Abstract
We assessed the interplay of artificial and natural selection in rice adaptation in low-input farming systems in West Africa. Using 20 morphological traits and 176 molecular markers, 182 farmer varieties of rice (Oryza spp.) from 6 West African countries were characterized. Principal component analysis showed that the four botanical groups (Oryza sativa ssp. indica, O. sativa ssp. japonica, O. glaberrima, and interspecific farmer hybrids) exhibited different patterns of morphological diversity. Regarding O. glaberrima, morphological and molecular data were in greater conformity than for the other botanical groups. A clear difference in morphological features was observed between O. glaberrima rices from the Togo hills and those from the Upper Guinea Coast, and among O. glaberrima rices from the Upper Guinea Coast. For the other three groups such clear patterns were not observed. We argue that this is because genetic diversity is shaped by different environmental and socio-cultural selection pressures. For O. glaberrima, recent socio-cultural selection pressures seemed to restrict genetic diversity while this was not observed for the other botanical groups. We also show that O. glaberrima still plays an important role in the selection practices of farmers and resulting variety development pathways. This is particularly apparent in the case of interspecific farmer hybrids where a relationship was found between pericarp colour, panicle attitude and genetic diversity. Farmer varieties are the product of long and complex trajectories of selection governed by local human agency. In effect, rice varieties have emerged that are adapted to West African farming conditions through genotype × environment × society interactions. The diversity farmers maintain in their rice varieties is understood to be part of a risk-spreading strategy that also facilitates successful and often serendipitous variety innovations. We advocate, therefore, that farmers and farmer varieties should be more effectively involved in crop development.
Introduction
West African farmers have cultivated two species of rice Oryza sativa (Asian rice) and Oryza glaberrima (African rice) for several centuries. Over much of the West African coastal zone, resource-poor farmers cultivate the two species as rainfed varieties in a range of ecologies, from lowland to upland. According to one view, Asian rice was introduced into coastal West Africa by Portuguese traders in the 16th century [1]. Another view is that it may have arrived earlier (perhaps around the beginning of the Common Era) via trans-Saharan trade routes and trade links between East Africa and India [2]. African rice (O. glaberrima) is thought to have been first domesticated in the swampy basins of the upper Niger River delta 3000–4000 years ago [3], [4]. Since its introduction into West Africa, Asian rice has tended to replace African rice, particularly in wetland cultivation. From the late 18th century onwards a second wave of introductions occurred from Asia and America, including both O. sativa ssp. indica and O. sativa ssp. japonica. This boosted the rate at which O. sativa replaced O. glaberrima [3], now including in dryland rice farming conditions. This accelerated replacement, alongside the enduring cultivation of O. glaberrima in certain pockets, is often explained as resulting from local variations in socio-cultural, political, ecological and geographical factors influencing farmers and their work [5]–[9]. O. glaberrima is widely believed to be well adapted to low-input farming conditions [10].
Oryza glaberrima has never been improved by agronomists or plant breeders. Professional opinion has been that the species has little to offer and that yields are invariably low. More recently, O. glaberrima has been seen as a useful genetic resource to improve O. sativa varieties [11], [12]. The two rice species are genetically isolated from each other by an F1 sterility barrier ([13]–[17], amongst others), although gene exchange can occur in the field [15], [17]–[21]. Recent research confirms that varieties with an interspecific background, resulting from introgressions, are regularly to be found in farmer fields along the Upper Guinea Coast from The Gambia down to Sierra Leone [22], [23]. Because backcrossing to either parent (to produces fertile progeny) results in parental phenotypical resemblance, it is difficult to detect hybrid derivatives; they look like either sativa or glaberrima [17], [23]. This means that four botanical clusters can be identified as co-existing in West Africa: these are O. sativa ssp. indica, O. sativa ssp. japonica, O. glaberrima and interspecific farmer varieties [23].
A recurrent idea in the literature is that although farmer varieties look very diverse morphologically, they are actually genetically rather uniform at gene pool level because of continuous selection on qualitative traits in the same gene pool [24] and because most farmer varieties are the result of recombination of existing farmer varieties [25]. A common, different view is that farmer varieties are made up of different genotypes, making them genetically quite diverse. Both views do not seem to apply to rice in West Africa. The first idea is countered by a study conducted by Nuijten et al. [23], and the second view may apply to other crops, but not to rice [6]. In West Africa the coexistence of Asian and African rice has resulted in an enlarged gene pool and the development of interspecific farmer varieties [23], [26], [27]. The main underlying factors are farmer selection and gene flow through cross pollination and seed exchange [6]. From seemingly isolated hamlets seed can travel long distances, through informal seeds networks, mostly based on extended family ties, and can diffuse across countries [7], [28]. These processes of seed diffusion have been traced over several centuries [29]. The time-depth and durability of this process prepares us to understand the finding that farmer varieties can embody greater levels of genetic diversity than formal varieties [30], challenging an assumption often made by plant breeders that the reverse is true on account of the access enjoyed by breeders to a world-wide spectrum of genetic resources [31]. The existence of farmer varieties with an interspecific background clearly shows that farmer crop development has more potential value as a complement to scientific breeding than is often assumed [23]. The value of these activities, by farmers in West African conditions, is further reinforced by recent research showing that farmer rice varieties can be adapted to a wide range of agro-ecological conditions [10].
Country-specific studies have been conducted to unravel the genetic variability of rice in West Africa (e.g. for Sierra Leone, Guinea and The Gambia, see [22], [30], [32], [33]). Nuijten et al. [23] then offered a regional perspective by analysing a large set of farmer varieties collected from seven countries across coastal West Africa, using molecular markers. To obtain a more complete understanding of the processes underlying the development and maintenance of genetic diversity, the present study now combines molecular and morphological characterization with socio-economic information concerning four botanical groups of rice from six West African countries. The aim is to explain how farmer practices have combined with environmental pressures to shape rice diversity in the case study countries. Reference to historical and socio-cultural data is made in order to better understand region-specific morphological traits.
Analysis directs attention to underlying processes regulating the development of genetic diversity in crops in low-input farming systems - processes not yet well understood. An important issue is to grasp the scope of the interplay of artificial and natural selection in crop adaptation. Our findings suggest (Figure 1) that there are multiple pathways for natural and artificial (farmer) selection to influence molecular and morphological markers. Correlations between morphological and molecular data may also vary among the botanical groups because of differences in genetic background, robustness and differential response to human or environmental selection pressures.
Figure 1. Schematic representation of the main aspects of our research and their interlinkages.
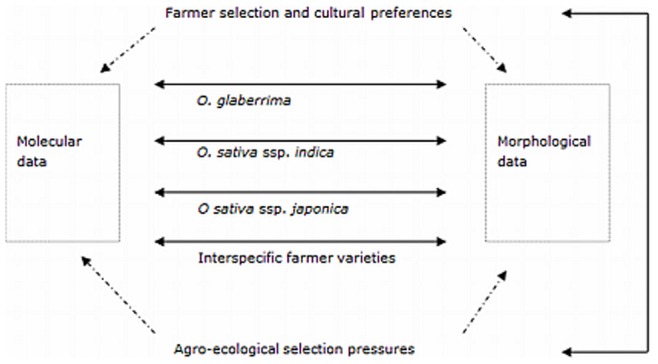
Our analysis confirms that rice varieties in West Africa are adapted to their conditions as a result of genotype × environment × society interactions. The rice diversity farmers appeared to maintain is probably part of a risk-spreading strategy that facilitates innovations in variety development.
Results and Discussion
Rice Diversity in West Africa at the Molecular Level
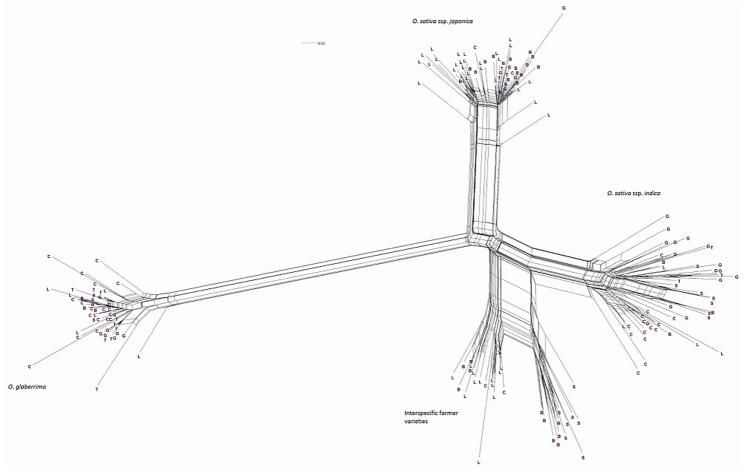
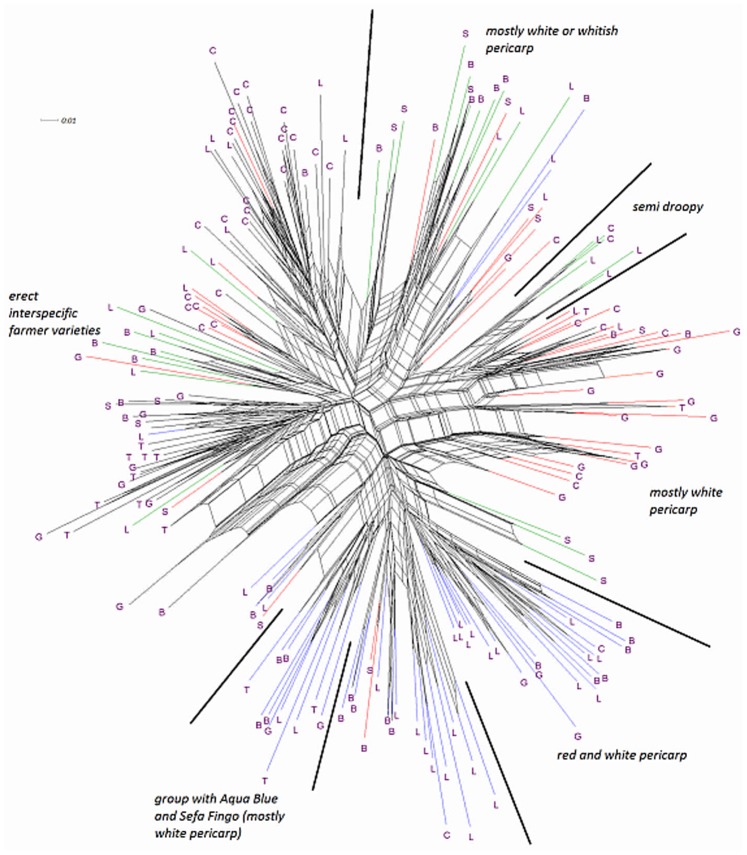
Figure 2 illustrates the phylogenetic relationships of materials studied in the field trial, as assessed during molecular analysis (cf. [23]). Four clusters are shown in detail. Three of these clusters correspond to the botanical groups O. glaberrima, O. sativa ssp. japonica and O. sativa ssp. indica. In between O. glaberrima and O. sativa ssp. indica is situated the group of interspecific farmer varieties sharing the genetic background of both O. glaberrima and O. sativa (see [23], hereafter referred to as Cluster 4).
Figure 2. Phylogenetic relationships based on molecular markers of the 182 materials included in the morphological analysis.
Country of collection is indicated by letters: B = Guinea Bissau, C = Guinea Conakry, G = Ghana, L = Sierra Leone, S = Senegal, T = Togo.
The genotypes comprising each cluster also tend to separate in sub-clusters (Figure 2). The genotypes of the O. glaberrima cluster split into O. glaberrima from the lower Guinea Coast and O. glaberrima from the upper Guinea Coast. The indica group splits into several sub-clusters in a complex way. Some sub-clusters only consist of genotypes from one country (indica from Ghana), while other sub-clusters are constituted by materials from different countries. The japonica cluster splits into one sub-cluster with japonica mainly from Sierra Leone and a sub-cluster with japonica mainly from Ghana and Guinea Bissau. The cluster of the farmer hybrids splits into one sub-cluster with genotypes that display erect and semi-erect panicles and a second sub-cluster with droopy panicles. Genotypes of the first sub-cluster (Cluster 4-1) were found in Sierra Leone, Guinea and Guinea Bissau while genotypes of the second sub-cluster were found in Guinea Bissau and Senegal (Cluster 4-2). The following sections explore the morphological diversity of the respective sub-clusters to see how they are related to the observations at molecular level and farming system level. Various historical and contextual explanations for these clusterings are discussed in Nuijten et al. [23] and Mouser et al. [29]. For example, the Ghana-Guinea Bissau japonica cluster could be interpreted as indicating a pathway of rice introduction from the East Indies via the important and long-established Portuguese coastal trading stations at Elmina (Ghana) and Cacheu (Guinea Bissau).
Morphological Diversity
Out of 17 principal components (PCs), the first four accounted for 73.57% of the variance among the traits studied (Table 1). The fifth component was not used in the biplots (Figures 3, 4, 5, 6, 7, and 8) because it had very little explanatory value for most traits. Table 2 presents the rotated principal components matrix and shows how traits contributed to the PCs. Traits commonly used to distinguish O. glaberrima from O. sativa contributed most to PC 1: ligule shape and length, panicle attitude of main axis (PAMA), and leaf blade pubescence. Traits that contributed most to PC 2 were leaf width, seed width, number of tillers and number of panicles. Traits that contributed most to PC 3 were culm length, plant height, panicle length and leaf length. Seed length contributed clearly to PC 4. Tables 3 and 4 show average values, standard deviations and coefficients of variation for 10 agronomic traits, by botanical groups and sub-groups.
Table 1. Initial eigenvalues and rotation sums of squared loadings of 17 principal components based on 17 morphological traits measured on 182 rice accessions.
| Principal Component | Initial eigenvalues | Rotation sums of squared loadings | ||||
| Total | % of variance | Cumulative % | Total | % of variance | Cumulative % | |
| 1 | 5.98 | 35.17 | 35.17 | 5.51 | 32.38 | 32.38 |
| 2 | 3.13 | 18.38 | 53.55 | 2.98 | 17.51 | 49.90 |
| 3 | 2.20 | 12.94 | 66.49 | 2.65 | 15.57 | 65.47 |
| 4 | 1.20 | 7.08 | 73.57 | 1.29 | 7.58 | 73.04 |
| 5 | 1.00 | 5.90 | 79.47 | 1.09 | 6.43 | 79.47 |
| 6 | 0.88 | 5.16 | 84.63 | |||
| 7 | 0.64 | 3.79 | 88.42 | |||
| 8 | 0.58 | 3.42 | 91.84 | |||
| 9 | 0.36 | 2.09 | 93.93 | |||
| 10 | 0.29 | 1.70 | 95.63 | |||
| 11 | 0.23 | 1.38 | 97.01 | |||
| 12 | 0.21 | 1.22 | 98.23 | |||
| 13 | 0.12 | 0.71 | 98.94 | |||
| 14 | 0.08 | 0.47 | 99.41 | |||
| 15 | 0.06 | 0.34 | 99.76 | |||
| 16 | 0.04 | 0.24 | 100.00 | |||
| 17 | 0.00 | 0.00 | 100.00 | |||
Extraction Method: Principal Component Analysis.
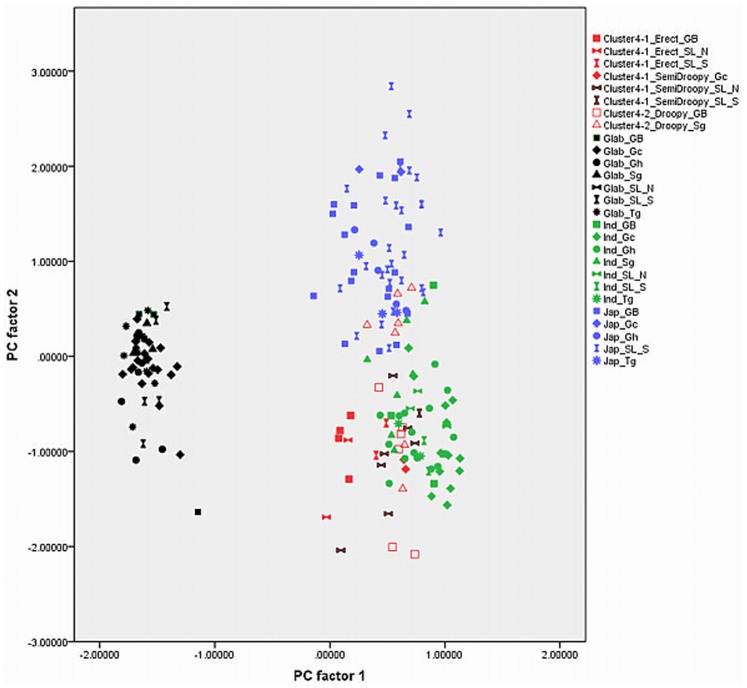
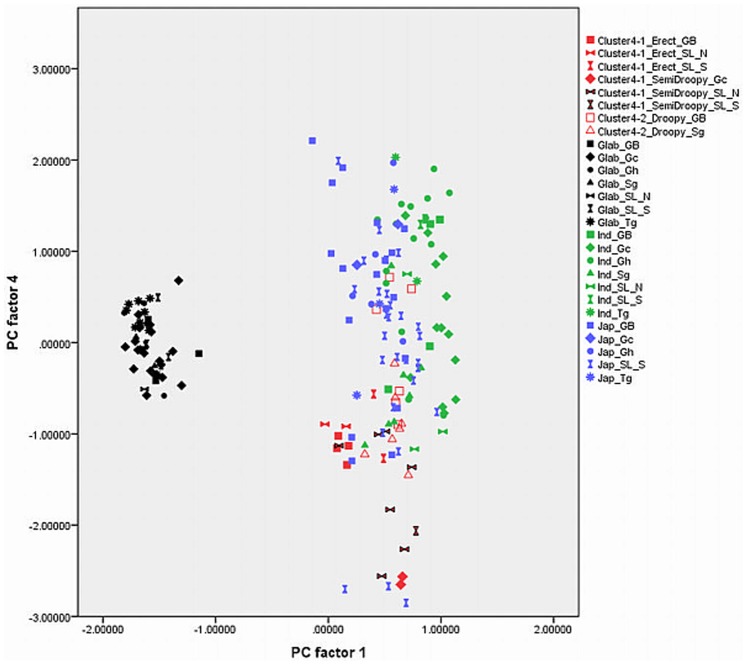
Figure 3. Graphical repartition of materials based on morphological data of PC 1 and 2.
Component 1: Ligule shape (0.97)*, Leaf blade pubescence (0.90), Ligule length (0.90), PAMA** (0.88), PAB*** (0.77), Rattoon potential (0.74), Leaf blade colour (0.65). Component 2: Leaf width (0.80), # tillers per plant (−0.79), # panicles per plant (−0.79), Seed width (0.71), Leaf blade colour (0.50). Glab: glaberrima, Ind: indica, Jap: japonica, Clusters 4-1 and 4-2: farmer hybrids. GB: Guinea Bissau, SL: Sierra Leone (north: N south: S), Gc: Guinea Conakry, Sg: Senegal, Gh: Ghana, Tg: Togo. *(): value of the correlation of the trait with the component. **: Panicle Attitude of Main Axis. ***: Panicle Attitude of Branches.
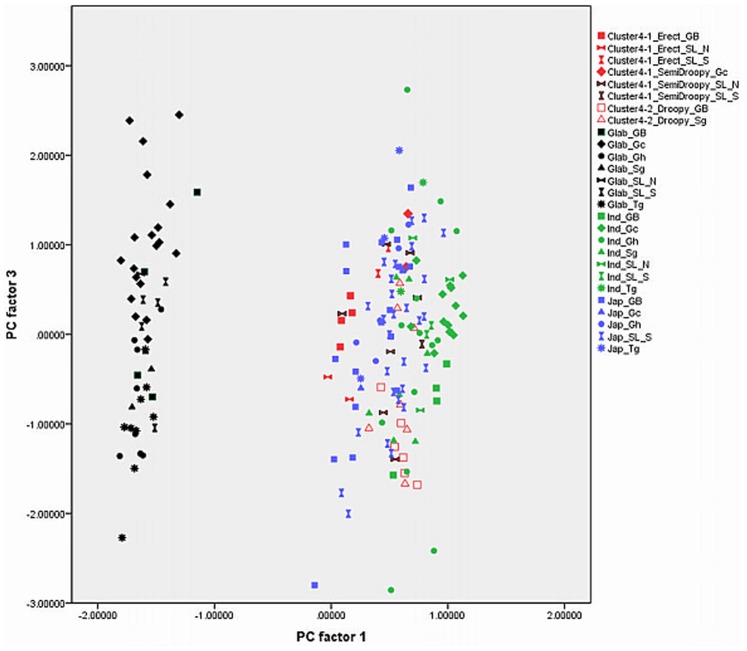
Figure 4. Graphical repartition of materials based on morphological data of PC 1 and 3.
Component 1: Ligule Shape (0.97)*, Leaf blade pubescence (0.90), Ligule length (0.90), PAMA** (0.88), PAB*** (0.77), Rattoon potential (0.74), Leaf blade colour (0.65). Component 3: Plant height (0.95), Culm length (0.88), Panicle length (0.70), Leaf length (0.60). GB: Guinea Bissau, SL: Sierra Leone (north: N south: S), Gc: Guinea Conakry, Sg: Senegal, Gh: Ghana, Tg: Togo. *(): value of the correlation of the trait with the component. **: Panicle Attitude of Main Axis. ***: Panicle Attitude of Branches.
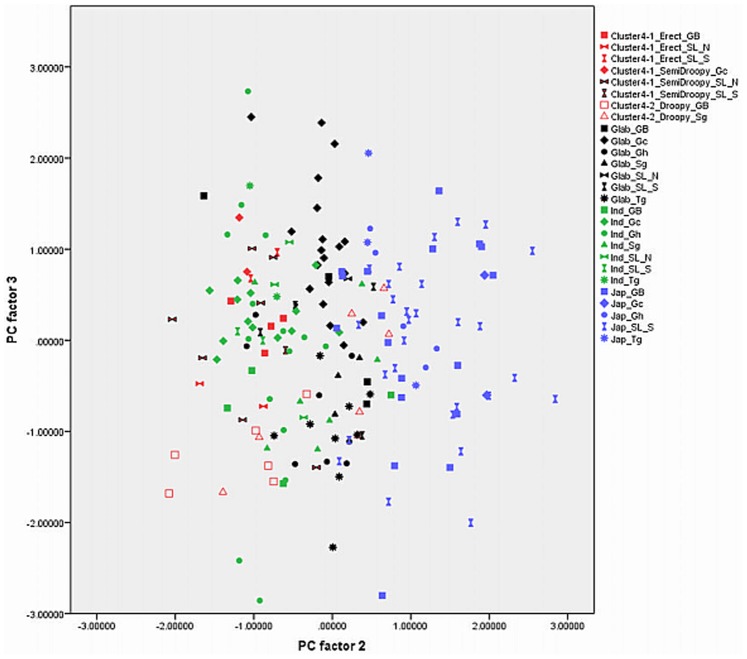
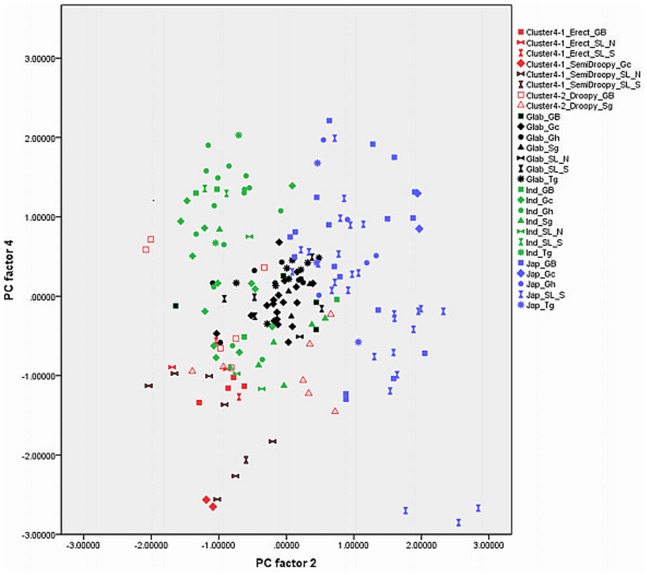
Figure 5. Graphically repartition of materials based on morphological data of PC 2 and 3.
Component 2: Leaf width (0.80)*, # tillers / plant (−0.79), # panicles/plant (−0.79), Seed width (0.71), Leaf blade colour (0.50). Component 3: Plant height (0.95), Culm length (0.88), Panicle length (0.70), Leaf length (0.60). GB: Guinea Bissau, SL: Sierra Leone (north: N south: S), Gc: Guinea Conakry, Sg: Senegal, Gh: Ghana, Tg: Togo. *(): value of the correlation of the trait with the component.
Figure 6. Graphically repartition of materials based on morphological data of PC 2 and 3.
Component 1: Ligule Shape (0.97)*, Leaf blade pubescence (0.90), Ligule length (0.90), PAMA** (0.88), PAB*** (0.77), Rattoon potential (0.74), Leaf blade colour (0.65). Component 4: Seed length (0.93), Seed width (−0.36). GB: Guinea Bissau, SL: Sierra Leone (north: N south: S), Gc: Guinea Conakry, Sg: Senegal, Gh: Ghana, Tg: Togo. *(): value of the correlation of the trait with the component. **: Panicle Attitude of Main Axis. ***: Panicle Attitude of Branches.
Figure 7. Graphically repartition of materials based on morphological data of PC 2 and 4.
Component 2: Leaf width (0.80)*, # tillers / plant(−0.79), # panicles/plant (−0.79), Seed width (0.71), Leaf Blade Colour (0.50). Component 4: Seed length (0.93), Seed width (−0.36). GB: Guinea Bissau, SL: Sierra Leone (north:N south: S), Gc: Guinea Conakry, Sg: Senegal, Gh: Ghana, Tg: Togo. *(): value of the correlation of the trait with the component.
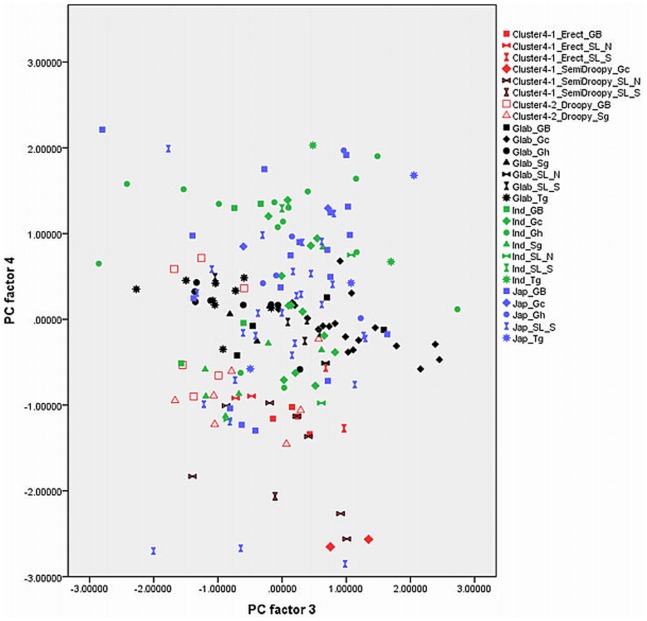
Figure 8. Graphically repartition of materials based on morphological data of PC 3 and 4.
Component 3: Plant height (0.95)*, Culm length (0.88), Panicle length (0.70), Leaf length (0.60). Component 4: Seed length (0.93), Seed width (−0.36). GB: Guinea Bissau, SL: Sierra Leone (north: N, south: S), Gc: Guinea Conakry, Sg: Senegal, Gh: Ghana, Tg: Togo. *(): value of the correlation of the trait with the component.
Table 2. Rotated Principal Components (PCs) of 17 morphological rice traits.
| Trait | Components | ||||
| 1 | 2 | 3 | 4 | 5 | |
| Leaf blade colour | 0.65 | 0.50 | −0.04 | −0.10 | −0.05 |
| Leaf blade pubescence | 0.90 | 0.02 | 0.01 | −0.16 | 0.07 |
| Culm length | −0.09 | 0.05 | 0.88 | −0.15 | 0.24 |
| Plant height | −0.09 | 0.02 | 0.95 | −0.10 | 0.12 |
| Panicle length | −0.04 | −0.09 | 0.70 | 0.17 | −0.40 |
| Leaf length | 0.25 | 0.37 | 0.60 | 0.14 | 0.20 |
| Leaf width | −0.37 | 0.80 | 0.25 | 0.05 | 0.12 |
| Ligule length | 0.90 | −0.22 | 0.05 | −0.02 | −0.01 |
| Ligule shape | 0.97 | 0.07 | −0.07 | −0.00 | 0.02 |
| # tillers / plant | −0.42 | −0.79 | −0.05 | −0.07 | 0.11 |
| # panicles / plant | −0.44 | −0.79 | −0.00 | −0.08 | 0.07 |
| Panicle attitude of mainaxis (PAMA) | 0.88 | 0.16 | −0.09 | 0.28 | −0.07 |
| Panicle attitude ofbranches (PAB) | 0.77 | 0.28 | −0.07 | 0.17 | −0.04 |
| Seed length | 0.10 | −0.07 | −0.05 | 0.93 | 0.09 |
| Seed width | −0.05 | 0.71 | −0.05 | −0.36 | 0.19 |
| Collar colour | 0.05 | 0.03 | 0.17 | 0.09 | 0.81 |
| Rattoon potential | 0.74 | 0.15 | 0.08 | 0.23 | 0.27 |
Extraction Method: Principal Component Analysis; Rotation Method: Varimax with Kaiser Normalization.
Table 3. Means, standard deviation (SD), coefficient of variation (CV) and t-test results (P, in bold) for the agronomic morphological traits for the different botanical groups from the Lower Guinea Coast (Lower) and the Upper Guinea Coast (Upper).
| Botanical group | Glaberrima | Indica | Japonica | Average | ||||
| Region | Lower | Upper | Lower | Upper | Lower | Upper | all groups | |
| Trait | N | 17 | 32 | 29 | 17 | 8 | 48 | 151 |
| Culm length (cm) | Mean | 79.1 | 86.3 | 81.0 | 82.5 | 89.2 | 82.4 | 83.4 |
| SD | 5.10 | 6.52 | 8.37 | 6.25 | 5.09 | 5.72 | 6.18 | |
| CV (%) | 6.45 | 7.55 | 10.33 | 7.57 | 5.71 | 6.94 | 7.43 | |
| P | 0.000 | 0.528 | 0.003 | |||||
| Plant height (cm) | Mean | 99.1 | 109.7 | 103.3 | 104.7 | 110.2 | 103.9 | 105.2 |
| SD | 5.22 | 7.09 | 9.21 | 6.17 | 6.27 | 6.52 | 6.75 | |
| CV (%) | 5.27 | 6.46 | 8.91 | 5.89 | 5.68 | 6.27 | 6.41 | |
| P | 0.000 | 0.561 | 0.014 | |||||
| Panicle length (cm) | Mean | 20.1 | 23.4 | 22.3 | 22.2 | 21.1 | 21.5 | 21.8 |
| SD | 1.01 | 1.29 | 1.44 | 1.01 | 1.95 | 2.23 | 1.49 | |
| CV (%) | 5.03 | 5.50 | 6.46 | 4.55 | 9.24 | 10.37 | 6.86 | |
| P | 0.000 | 0.800 | 0.611 | |||||
| Leaf length (cm) | Mean | 41.4 | 42.3 | 43.1 | 42.8 | 48.7 | 46.3 | 44.1 |
| SD | 2.80 | 2.52 | 4.17 | 2.65 | 3.22 | 3.91 | 3.21 | |
| CV (%) | 6.77 | 5.96 | 9.66 | 6.19 | 6.62 | 8.45 | 7.28 | |
| P | 0.234 | 0.761 | 0.107 | |||||
| Leaf width | Mean | 1.44 | 1.46 | 1.04 | 1.06 | 1.50 | 1.58 | 1.34 |
| SD | 0.07 | 0.12 | 0.10 | 0.14 | 0.08 | 0.20 | 0.12 | |
| CV (%) | 5.04 | 8.14 | 9.62 | 13.17 | 5.62 | 12.57 | 9.03 | |
| P | 0.140 | 0.572 | 0.261 | |||||
| # tillers / plant | Mean | 5.2 | 4.8 | 4.3 | 4.6 | 3.3 | 3.1 | 4.2 |
| SD | 0.80 | 0.80 | 0.58 | 0.64 | 0.46 | 0.59 | 0.65 | |
| CV (%) | 15.48 | 16.46 | 13.32 | 14.05 | 14.11 | 19.12 | 15.42 | |
| P | 0.599 | 0.208 | 0.485 | |||||
| # panicles / plant | Mean | 4.5 | 4.4 | 3.8 | 4.0 | 2.7 | 2.7 | 3.7 |
| SD | 0.56 | 0.70 | 0.52 | 0.60 | 0.30 | 0.47 | 0.53 | |
| CV (%) | 12.51 | 16.19 | 13.98 | 15.05 | 10.89 | 17.39 | 14.34 | |
| P | 0.621 | 0.123 | 0.738 | |||||
| Average 200 seed weight (g) | Mean | 4.63 | 4.17 | 4.67 | 4.77 | 5.29 | 4.82 | 4.73 |
| SD | 0.19 | 0.41 | 0.38 | 0.44 | 0.33 | 0.73 | 0.41 | |
| CV (%) | 4.10 | 9.83 | 8.07 | 9.12 | 6.29 | 15.08 | 8.75 | |
| P | 0.000 | 0.421 | 0.082 | |||||
| Seed length (mm) | Mean | 8.69 | 8.40 | 9.13 | 9.03 | 9.25 | 8.66 | 8.86 |
| SD | 0.16 | 0.29 | 0.68 | 0.60 | 0.49 | 0.82 | 0.51 | |
| CV (%) | 1.84 | 3.45 | 7.47 | 6.68 | 5.28 | 9.51 | 5.71 | |
| P | 0.001 | 0.629 | 0.058 | |||||
| Seed width (mm) | Mean | 3.07 | 3.03 | 2.93 | 2.96 | 3.18 | 3.25 | 3.07 |
| SD | 0.07 | 0.13 | 0.30 | 0.19 | 0.24 | 0.31 | 0.21 | |
| CV (%) | 2.28 | 4.29 | 10.27 | 6.26 | 7.50 | 9.52 | 6.69 | |
| P | 0.112 | 0.651 | 0.545 | |||||
| Average CV (%) | 5.9 | 7.6 | 8.9 | 8.0 | 7.0 | 10.5 | ||
Table 4. Means, standard deviations (SD), coefficients of variation (CV) and t-test results (P, in bold) for the agronomic morphological traits for the four different sub-groups within the Upper Guinea Coast.
| Botanical group | Glaberrima | Indica | Japonica | Cluster 4 | Cluster 4-1 | ||||||
| Region / sub-cluster | UpperGc | Upper-Other | UpperGc | Upper-Other | UpperGB | UpperSL | Cluster4-2 | Cluster4-1 | 4-1Erect | 4-1 Semi-droopy | |
| Trait | N | 19 | 13 | 13 | 12 | 18 | 28 | 13 | 18 | 8 | 10 |
| Culm length (cm) | Mean | 89.3 | 82.1 | 84.9 | 82.6 | 83.9 | 81.6 | 76.3 | 83.3 | 83.6 | 83.0 |
| SD | 5.66 | 5.32 | 4.76 | 5.39 | 6.83 | 4.98 | 5.23 | 5.32 | 4.13 | 6.33 | |
| CV (%) | 6.34 | 6.48 | 5.61 | 6.53 | 8.14 | 6.11 | 6.86 | 6.39 | 4.94 | 7.63 | |
| P | 0.001 | 0.260 | 0.198 | 0.001 | 0.826 | ||||||
| Plant height (cm) | Mean | 112.8 | 105.2 | 107.1 | 104.3 | 105.1 | 103.2 | 97.9 | 104.6 | 104.3 | 104.9 |
| SD | 6.25 | 5.80 | 4.70 | 5.84 | 7.95 | 5.68 | 5.76 | 6.04 | 4.51 | 7.28 | |
| CV (%) | 5.54 | 5.52 | 4.39 | 5.60 | 7.57 | 5.50 | 5.89 | 5.77 | 4.32 | 6.94 | |
| P | 0.001 | 0.192 | 0.349 | 0.004 | 0.834 | ||||||
| Paniclelength (cm) | Mean | 23.6 | 23.3 | 22.2 | 21.7 | 21.1 | 21.6 | 21.6 | 21.4 | 20.7 | 22.0 |
| SD | 1.08 | 1.57 | 1.05 | 1.17 | 2.06 | 2.23 | 0.98 | 1.43 | 1.13 | 1.44 | |
| CV (%) | 4.59 | 6.75 | 4.76 | 5.38 | 9.75 | 10.32 | 4.55 | 6.68 | 5.47 | 6.57 | |
| P | 0.543 | 0.352 | 0.479 | 0.642 | 0.060 | ||||||
| Leaf length (cm) | Mean | 43.1 | 41.1 | 43.6 | 41.5 | 46.1 | 46.3 | 38.5 | 45.6 | 48.2 | 43.6 |
| SD | 1.77 | 3.01 | 2.04 | 2.82 | 4.05 | 4.03 | 4.21 | 3.45 | 3.34 | 1.84 | |
| CV (%) | 4.10 | 7.32 | 4.68 | 6.79 | 8.77 | 8.70 | 10.93 | 7.55 | 6.94 | 4.22 | |
| P | 0.043 | 0.041 | 0.892 | 0.000 | 0.002 | ||||||
| Leaf width | Mean | 1.49 | 1.40 | 1.08 | 1.05 | 1.51 | 1.62 | 1.04 | 1.01 | 1.05 | 0.98 |
| SD | 0.13 | 0.07 | 0.15 | 0.14 | 0.23 | 0.18 | 0.18 | 0.09 | 0.09 | 0.09 | |
| CV (%) | 8.92 | 4.72 | 13.99 | 13.76 | 15.08 | 10.85 | 17.11 | 9.22 | 8.26 | 9.13 | |
| P | 0.030 | 0.633 | 0.087 | 0.672 | 0.104 | ||||||
| # tillers/plant | Mean | 4.7 | 5.0 | 4.7 | 4.3 | 3.2 | 3.1 | 4.2 | 4.7 | 4.9 | 4.6 |
| SD | 0.66 | 0.97 | 0.63 | 0.66 | 0.61 | 0.56 | 1.02 | 0.63 | 0.47 | 0.74 | |
| CV (%) | 13.85 | 19.51 | 13.62 | 15.31 | 19.32 | 18.05 | 24.44 | 13.45 | 9.73 | 16.14 | |
| P | 0.367 | 0.240 | 0.874 | 0.101 | 0.391 | ||||||
| # panicles/plant | Mean | 4.3 | 4.4 | 4.1 | 3.8 | 2.7 | 2.7 | 3.6 | 4.2 | 4.3 | 4.1 |
| SD | 0.43 | 1.00 | 0.57 | 0.54 | 0.48 | 0.44 | 0.92 | 0.72 | 0.57 | 0.84 | |
| CV (%) | 9.91 | 22.79 | 13.90 | 14.38 | 17.83 | 16.42 | 25.84 | 17.44 | 13.32 | 20.83 | |
| P | 0.808 | 0.128 | 0.819 | 0.049 | 0.529 | ||||||
| Average 200 seed weight (g) | Mean | 4.04 | 4.35 | 4.73 | 4.93 | 5.03 | 4.58 | 3.73 | 3.59 | 4.37 | 2.96 |
| SD | 0.20 | 0.54 | 0.49 | 0.21 | 0.67 | 0.61 | 0.61 | 0.79 | 0.30 | 0.39 | |
| CV (%) | 4.95 | 12.41 | 10.29 | 4.28 | 13.36 | 13.31 | 16.27 | 22.16 | 6.81 | 13.14 | |
| P | 0.064 | 0.196 | 0.024 | 0.580 | 0.000 | ||||||
| Seed length (mm) | Mean | 8.37 | 8.45 | 8.98 | 8.78 | 8.95 | 8.43 | 8.13 | 7.51 | 8.20 | 6.96 |
| SD | 0.32 | 0.25 | 0.54 | 0.66 | 0.72 | 0.84 | 0.51 | 0.82 | 0.25 | 0.68 | |
| CV (%) | 3.82 | 2.96 | 6.00 | 7.51 | 8.01 | 9.92 | 6.28 | 10.90 | 3.05 | 9.78 | |
| P | 0.469 | 0.425 | 0.035 | 0.015 | 0.000 | ||||||
| Seed width (mm) | Mean | 2.96 | 3.12 | 2.96 | 3.15 | 3.20 | 3.27 | 2.84 | 2.90 | 3.09 | 2.74 |
| SD | 0.08 | 0.14 | 0.15 | 0.28 | 0.31 | 0.32 | 0.40 | 0.21 | 0.04 | 0.16 | |
| CV (%) | 2.70 | 4.49 | 4.92 | 9.01 | 9.69 | 9.71 | 14.07 | 7.36 | 1.26 | 5.98 | |
| P | 0.002 | 0.062 | 0.469 | 0.632 | 0.000 | ||||||
| Average CV (%) | 5.9 | 8.5 | 7.5 | 8.0 | 10.7 | 9.9 | 12.0 | 9.7 | 5.8 | 9.1 | |
UpperSL = material from Sierra Leone; UpperGB = material from Guinea Bissau; UpperGc = material from Guinea; Cluster 4-1 = material belonging to the sub-cluster of Cluster 4 with erect and semi-droopy panicles; Cluster 4-2 = material belonging to the sub-cluster of Cluster 4 with droopy panicles; 4-1 Erect = material of Cluster 4-1 with erect panicle; 4-1 semi droopy = material belonging to Cluster 4-1 with semi-droopy panicle.
Comparison between botanical groups
Figures 3, 4, 5, 6, 7, and 8 represent the morphological diversity using different combinations of PC 1, 2, 3 and 4. The graphical representation of genotypes using PC 1 and 2 (53.6%) shows two clouds of genotypes (Figure 3), separating glaberrima distinctly from the other three groups. O. glaberrima has a rounded and short ligule, erect panicle, erect primary branches, generally displays little leaf blade pubescence and tends to have a rather light leaf blade colour. This separation agrees with separations achieved through the molecular analysis.
By contrast, the three other botanical groups are not as clearly separated as they are in the molecular analysis. The clusters japonica, and indica form two connected clouds distributed along PC 2. The japonicas produce fewer tillers and panicles, and wider leaves and seed compared to the indicas. The farmer hybrids overlap mostly with the indicas. The molecular analysis also suggested that farmer hybrids are more closely related to indicas than to japonicas (see Figure 2). Most of the farmer hybrids that are clearly separate from the indicas belong to Sub-cluster 4-1 (Sierra Leone and Guinea Bissau) and only a few to Sub-cluster 4-2 (Guinea Bissau).
The combination of PC 1 and 3 (Figure 4) shows a larger overlap between japonicas, indicas and the farmer hybrids along the third component while the glaberrima cluster is pulled apart along the third component. The genotypes of glaberrima studied here are thus highly differentiated from each other on traits represented by PC 3 (plant height, culm length, panicle length and leaf length). The genotypes from Togo and Ghana tend to sit toward the lower part of the cloud and those from Guinea and Sierra Leone sit in the upper part.
When combining PC 2 and 3 (31.3%) all botanical groups form a single cloud (Figure 5). Whereas PC 1 is based on traits that separate glaberrima from the other botanical groups, PC 2 and 3 are based on a majority of the agronomic traits included in this study. The glaberrimas, indicas and most of the farmer hybrids, except for most of those from Senegal, are situated towards the left of the scatter, while the japonicas are positioned towards the right. Also, Figure 5 shows that glaberrima varieties differ more in height-related traits and panicle length than in number of panicles and tillers and leaf and seed width.
Through combination of PC 1 and 4 (Figure 6) the glaberrima group is bunched into a concentrated cluster showing a large degree of uniformity in related traits. The indica and japonica genotypes, however, show an equally large range for seed length. The farmer hybrids (mostly the erect and semi-droopy types) are situated at the lower part of the shared cloud with indicas and japonicas, showing relatively short grain length.
Comparison within botanical groups
When combining PC 1 and 3 (Figure 4) the O. glaberrima varieties from the Upper Guinea Coast are found in the upper part of the cloud, with those from Guinea right at the top, and those from the Lower Guinea Coast further down. The glaberrimas from the Upper Guinea Coast are taller and have longer culms and panicles but have similar leaf length and ligule length, when compared to the glaberrimas from the Lower Guinea Coast (Table 3). Among the Upper Guinea Coast glaberrimas, the varieties from Guinea seem to constitute a special group, being taller, with longer culms, panicles and leaves (Table 4). This was also observed in several trials conducted in five countries by Mokuwa et al. [29]. That some glaberrima varieties from Senegal and Guinea Bissau sit with those from Ghana and Togo when combining PC1 and 2 (Figure 3) might imply a process of adaptation to agro-ecological conditions, such as amount of rainfall, since this is comparable in the two regions.
Table 3 shows that glaberrimas from the Lower Guinea Coast have longer and heavier seeds than those from the Upper Guinea Coast. The differences in seed and plant height-related traits might be ascribed to a process of adaptation to specific ecological and/or socio-cultural factors. Farmers on the Danyi Plateau in the Togo Hills stated that glaberrimas used to thrive well on relatively poor and acid soils, in which the availability of vital nutrients is restricted. The cultivation of rice under these acid conditions might have led to selection for shorter plants that produce heavier and longer grains. Roy et al. [34] showed that larger seeds germinate better and produce more vigorous seedlings than smaller seeds and are able to produce a deeper initial root system. Also farmers on the Danyi plateau indicated that larger seed is clearly preferred for culinary reasons (B. Teeken, unpublished data).
A few glaberrima varieties from Ghana, Sierra Leone, Guinea and Guinea Bissau separate (downwards) from the core glaberrima cluster (Figure 3). These varieties have more tillers and panicles but have narrower leaves and smaller seed width compared to the other glaberrimas. For these traits, these glaberrima varieties resemble the indica group.
Unlike the case for O. glaberrima, no separate clustering can be observed for O. sativa ssp. indica from the Upper and Lower Guinea Coast (Figures 3, 4, 5, 6, 7, and 8), nor are significant differences observed for the agronomic traits (Table 3). At molecular level, some indicas from Sierra Leone and the Maritime region of Guinea tend to cluster together. Likewise, the materials from Senegal and the Togo hills cluster. However, at the morphological level a different tendency can be observed. Within the indica group (Figure 3) those from Guinea are situated towards the right, and those from Senegal are situated in the upper part, of a cloud. The indicas from Togo, Ghana, Sierra Leone and Guinea Bissau sit together in the centre of the cloud. The indicas show similarity with the farmer hybrids, particularly the semi-droopy hybrids from Sierra Leone and Guinea, and the droopy hybrids from Guinea Bissau.
Within Figures 3, 4, and 5, the indicas from Guinea closely bunch together whereas those from Ghana and other countries are very scattered. One explanation is that the materials collected in Guinea represent a small range of indica varieties, whereas a wide range of indica varieties was collected from rather diverse ecologies (ranging from hydromorphic soils to pure upland ecologies) in Ghana. Only when combining PC 1 and 4 (Figure 6) and PC 2 and 4 (Figure 7) is the Guinea material pulled apart, reflecting diversity on seed width and length, but not on other traits. The Guinea materials do not differ from the other indica varieties from the upper Guinea Coast on agronomic traits, except slightly for leaf length (Table 4).
Our findings at morphological level suggest that farmers in Ghana, Guinea Bissau, Sierra Leone and Senegal tend to select indica varieties with a range of morphological features while farmers in Guinea have been selecting narrowly, favouring a particular group of indicas. In the rather difficult upland conditions of the Guinea case-study areas (adjacent to the Bena hills) only a limited range of indica varieties has proven to be locally well adapted.
As is the case with O. glaberrima, O. sativa ssp. japonica from the Togo hills tends to have heavier seeds than the japonica from the Upper Guinea Coast region, but unlike glaberrima the japonica from the Togo hills are taller plants (Table 3). Considering PC 1 and 2 (Figure 3) the genotypes from the Upper Guinea Coast (mostly from Sierra Leone and Guinea Bissau) are found throughout the whole of the japonica cluster, while genotypes from the Lower Guinea Coast (materials from Ghana and Togo) are only found in the lower part of the cluster. Japonica varieties situated in the upper part of the cluster have broader leaves, fewer panicles and tillers, broader seeds and darker leaves. Materials from Sierra Leone and Guinea Bissau showed equal (high) levels of variation for these traits (see also Table 4). The japonicas from Sierra Leone were only collected from the south of the country meaning that farmers in a specific area deal with a highly diverse set of japonicas. At molecular level the japonicas from Sierra Leone tend to cluster separately from those from the other countries. Such separation does not show clearly in Figures 3, 4, 5, 6, 7, and 8.
Mokuwa et al. [10] found that a group of japonicas from Sierra Leone were more niche adapted, whereas a group of japonicas from Guinea Bissau showed wide adaptation. Both the Sierra Leone materials and most of the materials from Guinea Bissau used in the experiments by Mokuwa et al. [10] are among the genotypes sitting in the upper part of the japonica cluster (PC 1 and 2; Figure 3). At molecular level one Sierra Leone variety (Nduluwai) clusters with the Guinea Bissau varieties. In Figures 9 and 10 these varieties are found in different sub-groups, clustering in idiosyncratic ways. What this suggests is that farmers in both regions have selected morphologically similar materials responsive to different agro-ecological conditions. This might reflect histories of adaptation and introduction for these japonicas [29]. Evidence supporting a different process of introduction and adaptation is the similarity of the varieties Aqua Blue (‘blue water’) from Ghana and Sefa Fingo (meaning ‘black type’ in Mandinka) from Guinea Bissau at molecular and morphological levels, perhaps indicating common origins via Portuguese trading networks. It is thought that Portuguese traders brought japonicas from Indonesia to Guinea Bissau from where they spread to other West African countries [35]. To emphasise the distinctiveness of this case, both varieties have a distinct colouration during flowering and maturation not observed in other varieties.
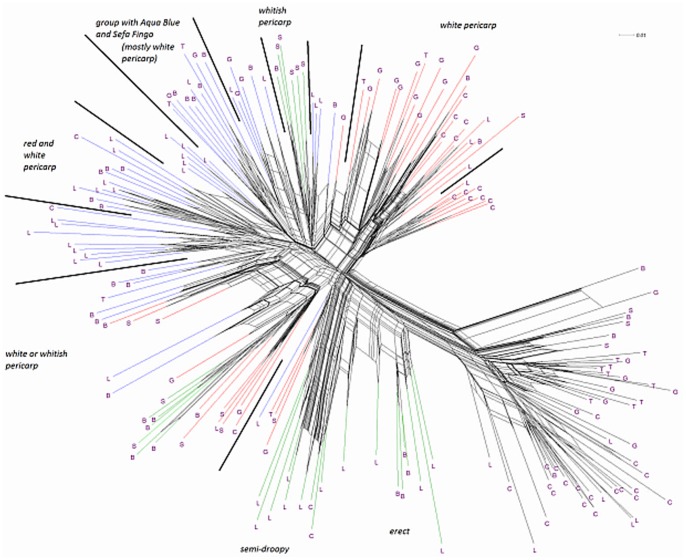
Figure 9. Phylogenetic relationships of 182 rice genotypes based on all morphological traits converted into dummy variables.
Botanical groups are indicated by colours: Black = O. glaberrima, red = O. sativa ssp. indica, blue = O. sativa ssp. japonica and green = interspecific farmer varieties. Country of collection is indicated by letters: B = Guinea Bissau, C = Guinea Conakry, G = Ghana, L = Sierra Leone, S = Senegal, T = Togo.
Figure 10. Phylogenetic relationships of 182 rice genotypes based on the agronomic traits converted into dummy variables.
Botanical groups are indicated by colours: Black = O. glaberrima, red = O. sativa ssp. indica, blue = O. sativa ssp. japonica and green = interspecific farmer varieties. Country of collection is indicated by letters: B = Guinea Bissau, C = Guinea Conakry, G = Ghana, L = Sierra Leone, S = Senegal, T = Togo.
Farmers from the Ghana side of the Togo hills have been selecting japonicas with relatively narrow leaves, high tillering and panicle production, slender and long grains similar to some indicas. (Figures 5 and 6). The long grain size could be explained by the large demand for long grained rice in the market.
In Figures 3, 4, 5, 6, and 7, farmer hybrids (Cluster 4) in general formed a large cloud suggesting they are diverse, confirming the molecular findings. Based on the panicle architecture (PAMA) most widely used to distinguish O. glaberrima from O. sativa varieties, the farmer hybrids were assigned to three sub groups: erect panicles, semi-droopy panicles and droopy panicles. In Figures 3, 4, 5, 6, and 7 the farmer hybrids with erect panicles did not clearly separate from the farmer hybrids with semi-droopy and droopy panicles, although they did in Figures 9 and 10. Table 4 shows statistically significant differences in seed weight, length and width between farmer hybrids with erect and semi-droopy panicles. For these two groups no clear difference was observed in the clustering based on molecular data.
Figure 4 (PC 1 and 3) shows that the farmer hybrids with erect panicles from Guinea Bissau cluster closely together, whereas those from Sierra Leone are more scattered. This agrees with the molecular analysis. Particularly, erect farmer hybrids from Northern Guinea Bissau sit together. These varieties were considered weeds by Mandinka farmers from northern Guinea Bissau; they referred to these interspecific varieties by names they also used for glaberrima. The one from Southern Guinea Bissau was brought from Guinea and sits somewhat separated. The scattering of the farmer hybrids with erect and semi-droopy panicles from Sierra Leone in Figure 4 points to active selection by farmers.
The erect farmer hybrids of Guinea Bissau and Sierra Leone are known to be four months in duration from germination to ripening. The semi-droopy farmer hybrids from Sierra Leone and Guinea are three months in duration. These semi-droopy farmer hybrids can be further divided into those with small and slender grains and those with short and bold grains. The latter are visible in Figure 6 down among the semi-droopy farmer hybrids from Sub-cluster 4-1. Farmers have been selecting ‘three month’ varieties as hunger breakers because they ripen about one and half or two month(s) before the major rice harvesting time. In this respect the three-month group of farmer hybrids has been replacing some of the short cycle glaberrima traditionally used as hunger breakers. Compared to the erect farmer hybrids these ‘three months’ interspecific farmer varieties vary more for husk colour and seed size (see also Table 4).
Compared to the limited diversity represented by the erect farmer hybrids from Guinea Bissau, the larger diversity in droopy farmer hybrids from Guinea Bissau and Senegal (Sub-cluster 4-2 in Table 4) agrees with interview data that farmers actively select for droopy farmer hybrids in these regions. The farmer hybrids with droopy panicles split into two groups largely reflecting their area of collection. Figure 3 (PC 1 and 2) shows that only farmer hybrids with droopy panicles from Senegal are found in the area where indica and japonica overlap. Interviews with farmers indicated that the farmer hybrids in Senegal have their origin in Guinea Bissau. The droopy varieties spread to Senegal particularly during the independence war in Guinea Bissau from 1963 to 1974. However, the farmer hybrids with droopy panicles collected in Guinea Bissau are situated in the lower part of the cloud of farmer hybrids. This suggests that over a period of approximately 40–50 years a selection process has taken place, and that farmers in the case study areas in Senegal and Guinea Bissau prefer farmer hybrids with area-specific morphological characteristics. Overall, morphological characterisation of farmer hybrids underlines a conclusion that Cluster 4-2 is highly variable and shares characteristics with japonica and indica. At the molecular level, however, the farmer hybrids are all closer to indica than to japonica.
Geographical and Climatic Clustering
Figure 9 shows an unrooted tree based on 20 morphological traits, and Figure 10 based on agronomic traits only (see Table 5). The clustering in Figure 9 is largely according to botanical groups, with glaberrima and japonica making well-defined clusters and indica and the farmer hybrids consisting of several clusters. The glaberrima clearly forms three sub-clusters for Guinea and Sierra Leone, Ghana and Togo, and north Guinea Bissau and Senegal. For the other botanical groups some country based clustering patterns can also be observed, although less clearly. Some clusters contain material from several botanical groups. In the case of japonica, two clusters hold mostly material from Sierra Leone, and another cluster groups material from various countries. In the case of indica from the Upper Guinea Coast some clustering based on seed colour can be observed, but not for the Lower Guinea Coast. Some white seeded indicas cluster with light-coloured droopy farmer hybrids from Guinea Bissau, and some red seeded indicas cluster with red-coloured semi-droopy farmer hybrids from Sierra Leone and Guinea.
Table 5. The 20 traits measured on the rice genotypes in a field trial in Sierra Leone in 2008. Ratings were based on five at randomly chosen plants per plot.
| Characteristic | Description and scale or unit | Type of determination | Stage of measurement |
| Agronomic traits | |||
| Culm length | Average length, from ground level to the base of thepanicle, in cm | Numerical | Physiological maturity |
| Plant height | Average height, from soil surface up to the tip of the tallestpanicle, in cm | Numerical | Physiological maturity |
| Leaf length | Average length of peninsulate leaf (leaf below flag leaf),from collar to tip of leaf, in cm | Numerical | Physiological maturity |
| Leaf width | Average width of peninsulate leaf (leaf below flag leaf),widest portion of the leaf, in cm | Numerical | Physiological maturity |
| Panicle length | Average length, of main panicle, from panicle baseto tip, in cm, | Numerical | Physiological maturity |
| Panicle number | Average number of panicles per plant | Numerical | Physiological maturity |
| Number of tillers | Average number of tiller(s) per plant | Numerical | Physiological maturity |
| Rattoon potential | Assessed after harvests: 0 = None; 1 = Low; 3 = Medium;5 = Vigorous; 7 = Very vigorous | Scale. | After harvest |
| Grain length | Average length of grain length, from base of lowermoststerile lemma to tip of fertile lemma or palea, in mm. | Numerical | Post-harvest |
| Grain width | Average width, measured at the widest portion, in mm. | Numerical | Post-harvest |
| 100-grain weight | Average weight of 100 filled seeds at 13% moisture content. | Numerical | Post-harvest |
| Botanical traits | |||
| Leaf blade colour | 0 = No green visible due to anthocyanin; 3 = Light green;5 = Medium green; 7 = Dark green | Visual assessment | Physiological maturity |
| Leaf blade pubescence | 1 = Glabrous (smooth); 2 = Intermediate; 3 = Pubescent | Ocular inspection and thenfingertip rub to class hairiness | Physiological maturity |
| Ligule length | Average length, on peninsulate leaf of main stem, from thebase of the collar to the tip, in mm | Numerical | Physiological maturity |
| Ligule shape | 0 = Absent; 1 = Truncate; 2 = Acute to acuminate; 3 = 2-cleft | Visual assessment | Physiological maturity |
| Panicle attitude of mainaxis (PAMA) | 1 = Upright; 2 = Semi-upright; 3 = Slightly drooping;4 = Strongly drooping | Visual assessment of the mainaxis of the panicle | Physiological maturity |
| Panicle attitude ofprimary branches (PAB) | 1 = Erect (compact panicle); 3 = Semi erect, semi-compactpanicle; 5 = Spreading (open panicle); 7 = Horizontal; 9 = Drooping | Visual assessment | Physiological maturity |
| Awn length | 0 = None (awn less); 1 = Very short (<5 mm);3 = Short (∼8 mm); 5 = Intermediate (∼15 mm);7 = Long (∼30 mm); 9 = Very long (>40 mm) | The awn was measured frombase to the tip, then translatedin scales | Post-harvest |
| Husk (lemma and palea) colour | 1 = White; 2 = Straw; 3 = Gold and gold furrows; 4 = Brown(tawny); 5 = Brown spots; 6 = Brown furrows; 7 = Purple;8 = Reddish to light purple; 9 = Purple spots; 10 = Purplefurrows; 11 = Black | Visual assessment | Post-harvest |
| Seed coat colour /pericarp colour | 1 = White; 2 = Light brown; 3 = Speckled brown; 4 = Brown;5 = Red; 6 = Variable purple; 7 = Purple | Visual assessment | Post-harvest |
The clustering in Figure 10 is complex, with material from the four botanical groups clustering in various ways. To some extent the patterns may reflect agro-ecological selection pressures. This is perhaps particularly true for the glaberrimas, where grouping reflects geographical factors. The glaberrimas from Senegal and northern Guinea Bissau, for instance, cluster more closely with the glaberrimas from Togo and Ghana, with both areas having similar amounts of rainfall. For the other botanical groups no such clear separation is apparent. Another apparent indicator of agro-ecological selection pressures is the extent to which material from various botanical groups from one country, or two neighbouring countries, clusters together. For example, the erect farmer hybrids, most coming from Sierra Leone, and the indicas from Guinea cluster closely with the glaberrimas from Guinea and Sierra Leone. However, clusters can also be found grouping material from all countries, as applies to subsets of indicas and japonicas. Also the droopy farmer hybrids from Senegal form several small independent clusters.
Pericarp Colour as a Selection Factor
Seed colour (pericarp) is an important characteristic often mentioned by farmers [9].
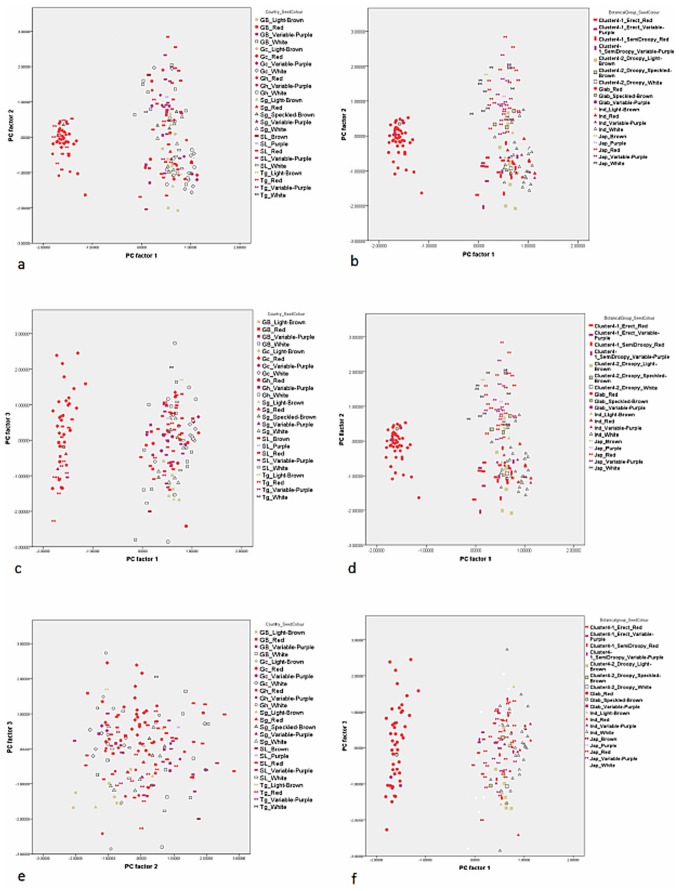
Depending on the farming system and social context, pericarp colour is a nutritional, gender, religious or cultural marker, and plays a role in the selection and acceptance of rice varieties [9]. Seed colour was not incorporated in the PCA because it could not be converted into a linear scale. Instead we labelled the materials of this study according to seed colour. Figure 11 shows the combination of factor PC 1 and 2, 1 and 3 and 2 and 3 marked according to seed colour, country of collection and botanical group. Only a few relationships between pericarp colour, molecular, and morphological data were found. The clearest relationship was among the farmer hybrids, where varieties with erect and semi-droopy panicles have a red pericarp, and those with a droopy panicle have a white or light brown pericarp. The varieties Pugulu ‘white’ and ‘red’ from Ghana were found in neighbouring clusters in Figure 9 (B. Teeken, unpublished). This is a case of farmers using the same name with the addition ‘white’ or ‘red’ for varieties that are genetically different.
Figure 11. Graphically repartition of materials based on morphological data of PC 1&2, 1&3 and 2&3 marked according to country of collection and seed colour* (11a, 11c and 11e) as well as botanical group and seed colour (11b, 11d and 11f).
Country of collection is indicated by letters: Gc = Guinea Conakry, GB = Guinea Bissau, Gh = Ghana, Sg = Senegal, SL = Sierra Leone, Tg = Togo. *According to the seed colour chart of the Bioversity rice descriptor version 2007 [49].
Farmers from whom we collected material have no fixed ideas about the ‘correct’ morphological traits of rice varieties [36]. Rather than focusing on a particular ideotype they sustain what might be termed a broad flexset (combining a range of ideotypes depending on the conditions). Seemingly, this is a way to optimize benefits of cognitive flexibility, to be understood in relation to a long history of in situ domestication. Gross et al. [37] indicate that pericarp colour might be a phenomenon rather independent of trajectory of domestication. In Ghana preference for white and red varieties was modulated by other traits, such as robustness, yield and intended usage. In the Ghanaian Togo hills, as well as in Sierra Leone, rice with a red pericarp is considered ‘heavier’ in the stomach (i.e. it digests more slowly than white rice, a valuable characteristic where sustained hard work has to be attempted). To make a meal last longer, white rice is sometimes mixed with some red rice before eating and in some cases (e.g. in Sierra Leone) it is sometimes mixed before sowing to allow easy milling. In Mandinka-dominated areas of Upper West Africa, red rice is regarded as “outmoded” and white rice is now preferred. There is also high demand for white rice in urban areas where people, because of their different labour pattern, tend to prefer rice that is more easily prepared.
Development of Genetic Diversity
Whereas the molecular data suggested that the indica group and farmer hybrids had greater genetic diversity than the japonica and glaberrima groups (see Table 6), Figures 3, 4, 5, 6, 7, and 8 suggest the differences in genetic diversity between the groups might be smaller than represented by the molecular analysis. Particularly for japonica, Figures 4, 5, 6, 7, and 8 and Figure 11 show a large dispersion, similar to indica, for all components. For glaberrima only Figures 4, 5, and 8 show a large dispersion. Calculations of genetic diversity based on morphological traits (Table 6) confirm that glaberrima have less diversity, but that japonica has a higher level of diversity (see also Tables 3 and 4).
Table 6. Level of genetic diversity of four botanical groups of rice in West Africa, calculated with Nei’s index (He) and the fraction of polymorphic markers (P-value), based on molecular markers and morphological traits converted into dummy variables.
| Botanicalgroup | N | Molecular markers | Morphological traits | ||
| He | P-value | He | P-value | ||
| O. glaberrima | 49 | 0.042 | 0.430 | 0.189 | 0.762 |
| Lower Guinea Coast | 17 | 0.027 | 0.181 | 0.131 | 0.438 |
| Upper Guinea Coast | 32 | 0.047 | 0.362 | 0.185 | 0.724 |
| Guinea | 19 | 0.052 | 0.305 | 0.154 | 0.610 |
| Other | 13 | 0.035 | 0.152 | 0.190 | 0.552 |
| O. sativa ssp. indica | 46 | 0.099 | 0.653 | 0.218 | 0.800 |
| Lower Guinea Coast | 17 | 0.102 | 0.410 | 0.195 | 0.619 |
| Upper Guinea Coast | 29 | 0.070 | 0.429 | 0.208 | 0.733 |
| Guinea | 13 | 0.055 | 0.276 | 0.173 | 0.581 |
| Other | 16 | 0.072 | 0.333 | 0.208 | 0.590 |
| O. sativa ssp. japonica | 56 | 0.054 | 0.481 | 0.238 | 0.819 |
| Lower Guinea Coast | 8 | 0.033 | 0.143 | 0.173 | 0.476 |
| Upper Guinea Coast | 48 | 0.053 | 0.400 | 0.240 | 0.810 |
| Guinea Bissau | 18 | 0.035 | 0.200 | 0.236 | 0.676 |
| Sierra Leone | 28 | 0.056 | 0.371 | 0.225 | 0.705 |
| Cluster 4 | 31 | 0.102 | 0.444 | 0.257 | 0.752 |
| Cluster 4-1 | 18 | 0.060 | 0.274 | 0.223 | 0.629 |
| Cluster 4-1erect | 8 | 0.038 | 0.143 | 0.149 | 0.400 |
| Cluster 4-1semi-droopy | 10 | 0.065 | 0.219 | 0.169 | 0.495 |
| Cluster 4-2 | 13 | 0.078 | 0.281 | 0.204 | 0.581 |
There seems to exist a relationship between the level of farmer selection and seed exchange and the level of diversity in botanical groups. Farmer accounts of the introduction or in situ development of new varieties related mainly to farmer hybrids, indica and to a lesser extent japonica. No such account related to glaberrima. In recent years farmers in Ghana have developed an idea that the morphology of glaberrima is fixed, and Mandinka people in Senegal and northern Guinea Bissau consider glaberrima to be a rice belonging to history. Only in a few areas (e.g. southern Guinea Bissau) are farmers actively re-introducing varieties of glaberrima [9]. This suggests there is little current active farmer variety development for glaberrima. By contrast, accounts concerning introductions and further development of farmer hybrids and/or indica are especially numerous in all countries where the research took place ([6], [9], [36]; A. Mokuwa, unpublished data).
Country-specific Findings
Sierra Leone
In Sierra Leone japonica varieties are extensively cultivated only in the southern half of the country while almost all upland varieties in the north are mainly farmer hybrids and indica, with a few glaberrima. This suggests that diversity of climate and agro-ecological conditions (upland and hydromorphic ecologies) is the main driver for selection of botanical groups [38]. The japonica in southern Sierra Leone and the farmer hybrids in northern Sierra Leone show considerable variation, suggesting that active cultivation plays a role in maintaining and developing genetic diversity. Close to the border with Guinea more extensive cultivation of glaberrima occurs with varieties that resemble varieties from Guinea, an area where glaberrima is still widely cultivated [7], [9]. An ethnic factor plays a part - the glaberrima were collected mainly among the Susu people who live on both sides of the border, linked by strong family ties and seed networking relationships.
Guinea
It is important to mention that in the Guinea case study area almost no japonica varieties are cultivated. In these conditions (soils of low pH) farmers mainly cultivate glaberrima and indica varieties. Among rice scientists it is thought that West African upland varieties are generally japonica rather than indica [35], [39], [40]. As a result, and in contrast to japonica, indica cultivars have yet to be fully evaluated regarding their adaptation to upland conditions in West Africa ([41]; [42], cited in [43]).
The indica varieties collected in Guinea showed less diversity compared to varieties collected from the other study countries. This limited diversity partly relates to a fieldwork circumstance - varieties were collected from an ethnically homogenous group (Susu) growing essentially the same set of varieties along a 120 km transect from the Sierra-Leone borders (Bassia) to Kindia. These Guinean indicas are morphologically strongly differentiated from both glaberrima as well as from japonica. This is despite the fact that in Guinea indica and glaberrima are cultivated in the same upland conditions. For Susu farmers, selecting morphologically distinct genotypes helps avoid variety mixtures in the field. This part of Guinea (the Benna region in particular) was historically involved in an international rice trade to Freetown when local slave-manned plantations replaced the Atlantic slave trade. The Freetown rice trade demanded white rice [29]. Keeping field homogeneity (a relic of this long-dormant trade) is a cultural and managerial value lingering for nearly two centuries in some of these Susu farming communities [7]. That indicas with white and red seed colour cluster differently helps to confirm the significance of these socio-economic and cultural selection preferences in influencing genetic make-up of rice.
That the glaberrima from Guinea also show much diversity, points to active selection of African rice in this region. Mouser et al. [29] suggest that this may be linked to the food security needs of newly founded maroon communities of self-emancipated slaves fleeing Susu rice plantations.
Senegal
Senegalese indica (and hybrid) varieties resemble japonica in having fewer tillers and panicles, broader leaves and seeds than the indica from the other countries in the study. The land farmers work in Senegal mostly comprises hydromorphic soils, but also some uplands. The low tiller number is probably related to the relative earliness of local varieties. All farmer hybrids collected in Senegal had a light coloured pericarp as farmers strongly selected against red pericarp. A few off-types (representing old varieties) rogued from collected samples clustered with red seeded varieties from Guinea and Sierra Leone. This can be taken as an indicator that localized farmer preferences changing over time can influence the genetic make-up represented by the varieties cultivated.
Guinea Bissau
The collected farmer hybrids cluster with indicas and have low similarity to japonica from Guinea Bissau. Japonicas were cultivated under upland conditions and the farmer hybrids tended to be more frequently cultivated in hydromorphic zones. However, respondents said that in the past, when more labour was available for bird scaring, farmer hybrids were also cultivated in the uplands. The droopy farmer hybrids are genetically different from the erect farmer hybrids. One reason is seed colour. Mandinka farmers are unlikely to select an off-type with an erect panicle to develop it into a variety since they associate erectness of the panicle with (undesirable) red pericarp. The glaberrima from northern Guinea Bissau clustered with those from Senegal while those from southern Guinea Bissau clustered with those from Guinea and Sierra Leone. Climatic conditions are clearly different between the case study villages in the north and south of the country, but account should also be taken of the fact that historical relationships differ. The north is oriented towards Senegal (Casamance) and the south is oriented towards Guinea.
Togo Hills (Ghana and Togo)
The relatively large diversity within the indica, japonica and glaberrima groups in the Togo Hills can be partly ascribed to the many different ecological niches found in a forested landscape that ranges from lowland to mountain where farmers take considerable advantage of intra-mountain basins for rice cultivation. These mountain basins offered the double advantage of more fertile soils and security. Rice diversity can thus be related to a history of refuge, displacement and enclaved social life in a region characterized by war and political instability. Seeking security, farmers strove to intensify farming on stony, acid and often sloping soils by emphasizing O. glaberrima, the only rice available at that time. More recent factors include the developments in farming over the past 50 years. Until the 1960s, the main rice producing ecology was upland, where mainly glaberrima varieties were cultivated. On the Ghana side of the range, farmers started to cultivate indica varieties in lowland areas from the 1960s onwards, while in the Togolese Togo Hills (mainly the Danyi Plateau) farmers continued - to this day- to cultivate solely glaberrima varieties, as no lowland varieties were available to them. Lowland rice farming in the foothills of the Ghanaian Togo Hills has meanwhile become a major activity. It has also resulted in farmers introducing some indica varieties to hydromorphic and upland conditions. The cultivation of glaberrima is still maintained today, especially for its role in customary rites, and as a significant part upland cultivation clearly continues to set criteria for the selection and development of indica rice varieties [9]. This also helps explain why no farmer hybrids are found in the Togo hills. Here O. glaberrima and O. sativa are separated in the landscape by altitude, not grown side by side, as they have been for centuries, in Upper West Africa. It should also be noted that local customary rites demand use of pure glaberrima for feeding and sacrifice. This acts as a disincentive to any farmer inclined to select off-types intermediate between O. glaberrima and O. sativa.
Conclusions and Implications
Main Conclusions
This paper has combined morphological and molecular data with socio-economic and cultural information to provide a better understanding of how cultivation practices combine with environmental pressures to shape rice diversity in six case study areas in coastal West Africa. Examples have been provided of how, per botanical group and case study area, these integrated data offer novel insights into the potential of neglected crop resources. The paper points both to the complexity of farmer rice genetic diversity management and to the significance of farmer innovation.
For O. glaberrima the molecular and morphological data largely agree with each other (see Figure 1). The morphological data showed clear differences in morphological features between glaberrima varieties from the Togo hills and the Upper Guinea Coast region, and between Guinea and the other countries of the Upper Guinea Coast. A relationship between genetic diversity and agro-ecology emerges. Farmers did not exchange glaberrima varieties over large distances, and we did not receive information about the development of new glaberrima varieties (the Guinea case excluded). What seems now to be true is that ethnic groups either stress the true-to-type maintenance of specific varieties or have abandoned the cultivation of glaberrima altogether.
For the other botanical groups a different picture emerges. Molecular and morphological data do not always agree. Particularly for the japonica group more diversity was observed at the morphological than at the molecular level. This could be caused by the possibility that the molecular markers used were more informative on other botanical groupings than on the japonicas. Taken in conjunction with the findings on differences in adaptation within japonica reported by Mokuwa et al. [10] the question arises about whether the japonica harbour more genetic diversity than observed. At the morphological level the three non-glaberrima botanical groups did not group geographically (by country, or groups of neighbouring countries). Particularly for indicas and the farmer hybrids, much evidence of recently introduced or newly developed varieties was recorded. Particularly with the farmer hybrids, seed colour has a clear relationship with the genetic make-up of rice varieties. Such a relationship is non-existent (or less clear) for the japonica group.
Apart from glaberrima, farmers seem ready to cultivate any variety of the other three botanical groups that meets a certain minimum set of criteria, such as plant height, time of ripening, seed colour and digestibility. Even these criteria are used flexibly, depending on the other advantages a variety may possess. For example, in general farmers prefer tall varieties. In Ghana this is because a long stem is considered easier for threshing. In southern Sierra Leone farmers mostly harvest by panicle and seek to avoid too much stooping and an aching back. Short plants, however, may not always be selected against if they have compensating advantages such as earliness [36].
For glaberrima, socio-cultural selection pressures seem to reduce diversity, particularly at a more local scale, while for the other botanical groups they seem to have an enhancing effect on genetic diversity. However, glaberrima still plays an important role in determining the selection criteria of farmers and shaping variety development pathways. For instance, farmers in northern Sierra Leone select farmer hybrids with erect panicles. This implies these farmer hybrids are selected according to standards established for glaberrima cultivation. Most japonicas in southern Sierra Leone have a red pericarp. This results from historically-specific socio-cultural selection pressures [29]. The farmer hybrids from Senegal and Guinea Bissau show much overlap with, respectively, the japonicas and the indicas in the PCA analysis. This is apparently related in part to shared agro-ecological conditions. The droopy panicle and light seed colour of farmer hybrids in this region also reflect a history of O. sativa cultivation. In sum, at a regional level, farmer hybrids combine (advantageous) traits from different botanical groups by embodying responses to different local cultural and ecological considerations.
Because farmers in West Africa embark on risk spreading practices - e.g. growing varieties mixed-in with other varieties and assigning sections of their fields to different varieties [44] – ‘in-situ’ experimentation and on-farm hybridization is facilitated. In Sierra Leone farmer hybrids are generally popular; they are said to perform well under low field management and when consumed enable farmers to sustain longer hours of work without hunger, and are thus similar to glaberrima. In Senegal, the farmer hybrids also perform well under low field management, but do not have a red pericarp and in that respect are regarded as being similar to the O. sativa varieties commonly planted. The farmer hybrids are a welcome enrichment of local planting resources since they are genetically rich and diverse and can be considered products of long trajectories of interaction between botanical groups, ecological, socio-cultural and economic factors.
Wider Societal Context and Implications
The present paper belongs to a group of three that report an interconnected set of findings: we first described the emergence of a new rice type of interspecific hybrid origin in West African farmers’ fields [23], then we analysed robustness and strategies of physiological adaptation within a large set of farmer varieties of African rice and Asian rice across West Africa [10] and third, this paper has compared morphological and molecular data with information on socio-economic seed selection factors, in order better to show how farmer practices and culture combine with environmental selection pressures to shape diversity in rice across coastal West Africa.
All three papers provide evidence that West African small-scale food-crop farmers conserve and develop valuable rice varieties, despite limitations of poverty, isolation, and formal education. A major implication of this result is that farmer practices and culture strengthen the conservation and development of genetic diversity. Modern varieties of many crops have little or no genetic disparity within cultivars. It has been estimated that as little as 20% [45] of the total diversity contained within the wild ancestors of rice, cassava, and soybeans is maintained through breeding of ‘modern elite’ varieties [46]–[48]. Our work shows, by contrast, that farmer innovation helps to protect this diversity and keep it ‘in play’ for future adaptation. Sustaining crop genetic diversity in situ is an especially important topic in an era of rapid climatic change. Our results, therefore, support calls for the protection and valorisation of farmer crop innovation processes, as a basis for addressing issues of rural food security in Africa.
Materials and Methods
Ethics Statement
We confirm that no specific permit was required for using the location where the field trial was conducted. The location was not protected in any way. The field study never involved endangered or protected species. Approval for the collection of socio-economic data using in-depth interviews and questionnaires was obtained from the Social Sciences Ethical Committee (SSEC) of Wageningen University. The research was carried out by researchers living in the country for at least several years and approved by village elders and farmer communities. Individual participants provided their verbal informed consent to participate in the interviews as part of the interview protocol. Written consent was not possible as most of the interviewees were illiterate. The SSEC approved this consent procedure. We thank the village elders, farmers and the land holding family at Fala Junction Kowa Chiefdom, Sierra Leone.
Variety Collection and Molecular Analysis
Variety collection was carried out from June to December 2007 in seven countries of Coastal West Africa: The Gambia, Ghana, Guinea, Guinea Bissau, Senegal, Sierra Leone and Togo (Figure 12). The purpose was to collect varieties of O. glaberrima and O. sativa cultivated by farmers in regions where O. glaberrima was known to be cultivated. In each country varieties were collected in a number of case study villages. In exceptional cases, varieties in other villages were collected if they had a clear relationship to the main case study villages, if there was an important ‘story’ related to them, or if they were morphologically intermediate between O. sativa and O. glaberrima. At harvest time a total of 231 accessions were collected. In February and March 2008 these accessions were analyzed molecularly using AFLP markers. In the research by Nuijten et al. [23] these data were then added to the 84 accessions analyzed by Nuijten and Van Treuren [30]. With the software package ‘Structure’ (version 2.2), materials with a probability equal to or higher than 91% were assigned to four clusters (glaberrima, indica, japonica and farmer hybrids (see Table 7). Materials assigned with a value lower than p = 0.91 were considered outliers. Farmer hybrids are farmer varieties of interspecific origin [23].
Figure 12. Case study areas are indicated by colours representing the most commonly cultivated botanical groups in those areas.
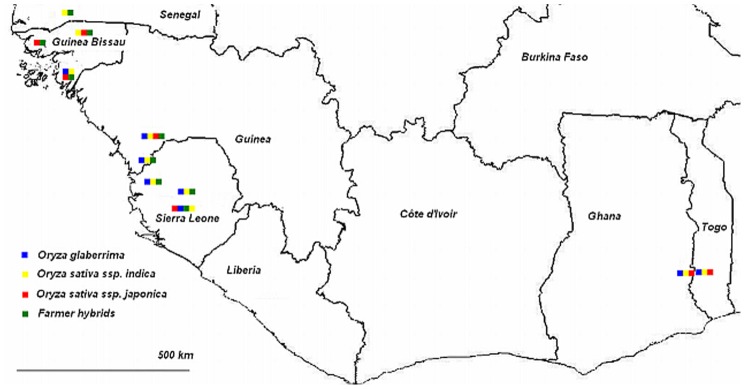
Table 7. Number of materials used in the molecular and morphological analysis according to their botanical group and their areas of collection.
| Botanical group | Senegal (Casamance) | Guinea Bissau | Guinea (Kindiaand Forecariah) | Sierra Leone (Central- N/West) | Ghana (Togo Hills,Volta region) | Togo (Togo Hills,Danyi plateau) | Total |
| O. glaberrima | 3 | 4 | 19 | 6 | 8 | 9 | 49 |
| O. sativa ssp. indica | 7 | 4 | 13 | 5 | 15 | 2 | 46 |
| O. sativa ssp. japonica | 0 | 18 | 2 | 28 | 5 | 3 | 56 |
| Farmer hybrids* | 7 | 10 | 2 | 12 | 0 | 0 | 31 |
| Total | 17 | 36 | 36 | 51 | 28 | 14 | 182 |
Interspecific farmer varieties with a combined background of O. glaberrima and O. sativa.
Choice and Types of Farmer Varieties
In this paper we consider only the materials that were assigned with a probability equal to or larger than 91% to the botanical groups O. glaberrima, O. sativa ssp. japonica, O. sativa ssp. indica and the farmer interspecific hybrids (Cluster 4). The focus of this study was on upland varieties. Apart from pure upland varieties also varieties from the upper part of the lowland-upland continuum were included. Typical lowland varieties were left out.
In addition, the number of materials collected from The Gambia in 2007 was too limited for a meaningful comparison and were left out. Because for some materials not enough seeds were available for the morphological analysis, we worked with a total of 182 varieties.
Trial set-up
Field evaluations were carried out in Sierra Leone from June to December 2008. The trial was set up under upland rain-fed conditions at Fala Junction, Kowa Chiefdom (8.14917 N, 11.90806 E, 58 m asl), in Moyamba District. The period of field evaluation corresponded to the cropping season. The average annual rainfall is between 2100–3000 mm and the rainy season lasts for 6 to 7 months. The selected site was flat. The soil was cleared and deeply plowed after 24 years of bush fallow. The soil was silt loam (Mende: tumui).
The seeds of each accession were sown in a randomized block design. Each plot was 1.5 m × 2.1 m and contained 70 pockets, spaced 30 cm between rows and 15 cm within rows. Three grains were sown in each pocket and pockets were thinned to one plant within four weeks after sowing. Sowing date was determined by following the farmer practices in the region. Excellent germination and growth were observed with low to moderate pest (rodent, termites, cut worms, stem borers) incidences, mostly with O. sativa ssp. japonica varieties. Traditional fencing and mesh wire were used to prevent damage by rodents. No fertilizer was applied.
Measurements
A total of 20 traits were measured (Table 5). Most traits were measured in all four replications, except a few qualitative traits which were measured only on the first replication, as these traits were not influenced by microenvironment. Measurements were done on five plants chosen randomly in each plot excluding the border rows. The accessions were characterized according to the descriptor list by Bioversity International (2007) [49] with the exception of rattooning potential.
Socio-economic Data Collection
Besides the collection of farmer accessions, socio-economic data were collected on all 182 varieties using in-depth interviews and questionnaires which mainly covered (i) household data, (ii) number of varieties grown, (iii) ecology of cultivation, (iv) the area under cultivation, (v) farmer reasons for growing the variety, (vi) seed source, (vii) on-farm seed management practices from harvest to sowing and farmer knowledge related to variety use.
Data Analysis
Principal component analysis (PCA) was used to describe the morphological data measured through a reduced number of variables shown in biplot as vectors. The genetic implications can be assessed from the eigenvalues ascribed to the different traits [50]. The values of the principal components per genotype correspond to a combination of traits explaining the variability. The closer the distance between genotypes in the biplots with the different principal components the closer the genotypes are related with respect to the traits represented by the principal components. PCA was conducted using SPSS/ PASW Statistics 18.
The morphological data were also analysed with the software Splitstree [51]. The measured data were translated into dummy variables. For the data with ordinal scales: for each value a column was created. For the numerical data, the number of categories was determined based on the difference between the maximum and minimum value divided by the standard deviation. The width of a category was determined by dividing the range by the number of categories multiplied with the factor 1.5. These data and the molecular data were analysed with the software Splitstree using the same method followed by Nuijten et al. [23].
Acknowledgments
The authors would like to acknowledge the contributions of all those involved in the research, in particular the staff of the research stations and universities in the countries involved, the farmers in the different countries, the research assistants at the different trial sites, and Rob van Treuren of the Centre for Genetic Resources, the Netherlands for contributing to the molecular analysis.
Funding Statement
Funding was provided by NWO WOTRO (Science for Global Development, part of the Netherlands Organisation for Scientific Research), CSG (Centre for Society and Genomics), NUFFIC (Netherlands Organisation for International Cooperation in Higher Education) and AfricaRice Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Portères R (1950) Ancient agricultures of intertropical Africa. Centres of origin and of primary varietal diversification and cradles of agriculture previous to the 16th century. Agron Trop 5: 489–507. [Google Scholar]
- 2.Harlan JR, Stemler ABL (1976) Races of sorghum in Africa. In: Harlan JR, de Wet JMJ, Stemler ABL, editors. Origins of African plant domestication. The Hague: Mouton Press. 465–478.
- 3.Portères R (1976) African cereals: Eleusine, fonio, black fonio, teff, Brachiaria, paspalum, Pennisetum, and African rice. In: Harlan JR, De Wet JMJ, Stemler AB, editors. Origins of African plant domestication. The Hague: Mounton Press. 409–452.
- 4. Li ZM, Zheng XM, Ge S (2011) Genetic diversity and domestication history of African rice (Oryza glaberrima) as inferred from multiple gene sequences. Theor Appl Gen 123(1): 21–31. [DOI] [PubMed] [Google Scholar]
- 5. Linares OF (2002) African rice (Oryza glaberrima): History and future potential. Proc Natl Acad Sci USA 99: 16360–16365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nuijten E (2005) Farmer management of gene flow: The impact of gender and breeding system on genetic diversity and crop improvement in The Gambia. Wageningen: PhD-thesis, Wageningen University, The Netherlands. 271 p.
- 7.Okry F (2011) Strengthening rice seed systems and agro- biodiversity conservation in West Africa. A socio-technical focus on farmers’ practices of rice seed development and diversity conservation in Susu cross border lands of Guinea and Sierra Leone. Wageningen: PhD thesis, Wageningen University, The Netherlands. 208 p.
- 8. Okry F, Van Mele P, Nuijten E, Struik PC, Mongbo RL (2011) Organizational Analysis of the Seed Sector of Rice in Guinea: Stakeholders, Perception and Institutional Linkages. Exp Agric 47: 137–157. [Google Scholar]
- 9. Teeken B, Nuijten E, Temudo MP, Okry F, Mokuwa A, et al. (2012) Maintaining or abandoning African rice: lessons for understanding processes of seed innovation. Human Ecol 40: 879–892. [Google Scholar]
- 10. Mokuwa A, Nuijten E, Okry F, Teeken B, Maat H, et al. (2013) Robustness and strategies of adaptation among farmer varieties of African rice (Oryza glaberrima) and Asian rice (Oryza sativa) across West Africa. PLoS ONE 8(3): e34801 doi:10.1371/journal.pone.0034801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones M, Dingkuhn M, Aluko GK, Semon M (1997) Interspecific Oryza sativa L. × O. glaberrima Steud. progenies in upland rice improvement. Euphytica 94: 237–246. [Google Scholar]
- 12. Dingkuhn M, Jones MP, Johnson DE, Sow A (1998) Growth and yield potential of Oryza sativa and O. glaberrima upland rice cultivars and their interspecific progenies. Field Crops Res 57: 57–69. [Google Scholar]
- 13. Chevalier A (1932) Nouvelle contribution à l’étude systématique des Oryza . Rev Bot Appl 136: 1014–1032. [Google Scholar]
- 14. Yabuno T (1977) Genetic studies on the interspecific cytoplasm substitution lines of japonica varieties of Oryza sativa L. and O. glaberrima Stued. Euphytica 26: 451–463. [Google Scholar]
- 15.Second G (1985) Relations Evolutives chez le genre Oryza et processus de domestication des riz. Etudes et Thèses, Paris: ORSTOM 190 p.
- 16. Sano Y, Chu YE, Oka HI (1980) Genetic studies of speciation in cultivated rice Character variations in backcross derivatives between Oryza sativa and O. glaberrima: M-V linkage and key characters. Jap J Gen 55: 19–39. [Google Scholar]
- 17. Sano Y (1989) The direction of pollen flow between two co-occurring rice species, Oryza sativa and O. glaberrima. Heredity 63: 353–357. [Google Scholar]
- 18. Chang TT (1976) The origin, evolution, cultivation, dissemination and diversification of Asian and African rices. Euphytica 25: 425–441. [Google Scholar]
- 19. Second G (1982) Origin of the genic diversity of cultivated rice (Oryza spp.): Study of the polymorphism scored at 40 isozyme loci. Jap J Genet 57: 25–57. [Google Scholar]
- 20. Pham JL, Bougerol B (1993) Abnormal segregations in crosses between two cultivated rice species. Heredity 70: 466–471. [Google Scholar]
- 21. Pham JL, Bougerol B (1996) Variation in fertility and morphological traits in progenies of crosses between the two cultivated rice species. Hereditas 124: 179–183. [Google Scholar]
- 22. Barry MB, Pham JL, Noyer JL, Billot C, Courtois B, et al. (2007) Genetic diversity of the two cultivated rice species (O. sativa & O. glaberrima) in Maritime Guinea. Evidence for interspecific recombination. Euphytica 154: 127–137. [Google Scholar]
- 23. Nuijten E, van Treuren R, Struik PC, Mokuwa A, Okry F, et al. (2009) Evidence for the emergence of new rice types of interspecific hybrid origin in West African farmers’ fields. PLoS ONE 4(10): e7335 doi:10.1371/journal.pone.0007335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox TS, Wood D (1999) The nature and role of crop biodiversity, Chapter 3. In: Wood D, editor. Agrobiodiversity: Characterization, utilization and management. Oxford: CABI Publishing. 464 p.
- 25. Wood D, Lenné JM (1997) The conservation of agrobiodiversity on-farm: questioning the emerging paradigm. Biodiv Conserv 6: 109–129. [Google Scholar]
- 26. Barry MB, Pham JL, Courtois B, Billot C, Ahmadi N (2007) Rice genetic diversity at farm and village levels and genetic structure of local varieties reveal need for in situ conservation. Gen Res Crop Evol 54: 1675–1690. [Google Scholar]
- 27. Semon M, Nielsen R, Jones MP, McCouch SR (2005) The population structure of African cultivated rice Oryza glaberrima (Steud.): evidence for elevated levels of linkage disequilibrium caused by admixture with O. sativa and ecological adaptation. Genetics 169: 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nuijten E, Richards P (2011) Pollen flows within and between rice and millet fields in relation to farmer variety development in The Gambia. Plant Gen Res 9: 361–374. [Google Scholar]
- 29.Mouser B, Okry F, Nuijten E, Richards P (2012) Commodity and anti-commodity: linked histories of slavery, emancipation and red and white rice at Sierra Leone, Commodities of Empire Working Papers WP19. 29 pp.
- 30. Nuijten E, Van Treuren R (2007) Spatial and temporal dynamics in genetic diversity in upland rice and late millet in The Gambia. Gen Res Crop Evol 54: 989–1009. [Google Scholar]
- 31.Smith S (2000) Perspectives on diversity from an international commercial plant breeding organization. In: Almekinders CJM, De Boef W, editors. Encouraging diversity. London: Intermediate Technology Publications. 175–179.
- 32.Jusu MS (1999) Management of genetic variability in rice (Oryza sativa L. and O. glaberrima Steud.) by breeders and farmers in Sierra Leone. Wageningen: PhD-thesis, Wageningen University, The Netherlands, 198 p.
- 33. Barry MB, Pham JL, Béavogui S, Ghesquière A, Ahmadi N (2008) Diachronic (1979–2003) analysis of rice genetic diversity in Guinea did not reveal genetic erosion. Gen Res Crop Evol 55: 723–733. [Google Scholar]
- 34. Roy SKS, Hamid A, Miah MG, Hashem A (1996) Seed size variation and its effects on germination and seedling vigour in rice. J Agron Crop Sci 176: 79–82. [Google Scholar]
- 35. Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Plant Mol Biol 35: 25–34. [PubMed] [Google Scholar]
- 36.Nuijten E, Temudo MP, Richards P, Okry F, Teeken B, et al.. (2013). Towards a new approach for understanding interactions of technology with environment and society in small-scale rice farming. In: Wopereis MCS, Johnson D, Ahmadi N, Tollens E, Jalloh A, editors. Realizing Africa’s Rice Promise. Wallingford: CABI (in press).
- 37. Gross BL, Steffen FT, Olsen KM (2010) The molecular basis of white pericarps in African domesticated rice: novel mutations at the Rc gene J Evol Biol. 23: 2747–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards P (1986) Coping with hunger: hazard and experiment in an African rice-farming system. London: Allen & Unwin. 192 p.
- 39. de Kochko A (1987) A classification of traditional rice varieties (Oryza sativa L.) from Africa using isozymic variability. Evol Trends in Plants 1: 105–110. [Google Scholar]
- 40.WARDA (West African Rice Development Association–Africa Rice Center) (2003–2004, 2004–2005) WARDA Annual reports. Cotonou Benin: WARDA.
- 41. Dalton TJ, Guei RG (2003) Productivity gains from rice genetic enhancements in West Africa: countries and ecologies. World Dev 31: 359–374. [Google Scholar]
- 42. Balasubramanian V, Sie M, Hijmans RJ, Otsuka K (2007) Increasing rice production in Sub-Saharan Africa: challenges and opportunities. Adv Agron 94: 55–133. [Google Scholar]
- 43. Saito K, Futakuchi K (2009) Performance of diverse upland rice cultivars in low and high soil fertility conditions in West Africa. Field Crops Res 111: 243–250. [Google Scholar]
- 44.Nuijten E, Richards R (2013) Gene flow in African rice farmers’ fields. In: Wopereis MCS, Johnson D, Ahmadi N, Tollens E, Jalloh A, editors. Realizing Africa’s Rice Promise. Wallingford: CABI (in press).
- 45. Pusadee T, Jamjod S, Chiang Y, Rerkasem B, Schaal BA (2009) Genetic structure and isolation by distance in a landrace of Thai rice. Proc Natl Acad Sci USA 106: 13880–13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Londo J, Chiang Y, Hung K, Chiang T, Schaal B (2006) Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa . Proc Natl Acad Sci USA 103: 9578–9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olsen K, Schaal A (2001) Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: Further evidence for a southern Amazonian origin of domestication. Am J Bot 88: 131–142. [PubMed] [Google Scholar]
- 48. Hyten D, Song Q, Zhu Y, Choi I, Nelson R, et al. (2006) Impact of genetic bottlenecks on soybean genome diversity. Proc Natl Acad Sci USA 103: 16666–16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bioversity International (2007) Descriptors for wild and cultivated rice (Oryza spp.) Rome: Bioverstiy International.
- 50. Lezzoni AF, Pritts MP (1991) Application of principal component analysis to horticultural research. Hortic Sci 26: 334–338. [Google Scholar]
- 51. Huson DH (1998) SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14: 68–73. [DOI] [PubMed] [Google Scholar]