Abstract
Background
Net atrioventricular compliance (Cn) has been reported to be an important determinant of pulmonary hypertension in mitral stenosis (MS). We hypothesized that, as Cn reflects hemodynamic consequences of MS, it may be useful in assessing prognosis. To date, limited data with an assumed Cn cutoff have indicated the need for larger prospective studies. This prospective study was designed to determine the impact of Cn on clinical outcome and its contribution to pulmonary pressure in MS. In addition, we aimed to identify a cutoff value of Cn for outcome prediction in this setting.
Methods and Results
A total of 128 patients with rheumatic MS without other significant valve disease were prospectively enrolled. Comprehensive echocardiography was performed and Doppler-derived Cn estimated using a previously validated equation. The endpoint was either mitral valve intervention or death. Cn was an important predictor of pulmonary pressure, regardless of classic measures of MS severity. During a median follow-up of 22 months, the endpoint was reached in 45 patients (35%). Baseline Cn predicted outcome, adding prognostic information beyond that provided by mitral valve area and functional status. Cn ≤ 4 mL/mmHg best predicted unfavorable outcome in derivation and validation sets. A subgroup analysis including only initially asymptomatic patients with moderate to severe MS without initial indication for intervention (40.6 % of total) demonstrated that baseline Cn predicted subsequent adverse outcome even after adjusting for classic measures of hemodynamic MS severity (hazard ratio [HR] 0.33, 95% confidence interval [CI] 0.14–0.79, p = 0.013).
Conclusions
Cn contributes to pulmonary hypertension beyond of stenosis severity itself. In a wide spectrum of MS severity, Cn is a powerful predictor of adverse outcome, adding prognostic value to clinical data and mitral valve area. Importantly, baseline Cn predicts a progressive course with subsequent need for intervention in initially asymptomatic patients. Cn assessment therefore has potential value for clinical risk stratification and monitoring in MS patients.
Keywords: mitral stenosis, net atrioventricular compliance, pulmonary hypertension, outcome
The presence of pulmonary hypertension (PH) is fundamental in the clinical decision-making process for mitral stenosis (MS).1, 2 It strongly expresses valve disease severity and conveys adverse effects on functional status, exercise tolerance, and prognosis.3–5 However, pulmonary pressure may not be uniquely determined by the stenotic lesion itself but by a combination of hemodynamic parameters.6, 7
Several factors may contribute to clinical presentation and outcome in MS. Left-heart compliance plays a crucial role in the occurrence of PH and symptoms.8–15 Net atrioventricular compliance (Cn) has been reported to be a major determinant of elevated pulmonary artery pressure in patients with MS,9, 10 independent of mitral valve (MV) area and transvalvular pressure gradients. Li et al10 found that Cn was the only Doppler echocardiographic variable that independently predicted pulmonary artery pressure.
Cn can be readily calculated by Doppler echocardiography. Cn was originally utilized for analysis of transmitral flow by Thomas et al,16 demonstrating that the pressure half-time to estimate MV area varies inversely with orifice area, but also directly with net left atrial and ventricular compliance and the square root of the peak transmitral gradient.
Flachskampf et al17 presented analytic and numeric evidence supporting the quantitative assessment of Cn from transmitral velocity profiles, deriving a simple equation that relates it to effective MV area and E-wave downslope. This equation has been validated in vitro and accurately predicts Cn. Subsequently, Schwammenthal et al9 showed that Cn can be calculated noninvasively and reproducibly in the clinical setting and correlates well with invasively determined values.
Although Cn seems to be an important determinant of cardiovascular performance in MS,9, 12, 13, 15 few data are available regarding its prognostic implications. To date, only one study12 has evaluated the relation between Cn and clinical events; that study was limited by the small number of patients enrolled and by an assumed cutoff value for Cn. The authors acknowledged the need for further prospective studies in more patients and the limitation of an assumed cutoff used for Cn in the analysis.
We hypothesized that Cn, which reflects the hemodynamic consequences of MS, will be a useful predictor of outcome, adding value to other well-established indices of stenosis severity. The present prospective study was designed to determine the incremental prognostic value of Cn and its independent contribution to pulmonary artery pressure in a substantial population of patients with MS. In addition, we aimed to identify an optimal cutoff value of Cn predictive of adverse outcomes for use in the clinical setting.
Methods
Study Population
Patients were recruited prospectively and consecutively from a tertiary center for heart valve disease among those routinely referred for management of rheumatic valve disease.
The study enrolled 140 patients (124 women and 16 men) with rheumatic MS from 2007 to 2011. Exclusion criteria were pregnancy, other hemodynamically significant valve disease, and congenital or myopathic lesions that could independently affect pulmonary artery pressure. Based on these criteria, 12 patients were excluded for pregnancy (n=1), moderate to severe aortic regurgitation (n=3), moderate mitral regurgitation (n=2), interatrial communication (n=1), aortic prosthesis (n=1), Schistosomiasis mansoni with pulmonary hypertension (n=1), acquired immunodeficiency syndrome (n=1), Chagas heart disease (n=1) and intestinal neoplasia with chemotherapy treatment (n=1). The study was approved by the Federal University of Minas Gerais in Brazil and the subjects gave informed consent.
Clinical Data
At entry, a complete clinical evaluation was performed on all patients. Only clinically stable patients were included, and functional status was determined using the New York Heart Association (NYHA) classification based on functional capacity and dyspnea.
Echocardiographic Evaluation
At the time of enrollment, two-dimensional and Doppler echocardiographic imaging was performed and analyzed according to the recommendations of the American Society of Echocardiography18 using a commercially available echocardiograph (GE Vivid 7, Horten, Norway or Philips ie33, Andover, MA). Left atrial (LA) volume was assessed by the biplane area-length method from apical 2- and 4-chamber views.18, 19
Mitral valve (MV) morphology was evaluated using the score of Wilkins and colleagues20 by grading valve leaflet thickness, mobility, calcification and subvalvular thickening. MV area was measured using planimetry and concurrently calculated using the pressure half-time method. Peak and mean transmitral diastolic pressure gradients were measured from Doppler profiles recorded in the apical four-chamber view. The continuous-wave Doppler tricuspid regurgitant velocity was used to determine systolic pulmonary artery pressure (SPAP) using the simplified Bernoulli equation. Mean right atrial pressure was estimated based on the diameter of the inferior vena cava and its respiratory change.18, 21
Global right ventricular (RV) function was quantitatively assessed using the RV myocardial performance index,22 peak systolic velocity at the tricuspid annulus using tissue Doppler imaging,23 and the tricuspid annular plane systolic excursion at the RV free wall obtained from 2-dimensionally-guided M-mode recordings.24 Each echocardiographic parameter was averaged from 3 consecutive cardiac cycles for patients in sinus rhythm or 5 consecutive cycles in atrial fibrillation. The echocardiographic measurements were performed by 2 independent observers who were blinded to the clinical information.
Assessment of Cn
Cn was determined noninvasively by means of Doppler echocardiography as previously described9, 12, 14, 17, 25: Cn (mL/mmHg) = 1270 × (planimetric MV area [cm2]/E-wave downslope [cm/s2]). In those patients with nonlinear diastolic flow, the mid-diastolic flow was used, which is felt to best represent the valve stenosis as opposed to early LA depressurization, and the slope was extrapolated back to obtain the initial maximal velocity.
Cn was also calculated invasively in 25 patients with pure MS in sinus rhythm who underwent percutaneous valvuloplasty to compare with its noninvasive value. As described by Thomas et al,26 mean left atrial compliance (Ca) was obtained by dividing the cardiac stroke volume by the systolic rise in left atrial pressure. The cardiac stroke volume was calculated using the Fick method. Similarly, mean left ventricular compliance (Cv) was estimated as the stroke volume divided by the diastolic rise in left ventricular pressure. Ventricular compliance was calculated using an LV catheter passed retrograde through the aortic valve. Fluid-filled catheters were used to measure the pressures. Cn was then calculated as (1/Ca + 1/Cv)−1.
Endpoint Definition
The primary outcome was composite endpoints of either MV intervention (percutaneous or surgical) or death related to MS. Follow-up data were obtained during clinical follow-up appointment or telephone interviews.
To satisfy the assumption of the independence of events, hospitalization for progressive dyspnea or overt pulmonary edema, during which valve intervention was recommended, was not defined as a separate endpoint.
Clinical management of the patients and decisions about valve intervention were made by their respective physicians, who were independent of the study and unaware of the results of the Cn measurement. Indications for valve intervention were according to the 2008 ACC/AHA guidelines1 based on a combination of functional limitation, severity of obstruction, and pulmonary hypertension: NYHA functional class III or IV; NYHA class II with moderate or severe MV area reduction and valve morphology favorable for percutaneous mitral valvuloplasty; or asymptomatic patients with pulmonary hypertension, moderate or severe stenosis, and valve morphology favorable for percutaneous intervention. The purpose of this study, therefore, was to determine which baseline measures best predicted this outcome.
Statistical Analysis
Baseline demographic features and echocardiographic variables are presented as mean ± standard deviation. All data have been tested for normality, and transformation performed when necessary.
A multivariable regression analysis was performed to identify the factors associated with pulmonary artery pressure, including all MV parameters and measures of right-sided function described above. The associated increase of R2 was assessed to identify the respective contribution of each variable to the variance of the pulmonary pressure in the multivariable model. Model fit was assessed by residual analysis. Residual plots were examined for relationship between residual and predicted values. The Shapiro-Wilk test was employed to assess the normality of residuals for the overall and the final model.
Multivariable Cox proportional-hazards analysis was used to identify risk factors for MS-related intervention. The potential predictive variables of outcome included in the Cox analysis were age, symptoms, atrial fibrillation, MV area, transvalvular gradients, RV myocardial performance index, LA volume, SPAP, and Cn. The variables were checked for collinearity and obviously interdependent covariates were not used simultaneously in any of the analyses. The interaction between Cn and MV area (product term) was also included in the multivariable analysis. The predictors of outcome by multivariable analysis were then tested for their incremental contribution to the model prediction of outcome using the likelihood ratio statistic, which follows a chi-square distribution.
Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff values of Cn that best predict binary outcome. Applying a randomized splitting technique, the cutoff value was compared in derivation and validation sets. Intervention-free survival rates were estimated by the Kaplan-Meier method and compared by the log-rank test. Reproducibility of Cn was assessed by the intra-class correlation coefficients for repeated measures in a random sample of 10 patients. Inter-method agreement (non-invasive vs invasive) was evaluated using the Bland-Altman method. A p value <0.05 was regarded as statistically significant. Statistical analysis was performed using the Statistical Package for Social Sciences for Windows, version 18.0 (SPSS Inc., Chicago, Illinois).
Results
Baseline Clinical Characteristics
The mean age was 42.6 ± 11.2 years, and 116 patients were women (90.6 %). The baseline clinical characteristics of the study population are summarized in Table 1. Sixty-eight patients (53%) were asymptomatic at the time of recruitment into the study while the remaining 60 patients (47%) had exertional dyspnea. Fifty-three patients (41%) had associated mild aortic valve rheumatic disease and 6 patients (5%) had rheumatic tricuspid valve disease.
Table 1.
Baseline Characteristics of the Study Population
| Variables | Value |
|---|---|
| Age (years) | 43 ± 11 |
| Heart rate (bpm) | 69 ± 13 |
| Systolic/ diastolic blood pressures (mmHg) | 118 ± 14/76 ± 10 |
| Left ventricular ejection fraction (%) | 58 ± 5 |
| Left atrial diameter (mm) | 49 ± 6 |
| Left atrial volume index (mL/m2) | 52 ± 16 |
| Transvalvular mitral peak /mean gradients (mmHg) | 18 ± 8/ 10 ± 6 |
| Mitral valve area by planimetry (cm2) | 1.2 ± 0.4 |
| Mitral valve area by pressure half-time (cm2) | 1.3 ± 0.3 |
| Pulmonary artery systolic pressure* (mmHg) | 44 ± 19 |
| Peak systolic velocity at the tricuspid annulus (cm/s) | 11 ± 2 |
| Tricuspid annular motion (mm) | 19 ± 4 |
| Right ventricular myocardial performance index | 0.3 ± 0.1 |
| Moderate-to-severe tricuspid regurgitation (n/%) | 17 (13) |
| Net atrioventricular compliance† (mL/mmHg) | 4.7 ± 1.3 |
Data are expressed as the mean value ± SD or number (percentage) of patients.
Pulmonary artery systolic pressure could not be assessed in 4 patients (3%).
Compliance could not be calculated in 3 patients (2%) due to extensive calcification of mitral valve leaflets hence inability to planimeter MV area accurately.
Mitral valvuloplasty had previously been performed in 35 patients (27%), including either percutaneous or surgical intervention. At the time of enrollment, 5 patients (4%) had had 2 or more valvuloplasties. The mean time between the last valve intervention and enrollment into the study was 5 years (range 1 to 14 years).
Atrial fibrillation was present in 18 patients (14%) at enrollment. The medications most frequently used were beta-blockers (59% of cases). Thirty-three patients (28%) were using penicillin benzathine for secondary prevention of rheumatic fever. Patients with atrial fibrillation and/or previous embolic events were taking anticoagulants.
Mitral valve area was 1.2 ± 0.4 (range, 0.6 to 2.0) cm2 with mild MS (area >1.5 cm2) found in 34 patients, moderate (area 1 to 1.5 cm2) in 52, and severe (area <1 cm2) in 42 based on MV area by PHT. The mitral valve morphology was suitable for percutaneous valvuloplasty in the majority of the patients, with a median Wilkins echocardiographic score of 7 (range, 4 to 12). There was no difference in transmitral gradients, pulmonary artery pressure or Cn between patients with atrial fibrillation and sinus rhythm. There was a weak negative correlation between LA volume index and Cn (r = −0.3, p=0.002).
Cn decreased with worsening NYHA functional class, with a mean Cn of 5.1 ± 1.1 mL/mmHg in asymptomatic patients and 4.1 ± 1.1 mL/mmHg in symptomatic patients (p=0.001).
Predictors of Pulmonary Artery Systolic Pressure
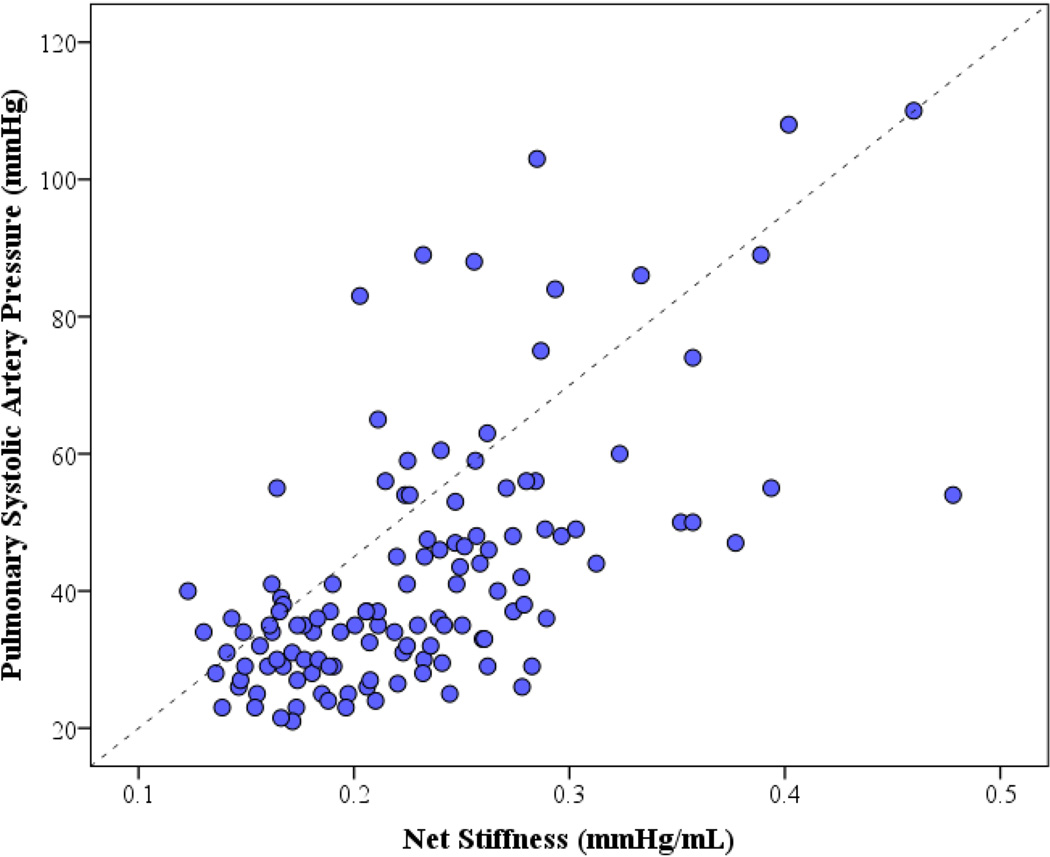
The predictors of pulmonary pressure in MS are shown in Table 2. There was a correlation between SPAP and measures of MS severity, including MV area and transvalvular pressure gradients. As expected, several measures of RV function also correlated with SPAP. Of note, although there was an inverse correlation between MS severity (valve area) and SPAP, there was a wide spectrum of SPAP in a subgroup of patients with severe MS, with a median of 50.5 mmHg [interquartile range 37.8 – 65.8, range 25 to 110 mmHg], indicating that additional variation in SPAP must be accounted for by other factors. Specifically, Cn inversely correlated with SPAP and directly correlated with net stiffness (y=170× 3.3, R2=0.39, p<0.001) (Figure 1). By multivariable analysis, mean transvalvular gradient, MV area, and Cn were identified as the most significant predictors of increased SPAP (Table 2). Cn was therefore a powerful predictor of pulmonary hypertension, which contributes beyond of MS severity itself.
Table 2.
Predictors of Pulmonary Artery Pressure in Mitral Stenosis
| Individual Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Variable | r | p Value | Regression coefficient |
p Value | R2 * |
| Age (years) | −0.18 | 0.029 | … | … | … |
| LA volume index (ml/m2) | 0.24 | 0.008 | … | … | … |
| Mean gradient (mmHg) | 0.68 | <0.001 | 0.26 | 0.021 | 0.46 |
| Mitral valve area (cm2) † | −0.62 | <0.001 | −0.30 | 0.001 | 0.50 |
| RVMPI | 0.33 | 0.001 | … | … | … |
| PSVTA (cm/s) | −0.28 | 0.004 | … | … | … |
| Cn (mL/mmHg) | −0.61 | <0.001 | −0.27 | 0.005 | 0.57 |
The R2 values are cumulative, and its value for the final multivariate model was 0.57.
Mitral valve area by planimetry
Cn = Net atrioventricular compliance; LA = Left atrial; PSVTA = Peak systolic velocity at the tricuspid annulus; RVMPI = Right ventricular myocardial performance index
Figure 1.
Scatter plot showing correlation between systolic pulmonary artery pressure (SPAP) and net stiffness (1/Cn). There is a nonlinear negative relationship between Cn and SPAP, and a positive correlation with stiffness.
Predictors of Outcome
During a median follow-up of 22 months (limits, 3 to 49), the endpoint was reached in 45 patients (35%): one patient died, 41 underwent percutaneous mitral valvuloplasty, and 3 underwent to MV replacement. The baseline clinical and echocardiographic variables associated with patient outcome are compared in Table 3. As expected, patients who reached the endpoint presented with more advanced functional class and more severe MS by echocardiography than medically treated patients. Of note, however, only 24% of patients who reached the endpoint had NYHA class III-IV functional status at baseline. By multivariable analysis, the baseline determinants predicting valve intervention were Cn, MV area, and NYHA functional class (Table 4). There was a significant interaction between MV area by planimetry and Cn (p=0.007), demonstrating that the prognostic impact of MV area is modulated by Cn. Therefore, patients with larger valve areas have worse prognosis if their Cn is lower.
Table 3.
Predictors of Adverse Outcome
| Baselines values | No events (n=83) |
Events (n=45) |
Hazard Ratio* (95% CI) |
p Value |
|---|---|---|---|---|
| Age (years) | 44.9 ± 10.8 | 37.9 ± 10.7 | 0.59 (0.43 – 0.80) | 0.001 |
| NYHA class III–IV (n/%) | 8 (10) | 11 (24) | 2.80 (1.40 – 5.58) | 0.003 |
| Loud P2 (n/%) | 27 (32) | 25 (55) | 3.92 (2.0 – 7.68) | <0.001 |
| Atrial fibrillation (n/%) | 13 (16) | 5 (11) | 0.66 (0.26 – 1.67) | 0.38 |
| LAV index (mL/m2) | 49.4 ± 15.5 | 56.9 ± 14.3 | 1.37 (1.04 – 1.79) | 0.010 |
| RVMPI | 0.29 ± 0.12 | 0.33 ± 0.11 | 1.41 (1.04 – 1.91) | 0.026 |
| Peak gradient (mmHg) | 15.2 ± 5.8 | 24.2 ± 9.2 | 2.19 (1.73 – 2.76) | <0.001 |
| Mean gradient (mmHg) | 8.2 ± 3.9 | 14.7 ± 6.4 | 2.09 (1.71 – 2.57) | <0.001 |
| MV area (cm2) | 1.4 ± 0.3 | 0.9 ± 0.2 | 0.13 (0.07 – 0.23) | <0.001 |
| SPAP (mmHg) | 36.9 ± 13.2 | 56.9 ± 22.6 | 1.93 (1.54 – 2.40) | <0.001 |
| Cn (mL/mmHg) | 5.1 ± 1.1 | 3.8 ± 1.2 | 0.30 (0.19 – 0.47) | <0.001 |
Data are expressed as the mean value ± SD or number (percentage) of patients.
hazard ratio per 1 SD increase
Cn = net atrioventricular compliance; LAV = left atrial volume; MV = mitral valve; P2 = pulmonic valve closure sound; RVMPI = Right ventricular myocardial performance index; SPAP = systolic pulmonary artery pressure
Table 4.
Multivariable Cox Proportional-Hazards Analysis for Predicting Adverse Outcome
| Variables* | Hazard Ratio † | (95% CI) | p Value |
|---|---|---|---|
| NYHA class III–IV | 2.87 | 1.27 – 6.49 | 0.011 |
| MV area (cm2) | 0.62 | 0.50 – 0.77 | <0.001 |
| Cn (mL/mmHg) | 0.23 | 0.06 – 0.87 | 0.031 |
| MV area and Cn † | 1.15 | 1.04 – 1.27 | 0.007 |
Abbreviations as in Table 3
hazard ratio per 1 SD increase
Interaction between MV area by planimetry and Cn
Although the classic markers of hemodynamic severity, particularly mean transvalvular pressure gradient and SPAP, were significant determinants on individual analysis, they were not predictors of outcome in a model including MV area and Cn.
To assess the additional value of low Cn in predicting adverse outcome, we selected a subgroup of patients with moderate to severe anatomic mitral stenosis without indication for mitral valve intervention at the time of recruitment into the study, which included asymptomatic patients with pulmonary artery systolic pressure at rest less than 50 mmHg. Of the 52 patients in this group (40.6% of the entire study population), 17 subsequently achieved criteria that warranted MV intervention.
In this subset of initially asymptomatic patients, multivariable analysis demonstrated that Cn predicted subsequent MV intervention (hazard ratio [HR] 0.33, 95% confidence interval [CI] 0.14–0.79, p = 0.013), after adjusting for classic measures of hemodynamic MS severity. Baseline Cn therefore had an additional and incremental effect for predicting adverse outcome, even after adjusting for valve area, pressure gradient and pulmonary artery pressure.
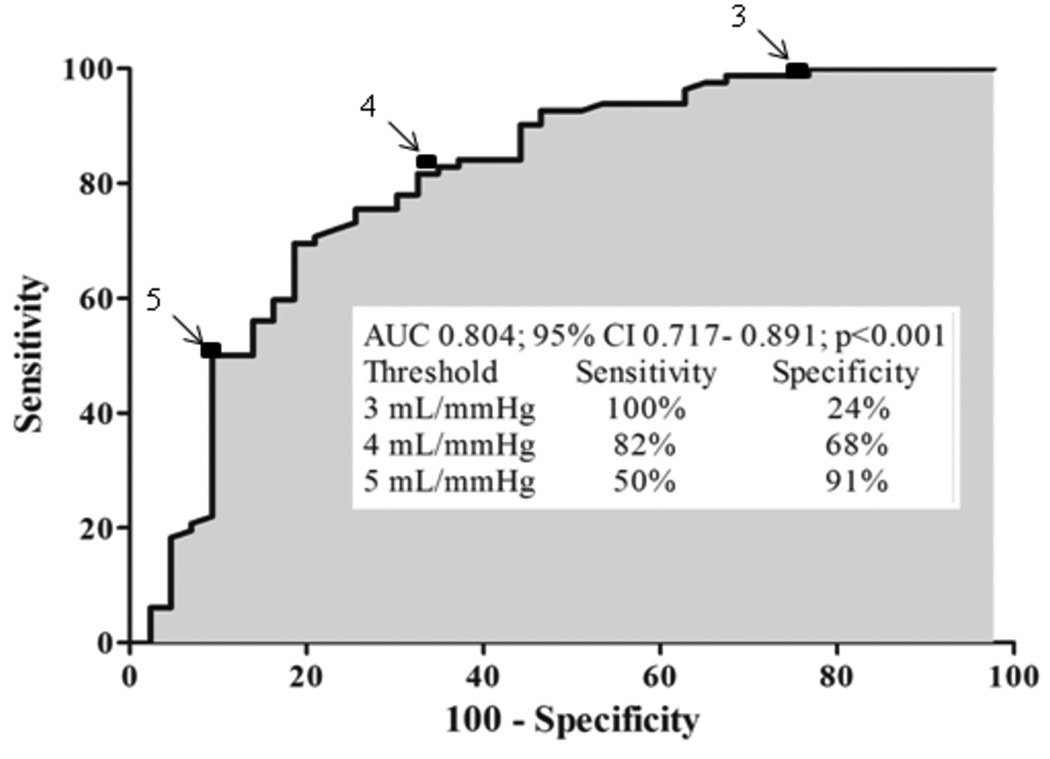
The cutoff level for Cn was set according to the ROC curve analysis. In order to validate this cutoff point, our population was split randomly into two portions. The first 65 patients were analyzed as the derivation set and the next 63 as the validation set, without significant differences in key measures between the 2 sets. In the overall population, different cutoff values of Cn to predict binary outcome were obtained (Figure 2). The best cutoff value of Cn for outcome prediction was 4 mL/mmHg, with sensitivity of 82% and specificity of 68%.
Figure 2.
Receiver operating characteristic curve for net atrioventricular compliance (Cn) in predicting mitral valve intervention. The best cutoff value of Cn for outcome prediction was 4 mL/mmHg, with sensitivity of 82% and specificity of 68%.
Of the patients with baseline values of Cn > 4 mL/mmHg, only 18% were referred for interventional therapy compared with 82% with values of Cn ≤ 4 mL/mmHg. Of note, although not an endpoint because it determined intervention, all 4 patients who developed acute pulmonary edema had Cn less than 4 mL/mmHg.
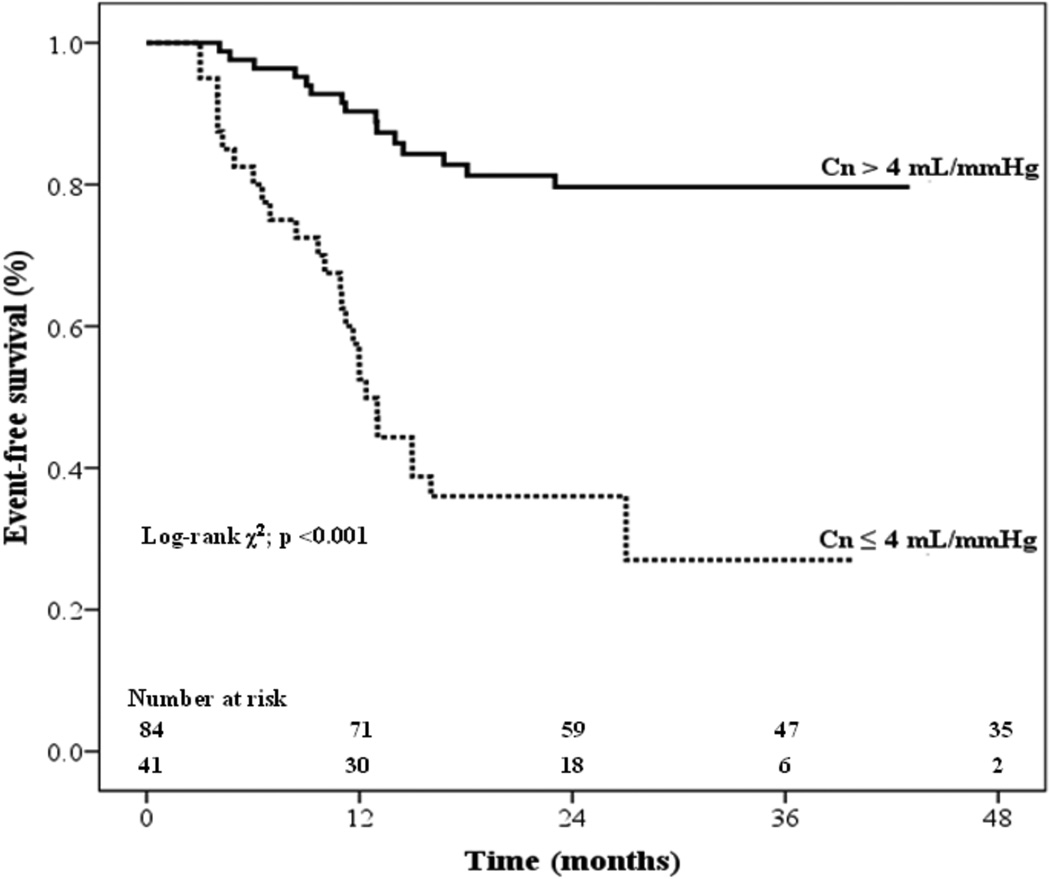
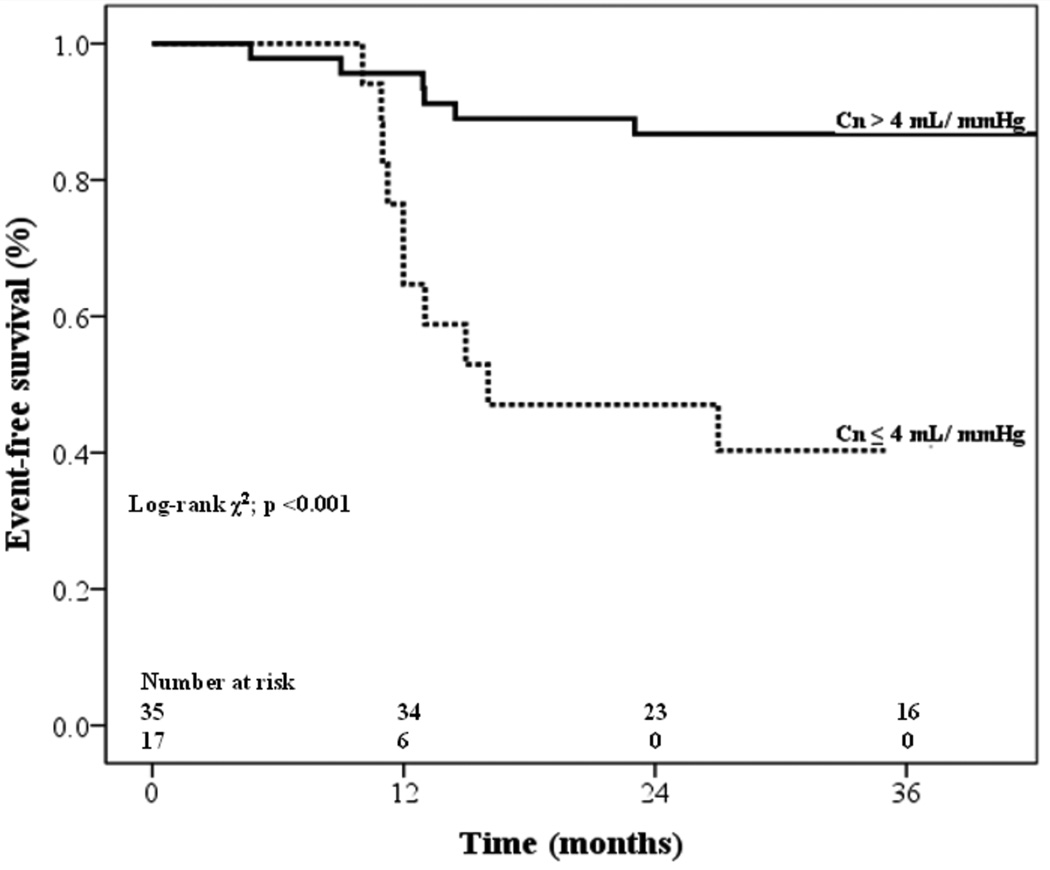
In the Kaplan-Meier analysis (Figure 3), the event-free survival rate was significantly higher in patients with Cn > 4 ml/mmHg than in patients with Cn ≤ 4 ml/mmHg (p <0.001). Furthermore, in the initially asymptomatic subgroup, of the patients with Cn > 4 mL/mmHg, 30% later underwent MV intervention compared with 69% of those with Cn ≤ 4 mL/mmHg (p=0.001). The Kaplan-Meier curve (Figure 4) showed that event-free survival rate was significantly higher in the patients with Cn > 4 ml/mmHg than in those with Cn ≤ 4 ml/mmHg (p <0.001), even though patients in this analysis did not have indication for a procedure at baseline.
Figure 3.
Kaplan-Meier intervention-free survival curves for patients stratified by net atrioventricular compliance (Cn) ≤ 4 compared to patients with Cn > 4 mL/mmHg (log-rank 30.5; p<0.001).
Figure 4.
Kaplan-Meier intervention-free survival curves for a subgroup of patients with moderate to severe anatomic mitral stenosis without indication for mitral valve intervention at baseline. The event-free survival rate was significantly higher in the patients with Cn > 4 ml/mmHg than in those with Cn ≤ 4 mL/mmHg (log-rank 15; p <0.001).
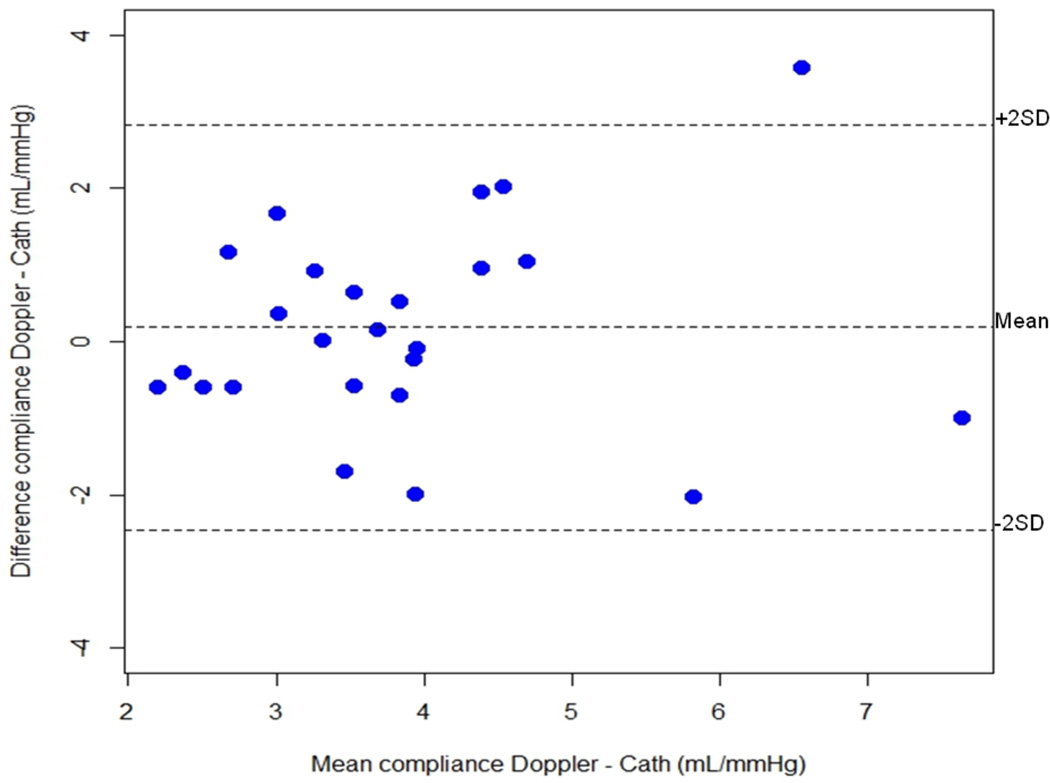
In 25 patients who underwent percutaneous valvuloplasty and in whom Cn was also measured by catheterization before the procedure, the catheter- and Doppler-derived values for Cn showed reasonable correlation (r=0.60; p=0.002). There was also a good inter-method agreement (non-invasive vs invasive) calculation of Cn as shown by Bland-Altman plot (Figure 5).
Figure 5.
Bland-Altman graph between invasively (Cn Cath) and noninvasively (Cn Doppler) determined net atrioventricular compliance (Cn) in a subgroup of 25 patients with mitral stenosis.
Reproducibility
For the Cn measurement, 2 independent observers achieved a high level of agreement with intra-class correlation coefficient of 0.93 for inter-observer and 0.97 for intra-observer variability.
Discussion
MS is a progressive disease, usually following a stable early course with subsequent progressive clinical evolution.7, 27 Its prognosis depends mainly on symptoms at presentation, and once limiting symptoms develop, survival is dismal.27 However, the clinical presentation of MS varies widely and symptoms may be inconsistent with the standard measurements of MS severity.28 MS is best described as a disease continuum, with no single value that can define its severity. More specifically, a wide variation in pulmonary pressures for the same range of mitral obstruction emphasizes the need to identify key factors implicated in the development of pulmonary hypertension and functional limitation in MS.5
This study addresses the value of Cn as a predictor of pulmonary artery pressure in rheumatic MS, and also evaluates the impact of baseline Cn on clinical outcome. The results show that Cn contributes not only to pulmonary hypertension, a basic determinant of functional capacity, but also adds incremental value in predicting clinical outcome beyond that provided by MV area and NYHA functional class.
Cn does not merely reflect severity of symptoms but identifies patients at high risk of ultimately requiring intervention within an intermediate follow-up period. Cn assessed in patients who at baseline were mostly asymptomatic or had mild exercise intolerance (NYHA class I or II) had prognostic importance, which highlighting that there are pathophysiologic predictors of adverse outcome apart from symptoms and reduction in valve area.
Previous studies have also indicated that Cn contributes to determining pulmonary hypertension in MS,3, 12–14, 17, 25, 29 but had several limitations. Li et al10 could not find any relation between parameters of MS severity and pulmonary pressure from the small number of patients studied. The rate of pressure decay across the MV, which ultimately determines the mean LA pressure that is transmitted to the lungs, also depends on MV area and flow, yielding transmitral gradient.30 Kim at al12 demonstrated the potential value of Cn in predicting MV intervention, but with relatively few patients and events, which limited analysis and indicated the need for confirmation by larger studies. Finally, the cutoff value of Cn used for clinical correlation in previous studies9, 10 was selected based on an assumed proportion of the statistical distribution of Cn in patients with MS. This cutoff, which might be used to guide patient management, required prospective determination without assumption, based on the ability of Cn to predict outcomes.
The present study assessing the role of Cn in patients with MS overcomes these previous limitations. It is prospective and includes a larger number of MS patients with a wide spectrum of severity to address current gaps in the field. The results of this study verify the important contribution of Cn to pulmonary pressure; establishes the prognostic value of Cn; and supports the use of a cutoff value for predicting outcomes, which have also been confirmed by comparing derivation and validation sets.
Rationale for Prognostic Significance of Net Atrioventricular Compliance
The decay rate of the atrioventricular pressure gradient is determined by orifice size and left-sided chamber pressures, which in turn are determined by flow and chamber compliance. For a given planimetered MV area, more rapid mitral deceleration (and consequent low Cn) indicates that the atrium is filling on a steeper portion of its pressure volume curve and is more likely to have higher mean pressure, regardless of the transmitral gradient. Therefore Cn contributes to the overall severity of the disease beyond anatomic valve obstruction itself.
The prognostic value of Cn in general and in asymptomatic patients can be understood in several ways: 1) Baseline Cn can predict pulmonary hypertension not only at rest but also during exercise or in situations of increased cardiac output in a way that would influence prognosis. 2) For a given orifice area, Cn determines left atrial pressure, which in turn influences pulmonary artery pressure. Compensatory mechanisms, however, including adjustments of heart rate, cardiac output, and pulmonary vasoreactivity, can delay the onset of symptoms. The ability of baseline Cn to predict subsequent need for intervention, even in initially asymptomatic patients, is consistent with the possibility that Cn can predict progressive deterioration of functional capacity otherwise masked by initial compensation. 3) It is conceivable that low Cn may also induce progressive worsening of function through repeated exertional elevation of left-sided filling pressures, promoting reactive pulmonary vasoconstriction and arteriolar obliteration that ultimately lead to indications for intervention. Additionally, low Cn can reflect atrial myocardial fibrosis and stiffening that may be progressive and lead to adverse outcomes in initially asymptomatic patients.
Left Atrial Size and Compliance
The influence of LA size on compliance has not been established. Previous studies found no correlation between Cn and LA size.9, 10, 13, 31 This study demonstrated a significant but weak correlation. This is reasonable because, as indicated by Schwammenthal et al,9 Cn not only reflects intrinsic compliance but also expresses the position of the left heart on its pressure-volume curve. LA compliance can also be altered as a consequence of atrial fibrillation. Kim et al29 showed higher Cn in atrial fibrillation; in contrast, in Guray et al13 and the current study, Cn was similar in patients with and without atrial fibrillation. Low Cn (and by implication low Ca) indicates a steeper portion of the pressure-volume curve, probably a shift to the right along an exponential curve as the LA enlarges.
Right Ventricular Function
Although the hemodynamic consequences of MS mainly affect the RV as mediated by pulmonary hypertension, the pathophysiologic mechanisms of RV dysfunction in MS are unclear.4, 32 RV dysfunction is not a simple expression of elevated pulmonary artery pressure.33 Pande et al4 showed that RV dysfunction was observed in all cases of rheumatic MS regardless of SPAP. Similarly, Sagie et al34 demonstrated that right heart disease can progress independent of MV area. In our study, echocardiographic indices of RV function weakly correlated with SPAP and had no independent effect on the clinical outcome. In fact, normal RV function does not reliably exclude significant pulmonary hypertension in MS.
Limitations of the Study
Cn is not equal to atrial compliance.9, 17 However, in considering SPAP determinants, Cn is the physiologically meaningful variable, not LA compliance alone, since Cn ultimately determines the transmitral pressure gradient and pressure-volume curve. In our relatively young patients, impaired ventricular myocardial relaxation is unlikely to have influenced on Cn.35. In addition, particularly in MS, the LV fills on the flat low-volume portion at the left end of the pressure-volume curve.
Although Cn is a predictor of pulmonary pressure during exercise, exercise echocardiography was not performed. However, even at rest, Cn was powerful enough to predict adverse events.
Clinical Implications
Risk stratification and decisions about valve intervention are most difficult in patients with moderate MS who have a similar anatomic severity of stenosis but varying functional status. In this subset, there may be discrepancies between symptoms and conventional echocardiographic parameters, requiring more complete evaluation of physiology. Baseline Cn may have its greatest utility in this subset of patients by providing an additional indication of impaired physiology and progression to the need for intervention in those initially asymptomatic patients with moderate MS and no significant pulmonary hypertension – 40.6% of our overall study population - in whom there is the greatest need for additional decision-making guidance.
Conclusions
In a wide spectrum of severity of MS, Cn contributes to pulmonary hypertension and is a powerful predictor of adverse outcome, adding prognostic information beyond that provided by clinical evaluation and mitral valve area. Importantly, baseline Cn may provide its greatest value by predicting a progressive course with subsequent need for intervention in initially asymptomatic patients. Cn assessment therefore has potential value for clinical risk stratification and monitoring in MS patients.
Supplementary Material
Acknowledgments
Sources of Funding
This study was partly supported by grants from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasília, Brazil), by NIH/NHLBI grants R01 HL092101 (JH), K24 HL67434 and R01 HL109506 (RAL), and by the Leducq Transatlantic Mitral Network (Paris, France; RAL).
Footnotes
Disclosures
None.
References
- 1.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. 2008 focused update incorporated into the acc/aha 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): Endorsed by the society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 2.Corte TJ, McDonagh TA, Wort SJ. Pulmonary hypertension in left heart disease: A review. Int J Cardiol. 2012;156:253–258. doi: 10.1016/j.ijcard.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Ha JW, Chung N, Jang Y, Kang WC, Kang SM, Rim SJ, Shim WH, Cho SY, Kim SS. Is the left atrial v. Wave the determinant of peak pulmonary artery pressure in patients with pure mitral stenosis? Am J Cardiol. 2000;85:986–991. doi: 10.1016/s0002-9149(99)00915-7. [DOI] [PubMed] [Google Scholar]
- 4.Pande S, Agarwal SK, Dhir U, Chaudhary A, Kumar S, Agarwal V. Pulmonary arterial hypertension in rheumatic mitral stenosis: Does it affect right ventricular function and outcome after mitral valve replacement? Interact Cardiovasc Thorac Surg. 2009;9:421–425. doi: 10.1510/icvts.2009.206607. [DOI] [PubMed] [Google Scholar]
- 5.Song JK, Kang DH, Lee CW, Lee SG, Cheong SS, Hong MK, Kim JJ, Park SW, Park SJ, Lee SJ. Factors determining the exercise capacity in mitral stenosis. Am J Cardiol. 1996;78:1060–1062. doi: 10.1016/s0002-9149(96)00539-5. [DOI] [PubMed] [Google Scholar]
- 6.Guazzi M, Arena R. Pulmonary hypertension with left-sided heart disease. Nat Rev Cardiol. 2010;7:648–659. doi: 10.1038/nrcardio.2010.144. [DOI] [PubMed] [Google Scholar]
- 7.Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009;374:1271–1283. doi: 10.1016/S0140-6736(09)60994-6. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E, Moscovitz HL, Amram SS, Lasser RP, Sapin SO, Himmelstein A, Ravitch MM, Gordon AJ. The hemodynamics of the left side of the heart as studied by simultaneous left atrial, left ventricular, and aortic pressures; particular reference to mitral stenosis. Circulation. 1955;12:69–81. doi: 10.1161/01.cir.12.1.69. [DOI] [PubMed] [Google Scholar]
- 9.Schwammenthal E, Vered Z, Agranat O, Kaplinsky E, Rabinowitz B, Feinberg MS. Impact of atrioventricular compliance on pulmonary artery pressure in mitral stenosis: An exercise echocardiographic study. Circulation. 2000;102:2378–2384. doi: 10.1161/01.cir.102.19.2378. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Dery JP, Dumesnil JG, Boudreault JR, Jobin J, Pibarot P. Usefulness of measuring net atrioventricular compliance by doppler echocardiography in patients with mitral stenosis. Am J Cardiol. 2005;96:432–435. doi: 10.1016/j.amjcard.2005.03.094. [DOI] [PubMed] [Google Scholar]
- 11.Ko YG, Ha JW, Chung N, Shim WH, Kang SM, Rim SJ, Jang Y, Cho SY, Kim SS. Effects of left atrial compliance on left atrial pressure in pure mitral stenosis. Catheter Cardiovasc Interv. 2001;52:328–333. doi: 10.1002/ccd.1076. [DOI] [PubMed] [Google Scholar]
- 12.Kim HK, Kim YJ, Hwang SJ, Park JS, Chang HJ, Sohn DW, Oh BH, Park YB. Hemodynamic and prognostic implications of net atrioventricular compliance in patients with mitral stenosis. J Am Soc Echocardiogr. 2008;21:482–486. doi: 10.1016/j.echo.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Guray Y, Demirkan B, Karan A, Guray U, Boyaci A, Korkmaz S. Left atrial compliance and pulmonary venous flow velocities are related to functional status in patients with moderate-to-severe mitral stenosis. Echocardiography. 2009;26:1173–1178. doi: 10.1111/j.1540-8175.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim HK, Kim YJ, Chang SA, Kim DH, Sohn DW, Oh BH, Park YB. Impact of cardiac rhythm on mitral valve area calculated by the pressure half time method in patients with moderate or severe mitral stenosis. J Am Soc Echocardiogr. 2009;22:42–47. doi: 10.1016/j.echo.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Choi EY, Shim J, Kim SA, Shim CY, Yoon SJ, Kang SM, Choi D, Ha JW, Rim SJ, Jang Y, Chung N. Value of echo-doppler derived pulmonary vascular resistance, netatrioventricular compliance and tricuspid annular velocity in determining exercise capacity in patients with mitral stenosis. Circ J. 2007;71:1721–1727. doi: 10.1253/circj.71.1721. [DOI] [PubMed] [Google Scholar]
- 16.Thomas JD, Weyman AE. Doppler mitral pressure half-time: A clinical tool in search of theoretical justification. J Am Coll Cardiol. 1987;10:923–929. doi: 10.1016/s0735-1097(87)80290-5. [DOI] [PubMed] [Google Scholar]
- 17.Flachskampf FA, Weyman AE, Guerrero JL, Thomas JD. Calculation of atrioventricular compliance from the mitral flow profile: Analytic and in vitro study. J Am Coll Cardiol. 1992;19:998–1004. doi: 10.1016/0735-1097(92)90284-t. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the american society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Ujino K, Barnes ME, Cha SS, Langins AP, Bailey KR, Seward JB, Tsang TS. Two-dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol. 2006;98:1185–1188. doi: 10.1016/j.amjcard.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: An analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–496. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 22.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 23.Meluzin J, Spinarova L, Bakala J, Toman J, Krejci J, Hude P, Kara T, Soucek M. Pulsed doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22:340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 24.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: A primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22:776–792. doi: 10.1016/j.echo.2009.04.027. quiz 861-772. [DOI] [PubMed] [Google Scholar]
- 25.Salem Omar AM, Tanaka H, AbdelDayem TK, Sadek AS, Raslaan H, Al-Sherbiny A, Yamawaki K, Ryo K, Fukuda Y, Norisada K, Tatsumi K, Onishi T, Matsumoto K, Kawai H, Hirata K. Comparison of mitral valve area by pressure half-time and proximal isovelocity surface area method in patients with mitral stenosis: Effect of net atrioventricular compliance. Eur J Echocardiogr. 2011;12:283–290. doi: 10.1093/ejechocard/jeq194. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JD, Wilkins GT, Choong CY, Abascal VM, Palacios IF, Block PC, Weyman AE. Inaccuracy of mitral pressure half-time immediately after percutaneous mitral valvotomy. Dependence on transmitral gradient and left atrial and ventricular compliance. Circulation. 1988;78:980–993. doi: 10.1161/01.cir.78.4.980. [DOI] [PubMed] [Google Scholar]
- 27.Selzer A, Cohn KE. Natural history of mitral stenosis: A review. Circulation. 1972;45:878–890. doi: 10.1161/01.cir.45.4.878. [DOI] [PubMed] [Google Scholar]
- 28.Hugenholtz PG, Ryan TJ, Stein SW, Abelmann WH. The spectrum of pure mitral stenosis. Hemodynamic studies in relation to clinical disability. Am J Cardiol. 1962;10:773–784. doi: 10.1016/0002-9149(62)90171-6. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, Kim YJ, Shin JI, Hwang SJ, Jo SH, Park JS, Chang HJ, Sohn DW, Oh BH, Park YB, Choi YS. Echocardiographic and hemodynamic findings in patients with mitral stenosis undergoing percutaneous mitral commissurotomy comparing those with chronic atrial fibrillation versus those with normal sinus rhythm. Am J Cardiol. 2007;100:1153–1156. doi: 10.1016/j.amjcard.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JD, Weyman AE. Fluid dynamics model of mitral valve flow: Description with in vitro validation. J Am Coll Cardiol. 1989;13:221–233. doi: 10.1016/0735-1097(89)90575-5. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor A, Kumar S, Shukla A, Tewari S, Garg N, Goel P, Sinha N. Determinants of left atrial pressure in rheumatic mitral stenosis: Role of left atrial compliance and "atrial stiffness". Indian Heart J. 2004;56:27–31. [PubMed] [Google Scholar]
- 32.Sade LE, Ozin B, Ulus T, Acikel S, Pirat B, Bilgi M, Ulucam M, Muderrisoglu H. Right ventricular contractile reserve in mitral stenosis: Implications on hemodynamic burden and clinical outcome. Int J Cardiol. 2009;135:193–201. doi: 10.1016/j.ijcard.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 33.Morrison DA, Lancaster L, Henry R, Goldman S. Right ventricular function at rest and during exercise in aortic and mitral valve disease. J Am Coll Cardiol. 1985;5:21–28. doi: 10.1016/s0735-1097(85)80080-2. [DOI] [PubMed] [Google Scholar]
- 34.Sagie A, Freitas N, Padial LR, Leavitt M, Morris E, Weyman AE, Levine RA. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: Valve area and right heart disease. J Am Coll Cardiol. 1996;28:472–479. doi: 10.1016/0735-1097(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 35.Abascal VM, Moreno PR, Rodriguez L, Monterroso VM, Palacios IF, Weyman AE, Davidoff R. Comparison of the usefulness of doppler pressure half-time in mitral stenosis in patients < 65 and > or = 65 years of age. Am J Cardiol. 1996;78:1390–1393. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.