Abstract
The heart has a high rate of ATP production and turnover which is required to maintain its continuous mechanical work. Perturbations in ATP generating processes may therefore affect contractile function directly. Characterizing cardiac metabolism in heart failure revealed several metabolic alterations termed metabolic remodeling, ranging from changes in substrate utilization to mitochondrial dysfunction, ultimately resulting in ATP deficiency and impaired contractility. However, ATP depletion is not the only relevant consequence of metabolic remodeling during heart failure. By providing cellular building blocks and signaling molecules, metabolic pathways control essential processes such as cell growth and regeneration. Thus, alterations in cardiac metabolism may also affect the progression to heart failure by mechanisms beyond ATP supply. Our aim is therefore to highlight that metabolic remodeling in heart failure not only results in impaired cardiac energetics, but also induces other processes implicated in the development of heart failure such as structural remodeling and oxidative stress. Accordingly, modulating cardiac metabolism in heart failure may have significant therapeutic relevance that goes beyond the energetic aspect.
Keywords: Metabolism, mitochondria, hypertrophy/remodeling, heart failure
PREAMBLE
Heart failure (HF) is a clinical syndrome in which myocardial pump function is inadequate for maintaining and supporting an individual's physiological requirements. The clinical presentation is characterized by pulmonary congestion, dyspnea, and fatigue. Historically, HF was suspected when systolic function was impaired. Stages of cardiac hypertrophy without systolic dysfunction have mostly been termed “compensated hypertrophy”. More recently, it has become clear that HF symptoms can also exist despite relatively preserved systolic function. This condition is mostly caused by diastolic dysfunction that may even be present in conditions historically termed compensated hypertrophy. Thus, moving forward, metabolic research will need to take this aspect into account. It is possible that distinct changes in cardiac metabolism may mediate these two forms of cardiac dysfunction. For the sake of simplicity and because most studies on cardiac metabolism in HF did not investigate diastolic function, we use the term “compensated hypertrophy” for all conditions associated with normal systolic function, and the term heart failure to indicate the presence of systolic dysfunction. Metabolic changes that specifically impact or contribute to diastolic dysfunction will be addressed if information is available.
In this review, we will focus primarily on metabolic adaptations in chronic HF. Acute changes such as those that develop following myocardial stunning or ischemia/reperfusion have been addressed elsewhere. To understand metabolic alterations in HF, an overview of basic metabolic processes in the healthy heart is necessary.
METABOLISM IN THE NORMAL HEART
Under normoxic conditions, more than 95% of ATP generated in the heart is derived from oxidative phosphorylation in the mitochondria. The remaining 5% mainly comes from glycolysis and to a lesser extent from the citric acid cycle (Krebs cycle).1, 2 The heart utilizes about 60-70% of generated ATP to fuel contraction and the remaining 30-40% for various ion pumps, especially the Ca2+ -ATPase in the sarcoplasmic reticulum.3, 4 The energy pool of the heart includes ATP (~ 5 μmol/g wet wt) and phosphocreatine (~ 8 μmol/g wet wt) with the latter serving as an ATP transport and buffer system.5 In the mitochondria, the high-energy phosphate bond in ATP can be transferred to creatine by mitochondrial creatine kinase (CK) to form phosphocreatine (PCr). With a smaller molecular weight than ATP, PCr can easily diffuse through the mitochondrial membrane into the cytosol. Here, it can be used to generate ATP from ADP through reactions catalyzed by the cytosolic CK.6 Due to its continuous mechanical work, the heart has a high rate of ATP hydrolysis (~ 0.5 μmol/g wet wt/s). Accordingly, the high-energy-phosphate pool in the heart is relatively small and can be exhausted within a few seconds. Therefore, cardiac work strongly depends on ATP generation and impairments in this process can rapidly induce contractile dysfunction. Approximately 70% to 90% of cardiac ATP is produced by the oxidation of fatty acids (FA). The remaining 10% to 30% comes from the oxidation of glucose and lactate, as well as small amounts of ketone bodies and certain amino acids.2, 7, 8 However, it is also important to note that cardiac substrate selection in human studies has mostly been assessed in the fasted state. In the fed state, when plasma levels of glucose and insulin rise, the contribution of glucose utilization to cardiac ATP production increases.
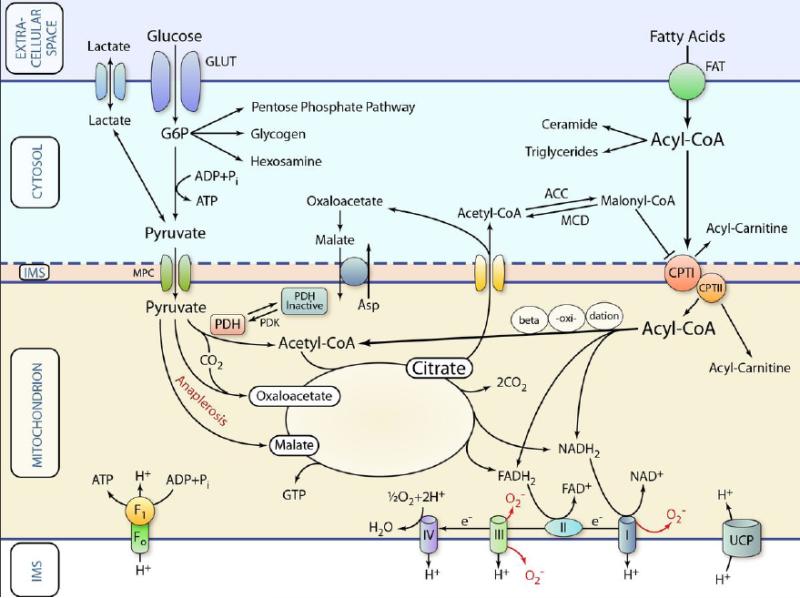
Figure 1 is a schematic representation of well-described metabolic processes, focused on the generation of ATP from glucose and fatty acids.
Figure 1. Schematic representation of classic pathways of cardiac metabolism.
Substrates are transported across the extracellular membrane into the cytosol and are metabolized in various ways. For oxidation, the respective metabolic intermediates (e.g., pyruvate or acyl-CoA) are transported across the inner mitochondrial membrane by specific transport systems. Once inside the mitochondrion, substrates are oxidized or carboxylated (anaplerosis) and fed into the Krebs cycle for the generation of reducing equivalents (NADH2 and FADH) and GTP. The reducing equivalents are used by the electron transport chain to generate a proton gradient, which in turn is used for the production of ATP. This principal functionality can be affected in various ways during HF thereby limiting ATP production or affecting cellular function in other ways (see text and further Figures for details). IMS: mitochondrial intermembrane space; GLUT: glucose transporter; FAT: fatty acid transporter. MPC: mitochondrial pyruvate transporter. (Illustration Credit: Ben Smith)
Fatty acid utilization
Fatty acid (FA) utilization can be divided into 3 steps: uptake into the cytosol, transport across the mitochondrial membrane and oxidation within the mitochondria. Although FAs can traverse the plasma membrane (flip flop), their transport is facilitated by transport proteins including fatty acid translocase (FAT/CD36) and the plasma membrane fatty acid binding protein (FABPpm).9, 10 In the cytosol, free fatty acids are esterified to fatty acyl-CoA, which is either esterified to triglyceride or converted to long-chain acylcarnitine by carnitine palmitoyltransferase I (CPT I) located in the outer mitochondrial membrane prior to entering the mitochondria.9, 11 The turnover of the myocardial triglyceride pool is high and represents an important source of fatty acyl-CoA that subsequently is metabolized by the mitochondria.12 Long-chain acylcarnitine is then transported into the mitochondrial matrix and converted back to long-chain acyl-CoA by carnitine palmitoyl transferase II (CPT II). Acyl-CoA then enters β-oxidation, generating acetyl-CoA and the reducing equivalents NADH and FADH2.
Fatty acyl-CoA, particularly palmitoyl-CoA can be used for de novo synthesis of ceramides, which has pleiotropic effects on cellular function.13, 14 Incomplete FA oxidation, particularly in skeletal muscle may result in release of acylcarnitines from mitochondria into the cytosol and subsequently into the circulation. These acylcarnitines have been implicated in the pathophysiology of insulin resistance.15
The reaction catalyzed by CPT I represents a critical regulatory node that determines FA oxidation rates. In the normal adult heart, the muscle isoform (CPT Ib or M-CPT I) is predominant.16 In contrast, the expression of the liver isoform (CPT Ia or L-CPT I), which is abundantly expressed in the fetal heart, has been shown to increase in the hypertrophied heart.17 The activity of CPT I is inhibited by malonyl-CoA,18, 19 which is generated by carboxylation of cytosolic acetyl-CoA, by the enzyme acetyl-CoA carboxylase (ACC). Malonyl CoA is converted back to acetyl-CoA by malonyl-CoA decarboxylase (MCD)20, 21 (See Figure 1). Although this pathway has been evaluated as a therapeutic target in the setting of ischemic heart disease,22, 23 recent evidence has also suggested that CPT-I activity may be regulated via mechanisms that are independent of changes in malonyl-CoA.24
Glucose utilization
Glucose that is utilized by the heart either comes from uptake of exogenous glucose or is derived from glycogen stores. The heart has a relatively small glycogen pool (~ 30 μmol/g wet wt, which is 20 % of that of skeletal muscle), but rates of glycogen turnover are high. In the heart, glycogen-derived glucose may contribute up to 40 % of glucose mediated ATP production in rats.25 Glucose is transported into the cytosol by glucose transporters including GLUT1 and GLUT4. While GLUT1 is the major glucose transporter in the fetal heart and contributes to constitutive glucose uptake, in the adult heart GLUT4 is the predominant isoform and mediates the bulk of basal myocardial glucose uptake.26, 27
Following uptake, free glucose is rapidly phosphorylated to glucose 6-phosphate (G6P), which subsequently enters many metabolic pathways. Glycolysis represents the major pathway in glucose utilization. Glycolysis generates pyruvate, NADH and a small amount of “substrate level” ATP. This glycolytic ATP appears to be important for Ca2+ uptake into the sarcoplasmic reticulum and diastolic relaxation.28, 29 In the cytosol, pyruvate can be converted to lactate. If transported into the mitochondrial matrix, pyruvate undergoes oxidation to acetyl-CoA or carboxylation to form oxaloacetate or malate. Pyruvate oxidation is catalyzed by the multienzyme complex pyruvate dehydrogenase (PDH), whose activity is highly regulated by its products (acetyl-CoA, NADH) and by phosphorylation of its E1 subunit.30 The carboxylation of pyruvate is a major anaplerotic pathway (see below).
Accessory pathways of glucose metabolism
Beside glycolysis, G6P may also be channeled into glycogen synthesis or the pentose phosphate pathway (PPP). The PPP is an important source of NADPH, which plays a critical role in regulating cellular oxidative stress and is required for lipid synthesis 31 and anaplerosis (see below). The key oxidative enzyme of the PPP is glucose 6-phosphate dehydrogenase (G6PDH), which catalyzes the first reaction of the pathway to generate NADPH. In addition to the PPP, a small amount of G6P can enter the hexosamine biosynthetic pathway (HBP) leading to the formation of uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc), a monosaccharide donor for O-GlcNAcylation of various proteins.12 Since the HBP requires not only glucose but also acetyl-CoA and glutamine, it may serve as a metabolic sensor linking metabolic status to several cellular processes. While flux through the PPP and the HBP in the normal heart is relatively small, recent evidence has suggested a role for these pathways in the pathophysiology of heart disease (see below).
The Krebs cycle and anaplerosis
Acetyl-CoA, the common end product of glucose and FA oxidation, enters the citric acid cycle (a.k.a. tricarboxylic acid cycle or Krebs cycle) to generate GTP (or ATP), CO2 and reducing equivalents NADH2.2 Since Krebs cycle intermediates can be used for many biosynthetic pathways (e.g. amino and nucleic acids), they are constantly removed from the cycle and therefore need to be replaced. The replenishment of the Krebs cycle intermediate pool through pathways independent of acetyl-CoA is termed “anaplerosis”.32, 33 The carboxylation of pyruvate to malate is an important anaplerotic reaction. This requires NADPH and therefore links anaplerosis to processes that generate (PPP) or consume (lipogenesis, anti-oxidative defense) NADPH. Anaplerosis is a crucial process in the heart, whose impairment rapidly causes contractile dysfunction.34 Changes in anaplerotic pathways have recently been implicated in heart disease (see below).
Oxidative phosphorylation and the generation of reactive oxygen species (ROS)
The reducing equivalents (NADH2 and FADH) enter the electron transport chain (ETC) for oxidative phosphorylation. A byproduct of this activity is the generation of ROS arising mainly from complexes I and III.35 Mitochondrial ROS production has been implicated in a wide variety of cellular processes, ranging from protective mechanisms such as preconditioning to detrimental mechanisms such as oxidative damage and activation of adverse ventricular remodeling.36
Short-term regulation of cardiac substrate utilization
FA and glucose utilization is tightly linked and co-regulated. Utilization of one substrate may directly inhibit the utilization of the other (the “Randle cycle”).37 Complete metabolism of glucose is more oxygen-efficient than that of FAs.38 Therefore, substrate selection and the interaction between glucose and FA utilization in the heart have been considered highly relevant in cardiac disease.39 Substrate use and selection in the heart is also modulated by hormones such as insulin, glucagon-like peptides and catecholamines. Although important, these regulatory mechanisms lie outside of the scope of this review.
Long-term regulation of cardiac metabolism
Expression levels of metabolic enzymes and their posttranslational modifications may strongly affect cardiac metabolic activity. Peroxisome proliferator-activated receptor (PPAR) nuclear receptors are important transcriptional regulators of FA utilization. Although PPARα is the predominant isoform in the heart, PPARδ and PPARγ may also modulate FA metabolism.11 Cardiac FA utilization is also regulated by the estrogen-related receptor ERRα. In addition to sharing common targets with PPARα, ERRα also stimulates the transcription of genes involved in glucose metabolism and oxidative phosphorylation.40, 41
The transcriptional activities of PPARs and ERRs are potently induced by interacting with members of the PPAR-γ coactivator-1 (PGC-1) family. PGC-1α binds to and coactivates PPARα and ERRα leading to increased capacity of FA uptake and oxidation.42 Although less extensively studied, PGC-1β may also play an important role in the regulation of cardiac energy metabolism.43 PGC-1 proteins are powerful activators of mitochondrial biogenesis. This is mediated in part by induction of the nuclear respiratory factor NRF-1 and the mitochondrial transcription factor A (Tfam).44 The activity of PGC-1α is highly regulated by both its expression levels and posttranslational modifications such as phosphorylation, acetylation or methylation.42
AMP-activated protein kinase (AMPK) is another important mediator of metabolic adaptation that promotes increased ATP production and inhibition of energy-consuming biosynthetic pathways under conditions of hemodynamic stress or ischemia. AMPK boosts ATP production by acutely stimulating both FA oxidation and glycolysis while slowing down ATP consuming processes such as lipid and protein synthesis. Furthermore, AMPK may also improve cardiac energetics in a long-term manner by activating PGC-1α, leading to increased mitochondrial biogenesis and oxidative capacity.45
Metabolism beyond ATP production
Metabolic intermediates may act as regulators of many pathways not directly related to ATP production. Table 1 summarizes classic metabolites that have been recognized as signal transducers. While much of our discussion so far has focused on substrate oxidation and ATP production in the heart, it is equally important to consider that cardiac metabolism also involves anabolic processes which are essential for maintaining cellular activities and promoting cell growth and proliferation (Cai et al.46 and Ward et al.47 have provided detailed reviews). The development of HF is characterized by profound changes in cardiac structure and function. Given the fundamental role of metabolism in all of these processes, it is reasonable to assume that cardiac metabolism occupies a central position in the pathophysiology of HF.
Table 1.
“Classic” metabolic intermediates that have been recognized as “signal transducers”
| Metabolic intermediates | Regulatory effects |
|---|---|
| Fatty acids | Activation of PPARs,145 modulation of ion channels by palmitoylation.146 |
| Acylcarnitines | Activation of Ca+ channels,147 induction of insulin resistance.15 |
| Ceramides | Activation of PP2A, PKCζ → inhibition of insulin signaling,14 induction of mitochondrial and ER stress and apoptosis.148 |
| Pyruvate | Stimulation of mitochondrial biogenesis,149 regulation of PGC−1α expression.150 |
| Acetyl-CoA | Induction of cell growth and proliferation by promoting the acetylation of histones.151 |
| Hexosamine | Multiple cellular effects via O-GlcNAcylation of regulatory proteins.152 |
| NAD(P)+/NAD(P)H | Modulation of activity of metabolic enzymes, numerous additional effects via regulation of redox state and sirtuins.31, 36 |
| ROS | Regulation of redox state, enzyme activity, high levels induce apoptosis, hypertrophy, inflammation.36, 153 |
| AMP | Diverse effects on metabolism and cell growth through activation of AMPK.45 |
| BCAA | Stimulation of protein synthesis154 and various additional effects via activation of mTOR and inhibition of autophagy.155 |
PP2A: protein phosphatase; PKC: protein kinase C; ER: endoplasmic reticulum; BCAA: branched-chain amino acids
CARDIAC METABOLISM IN HEART FAILURE
There are two major points that should be considered when interpreting study results on metabolic alterations in HF. First, metabolic phenotypes and their mechanisms differ between HF of different etiologies. Second, because the progression to HF is often long and complex, the time point of assessment (i.e., compensated hypertrophy with or without diastolic dysfunction vs. manifest systolic dysfunction) will influence metabolic adaptations that are observed. Despite these caveats, findings in humans and animal models provide clear evidence indicating severe metabolic alterations in the failing heart. Table 2 summarizes reported changes in substrate utilization and energy production in various models of HF.
Table 2.
Changes in substrate oxidation and mitochondrial function in various models of cardiac hypertrophy and failure
| Model of heart disease | Morphological and functional characteristics | FA metabollism | Glucose metabolism | Mitochondrial changes and energy status | Reference No. |
|---|---|---|---|---|---|
| Aortic constriction in rats | Compensated hypertrophy without/with diastolic dysfunction; systolic dysfunction | FAO and mRNA expression of genes regulating FAO decreased in compensated hypertrophy and systolic dysfunction | GO decreased in systolic dysfunction | State-3 respiration initially increased but decreased in systolic dysfunction | 50 |
| Aortic constriction in rats | Hypertrophy with diastolic and systolic dysfunction | FAO, mRNA and protein expression of FAO enzymes decreased | GO decreased, protein expression of PDH complex increased | Dysorganized mitochondrial cristae, mitochondrial volume density and levels of most ETC proteins decreased, state-3 respiration decreased | 66 |
| Aortic constriction in mice | Hypertrophy with systolic dysfunction | FAO unchanged | Glycolysis and GO increased | Mitochondrial dysfunction, decreased respiration and ATP content | 43 |
| Aortic constriction in mice | Hypertrophy with diastolic and systolic dysfunction | FAO unchanged | GU, glycogen synthesis, glucose and lactate oxidation decreased | 57 | |
| Abdominal aortic constriction in rats | Mild, compensated hypertrophy | FAO and mRNA expression of genes regulating FAO unchanged | Glycolysis increased modestly | 59 | |
| Abdominal aortic constriction in rats | Mild, compensated hypertrophy | FAO and mRNA expression of genes regulating FAO decreased | GO unchanged | 56 | |
| Abdominal aortic constriction in rats | Hypertrophy, heart function ex vivo unchanged | FAO decreased | Glycolysis increased, GO unchanged | 55 | |
| Dahl salt-sensitive rats | Compensated hypertrophy; systolic dysfunction | FA uptake and mRNA expression of genes regulating FA utilization decreased in systolic dysfunction | GU increased, mRNA expression of genes regulating glycolysis and GO decreased; pentose phosphate pathway flux increased in systolic dysfunction | mRNA and protein expression of genes regulating mitochondrial biogenesis decreased in systolic dysfunction; PCr/ATP ratio decreased | 48 |
| Spontaneously hypertensive rats (Sprague Dawley rats as controls) | Hypertrophy, heart function ex vivo unchanged | FAO lower. (Note that some strains of SHRs have a mutation in CD36, which could independently modulate FAO). | GO higher | 54 | |
| Spontaneously hypertensive rats (Wistar rats as controls) | Hypertrophy, minor differences in diastolic and systolic function | Flux through PDH higher | PCr/ATP ratio not different | 61 | |
| Myocardial infarction in rats | Compensated hypertrophy; systolic dysfunction | mRNA expression of genes regulating FA utilization decreased in systolic dysfunction | mRNA and protein expression of GLUT-1 increased in systolic dysfunction | 52 | |
| Myocardial infarction in rats | No hypertrophy, left ventricular dilatation, systolic dysfunction | FAO and mRNA expression of genes regulating FAO decreased | GU and GO unchanged | mRNA expression of genes regulating mitochondrial biogenesis decreased | 58 |
| Myocardial infarction in rats | Mild hypertrophy, systolic dysfunction | FA utilization decreased, protein expression of fatty acid transporters decreased | 51 | ||
| Pacing-induced HF in dogs | No hypertrophy, left ventricular dilatation, systolic dysfunction | FA uptake and oxidation, activity of CPT-1 and MCAD decreased | GO increased | 49 | |
| HF patients with IDCM or ischemic heart disease | Systolic dysfunction | mRNA expression of MCAD, LCAD lower | 53 | ||
| HF patients with IDCM | Hypertrophy, systolic dysfunction | FA utilization lower | Glucose utilization higher | 62 | |
| Patients with hypertensive heart disease (HHD), aortic stenosis (AS) or dilated cardiomyopathy (DCM) | Hypertrophy with normal systolic function in HHD and AS patients; left ventricular dilatation and systolic dysfunction in DCM patients | PCr/ATP ratio not different among groups; PCr content lower in AS and DCM; ATP content lower in DCM | 5 |
HHD: hypertensive heart disease; AS: aortic stenosis; DCM: dilated cardiomyopathy; IDCM: idiopathic dilated cardiomyopathy; FA: fatty acid; FAO: fatty acid oxidation; GO: glucose oxidation; GU: glucose uptake; MCAD: medium-chain acyl-CoA dehydrogenase; LCAD: long-chain acyl-CoA dehydrogenase; SHRs: spontaneously hypertensive rat
Fatty acid utilization in HF
Most studies reveal reduced cardiac FA utilization. FA uptake is reduced in concert with high-salt diet-induced HF,48 and by rapid pacing.49 In agreement with these findings, studies in other models of HF also observed a reduction in mRNA and protein expression of FA transporters when systolic dysfunction was present.48, 50-52 We found that FA oxidation rate as well as the expression of FA oxidation enzymes were already decreased in early (compensated) stages of left ventricular hypertrophy.50 These results are also consistent with findings in spontaneously hypertensive rats,53, 54 and in rats with abdominal constriction,55, 56 but not with those in Dahl salt-sensitive rats 48 and in rats with myocardial infarction.52 However, by the time that systolic dysfunction manifests, there is clear evidence from studies in animal models and human subjects that myocardial FA oxidation is decreased. Fig. 2A summarizes the fate of FAs in HF and how these changes may lead to decreased ATP production.
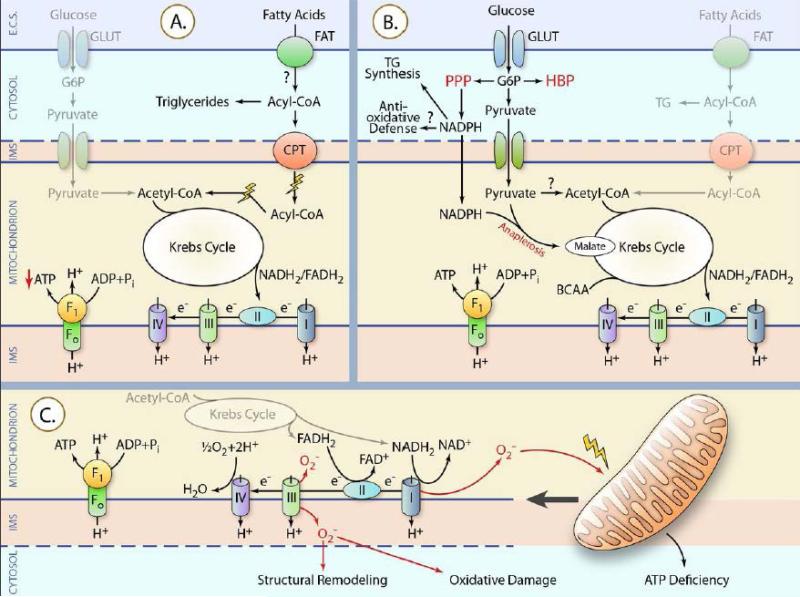
Figure 2. Alterations in substrate metabolism and mitochondria during the development of HF.
Bold lines indicate pathways reported to be activated. Thin lines represent pathways reported decreased. The question marks imply unknown changes or inconsistent observations. IMS: mitochondrial intermembrane space; GLUT: glucose transporter; FAT: fatty acid transporter. (A) Fatty acid oxidation is impaired in cardiac hypertrophy and failure leading to reduced ATP production. (B) HBP: hexosamine biosynthetic pathway; PPP: pentose phosphate pathway; TG: triglyceride. Most evidence suggests that glucose oxidation is unchanged in compensated hypertrophy and decreased in HF, but discrepancies exist. In contrast, several non-ATP-generating pathways of glucose metabolism (HBP, PPP, anaplerosis) are induced. (C) Increased generation of mitochondrial ROS (perhaps due to changes in the electron transport chain) causes direct mitochondrial damage, which may further increase mitochondrial ROS production to create a vicious cycle. Mitochondrial damage results in ATP deficiency. Mitochondrial ROS may also cause oxidative damage to other cellular components and may contribute to adverse structural remodeling. (Illustration Credit: Ben Smith)
Glucose utilization in HF
In contrast to FA utilization, data on cardiac glucose utilization are less consistent (summarized in Table 2). In the presence of systolic dysfunction, cardiac glucose uptake was found to be decreased following aortic constriction in mice57 but unchanged in rats with myocardial infarction,58 and increased in Dahl salt-sensitive rats.48 In compensated hypertrophy, induced by abdominal aortic constriction, glycolysis was modestly increased without changes in glucose oxidation.56, 59 By assessing substrate oxidation at various time points following aortic constriction in rats, we observed that cardiac glucose oxidation tended to increase initially, but was unchanged relative to controls in the phase of compensated hypertrophy and ultimately decreased when systolic dysfunction occurred.50 The impaired glucose oxidation that parallels systolic dysfunction might be due in part to mitochondrial dysfunction or by reduced expression of genes involved in glycolysis and glucose oxidation or to decreased abundance of the PDH complex.48, 60 However, in infarcted rat hearts, we observed no change in glucose oxidation rate despite manifest systolic dysfunction.58 Interestingly, spontaneously hypertensive rats, at the cardiac hypertrophy stage and prior to HF were found to have higher glucose oxidation rates54 or increased flux through the PDH complex61 relative to control rats. In addition, Osorio et al. showed increased glucose oxidation rates in failing dog hearts induced by rapid pacing,49 and Dávila-Román et al. demonstrated higher total rates of glucose utilization in patients with idiopathic dilated cardiomyopathy.62 It is unlikely that these differences can be explained by methodological differences. We therefore suggest that in contrast to changes in FA oxidation, glucose oxidation does not correlate with contractile function in HF, but that changes in glucose oxidation may depend on both the stage and the etiology of HF.
Anaplerosis in HF
Another factor that may influence the interpretation of glucose oxidation results is the fact that pyruvate may be channeled into anaplerotic pathways (Fig. 2B). In hypertrophied rat hearts induced by aortic constriction, Sorokina et al. showed that glucose oxidation by PDH is unchanged despite increased glycolysis. More importantly, they identified an increase in anaplerotic flux into the Krebs cycle probably via pyruvate carboxylation by malic enzyme.17 The increase in this alternative pathway of pyruvate utilization may account for the mismatch between glycolysis and glucose oxidation that is commonly seen in models of pressure-overload induced cardiac hypertrophy. This hypertrophy-associated anaplerotic change has also been confirmed in mice with aortic constriction 63 and in hyperthyroid rats.64 Thus, induction of anaplerotic pathways appears to be a hallmark of metabolic remodeling in cardiac hypertrophy. Considering that hypertrophic growth requires increased supply of amino acids and nucleic acids derived from precursors in the Krebs cycle, then activation of anaplerotic pathways may be required to maintain Krebs cycle function. However, this compensatory mechanism might also have unfavorable consequences (see Fig. 2B and 3 for illustration). For example, increased flux of pyruvate through anaplerotic pathways reduces its availability for oxidation by the PDH complex. As a result, pyruvate oxidation might not sufficiently compensate for the impaired FA oxidation, leading to energetic inefficiency of the Krebs cycle.17 Furthermore, increased flux through malic enzyme may also affect cardiac function by consuming NADPH,65 which is required for triglyceride synthesis and for defending against oxidative stress. Therefore, the role of anaplerotic changes particularly in relation to the nature of cardiac hypertrophy remains incompletely understood. This knowledge gap is due in part to the technical challenges inherent in measuring anaplerosis.
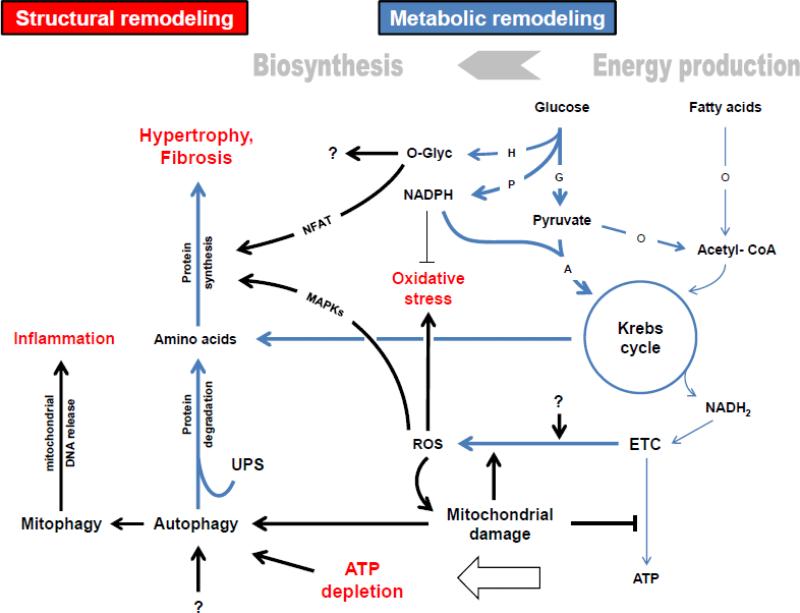
Figure 3. Overview of metabolic remodeling and proposed mechanisms linking it to other processes in the progression to HF.
H: Hexosamine biosynthetic pathway (HBP); P: Pentose phosphate pathway (PPP); G: Glycolysis; A: Anaplerosis; O: Oxidation; ETC: Electron transport chain; ROS: reactive oxygen species; UPS: ubiquitin-proteasome system. Metabolic pathways are blue. Bold lines indicate pathways/ processes that are increased or dominant. Thin lines represent pathways/ processes that are decreased. The question marks imply unknown causes/ effects. In general, metabolic remodeling in cardiac hypertrophy and failure is characterized by a shift away from energy production to activation of biosynthetic pathways required for structural remodeling processes such as ventricular hypertrophy and fibrosis. Particularly, fatty acid oxidation is decreased and may not be sufficiently compensated given the lack of increase in glucose oxidation. These alterations and further mitochondrial defects result in ATP depletion. Instead of being oxidized, pyruvate may be preferentially used for anaplerosis to maintain Krebs cycle moieties, which might be increasingly channeled into protein synthesis. Hypertrophic mediators such as MAPKs and NFAT are activated as a result of increased mitochondrial ROS and flux through the HBP, respectively. Overproduction of mitochondrial ROS causes oxidative damage. Although the flux through the PPP is increased, anti-oxidative defense might be inadequate due to the consumption of NADPH by the anaplerotic malic enzyme. Mitochondrial damage and ATP depletion may stimulate autophagy. Increased activity of autophagy and the UPS may contribute to hypertrophy by providing amino acids and other metabolites. Increase in mitophagy may trigger myocardial inflammation by releasing mitochondrial DNA.
Mitochondrial biogenesis and function in HF
The above described alterations in substrate oxidation and anaplerosis occur at the level of mitochondria. It is therefore not surprising that many studies have described significant mitochondrial changes in HF (Table 2). In rat hearts with systolic dysfunction induced by aortic constriction, we identified abnormal mitochondrial morphology, reduced mitochondrial volume density and altered levels of most ETC proteins with the majority being decreased. 66 Mitochondrial proteomic remodeling in HF has also been demonstrated in mice with pressure overload, which is primarily characterized by altered abundance of proteins involved in ETC and substrate metabolism.60 These results suggest that perturbed mitochondrial biogenesis is an important feature of pressure overload-induced HF. Consistent with this notion, expression of PGC-1α is decreased in systolic HF as has been shown by us 43, 66 and others.67, 68 There is limited evidence for mitochondrial alterations in other models of HF. In failing rat hearts caused by myocardial infarction, we found normal mRNA expression of PGC-1α. However, the expression of p38 MAPK, which could modulate PGC-1α activity was reduced.58 In HF patients of diverse etiologies, Karamanlidis et al. observed decreased mitochondrial DNA (mtDNA) content accompanied by reductions in mtDNA-encoded proteins. Of note, these changes were associated with increased abundance of PGC-1α protein but decreased expression of ERRα and Tfam both at mRNA and protein levels. 69 These findings suggest that mitochondrial biogenesis signaling is depressed in advanced HF and that posttranslational modulation of PGC-1α may play a role.
Little is known about the regulation of mitochondrial biogenesis in compensated hypertrophy. We found a significant decline in mRNA expression of PGC-1α, ERRα and Tfam at times when left ventricular ejection fraction was still preserved (unpublished observations), suggesting repressed mitochondrial biogenesis pathways in compensated hypertrophy. By contrast, in hypertrophied mouse hearts with normal function induced by chronic angiotensin II infusion, Dai et al. reported increased mRNA expression of PGC-1α, ERRα and Tfam. However, these hearts exhibited increased mitochondrial damage and mitophagy.70 Therefore, the induction of mitochondrial biogenic signaling could represent a compensatory mechanism triggered by mitochondrial injury and loss. Collectively, although the regulation of mitochondrial biogenesis and its signaling in cardiac hypertrophy and failure is still incompletely understood, available evidence supports the concept that cardiac mitochondria are affected early and undergo progressive remodeling during the development of cardiac hypertrophy and failure.
Mitochondrial dysfunction as evidenced by decreased mitochondrial respiration and reduced ATP production is consistently observed when systolic dysfunction occurs in various models of HF.50, 71, 72 Interestingly, we and others found that mitochondrial respiration in pressure-overloaded hearts initially increased and did not fall until systolic dysfunction developed.50 Considering that mitochondrial damage is probably an early event in the progression to HF, this biphasic response of mitochondrial respiratory capacity might reflect unknown compensatory mechanisms, which can sustain oxidative phosphorylation during the stage of compensated hypertrophy but are lost as cardiac dysfunction develops.
Cardiac energetics in HF
Mitochondrial dysfunction in advanced HF has been linked to impaired myocardial energetics. Studies in animal models and humans have reported a decrease in the PCr/ATP ratio, ATP content, and the ATP flux through CK in advanced HF with reduced EF. Given that sufficient ATP supply is essential for normal cardiac function, this change has been suggested to be responsible for the transition to systolic dysfunction.6, 73 If this were true, it would be reasonable to hypothesize that ATP depletion should be detectable in compensated hypertrophy just prior to the onset of systolic dysfunction. Kato et al. showed a 12% decrease in the PCr/ATP ratio in compensated hypertrophy in Dahl salt-sensitive rats.48 In contrast, Dodd et al. found that both absolute content of high-energy phosphate metabolites and the PCr/ATP ratio were normal in the hypertrophied heart of spontaneously hypertensive rats.61 In hearts of hypertensive heart disease patients with normal EF, the PCr/ATP ratio was found to be decreased74 but the absolute content of ATP and PCr have been shown to be preserved.5, 75 Thus, data in hypertrophied hearts with normal systolic function do not support a model that impaired ATP-producing capacity might be an antecedent to the transition to decompensated HF. It is possible that the lack of a clear conclusion could be due in part to methodological limitations in using PCr/ATP ratios to quantify myocardial energetics. However, an alternative conclusion is that decreased levels of high-energy phosphates do not cause HF per se, but represent an adaptation to decreased pump function.
In advanced HF, there is consistent evidence of decreased myocardial ATP availability, but it remains unclear if the reduction in ATP abundance contributes to contractile dysfunction. Approaches that would directly enhance myocardial ATP abundance in the failing heart are required to address this question. In a recent study, the xanthine oxidase inhibitor allopurinol was shown to acutely improve the relative and absolute concentrations of myocardial high-energy phosphates and ATP flux through CK in the failing human heart. Nevertheless, whether cardiac function changed in response to this energetic improvement was unknown.76 Furthermore, in a retrospective study in a large cohort of HF patients, Struthers et al. found no beneficial effects of long-term allopurinol treatment on cardiovascular mortality.77 Of note, since allopurinol may exert complex systemic effects, these negative results can neither confirm nor refute a potential role of energetic impairment in HF pathogenesis. Although the correlation between cardiac energy status and survival in HF patients is striking,78 this correlation could also represent worsening mitochondrial function that occurs in concert with progression of HF severity. Although this alternative hypothesis remains to be disproved, studies on cardiac energetics in HF are attractive, because they may provide not only mechanistic insights and potential new therapeutic strategies but also valuable prognostic information.
Normal cardiac function requires the coordinated regulation and interaction of several physiological and signaling systems in addition to energy supply. In rats developing HF due to pressure overload, we calculated the ATP amount generated from substrate oxidation and found that the ratio of cardiac power to ATP was consistently reduced as soon as cardiac hypertrophy developed.50 This result provides indirect evidence for inefficient transduction from ATP into mechanical power. Before the onset of systolic dysfunction, the heart undergoes severe structural changes (e.g. hypertrophy, fibrosis, dilatation). It is therefore likely that such profound remodeling of the contractile apparatus may involve mechanisms that significantly affect cardiac function independently of ATP supply. Potential mechanisms range from changes in the extracellular matrix (myocardial fibrosis, inflammation)79 to impaired calcium handling80 or modification of sarcomeric proteins,81 which have all been reviewed by others.
Mitochondrial ROS and its link to cardiac metabolism in HF
While metabolic and structural alterations in the failing heart have been increasingly well characterized, little is known about mechanisms that drive the remodeling process in HF. Increased cardiac ROS levels have been implicated in HF, but the role of ROS in the pathogenesis of HF was questioned as a result of disappointing outcomes of antioxidant interventions in human studies.82, 83 However, in a recent animal study, Dai et al. demonstrated that angiotensin II increased mitochondrial ROS, leading to mitochondrial damage, activation of mitogen-activated protein kinases and finally cardiac hypertrophy and fibrosis, 70 Another study by the same group also showed that mitochondria-targeted antioxidant treatment but not non-targeted ROS scavenging with N-acetyl cysteine ameliorated cardiomyopathy induced by chronic angiotensin II infusion.84 Of note, mouse hearts in this model of pressure overload presented mild diastolic dysfunction without changes in left ventricular ejection fraction,70 indicating an early stage of compensated hypertrophy. These results suggest that increased mitochondrial ROS could be an early event triggering structural remodeling and mitochondrial defects in hypertensive heart disease. Figure 2C illustrates the generation of mitochondrial ROS and its potential impact on energy metabolism and other pathological processes.
The regulation of cellular ROS homeostasis is complex and only partially understood. In the mitochondria, ROS are produced mainly from the electron transport chain (ETC) particularly from complex I and III. Therefore, changes in the ETC may favor “electron leakage” and consequently ROS formation.36 The elevation in ROS production may further affect the function of the ETC leading to a vicious cycle (Figures 2C, 3). Studies in various tissues have linked FA utilization to increased ROS levels.85, 86 In diabetic cardiomyopathy, ROS have also been associated with myocardial lipid accumulation, lipotoxicity and decreased cardiac efficiency on the basis of mitochondrial uncoupling.87 The failing heart is believed to be subjected to higher levels of free fatty acids, probably as a result of increased lipolysis.39 In addition, models of HF have been associated with myocardial lipid accumulation.88, 89 As a result, FA utilization has been considered unfavorable for the stressed heart because of increasing ROS production.39 However, in animal models with a cardiac specific loss of ACC-2, increased myocardial FA utilization did not adversely impact left ventricular remodeling following pressure overload. This might represent a scenario of complete substrate oxidation without increased generation of mitochondrial ROS.63 Furthermore, cardiac FA oxidation is depressed during HF progression and there is little direct evidence in HF models that FA utilization may increase ROS production. Thus, the causes of increased mitochondrial ROS in the progression to HF remain unclear and warrant further investigation.
Changes in the pentose phosphate pathway in HF
The regulation of the cellular redox environment is tightly linked to substrate metabolism via the PPP as an important source of NADPH. NADPH is required for the generation of cytosolic ROS, which at low levels, are involved in proliferation and survival signaling. Conversely, NADPH also maintains the pool of reduced antioxidants such as glutathione, which are crucial defenses against oxidative stress. 36 Hence, the PPP may play a dual role in the regulation of redox homeostasis. While much effort has been made to characterize glycolysis and glucose oxidation, little is known about the regulation of the PPP in HF. In dogs subjected to pacing-induced HF, increased superoxide levels were attributed to increased activity of G6PD, the key oxidative enzyme of the PPP.90 If the effects of superoxide are indeed damaging, this activation of the PPP in the failing heart could be considered detrimental. However, G6PD deficient mice developed higher oxidative stress and worsened contractile function following myocardial infarction or aortic constriction.91 In Dahl salt-sensitive rats, Kato et al. also found that the flux through the PPP progressively increased during the development of HF. Importantly, they demonstrated that treatment with dichloroacetate (DCA) further increased this flux, which was associated with improved cardiac function.48 Taken together, the PPP is activated in HF models. Although superoxide production may consequently increase, currently available data support the notion that higher flux through the PPP in HF may represent a compensatory mechanism whose further activation could be of therapeutic relevance.
Changes in the hexosamine biosynthetic pathway in HF
The HBP, which is linked to metabolism of glucose, FAs and amino acids, has been implicated in various models of heart disease. The HBP and HBP-dependent O-GlcNAcylation are induced in the diabetic heart, which may result in increased apoptosis,92 mitochondrial dysfunction93 and impaired Ca2+ cycling.94 Recently, the role of the HBP in HF has also received special interest. By employing various models of HF including aortic constriction, myocardial infarction, and hypertensive rats, Lunde et al. demonstrated that global O-GlcNAcylation was increased by at least 40% in hypertrophied and failing hearts. They also confirmed these findings in patients with aortic stenosis.95 In endothelial cells, increased flux through the HBP has been attributed to overproduction of mitochondrial superoxide.96 Considering that mitochondrial ROS formation also increases in cardiac hypertrophy,70 mitochondrial ROS might represent a potential mechanism for activation of the HBP in the heart.
Whereas the induction of O-GlcNAcylation in the progression of HF is well described, less is known about the functional relevance of these changes. In a cardiomyocyte model of hypertrophy, Facundo et al. found that increased flux through the HBP and the resulting increase in O-GlcNAcylation were responsible for hypertrophic growth via activation of the nuclear factor of activated T-cells (NFAT).97 This finding is highly relevant because it indicates that induction of the HBP is an early event that triggers myocardial remodeling. Because NFAT signaling has been associated with pathological hypertrophy,98 one might consider this activation of the HBP to be detrimental. However, reducing O-GlcNAcylation in mice with myocardial infarction exacerbated ventricular dysfunction.99 Thus, although the HBP activates signaling pathways that initiate cardiac remodeling, additional signaling pathways linked to the HBP may also promote favorable effects. Further studies are therefore needed that focus on the characterization and targeted modulation of intracellular signaling pathways that are regulated by HBP, in the context of clinical and experimental models of HF.
Autophagy and its link to cardiac metabolism in HF
Metabolic remodeling in HF is also associated with changes in autophagy. Autophagy is a highly conserved process by which organelles and large cellular components are degraded. The products of autophagy (amino acids, fatty acids, sugars, and nucleosides) may then be channeled into both energy generating and biosynthesis pathways. Under normal conditions, basal autophagy is crucial by eliminating damaged organelles and misfolded proteins. In states of nutrient deprivation such as starvation or ischemia, autophagic activity is increased, which may support cell function by mobilizing endogenous nutritional sources. 100 In models of HF including pressure overload101 and myocardial infarction,102 autophagy has also been shown to be induced. Mechanisms for the activation of autophagy in HF are less clear. A number of changes observed in the hypertrophied and failing heart such as energy depletion, AMPK activation,103 mitochondrial ROS overproduction and damaged mitochondria are strong mediators of autophagy in various settings.100, 104 However, the potential contribution of these events to autophagic activation in HF remains to be verified.
Autophagy has also been implicated in ventricular remodeling. In an elegant study, Cao et al. provided evidence indicating that the activation of autophagy, although degradative in nature, is essential for cardiac hypertrophy.105 Since cell growth requires stimulation of biosynthesis pathways, these data may appear paradoxical at first glance. However, they are supported by earlier studies showing that blunting of protein degradation mediated by the UPS may attenuate cardiac hypertrophy.106, 107 Together, these data indicate that cardiac hypertrophy and remodeling are dynamic reconstructive processes, in which degradation of certain cellular components by autophagy and the UPS is an important antecedent that might stimulate the biosynthesis of new structures.
Due to the essential role of autophagy in ventricular remodeling, one might expect that inhibiting autophagy may prevent cardiac hypertrophy and failure. However, studies in knockout models with suppressed autophagy have delivered inconsistent results.108, 109 Given the highly complex regulation of autophagy, it is reasonable to assume that specific changes in the induction or targeting of autophagy but not the autophagic flux itself may affect functional outcome. For example, Oka et al. recently demonstrated in mice with aortic constriction that mitochondrial DNA that escapes from autophagy causes cardiac inflammation and failure.110 Therefore, the regulation and the role of autophagy in HF remain to be fully elucidated. Because both the induction of autophagy and the processing of autophagic products are linked to metabolism, the relationship between metabolic remodeling in HF and the regulation of autophagy is an attractive target for future studies.
Figure 3 schematically illustrates the interaction between metabolic and structural remodeling of the heart in the progression to HF. Metabolic remodeling in HF is characterized by defects in energy production and changes in metabolic pathways that are involved in the regulation of essential cellular functions. The figure attempts to illustrate that the inability to produce sufficient ATP is only one aspect of metabolic remodeling. This model promotes the hypothesis that during HF, other non ATP-producing processes, which might play a relatively small role in the non-stressed heart, may gain more importance and directly affect contractile function as illustrated in Figure 4. This schematic also provides a roadmap towards identifying targets that could be translated to HF therapeutics.
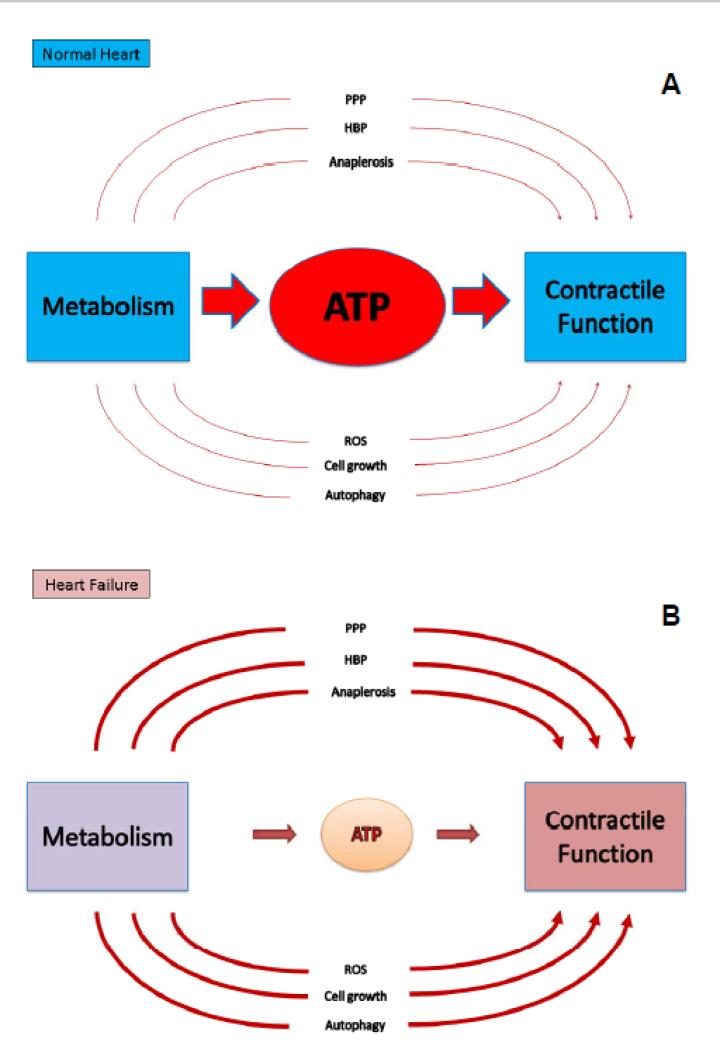
Figure 4. Simplified illustration of the relationship between cardiac metabolism and contractile function, reflecting the role of ATP production and non-ATP producing processes.
In the normal heart under steady state conditions (A), the main metabolic task is to produce ATP for contractile function. Alternative pathways such as the pentose phosphate pathway (PPP), the hexosamine biosynthetic pathway (HBP), the activation of autophagy or the production of reactive oxygen species (ROS) and others play a minor role. In the progression to HF (B), ATP producing capacity is reduced. In addition, the alternative pathways may be activated to various degrees. Such activation may provide additional metabolic mechanisms influencing contractile function that could represent potential new metabolic targets for therapy.
POTENTIAL METABOLIC THERAPY FOR HEART FAILURE
Modulation of cardiac fatty acid metabolism
Modulation of cardiac FA utilization has been a target of metabolic therapy in HF for the past decade. The failing heart has been suggested to be in a state of “fatty-acid overload”, suffering from “increased oxygen wastage” and oxidative stress or from accumulation of cardiotoxic lipid derivatives.39 Consequently, many pharmacological approaches sought to reduce cardiac FA utilization and some studies revealed beneficial effects.111 However, the concept of inhibiting FA utilization has now been challenged by contradictory results of studies in animal models of HF subjected to high-fat diets.112 For example, Raher et al. showed that high-fat diet in mice induced myocardial insulin resistance and exacerbated left ventricular remodeling caused by aortic constriction.113 In contrast, Okere et al. reported decreased cardiac hypertrophy and improved contractile function following treatment with high-fat diet in hypertensive Dahl salt-sensitive rats.114 In addition, acute inhibition of FA oxidation in a population of patients with end stage HF led to significant worsening of left ventricular function.115 The plethora of studies on the modulation of cardiac FA utilization and its effects on contractile function have been extensively reviewed and discussed elsewhere.111, 112, 116 Although a mechanistic link between cardiac FA metabolism and contractile function remains controversial, because some therapeutic strategies targeting cardiac FA utilization have suggested functional consequences of manipulating this pathway, additional investigation of therapies that modulate FA metabolism in the failing heart are warranted.
Modulation of cardiac glucose metabolism
A small number of studies suggest that direct modulation of glucose utilization could also be beneficial. In mice subjected to pressure overload, life-long cardiac-specific overexpression of GLUT-1 increased glucose uptake and glycolysis and improved cardiac energetics and function.117 Although the enhanced ATP production may account for the improved functional outcome in these mice, possible changes in accessory pathways of glucose metabolism such as the PPP and the HBP could also play a role but were not evaluated. Because the increase in glucose uptake in this model preceded the induction of pressure overload, it remains to be determined if increasing glucose uptake after the onset of pressure overload or prior to the transition to HF will have any effect.
Among the limited pharmacological strategies to stimulate glucose utilization directly in HF, DCA has been the most widely studied compound. DCA indirectly activates the PDH complex and thus glucose oxidation, which is considered the mechanism of cardioprotection in ischemia-reperfusion models.118, 119 The use of DCA to treat HF has been tested in some animal models and humans. In patients with advanced HF, Bersin et al. showed that a 30-minute-infusion of DCA stimulated myocardial lactate consumption, which was accompanied by decreased oxygen consumption and increased cardiac work.120 However, not all human studies have demonstrated short-term benefit121 and long-term clinical trials have never been performed in HF perhaps in part because of neuropathy that has been reported when DCA was used long-term in cases of inherited mitochondrial disorders.122 In Dahl salt-sensitive rats with hypertension, DCA treatment reduced ventricular hypertrophy, improved cardiac function and survival, which was associated with increased myocardial glucose uptake and energy reserves. Of note, treatment with DCA also enhanced flux through the PPP, which was shown to be the mechanism by which DCA reduced oxidative damage and apoptosis in cardiomyocytes.48 Interestingly, long-term DCA treatment also increased flux through PDH and reduced cardiac hypertrophy in rats with hyperthyroidism.64 Collectively, these results suggest that augmenting cardiac glucose utilization could represent a potential strategy to treat HF, which may not only improve cardiac energetics, but also attenuate oxidative stress and structural remodeling. However, additional studies with less toxic agents are required.
Modulation of cardiac anaplerosis
As described above, anaplerotic flux through pyruvate carboxylation is elevated in compensated hypertrophy, which may affect cardiac lipogenesis and antioxidant defense mechanisms by consuming NADPH. In hypertrophied rat hearts, Pound et al. demonstrated that acute activation of PDH by DCA competed for pyruvate and partially reduced its flux through malic enzyme, which led to normalization of the myocardial triglyceride pool and improved contractile function.65 These results may suggest therapeutic relevance of inhibiting pyruvate carboxylation in cardiac hypertrophy. Furthermore, they provide an additional protective mechanism beyond the energetic aspect of improving cardiac glucose oxidation. However, the role of long-term inhibition of anaplerotic pathways in cardiac hypertrophy and failure remains to be elucidated.
Cardiac function may also be enhanced by targeting other anaplerotic routes. If the induction of anaplerosis through pyruvate carboxylation is a compensatory mechanism to maintain Krebs cycle moieties,17 early supplementation with an anaplerotic substrate may allow pyruvate to be oxidized by PDH. We found that feeding aortic-constricted rats with triheptanoin-enriched diets, which may exert anaplerotic effects by providing the Krebs cycle with propionyl-CoA, attenuated ventricular hypertrophy and diastolic dysfunction. Importantly, these effects were accompanied by increased cardiac glucose oxidation.123 Although the mechanistic role of anaplerosis in these treatments remains to be substantiated, the results again point to anaplerotic pathways as a potential therapeutic target that may modulate metabolic and structural remodeling in HF.
AMPK activation
Cardiac hypertrophy and failure are associated with increased activity of AMPK, which may be attributable to increased AMP/ATP ratio, ROS or Ca2+ load.124 Pharmacological interventions that further increase AMPK activity in HF models have resulted in reduced ventricular remodeling and improved cardiac function.72, 124 These findings suggest that the activation of AMPK in HF may be adaptive and amenable to pharmacological manipulation. Activation of AMPK induces a wide range of effects that coordinately improve cardiac energetics and counteracts ventricular remodeling. For example, AMPK activation may acutely stimulate both glucose and fatty acid oxidation, thereby enhancing energy production. This mechanism has been shown to exist in normal hearts or following ischemia and reperfusion 125-127 and may also apply to HF. Activation of AMPK could also increase energy metabolism by increasing PGC-1α activity,128 which would induce genes of fatty acid utilization. Furthermore, a PGC-1α mediated increase in mitochondrial biogenesis could potentially compensate for mitochondrial damage and loss in HF. This potential mechanism has been suggested in mice with myocardial infarction72 but further studies in other models of HF are warranted.
Activation of AMPK also alleviates ventricular remodeling in terms of hypertrophy, myocardial fibrosis and inflammation.72, 129 Various potential mechanisms may account for these beneficial effects. AMPK reduces ROS-related oxidative stress in endothelial cells by inducing the antioxidant thioredoxin.130 Since ROS can lead to mitochondrial damage and cardiac structural remodeling,70 inhibition of ROS signaling by AMPK, could potentially attenuate these pathological processes. Similarly, AMPK plays an important role in the regulation of autophagy, which has been implicated in ventricular hypertrophy 105 and inflammation.110 Given that ventricular remodeling may occur early in the development of HF, studies examining the impact of early AMPK activation on pathological remodeling are therefore warranted.
AMPK can be activated by a number of drugs. A long-used agonist is AICAR, which activates AMPK in various tissues including the heart by forming the AMP-mimetic “ZMP”.45 Although AICAR has been extensively used in animal studies, its role in humans is still unclear.131 The anti-diabetic drug metformin has also been shown to activate AMPK in the heart. Although metformin was initially contraindicated in advanced HF due to the risk of lactic acidosis, recent studies have shown beneficial effects of early administration of metformin in models of HF.72, 132, 133 Metformin is believed to activate AMPK by inhibiting complex I leading to increased cellular AMP levels.134, 135 However, complex I-independent mechanisms have also been proposed.136 Because metformin may also act independently of AMPK,137, 138 caution is warranted when attributing treatment effects of metformin to AMPK activation.
Activation of cardiac glucagon-like peptide-1 (GLP-1) receptors
GLP-1 is an incretin hormone that is secreted by neuroendocrine cells in the gut in response to feeding. Once secreted, GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4). DPP-4 inhibitors and GLP-1 agonists are now commonly used in the treatment of diabetes as increased GLP-1 or activation of its receptors in the beta cell or GI tract stimulates insulin secretion and delays gastric emptying thereby improving metabolic homeostasis.139
GLP-1 receptors are expressed in the heart. Activating cardiac GLP-1 receptors in models of ischemia reperfusion have consistently shown beneficial effects in terms of reduced infarct size and improved contractile function.140 In pacing-induced HF in dogs, Nikolaidis et al. found a significant improvement in ventricular function following a 48-hour infusion of GLP-1, which was associated with increased cardiac glucose uptake and insulin sensitivity.141 In spontaneously hypertensive rats receiving a chronic infusion of GLP-1, Poornima et al. also reported improved survival and cardiac function.142 Since GLP-1 treatment increased insulin secretion in these rats, it is unclear whether GLP-1 or insulin accounted for these effects. In contrast, in our rat model of pressure overload, we also observed improved functional outcome and preserved cardiac glucose oxidation without changes in insulin levels,143 which suggest insulin-independent effects of GLP-1. There is also evidence that GLP1 might mediate cardiovascular effects via GLP-1 receptor independent mechanisms.144
While the protective effects of GLP-1 have been demonstrated by numerous studies, less is known about the underlying molecular mechanisms. In HF models, the protective effects of GLP-1 are mostly associated with improved glucose utilization. However, the mechanisms by which GLP-1 influences glucose metabolism and the relationship between changes in glucose metabolism and cardiac function in this context is incompletely understood.
CONCLUSIONS
Heart failure is associated with profound changes in cardiac metabolism. Metabolic remodeling in HF is characterized by decreased cardiac energy production that may result from progressive impairments in substrate utilization and mitochondrial biogenesis and function. In addition to ATP deficiency, metabolic remodeling involves changes in metabolic pathways that regulate essential, non-ATP-generating cellular processes such as growth, redox homeostasis and autophagy. Therefore, modulating cardiac metabolism may also affect these critical processes and improve cardiac function by mechanisms beyond ATP production. Nevertheless, the exact mechanisms linking metabolic changes to other pathological processes in HF are still poorly understood. Future mechanistic investigations are therefore needed to decipher this complex network and improve the effectiveness of metabolic therapies for HF.
ACKNOWLEDGEMENTS
We wish to thank Dr. Michael Schwarzer for help in reviewing the vast literature and the many discussions.
SOURCES OF FUNDING
TD is supported by grant DO602/9-1 from the Deutsche Forschungsgemeinschaft. EDA is supported by grant R01HL108379 from the National Institutes of Health.
Non-standard Abbreviations and Acronyms
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- CK
creatine kinase
- CPT
carnitine palmitoyltransferase
- DCA
dichloroacetate
- ERR
estrogen-related receptors
- ETC
electron transport chain
- FA
fatty acid
- G6P
glucose 6-phosphate
- G6PDH
glucose 6-phosphate dehydrogenase
- GLP-1
glucagon-like peptide-1
- GLUT
glucose transporter
- HBP
hexosamine biosynthetic pathway
- HF
heart failure
- MAPK
mitogen-activated protein kinase
- MCD
malonyl-CoA decarboxylase
- NFAT
nuclear factor of activated T-cells
- NRF
nuclear respiratory factors
- PCr
phosphocreatine
- PDH
pyruvate dehydrogenase
- PGC-1
peroxisome proliferator-activated receptor γ coactivator 1
- PPAR
peroxisome proliferator-activated receptor
- PPP
pentose phosphate pathway
- ROS
reactive oxygen species
- Tfam
mitochondrial transcription factor A
- UPS
ubiquitin-proteasome system
Footnotes
DISCLOSURES
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ingwall JS. Atp and the heart. Kluwer Academic; 2002. [Google Scholar]
- 2.Opie LH. Heart physiology: From cell to circulation. Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 3.Gibbs CL. Cardiac energetics. Physiol Rev. 1978;58:174–254. doi: 10.1152/physrev.1978.58.1.174. [DOI] [PubMed] [Google Scholar]
- 4.Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 5.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)p-sloop magnetic resonance spectroscopy. Journal of the American College of Cardiology. 2002;40:1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 7.Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Craig JC. Dual carbon-labeled isotope experiments using d-[6-14c] glucose and l-[1,2,3-13c3] lactate: A new approach for investigating human myocardial metabolism during ischemia. Journal of the American College of Cardiology. 1985;5:1138–1146. doi: 10.1016/s0735-1097(85)80016-4. [DOI] [PubMed] [Google Scholar]
- 8.Gertz EW, Wisneski JA, Stanley WC, Neese RA. Myocardial substrate utilization during exercise in humans. Dual carbon-labeled carbohydrate isotope experiments. J Clin Invest. 1988;82:2017–2025. doi: 10.1172/JCI113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovascular research. 2000;45:279–293. doi: 10.1016/s0008-6363(99)00263-1. [DOI] [PubMed] [Google Scholar]
- 10.Glatz JF, Luiken JJ, Bonen A. Involvement of membrane-associated proteins in the acute regulation of cellular fatty acid uptake. J Mol Neurosci. 2001;16:123–132. doi: 10.1385/JMN:16:2-3:123. discussion 151-127. [DOI] [PubMed] [Google Scholar]
- 11.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 12.Banke NH, Wende AR, Leone TC, O'Donnell JM, Abel ED, Kelly DP, Lewandowski ED. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor pparalpha. Circulation research. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell metabolism. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell metabolism. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Cook GA, Edwards TL, Jansen MS, Bahouth SW, Wilcox HG, Park EA. Differential regulation of carnitine palmitoyltransferase-i gene isoforms (cpt-i alpha and cpt-i beta) in the rat heart. J Mol Cell Cardiol. 2001;33:317–329. doi: 10.1006/jmcc.2000.1304. [DOI] [PubMed] [Google Scholar]
- 17.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase i activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 18.McGarry JD, Mills SE, Long CS, Foster DW. Observations on the affinity for carnitine, and malonyl-coa sensitivity, of carnitine palmitoyltransferase i in animal and human tissues. Demonstration of the presence of malonyl-coa in non-hepatic tissues of the rat. Biochem J. 1983;214:21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zammit VA, Fraser F, Orstorphine CG. Regulation of mitochondrial outer-membrane carnitine palmitoyltransferase (cpt i): Role of membrane-topology. Adv Enzyme Regul. 1997;37:295–317. doi: 10.1016/s0065-2571(96)00015-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim YS, Kolattukudy PE. Purification and properties of malonyl-coa decarboxylase from rat liver mitochondria and its immunological comparison with the enzymes from rat brain, heart, and mammary gland. Arch Biochem Biophys. 1978;190:234–246. doi: 10.1016/0003-9861(78)90273-4. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton C, Saggerson ED. Malonyl-coa metabolism in cardiac myocytes. Biochem J. 2000;350(Pt 1):61–67. [PMC free article] [PubMed] [Google Scholar]
- 22.Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K, Lopaschuk GD. Absence of malonyl coenzyme a decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114:1721–1728. doi: 10.1161/CIRCULATIONAHA.106.642009. [DOI] [PubMed] [Google Scholar]
- 23.Ussher JR, Lopaschuk GD. Targeting malonyl coa inhibition of mitochondrial fatty acid uptake as an approach to treat cardiac ischemia/reperfusion. Basic Res Cardiol. 2009;104:203–210. doi: 10.1007/s00395-009-0003-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Huang H, Yuan CL, Keung W, Lopaschuk GD, Stanley WC. Metabolic response to an acute jump in cardiac workload: Effects on malonyl-coa, mechanical efficiency, and fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2008;294:H954–960. doi: 10.1152/ajpheart.00557.2007. [DOI] [PubMed] [Google Scholar]
- 25.Henning SL, Wambolt RB, Schonekess BO, Lopaschuk GD, Allard MF. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation. 1996;93:1549–1555. doi: 10.1161/01.cir.93.8.1549. [DOI] [PubMed] [Google Scholar]
- 26.Aerni-Flessner L, Abi-Jaoude M, Koenig A, Payne M, Hruz PW. Glut4, glut1, and glut8 are the dominant glut transcripts expressed in the murine left ventricle. Cardiovasc Diabetol. 2012;11:63. doi: 10.1186/1475-2840-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abel ED. Glucose transport in the heart. Front Biosci. 2004;9:201–215. doi: 10.2741/1216. [DOI] [PubMed] [Google Scholar]
- 28.Entman ML, Bornet EP, Van Winkle WB, Goldstein MA, Schwartz A. Association of glycogenolysis with cardiac sarcoplasmic reticulum: Ii. Effect of glycogen depletion, deoxycholate solubilization and cardiac ischemia: Evidence for a phorphorylase kinase membrane complex. J Mol Cell Cardiol. 1977;9:515–528. doi: 10.1016/s0022-2828(77)80367-2. [DOI] [PubMed] [Google Scholar]
- 29.Kusuoka H, Marban E. Mechanism of the diastolic dysfunction induced by glycolytic inhibition. Does adenosine triphosphate derived from glycolysis play a favored role in cellular ca2+ homeostasis in ferret myocardium? J Clin Invest. 1994;93:1216–1223. doi: 10.1172/JCI117075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 31.Ussher JR, Jaswal JS, Lopaschuk GD. Pyridine nucleotide regulation of cardiac intermediary metabolism. Circulation research. 2012;111:628–641. doi: 10.1161/CIRCRESAHA.111.246371. [DOI] [PubMed] [Google Scholar]
- 32.Kornberg HL. The regulation of anaplerotic enzymes in e. Coli. Bull Soc Chim Biol (Paris) 1967;49:1479–1490. [PubMed] [Google Scholar]
- 33.Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: Food for thought. Cardiovascular research. 2011;90:210–219. doi: 10.1093/cvr/cvr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell RR, 3rd, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest. 1991;87:384–390. doi: 10.1172/JCI115008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circulation research. 2012;111:1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 37.Randle PJ. Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes Metab Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. Journal of the American College of Cardiology. 2009;54:1637–1646. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wende AR, Huss JM, Schaeffer PJ, Giguere V, Kelly DP. Pgc-1alpha coactivates pdk4 gene expression via the orphan nuclear receptor erralpha: A mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol. 2005;25:10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowe GC, Jiang A, Arany Z. Pgc-1 coactivators in cardiac development and disease. Circulation research. 2010;107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, Doenst T, Medina-Gomez G, Litwin SE, Lelliott CJ, Vidal-Puig A, Abel ED. Pgc-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circulation research. 2011;109:783–793. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: A question of balance. J Clin Invest. 2005;115:547–555. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaha VG, Young LH. Amp-activated protein kinase regulation and biological actions in the heart. Circulation research. 2012;111:800–814. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai L, Tu BP. Driving the cell cycle through metabolism. Annu Rev Cell Dev Biol. 2012;28:59–87. doi: 10.1146/annurev-cellbio-092910-154010. [DOI] [PubMed] [Google Scholar]
- 47.Ward PS, Thompson CB. Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol. 2012;4:a006783. doi: 10.1101/cshperspect.a006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circulation. Heart failure. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 49.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid x receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 50.Doenst T, Pytel G, Schrepper A, Amorim P, Farber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovascular research. 2010;86:461–470. doi: 10.1093/cvr/cvp414. [DOI] [PubMed] [Google Scholar]
- 51.Heather LC, Cole MA, Lygate CA, Evans RD, Stuckey DJ, Murray AJ, Neubauer S, Clarke K. Fatty acid transporter levels and palmitate oxidation rate correlate with ejection fraction in the infarcted rat heart. Cardiovascular research. 2006;72:430–437. doi: 10.1016/j.cardiores.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction heart failure in rats is associated with upregulation of glut-1 and downregulation of genes of fatty acid metabolism. Cardiovascular research. 2001;52:407–416. doi: 10.1016/s0008-6363(01)00393-5. [DOI] [PubMed] [Google Scholar]
- 53.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 54.Christe ME, Rodgers RL. Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol. 1994;26:1371–1375. doi: 10.1006/jmcc.1994.1155. [DOI] [PubMed] [Google Scholar]
- 55.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to atp production in hypertrophied hearts. The American journal of physiology. 1994;267:H742–750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 56.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Molecular and cellular biochemistry. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 57.Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovascular research. 2013;97:676–685. doi: 10.1093/cvr/cvs424. [DOI] [PubMed] [Google Scholar]
- 58.Amorim PA, Nguyen TD, Shingu Y, Schwarzer M, Mohr FW, Schrepper A, Doenst T. Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. The Journal of thoracic and cardiovascular surgery. 2010;140:1160–1167. doi: 10.1016/j.jtcvs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Degens H, de Brouwer KF, Gilde AJ, Lindhout M, Willemsen PH, Janssen BJ, van der Vusse GJ, van Bilsen M. Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol. 2006;101:17–26. doi: 10.1007/s00395-005-0549-0. [DOI] [PubMed] [Google Scholar]
- 60.Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT, MacCoss MJ, Rabinovitch PS. Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovascular research. 2012;93:79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dodd MS, Ball DR, Schroeder MA, Le Page LM, Atherton HJ, Heather LC, Seymour AM, Ashrafian H, Watkins H, Clarke K, Tyler DJ. In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovascular research. 2012;95:69–76. doi: 10.1093/cvr/cvs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davila-Roman VG, Vedala G, Herrero P, de las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. Journal of the American College of Cardiology. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 63.Kolwicz SC, Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl coa carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circulation research. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atherton HJ, Dodd MS, Heather LC, Schroeder MA, Griffin JL, Radda GK, Clarke K, Tyler DJ. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: A combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation. 2011;123:2552–2561. doi: 10.1161/CIRCULATIONAHA.110.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O'Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: Attenuating upregulated anaplerosis in hypertrophy. Circulation research. 2009;104:805–812. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, Nguyen TD, Mohr FW, Khalimonchuk O, Weimer BC, Doenst T. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovascular research. 2010;85:376–384. doi: 10.1093/cvr/cvp344. [DOI] [PubMed] [Google Scholar]
- 67.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking ppar-gamma coactivator 1alpha. Proc Natl Acad Sci U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circulation research. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and galphaq overexpression-induced heart failure. Circulation research. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faerber G, Barreto-Perreia F, Schoepe M, Gilsbach R, Schrepper A, Schwarzer M, Mohr FW, Hein L, Doenst T. Induction of heart failure by minimally invasive aortic constriction in mice: Reduced peroxisome proliferator-activated receptor gamma coactivator levels and mitochondrial dysfunction. The Journal of thoracic and cardiovascular surgery. 2011;141:492–500. 500, e491. doi: 10.1016/j.jtcvs.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 72.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of amp-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circulation research. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. 2013;55:31–41. doi: 10.1016/j.yjmcc.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van der Wall EE, de Roos A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation. 1999;99:2261–2267. doi: 10.1161/01.cir.99.17.2261. [DOI] [PubMed] [Google Scholar]
- 75.Okada M, Mitsunami K, Inubushi T, Kinoshita M. Influence of aging or left ventricular hypertrophy on the human heart: Contents of phosphorus metabolites measured by 31p mrs. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1998;39:772–782. doi: 10.1002/mrm.1910390515. [DOI] [PubMed] [Google Scholar]
- 76.Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. Journal of the American College of Cardiology. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Struthers AD, Donnan PT, Lindsay P, McNaughton D, Broomhall J, MacDonald TM. Effect of allopurinol on mortality and hospitalisations in chronic heart failure: A retrospective cohort study. Heart. 2002;87:229–234. doi: 10.1136/heart.87.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-atp ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 79.Diez J, Gonzalez A, Lopez B, Querejeta R. Mechanisms of disease: Pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005;2:209–216. doi: 10.1038/ncpcardio0158. [DOI] [PubMed] [Google Scholar]
- 80.Marks AR. Calcium cycling proteins and heart failure: Mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJ, van der Velden J. Sarcomeric dysfunction in heart failure. Cardiovascular research. 2008;77:649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 82.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin e supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 83.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin e supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 84.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. Journal of the American College of Cardiology. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase c--dependent activation of nad(p)h oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 86.Schonfeld P, Wojtczak L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochimica et biophysica acta. 2007;1767:1032–1040. doi: 10.1016/j.bbabio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 88.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 89.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T, Krek W. Activation of a hif1alpha-ppargamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell metabolism. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived nadph fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Hecker PA, Lionetti V, Ribeiro RF, Jr., Rastogi S, Brown BH, O'Connell KA, Cox JW, Shekar KC, Gamble DM, Sabbah HN, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circulation. Heart failure. 2013;6:118–126. doi: 10.1161/CIRCHEARTFAILURE.112.969576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rajamani U, Essop MF. Hyperglycemia-mediated activation of the hexosamine biosynthetic pathway results in myocardial apoptosis. American journal of physiology. Cell physiology. 2010;299:C139–147. doi: 10.1152/ajpcell.00020.2010. [DOI] [PubMed] [Google Scholar]
- 93.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, Dillmann WH. Regulation of mitochondrial morphology and function by o-glcnacylation in neonatal cardiac myocytes. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R1296–1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear o-glcnacylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 95.Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR. Cardiac o-glcnac signaling is increased in hypertrophy and heart failure. Physiological genomics. 2012;44:162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 96.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-glcnac signaling is essential for nfat-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2012;302:H2122–2130. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/nfat coupling participates in pathological, but not physiological, cardiac hypertrophy. Circulation research. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 99.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-n-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circulation research. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanamori H, Takemura G, Goto K, Maruyama R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovascular research. 2011;91:330–339. doi: 10.1093/cvr/cvr073. [DOI] [PubMed] [Google Scholar]
- 103.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 104.Huang J, Lam GY, Brumell JH. Autophagy signaling through reactive oxygen species. Antioxid Redox Signal. 2011;14:2215–2231. doi: 10.1089/ars.2010.3554. [DOI] [PubMed] [Google Scholar]
- 105.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (hdac) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. 2006;114:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- 107.Hedhli N, Depre C. Proteasome inhibitors and cardiac cell growth. Cardiovascular research. 2010;85:321–329. doi: 10.1093/cvr/cvp226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 109.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovascular research. 2011;90:202–209. doi: 10.1093/cvr/cvr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stanley WC, Dabkowski ER, Ribeiro RF, Jr., O'Connell KA. Dietary fat and heart failure: Moving from lipotoxicity to lipoprotection. Circulation research. 2012;110:764–776. doi: 10.1161/CIRCRESAHA.111.253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol. 2008;295:H2495–2502. doi: 10.1152/ajpheart.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clinical and experimental pharmacology & physiology. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 115.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 116.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation--a novel therapeutic intervention in the ischemic and failing heart. Biochimica et biophysica acta. 2011;1813:1333–1350. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 117.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of glut1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 118.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular ph and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. Journal of the American College of Cardiology. 2002;39:718–725. doi: 10.1016/s0735-1097(01)01803-4. [DOI] [PubMed] [Google Scholar]
- 119.Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, Wagg CS, Jaswal JS, Harris RA, Clanachan AS, Dyck JR, Lopaschuk GD. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovascular research. 2012;94:359–369. doi: 10.1093/cvr/cvs129. [DOI] [PubMed] [Google Scholar]
- 120.Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. Journal of the American College of Cardiology. 1994;23:1617–1624. doi: 10.1016/0735-1097(94)90665-3. [DOI] [PubMed] [Google Scholar]
- 121.Lewis JF, DaCosta M, Wargowich T, Stacpoole P. Effects of dichloroacetate in patients with congestive heart failure. Clin Cardiol. 1998;21:888–892. doi: 10.1002/clc.4960211206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database Syst Rev. 2012;4:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T. Triheptanoin diet alleviates hypertrophy and diastolic dysfunction and preserves cardiac glucose oxidation in rats with pressure overload. Clin Res Cardiol. 2013;102(Suppl 1):V577. [Google Scholar]
- 124.Beauloye C, Bertrand L, Horman S, Hue L. Ampk activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovascular research. 2011;90:224–233. doi: 10.1093/cvr/cvr034. [DOI] [PubMed] [Google Scholar]
- 125.Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial glut-4 and increased glucose uptake through activation of ampk by aicar. The American journal of physiology. 1999;277:H643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 126.Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/cd36 translocation in rat cardiac myocytes is mediated through amp-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- 127.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-coa levels due to an increase in 5'-amp-activated protein kinase inhibition of acetyl-coa carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 128.Jager S, Handschin C, St-Pierre J, Spiegelman BM. Amp-activated protein kinase (ampk) action in skeletal muscle via direct phosphorylation of pgc-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McGaffin KR, Witham WG, Yester KA, Romano LC, O'Doherty RM, McTiernan CF, O'Donnell CP. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovascular research. 2011;89:60–71. doi: 10.1093/cvr/cvq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, Shen YH. Activation of the ampk-foxo3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Newman MF, Ferguson TB, White JA, Ambrosio G, Koglin J, Nussmeier NA, Pearl RG, Pitt B, Wechsler AS, Weisel RD, Reece TL, Lira A, Harrington RA. Effect of adenosine-regulating agent acadesine on morbidity and mortality associated with coronary artery bypass grafting: The red-cabg randomized controlled trial. JAMA. 2012;308:157–164. doi: 10.1001/jama.2012.7633. [DOI] [PubMed] [Google Scholar]
- 132.Cittadini A, Napoli R, Monti MG, Rea D, Longobardi S, Netti PA, Walser M, Sama M, Aimaretti G, Isgaard J, Sacca L. Metformin prevents the development of chronic heart failure in the shhf rat model. Diabetes. 2012;61:944–953. doi: 10.2337/db11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circulation. Heart failure. 2011;4:53–58. doi: 10.1161/CIRCHEARTFAILURE.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex i. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 135.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of amp-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ouyang J, Parakhia RA, Ochs RS. Metformin activates amp kinase through inhibition of amp deaminase. J Biol Chem. 2011;286:1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the lkb1/ampk pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the tgfbeta1-smad3 signalling pathway. Cardiovascular research. 2010;87:504–513. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 139.Drucker DJ. The biology of incretin hormones. Cell metabolism. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 140.Ravassa S, Zudaire A, Diez J. Glp-1 and cardioprotection: From bench to bedside. Cardiovascular research. 2012;94:316–323. doi: 10.1093/cvr/cvs123. [DOI] [PubMed] [Google Scholar]
- 141.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 142.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circulation. Heart failure. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nguyen TD, Shingu Y, Amorim PA, Schwarzer M, Doenst T. Chronic glp-1 treatment preserves diastolic function and improves survival in rats with pressure overload. European Heart Journal. 2011;32:783, P4391. [Google Scholar]
- 144.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 145.Hihi AK, Michalik L, Wahli W. Ppars: Transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci. 2002;59:790–798. doi: 10.1007/s00018-002-8467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shipston MJ. Ion channel regulation by protein palmitoylation. J Biol Chem. 2011;286:8709–8716. doi: 10.1074/jbc.R110.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spedding M, Mir AK. Direct activation of ca2+ channels by palmitoyl carnitine, a putative endogenous ligand. British journal of pharmacology. 1987;92:457–468. doi: 10.1111/j.1476-5381.1987.tb11343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase czeta before the formation of a pro-apoptotic complex with par-4 in differentiating stem cells. The Journal of biological chemistry. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- 149.Wilson L, Yang Q, Szustakowski JD, Gullicksen PS, Halse R. Pyruvate induces mitochondrial biogenesis by a pgc-1 alpha-independent mechanism. American journal of physiology. Cell physiology. 2007;292:C1599–1605. doi: 10.1152/ajpcell.00428.2006. [DOI] [PubMed] [Google Scholar]
- 150.Philp A, Perez-Schindler J, Green C, Hamilton DL, Baar K. Pyruvate suppresses pgc1alpha expression and substrate utilization despite increased respiratory chain content in c2c12 myotubes. American journal of physiology. Cell physiology. 2010;299:C240–250. doi: 10.1152/ajpcell.00438.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cai L, Sutter BM, Li B, Tu BP. Acetyl-coa induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-glcnac signaling in the cardiovascular system. Circulation research. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in tnfr1-associated periodic syndrome (traps). The Journal of experimental medicine. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chua B, Siehl DL, Morgan HE. Effect of leucine and metabolites of branched chain amino acids on protein turnover in heart. J Biol Chem. 1979;254:8358–8362. [PubMed] [Google Scholar]
- 155.Huang Y, Zhou M, Sun H, Wang Y. Branched-chain amino acid metabolism in heart disease: An epiphenomenon or a real culprit? Cardiovascular research. 2011;90:220–223. doi: 10.1093/cvr/cvr070. [DOI] [PMC free article] [PubMed] [Google Scholar]