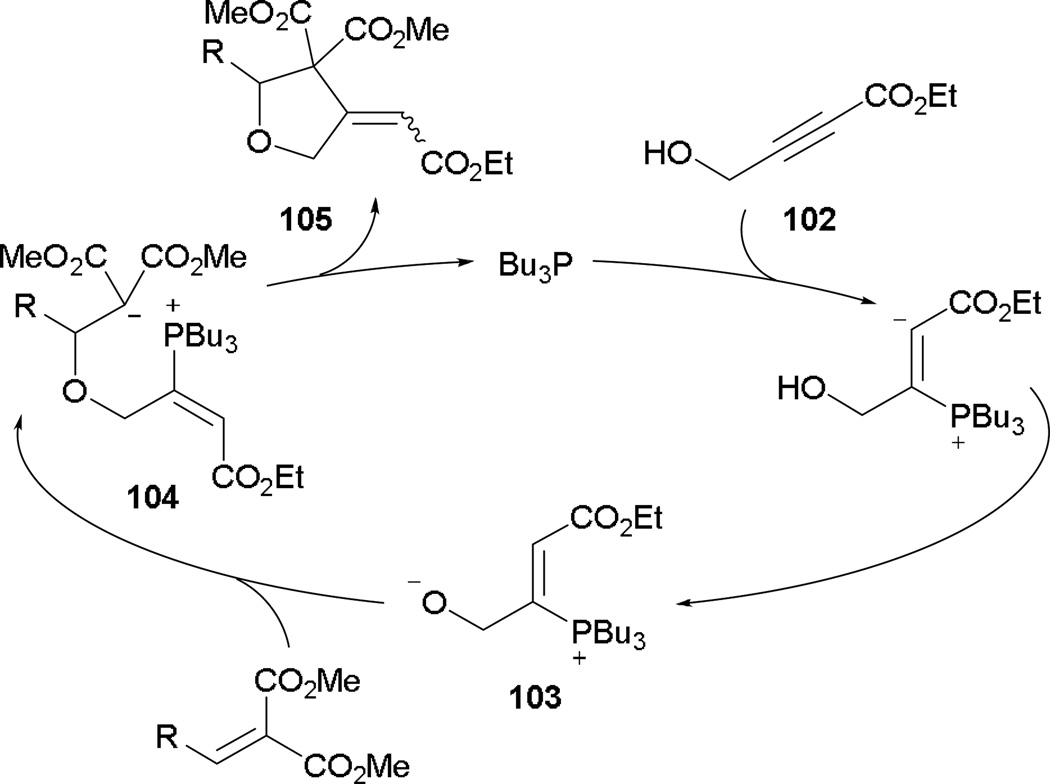
Generally, treatment of activated alkynes featuring γ-protons triggers Lu’s [3 + 2] annulation with electrophiles, because both 2-butynoate and 2,3-butadienoate are converted to the common phosphonium dienolate 17 intermediate in the presence of a phosphine. In an unusual setting, Williamson reported that with the use of 4-hydroxy-2-butynoate as a reaction partner, tetrahydrofurans are formed via two-step Michael–Michael addition (Scheme 65).58 Treating the 4-alkoxy-3-phosphonium enoate 102 with a phosphine induces the formation of the γ-hydroxyphosphonium dienolate 103. In the presence of an activated alkene, Michael addition occurs immediately, generating the intermediate 104 for the subsequent, second Michael addition. The functionalized tetrahydrofuran 105 is synthesized after elimination of the catalyst.
Scheme 65.
Proposed mechanism for the formation of tetrahydrofurans.
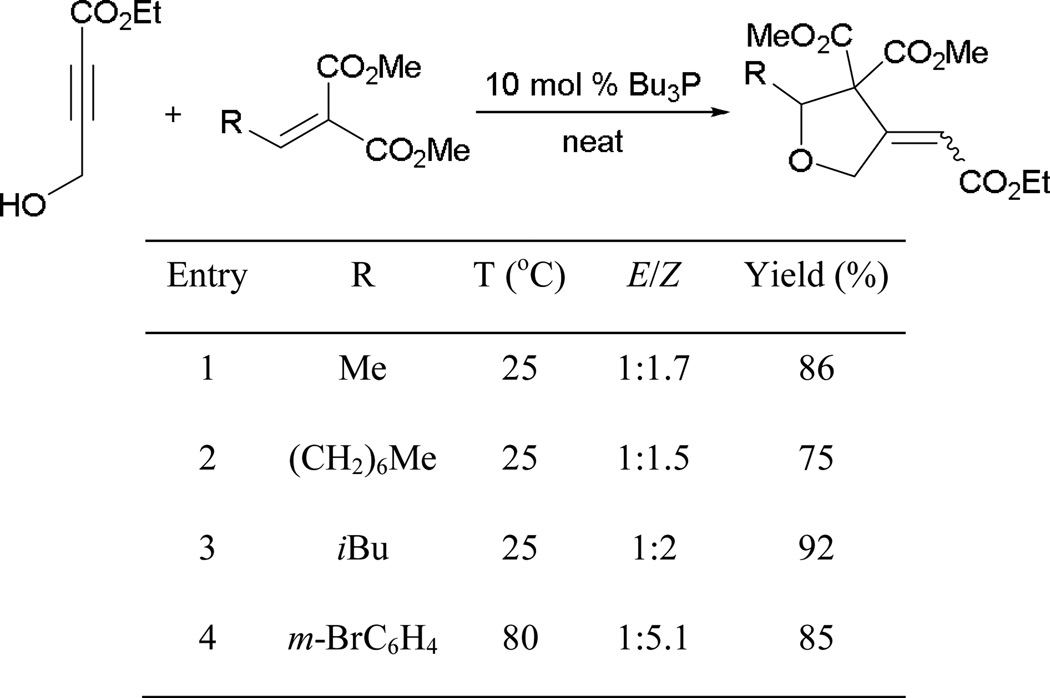
The reaction is performed with the highly nucleophilic tributylphosphine, because undesired side reactions occur with less-nucleophilic phosphines (Scheme 66). Different alkylidenemalonates undergoes annulation smoothly under the reaction conditions, providing several tetrahydrofurans in high yields (entries 1–3). Although good yields are obtained with alkylidenemalonates, stereoselectivities are poor unless an arylidenemalonate is applied (entry 4).
Scheme 66.
Synthesis of tetrahydrofurans through Michael–Michael annulation.
4.5. Reactions of Alkynes with Electrophile–Nucleophiles
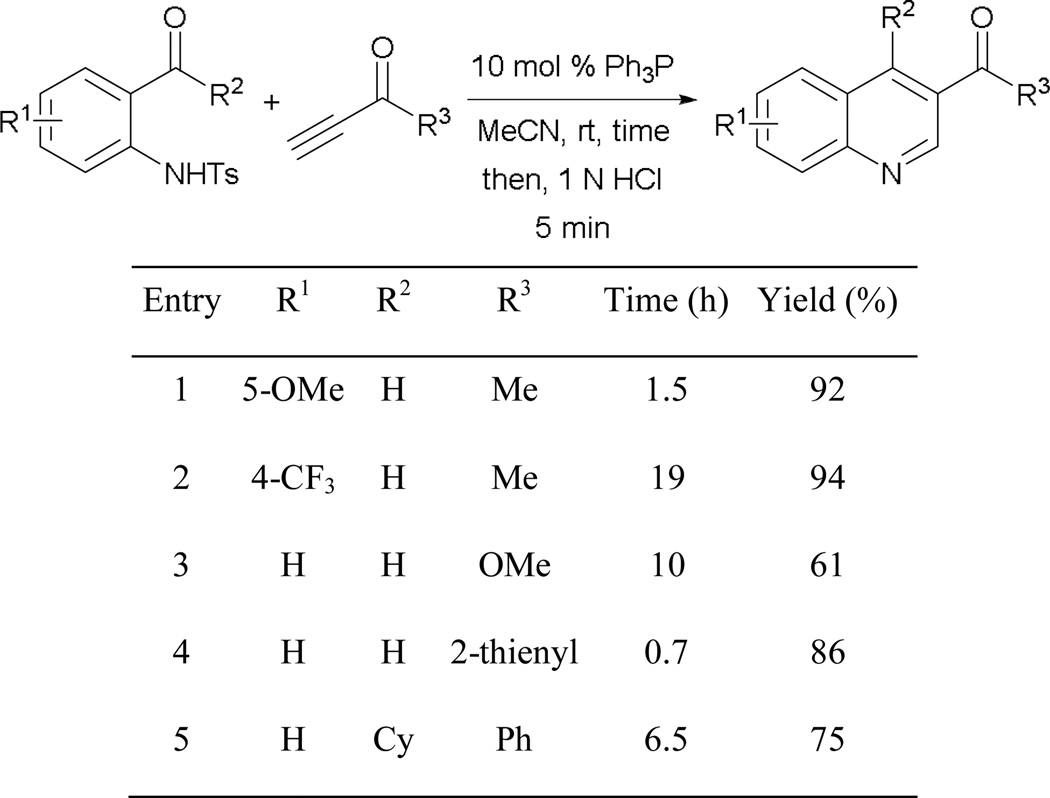
Recently, Khong and Kwon disclosed the formation of functionalized quinolines by employing o-tosylamidobenzaldehydes and o-tosylamidophenones as coupling partners (Scheme 67).59 The annulation sequence is speculated to involve an initial Michael addition followed by an intramolecular aldol reaction. Subsequent work-up of the resulting N-tosyl-4-hydroxydihydroquinolines with aqueous HCl provides the quinolines. Under the reaction conditions, excellent yields of quinolines can be obtained across different substitution patterns. The reaction takes longer to complete with aminobenzaldehydes bearing electron-withdrawing functionalities (entry 2). Although less-activated methyl propiolate can be used, the reaction is prolonged and the yield is affected (entry 3). Notably, a sterically encumbered cyclohexylketone is also well suited to the reaction with only a minor loss in yield (entry 5).
Scheme 67.
Formation of functionalized quinolines.
5. Phosphine Catalysis of Morita–Baylis–Hillman Alcohol Derivatives (MBHADs)
5.1. Reactions of MBHADs with Nucleophiles
An emerging field in phosphine catalysis is the use of derivatives of β'-hydroxyacrylates, products of the MBH reaction, to introduce novel phosphonium species.60 These new phosphonium species grant access to new reaction pathways with nucleophiles, electrophiles, and electrophile–nucleophiles.
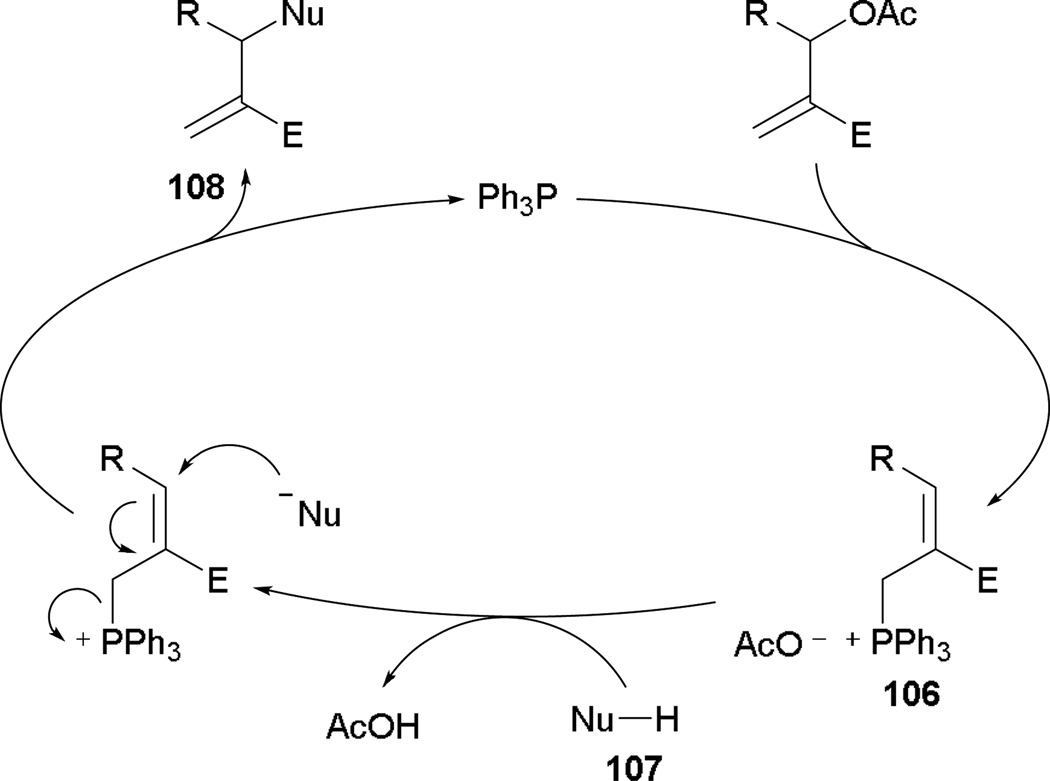
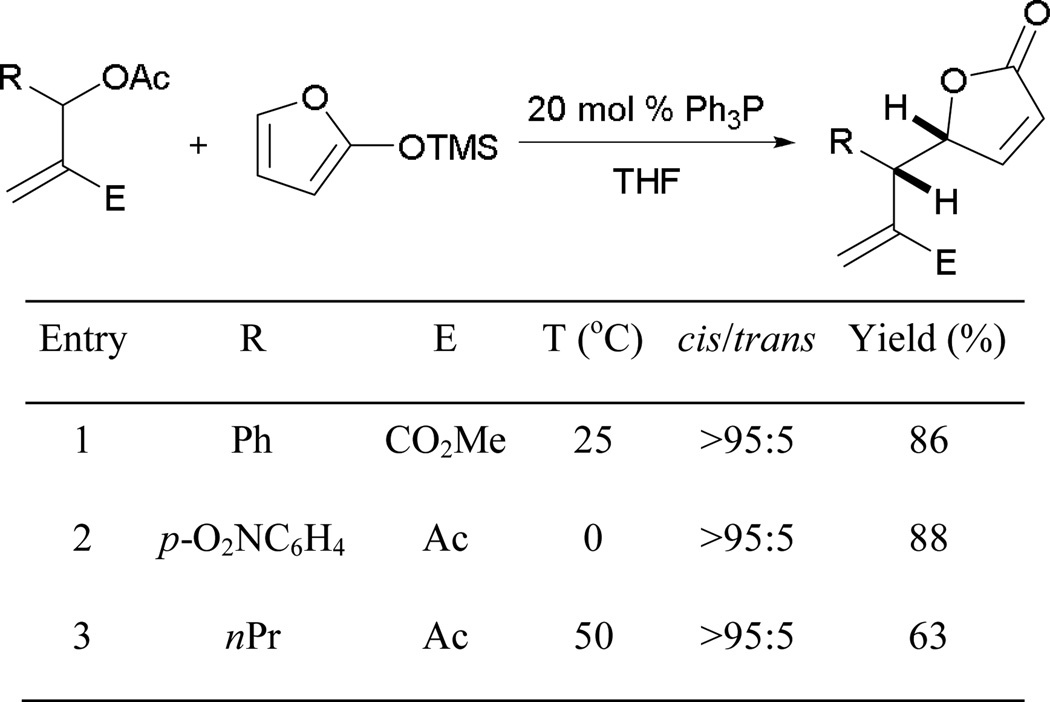
When nucleophiles are used with MBHADs, the main reactivity mode is substitution. Krische employed MBHADs protected by acetate groups and 2-(trimethylsiloxy)furan to synthesize functionalized γ-butenolides.61 With the use of MBHADs, triphenylphosphine undergoes SN2′ displacement forming the phosphonium species 106 (Scheme 68). The extruded acetate serves as an in situ generated base to activate the pronucleophile 107 for immediate addition, providing the functionalized γ-butenolides 108.
Scheme 68.
Plausible mechanism for γ-butenolide formation.
In this reaction, 2-(trimethylsiloxy)furan serves as an ideal pronucleophile and surrogate for introducing a butenolide group (Scheme 69). The reaction behaves well with different β'-aryl substituents, providing good yields of γ-butenolides with excellent diastereoselectivities (entries 1 and 2). If an alkyl substituent is placed in the substrate, however, the product yield is lowered (entry 3).
Scheme 69.
Synthesis of γ-butenolides from MBHADs.
5.2. Reactions of MBHADs with Electrophiles
5.2.1. Phosphine-Catalyzed MBHAD–Alkene [3 + 2] Annulation
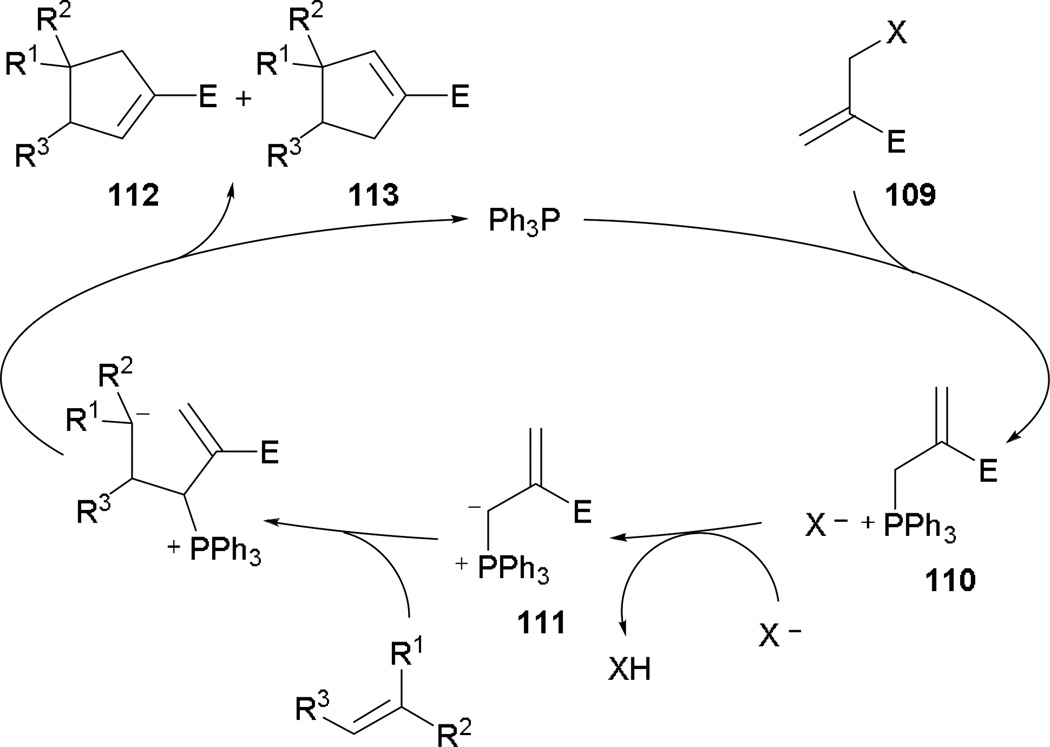
The idea of employing acetate/tert-butylcarbonate-protected β'-hydroxymethylacrylates in phosphine catalysis was first introduced by Lu in 2003.60 By installing a β'-acetate or β'-tert-butylcarbonate group, novel phosphonium species are obtained through new annulation pathways. The mechanism proceeds with conjugate addition into the MBHAD 109 with the ejection of the β'-leaving group, forming the phosphonium species 110 (Scheme 70). The expelled acetate or tert-butoxide acts as base to activate and generate the phosphonium ylide 111. In the presence of an activated alkene, annulation occurs to yield a mixture of the cyclopentenes 112 and 113.
Scheme 70.
Proposed mechanism for MBHAD–alkene [3 + 2] annulation.
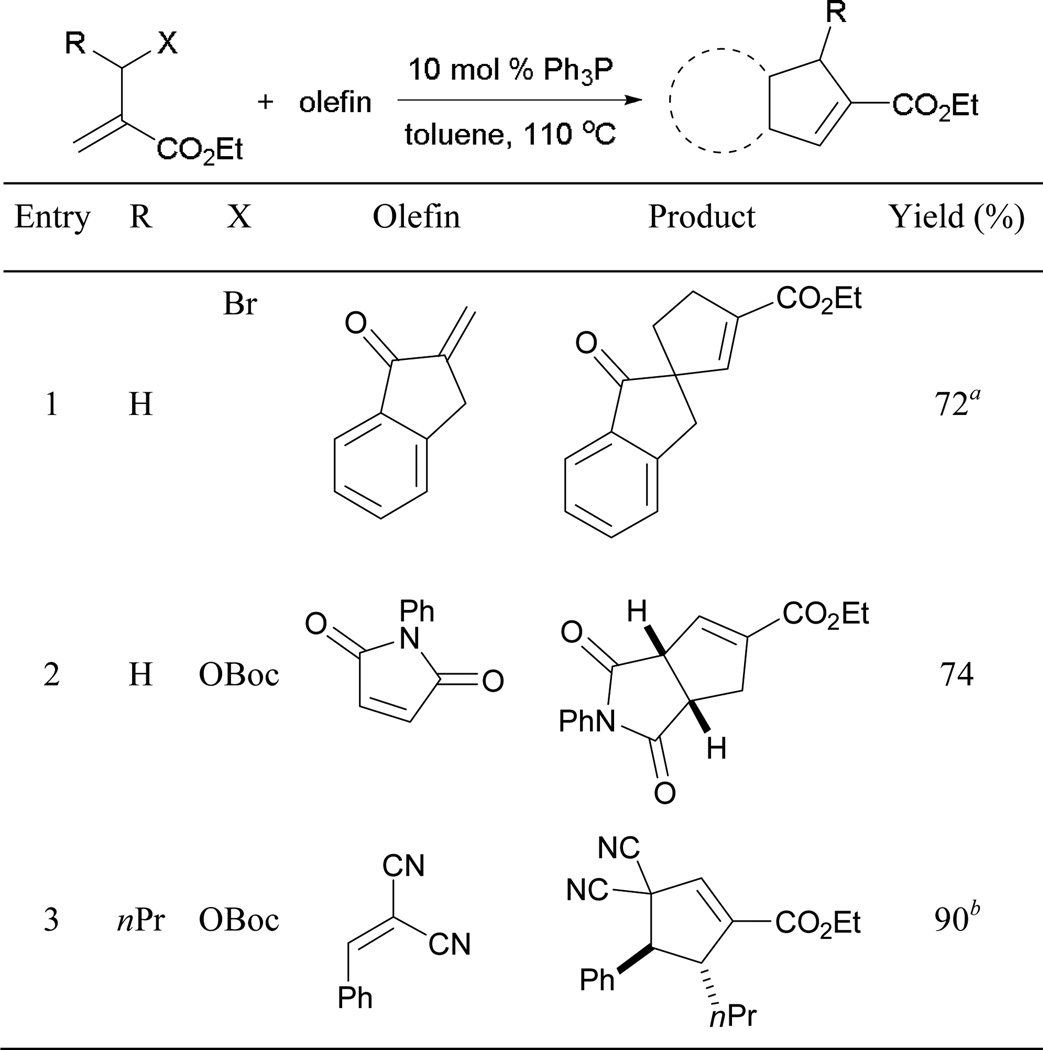
Reminiscent of Lu’s original allene–alkene [3 + 2] annulation, the MBHAD–alkene [3 + 2] reaction is a versatile and powerful means to access cyclopentenes (Scheme 71). Good yields of cyclopentenes are obtained with either bromo or carbon dioxide and tert-butoxide as the leaving groups (entries 1 and 2). Notably, no external base is required when tert-butylcarbonate is installed on the MBHADs, due to the in situ generation of tert-butoxide (entry 2). Furthermore, the issue of regiochemical selectivity can be tuned to select for one regioisomer when alkylidene or arylidenemalononitrile is used (entry 3).62
Scheme 71.
Synthesis of cyclopentenes via MBHAD–alkene [3 + 2] annulation.
a 1.5 eq. K2CO3 employed as additive. b Reaction performed at rt.
5.2.2. Phosphine-Catalyzed Intramolecular MBHAD–Alkene [3+2] Annulation
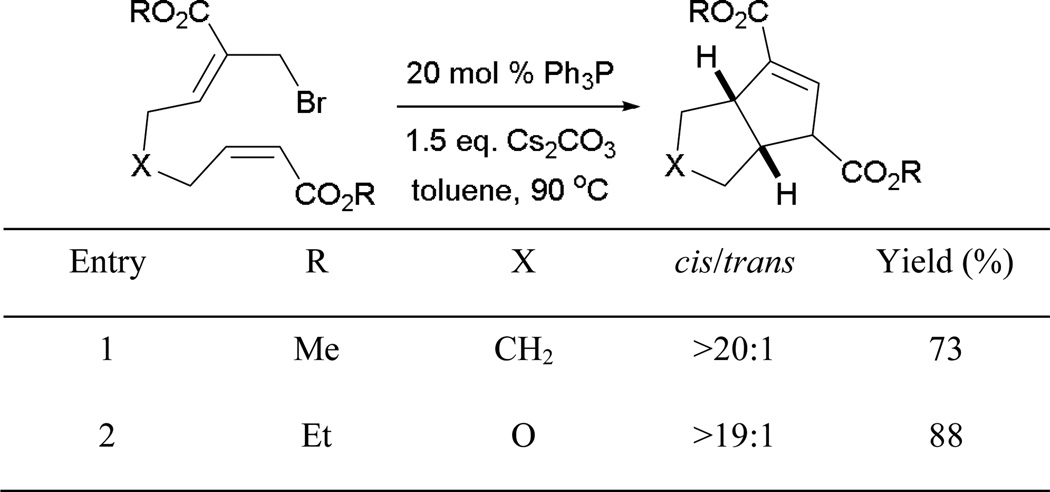
Shortly after Lu’s discovery of the MBHAD–alkene [3 + 2] annulation, Tang reported an intramolecular variant of the [3 + 2] annulation using tethered systems to synthesize diquinane and tetrahydrocyclopenta[c]furan derivatives.63 Bicyclic ring structures are formed in good yields and with good diastereoselectivities (Scheme 72).
Scheme 72.
Intramolecular MBHAD–alkene [3+2] annulation.
5.2.3. Phosphine-Catalyzed MBHAD–Imine [3 + 2] Annulation
Similar to the formation of dihydropyrroles through Lu’s allene–imine annulation, good yields of dihydropyrrole derivatives can also be obtained when using MBHADs with imines (Scheme 73).64 The annulation products are prepared in good yields, favoring cis-isomers (entries 1 and 2). A consistent result is seen when the imine bears an electron-donating substituent (entry 3). This approach is not just an alternative route to dihydropyrroles: a functionalized dihydropyrrole bearing a 2-ethyl substituent can be achieved, albeit in low yield (entry 4).
Scheme 73.
Preparation of dihydropyrroles from MBHADs.
5.3. Reaction of MBHADs with Electrophile–Nucleophiles
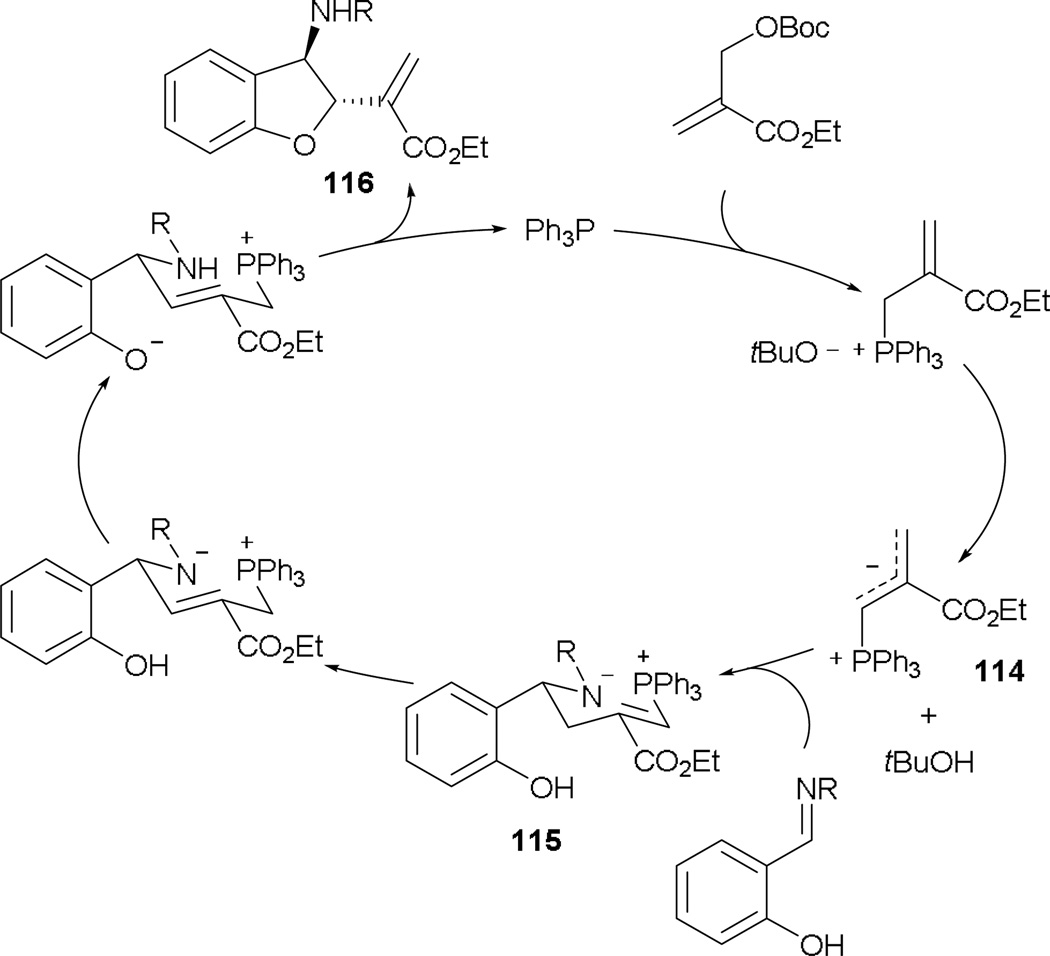
The application of MBHADs in reactions with electrophile–nucleophiles has also been explored by Huang and Chen, who are among the pioneers of the development of electrophile–nucleophile systems. They demonstrated the formation of 3-amino-2,3-dihydrobenzofurans after treating MBHADs with salicyl aldimines in the presence of a phosphine.65 The catalytic cycle begins with displacement of the β'-leaving group with activation to afford the phosphonium ylide 114 (Scheme 74). Nucleophilic addition occurs in the presence of a tethered electrophile–nucleophile, giving the intermediate 115. After a series of proton transfers and olefin isomerization, elimination of the phopshine yields the 3-amino-2,3-dihydrobenzofuran 116.
Scheme 74.
Suggested mechanism for the formation of 3-amino-2,3-dihydrobenzofurans.
This highly efficient reaction provides functionalized 3-amino-2,3-dihydrobenzofurans (Scheme 75). In general, the reaction reaches completion more rapidly when the salicyl aldimines possess electron-withdrawing substituents (entry 1). On the other hand, higher diastereoselectivities are discerned with substrates bearing electron-donating groups (entries 2). Although an MBHAD with an aryl substituent prolongs the reaction time substantially, the reaction’s high efficiency is maintained (entry 4). Notably, a relatively low loading of triphenylphosphine is needed to ensure a good conversion.
Scheme 75.
Preparation of 3-amino-2,3-dihydrobenzofuran derivatives.
6. Enantioselective Phosphine Catalysis
Traditionally, chiral phosphines have been employed in transition metal catalysis to serve mainly as chiral ligands. It was not until the end of the 20th century that the first asymmetric phosphine catalysis was reported. While Vedejs has performed pioneering studies into the enantioselective acylation of secondary alcohols using chiral phosphines,66 our focus in the Article is on asymmetric variants of the phosphine catalysis reactions that we have discussed above. To assist the study of the structures and reactivities of chiral phosphines, our report is categorized into two subsections: chiral phosphines without additional functionality and multi-functional chiral phosphines.
6.1. Chiral Phosphines without Additional Functionality
In the first category, the way of rendering asymmetry is mostly through chiral phosphorus centers or the chiral backbones of the phosphorus-containing catalysts. Reactions catalyzed by chiral phosphines without extra assistance through hydrogen bonding from the appending functional groups are covered in this Section.
6.1.1. Asymmetric Phosphine-Catalyzed Allene–Alkene [3 + 2] Annulation
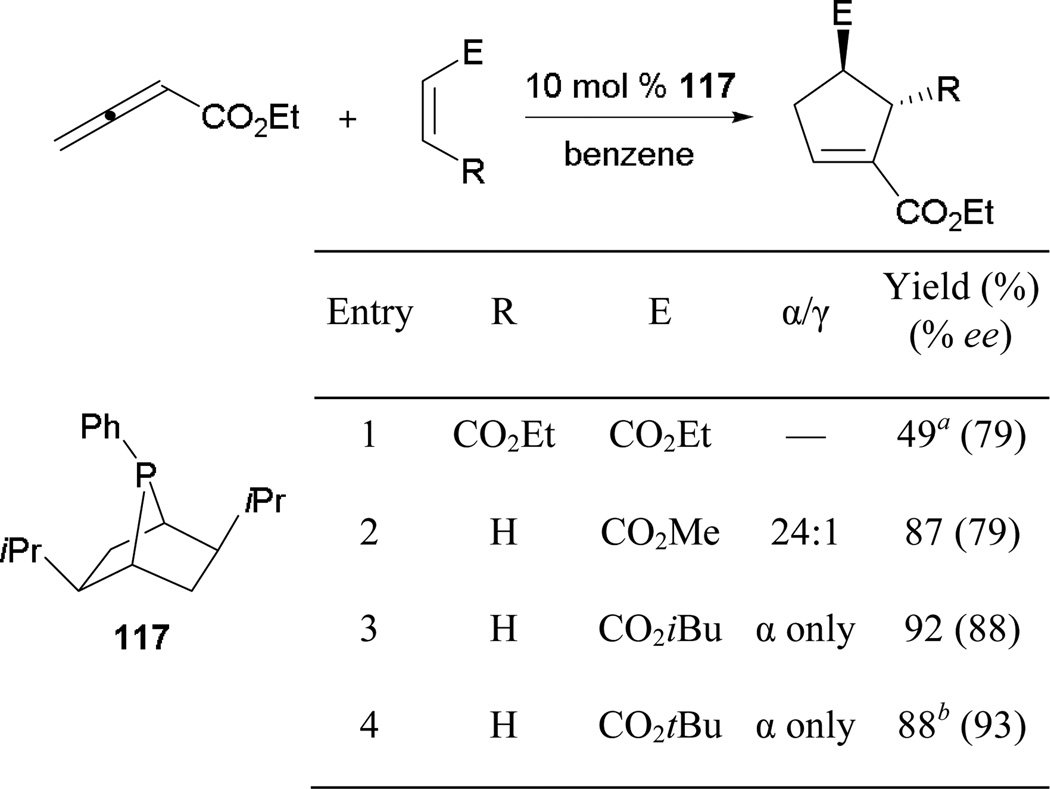
Zhang and co-workers demonstrated the first example of asymmetric allene–alkene [3 + 2] annulation using the chiral phosphabicyclo[2.2.1]heptane 117.67 The isopropyl groups provide a steric barrier to block the approach of activated alkenes, thereby inducing asymmetry (Scheme 76). Alkyl acrylates bearing larger alkyl groups furnish greater yields and enantioselectivities (entries 2–4). With Zhang’s success in asymmetric induction of [3 + 2] annulation, many research groups have been attracted to the realm of asymmetric phosphine catalysis.
Scheme 76.
Asymmetric formation of cyclopentenes using phosphabicyclo[2.2.1]heptane.
a Reaction performed at 0 °C. b Reaction performed in toluene at 0 °C
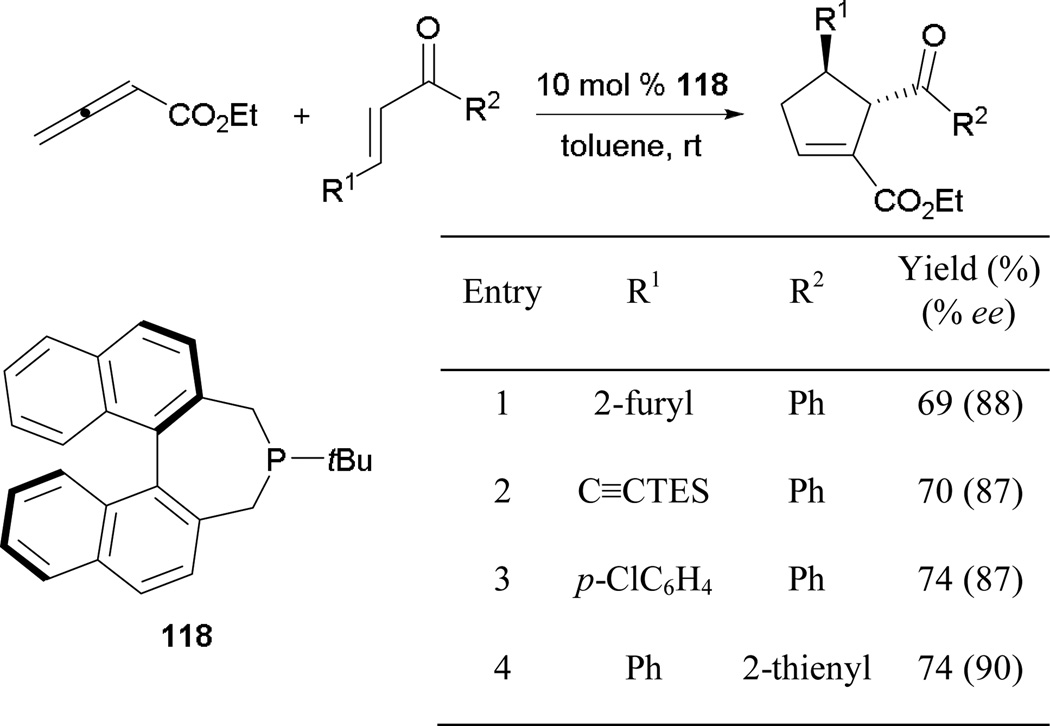
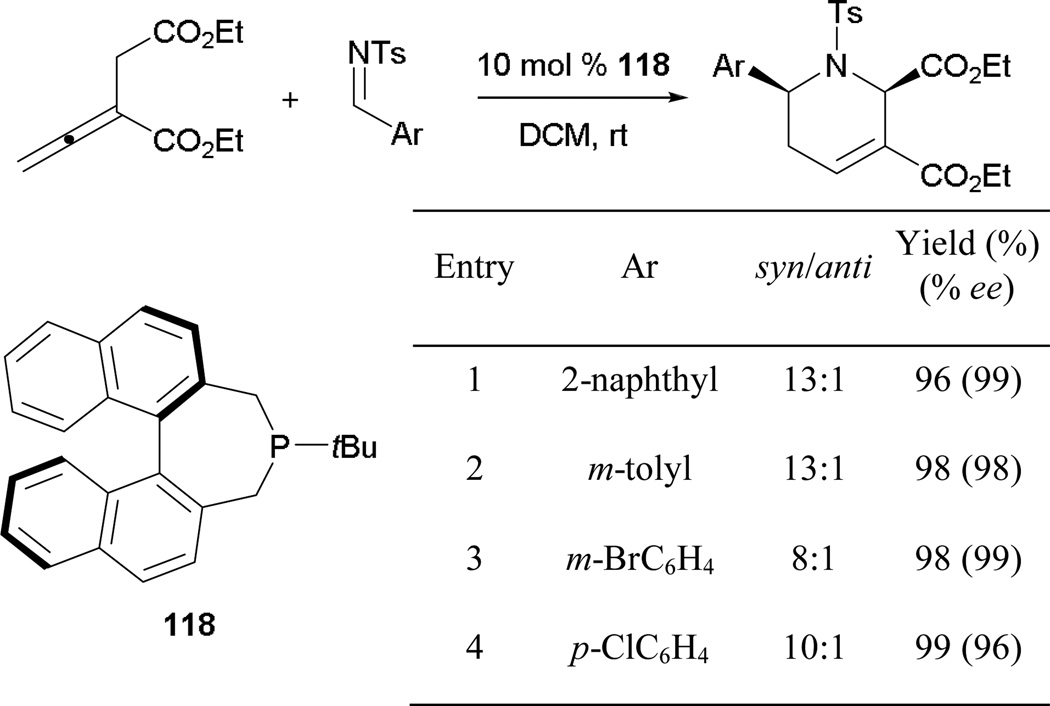
About a decade after Zhang’s seminal report on asymmetric [3 + 2] annulation, Fu’s group expanded the scope of the enantioselective allene–alkene [3 + 2] annulation through the employment of Gladiali’s phosphepine 118 (Scheme 77).68 They formed several cyclopentenes in good yields and with high enantiomeric excesses. Interestingly, unlike the case in many other [3 + 2] annulations, cyclopentenes were isolated in favor of the γ-addition products, presumably because of unfavorable steric interactions between the allenyl ester and the β-substituent (R1) of the enone.
Scheme 77.
Asymmetric formation of cyclopentenes, catalyzed by Gladiali’s phosphepine 118.
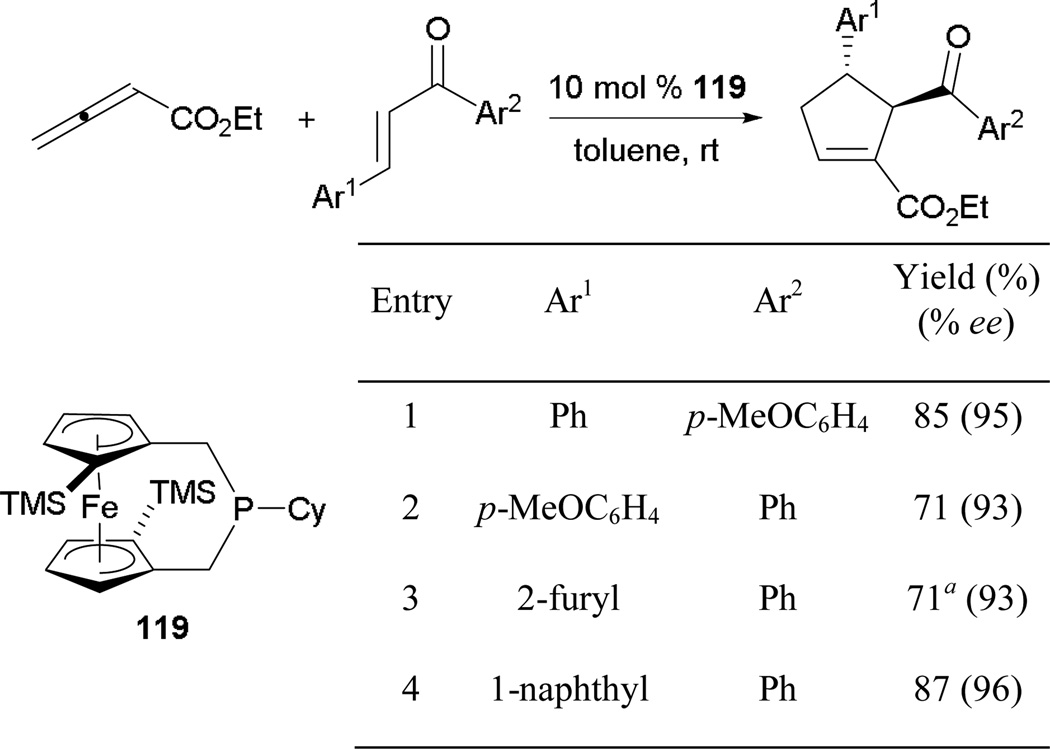
More recently, Marinetti and co-workers employed a chiral ferrocene as a backbone upon which to construct the FerroPHANE 119 catalyst, successfully achieving great asymmetric induction when forming cyclopentenes (Scheme 78).69 Similar to Fu’s study, they isolated cyclopentenes in great yields after exclusive γ-addition, with excellent enantioselectivities. The use of an arylidene ketone as a reaction partner is important in controlling the regiochemistry of the reaction. Almost an equal mixture of both the α and γ adducts was obtained when a vinyl ketone was used as the electrophile.
Scheme 78.
Asymmetric formation of cyclopentenes using FerroPHANE 119.
a Reaction performed in acetone.
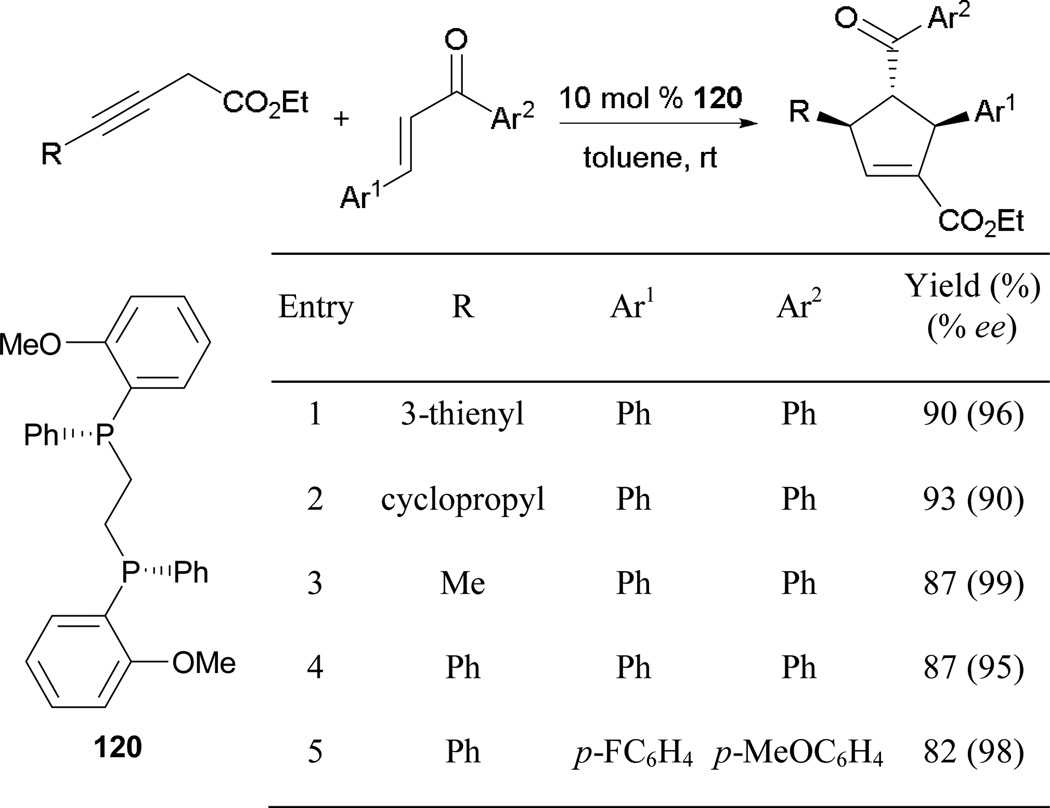
When it comes to enantioselective phosphine catalysis, most of the tested chiral catalysts have been monodentate phosphines. From a mechanistic point of view, reactions based on phosphine catalysis have typically involved only one phosphine molecule and the use of bidentate phopshines would appear to offer no clear advantage. Although Kwon had reported enantioselective allene–imine [4 + 2] annulation using (S,S)-DIPAMP at 34% ee,36 it was not until 2010 that Sampath and Loh disclosed the use of (R,R)-DIPAMP 120 as a phosphine catalyst, en route to functionalized cyclopentenes in great efficiencies (Scheme 79).70 Notably, they employed 3-alkynoates as reaction partners, instead of the traditional 2-butynoates or 2,3-butadienoates. Furthermore, they obtained the α-adducts of the cyclopentenes exclusively in excellent yields and with excellent enantiomeric ratios. Remarkably, this route also affords highly functionalized tetra-substituted cyclopentenes, which had been unobtainable using previous strategies.
Scheme 79.
Asymmetric formation of cyclopentenes, catalyzed by (R,R)-DIPAMP.
6.1.2. Asymmetric Phosphine-Catalyzed MBHAD–Alkene [3 + 2] Annulation
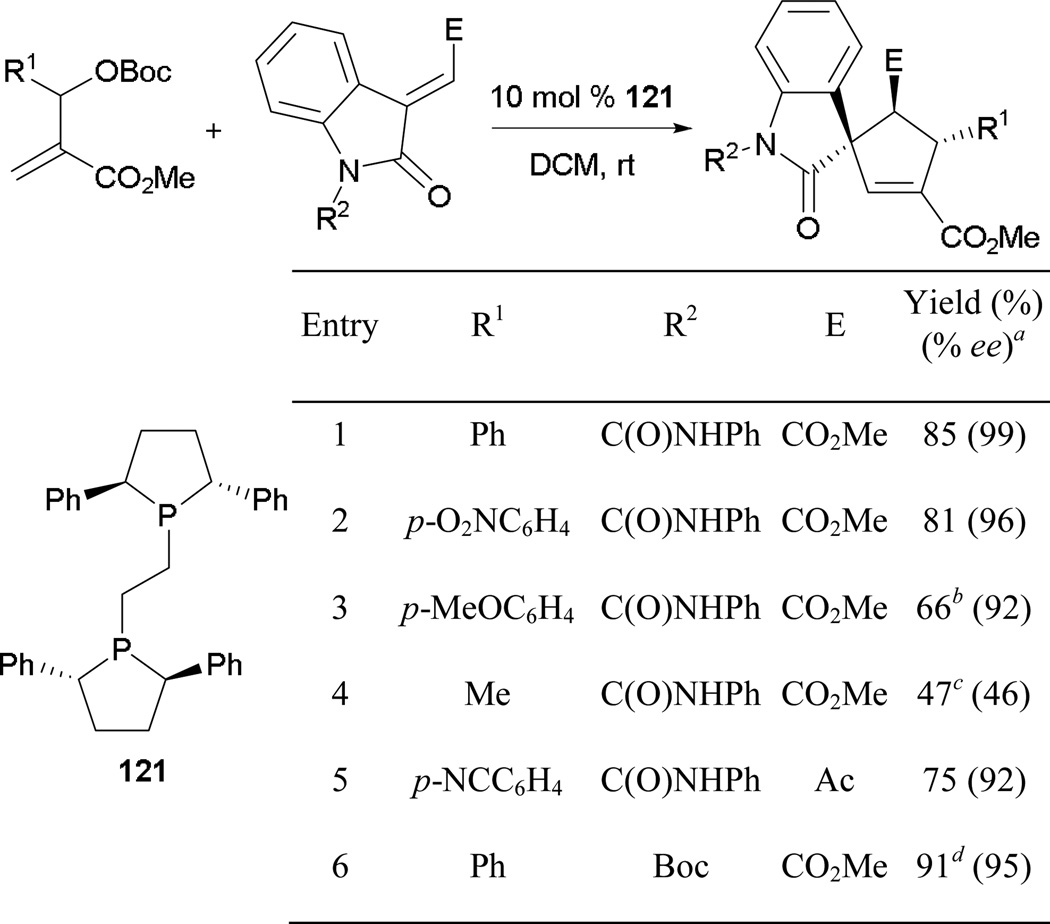
An asymmetric variant of the MBHAD–alkene [3 + 2] annulation was also developed by the Barbas group.71 To access functionalized spirocyclopenteneoxindoles, Barbas and co-workers utilizes MBHADs to undergo annulation with various 2-oxindolylidene esters in the presence of (+)-Ph-BPE 121 as the catalyst (Scheme 80). Generally, they achieved high yields and excellent enantioselectivities when aryl MBHADs were reaction partners (entries 1 and 2). Significant drops in both product yield and enantiomeric excess occured with an MBHAD bearing an alkyl substituent (entry 4). The urea functionality from the protected 2-oxindolylidene ester forms an intramolecular hydrogen bond with the adjacent carbonyl group, prompting an increase in enantioselectivity. In the absence of such a rigidifying element, the reaction must be performed at a much lower temperature to maintain the same level of efficiency (entry 6).
Scheme 80.
Asymmetric formation of spirocyclopenteneoxindoles, catalyzed by (+)-Ph-BPE.
a Yield and ee of the major diastereoisomer. b Dr 3.7:1:0.7:0.04. c Dr 2:1:0.2:0.2. d Reaction performed with 20 mol % 121 at −20 °C.
6.1.3. Asymmetric Phosphine-Catalyzed Allene–Imine [4 + 2] Annulation
Asymmetric allene–imine [4 + 2] has also been studied by Fu.72 Gladiali’s phosphepine 118, which had been used to induce asymmetry for Lu’s [3 + 2] annulation, also serves as an ideal catalyst for Kwon’s [4 + 2] annulation, affording functionalized tetrahydropyridines in excellent enantiomeric excess (Scheme 81). Across a range of aryl aldimines bearing different substituents with varying electronic properties, tetrahydropyridines were isolated in exceptional yields and enantioselectivities, although 2-vinylidenesuccinate was required for great results.
Scheme 81.
Asymmetric formation of tetrahydropyridines, catalyzed by Gladiali’s phosphepine 118.
6.1.4. Asymmetric Phosphine-Catalyzed γ-Umpolung Addition
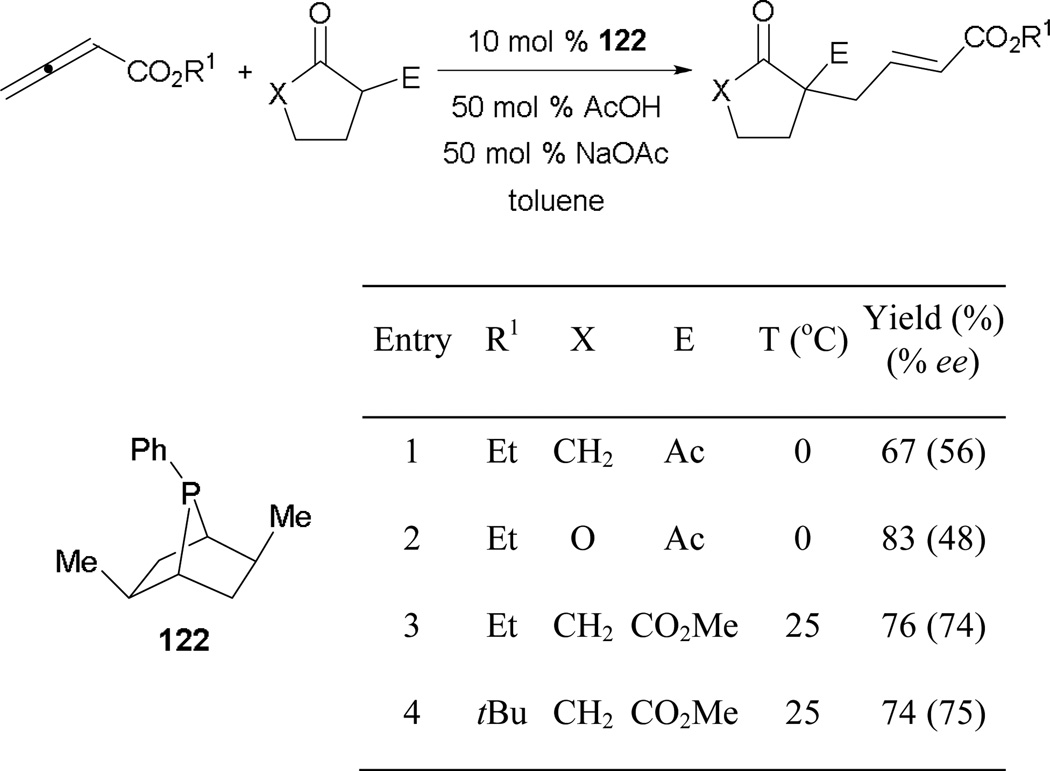
Soon after the report of an asymmetric version of Lu’s [3 + 2] annulation, the Zhang group utilized an analogue of catalyst 117 to mediate the first γ-umpolung addition enantioselectively.73 Good results have been reported when using the chiral phosphabicyclo[2.2.1]heptane 122 in conjunction with several 1,3-dicarbonyl pronucleophiles (Scheme 82). Reminiscent of Trost’s results, a buffer system of acetic acid/sodium acetate was employed to facilitate proton transfers. Unlike the situation in the formation of cyclopentenes, the steric bulk of the alkoxide group of the allenoate plays no role in influencing the selectivity of this reaction (entry 4).
Scheme 82.
Asymmetric formation of γ-substituted enoates catalyzed by the phosphabicyclo[2.2.1]heptane 122.
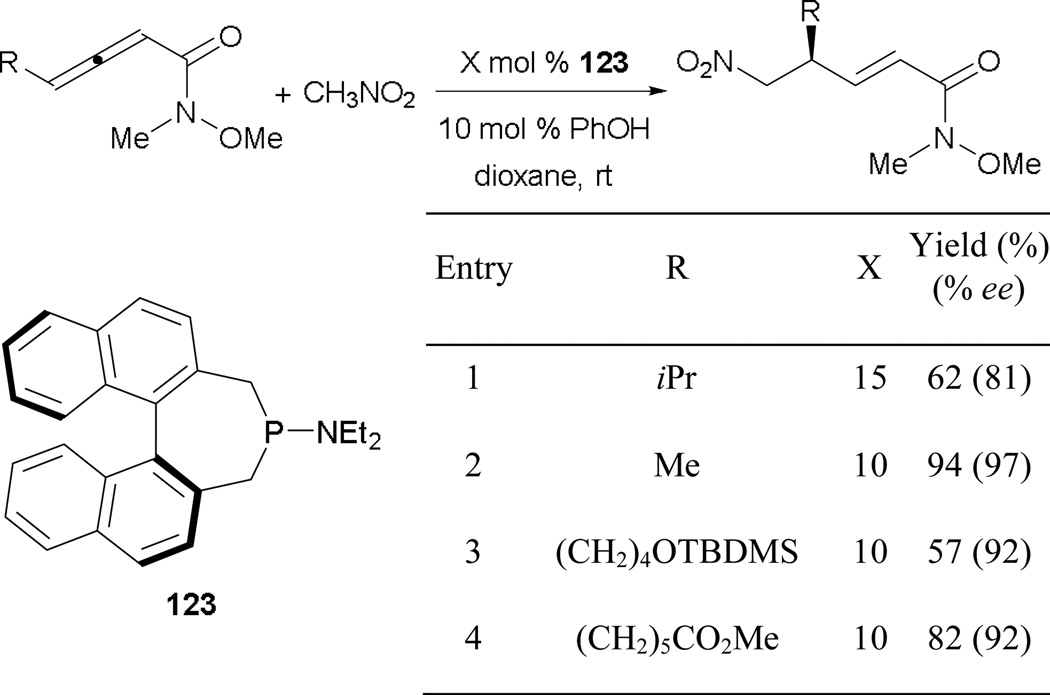
Aside from Zhang’s initial paper in 1998, there have been no reports of highly efficient enantioselective γ-umpolung additions. Recently, Fu and co-workers established an effective route for nitromethane to undergo γ-umpolung addition in a highly efficient manner.74 There are a few features in their report that differ from many of the other γ-umpolung reactions (Scheme 83). First, the reaction employs an uncommon phosphinamine 123 as the catalyst. Second, a Weinreb amide group is installed as an activating functionality on the allene. Third, this strategy utilizes phenol as an additive to assist the proton transfer process, instead of the traditional acetic acid/sodium acetate system.
Scheme 83.
Asymmetric formation of γ-substituted acrylamides catalyzed by the phosphinamine 123.
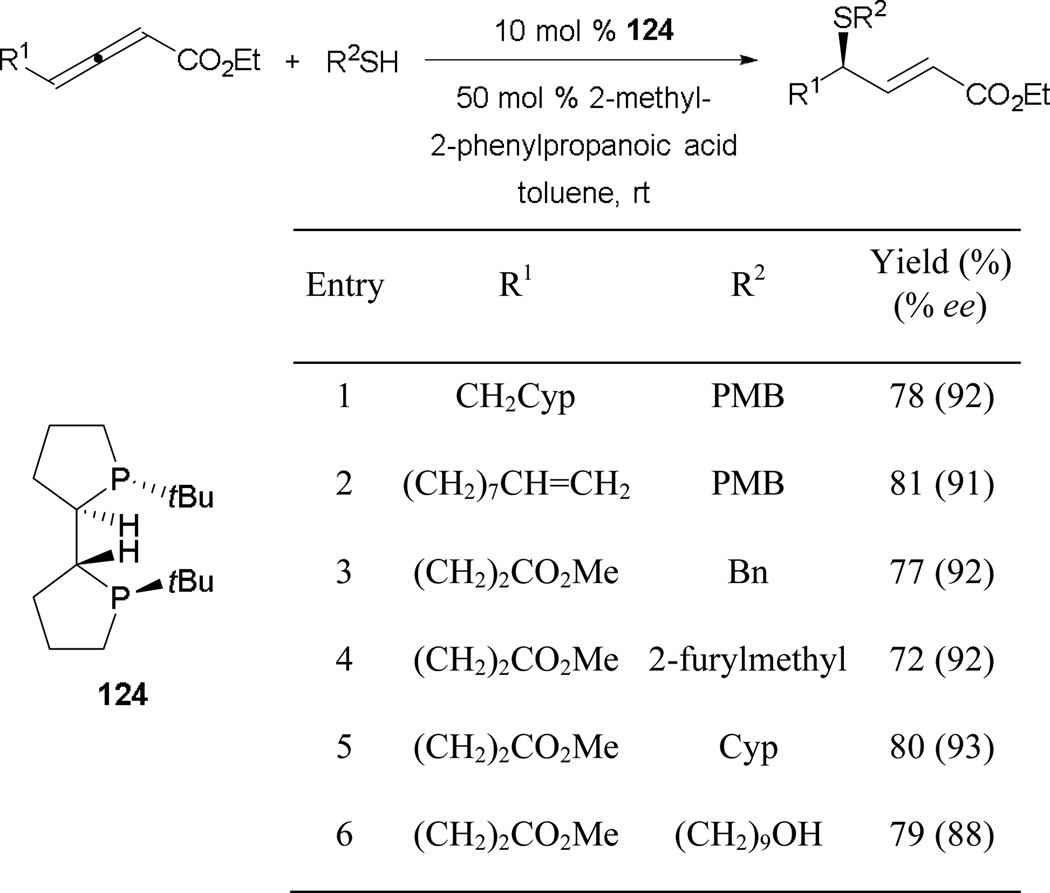
Shortly after their initial success in performing γ-umpolung addition with nitromethane, the Fu group demonstrated that efficient γ-umpolung additions with thiol-based pronucleophiles are also feasible.75 They adopted the less-common bidentate phosphine TangPhos 124 (Scheme 84). This catalyst, designed by Zhang as a ligand on rhodium for asymmetric hydrogenation,76 is well suited to catalyzing γ-umpolung reactions of thiols. Similar to the case in the γ-umpolung addition of nitromethane, they used an uncommon additive, 2-methyl-2-phenylpropanoic acid, to facilitate proton transfer. The reaction is widely compatible with several functionalities, including alkene, ester, and heteroaryl groups. Exclusive addition at the thiol-terminus is observed when 7-mercaptoheptanol is employed (entry 6).
Scheme 84.
Asymmetric formation of γ-substituted enoates, catalyzed by TangPhos.
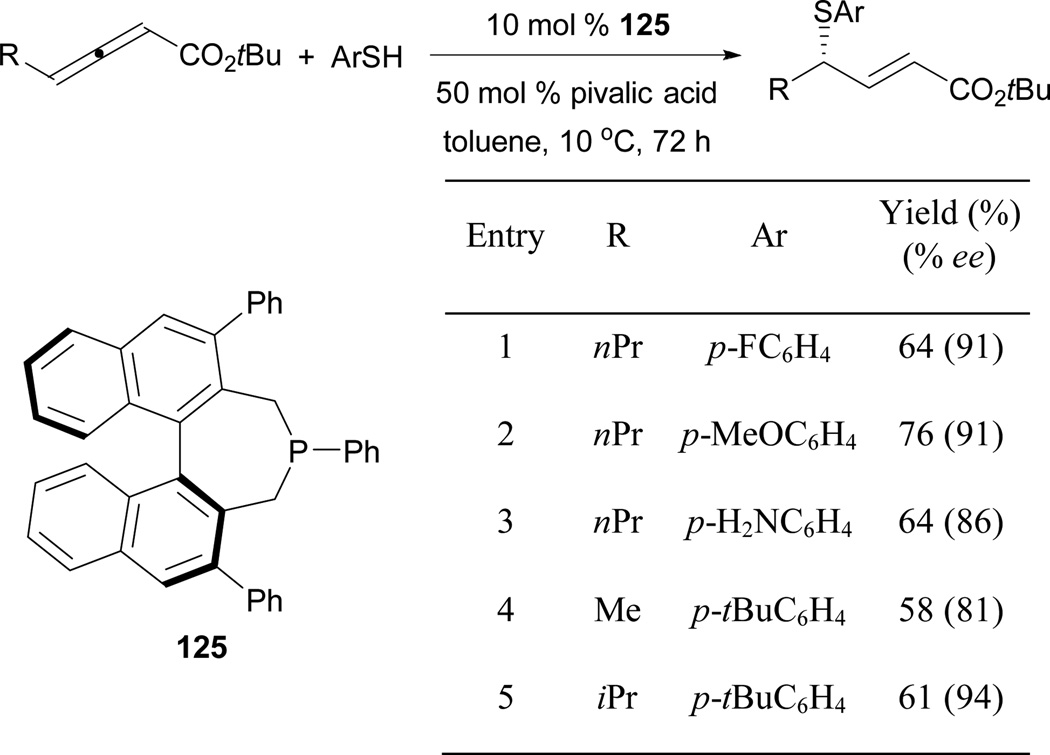
The Fu group also reported γ-umpolung additions of aryl thiols when using the chiral phosphepine 125 (Scheme 85).77 Here, they employed the highly sterically demanding chiral phosphepine 125 in conjunction with pivalic acid to enhance the proton transfers, providing γ-substituted enoates in moderate yields. They also decreased the reaction temperature to ensure optimal efficiency.
Scheme 85.
Asymmetric formation of γ-substituted enoates, catalyzed by the phosphepine 125.
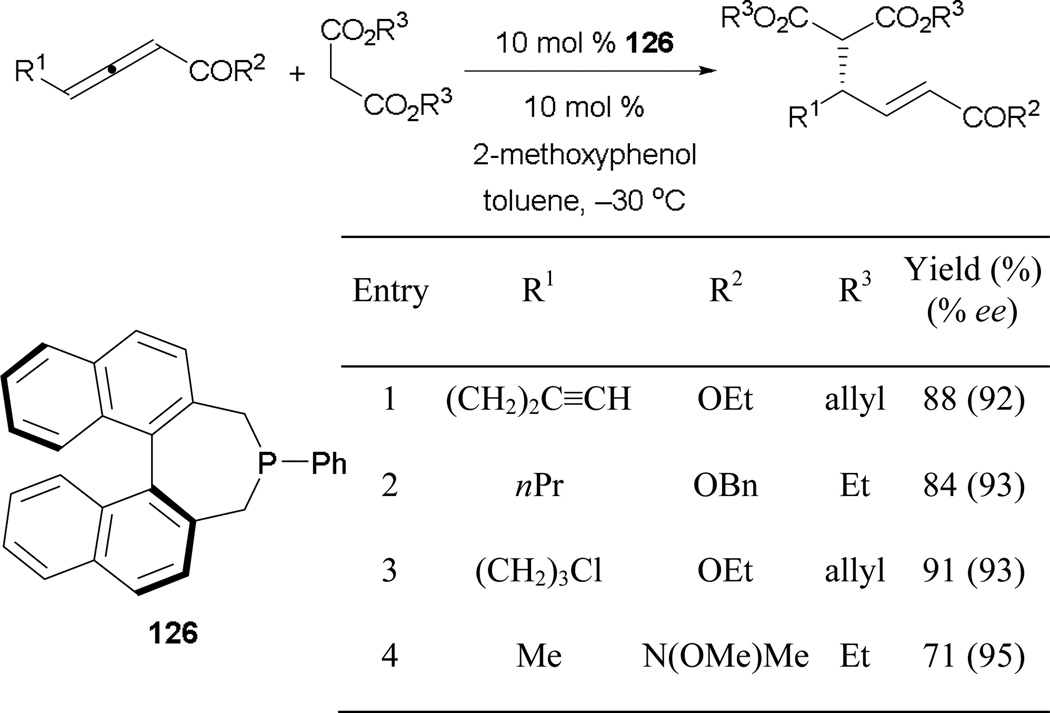
To further expand the scope of carbon-centered pronucleophiles in γ-umpolung reactions, the Fu group demonstrated an efficient transformation involving alkyl malonates adding into allenoates and allenamides with high enantioselective control, mediated by chiral 126 (Scheme 86).78 Unlike previous examples, promoting efficient proton transfer required only a small amount (10 mol %) of 2-methoxyphenol and the reaction could be conducted at much lower temperatures to ensure good enantiocontrol.
Scheme 86.
Asymmetric formation of γ-substituted acrylates and acrylamides, catalyzed by the phosphepine 126.
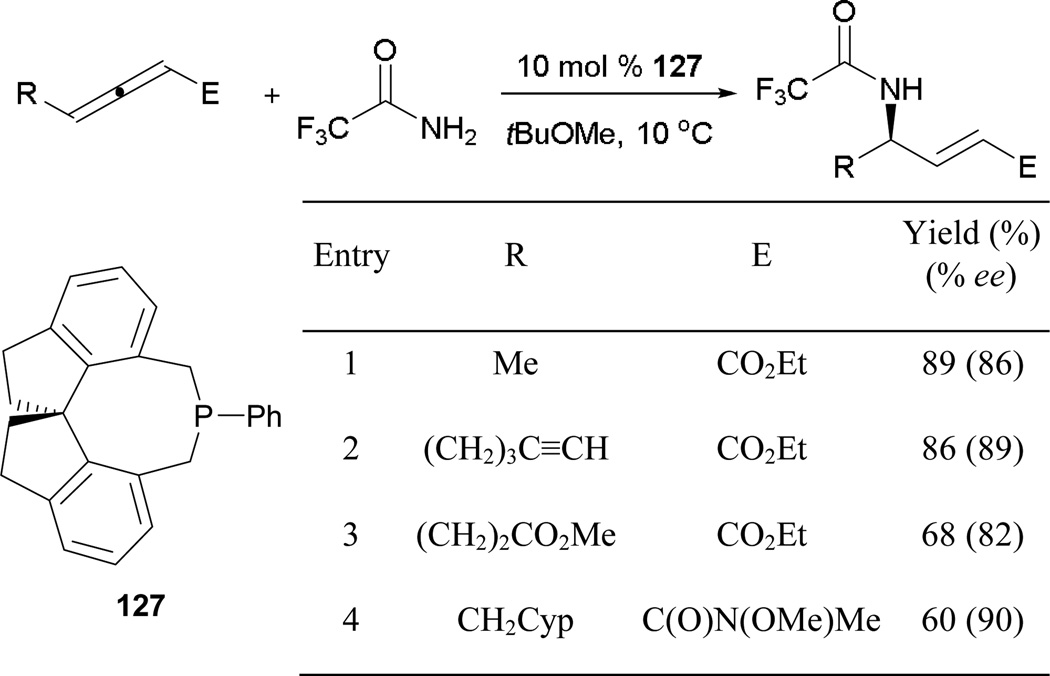
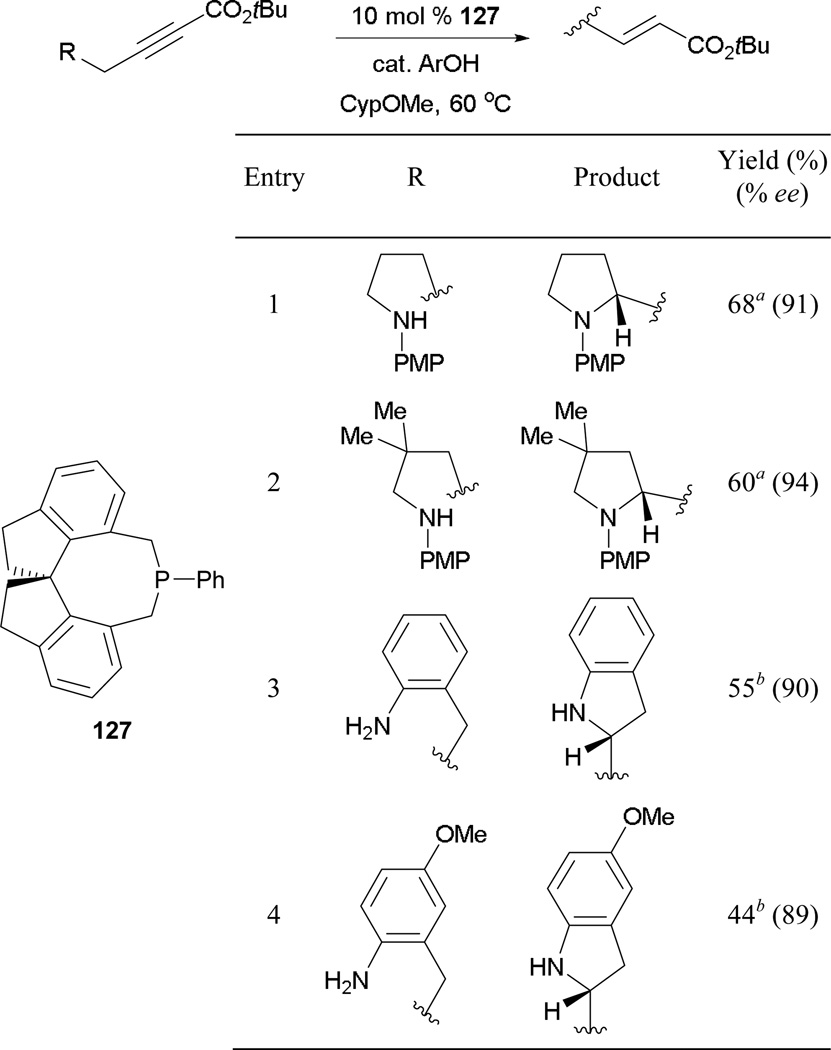
Recently, Fu and co-workers reported the first examples of γ-umpolung reactions between allenoates and nitrogen pronucleophiles.79 Here, they employed the chiral spirophosphepine 127 as the catalyst, forming functionalized γ-amino acrylates (Scheme 87). There are a few interesting features of this study. First, it is the first report of the spirophosphepine 127 acting as the optimal catalyst for a γ-umpolung reaction. Second, the reaction did not require the use of an external acidic additive to promote efficient proton transfers. Several γ-amino acrylates were prepared in high yields and with high enantio-control (entries 1 and 2). Although an allenoate bearing a distal methyl ester group or an allenamide resulted in lower yields, enantioselectivities remained high (entries 3 and 4).
Scheme 87.
Asymmetric formation of γ-amino acrylates and acrylamides, catalyzed by the spirophosphepine 127.
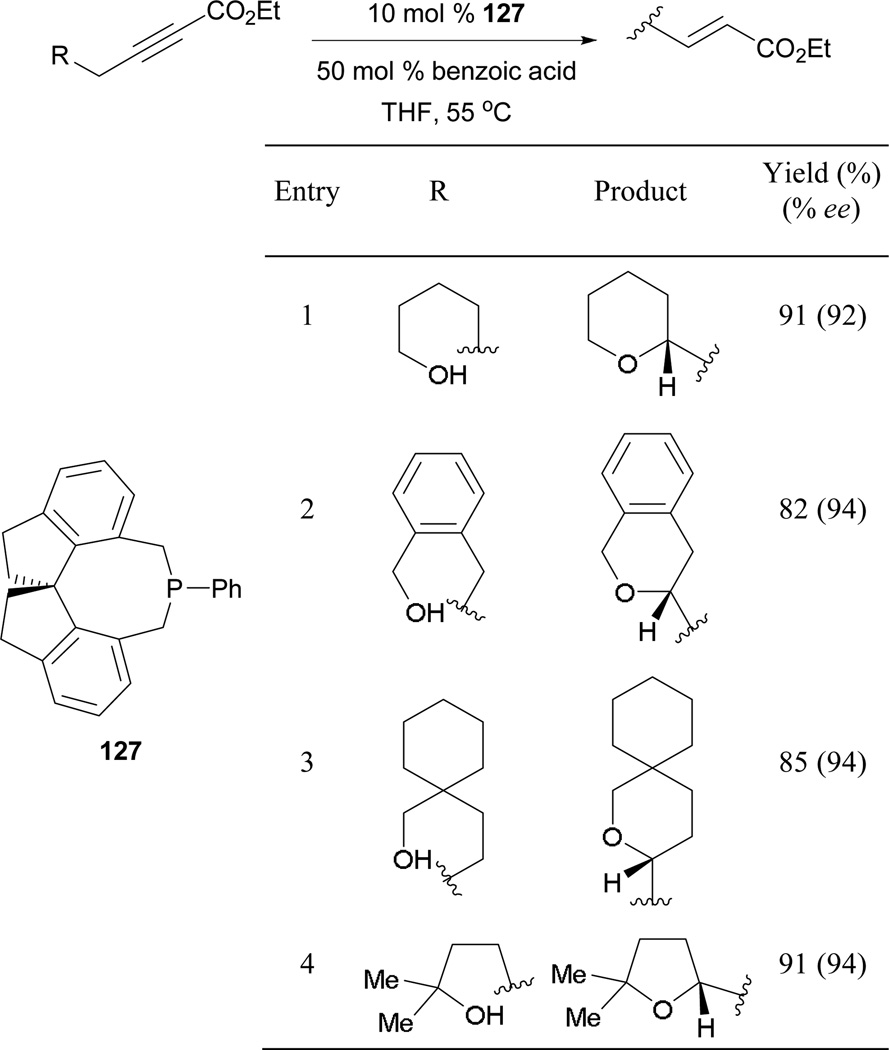
6.1.5. Asymmetric Phosphine-Catalyzed Intramolecular γ-Umpolung Addition
Since Trost’s initial demonstration of intramolecular γ-umpolung addition, there have been no reports of asymmetric variants until the Fu group reported one in 2009.80 Utilizing the spirophosphepine 127 as the catalyst, they obtained high yields of tetrahydropyran and tetrahydrofuran derivatives with high enantioselectivities (Scheme 88). One notable feature of this approach is the use of the monodentate spirophosphepine 127 instead of the original bidentate phosphine used by Trost to prevent alkyne isomerization.
Scheme 88.
Asymmetric formation of tetrahydropyrans and tetrahydrofurans, catalyzed by the spirophosphepine 127.
Remarkably, the same spirophosphepine 127 can also be used for efficient intramolecular γ-umpolung additions of tethered aniline-based pronucleophiles.79 Moderate to good yields of pyrrolidines and 2,3-dihydroindoles have been isolated with excellent enantiomeric excesses (Scheme 89). Good yields of pyrrolidines were isolated with excellent enantiocontrol (entries 1 and 2). In the case of 2,3-dihydroindoles, the annulation products were obtained in lower yields while maintaining the same level of enantioselectivity (entries 3 and 4).
Scheme 89.
Asymmetric formation of pyrrolidines and 2,3-dihydroindoles, catalyzed by the spirophosphepine 127.
a Reaction performed with 50 mol % of 2,4- dimethoxyphenol. b Reaction performed with 20 mol % of 2-fluoro-6-methoxyphenol.
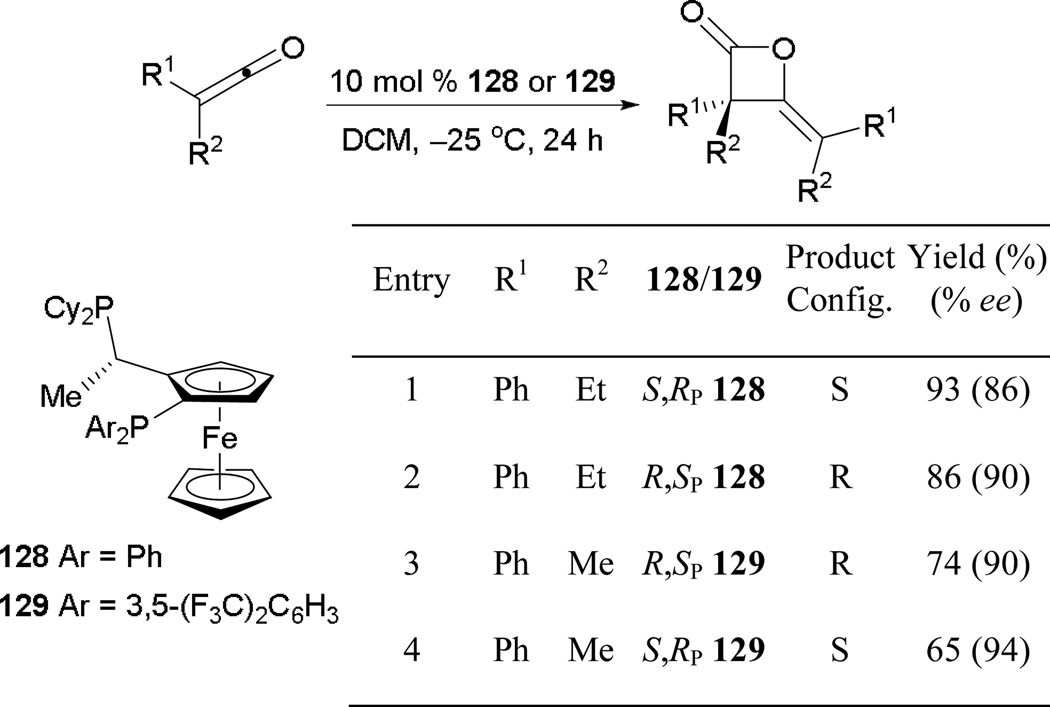
6.1.6. Asymmetric Phosphine-Catalyzed Homodimerization of Ketoketenes
Other than asymmetric annulations and umpolung additions, homodimerization of ketoketenes with chiral phosphines hsas also been developed. Kerrigan and co-workers performed the successful dimerization of ketoketenes (Scheme 90).81 Under the reaction conditions, they formed 4-arylidene β-lactones in good yields with exclusive Z-olefin geometry and high enantioselectivities. Furthermore, the configuration of the β-lactones was controlled well by the catalyst.
Scheme 90.
Asymmetric formation of β-lactones, catalyzed by Josiphos.
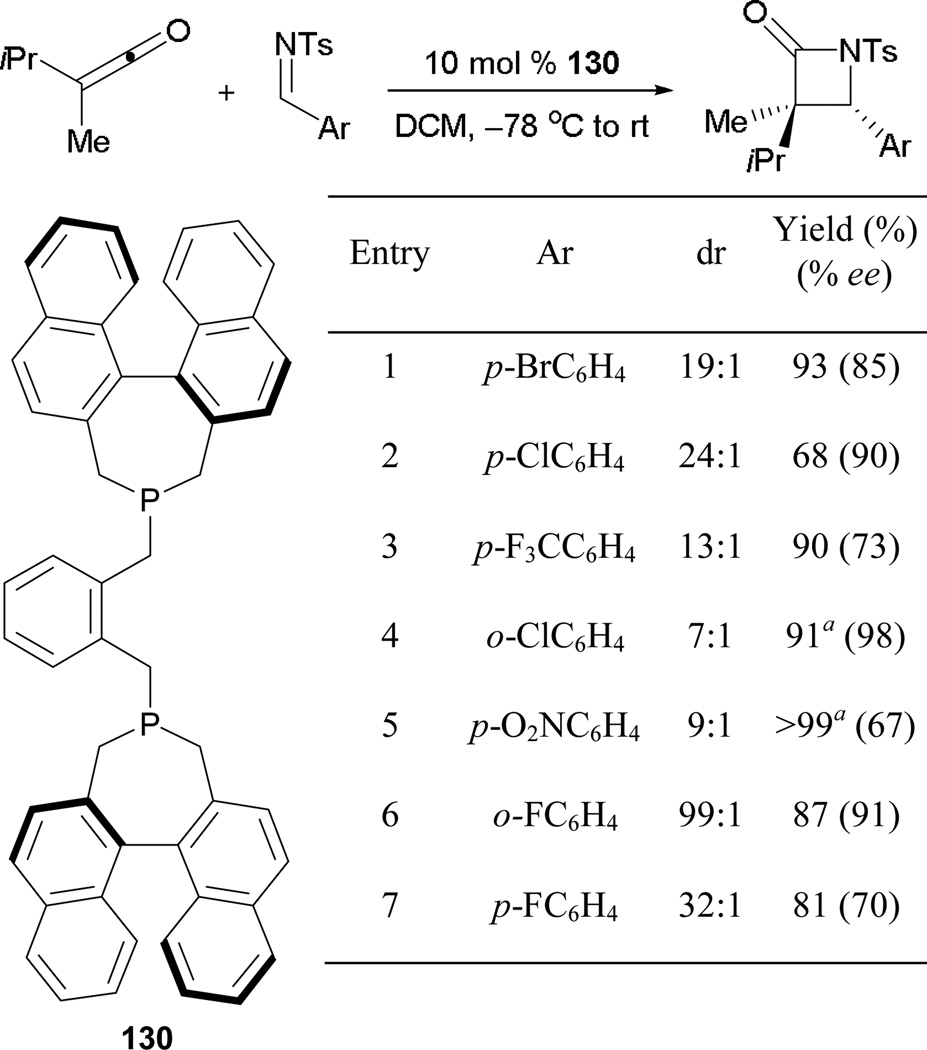
6.1.7. Asymmetric Phosphine-Catalyzed Ketoketene–Imine [2 + 2] Annulation
Shortly after the report of ketoketene dimerization, Kerrigan demonstrated the formation of β-lactam derivatives from ketoketenes and imines (Scheme 91).82 When using imines bearing electron-poor substituents, they synthesized functionalized β-lactams in high yields, accompanied by good diastereo- and enantiocontrol. The presence of a strongly electron withdrawing nitro group led to a quantitative yield of the product, but with a significant drop in enantioselectivity (entry 5).
Scheme 91.
Asymmetric formation of β-lactams using BINAPHANE.
a Reaction performed with 15 mol % of 130
6.2. Chiral Multifunctional Phosphines
Most of the chiral phosphines covered in this Section stabilize the reaction intermediates through hydrogen bonding interactions as an additional means of molecular structural reinforcement. The majority of these chiral phosphines are derived from α-amino acids as the source of chirality. Furthermore, methods for preparing chiral phosphines from amino acids are also well established, allowing the rapid generation of novel multifunctional phosphine catalysts.
6.2.1. Asymmetric Phosphine-Catalyzed Allene–Alkene [3 + 2] Annulation
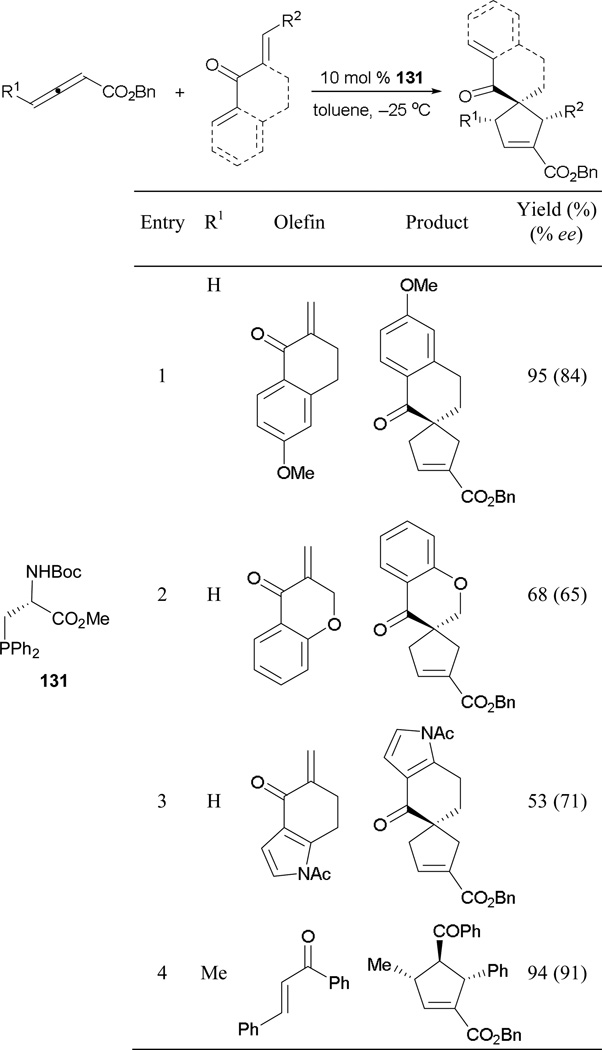
The first use of a multifunctional chiral phosphine was reported by Miller in 2007.83 Although the alanine-based phosphine was first developed by Gilbertson for metal catalysis,84 this catalyst was well suited to induce asymmetry in Lu’s [3 + 2] annulation. Envisioned as providing an enzyme-like binding pocket, the phosphinyl alanine 131 furnished cyclopentenes in great yields and with excellent enantioselectivities (Scheme 92). Here, the use of sterically hindered benzyl allenoates enhances regio- and enantiocontrol. Although the annulation product could be obtained from a heteroaryl tetralone, a noticeable drop in enantioselectivity occured (entry 2). Unlike Marinetti’s case where an equal mixture of α- and β-adducts was obtained, here a single regioisomer was isolated when using benzyl-2,3-pentadienaote and chalcone as reaction partners (entry 4).
Scheme 92.
Asymmetric formation of cyclopentenes, catalyzed by the phosphinyl alanine 131.
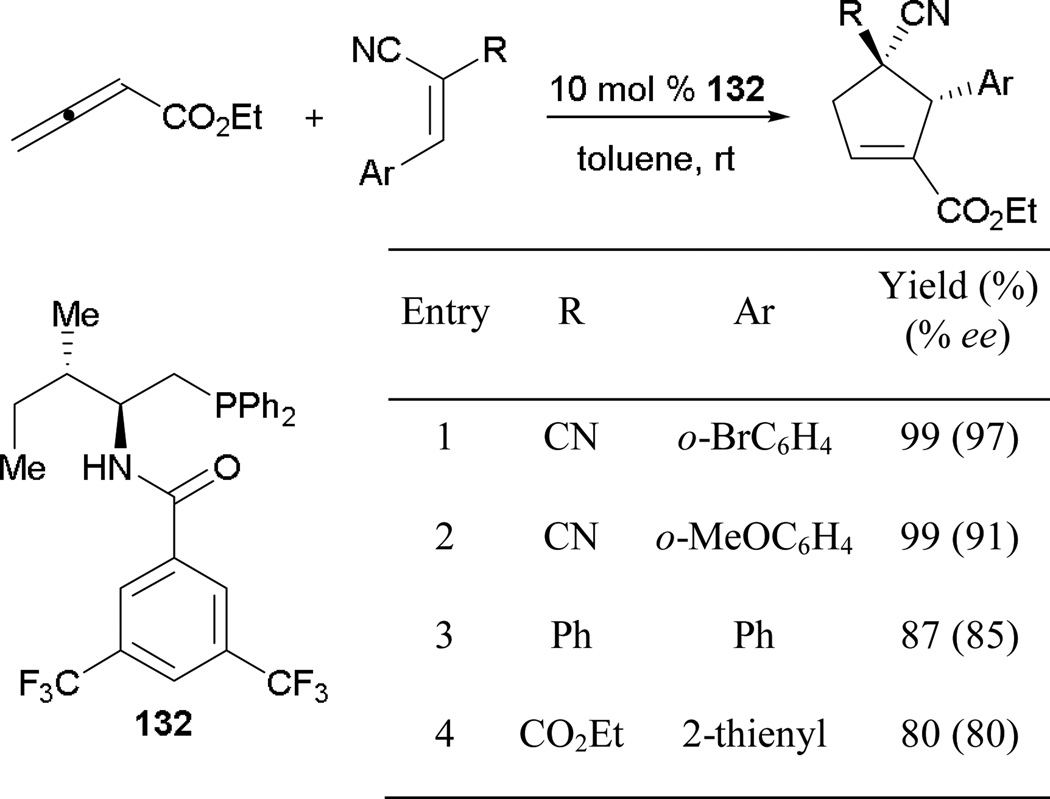
Three years after Miller’s reported use of an amino acid-based phosphine, Zhao provided a gateway for access to many cyclopentenes with high enantiopurity (Scheme 93).85 Although Zhao’s catalyst 132 is also derived from amino acids, it differs from Miller’s approach in that the carboxylic acid is converted into a diphenylphosphinylmethyl group. This method allows reactions to be performed at room temperature, simplifying synthetic operations. Consistent results, with excellent yields and selectivities, were obtained when using arylidenemalononitriles bearing either electron-rich or -poor substituents (entries 1 and 2). Slight erosions of both yield and enantiomeric excess occurred when employing ethyl-2-thienylmethylidenecyanoacetate as a reaction partner (entry 4).
Scheme 93.
Asymmetric formation of cyclopentenes using N-acyl amino phosphine.
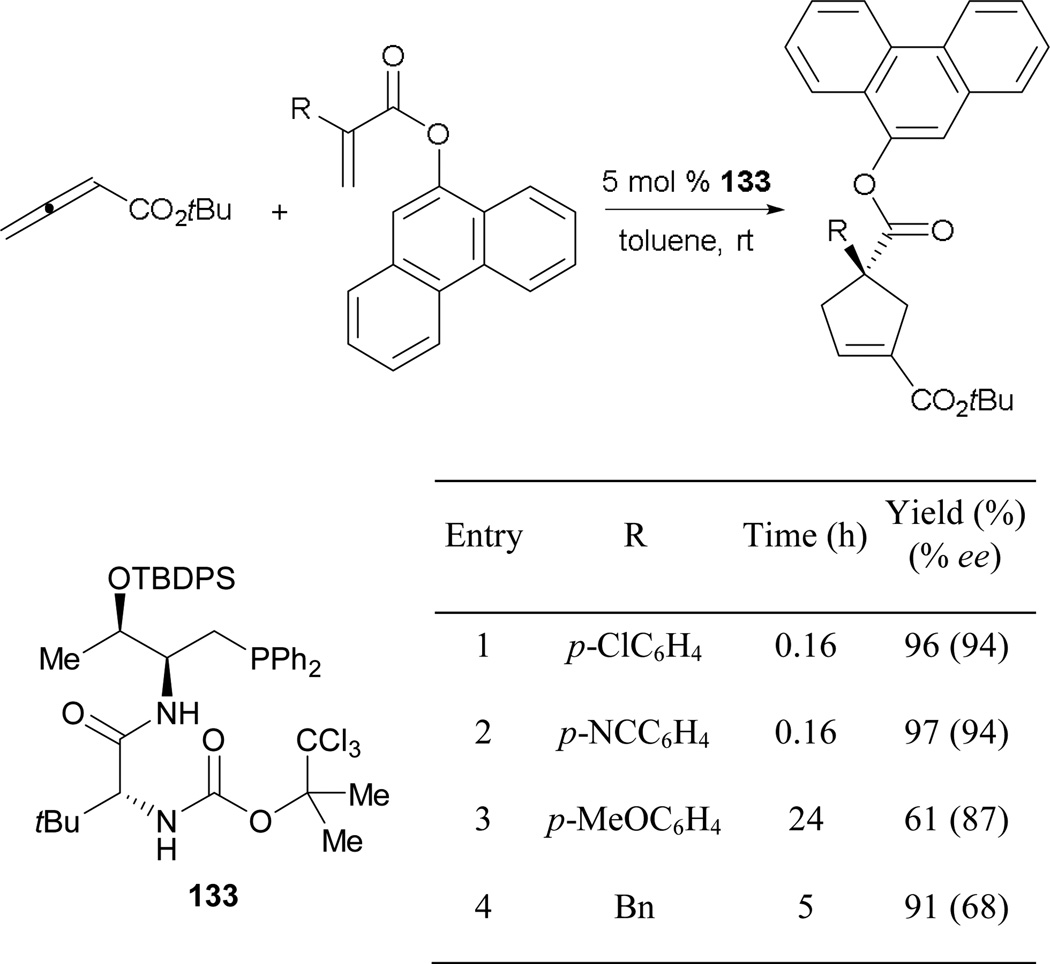
Expanding the repertoire of asymmetric phosphine catalysis with multifunctional phosphines, Lu demonstrated the formation of 4,4'-disubstituted cyclopentenes catalyzed by the dipeptide-based chiral amino phosphine 133 (Scheme 94).86 Similar to Zhao’s catalyst, the phosphorus center is installed at the carboxylic acid terminus for ease of generating libraries of catalysts. In this system, the electronic properties of the activated olefins have drastic effects on the reaction time, yield, and enantioselectivity. Higher efficiencies are achieved when the acrylates feature electron-poor aryl units (entries 1 and 2). With a strongly donating methoxy group, the yield of the reaction decreases, with a minor drop in enantiomeric excess (entry 3). With 2-benzylacrylate as a reaction partner, the corresponding cyclopentene was isolated in high yield, but with moderate enantiomeric excess (entry 4).
Scheme 94.
Asymmetric formation of cyclopentenes, catalyzed by the chiral dipeptide phosphine 133.
6.2.2. Asymmetric Phosphine-Catalyzed Allene–Imine [3 + 2] Annulation
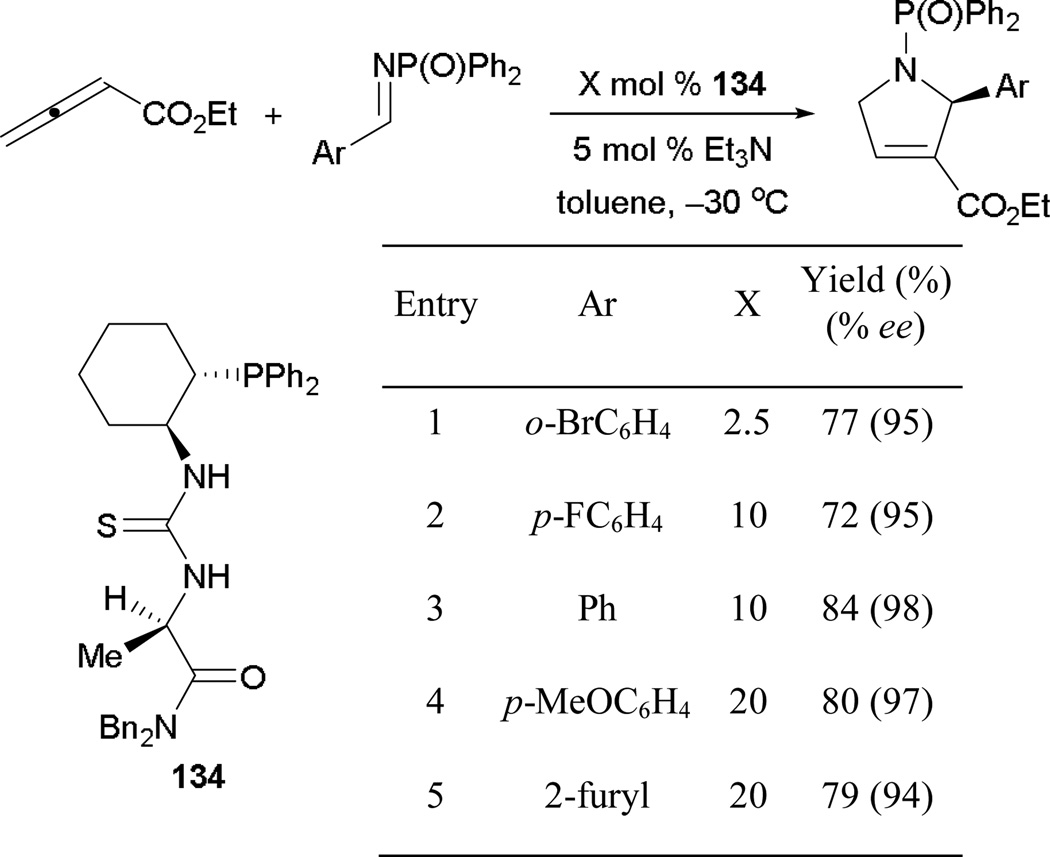
Although many studies of asymmetric allene–alkene [3 + 2] annulations have been reported, enantioselective formation of dihydropyrroles remains difficult and without significant accomplishment. The use of both monodentate and bidentate phosphines has led to minor asymmetric inductions with low yields.87 It was not until 2008, when Jacobsen and co-workers introduced their thiourea-based multifunctional chiral phosphine, that high yields and enantiocontrol could be achieved in forming functionalized dihydropyrroles (Scheme 95).88 With the chiral phosphinothiourea 134 and sterically demanding N-diphenylphosphinoyl imines, they synthesized dihydropyrroles bearing both electron-rich or -poor substituents in high yields and with excellent enantiomeric excesses. Arylimines with electron-donating functionalities required a higher catalyst loading to maintain good reaction efficiencies (entries 4 and 5). Interestingly, the addition of water and triethylamine accelerated the reaction, while suppressing the formation of undesired side products. Increasing reaction efficiencies through the addition of water has been studied computationally by Yu, Dudding, and Kwon.20a,89
Scheme 95.
Asymmetric formation of dihydropyrroles, catalyzed by the chiral phosphinothiourea 134.
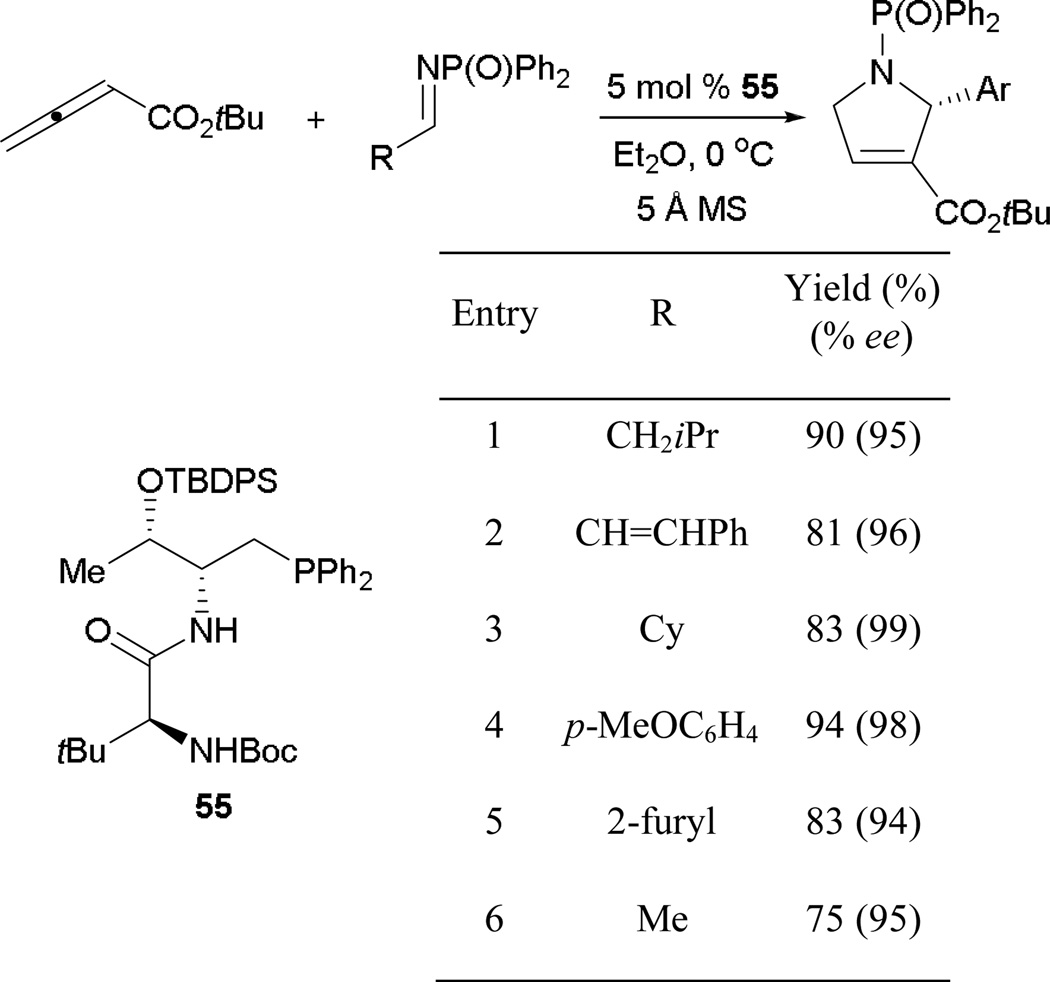
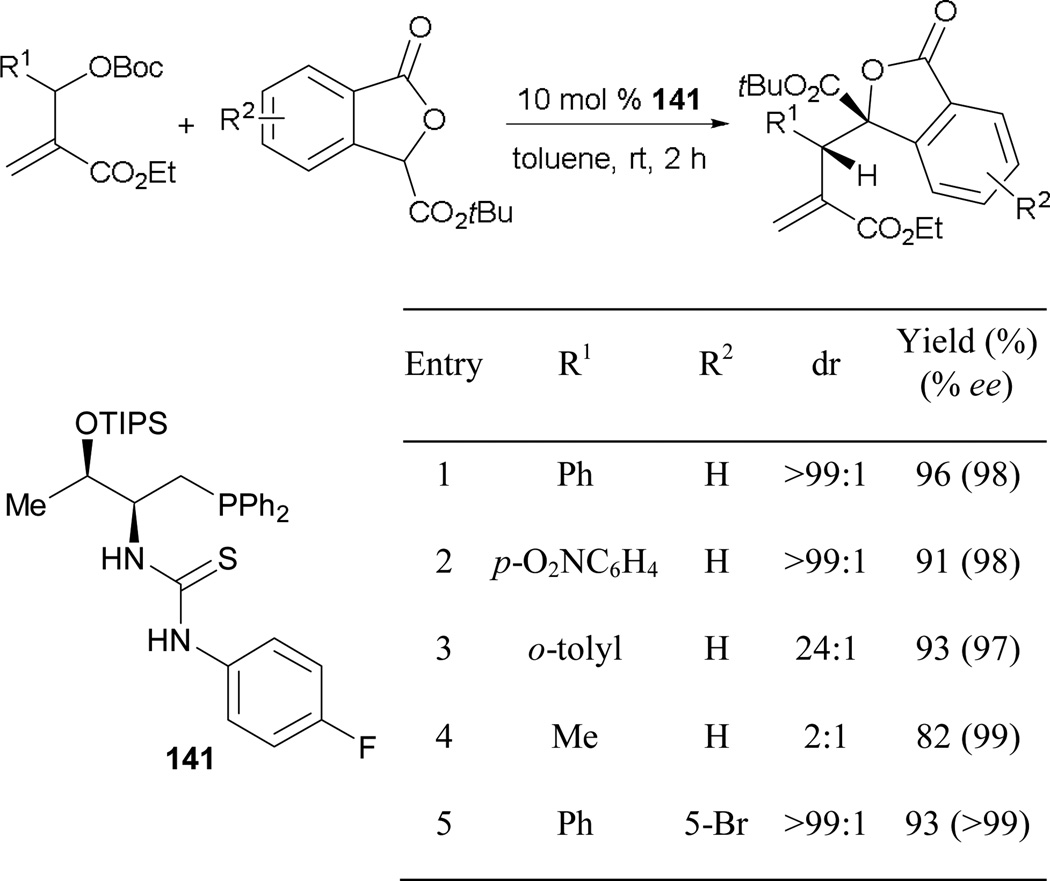
6.2.3. Asymmetric Phosphine-Catalyzed Allene–Alkylimine [3 + 2] Annulation
More recently, the Lu group expanded the use of chiral dipeptide phosphines to catalyzing allene–alkylimine [3 + 2] annulations.33 Similar to Jacobsen’s work, they employed sterically encumbered diphenylphosphinoyl imines to enhance enantioselectivity (Scheme 96). High degrees of enantiomeric excess were achieved across various alkylimines along with high isolated yields (entries 1–3). Arylimines are also compatible under the reaction conditions providing the same levels of efficiency (entries 4 and 5). Remarkably, simple acetylimine also undergoes annulation with only a minor erosion in yield (entry 6).
Scheme 96.
Asymmetric formation of dihydropyrroles, catalyzed by the chiral dipeptide phosphine 55.
6.2.4. Asymmetric Phosphine-Catalyzed MBHAD–Alkene [3 + 2] Annulation
The reactions between MBHADs and alkenes can also be catalyzed by chiral multifunctional catalysts. Shi and co-workers demonstrated that trifluoroethylidene malonates as coupling partners undergo asymmetric [3 + 2] annulations in the presence of the chiral thiourea phosphine 135 (Scheme 97).90 The transformation proceeds smoothly to give several cyclopentenes in high yields and enantiomeric excesses for MBHADs bearing electron-poor aryl groups (entries 1–3). No product was isolated, however, when a less-activated enoate system was employed (entry 5).
Scheme 97.
Asymmetric formation of cyclopentenes, catalyzed by the chiral thiourea phosphine 135.
In addition to Barbas’s example using (+)-Ph-BPE (121), spirocyclopenteneoxindoles can also be synthesized asymmetrically using the chiral thiourea phosphine 136 reported by Lu (Scheme 98).5eeld (%) (% ide phosphine.are employed to generate functionalized cyclohexenes with excellent efficiencies (Scheme 80).s. and e91 MBHADs bearing p-tolyl, 2-thienyl, and p-cyanophenyl groups undergo annulations, affording their products in high yields (entries 1–3). For the 2-thienyl substituent, however, an equal mixture of α- and γ-adducts was acquired (entry 3).
Scheme 98.
Asymmetric formation of spirocyclopenteneoxindoles, catalyzed by the chiral dipeptide phosphine 136.
6.2.5. Asymmetric Phosphine-Catalyzed Allene–Imine [4 + 2] Annulation
Zhao demonstrated excellent conversions in the asymmetric formation of tetrahydropyridines, catalyzed by the N-acyl amino phosphine 132 (Scheme 99).92 Reminiscent of Fu’s results with Gladiali’s phosphepine 118, excellent conversions were obtained with 2-vinylidenesuccinate as a reaction partner. Across several arylimines bearing electron-donating or -withdrawing groups, great yields and high levels of enantioselectivity were obtained from isolated tetrahydropyridines (entries 1–3). Although a slight erosion in diastereoselectivity occurred for the reaction of an imine containing an o-chloro group, the reaction efficiency remained unaltered (entry 4).
Scheme 99.
Asymmetric formation of tetrahydropyridines, catalyzed by the N-acyl amino phosphine 132.
6.2.6. Asymmetric Phosphine-Catalyzed Allene–Alkene [4 + 2] Annulation
Lu and co-workers introduced chiral amino phosphine 137 in the first asymmetric allene–alkene [4 + 2] annulation in 2012.93 They employed different combinations of arylidenemalononitriles and 2-vinylidenesuccinate as reaction partners to generate functionalized cyclohexenes with excellent efficiencies (Scheme 100). In general, the reaction is well suited for arylidenemalononitriles bearing substituents of various electronic properties, providing excellent yields and selectivities. Overall, the presence of the sterically demanding tBu group favors the generation of the cis-isomer over the trans form, except in the case with 2-furylmethylidenemalononitrile (entry 3).
Scheme 100.
Asymmetric formation of cyclohexenes, catalyzed by the chiral dipeptide phosphine 137.
Also working in the realm of asymmetric cyclohexene formation, Zhao documented the use of the N-acyl amino phosphine 138 as the ideal catalyst for smooth [4 + 2] annulations. With the 3,5-difluorobenzoyl group, catalyst 138 performed to render better efficiencies than catalyst 132 (Scheme 101).94 By varying the reaction conditions, several cyclohexene derivatives could be synthesized in great yields and with high enantiocontrol. In contrast to other examples of allene–alkene [4 + 2] annulations, Zhao employed novel arylidenecyanoacetates as well as an isobutylidenecyanoacetate in the first successful example of an aliphatic substrate undergoing annulation (entry 5).
Scheme 101.
Asymmetric formation of cyclohexenes, catalyzed by the N-acyl amino phosphine 138.
Lu also reported the asymmetric formation of functionalized spirocyclohexeneoxindoles using the same dipeptide catalyst 55 employed in the asymmetric preparation of alkyl-dihydropyrroles (Scheme 102).93 Notably, 4-Å molecular sieves enhanced the product conversion and degree of enantioselectivity. Generally, the reaction is highly diastereoselective, giving annulation adducts in high yields with excellent enantiocontrol.
Scheme 102.
Asymmetric formation of spirocyclohexeneoxindoles, catalyzed by the chiral dipeptide phosphine 55.
6.2.7. Asymmetric Phosphine-Catalyzed Michael Addition
Recently, Lu disclosed the first asymmetric phosphine-catalyzed Michael addition mediated by a chiral dipeptide phosphine (Scheme 103).95 Because phosphine-catalyzed Michael addition is believed to operate through a mechanism involving general-base catalysis, transferring chirality from the chiral phosphine to the reaction intermediate is difficult. To this end, Lu employed a multifunctional phosphine to overcome this challenge, promoting additional catalyst–substrate interactions through hydrogen bonding. The generality of the reaction is broad, across oxindoles presenting various substituents. Most often with 3-aryl oxindoles, the reaction leads to excellent yields and enantiocontrol. Lower yields and decreased enantioselectivity are observed with 3-alkyl oxindoles (entry 6). Interestingly, the catalyst 139 is closely related, in structure, to Zhao’s privileged N-acyl amino phosphine 132 with a shorter alkyl chain.
Scheme 103.
Asymmetric formation of 3,3-disubstituted oxindoles, catalyzed by the chiral dipeptide phosphine 139.
6.2.8. Asymmetric Phosphine Catalysis: Reactions of MBHADs with Nucleophiles
Among the pioneers in the field of asymmetric phosphine catalysis, Shi and co-workers introduced the use of multifunctional binaphthyl phosphines to access γ-butenolides asymmetrically.96 Although they first used 2-(trimethylsiloxy)furan as the pronucleophile, similar reactions can also be catalyzed by different chiral binaphthyl phosphines, bearing either amide or thiourea units as hydrogen bond donors, to form oxazolidinones,97a phthalimides,97b benzofuran-2-ones,97c and oxindoles.97c
Enantiomerically enriched γ-butenolides can be synthesized from the reactions of MBHADs and 2-(trimethylsiloxy)furan mediated by the chiral phosphine 140 (Scheme 104). The scope of the reaction appears to be general across MBHADs bearing either electron-poor or -rich aryl rings, providing excellent yields and selectivities (entries 1 and 2). Although an alkyl substituent is tolerated under the reaction conditions, decreased yields and enantiocontrol were observed with prolonged reaction times (entry 3). Interestingly, a dramatic drop in product conversion occured when an electronically neutral phenyl group was in place (entry 4).
Scheme 104.
Asymmetric formation of γ-butenolides, catalyzed by the chiral binaphthyl phosphine 140.
Recently, the Lu group documented the enantioselective formation of functionalized phthalides, mediated by the chiral dipeptide phosphine 141.98 Using 3-carboxylate phthalides as pronucleophiles, they formed 3,3-disubstituted phthalides in excellent yields and with high enantiomeric excess (Scheme 105). The reaction is well suited for MBHADs and 3-carboxylate phthalides bearing various substituents, providing high yields along with excellent levels of enantiocontrol. Although the reaction with an alkyl MBHAD exhibited decreased diastereoselectivity, good product conversion was obtained (entry 4). Reactions with alkyl MBHADs have also been reported previously to provide sluggish yields and low enantioselectivity,96 making this method more general and effective.
Scheme 105.
Asymmetric formation of 3,3-disubstituted phthalides, catalyzed by the chiral dipeptide phosphine 141.
Conclusions
The field of phosphine catalysis has grown tremendously since the first disclosures by Price, Rauhut–Currier, and Morita in the 1960s. Different modes of reactivity have been discovered involving various phosphonium species: β-phosphonium enolates, β-phosphonium dienolates, β-phosphonium enoates, and vinyl phosphonium ylides. These phosphonium species react with nucleophiles, dinucleophiles, electrophiles, and electrophile–nucleophiles, furnishing a wide array of heterocycles and carbocycles from readily accessible starting materials. In the past two decades, the area of phosphine catalysis has expanded greatly with the discovery of many novel annulation processes and producing compounds with complex molecular architectures.
Furthermore, many research groups have joined the field of phosphine catalysis—especially asymmetric phosphine catalysis—in the quest to achieve efficient enantioselection. Nevertheless, the area of enantioselective phosphine catalysis remains in a development stage. Although many great studies have been performed, there is still much to be discovered.
While there are many wonderful examples of asymmetric phosphine catalysis in regard to Lu’s [3 + 2] annulation and γ-umpolung reactions, asymmetric inductions remain unknown in β'-umpolung additions, mixed double-Michael reactions, and reactions of aldehydes and allenoates. Also, there are only a few reports of asymmetric [4 + 2] annulations, and only one describing an enantioselective phosphine-catalyzed Michael addition.
Having more people working on the development of new chiral phosphines and with the emerging area of chiral peptide-based phosphines, new discoveries and opportunities await in the future, granting access to a better understanding of the nature of reactions catalyzed by phosphines, thereby further expanding the horizons of nucleophilic phosphine catalysis. Such great momentum will surely stimulate the field of phosphine catalysis to grow and thrive with novel innovations.
Acknowledgements
Financial support from the NIH (GM071779) is gratefully acknowledged.
Biographies

Yi Chiao Fan
Yi Chiao Fan was born in 1986 in Taiwan. He received his BA degree in chemistry from University of California, Davis in 2008. He then joined the PhD program at University of California, Los Angeles. He is currently a PhD candidate researching under the instruction of Professor Ohyun Kwon working on tandem phosphine–palladium catalysis.

Ohyun Kwon
Ohyun Kwon, Professor of Chemistry and Biochemistry at UCLA, received her BS and MS degrees from Seoul National University in 1991 and 1993, respectively. After her PhD at Columbia University in 1998 and a postdoctoral stint at Harvard University, Kwon started her independent career at UCLA in 2001. She has played key roles in establishing phosphinocatalysis as one of the main areas of organocatalysis research. She is recognized as one of the key leaders in the field.
Notes and references
- 1.Takashina N, Price CC. J. Am. Chem. Soc. 1962;84:489. [Google Scholar]
- 2.Rauhut MM, Currier H. US 3 074 999, 1963. Chem. Abstr. 1963;58:66109. [Google Scholar]
- 3.(a) Morita K, Suzuki Z, Hirose H. Bull. Chem. Soc. Jpn. 1968;41:2815. [Google Scholar]; (b) Baylis AB, Hillman MED. DE 2 155 113, 1972. Chem. Abstr. 1972;77:34174. [Google Scholar]
- 4.White DA, Baizer MM. Tetrahedron Lett. 1973;14:3597. [Google Scholar]
- 5.Stewart IC, Bergman RG, Toste FD. J. Am. Chem. Soc. 2003;125:8696. doi: 10.1021/ja035232n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Langer P. Angew. Chem. Chem., Int. Ed. 2000;39:3049. doi: 10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]; (b) Basavaiah D, Satyanarayana T. Chem. Rev. 2003;103:811. doi: 10.1021/cr010043d. [DOI] [PubMed] [Google Scholar]; (c) Masson G, Housseman C, Zhu J. Angew. Chem., Int. Ed. 2007;46:4614. doi: 10.1002/anie.200604366. [DOI] [PubMed] [Google Scholar]; (d) Shi Y-L, Shi M. Eur. J. Org. Chem. 2007:2905. [Google Scholar]; (e) Basavaiah D, Rao KV, Reddy RJ. Chem. Soc. Rev. 2007;36:1581. doi: 10.1039/b613741p. [DOI] [PubMed] [Google Scholar]; (f) Singh V, Batra S. Tetrahedron. 2008;64:4511. [Google Scholar]; (g) Declerck V, Martinez J, Lamaty F. Chem. Rev. 2009;109:1. doi: 10.1021/cr068057c. [DOI] [PubMed] [Google Scholar]; (h) Yin W, Shi M. Acc. Chem. Res. 2010;43:1005. doi: 10.1021/ar900271g. [DOI] [PubMed] [Google Scholar]; (i) Basavaiah D, Reddy BS, Badsara SS. Chem. Rev. 2010;110:5447. doi: 10.1021/cr900291g. [DOI] [PubMed] [Google Scholar]; (j) Cheong PH-Y, Legault CY, Um JM, Çelebi-Ölçüm N, Houk KN. Chem. Rev. 2011;111:5042. doi: 10.1021/cr100212h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Basavaiah D, Veeraraghavaiah G. Chem. Soc. Rev. 2012;41:68. doi: 10.1039/c1cs15174f. [DOI] [PubMed] [Google Scholar]; (l) Fan YC, Kwon O. Phosphine Catalysis. In Science of Synthesis; B. List, Ed.; Asymmetric Organocatalysis, Vol 1, Lewis Base and Acid Catalysts; Georg Thieme: Stuttgart. 2012:723. [Google Scholar]; (m) Gomez C, Betzer J-F, Voituriez A, Marinetti A. ChemCatChem. 2013;5:1055. [Google Scholar]; (n) Wei Y, Shi M. Chem. Rev. 2013;113:6659. doi: 10.1021/cr300192h. [DOI] [PubMed] [Google Scholar]; (o) Xie P, Huang Y. Eur. J. Org. Chem. 2013:6213. [Google Scholar]
- 7.Aroyan CE, Dermenci A, Miller SJ. Tetrahedron. 2009;65:4069. [Google Scholar]
- 8.Thalji RK, Roush WR. J. Am. Chem. Soc. 2005;127:16778. doi: 10.1021/ja054085l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuler M, Duvvuru D, Retailleau P, Betzer J-F, Marinetti A. Org. Lett. 2009;11:4406. doi: 10.1021/ol901758k. [DOI] [PubMed] [Google Scholar]
- 10.Ay S, Gerard EMC, Shi M, Brase S. Synlett. 2010:128. [Google Scholar]
- 11.Cristau HJ, Viala J, Christol H. Tetrahedron Lett. 1982:1569. [Google Scholar]
- 12.(a) Trost BM, Li C-J. J. Am. Chem. Soc. 1994;116:3167. [Google Scholar]; (b) Trost BM, Li C-J. J. Am. Chem. Soc. 1994;116:10819. [Google Scholar]
- 13.Andrews IP, Blank BR, Kwon O. Chem. Commun. 2012;48:5373. doi: 10.1039/c2cc31347b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin TJ, Vakhshori VG, Kwon O. Org. Lett. 2011;13:2586. doi: 10.1021/ol200697m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan X-Y, Wei Y, Shi M. Eur. J. Org. Chem. 2011:2673. [Google Scholar]
- 16.Lu C, Lu X. Org. Lett. 2002;4:4677. doi: 10.1021/ol0270733. [DOI] [PubMed] [Google Scholar]
- 17.Szeto J, Sriramurthy V, Kwon O. Org. Lett. 2011;13:5420. doi: 10.1021/ol201730q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Yang L, Tong X. J. Am. Chem. Soc. 2010;132:2550. doi: 10.1021/ja100432m. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Lu X. J. Org. Chem. 1995;60:2906. [Google Scholar]
- 20.(a) Xia Y, Liang Y, Chen Y, Wang M, Jiao L, Huang F, Liu S, Li Y, Yu Z-X. J. Am. Chem. Soc. 2007;129:3470. doi: 10.1021/ja068215h. [DOI] [PubMed] [Google Scholar]; (b) Dudding T, Kwon O, Mercier E. Org. Lett. 2006;8:3643. doi: 10.1021/ol061095y. [DOI] [PubMed] [Google Scholar]
- 21.Wang J-C, Ng S-S, Krische MJ. J. Am. Chem. Soc. 2003;125:3682. doi: 10.1021/ja030022w. [DOI] [PubMed] [Google Scholar]
- 22.Wang J-C, Krische MJ. Angew. Chem., Int. Ed. 2003;42:5855. doi: 10.1002/anie.200352218. [DOI] [PubMed] [Google Scholar]
- 23.Henry CE, Kwon O. Org. Lett. 2007;9:3069. doi: 10.1021/ol071181d. [DOI] [PubMed] [Google Scholar]
- 24.Guan XY, Shi M. J. Org. Chem. 2009;74:1977. doi: 10.1021/jo802489t. [DOI] [PubMed] [Google Scholar]
- 25.Wallace DJ, Sidda RL, Reamer RA. J. Org. Chem. 2007;72:1051. doi: 10.1021/jo062170l. [DOI] [PubMed] [Google Scholar]
- 26.Sampath M, Loh T-P. Chem. Commun. 2009:1568. doi: 10.1039/b819959k. [DOI] [PubMed] [Google Scholar]
- 27.Du Y, Lu X. J. Org. Chem. 2003;68:6463. doi: 10.1021/jo034281f. [DOI] [PubMed] [Google Scholar]
- 28.Jones RA, Krische MJ. Org. Lett. 2009;11:1849. doi: 10.1021/ol900360h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Z, Lu X. Tetrahedron Lett. 1997;38:3461. [Google Scholar]
- 30.(a) Zhu XF, Henry CE, Kwon O. Tetrahedron. 2005;61:6276. [Google Scholar]; (b) Meng LG, Cai P, Guo Q, Xue S. J. Org. Chem. 2008;73:8491. doi: 10.1021/jo801687v. [DOI] [PubMed] [Google Scholar]
- 31.Castellano S, Fiji HDG, Kinderman SS, Watanabe M, de Leon P, Tamanoi F, Kwon O. J. Am. Chem. Soc. 2007;129:5843. doi: 10.1021/ja070274n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampath M, Lee P-YB, Loh T-P. Chem. Sci. 2011;2:1988. [Google Scholar]
- 33.Han X, Zhong F, Wang Y, Lu Y. Angew. Chem., Int. Ed. 2012;51:767. doi: 10.1002/anie.201106672. [DOI] [PubMed] [Google Scholar]
- 34.Andrews IP, Kwon O. Chem. Sci. 2012;3:2510. doi: 10.1039/C2SC20468A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Na R, Jing C, Xu Q, Jiang H, Wu X, Shi J, Zhong J, Wang M, Benitez D, Tkatchouk E, Goddard WA, III, Guo H, Kwon O. J. Am. Chem. Soc. 2011;133:13337. doi: 10.1021/ja200231v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X-F, Lan J, Kwon O. J. Am. Chem. Soc. 2003;125:4716. doi: 10.1021/ja0344009. [DOI] [PubMed] [Google Scholar]
- 37.Lu K, Kwon O. Org. Synth. 2009;86:212. doi: 10.1002/0471264229.os086.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(a) Choi J, Mouillesseauex K, Wang Z, Fiji HDG, Kinderman SS, Otto GW, Geisler R, Kwon O, Chen J-N. Development. 2011;138:1173. doi: 10.1242/dev.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Florian AE, Lepensky CK, Kwon O, Haynes MK, Sklar LA, Zweifach A. J. Biomolecular Screening. 2013;18:420. doi: 10.1177/1087057112466697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.(a) Watanabe M, Fiji HDG, G L, Umetsu A, Kinderman SS, Slamon DJ, Kwon O, Tamanoi F. J. Biol. Chem. 2008;283:9571. doi: 10.1074/jbc.M706229200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lu J, Chan L, Fiji HDG, Dahl R, Kwon O, Tamanoi F. Mol. Cancer. Ther. 2009;8:1218. doi: 10.1158/1535-7163.MCT-08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zimonjic DB, Chan LN, Tripathi V, Lu J, Kwon O, Popescu NC, Lowy DR, Tamanoi F. BMC Cancer. 2013;13:198. doi: 10.1186/1471-2407-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chan LN, Fiji HDG, Watanabe M, Kwon O, Tamanoi F. PLoS ONE. 2011;6(10):e26135. doi: 10.1371/journal.pone.0026135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Chan LN, Watanabe M, Kwon O, Tamanoi F. Small molecule inhibitors of GGTase-I from the heterocycle library derived from phosphine catalysis. In: Hrycyna C, Bergo M, Tamanoi F, editors. Enzymes Protein Prenylation Part B. Volume 30. Academic Press/Elsevier: Amsterdam; 2011. p. 165. [Google Scholar]
- 40.Wang Z, Castellano S, Kinderman SS, Argueta CE, Beshir AB, Fenteany G, Kwon O. Chem. Eur. J. 2011;17:649. doi: 10.1002/chem.201002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz D, Wang Z, Kibbie J, Modlin R, Kwon O. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6769. doi: 10.1073/pnas.1015254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran YS, Kwon O. Org. Lett. 2005;7:4289. doi: 10.1021/ol051799s. [DOI] [PubMed] [Google Scholar]
- 43.Barcan GA, Patel A, Houk KN, Kwon O. Org. Lett. 2012;14:5388. doi: 10.1021/ol302265z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villa RA, Xu Q, Kwon O. Org. Lett. 2012;14:4634. doi: 10.1021/ol302077n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran YS, Kwon O. J. Am. Chem. Soc. 2007;129:12632. doi: 10.1021/ja0752181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, Ye S. Org. Lett. 2010;12:4168. doi: 10.1021/ol101762z. [DOI] [PubMed] [Google Scholar]
- 47.(a) Zhu X-F, Henry CE, Wang J, Dudding T, Kwon O. Org. Lett. 2005;7:1387. doi: 10.1021/ol050203y. [DOI] [PubMed] [Google Scholar]; (b) Zhu X-F, Schaffner AP, Li RC, Kwon O. Org. Lett. 2005;7:2977. doi: 10.1021/ol050946j. [DOI] [PubMed] [Google Scholar]; (c) Creech GS, Kwon O. Org. Lett. 2008;10:429. doi: 10.1021/ol702462w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Creech GS, Zhu X-F, Fonovic B, Dudding T, Kwon O. Tetrahedron. 2008;64:6935. doi: 10.1016/j.tet.2008.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.(a) Meng X, Huang Y, Chen R. Org. Lett. 2009;11:137. doi: 10.1021/ol802453c. [DOI] [PubMed] [Google Scholar]; (b) Meng X, Huang Y, Zhao H, Xie P, Ma J, Chen R. Org. Lett. 2009;11:991. doi: 10.1021/ol802917d. [DOI] [PubMed] [Google Scholar]
- 49.Ma R, Xu S, Tang X, Wu G, He Z. Tetrahedron. 2011;67:1053. [Google Scholar]
- 50.Sun YW, Guan XY, Shi M. Org. Lett. 2010;12:5664. doi: 10.1021/ol102461c. [DOI] [PubMed] [Google Scholar]
- 51.Trost BM, Kazmaier U. J. Am. Chem. Soc. 1992;114:7933. [Google Scholar]
- 52.Inanaga J, Baba Y, Hanamoto T. Chem. Lett. 1993;2:241. [Google Scholar]
- 53.Grossman RB, Comesse S, Rasne RM, Hattori K, Delong MN. J. Org. Chem. 2003;68:871. doi: 10.1021/jo026425g. [DOI] [PubMed] [Google Scholar]
- 54.Trost BM, Dake GR. J. Am. Chem. Soc. 1997;119:7595. [Google Scholar]
- 55.Fan YC, Kwon O. Org. Lett. 2012;14:3264. doi: 10.1021/ol301154f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.(a) Sriramurthy V, Barcan GA, Kwon O. J. Am. Chem. Soc. 2007;129:12928. doi: 10.1021/ja073754n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fan YC, Kwon O. Molecules. 2011;16:3802. doi: 10.3390/molecules16053802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sriramurthy V, Kwon O. Org. Lett. 2010;12:1084. doi: 10.1021/ol100078w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedduri Y, Williamson JS. Tetrahedron Lett. 2008;49:6009. [Google Scholar]
- 59.Khong S, Kwon O. J. Org. Chem. 2012;77:8257. doi: 10.1021/jo3015825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Y, Lu X, Zhang C. Angew. Chem., Int. Ed. 2003;42:1035. doi: 10.1002/anie.200390266. [DOI] [PubMed] [Google Scholar]
- 61.(a) Cho CW, Krische MJ. Angew. Chem., Int. Ed. 2004;43:6689. doi: 10.1002/anie.200461381. [DOI] [PubMed] [Google Scholar]; (b) Cho CW, Kong JR, Krische MJ. Org. Lett. 2004;6:1337. doi: 10.1021/ol049600j. [DOI] [PubMed] [Google Scholar]; (c) Park H, Cho CW, Krische MJ. J. Org. Chem. 2006;71:7892. doi: 10.1021/jo061218s. [DOI] [PubMed] [Google Scholar]
- 62.Feng J, Lu X, Kong A, Han X. Tetrahedron. 2007;63:6035. [Google Scholar]
- 63.Ye LW, Sun XL, Wang QG, Tang Y. Angew. Chem., Int. Ed. 2007;46:5951. doi: 10.1002/anie.200701460. [DOI] [PubMed] [Google Scholar]
- 64.Zheng SQ, Lu X. Org. Lett. 2008;10:4481. doi: 10.1021/ol801661y. [DOI] [PubMed] [Google Scholar]
- 65.Xie P, Huang Y, Chen R. Org. Lett. 2010;12:3768. doi: 10.1021/ol101611v. [DOI] [PubMed] [Google Scholar]
- 66.(a) Vedejs E, Daugulis O, Diver ST. J. Org. Chem. 1996;61:430. doi: 10.1021/jo951661v. [DOI] [PubMed] [Google Scholar]; (b) Vedejs E, Daugulis O. J. Am. Chem. Soc. 1999;121:5813. doi: 10.1021/ja021224f. [DOI] [PubMed] [Google Scholar]; (c) Vedejs E, Daugulis O, MacKay JA, Rozners E. Synlett. 2001:1499. [Google Scholar]
- 67.Zhu G, Chen Z, Jiang Q, Xiao D, Cao P, Zhang X. J. Am. Chem. Soc. 1997;119:3836. [Google Scholar]
- 68.Wilson JE, Fu GC. Angew. Chem., Int. Ed. 2006;45:1426. doi: 10.1002/anie.200503312. [DOI] [PubMed] [Google Scholar]
- 69.Voituriez A, Panossian A, Fleury-Brégeot N, Retailleau P, Marinetti A. J. Am. Chem. Soc. 2008;130:14030. doi: 10.1021/ja806060a. [DOI] [PubMed] [Google Scholar]
- 70.Sampath M, Loh TP. Chem. Sci. 2010;1:739. [Google Scholar]
- 71.Tan B, Candeias NR, Barbas CF., III J. Am. Chem. Soc. 2011;133:4672. doi: 10.1021/ja110147w. [DOI] [PubMed] [Google Scholar]
- 72.Wurz RP, Fu GC. J. Am. Chem. Soc. 2005;127:12234. doi: 10.1021/ja053277d. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. J. Org. Chem. 1998;63:5631. doi: 10.1021/jo9623548. [DOI] [PubMed] [Google Scholar]
- 74.Smith SW, Fu GC. J. Am. Chem. Soc. 2009;131:14231. doi: 10.1021/ja9061823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun J, Fu GC. J. Am. Chem. Soc. 2010;132:4568. doi: 10.1021/ja101251d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang W, Zhang X. Angew. Chem., Int. Ed. 2002;41:1612. doi: 10.1002/1521-3773(20020503)41:9<1612::aid-anie1612>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 77.Fujiwara Y, Sun J, Fu GC. Chem. Sci. 2011;2:2196. doi: 10.1039/c1sc00414j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sinisi R, Sun J, Fu GC. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20652. doi: 10.1073/pnas.1003597107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundgren RJ, Wilsily A, Marion N, Ma C, Chung YK, Fu GC. Angew. Chem., Int. Ed. 2013;52:2525. doi: 10.1002/anie.201208957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung YK, Fu GC. Angew. Chem., Int. Ed. 2009;48:2225. doi: 10.1002/anie.200805377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibrahim AA, Wei P-H, Harzmann GD, Kerrigan NJ. J. Org. Chem. 2010;75:7901. doi: 10.1021/jo101867m. [DOI] [PubMed] [Google Scholar]
- 82.Chen S, Salo EC, Wheeler KA, Kerrigan NJ. Org. Lett. 2012;14:1784. doi: 10.1021/ol3003783. [DOI] [PubMed] [Google Scholar]
- 83.Cowen BJ, Miller SJ. J. Am. Chem. Soc. 2007;129:10988. doi: 10.1021/ja0734243. [DOI] [PubMed] [Google Scholar]
- 84.Agarkov A, Greenfield S, Xie D, Pawlick R, Starkey G, Gilbertson SR. Biopolymers: Pept. Sci. 2006;84:48. doi: 10.1002/bip.20395. [DOI] [PubMed] [Google Scholar]
- 85.Hua X, Chai Z, Zheng C-W, Yang Y-Q, Liu W, Zhang J-K, Zhao G. Angew. Chem., Int. Ed. 2010;49:4467. doi: 10.1002/anie.201000446. [DOI] [PubMed] [Google Scholar]
- 86.Han X, Wang Y, Zhong F, Lu Y. J. Am. Chem. Soc. 2011;133:1726. doi: 10.1021/ja1106282. [DOI] [PubMed] [Google Scholar]
- 87.(a) Jean L, Marinetti A. Tetrahedron Lett. 2006;47:2141. [Google Scholar]; (b) Fleury-Bregeot N, Jean L, Retailleau P, Marinetti A. Tetrahedron. 2007;63:11920. [Google Scholar]; (c) Panossian A, Fleury-Bregot N, Marinetti A. Eur. J. Org. Chem. 2008:3826. [Google Scholar]; (d) Pinto N, Fleury-Bregot N, Marinetti A. Eur. J. Org. Chem. 2009:146. [Google Scholar]
- 88.Fang Y-Q, Jacobsen EN. J. Am. Chem. Soc. 2008;130:5660. doi: 10.1021/ja801344w. [DOI] [PubMed] [Google Scholar]
- 89.(a) Mercier E, Fonovic B, Henry CE, Kwon O, Dudding T. Tetrahedron Lett. 2007;48:3617. [Google Scholar]; (b) Liang Y, Liu S, Xia Y, Li Y, Yu Z-X. Chem. Eur. J. 2008;14:4361. doi: 10.1002/chem.200701725. [DOI] [PubMed] [Google Scholar]; (c) Liang Y, Liu S, Yu Z-X. Synlett. 2009:905. [Google Scholar]
- 90.Deng H-P, Wei Y, Shi M. Adv. Synth. Catal. 2012;354:783. [Google Scholar]
- 91.Zhong F, Han X, Wang Y, Lu Y. Angew. Chem., Int. Ed. 2011;50:7837. doi: 10.1002/anie.201102094. [DOI] [PubMed] [Google Scholar]
- 92.Xiao H, Chai Z, Wang H-F, Wang D-D, Cao X-W, Liu W, Lu Y-P, Yang Y-Q, Zhao G. Chem. Eur. J. 2011;17:10562. doi: 10.1002/chem.201100850. [DOI] [PubMed] [Google Scholar]
- 93.Zhong F, Han X, Wang Y, Lu Y. Chem. Sci. 2012;3:1231. [Google Scholar]
- 94.Xiao H, Chai Z, Cao D-D, Wang H, Chen J, Zhao G. Org. Biomol. Chem. 2012;10:3195. doi: 10.1039/c2ob25295c. [DOI] [PubMed] [Google Scholar]
- 95.Zhong F, Dou X, Han X, Yao W, Zhu Q, Meng Y, Lu Y. Angew. Chem., Int. Ed. 2013;52:943. doi: 10.1002/anie.201208285. [DOI] [PubMed] [Google Scholar]
- 96.(a) Jiang Y-Q, Shi Y-L, Shi M. J. Am. Chem. Soc. 2008;130:7202. doi: 10.1021/ja802422d. [DOI] [PubMed] [Google Scholar]; (b) Wei Y, Ma G-N, Shi M. Eur. J. Org. Chem. 2011:5146. [Google Scholar]
- 97.(a) Yang Y-L, Pei C-K, Shi M. Org. Biomol. Chem. 2011;9:3349. doi: 10.1039/c1ob00017a. [DOI] [PubMed] [Google Scholar]; (b) Deng H-P, Wei Y, Shi M. Eur. J. Org. Chem. 2011:1956. [Google Scholar]; (c) Ma G-N, Caonames M. Tetrahedron: Asymmetry. 2009;20:1086. [Google Scholar]; (d) Wang D, Yang Y-L, Jiang J-J, Shi M. Org. Biomol. Chem. 2012;10:7158. doi: 10.1039/c2ob25694k. [DOI] [PubMed] [Google Scholar]
- 98.Zhong F, Lou J, Chen G-Y, Dou X, Lu Y. J. Am. Chem. Soc. 2012;134:10222. doi: 10.1021/ja303115m. [DOI] [PubMed] [Google Scholar]