Abstract
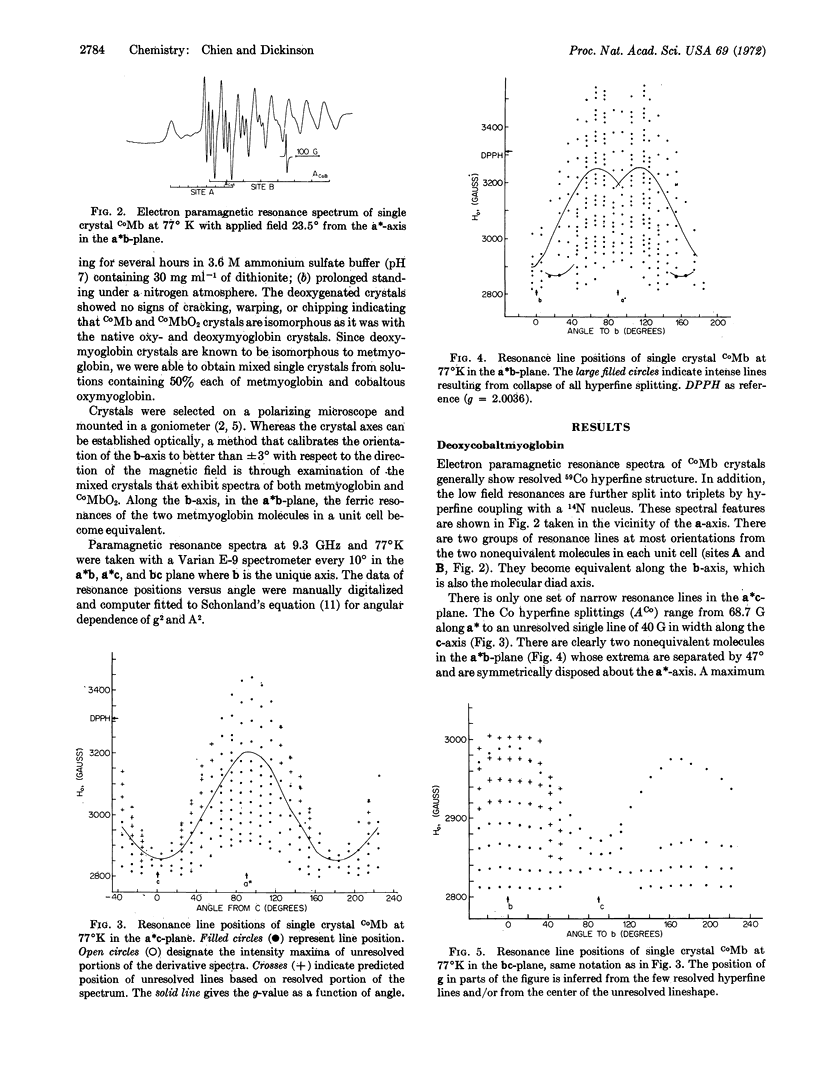
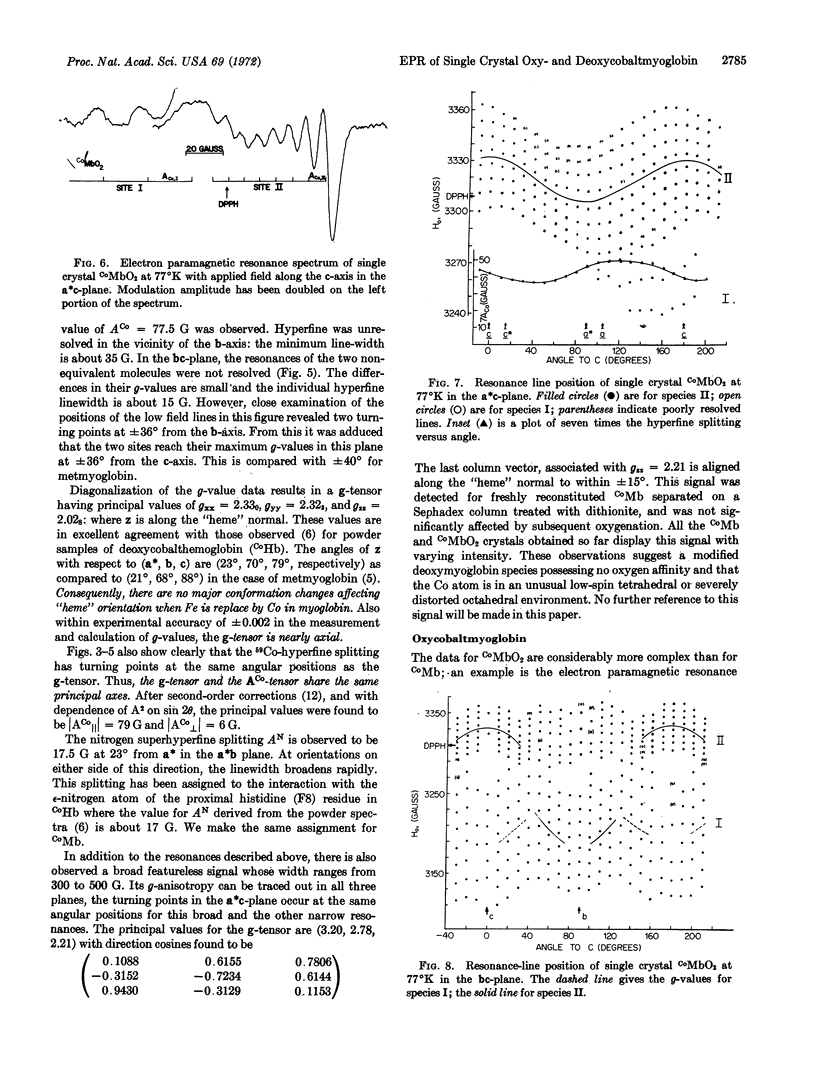
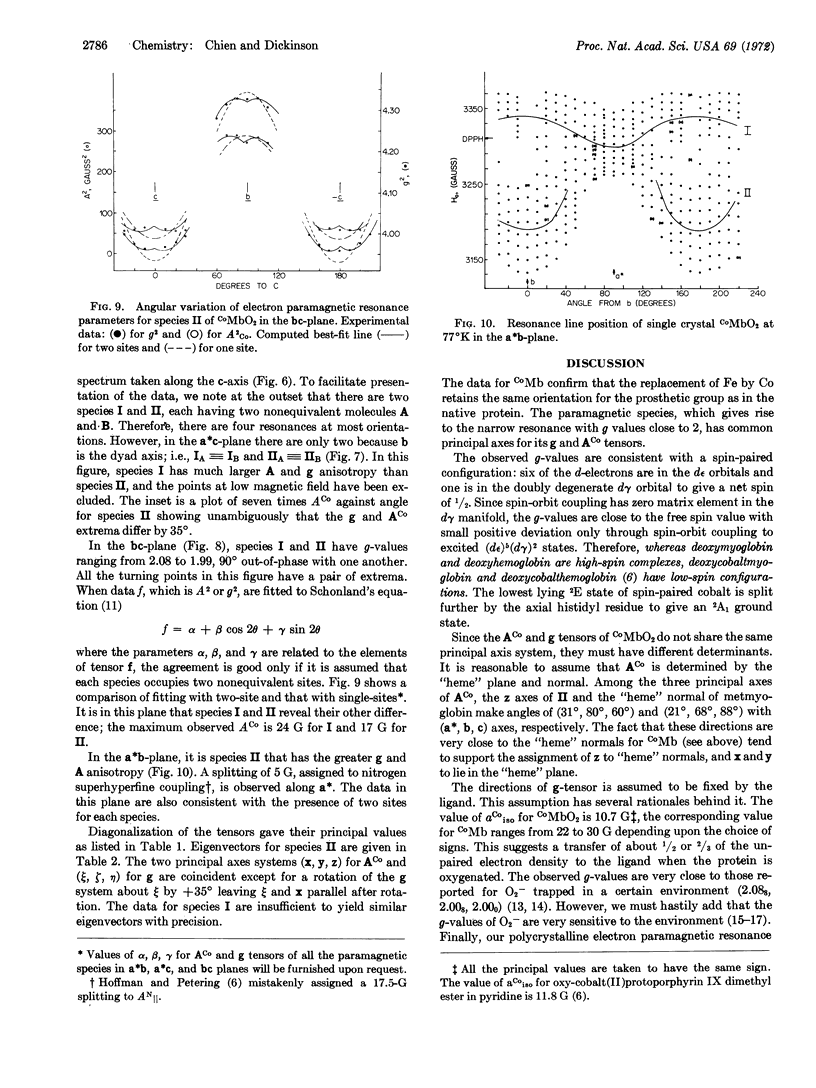
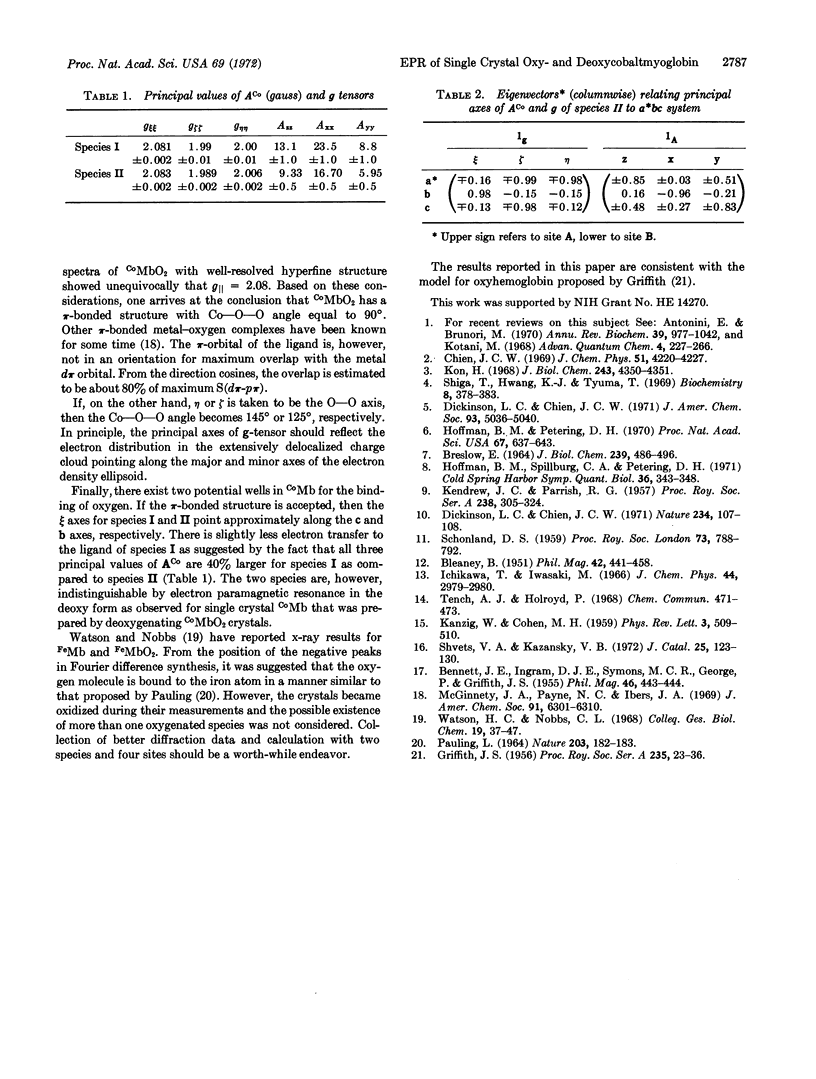
Single crystals of oxycobaltmyoglobin and deoxycobaltmyoglobin have been prepared and found to be isomorphous. The paramagnetic resonance spectra of deoxycobaltmyoglobin yield gxx = 2.330, gyy = 2.323, gzz = 2.028 with the z-axis parallel to the “heme” normal. The ACo and g tensors share the same principal axes with|ACo∥| = 79 G and|ACo[unk]| = 6 G. A value of AN = 17.5 G was also obtained for the ε-N atom of the F8 histidine. There are two paramagnetic species in oxycobaltmyoglobin having apparently identical g-tensors (gξξ = 2.083, gηη = 2.006, and gζζ = 1.989) but different ACo-tensors. Furthermore, g values ACo do not share the same principal axes. The ACo-values for one of the species are (Axx = 16.7 G, Ayy = 5.95 G, Azz = 9.3 G), they are all 40% larger for the other species. The two species are oriented 90° to each other in the crystal. The results from electron paramagnetic resonance studies are consistent with a π-bounded structure for CoMbO2.
Keywords: sperm-whale myoglobin, apomyoglobin, paper electrophoresis
Full text
PDF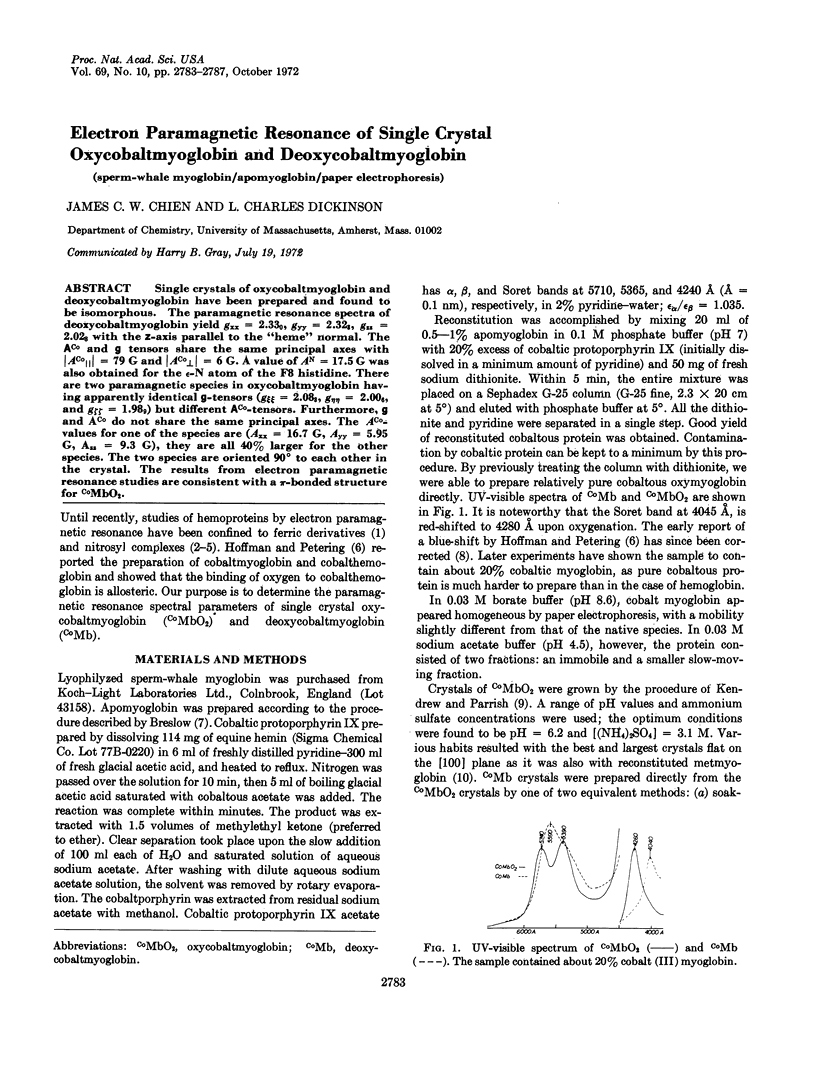
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Brunori M. Hemoglobin. Annu Rev Biochem. 1970;39:977–1042. doi: 10.1146/annurev.bi.39.070170.004553. [DOI] [PubMed] [Google Scholar]
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Chien J. C. Electron paramagnetic resonance study of the stereochemistry of nitrosylhemoglobin. J Chem Phys. 1969 Nov 15;51(10):4220–4227. doi: 10.1063/1.1671782. [DOI] [PubMed] [Google Scholar]
- Dickinson L. C., Chien J. C. An electron paramagnetic resonance study of nitrosylmyoglobin. J Am Chem Soc. 1971 Oct 6;93(20):5036–5040. doi: 10.1021/ja00749a011. [DOI] [PubMed] [Google Scholar]
- Dickinson L. C., Chien J. C. Crystallization of reconstituted sperm whale myoglobins. Nat New Biol. 1971 Nov 24;234(47):107–107. doi: 10.1038/newbio234107a0. [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Petering D. H. Coboglobins: oxygen-carrying cobalt-reconstituted hemoglobin and myoglobin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):637–643. doi: 10.1073/pnas.67.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. M., Spilburg C. A., Petering D. H. Coboglobins: cobalt substitution and the nature of the prosthetic group--apoprotein interaction in hemoglobin and myoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:343–348. doi: 10.1101/sqb.1972.036.01.045. [DOI] [PubMed] [Google Scholar]
- Kon H. Paramagnetic resonance study of Nitric Oxide hemoglobin. J Biol Chem. 1968 Aug 25;243(16):4350–4357. [PubMed] [Google Scholar]
- Shiga T., Hwang K. J., Tyuma I. Electron paramagnetic resonance studies of nitric oxide hemoglobin derivatives. I. Human hemoglobin subunits. Biochemistry. 1969 Jan;8(1):378–383. doi: 10.1021/bi00829a052. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Jul 11;203:182–183. [PubMed] [Google Scholar]



