Abstract
Brown adipose tissue (BAT) burns calories to produce heat, and is thus relevant to energy balance. Interscapular BAT (IBAT) of donor mice was transplanted into recipient mice (transBATation). To test whether transBATation counteracts high-fat diet (HFD)-induced obesity, some sham-operated and recipient mice were fed a HFD (HFD-sham, HFD-trans) while others remained on a standard chow (chow-sham, chow-trans). HFD-trans mice had lower body weight and fat, greater energy expenditure, but similar caloric intake compared with HFD-sham mice. We hypothesized that HFD-trans mice had elevated sympathetic activity compared with HFD-sham mice, contributing to increased energy expenditure and fuel mobilization. This was supported by findings that HFD-trans mice had greater energy expenditure during a norepinephrine challenge test and higher core temperatures after cold exposure than did HFD-sham mice, implicating enhanced whole-body metabolic response and elevated sympathetic activity. Additionally, transBATation selectively increased sympathetic drive to some, but not all, white adipose tissue depots and skeletal muscles, as well as the endogenous IBAT, heart, and liver. Collectively, transBATation confers resistance to HFD-induced obesity via increase in whole-body sympathetic activity, and differential activation of sympathetic drive to some of the tissues involved in energy expenditure and fuel mobilization.
Keywords: visceral white adipose tissue, energy expenditure, norepinephrine challenge, cold exposure, tyrosine hydroxylase, norepinephrine turnover
1. Introduction
Positive energy balance results in the accumulation of excess lipids in white adipose tissue (WAT). In contrast, brown adipose tissue (BAT) of mammals burns calories and expends energy to generate heat via non-shivering thermogenesis to maintain body temperature, without animals expending any effort [1]. BAT contains a large number of mitochondria that utilize glucose and fatty acids [1, 2] and express uncoupling protein 1 (UCP1) that uncouples oxidative phosphorylation, leading to inefficient ATP production, and causing chemical energy to be dissipated as heat. Cold- or diet-induced thermogenesis is thus engaged to maintain normal body temperature and modulate energy balance. Indeed, BAT is the major thermogenic organ, consuming nearly 50% of total oxygen consumption and generating 60% of animal's heat of cold-acclimated rats [3].
BAT is abundant in mammals with thermoregulatory demands, including adult humans [4]. The amount of metabolically active BAT is inversely correlated with BMI and adiposity in adult humans [5, 6]. Importantly, BAT has beneficial effects on whole-body metabolism by increasing energy expenditure and heat production, raising the possibility that increasing the amount of BAT might have therapeutic benefits. Indeed, subcutaneous [7, 8] or visceral [9] BAT transplantation (referred to as transBATation) increases physical activity and oxygen consumption, as well as fatty acid oxidation in endogenous BAT and skeletal muscles [8]. TransBATation thus counteracts HFD-induced weight and fat gain [7, 8], reverses preexisting obesity [8], and recovers HFD-induced increases in circulating triglycerides and hepatic steatosis to the same levels of low-fat diet-fed animals [8]. Additionally, subcutaneous or visceral transBATation improves glucose tolerance and insulin sensitivity in HFD-induced type 2 diabetes [8, 9] by increasing glucose uptake into metabolic tissues such as the heart, visceral WAT, and endogenous IBAT [9], and by increasing Akt phosphorylation in WAT [8]. These studies collectively suggest that BAT is critical to maintaining a metabolically healthy phenotype.
The sympathetic nervous system (SNS) modulates energy balance via its regulation of energy storage and utilization. SNS activation reduces food intake [10], contributes to thermic effect of food via β-adrenergic stimulation [11], increases BAT thermogenic activity [4] and glucose uptake [12], maintains resting metabolic rate [13], increases spontaneous physical activity [14], stimulates lipolysis in WAT [15, 16], and promotes fatty acid oxidation and glucose uptake in skeletal muscle [17] and liver [18]. Reduced SNS activity leads to increased energy storage and adiposity, and is thus a risk factor for obesity development, as overweight people tend to have overall decreased SNS activity [19, 20]. Importantly, SNS dysfunction increases the risk of developing diabetes, independent of other risk factors like BMI [21]. This is in line with Bray's “MONA LISA” hypothesis (“most obesities known are low in sympathetic activity”) [22]. While elevation of SNS activity also accompanies obesity [23], this is thought to be a compensatory reaction to the obese state that contributes to the development of cardiovascular disease [24] and insulin resistance [25]. Dampened SNS response to challenge, such as physical exercise [26, 27] and non-physical mental stress [28], is also seen in obesity.
The ability of SNS drive and transBATation to induce similar metabolic alterations suggests that transBATation may be elevating SNS activity. Elevated SNS drive has predictable effects on multiple metabolic systems resulting from transBATation, including elevated glucose uptake and fat oxidation, and increased thermogenic and physical activity [8, 9]. We hypothesize that transBATation increases whole-animal and tissue-specific SNS activity to counteract HFD-induced obesity. The aim of the study was therefore to first investigate whether transBATation elevated SNS activity by measuring whole-animal energy expenditure during obesity development, and response to SNS-activated conditions such as norepinephrine (NE) challenge and cold exposure tests. Second, we investigated whether transBATation altered SNS drive to individual metabolic tissues by measuring NE turnover (NETO) rates in multiple tissues.
2. Methods
2.1 Animals, diets, and surgeries
Eight-week old male C57BL/6J mice (Jackson Laboratory) were individually housed with a 12:12 light:dark cycle, 22-24 °C temperature, and unrestricted access to food and water except during the cold-exposure experiment. Mice were fed a standard low-fat chow (3.003 kcal/g, 14% kcal from fat, Teklad 8604) or a HFD (4.728 kcal/g, 45% kcal from fat, D12451, Research Diets Inc.). Mice had either sham or transBATation surgeries. TransBATation was performed using IBAT of chow-fed donors. After euthanized by CO2 asphyxia, IBAT of donors was dissected (~ 0.100 g), its surrounding peripheral white fat was excluded, the center remaining BAT (~ 0.060 g) was placed in sterile warm (37°C) saline, cut into two pieces, and transplanted into recipients as quickly as possible under isoflurane anesthesia. IBAT pieces (~0.03 g/piece, two pieces / recipient) were transplanted underneath skin on each side of dorsal region of recipients, through bilateral small incisions of 2-3 mm in length beginning at the proximal end of the hindlimb. The skin and fascia were loosened and a subcutaneous pocket was created by blunt dissection. BAT lobules were introduced into the pocket and pushed rostrally, with one piece on each side. At sacrifice, we found that transplanted BAT was not adjacent to the endogenous IBAT, and thus could be easily differentiated. Mice that were sham operated underwent the same procedure, but differed from transBATation in that no tissue was added in the former. All animal procedures were approved by the Institutional Animal Care and Use Committee of Miami University Ohio.
2.2 Experimental procedure
2.2.1 Exp 1: Did transplanted BAT tissue survive?
Ten chow-fed mice were used, 5 donors and 5 recipients. Eight weeks after transBATation, endogenous and transplanted BAT were collected for gene expression analysis for peroxisome proliferator-activated receptor gamma coactivator-1α (Pgc1α) and Ucp1. Total RNA was extracted and reverse transcribed into cDNA using 1 μg total RNA. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as a reference gene (Gapdh mRNA levels were similar in endogenous and transplanted BAT). PCR was run in triplicates with iQ SYBR Green Supermix and an iCycler (Bio-Rad), using a 2-step cycle of amplification (95 °C for 10s) and annealing (60 °C for 30 s) for 40 cycles. Amplified products were confirmed via gel electrophoresis and melt curve analysis. Results were calculated by a 2−ΔΔCt method [29], and presented using endogenous IBAT as 100%.
2.2.2 Exp 2: How did transBATed mice respond to HFD-induced obesity?
Six groups of mice were used (n=6-10). IBAT of donors was transplanted into two recipient groups (trans), and two groups were sham-operated (sham). To test whether transBATation alters energy balance during HFD-induced obesity, immediately after surgeries, one sham and one trans groups were switched to a HFD (HFD-sham, HFD-trans) for 8 weeks, and the others were maintained on chow (chow-sham, chow-trans) for 8 weeks. Body weight was measured weekly. Body fat and lean mass were assessed using an EchoMRI-900™ whole body composition analyzer (EchoMedical Systems) at postsurgery week 8. Cumulative caloric intake during eight weeks after surgeries was calculated, accounting for spillage. Whole-animal oxygen consumption (VO2, ml/kg/min) was assessed in an indirect calorimetry Physioscan System (Accuscan Instruments) during postsurgery week 8. Energy expenditure (EE) was calculated using the Weir equation: EE(J) = 15.818 VO2 + 5.176 VCO2 [30].
2.2.3 Exp 3: How did transBATed mice respond to NE challenge test?
We hypothesize that transBATation increases SNS activity, which would contribute to reduced body fat and increased EE seen in Exp 2. NE-stimulated EE is used as an indicator of whole-body SNS activity involving multiple sympathetically regulated tissues [30]. HFD-sham and HFD-trans mice were tested for differential response to NE challenge 3 weeks after surgery when their body weights just began to differ. Mice were acclimated in individual metabolic chambers for 24 hours with ad libitum access to water and HFD. VO2 of HFD-sham and HFD-trans mice was measured before and continuously for 20 minutes immediately after intraperitoneal injection of NE (Sigma Aldrich) at a dose of 2.53 * body mass ^ (−0.4) [31].
2.2.4 Exp 4: How did transBATed mice respond to cold exposure?
A 4 h 4°C cold exposure test was performed 8 weeks postsurgically to identify whole-animal response and BAT-specific alterations in thermogenic capacity. Mice were individually placed in clean cages without bedding, with access to water but not food, to eliminate cold-induced increases in food intake and diet-induced thermogenesis. Core temperature was measured rectally before and after cold exposure using a thermometer with a rectal probe (HH806AU, Omega). To directly measure BAT temperature, an Implantable Programmable Temperature Transponder (IPTT-300; BioMedic Data Systems) was attached under left side IBAT, and was scanned at a distance of ~3 inches every 15 minutes, without touching the mice. All mice were euthanized at the end of cold exposure, and endogenous IBAT and transplanted BAT were collected for immunohistochemical staining for tyrosine hydroxylase (TH), the rate-limiting enzyme for catecholamine biosynthesis.
The collected endogenous and grafted BAT were fixed in 4% paraformaldehyde and paraffin-embedded for immunohistochemistry. Tissues were sectioned at 7 μm. For each tissue, slides were grouped into levels of approximately 200 μm, and one slide from each level was used for immunohistochemistry [32]. Briefly, after blocking endogenous peroxidase activity and nonspecific staining, sections were incubated overnight at 4°C with 1:300 polyclonal rabbit anti-TH (Millipore), followed by 1:500 IgG biotinylated anti-rabbit, 1:100 avidin-biotin horseradish peroxidase complex, and 3,3-diaminobenzidine tetrahydrochloride peroxidase substrate solution (Vector Laboratories), and then counterstained with hematoxylin. Five microscopic fields from each slide with most TH-immunoreactivity (ir) were used for quantification. Total TH-ir from all five fields of each slide was averaged (TH-ir/slide) to represent TH-ir for each tissue.
2.2.4 Exp 5: Did transBATation change sympathetic activity of metabolic tissues?
Individual metabolic tissues, including those involved in energy expenditure, i.e., heart, IBAT, lateral and medial gastrocnemius (LGAS, MGAS), and extensor digitorum longus (EDL), and those involved in fuel storage and release, i.e., liver, visceral mesenteric WAT (MWAT), intra-abdominal retroperitoneal WAT (RWAT), gonadal epididymal WAT (EWAT), and subcutaneous inguinal WAT (IWAT), may have elevated sympathetic activity, which could contribute to the transBATation-induced metabolic alterations see here. This was assessed by measuring NETO using α-methyl-p-tyrosine (α-MPT) methyl-ester hydrochloride (Sigma Aldrich) [33] using our previously published method [34]. α-MPT, a competitive TH inhibitor, blocks synthesis of new catecholamines, including NE, depleting NE in nerve endings. NETO (i.e., the rate of NE disappearance) indicates sympathetic activity. Half of mice from each group were untreated and killed at 0 h; the other half of mice were injected intraperitoneally with α-MPT (250 mg/kg) at 0 h and at 2 h (a supplemental dos ofe 125 mg/kg), and killed 4 h after the initial α-MPT injection. All tissues specified above were collected rapidly, frozen, and stored at −80 °C.
Approximately 100 mg tissue was homogenized in 0.2 M perchloric acid with 1 mg/ml ascorbic acid (PCA/AA) solution containing internal standard dihydroxybenzylamide (DHBA). Catecholamines were extracted from the homogenate with alumina and eluted into the PCA/AA. The catecholamines were measured using reverse-phase HPLC system with electrochemical detection (ESA). Whole-tissue NETO was used to indicate the overall SNS drive for each tissue: k=(lg[NE]0-lg[NE]4)/(0.434*4) and K = k[NE]0, where k is the rate constant of NE efflux, [NE]0 is the initial NE concentration, [NE]4 is the final NE concentration, and K is NETO [35].
2.3 Statistics
An unpaired two-tailed t test was used to compare gene expression between endogenous and transplanted BAT (Exp 1) and NETO of each tissue between HFD-sham and HFD-trans groups (Exp 5). A two-factorial (surgery × diet) analysis of variance (ANOVA), with Bonferroni post-hoc tests, was used to compare fat and lean mass, cumulative postsurgical caloric intake, and average VO2 and EE (Exp 2). Weekly body weights, hourly VO2, and EE during normal condition (Exp 2), VO2 and EE during NE challenge test (Exp 3), core temperature before and after cold exposure, and IBAT temperature during cold exposure (Exp 4) were analyzed by a two-factorial (treatment × time) ANOVA for repeated measures with Bonferroni post-tests. Quantification of TH-ir (Exp 4) was analyzed using a one-way ANOVA with Tukey post-hoc test. A P value < 0.05 was considered to be statistically significant.
3. Results
3.1 Exp 1: Did transplanted BAT tissue survive?
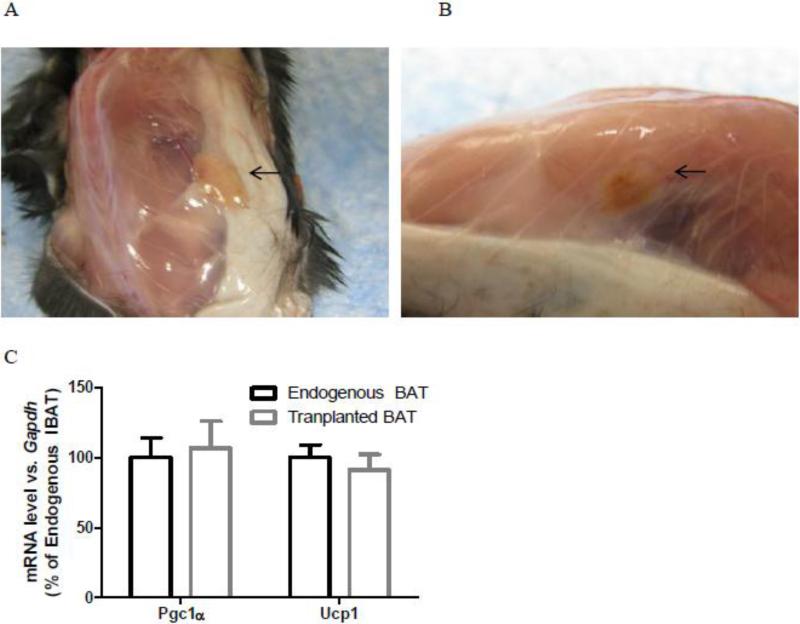
Transplanted BAT had normal appearance with blood vessels entering transplanted BAT eight weeks after surgeries (Fig. 1A-B), and similar mRNA levels of thermogenic genes Ucp1 and Pgc1α as endogenous IBAT (Fig. 1C), suggesting normal sympathetically regulated thermogenic molecular characteristics of tranplanted BAT.
Fig.1.
Transplanted BAT (arrows) had normal appearance (A, B) and similar gene expressions of Pgc1α and Ucp1 as endogenous IBAT (C) in chow-fed male recipient mice (Exp 1).
BAT: brown adipose tissue
Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) - reference gene:
F: 5’-TGC GAC TTC AAC AGC AAC TC-3’, R: 5’-GCC TCT CTT GCT CAG TGT CC-3’.
Peroxisome proliferator-activated receptor gamma coactivator-1α (Pgc1α) - a key transcriptional co-activator that stimulates BAT mitochondrial biogenesis and thermogenic program:
F: 5’-ATGTGTCGCCTTCTTGCTCT-3’, R: 5’-ATCTACTGCCTGGGGACCTT-3’.
Uncoupling protein 1 (Ucp1) - a major thermogenic gene:
F: 5’- GGGCCCTTGTAAACAACAAA -3’, R: 5’- GTCGGTCCTTCCTTGGTGTA-3’.
Endogenous and transplanted BAT: n=5.
3.2 Exp 2: How did transBATed mice respond to HFD-induced obesity?
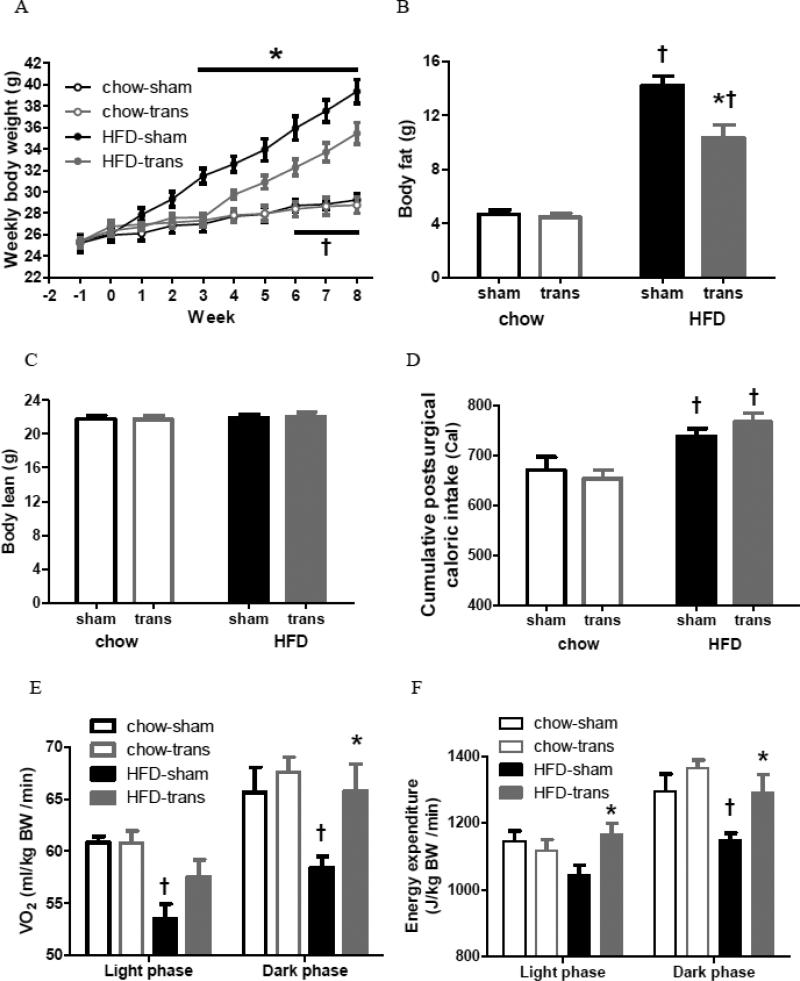
Body weights were similar in all groups before surgery (Fig. 2A). Both HFD groups gained more weight than chow groups. Although chow-sham and chow-trans had similar body weights throughout this experiment, HFD-sham mice gained greater body weights than HFD-trans mice beginning at postsurgical week 3 [*P<0.05], and HFD-trans mice had greater body weights than chow groups after postsurgical week 6 [†P<0.05] (Fig. 2A). At postsurgical week 8, HFD groups had greater adiposity than their chow-fed counterparts [†chow-sham vs. HFD-sham: t=9.975, chow-trans vs. HFD-trans: t=6.134; P<0.001]. Chow-sham and chow-trans groups had similar body fat, whereas HFD-trans mice had less adiposity compared with HFD-sham mice [*t=4.262, P<0.001] (Fig. 2B). In contrast to fat mass, the lean mass was similar between all chow and HFD groups (Fig. 2C). Thus, transBATation reduced HFD-induced weight gain at as early as postsurgical week 3, and reduced fat mass, but only in the HFD-fed groups.
Fig. 2.
Obesity development of sham-operated and BAT-transplanted mice (Exp 2). Weekly body weights (A), body fat and lean mass (B, C), cumulative caloric intake (D), and oxygen consumption (VO2, E) and energy expenditure (EE, F) during light and dark phases.
Chow-sham: n=6; chow-trans: n=8; HFD-sham: n=10; HFD-trans: n=7.
* Significant difference between sham and trans within the same diet.
† Significant difference between chow and HFD within the same surgery.
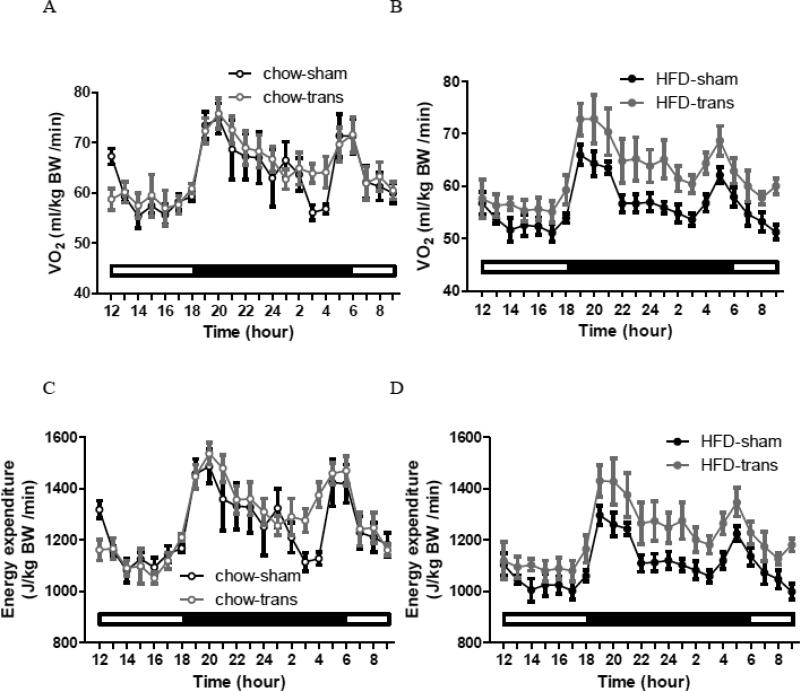
HFD-sham mice consumed more calories than chow groups after surgery [†sham: t=2.589, P<0.05; trans: t=4.302, P<0.001], but cumulative caloric intake was not different between sham and trans groups within chow or HFD (Fig. 2D). The lower body weight and fat of transBATed mice without differing HFD intake implicated alterations in energy expenditure. VO2 and EE were similar between two trans groups [P>0.05] (Fig. 2E-F). VO2 was greater in the chow-sham group than the HFD-sham group during the light [†t=3.267, P<0.01] and dark [†t=2.806, P<0.05] phases (Fig. 2E). VO2 was greater in HFD-trans than HFD-sham mice during the dark phase only [*t=2.974, P<0.05]; whereas it was similar between two chow groups (Fig. 2E). EE was greater in the chow-sham than the HFD-sham group during the dark phase [†t=2.87, P<0.05], and was treater in the HFD-trans than the HFD-sham group during both the light [*t=2.864, P<0.05] and dark [*t=2.916, P<0.05] phases (Fig 2F). Thus, HFD-trans mice had lower body weight and less fat than HFD-sham mice, with similar energy intake but greater energy expenditure. Hourly patterns indicated that, for the chow groups, surgery did not affect VO2 or EE during the light [VO2: F1,12=0.01, P=0.9734; EE: F1,12=0.17, P=0.6845] or dark phase [VO2: F1,12=0.52, P=0.4837; EE: F1,12=1.73, P=0.2132], whereas for the HFD groups, surgery affected VO2 and EE during the dark phase [F1,15=8.56, P=0.0104; EE: F1,15=8.09, P=0.0123] but not light phase [VO2: F1,15=3.47, P=0.0822; EE: F1,15=3.28, P=0.0903] (Fig. 3).
Fig. 3.
Hourly patterns of oxygen consumption (VO2) and energy expenditure (EE) of chow-sham and chow-trans mice (A and C) and of HFD-sham and HFD-trans mice (B and D) (Exp 2).
Chow-sham: n=6; chow-trans: n=8; HFD-sham: n=10; HFD-trans: n=7.
3.3 Exp 3: How did transBATed mice respond to NE challenge test?
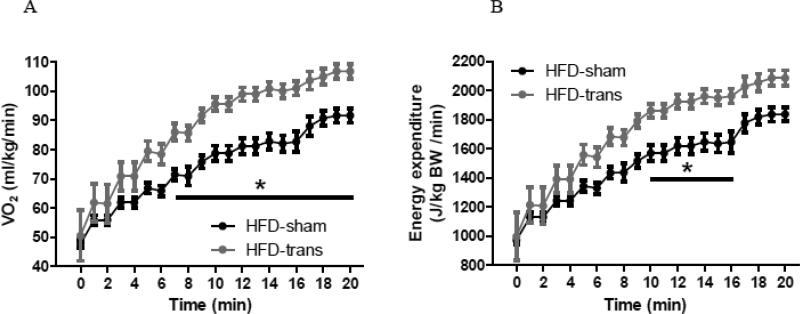
NE increased VO2 and EE in both HFD-sham and HFD-trans mice, with HFD-trans mice having greater VO2 than HFD-sham mice at 7 minutes (Fig. 4A) and greater EE at 10-16 min, after NE injection (Fig. 4B). VO2 and EE were affected by both time [VO2: F20,120=92.99, EE: F20,120=90.66; P<0.0001] and surgery [VO2: F1,6=15.05, P=0.0082; EE: F1,6=10.28, P=0.0184], with a significant interaction between time and surgery [VO2: F20,120=2.25, P=0.0038; EE: F20,120=2.00, P=0.0118]. Thus, NE-stimulated EE was elevated to a greater extent in HFD-trans mice than HFD-sham mice, indicating that HFD-trans mice were more sensitive to NE with an elevated whole-animal SNS activity in the sympathetically-activated condition.
Fig. 4.
Response to sympathetically activated condition of HFD-sham and HFD-trans mice during norepinephrine test (Exp 3). VO2 and EE increased to a greater extent in HFD-trans mice than in HFD-sham mice over time (min after NE injection).
HFD-sham: n=4; HFD-trans: n=4.
* Significant difference between HFD-sham and HFD-trans groups.
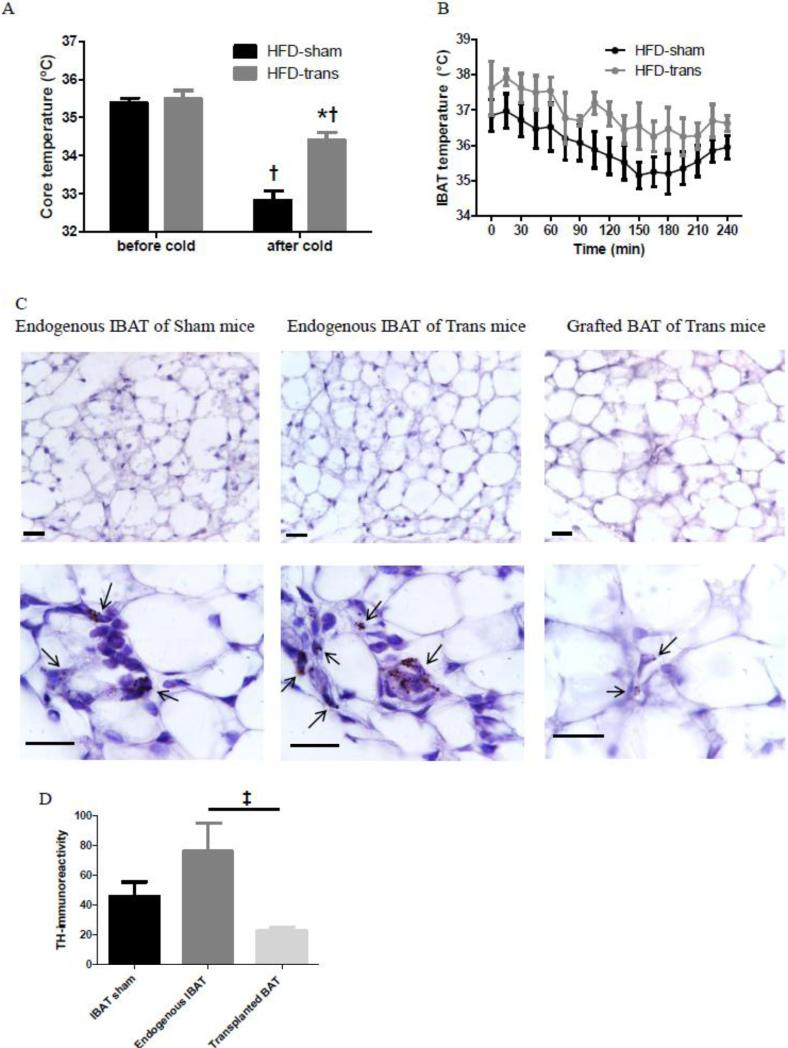
3.4 Exp 4: How did transBATed mice respond to cold exposure?
Both HFD-sham and HFD-trans mice had similar baseline core temperatures and reduced core temperatures the end of 4 h cold exposure [†HFD-sham: t=7.645, P<0.001; HFD-trans: t=3.298, P<0.05]. HFD-trans mice had higher core temperatures than HFD-sham mice [*t=5.382, P<0.001] (Fig. 5A), thus HFD-trans mice were better able to maintain their core temperature than were HFD-sham mice. Both time [F1,6=59.88, P=0.0002] and surgery [F1,6=24.08, P=0.0027] affected the core temperature, and there was an interaction between time and surgery [F1,6=9.45, P=0.0218]. HFD-sham and HFD-trans mice had similar IBAT temperatures throughout the cold exposure, while the pattern of change in IBAT temperature differed between groups, with HFD-trans group showing a delayed decline (Fig. 5B). Histology showed that all BAT tissues had mixed unilocular and multilocular brown adipocytes. It's noteworthy that these unilocular brown adipocytes were smaller, 20-50 μm in diameter, compared to unilocular white adipocytes that were 80-100 μm in diameter. Endogenous IBAT of HFD-sham and HFD-trans mice had similar gross morphology, but unilocular brown adipocytes were more prominent in transplanted BAT than in endogenous IBAT (Fig. 5C). TH-ir was different among the endogenous and transplanted BAT tissues of sham and trans mice [F2=4.699, P<0.05], and posthoc Tukey's comparison test indicated less TH staining of transplanted BAT [‡t=4.322, P<0.05] (Fig. 5D).
Fig. 5.
Whole-animal core temperature response (A), temperature response of endogenous IBAT (B) of HFD-sham and HFD-trans mice during 4-hour 4°C cold exposure, and immunohistochemical staining of tyrosine hydroxylase (TH) of endogenous IBAT of HFD-sham mice, and endogenous IBAT and transplanted BAT of HFD-trans mice (C, D) (Exp 4). Scale bar is 20 μm. Arrows indicate representative TH-positive staining.
HFD-sham: n=4; HFD-trans: n=4.
* Significant difference between HFD-sham and HFD-trans groups.
† Significant difference before and after cold exposure.
‡ Significant difference between endogenous and transplanted BAT of transBATed mice.
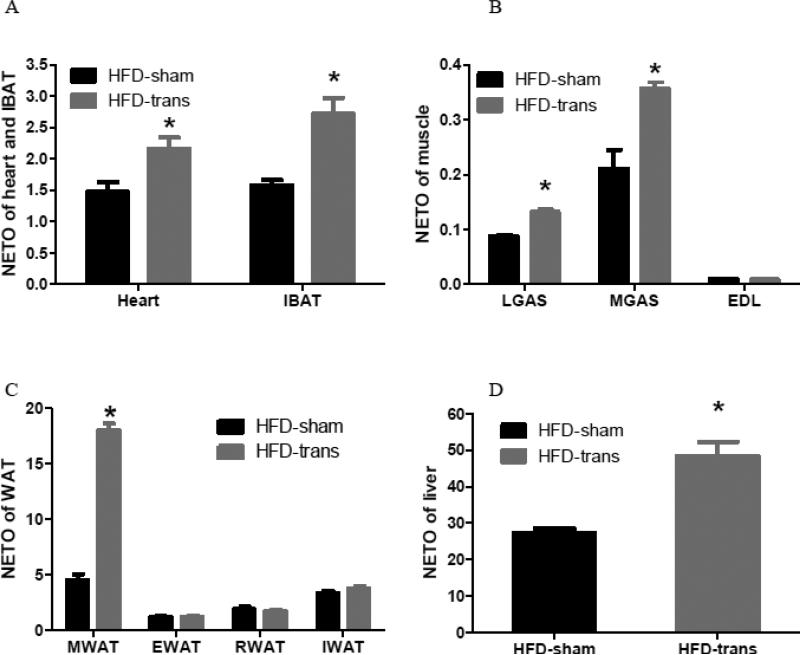
3.5 Exp 5: Did transBATation change sympathetic activity of metabolic tissues?
Mice with transBATation showed significantly elevated NETO in the heart [t=2.783, P=0.0388], IBAT [t=3.645, P=0.0148] (Fig. 6A), LGAS [*t=7.927, P=0.0005], MGAS [*t=4.680, P=0.0054], but not EDL (Fig. 6B). NETO in MWAT [*t=16.89, P<0.0001], but not EWAT, RWAT, or IWAT, was increased following transBATation (Fig. 6C). TransBATation increased NETO differentially across skeletal muscles and WAT assayed. The liver NETO of HFD-trans mice was increased compared with HFD-sham mice [*t=4.405, P=0.0070] (Fig. 6D).
Fig. 6.
Sympathetic activity as indicated by NE turnover (NETO) of the heart and endogenous IBAT (A), skeletal muscles (B), visceral and subcutaneous WAT (C), and liver (D) of HFD-sham or HFD-trans mice (Exp 5).
HFD-sham: n=6; HFD-trans: n=8.
* Significant difference between HFD-sham and HFD-trans groups.
4. Discussion
It has long been recognized that human infants have substantial amounts of BAT, a mitochondria-rich and highly thermogenic type of adipose tissue [4]. While BAT was previously not thought to play an important role in adult humans, recent studies have conclusively demonstrated that BAT is metabolically active and is relevant to energy balance in adult humans [5, 6]. Given the ability of BAT to burn calories, there is interest in understanding regulation of BAT metabolism, in search of an effective way of expending calories. Recent transBATation studies implicate BAT in whole-body energy balance and glucose metabolism in HFD-induced obesity and diabetes [7-9, 36]. Here, we demonstrate that transBATation counteracts HFD-induced obesity via increased energy expenditure without changing energy intake, with increased whole-body sympathetic activity and elevated sympathetic drive to the heart, liver, IBAT, oxidative gastrocnemius muscle, and visceral WAT. Thus, reduced energy expenditure and dampened sympathetic activity in obese state [22] was improved by transBATation.
It is likely that the amount of transplanted BAT, rather than its location, is critical to counteracting obesity. BAT is mainly distributed and most frequently described in subcutaneous areas in the supraclavicular and neck regions in humans and in subcutaneous interscapular areas in rodents. Although not always found, some BAT is located in the paraaortic region within the thoracic cavity, and in the suprarenal region within the abdominal cavity [37]. We and some other groups have performed subcutaneous transBATation that mimicked natural BAT distribution [7, 8, 36], while one group has performed visceral transBATation [9]. Both models lead to reduced HFD-induced weight and fat gain [7-9, 36]. Although Stanford et al. reported beneficial effects on glucose metabolism following visceral but not subcutaneous BAT transplantation [9], improved glucose regulation following subcutaneous BAT transplantation was reported by other groups [8, 36]. These studies suggest that amount of BAT plays a more critical role than distribution of BAT in the regulation of metabolism and energy balance.
Because transplanted BAT is re-vascularized, as indicated by normal levels of the angiogenic protein CD31 in transplanted BAT [9], humoral mechanisms involving circulating ‘BATokines’ released from transplanted BAT, such as IL-6 or FGF 21, have the potential to impact metabolism [9]. Indeed, circulating IL-6 level increases following visceral transBATation, and mice transplanted with BAT from IL-6 KO mice do not exhibit metabolic improvement [9]. IL-6 may not be critical for the beneficial effects following subcutaneous transBATation, since IL-6 level does not change [8], or even decreases IL-6 in endogenous WAT [36]. While transplanted BAT tissue progressively decreases multilocular appearance of typical brown adipocytes and shows more unilocular white adipocyte-like appearance at 12 weeks after surgery [9], metabolic improvement is sustained for 20 weeks [8], and euglycemia remains normalized for 5 months in a type 1 diabetes model [36]. This implicates humoral factors from other tissues/organs, rather than from transplanted BAT tissue, and/or neural factors, as a potential mechanism for modulating long-term metabolism after transBATation.
Transplanted BAT expresses UCP1 and TH mRNAs and proteins, although at reduced levels compared with endogenous IBAT [8, 9], suggesting partial sympathetic re-innervation of transplanted BAT. This is consistent with our findings that, while endogenous and transplanted BAT of lean recipients had similar Ucp1 and Pgc1α expression, transplanted BAT of obese recipients had lower TH-ir following cold exposure, a sympathetically-activated condition. Changes in Ucp1 or Pgc1α mRNA levels do not directly correlate with BAT thermogenic capacity [38]. Collectively, transplanted BAT has normal revascularization, but less sympathetic innervation and less thermogenic molecular characteristics, compared with endogenous IBAT of the recipients. Interestingly, we found such partial sympathetic innervation of transplanted BAT is somewhat WAT-like, with sparse TH-ir to unilocular brown adipocytes, different from compacted TH-staining from endogenous IBAT (Fig. 5C). This partial re-innervation of transplanted BAT might alter sympathetic drive to other metabolic tissues, as manipulating innervation of one adipose depot by lipectomy [34] or denervation [39] would alter sympathetic drive to other tissues,. It is possible that such WAT-like re-innervation provides a neural mechanism that “informs” animals an increase in energy storage. This is consistent with enhanced EE (Exp 2), whole-body response to sympathetic activation (Exp 3), and non-shivering thermogenesis and Ucp1 expression of the endogenous IBAT (downstream of NE-mediated activation of β3-adrenoceptor) upon cold exposure following subcutaneous [8] or visceral [9] transBATation. TransBATed mice were better capable of maintaining core body temperature and thus core temperature was reduced to a lesser extent during cold exposure (Exp 4), despite the fact that cold-induced SNS activation of endogenous IBAT was not enhanced after transBATation, as shown by similar endogenous IBAT temperature and TH-ir between HFD-sham and HFD-trans groups (Exp 4). HFD-trans mice may have generated heat from other sources, as they had heightened core, but not IBAT, temperature, suggesting other mechanisms of heat generation or mitigated heat loss.
The SNS plays a considerable role in the regulation of energy balance, and glucose and lipid metabolism in the liver [40], adipose tissue [41, 42], and skeletal muscle [43, 44]. SNS has a distinct organization in different tissues, and differential sympathetic activation is tissue/organ-specific through discrete sympathetic projections [41], permitting finely tuned control of metabolism. NETO data from this study indicated elevated sympathetic drive to the endogenous IBAT, heart, and visceral WAT, consistent with increased glucose uptake into these tissues [9]; to the oxidative lateral and medial gastrocnemius muscles, consistent with increased muscle fatty acid oxidation-related gene expression [8]; and to the liver, consistent with reduced hepatic steatosis [8]. TransBATation increases plasma NE level [9], liberating energy substrates from storage organs, such as the liver and WAT, and promoting usage of glucose and free fatty acids, counteracting HFD-induced obesity and insulin resistance. Here, we demonstrate that NETO in WAT was not uniform across depots, with significant increase of NETO in visceral MWAT but not non-visceral EWAT, RWAT, or IWAT. The mechanism underlying WAT-specific differences in NETO across WAT depots may involve divergent SNS outflow circuits with separate postganglionic SNS innervation from the CNS to WAT depots in different locations [15, 45]. For example, visceral WAT and liver may be controlled by the same neurons, whereas subcutaneous WAT receives input from a distinct set of autonomic neurons [45], which provides the neuroanatomical basis for selective changes of different WAT depots. The current findings fit nicely with previous studies that lipid mobilization from regional WAT depots is not uniform under energetically demanding conditions [46].
NETO increased in oxidative LGAS and MGAS, but not glycolytic EDL, following transBATation. Oxidative and glycolytic muscle fiber types differ in mitochondrial respiratory capacity, locomotor and metabolic demands, response to physiological and pathological conditions. Intramyocellular lipid levels in oxidative muscle, but not in glycolytic muscle, predict the degree of insulin resistance in humans [47]. Additionally, oxidative and glycolytic muscles respond differentially to physiological and pathological conditions. For example, in obese diabetic db/db mice, mitochondrial respiratory capacity of oxidative muscle is reduced, whereas it is enhanced in glycolytic muscle with increased mitochondrial biogenesis [48]. Muscle fiber type-specific sympathetic activation following transBATation may reflect increase in mitochondrial respiration and fatty acid oxidation in oxidative muscle.
Circulating humoral signals distribute information uniformly within the body, whereas neural signals receive information from and deliver message to individually-targeted tissues with different locations in a selective manner. If only humoral mechanisms are involved, all tissues that possess receptors for the humoral signals would simultaneously change their sympathetic activity nonselectively. The tissue/depot-specific changes in NETO implicate neural processing of hypothesized humoral signals from transplanted BAT.
Collectively, these data demonstrate for the first time that transBATation counteracts HFD-induced obesity, in the absence of change in caloric intake, via elevating whole-body sympathetic activity and sympathetic drive to multiple targets involved in fuel mobilization and use. Such changes consequently promote glucose metabolism and lipid reduction through increased glucose mobilization and fat oxidation, and enhanced energy expenditure via increased BAT thermogenesis and muscle activity. Importantly, such change is tissue depot- and subtype-specific, with NETO increasing only in visceral WAT and preferentially in oxidative rather than glycolytic muscle, consistent with the notion of distinct sympathetic drives to peripheral tissues. Finally, current finding suggest that dampened sympathetic activity in the obese state is improved at whole-animal and tissue levels following transBATation using both humoral and neural signals.
Highlights.
TransBATation counteracted HFD-induced obesity via increased energy expenditure without changing energy intake.
TransBATation elevated whole-body sympathetic activity.
TransBATation elevated sympathetic drive to multiple targets in a depot- and tissue subtype-specific manner.
Acknowledgments
This study was supported by Summer Research Fellowship from the Endocrine Society (EGS), NIH R15 DK090823 (HS), AHA 12GRNT12050566 (CMN), and NIH R15 DK097644 (CMN).
Abbreviations
- BAT
brown adipose tissue
- EDL
extensor digitorum longus
- EE
energy expenditure
- EWAT
epididymal white adipose tissue
- HFD
high-fat diet
- IBAT
interscapular brown adipose tissue
- IWAT
inguinal white adipose tissue
- LGAS
lateral gastrocnemius
- MGAS
medial gastrocnemius
- MWAT
mesenteric white adipose tissue
- NETO
norepinephrine turnover
- PBS
phosphate-buffered saline
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator-1α
- RWAT
retroperitoneal white adipose tissue
- SNS
sympathetic nervous system
- TH
tyrosine hydroxylase
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gesta S, Tseng Y-H, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 3.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: The dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol. 1979;57:257–70. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 4.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 5.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio- Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spicer EG, Shi H. Mice with brown fat transplantation partially resist to diet-induced obesity and glucose intolerance. Appetite. 2010;54:676. [Google Scholar]
- 8.Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23:851–4. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray GA. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S8–17. doi: 10.1038/sj.ijo.0801269. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz RS, Jaeger LF, Silberstein S, Veith RC. Sympathetic nervous system activity and the thermic effect of feeding in man. Int J Obes. 1987;11:141–9. [PubMed] [Google Scholar]
- 12.Shimizu Y, Kielar D, Minokoshi Y, Shimazu T. Noradrenaline increases glucose transport into brown adipocytes in culture by a mechanism different from that of insulin. Biochem J. 1996;314:485–90. doi: 10.1042/bj3140485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad MF, Alger SA, Zurlo F, Young JB, Bogardus C, Ravussin E. Ethnic differences in sympathetic nervous system-mediated energy expenditure. Am J Physiol. 1991;261:E789–E94. doi: 10.1152/ajpendo.1991.261.6.E789. [DOI] [PubMed] [Google Scholar]
- 14.Christin L, O'Connell M, Bogardus C, Danforth E, Jr, Ravussin E. Norepinephrine turnover and energy expenditure in Pima Indian and white men. Metabolism. 1993;42:723–9. doi: 10.1016/0026-0495(93)90239-k. [DOI] [PubMed] [Google Scholar]
- 15.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 1995;268:R744–R51. doi: 10.1152/ajpregu.1995.268.3.R744. [DOI] [PubMed] [Google Scholar]
- 16.Welle SL, Thompson DA, Campbell RG. Beta-adrenergic blockade inhibits thermogenesis and lipolysis during glucoprivation in humans. Am J Physiol. 1982;243:R379–R82. doi: 10.1152/ajpregu.1982.243.3.R379. [DOI] [PubMed] [Google Scholar]
- 17.Peters SJ, Dyck DJ, Bonen A, Spriet LL. Effects of epinephrine on lipid metabolism in resting skeletal muscle. Am J Physiol. 1998;275:E300–E9. doi: 10.1152/ajpendo.1998.275.2.E300. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman DH, Cherrington AD. Hepatic fuel metabolism during muscular work: role and regulation. Am J Physiol. 1991;260:E811–E24. doi: 10.1152/ajpendo.1991.260.6.E811. [DOI] [PubMed] [Google Scholar]
- 19.Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730–5. doi: 10.1172/JCI116760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med. 1988;318:1077–83. doi: 10.1056/NEJM198804283181701. [DOI] [PubMed] [Google Scholar]
- 21.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987-1998. Circulation. 2003;107:2190–5. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- 22.Bray GA, York DA. The MONA LISA hypothesis in the time of leptin. Recent Prog Horm Res. 1998;53:95–117. discussion -8. [PubMed] [Google Scholar]
- 23.Lambert GW, Straznicky NE, Lambert EA, Dixon JB, Schlaich MP. Sympathetic nervous activation in obesity and the metabolic syndrome—Causes, consequences and therapeutic implications. Pharmacol Ther. 2010;126:159–72. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 24.van Baak MA. The peripheral sympathetic nervous system in human obesity. Obes Rev. 2001;2:3–14. doi: 10.1046/j.1467-789x.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 25.Sayer JW, Marchant B, Gelding SV, Cooper JA, Timmis AD. Autonomic dysfunction is related to impaired pancreatic β cell function in patients with coronary artery disease. Heart. 2000;83:210–6. doi: 10.1136/heart.83.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvadori AM, Fanari PM, Giacomotti EM, Palmulli PM, Bolla GM, Tovaglieri IM, et al. Kinetics of catecholamines and potassium, and heart rate during exercise testing in obese subjects. Eur J Nutr. 2003;42:181–7. doi: 10.1007/s00394-003-0409-3. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour G, Lemoine-Morel S, Casazza GA, Hala Y, Moussa E, Zouhal H. Catecholamine response to exercise in obese, overweight, and lean adolescent boys. Med Sci Sports Exerc. 2011;43:408–15. doi: 10.1249/MSS.0b013e3181f1bef3. [DOI] [PubMed] [Google Scholar]
- 28.Flaa A, Sandvik L, Kjeldsen SE, Eide IK, Rostrup M. Does sympathoadrenal activity predict changes in body fat? An 18-y follow-up study. Am J Clin Nutr. 2008;87:1596–601. doi: 10.1093/ajcn/87.6.1596. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Virtue S, Vidal-Puig A. Assessment of brown adipose tissue function. Front Physiol. 2013 doi: 10.3389/fphys.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wunder BA, Gettinger RD. Effects of body mass and temperature acclimation on the nonshivering thermogenic response of small mammals. In: Geiser F, Hulbert A, Nicol S, editors. Adaptations to the Cold: Tenth International Hibernation Symposium. University of New England Press; Armidale: 1996. pp. 131–9. [Google Scholar]
- 32.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1028–37. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 33.Spector S, Sjoerdsma A, Udenfriend S. Blockade of endogenous norepinephrine synthesis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther. 1965;147:86–95. [PubMed] [Google Scholar]
- 34.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav. 2004;81:535–42. doi: 10.1016/j.physbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Brodie BB, Costa E, Dlabac A, Neff NH, Smookler H. Applicaiton of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966;154:493–8. [PubMed] [Google Scholar]
- 36.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–82. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E52. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 38.Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013;1831:943–9. doi: 10.1016/j.bbalip.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring) 2012;20:1355–64. doi: 10.1038/oby.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–6. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- 41.Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci. 2010;1212:114–29. doi: 10.1111/j.1749-6632.2010.05800.x. [DOI] [PubMed] [Google Scholar]
- 42.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1399–R411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 43.Kurpad AV, Khan K, Calder AG, Elia M. Muscle and whole body metabolism after norepinephrine. Am J Physiol. 1994;266:E877–84. doi: 10.1152/ajpendo.1994.266.6.E877. [DOI] [PubMed] [Google Scholar]
- 44.Abe H, Minokoshi Y, Shimazu T. Effect of a β3-adrenergic agonist, BRL35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J Endocrinol. 1993;139:479–86. doi: 10.1677/joe.0.1390479. [DOI] [PubMed] [Google Scholar]
- 45.Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–7. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 46.Jensen MD. Lipolysis: Contribution from regional fat. Annu Rev Nutr. 1997;17:127–39. doi: 10.1146/annurev.nutr.17.1.127. [DOI] [PubMed] [Google Scholar]
- 47.Roden M. Muscle triglycerides and mitochondrial function: possible mechanisms for the development of type 2 diabetes. Int J Obes (Lond) 2005;29:S111–5. doi: 10.1038/sj.ijo.0803102. [DOI] [PubMed] [Google Scholar]
- 48.Maria HH, Eduardo I-G, Juleen RZ, Pablo MG-R. Tissue-specific control of mitochondrial respiration in obesity-related insulin resistance and diabetes. Am J Physiol Endocrinol Metab. 2012;302:E731–E9. doi: 10.1152/ajpendo.00159.2011. [DOI] [PubMed] [Google Scholar]