Abstract
Objectives
Precursors to 1/3 of colorectal cancer (CRC), serrated polyps have been under-detected by screening due to their inconspicuous, non-hemorrhagic, and proximal nature. A new multi-target stool DNA test (multi-target sDNA) shows high sensitivity for both CRC and advanced adenomas. Screen detection of serrated polyps by this approach requires further validation. We sought to assess and compare noninvasive detection of sessile serrated polyps (SSP) ≥1 cm by sDNA and an occult blood fecal immunochemical test (FIT).
Methods
In a blinded prospective study, a single stool sample used for both tests was collected from 456 asymptomatic adults prior to screening or surveillance colonoscopy (criterion standard). All 29 patients with SSP≥1 cm were included as cases and all 232 with no neoplastic findings as controls. Buffered stool samples were processed and frozen on receipt; Exact Sciences performed sDNA in batches using optimized analytical methods. The sDNA multi-marker panel targets methylated BMP3 (mBMP3) and NDRG4, mutant KRAS, β-actin, and hemoglobin. FIT (Polymedco OC-FIT Check) was performed in separate lab ≤2 days post defecation and evaluated at cutoffs of 50 (FIT-50) and 100 ng/ml (FIT-100).
Results
Median ages: cases 61 (range 57–77), controls 62 (52–70), p = NS. Women comprised 59% and 51%, p = NS, respectively. SSP median size was 1.2 cm (1–3 cm), 93% were proximal, and 64% had synchronous diminutive polyps. Among multi-target sDNA markers, mBMP3 proved highly discriminant for detection of SSP≥1 cm (AUC = 0.87, p<0.00001); other DNA markers provided no incremental sensitivity. Hemoglobin alone showed no discrimination (AUC = 0.50, p = NS). At matched specificities, detection of SSP≥1 cm by stool mBMP3 was significantly greater than by FIT-50 (66% vs 10%, p = 0.0003) or FIT-100 (63% vs 0%, p<0.0001).
Conclusions
In a screening and surveillance setting, SSP≥1 cm can be detected noninvasively by stool assay of exfoliated DNA markers, especially mBMP3. FIT appears to have no value in SSP detection.
Introduction
Prevention of colorectal cancer (CRC) by screening rests on effective detection of the critical precursor lesions. Based on the assumption that CRC evolves primarily via the established adenoma-to-carcinoma pathway [1], screening has historically focused on detecting adenomas as the exclusive precursor lesion. However, it has become increasingly apparent in recent years that many CRCs arise from serrated polyps, which differ biologically and clinically from conventional adenomas [2], [3].
Serrated polyps, often classified in the past as innocuous hyperplastic polyps and disregarded, are now thought to represent the essential progenitor of up to 1/3 of all CRC [4]–[6]. Sessile serrated adenomas or polyps (SSPs) represent the subset of serrated polyps at greatest risk of CRC progression and may grow more rapidly than conventional adenomas [4]–[6]. SSPs are typically right-sided, often flat and endoscopically inconspicuous, and relatively more common in women and the elderly [6]–[9]. Unlike conventional adenomas, SSPs are commonly associated with BRAF mutations [3], [10]; while both SSPs and conventional adenomas frequently harbor aberrantly methylated genes [6], [11], [12]. SSPs that progress often acquire dysplasia, a transition accompanied by microsatellite instability [4], [8], [13], [14]; and remnants of SSP tissue can be demonstrated at margins of right-sided CRC [13], [15].
Data on SSP detection by conventional screening tools are limited, but indirect evidence suggests that their effectiveness is suboptimal. Despite trends of increasing polypectomy rates and reductions in left-sided CRC, population-based studies show that intensified screening has had proportionately less effect on the incidence of right-sided CRC [16]. Sigmoidoscopy is inherently limited to visualization of the left colorectum, and its programmatic application has little impact on proximal CRC [17], [18]. Fecal blood testing, whether by guaiac [19], [20] or immunochemical [21], [22] methods, exhibits low detection rates for adenomas, especially proximal ones; large screening studies reveal that guaiac-type fecal blood testing has had negligible or minimal effect on CRC incidence [20], [21]; and it is not known if they can detect SSPs which are largely non-hemorrhagic. Even colonoscopy appears to detect right-sided precursor lesions less well than left-sided ones, despite its capacity to directly inspect the entire colorectum [23]–[25]. The relatively lower effectiveness of current screening approaches to affect proximal colon cancer may relate to the nature of right-sided precursor lesions and to our difficulties in detecting them with current tools.
Stool DNA testing represents a biologically rational candidate approach to the screen detection of SSPs that merits further evaluation. With the improved analytical sensitivity of next generation assays, stool DNA testing has been shown to detect advanced adenomas at high rates [26]–[29], and neoplasm site does not appear to affect test sensitivity [20], [27]. Preliminary reports on selected patients suggest that stool DNA testing detects large SSPs at sensitivities comparable to those for adenomas. In one small study using archived stool specimens [30], both mutant BRAF and selected methylated gene markers were elevated in stools from patients harboring large SSPs. In the other preliminary report [31], an optimized pre-commercial multi-marker stool DNA test (sDNA), which targets aberrantly methylated BMP3 and NDRG4 genes, detected 60% of SSP≥1 cm at 90% specificity.
In the present prospective study, we sought to assess the noninvasive detection of SSP by stool DNA testing in asymptomatic persons undergoing screening or surveillance colonoscopic examination of the colon. Our specific aims were to 1) evaluate the performance of a pre-commercial multi-target stool DNA test (multi-target sDNA) for detection of SSP≥1 cm, 2) determine which multi-target sDNA markers contribute most to SSP detection based on stool and tissue analyses, and 3) compare SSP detection rates by assay of exfoliated markers using stool DNA testing with those by assay of occult bleeding using a quantitative fecal immunochemical test (FIT). We hypothesized that asymptomatic SSP lesions ≥1 cm would exfoliate markers at rates sufficient to be detected by sDNA testing but would rarely bleed and not be meaningfully detected by FIT.
Methods
This blinded cross-sectional study was approved by the Mayo Clinic Institutional Review Board on September 7, 2010 and conducted at Mayo Clinic facilities in Scottsdale AZ and Rochester MN. All stool and tissue assays were performed by technicians unaware of clinical source of samples. The Mayo Clinic Institutional Review Board deemed this study of de-identified biospecimens to be of minimal risk. Using a form approved by the Mayo Clinic Institutional Review Board, written informed consent was obtained prior to specimen collection.
Participants in Stool Study
Participants comprised consenting asymptomatic adults scheduled during 2011–2012 for a screening or polyp surveillance colonoscopy, which served as the criterion standard. Patients were excluded if they had (1) a prior colorectal resection, (2) inflammatory bowel disease, Lynch syndrome, familial adenomatous polyposis, or other high risk conditions for CRC, (3) colonoscopy that was incomplete or associated with a poor preparation, or (4) a prior screening examination was done within 5 years. All patients found to have a SSP≥1 cm without a synchronous advanced adenoma or CRC were designated as cases. The presence of synchronous small or diminutive polyps did not exclude cases; rather, stratified stool data analyses were performed on patient subsets with and without synchronous small or diminutive polyps. Data were not included on isolated lesions smaller than 1 cm in this proof-of-concept study. All patients found to be free of colorectal polyps or tumors were designated as controls.
Stool Collection and Storage
A single stool from each participant was prospectively collected prior to cathartic cleansing for colonoscopy using a bucket container mounted to the toilet seat. Patients sampled stools for FIT analyses using probe devices; and then added a preservative solution to the whole stool and promptly mailed the sealed container along with FIT tubes to the processing laboratories, as described [28].
Upon receipt, stools were homogenized, aliquoted, and frozen at −80°C for subsequent batch performance of the multi-marker sDNA assay. The commercial FIT assay was performed upon receipt (see below). Stools received >3 days after defecation were disqualified and not tested.
Stool Assay Methods
Stool DNA tests
Stool processing, assay methods, and primer sequences have been described in detail [28], [29], [31], [32]. This pre-commercial multi-target sDNA assay was performed at Exact Sciences (Madison WI) and included the following recent innovations: automation, direct gene capture from fecal supernatant, an optimized rapid bisulfite treatment process, a panel of broadly informative DNA markers (mBMP3, mNDRG4, mutant KRAS, and β-actin) assayed in multi-plex by the analytically-sensitive quantitative allele-specific real-time target and signal amplification (QuARTS) method, a proprietary quantitative fecal hemoglobin assay, and use of a logistic regression model for analysis. Results for the sDNA test were designated as “positive” or “negative” by the manufacturer based on their pre-established logistic algorithm. Individual tumor markers from the multi-target sDNA panel were normalized to stool β-actin (a marker of total human DNA) and evaluated separately as well; specificity cutoffs were selected to match those of FIT in the comparison studies.
Fecal Immunochemical Test for occult blood (FIT)
A quantitative commercially available FIT (OC-FIT CHEK, Polymedco, NY) was performed at Mayo Clinic using an automated reading device. FIT results were evaluated at the manufacturer’s recommended specificity cutoff of 100 ng hemoglobin/ml buffer (FIT-100) and also at a cutoff of 50 ng/ml (FIT-50).
Tissue analyses
To determine whether SSP lesions represent the likely origin of methylated markers in stool, a blinded independent tissue study was performed on SSP lesions ≥1 cm colonoscopically-removed from 20 unique case patients [median age 61 (range 30–83), 68% women] and on normal colon mucosa biopsies from 20 unique control patients without visible colorectal lesions [median age 54 (range 30–81), 70% women]. Following micro-dissection of paraffin or frozen tissue slides, DNA was extracted in usual fashion. Bisulfite treatment and marker assay methods were as described above for stool. Quantitative levels were normalized to β-actin.
Statistical Analysis
Associations between test positivity and clinical characteristics were assessed using the Chi-square test. Comparison of sensitivities between tests was done at matched specificities using McNemar’s test for paired proportion. The non-parametric Wilcoxon Rank Sums Test was used to test the association between continuous marker values and clinical characteristics. The discriminant accuracy of each marker was estimated as the area under the ROC curve (AUC) [33]. Linear combination of markers in the multi-target sDNA test for the prediction of disease status was assessed using logistic regression. To avoid over fitting of the data, the final set of predictors used within the logistic regression model was determined from fitting an Elastic Net regression model with all the markers; and the most discriminant marker or set of markers was selected to have the lowest cross-validated prediction error [34].
Results
Patient and Lesion Characteristics for Stool Study
From 456 asymptomatic patients undergoing screening or surveillance colonoscopy, we identified 29 individuals with SSP≥1 cm who served as cases and 232 free of polyps who served as controls. Age and sex distributions were similar between groups (Table 1).
Table 1. Patient and Lesion Characteristics for Stool Study.
| SSP Cases (29)a | Normal Controls(232)b | |
| Patient Demographics | ||
| Age in years, median (range) | 62 (57–77) | 61 (52–70) |
| Sex, % women | 59 | 52 |
| SSP Features | ||
| Size in cm, median (range) | 1.4 (1.0–3.0) | – |
| Right sided, %c | 93 | – |
| Dysplasia present, % | 3 | – |
| Synchronous small polyps, %d | 64 | – |
Abbreviation: –, not applicable.
a Cases comprised patients with at least one SSP (sessile serrated polyp) ≥1 cm found on screening or surveillance colonoscopy and without synchronous advanced adenomas or CRC.
b Control patients had no pathology (no CRC, colorectal polyps, hemorrhagic lesions, or inflammation) on screening or surveillance colonoscopy.
c Right-sided location was defined as proximal to the splenic flexure.
d Patients found to harbor synchronous polyps (adenomatous or serrated) <1 cm in size were included as cases.
In cases, median SSP size was 1.2 cm (range 1–3), 28/29 (93%) were located proximal to the splenic flexure, and only one SSP contained dysplasia (Table 1). Synchronous small or diminutive polyps were present in 64% of cases.
SSP Detection by the Multi-target Stool DNA Test
Test accuracy
Using the manufacturer’s current cutoff, the sensitivity of the sDNA test for detection of SSP≥1 cm was 55% (95% CI: 36–74) and the specificity was 91% (95% CI: 87–94).
Effect of covariates
The SSP detection rate did not differ significantly between case subsets with and without synchronous small or diminutive polyps; sensitivities were 60% and 50%, respectively (p = 0.4). Within the narrow size range of SSP cases identified, the detection rate was 55% for the 11 patients with lesions 1–1.4 cm and 53% for those 17 with lesions 1.5–3 cm, p = 0.9. Neither age nor sex influenced SSP detection rates (data not shown).
Contribution of component markers to SSP detection
Among the multi-target sDNA markers, mBMP3 proved most discriminant for SSP detection, with an AUC of 0.87 (95% CI: 0.80–0.95), p<0.0001. The other informative DNA markers, mNDRG4 (AUC 0.79; 95% CI; 0.70–0.88; p<0.0001) and mutant KRAS (AUC 0.64; 95% CI: 0.53–0.75; p = 0.0068), did not provide statistically significant incremental sensitivity in this study above that provided by mBMP3. Of note, fecal hemoglobin alone showed no discrimination (AUC = 0.50; 95% CI: 0.40–0.61; p = 0.4724).
Tissue Confirmation of SSP Discrimination by Methylation Markers
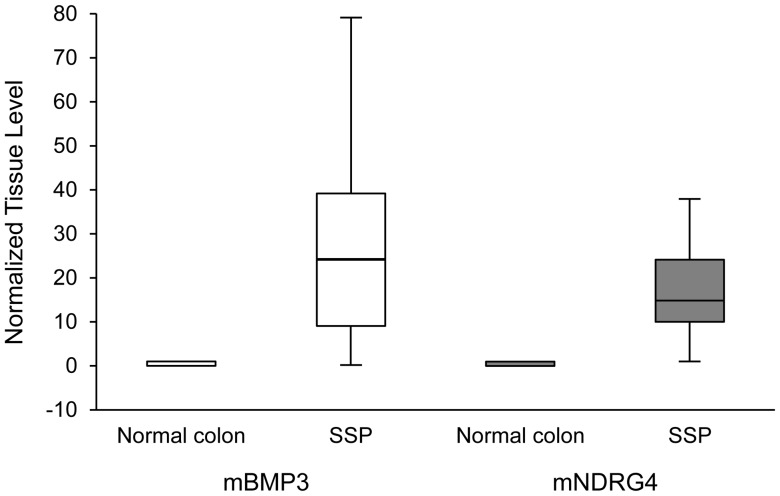
At the tissue level, each methylated marker alone discriminated SSP from normal colon mucosa almost completely (Figure 1). The median mBMP3 level in SSP lesions (24 relative units (range 0–78) was >200 times greater than in normal colon mucosa (0.11 (range 0–0.11), p<0.0001. The median mNDRG4 level in SSP lesions (15 (range 0.9–47)) was >70 times higher than in normal colon mucosa (0.21 (0–0.8)), p<0.0001.
Figure 1. Tissue levels of aberrantly methylated genes.
Tissue levels of methylated BMP3 (mBMP3) and NDRG4 (mNDRG4) genes are compared in normal colorectal mucosa, n = 20 unique control patients, and sessile serrated polyps (SSP), n = 20 unique case patients. Marker levels are normalized to β-actin (a marker of total human DNA) and expressed in relative units. Levels were substantially and significantly higher in SSP than normal colon for both mBMP3 (p<0.0001) and mNDRG4 (p<0.0001).
Comparison of Stool DNA Testing and FIT for SSP Detection
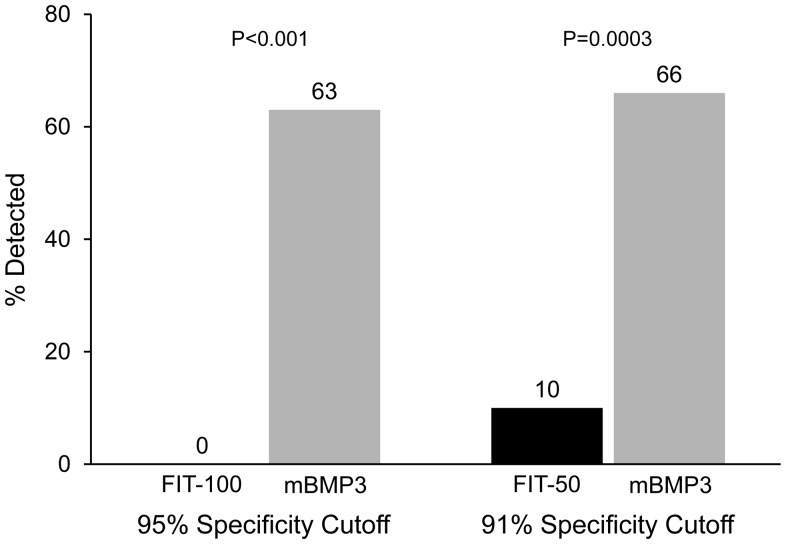
Stool assay of mBMP3 (an exfoliated DNA marker) was selected for comparison against FIT, which measures fecal hemoglobin (an occult bleeding marker), to best evaluate the diagnostic yield of these markers that enter stool via biologically distinct mechanisms. At matched specificities, stool assay of mBMP3 was substantially and significantly more sensitive for detection of SSP≥1 cm than FIT (Figure 2). At 95% specificity cutoffs, stool assay of mBMP3 detected 63% of SSP lesions compared to 0% by FIT-100, p<0.0001; at 91% specificities, stool assay of mBMP3 detected 66% compared to 10% by FIT-50, p = 0.0003.
Figure 2. Detection of SSP by stool assay of mBMP3 and by fecal immunochemical testing (FIT).
FIT sensitivity was evaluated at the conventional cutoff of 100/ml buffer (FIT-100) and at 50 ng/ml (FIT-50). Specificity cutoffs for stool DNA marker mBMP3 were selected to match those for both FIT-100 and FIT-50 so that sensitivities could be most meaningfully compared.
Discussion
Based on this prospective case-control study on asymptomatic patients undergoing screening or surveillance colonoscopy, we demonstrate that stool DNA testing represents a feasible approach to the noninvasive detection of SSP≥1 cm. Stool assay of mBMP3, a component of the multi-target stool DNA test, primarily accounted for the high SSP detection rates. Given our prior demonstrations that advanced adenomas can be detected at high rates by a next generation multi-target stool DNA test [26]–[29], [31], [32], these new findings suggest broadened value of this tool for CRC prevention through the detection of critical precursor lesions from both major molecular pathways of carcinogenesis.
In contrast to stool DNA testing, FIT essentially failed to detect SSPs. This finding is perhaps not surprising given the biology of these precursor lesions. SSPs are typically sessile or flat, non-ulcerated, and without hemorrhagic features [5], [6], [9]. In contrast, while SSPs may not bleed, our findings demonstrate that they do exfoliate at rates sufficient enough to allow their noninvasive detection by assay of altered DNA in stool.
The specific point sensitivities by sDNA testing for detection of SSP≥1 cm that we observed could translate to substantial practical value. Repeat screening with a test having a point sensitivity of 55–67% yields a potential program sensitivity exceeding 90% after the third screening round [35]. These programatic detection rates may compare favorably with conventional colonoscopy done every 10 years, particularly given the reported wide range in operator variation with colonoscopy [36].
Accurate detection of gastrointestinal neoplasms by stool assay of exfoliated markers requires high analytical sensitivity and discriminant markers. The QuARTS assay method used in this study achieves more than 100-fold higher sensitivity than earlier generation stool DNA assays [26]-[29], [31], [32] and, as we observed in this study, provides the critical analytical power to detect low-abundance DNA markers exfoliated into stool from SSPs. SSP detection by the multi-target stool DNA test was primarily accounted for by mBMP3. Consistent with the stool observations, we found in our tissue study that mBMP3 levels highly discriminated SSP from normal colon mucosa. Perhaps because of the low background levels of mBMP3 in normal control stools, assay of this marker alone achieved slightly higher SSP detection rates than did assay of the full marker panel in this study.
Strengths of this study included prospective stool collections from well-characterized patients undergoing colonoscopy for average risk screening or polyp surveillance, a blinded design, state-of-the-art assay technology, and use of discriminant methylation markers. Study limitations comprised the relatively narrow and small SSP size range, as the median size was only 1.2 cm, and a paucity of lesions containing dysplasia which likely represent those at greatest risk of progression. Despite these SSP characteristics, the majority were detected by the multi-target sDNA test. Furthermore, the observed differences in SSP detection rates between the stool DNA testing and FIT were substantial and highly significant. Sample size in this study did not permit robust covariate analyses.
We demonstrate proof-of-concept for SSP detection by stool DNA testing. Clinical applications of these findings can be considered and further explored. Incorporating markers specific for SSP into stool DNA tests designed for general CRC screening has potential to expand the effectiveness of this approach for CRC prevention, as both major types of precursor lesions could be targeted. Furthermore, given that colonoscopy has had a proportionately lower impact on incidence and mortality from right-sided than left-sided CRC [23]–[25] and that the majority of interval CRC cases are right-sided [37], a rationale could be made for the complementary use of stool DNA testing between screening colonoscopies, which are recommended at a frequency of every 10 years. While an economic analysis of an enhanced colorectal neoplasia detection strategy is beyond the scope of this study, once accurate assumptions of costs and test performance characteristics are available, modeling of new approaches will be instructive. The multi-target sDNA test is currently being evaluated in a prospective study involving >10,000 patients from the screen setting using colonoscopy as the criterion standard (the DEEP-C Study), and data from this large study should provide important and robust validation of this noninvasive screening approach.
Funding Statement
This study was funded by grants from the Charles Oswald Foundation, Helen Vandendriesche, and Exact Sciences Corporation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61: 759–767. [DOI] [PubMed] [Google Scholar]
- 2. Jass JR (2001) Serrated route to colorectal cancer: back street or super highway? J Pathol 193: 283–285. [DOI] [PubMed] [Google Scholar]
- 3. Jass JR (2005) Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol 2: 398–405. [DOI] [PubMed] [Google Scholar]
- 4. Snover DC (2011) Update on the serrated pathway to colorectal carcinoma. Hum Pathol 42: 1–10. [DOI] [PubMed] [Google Scholar]
- 5. Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP (2005) Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol 124: 380–91. [DOI] [PubMed] [Google Scholar]
- 6. Noffsinger AE (2009) Serrated polyps and colorectal cancer: new pathway to malignancy Annu Rev Pathol. 4: 343–364. [DOI] [PubMed] [Google Scholar]
- 7. Lazarus R, Junttila OE, Karttunen TJ, Makinen MJ (2005) The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol 123: 349–359. [DOI] [PubMed] [Google Scholar]
- 8. Lash RH, Genta RM, Schuler CM (2010) Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 63: 681–686. [DOI] [PubMed] [Google Scholar]
- 9. Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. (2012) Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 107: 1315–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, et al. (2006) High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 131: 1400–1407. [DOI] [PubMed] [Google Scholar]
- 11. Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, et al. (2003) Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol 162: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC 3rd, et al. (2007) Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol 5: 111–117. [DOI] [PubMed] [Google Scholar]
- 13. Sheridan TB, Fenton H, Lewin MR, Burkart AL, Iacobuzio-Donahue CA, et al. (2006) Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act.”. Am J Clin Pathol 126: 564–571. [DOI] [PubMed] [Google Scholar]
- 14. Oka S, Tanaka S, Hiyama T, Ito M, Kitadai Y, et al. (2004) Clinicopathologic and endoscopic features of colorectal serrated adenoma: differences between polypoid and superficial types. Gastrointest Endosc 59: 213–219. [DOI] [PubMed] [Google Scholar]
- 15. Makinen MJ, George SM, Jernvall P, Makela J, Vihko P, et al. (2001) Colorectal carcinoma associated with serrated adenoma: prevalence, histological features, and prognosis. J Pathol 193: 286–294. [DOI] [PubMed] [Google Scholar]
- 16. Gupta AK, Melton LJ 3rd, Petersen GM, Timmons LJ, Vege SS, et al. (2005) Changing trends in the incidence, stage, survival, and screen-detection of colorectal cancer: a population-based study. Clin Gastroenterol Hepatol 3: 150–158. [DOI] [PubMed] [Google Scholar]
- 17. Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, et al. (2010) Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375: 1624–1633. [DOI] [PubMed] [Google Scholar]
- 18. Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, et al. (2012) Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 366: 2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, et al. (2004) Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 351: 2704–2714. [DOI] [PubMed] [Google Scholar]
- 20. Ahlquist DA, Sargent DJ, Loprinzi CL, Levin TR, Rex DK, et al. (2008) Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med 149: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, et al. (2005) A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 129: 422–428. [DOI] [PubMed] [Google Scholar]
- 22. Park DI, Ryu S, Kim YH, Lee SH, Lee CK, et al. (2010) Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol 105: 2017–2025. [DOI] [PubMed] [Google Scholar]
- 23. Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, et al. (2010) Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst 102: 89–95. [DOI] [PubMed] [Google Scholar]
- 24. Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M (2011) Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 54: 22–30. [DOI] [PubMed] [Google Scholar]
- 25. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, et al. (2009) Association of colonoscopy and death from colorectal cancer. Ann Intern Med 150: 1–8. [DOI] [PubMed] [Google Scholar]
- 26. Zou H, Taylor WR, Harrington JJ, Hussain FT, Cao X, et al. (2009) High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology 136: 459–470. [DOI] [PubMed] [Google Scholar]
- 27.Ahlquist D ZH, Domanico M, Mahoney D, Lidgard G (2010) Next generation stool DNA testing for detection of colorectal neoplasia: Early marker evaluation. Presented at AACR Meeting, Philadelphia, PA.
- 28. Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, et al. (2012) Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology 142: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahlquist DA, Taylor WR, Mahoney DW, Zou H, Domanico M, et al. (2012) The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol 10: 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain FT, Yab TC, Harrington JJ, et al.. (2010) Noninvasive Detection of Serrated Colorectal Polyps by Stool Assay of Methylated Vimentin and Mutant BRAF Genes. In: DDW.
- 31.Lidgard GP DM, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, et al.. (2012) An Optimized Molecular Stool Test for Colorectal Cancer Screening: Evaluation of an Automated Analytic Platform and Logistic Algorithm. In: AACR Frontiers in Cancer Prevention; 2012; San Francisco, CA.
- 32. Lidgard GP DM, Bruinsma JJ, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, et al. (2012) An optimized multi-marker stool test for colorectal cancer screening: initial clinical appraisal. Gastroenterology 142: S1. [Google Scholar]
- 33. DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: 837–845. [PubMed] [Google Scholar]
- 34. Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Statist Soc B 67: 301–320. [Google Scholar]
- 35. Berger BM, Ahlquist DA (2012) Stool DNA screening for colorectal neoplasia: biological and technical basis for high detection rates. Pathology 44: 80–88. [DOI] [PubMed] [Google Scholar]
- 36. Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK, et al. (2011) Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 9: 42–46. [DOI] [PubMed] [Google Scholar]
- 37. Singh H TD, Xue L, Targownik LE, Bernstein CN (2006) Risk of Developing Colorectal Cancer Following a Negative Colonoscopy Examination: evidence for a 10-year interval between colonoscopies. JAMA 295: 2366–2373. [DOI] [PubMed] [Google Scholar]