Abstract
Dopamine receptors are implicated in the reinforcing effects of food and drug reinforcement. The purpose of this study was to evaluate whether blocking D2 dopamine receptors during extinction (secondary reinforcement) would affect reacquisition of responding for food pellets (primary reinforcement). Food-restricted rats self-administered (FR1) food pellets in 1-h daily sessions for seven days. For the next seven days rats responded in extinction conditions. Prior to each extinction session rats were injected with saline or the dopamine D2 antagonist eticlopride (0.03 mg/kg, cs.c.). After the extinction phase, rats were allowed to reacquire food pellet self-administration in seven daily sessions, and received saline or eticlopride prior to each session. Four treatment groups were represented: (saline extinction, saline reacquisition); (eticlopride extinction, saline reacquisition); (saline extinction, eticlopride reacquisition); (eticlopride extinction, eticlopride reacquisition). Locomotor activity did not differ between eticlopride-treated and saline-treated rats throughout the study. Extinction was accelerated in eticlopride-treated rats. Eticlopride also delayed reacquisition of food self-administration compared to saline-treated rats. Rats administered eticlopride during extinction showed delayed reacquisition and a decreased response rate for food during the reacquisition phase. Indirectly reducing the value of a reinforcer in this way may provide a novel approach for reducing addiction-related food or drug self-administration behaviors.
Keywords: dopamine, extinction, food, obesity, rat, reinforcement
Introduction
Given the severity of the obesity epidemic (WHO, 2013), further evaluation of factors that maintain feeding behaviors, such as the role of specific neurotransmitters, is warranted. Dopamine (DA) is important in food reinforcement (Ettenberg, 1989; Wise, 2004). Depending on the schedule of reinforcement, DA antagonists decrease responding in the presence of food or in the presence of food-paired cues (Wise et al., 1978a; Gray and Wise, 1980; Mason et al., 1980; Spyraki et al., 1982; Ettenberg and Camp, 1986; Wise and Raptis, 1986; Beninger et al., 1987; Asin and Wirtshafter, 1990; Duarte et al., 2003).
Exemplifying these findings are data from Wise et al., (1978a). Wise and colleagues found that the DA receptor blocker pimozide reduced the “reward quality” of food without impairing the animals’ physical ability to lever press. Rats were first trained to press a lever for food and were then either administered pimozide prior to operant conditioning sessions or put through several sessions of extinction. Rats that received pimozide prior to food-reinforced responding had reduced response rates for food in a manner similar to that of rats responding in extinction. Wise and colleagues suggested that, in both cases, rats reduced responding because they were no longer experiencing the reinforcing effects of the food.
That being said, it has been debated whether extinction of responding in the absence of food is equivalent to food-reinforced responding diminished by a DA antagonist (Gray and Wise, 1980; Mason et al., 1980; Salamone, 1986). For example, giving rats either pimozide or the DA receptor antagonist haloperidol during extinction trials can accelerate extinction if rats had been trained on a continuous reinforcement schedule (Phillips and Fibiger, 1979; Feldon and Weiner, 1991). Acceleration of extinction would not be predicted if DA antagonism was expected only to block primary reinforcement, as extinction responding is technically extinction of secondary reinforcement (Phillips and Fibiger, 1979), the secondary reinforcer(s) being the click of the lever and perhaps the act of lever pressing itself (Bindra, 1972; Grimm et al., 2000). One explanation for this apparent discrepancy is that DA antagonism reduces both primary and secondary reinforcement by reducing their incentive values (Wise, 2004).
Therefore, in the present study we examined whether extinction with DA receptor antagonism, compared to extinction alone, would lead to a change in the responding for food when it again became available. If DA antagonism reduces the incentive value of lever responding in the self-administration context, it would be expected that such an effect would be apparent even when primary reinforcement is again provided.
Rats were first allowed to respond for food pellets on a continuous reinforcement schedule for seven days. They were then split into two groups and allowed to respond in extinction conditions for seven days. One group was pretreated daily with saline while the other was pretreated with the specific DA D2 (DAD2) receptor antagonist eticlopride. Studies have shown the D2 receptor subtype to mediate food consumption (Beninger et al., 1987; Baldo et al., 2002; Duarte et al., 2003; Johnson and Kenny, 2010). After seven extinction sessions, the two extinction groups were further sub-divided. From the saline-treated extinction group, one subgroup continued to receive daily saline pre-treatments while the other received daily eticlopride pre-treatments. From the eticlopride-treated extinction group, one sub-group continued to receive daily eticlopride pre-treatments while the other received daily saline pre-treatments. Rats were again allowed to respond for food pellets for seven days. The effects of DA antagonism on food self-administration were evaluated to confirm a role of DAD2 receptors in food reinforcement. Inactive lever responses and photobeam breaks (a locomotor activity measure) were recorded in all sessions, as a means of identifying any response generalization (inactive lever) or motor-related (inactive lever and locomotor activity) effects of DA antagonist treatment.
Methods
Subjects
Forty-three male Long-Evans rats (approximately 4 months old, 452.6 ± 0.2 g at start of study), bred in the Western Washington University vivarium, were used for this experiment. Rats were housed individually under a 12-h reverse day/night cycle with lights off at 07.00 h. Rats were food restricted (20 g per day post session, Purina Mills Inc. Mazuri Rodent Pellets, Saint Louis, MO, USA) and their weights were recorded every Monday, Wednesday, and Friday for the duration of the study. Food restriction began 48 h prior to the first day of training. All procedures followed the guidelines outlined in the “Principles of laboratory animal care” (NIH publication no. 85-23) and were approved by the Western Washington University Institutional Animal Care and Use Committee.
Apparatus
Operant procedures took place in operant conditioning chambers (30 × 20 × 24 cm; Med Associates, Vermont, USA) equipped with two retractable levers on either side of the tray where food pellets were dispensed. Each chamber was also equipped with four infrared photobeams (Med Associates) that crossed the chamber. The operant conditioning chambers were enclosed in sound-attenuating chambers equipped with fans to provide air flow and white noise, and included a red houselight on the wall opposite the levers that was illuminated during operant sessions.
Materials
Subjects’ active lever presses were reinforced with 45mg food pellets (Rodent Tablets AIN-76A, Test Diet, IN, USA). During the Extinction and Reacquisition phases, rats were injected with either saline (1 ml/kg) or the dopamine DAD2 receptor antagonist S-(-)-eticlopride hydrochloride (Sigma-Aldrich, Saint Louis, MO, USA; 0.03 mg/kg in 1 ml/kg saline, SC). Injections were given 15 minutes prior to the start of a session. Rats were injected and remained in home cages until immediately prior to a session. Eticlopride was used as it has a higher affinity for the D2 receptor (Ki DAD2 = 0.06 nM) compared to compounds that have often been used to examine a role of dopamine in food-maintained responding, including pimozide (Ki DAD2 = 2.5 nM) and haloperidol (Ki DAD2 = 0.5 nM) (Ki values derived from [3H]spiperone competition assays (Roth et al., 1995; Tang et al., 1994)). Eticlopride is also more specific for DAD2 receptors as compared to pimozide or haloperidol. For example, pimozide has affinity for 5-HT7 receptors (Ki = 0.5 nM (Roth et al., 1994) and haloperidol inhibits NMDA receptors (Lynch and Gallagher, 1996). The dose of eticlopride was chosen based upon previous reports in which the drug altered operant behavior without obvious motor side-effects (Barrett et al., 2004; Botly et al., 2008).
General procedures
Training phase
Rats underwent 7 daily 1-h sessions (days 1–7) wherein they learned to press the active lever for a food pellet. Active lever presses (left lever) were reinforced under a fixed-ratio 1 schedule. No cues (e.g. light + tone) were associated with active lever presses and there was no time-out between food pellet delivery opportunities. Inactive lever presses elicited no response and were recorded as a control for discriminated responding and motor activity. On the last two days of Training the rats were administered saline handling injections (1 ml/kg, s.c.) in the vivarium approximately 1 h after the session.
Extinction Phase
Following the final day of Training, rats were randomly assigned to one of four groups: Saline-Saline (SAL/SAL), Saline-Eticlopride (SAL/ETIC), Eticlopride-Saline (ETIC/SAL), and Eticlopride-Eticlopride (ETIC/ETIC). Group designations indicated whether rats would receive saline or eticlopride prior to Extinction or Reacquisition sessions. For example, ETIC/SAL rats received eticlopride prior to each Extinction session and saline prior to each Reacquisition session.
Starting the next day, rats underwent 7 daily 1-h extinction sessions (days 8–14) during which active presses no longer elicited food pellets. The pellet dispenser was activated by lever presses, but it was empty. Rats were pre-treated with saline (1 ml/kg, s.c.) or eticlopride (0.03 mg/kg, s.c.) 15 minutes prior to sessions, according to their group designations.
Reacquisition Phase
Starting the day following the last day of Extinction, rats underwent 7 daily 1-h reacquisition sessions (days 15–21). During these sessions, active presses were once again reinforced with food pellets. The pre-session injections continued according to the group designations defined above.
Statistical analyses
Active (equivalent to # of pellets earned) and inactive lever presses, and photobeam breaks, were analyzed separately. Training, Extinction, and Reacquisition data were also analyzed separately. Body weight data were analyzed across the entire study. Data totals were analyzed using mixed-model repeated-measures analysis of variance (RM ANOVA). The repeated measure was Time (TIME) and the between-groups variables were Extinction Drug (EXTDRUG) and Reacquisition Drug (REACQDRUG). To identify whether any overall differences in responding on the first day of Extinction and the first day of Reacquisition occurred at the start of these sessions rather than appearing later, ANOVA was conducted on the first 2 minute bins of active lever responding on these days, using the variables EXTDRUG and REACQDRUG. Post-hoc comparisons for all ANOVAs were made using Fisher’s LSD tests with a Bonferroni-corrected alpha. Other than these corrected alphas, the criterion for statistical significance was P < 0.05. For brevity, in most instances only statistics for significant main effects and interactions are noted in the text. Means ± standard error of the mean (SEM) are indicated in the text and on the Figures.
Results
Body weight
Body weights increased over the course of the study,, as indicated by a significant effect of TIME [F(8,312) = 8.9, P < 0.001]. There were no significant effects for EXTDRUG or REACQDRUG or interactions between these variables and TIME, indicating that groups did not differ a priori, and neither did the weights of the rats in the various conditions differ over the course of the study. After visual inspection of the weight increase over TIME, a curve fit was conducted in SPSS using the averaged weights for each day. As expected from the RM ANOVA result, the increase in weight was described by a linear function [F(1,8) = 8.6, P < 0.05, R2 = 0.5]. It was also described by a cubic function [F(3,8) = 6.3, P < 0.05, R2 = 0.8]. These findings indicate that rats gained weight over the Training phase (Day 6: 469.8 ± 7.1 g), lost weight over the Extinction phase (Day 13: 461.5 ± 6.5 g), and then again gained weight over the Reacquisition phase (Day 20: 476.7 ± 6.5 g). As rats were maintained on the same daily amount of food in the home cage over the course of the study (20 g per day), these results indicate that the brief access to food pellets during self-administration (Training and Reacquisition) had a small impact on body weight.
Training phase
As acquisition of responding was highly variable over the first 4 days of Training, RM ANOVAs were only conducted for days 5–7. Rats responded on the active lever at stable rates over these three days, as there was no significant effect of TIME. There were also no significant effects or interactions for EXTDRUG or REACQDRUG, indicating that rats that were subsequently assigned to these conditions did not differ in response rates beforehand. Also, there were no significant effects or interactions for TIME, EXTDRUG, or REACQDRUG for inactive lever responding or photobeam breaks. Training data are depicted in Fig. 1.
Fig. 1.
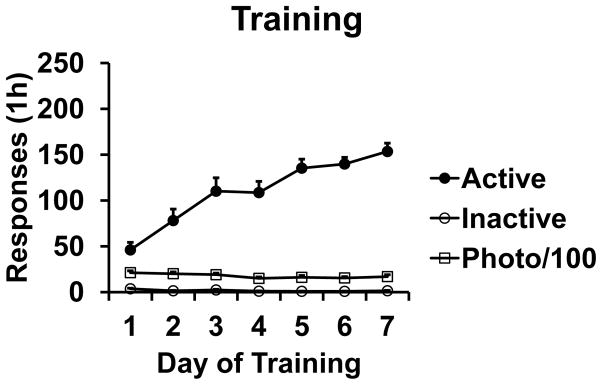
Acquisition of food pellet self-administration. N = 43.
Extinction Phase
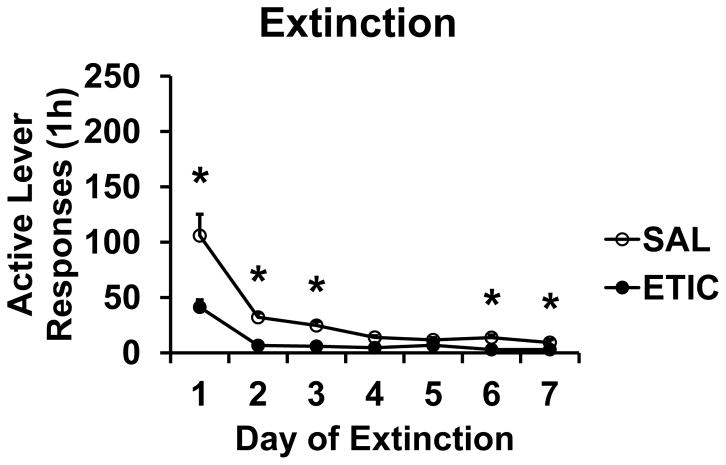
There were 21 rats assigned to the SAL condition and 22 rats assigned to the ETIC condition. For active lever responding there were significant effects of TIME [F(6,234) = 39.3] and EXTDRUG [F(1,39) = 25.3], and a significant TIME X EXTDRUG interaction [F(6,234) = 7.6; all P’s < 0.001]. Eticlopride accelerated extinction responding across the seven days of the Extinction phase. Active lever responses are shown in Fig. 2. There were no significant effects or interaction for inactive lever responding or photobeam breaks. Inactive presses in the SAL and ETIC groups averaged 2.1 ± 0.5 and 1.2 ± 0.5 presses per session, respectively. Photobeam breaks in the SAL and ETIC groups averaged 1555.2 ± 152.4 and 1522.4 ± 115.4 breaks per session, respectively. Active lever responding in the first 2-minute bin of the first day of Extinction was not significantly different across conditions (SAL 15.0 ± 1.4, ETIC 13.0 ± 1.9 responses).
Fig. 2.
Extinction of responding on lever that previously was associated with food pellet self-administration. SAL n = 21; ETIC n = 22. * indicates significant difference from ETIC group on that day, P < 0.043.
Reacquisition Phase
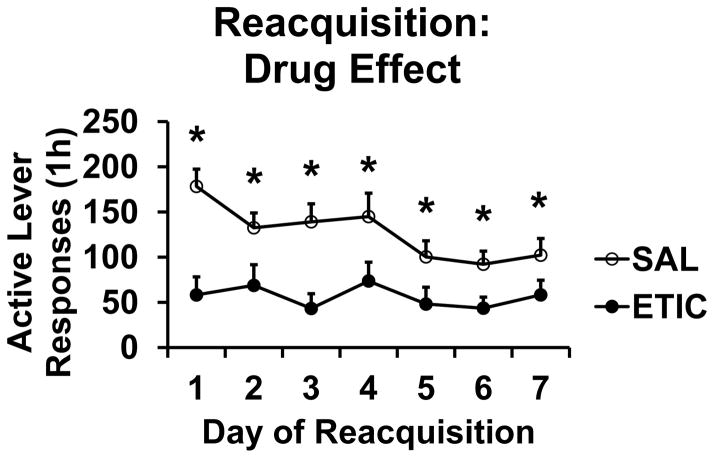
Group sizes at the start of the Reacquisition Phase were SAL/SAL n = 10, SAL/ETIC n = 11, ETIC/SAL n = 13, and ETIC/ETIC n = 9. For active lever responding there were significant effects of TIME [F(6,234) = 5.8, P < 0.001], EXTDRUG [F(1,39) = 10.7, P < 0.01], REACQDRUG [F(1,39) = 45.0, P < 0.001], and significant TIME X EXTDRUG [F(6,234) = 2.8, P < 0.05], and TIME X REACQDRUG [F(6,234) = 3.9, P < 0.01] interactions. The three-way (TIME X EXTDRUG X REACQDRUG) interaction was not statistically significant [F(6,234) = 0.83, NS].
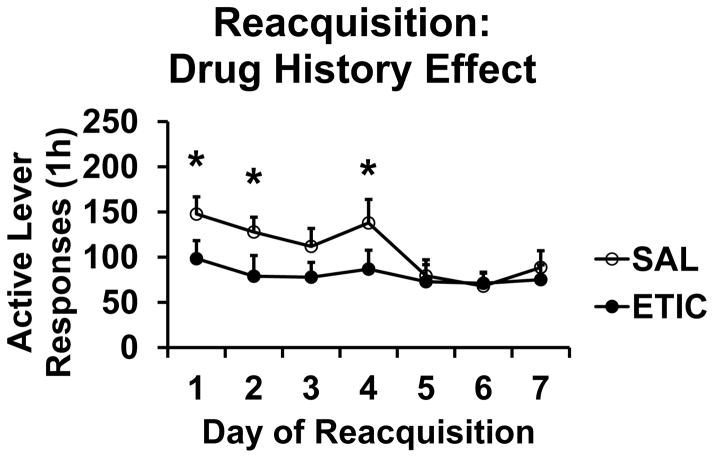
Figs. 3 and 4 s active lever response data organized according to the significant two-way interaction terms. Fig. 3 presents the TIME X REACQDRUG interaction. The effect of eticlopride to decrease responding for food pellets was apparent on the first day of Reacquisition and continued for the seven daily sessions. The effect was not immediate, however, as active lever responding in the first 2-minute bin of the first day of Extinction was not significantly different across conditions (SAL/SAL 9.5 ± 3.8, SAL/ETIC 7.4 ± 3.1, ETIC/SAL 4.7 ± 1.7, and ETIC/ETIC 2.4 ± 1.4 responses). Fig. 4 presents the TIME X EXTDRUG interaction. Rats with a history of eticlopride during Extinction responded less for food pellets over the first four days of Reacquisition sessions. This drug history effect was not explained by the response rates of rats on the last day of Extinction. We found this to be the case according to two statistical tests. First, response rate on the last day of Extinction was not significant when used as a covariate in the active lever RM ANOVA for Reacquisition [F(1,38) = 0.012, NS]. Second, responding on the last day of Extinction did not correlate with that on the first day of Reacquisition [Pr = 0.067, P = 0.67].
Fig. 3.
Reacquisition of food pellet self-administration: TIME X REACQDRUG interaction (Drug Effect). The Figure depicts the effect of daily eticlopride injections on food pellet self-administration. SAL n = 23; ETIC n = 20. * significant difference from ETIC group on that day, P < 0.043. Symbols represent means ± SEMs.
Fig. 4.
Reacquisition of food pellet self-administration: TIME X EXTDRUG interaction (Drug History Effect). The Figure depicts the effect of daily eticlopride injections during Extinction on food pellet self-administration during Reacquisition. SAL n = 21; ETIC n = 22. * significant difference from ETIC group on that day, P < 0.043. Symbols represent means ± SEMs.
To confirm the effects of Drug and Drug History, we performed planned comparisons for each day of Reacquisition, first between the SAL/SAL and SAL/ETIC groups (Drug Effect) and then between the SAL/SAL and ETIC/SAL groups (Drug History Effect). Active lever responding for all conditions and the statistically significant comparisons are show in Fig. 5, where the same patterns of results from Figs. 3 and 4 were observed when only considering rats from the complete control condition (SAL/SAL) vs. either the test for the Drug Effect (SAL/ETIC) or the test for the Drug History Effect (ETIC/SAL). The general replication of findings indicates that the effects observed in Figs. 3 and 4 were not due to collapsing groups across conditions; specifically, the ETIC/ETIC condition did not account for most of the reduced responding in Figs. 3 and 4.
Fig. 5.
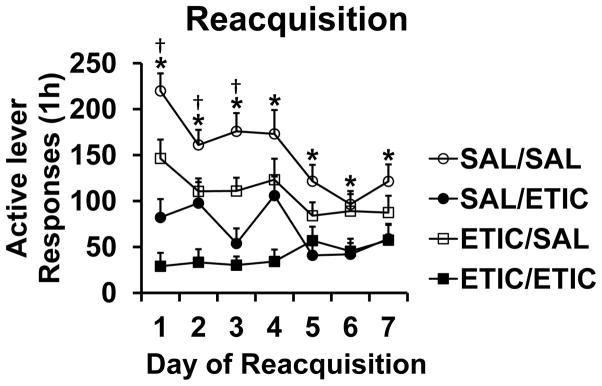
Reacquisition of food pellet self-administration with planned comparisons confirming the effects of Drug and Drug History indicated in Figs. 3 and 4. SAL/SAL n = 10; SAL/ETIC n = 11; ETIC/SAL n = 13; ETIC/ETIC n = 9. Comparisons were made for each day between SAL/SAL and SAL/ETIC (Drug Effect) and between SAL/SAL and ETIC/SAL (Drug History Effect). * significant difference between SAL/SAL and SAL/ETIC; † significant difference between SAL/SAL and ETIC SAL, P < 0.043. Symbols represent means ± SEMs.
Finally, there was a significant effect of REACQDRUG for inactive lever presses [F(1,39) = 9.2, P < 0.01]. Eticlopride reduced inactive lever responses from an across-sessions average of 1.5 ± 0.2 (SAL) to 0.5 ± 0.1 (ETIC) responses/h. Inactive presses in the various groups averaged across the seven days of Reacquisition were SAL/SAL 1.5 ± 0.2, SAL/ETIC 0.8 ± 0.2, ETIC/SAL 1.6 ± 0.3, and ETIC/ETIC 0.2 ± 0.1. There were no significant effects or interaction for photobeam breaks, which, averaged across the seven days of Reacquisition, were SAL/SAL 1684.7 ± 131.1, SAL/ETIC 1337.4 ± 152.8, ETIC/SAL 1790.7 ± 122.0, and ETIC/ETIC 1554.2 ± 163.4.
Discussion
The rats that were injected with eticlopride during the Extinction phase responded less on the active lever than those that were injected with saline. Similarly, eticlopride administration decreased lever pressing for food pellets in the Reacquisition phase. Finally, rats with a history of eticlopride administered during Extinction also pressed less during reacquisition of responding for food pellets compared to the rats that were given saline during Extinction. Overall, these findings support a role for DAD2 receptors in the primary and secondary reinforcing effects involved in lever pressing for food pellet reinforcement.
The ability of DAD2 antagonists to accelerate extinction responding and reduce operant responding for food is well documented (Wise et al., 1978a; Phillips and Fibiger, 1979; Feldon and Weiner, 1991). The first part of the present study replicates these findings by showing that active lever pressing decreased at a significantly faster rate in Extinction when rats were administered eticlopride than when they were administered saline (Fig. 2). Injecting rats with eticlopride during reacquisition of food pellet reinforcement also resulted in an overall decreased response rates for food pellets compared to controls (Figs. 3 and 5). This is consistent with previous findings, as noted in the Introduction, where DAD2 receptor-preferring antagonists reduced responding for food (Phillips and Fibiger, 1979; Wise et al., 1978a; Beninger et al., 1987; Wise, 2004). DAD2 receptor-preferring antagonists have also been found to decrease responding for other incentives, including water, saccharin, sucrose, brain stimulation, and drugs of abuse (Yokel and Wise, 1975; Wise et al., 1978b; Gerber et al., 1981; Wise, 2004; Smith and Smith, 2010).
Potential explanations for the response-decreasing effects of eticlopride on primary and secondary reinforcement include eticlopride-mediated motor impairment or changes in hunger. However, the acceleration of extinction responding by eticlopride was not attributable to motor impairment for three reasons. First, in the initial two minutes of the first day of extinction there was no difference in active lever responding between saline and drug-treated groups. Second, inactive lever responding did not differ between groups. And third, there was no difference in locomotor activity between groups. During Reacquisition, the response-decreasing effect of eticlopride was also most likely not attributable to a motor-impairing effect of the drug: there was no difference across treatment conditions in the first two minutes of responding on the first day of reacquisition, nor were there group differences in locomotor activity across the reacquisition sessions. There was a significant effect of eticlopride on inactive lever responding, but the overall difference between SAL and ETIC groups was approximately 1 lever press/h. Given the already very low response rate on the inactive lever, the lack of effect of eticlopride on locomotor activity, and the lack of active lever group differences in the first two minutes of the first day of Reacquisition, it is unlikely that the eticlopride-mediated decrease in active lever responding during Reacquisition was due to motor impairment.
It is also unlikely that eticlopride reduced food-reinforced responding during Reacquisition by reducing “hunger.” While we did not directly examine this hypothesis in the present study, findings from other studies lead us to conclude that eticlopride is not an anorectic agent. Previous studies have demonstrated that DAD2 antagonists reduce operant responding for food but are without effect on free feeding (Salamone et al., 2005; Randall et al., 2012). If DAD2 antagonism reduced hunger, rats would be expected to reduce their intake of food in both operant and free-feeding conditions.
Motivation to respond in extinction or for food pellets must have been reduced by some other factor. We propose that eticlopride reduced responding by reducing primary and secondary reinforcement, at least within the context of the operant conditioning chamber. The acceleration of responding in Extinction would therefore have been due to a decrease in the conditioned incentive value (Bindra and Palfai, 1967; Stewart and De Wit, 1987) of the operant conditioning chamber, including the lever that had previously been associated with food-pellet delivery. The reduction in food-reinforced responding in eticlopride-treated rats would then have been due to a drug-induced decrease in the incentive qualities of food pellets. This would require the rats to have some initial experience of responding for food under the effects of DAD2 antagonism to “learn” that the reinforcing efficacy of a food pellet was reduced. This appeared to be the case in the present study, as response rates in the first 2 minutes of the first reacquisition day were not different across treatment conditions. A similar effect has been reported previously: Wise et al. (1978a) reported that the motivation of rats to respond for food pellets was not reduced until after experiencing response-dependent food pellets under the dopamine receptor blocking effects of pimozide. Similar patterns of learning where the incentive value of a reinforcer has been diminished by DA receptor blockage have been reported in runway procedures (Wise et al., 1978a; Ettenberg and Camp, 1986).
The most novel finding of the present study is that the apparent decrease in the incentive value of the self-administration context by eticlopride in Extinction “transferred” to a diminished ability of food pellets to reinforce lever responding in Reacquisition. Specifically, rats that received eticlopride during the Extinction phase showed decreased responding for food pellets, compared to controls, during Reacquisition (Figs. 4 and 5). This was not explained by rate of responding on the last day of extinction (Fig. 2; see ANCOVA and correlation values in the Results section). In addition, this long-lasting effect (4 days) cannot be explained by a lingering effect of eticlopride itself, as the drug has a half-life of approximately 1h (Norman et al., 2011).
We are not certain as to the prevailing cause of this “history” effect, but we speculate that it may occurred because DAD2 antagonism changed the meaning of the self-administration context, including the active lever response, for the subjects. For example, eticlopride administration during Extinction could have decreased or even negated the incentive value of the active lever, such that, in Reacquisition, rats would need more time for the value of the lever to be re-established by contingent food delivery. This hypothesis fits with the pattern of reacquisition by rats with a history of eticlopride during Extinction, which took 5 days to reach the level of food self-administration of rats with a history of saline during Extinction. A second possibility, which also considers the role of the meaning of the self-administration context in the vigor of responding in Reacquisition, is that DAD2 antagonism during Extinction changed the meaning of the self-administration context, such that it became an “inhibitory” signal for responding. This hypothesis also fits with the pattern of reacquisition by rats with a history of eticlopride during Extinction. This inhibitory signal hypothesis somewhat maps onto results obtained by other investigators utilizing the “renewal” procedure where, typically, responding is first reinforced in one context (A), extinguished in another (B), and then renewed upon return to the first context (A) (Bouton et al., 2012). In one especially relevant study utilizing this procedure, reacquisition of food self-administration was slower for rats that reacquired in the context in which their responding had been extinguished (ABB) compared to the reacquisition of rats in the context where extinction had not occurred (ABA) (Todd et al., 2012).
Future studies are needed to clarify whether the history effect is mediated by the changes in the incentive value and/or response-appropriate signaling properties of the self-administration context, including the response requirement and/or manipulandum. For example, a reacquisition comparison group could be to have rats reacquire self-administration using a novel response such as a nose poke. A comparison to investigate the role of the signaling properties of the context could be to have rats reacquire self-administration in a novel context. If the history effect were to be maintained in both of these comparisons, it might then be concluded that experience of DAD2 antagonism during Extinction literally transferred to food pellets, devaluing their incentive value. Such transfer effects between primary and secondary reinforcers have been reported previously (Harkness et al., 2010).
To better understand the effects observed in the present study, future studies could also explore the neuroanatomical loci of DAD2 antagonism effects on primary and secondary reinforcement. Several studies have shown that the reinforcing effects of certain behaviors, including responding for food, are mediated by the nucleus accumbens (Kelley, 2004; Segovia et al., 2012; Nunes et al., 2013). For example, direct injections of DAD1 and DAD2 antagonists into the nucleus accumbens (both core and shell) of rats reduced responding for food reinforcement (Baldo et al., 2002). To determine the neuroanatomical locus of the eticlopride-mediated effects in the present study, it would be necessary to similarly direct intracranial injections of a DAD2 antagonist. In addition, given the findings of Baldo et al. (2002) and our previous findings of reduced food (sucrose) cue reactivity following accumbal DAD1 antagonist injections (Grimm et al., 2011), it would be of interest to compare the response-reducing effect of DAD2 antagonists with those of DAD1-specific antagonists. Given the results of the present study it would also be of interest to examine whether similar site-specific injections reduce extinction responding and, if so, whether such devaluation of the extinction context transfers over to reacquisition of responding for food. Further studies might also explore the generality of the effects observed in the present study by conducting similar studies with sucrose or a drug of abuse such as cocaine.
Concluding remarks
Eticlopride reduced the response rates of rats for food pellets during both the Extinction and Reacquisition phase. Also, eticlopride was able to indirectly reduce response rates in the Reacquisition phase if had been administered during the Extinction phase. Overall these results support a role for DAD2 receptors in both primary and secondary reinforcement. The eticlopride drug history effect may be particularly useful for the design of effective behavioral pharmacotherapies related to excessive food consumption (obesity) and, perhaps, drug addiction.
Acknowledgments
This study was supported by NIDA/NIH grant R15 DA016285-03 and Western Washington University.
The authors wish to thank Rachel Weber, Kindsey North, Stefan Collins, and Edwin Glueck for help with data collection.
References
- Asin KE, Wirtshafter D. Evidence for dopamine involvement in reinforcement obtained using a latent extinction paradigm. Pharmacol Biochem Behav. 1990;36:417–20. doi: 10.1016/0091-3057(90)90426-i. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–77. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Cheng M, Hahn BL, Hoffman DC, Mazurski EJ, Morency MA, Ramm P, Stewart RJ. Effects of extinction, pimozide, SCH 23390, and metoclopramide on food-rewarded operant responding of rats. Psychopharmacology (Berl) 1987;92:343–9. doi: 10.1007/BF00210842. [DOI] [PubMed] [Google Scholar]
- Bindra D. A unified account of classical conditioning and operant training. In: Black HH, Prokasy WF, editors. Classical conditioning II: Current research and theory. Appleton-Century-Crofts; New York: 1972. pp. 453–481. [Google Scholar]
- Bindra D, Palfai T. Nature of positive and negative incentive-motivational effects on general activity. J Comp Physiol Psychol. 1967;63:288–97. doi: 10.1037/h0024371. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Winterbauer NE, Todd TP. Relapse processes after the extinction of instrumental learning: renewal, resurgence, and reacquisition. Behav Processes. 2012;90:130–141. doi: 10.1016/j.beproc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botly LC, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology (Berl) 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Duarte C, Biala G, Le Bihan C, Hamon M, Thiebot MH. Respective roles of dopamine D2 and D3 receptors in food-seeking behaviour in rats. Psychopharmacology (Berl) 2003;166:19–32. doi: 10.1007/s00213-002-1310-0. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Dopamine, neuroleptics and reinforced behavior. Neurosci Biobehav Rev. 1989;13:105–11. doi: 10.1016/s0149-7634(89)80018-1. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. Haloperidol induces a partial reinforcement extinction effect in rats: implications for a dopamine involvement in food reward. Pharmacol Biochem Behav. 1986;25:813–21. doi: 10.1016/0091-3057(86)90392-8. [DOI] [PubMed] [Google Scholar]
- Feldon J, Weiner I. Effects of haloperidol on the multitrial partial reinforcement extinction effect (PREE): evidence for neuroleptic drug action on nonreinforcement but not on reinforcement. Psychopharmacology (Berl) 1991;105:407–14. doi: 10.1007/BF02244437. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Sing J, Wise RA. Pimozide attenuates lever pressing for water reinforcement in rats. Pharmacol Biochem Behav. 1981;14:201–5. doi: 10.1016/0091-3057(81)90243-4. [DOI] [PubMed] [Google Scholar]
- Gray T, Wise RA. Effects of pimozide on lever pressing behavior maintained on an intermittent reinforcement schedule. Pharmacol Biochem Behav. 1980;12:931–5. doi: 10.1016/0091-3057(80)90455-4. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–86. [Google Scholar]
- Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology (Berl) 2011;216:219–33. doi: 10.1007/s00213-011-2210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness JH, Webb S, Grimm JW. Abstinence-dependent transfer of lithium chloride-induced sucrose aversion to a sucrose-paired cue in rats. Psychopharmacology (Berl) 2010;208:521–530. doi: 10.1007/s00213-009-1755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Gallagher MJ. Inhibition of N-methyl-D-aspartate receptors by haloperidol: developmental and pharmacological characterization in native and recombinant receptors. J Pharmacol Exp Ther. 1996;279:154–61. [PubMed] [Google Scholar]
- Mason ST, Beninger RJ, Fibiger HC, Phillips AG. Pimozide-induced suppression of responding: evidence against a block of food reward. Pharmacol Biochem Behav. 1980;12:917–23. doi: 10.1016/0091-3057(80)90453-0. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tabet MR, Norman MK, Tsibulsky VL. Using the self-administration of apomorphine and cocaine to measure the pharmacodynamic potencies and pharmacokinetics of competitive dopamine receptor antagonists. J Neurosci Methods. 2011;194:252–8. doi: 10.1016/j.jneumeth.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: Effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Decreased resistance to extinction after haloperidol: implications for the role of dopamine in reinforcement. Pharmacol Biochem Behav. 1979;10:751–60. doi: 10.1016/0091-3057(79)90328-9. [DOI] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, Lopez Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Muller CE, Correa M, Salamone JD. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One. 2012;7:e47934. doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–10. [PubMed] [Google Scholar]
- Roth BL, Tandra S, Burgess LH, Sibley DR, Meltzer HY. D4 dopamine receptor binding affinity does not distinguish between typical and atypical antipsychotic drugs. Psychopharmacology (Berl) 1995;120:365–8. doi: 10.1007/BF02311185. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Different effects of haloperidol and extinction on instrumental behaviours. Psychopharmacology (Berl) 1986;88:18–23. doi: 10.1007/BF00310507. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Correa M, Lennington JB, Conover JC, Salamone JD. Changes in nucleus accumbens and neostriatal c-Fos and DARPP-32 immunoreactivity during different stages of food-reinforced instrumental training. Eur J Neurosci. 2012;35:1354–67. doi: 10.1111/j.1460-9568.2012.08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP, Smith JC. The inhibitory potency of SCH 23390 and raclopride on licking for sucrose increases across brief-access tests. Physiol Behav. 2010;101:315–9. doi: 10.1016/j.physbeh.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Attenuation by haloperidol of place preference conditioning using food reinforcement. Psychopharmacology (Berl) 1982;77:379–82. doi: 10.1007/BF00432775. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H. Reinstatement of drug-taking behavior as a method of assessing incentive motivational properties of drugs. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer; New York: 1987. pp. 211–227. [Google Scholar]
- Tang L, Todd RD, Heller A, O’Malley KL. Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J Pharmacol Exp Ther. 1994;268:495–502. [PubMed] [Google Scholar]
- Todd TP, Winterbauer NE, Bouton ME. Contextual control of appetite. Renewal of inhibited food-seeking behavior in sated rats after extinction. Appetite. 2012;58:484–489. doi: 10.1016/j.appet.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . [Accessed May 29, 2013];Obesity and overweight. 2013 Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Raptis L. Effects of naloxone and pimozide on initiation and maintenance measures of free feeding. Brain Res. 1986;368:62–8. doi: 10.1016/0006-8993(86)91042-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978a;201:262–4. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, Legault L. Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Can J Psychol. 1978b;32:77–85. doi: 10.1037/h0081678. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–9. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]