Abstract
Purpose
TBI plays an important role in patients undergoing HCT, but is associated with significant toxicities. Targeted TBI using helical tomotherapy (HT) results in reduced doses to normal organs, which predict for reduced toxicities compared to standard TBI.
Methods and Materials
Thirteen patients with multiple myeloma (MM) were treated on an autologous tandem transplant Phase I trial with high dose melphalan, followed 6 weeks later by TMI to skeletal bone. Doses levels were 10, 12, 14, and 16 Gy at 2 Gy QD/BID. On a separate allogeneic HCT trial, 8 patients (5 AML, 1 ALL, 1 NHL, 1 MM) were treated with TMI+TLI + splenic RT to 12 Gy (1.5 Gy BID) combined with fludarabine/melphalan.
Results
For the 13 patients on the tandem autoHCT trial, median age was 54 (42–66). Median organ doses were 15–65% that of the GTV dose. Grade 1–2 acute toxicities were primarily observed. Six reported no vomiting, 9 no mucositis, 6 no fatigue, and 8 no diarrhea. For the 8 patients on the alloHCT trial, the median age was 52 (24–61). Grade 2–3 nausea, vomiting, mucositis and diarrhea were observed. On both trials no grade 4 non-hematologic toxicity was observed and all patients engrafted successfully.
Conclusions
This study demonstrates that TMI using HT is clinically feasible. Reduced acute toxicities observed compare favorably to those seen with standard TBI. Initial results are encouraging and warrant further evaluation as a method to dose escalate with acceptable toxicity or to offer TBI containing regimens to patients unable to tolerate standard approaches.
Keywords: helical tomotherapy, IMRT, bone marrow transplantation, multiple myeloma, leukemia
INTRODUCTION
Radiation therapy in the form of total body irradiation (TBI) continues to be an important part of conditioning regimens in patients undergoing hematopoietic cell transplantation (HCT). Several randomized trials have demonstrated superior outcomes using TBI compared to non-TBI containing regimens (1–4). Randomized trials have also demonstrated reduced relapse rates in AML and CML with higher TBI doses (5,6). However, overall survival was unchanged due to an increase in toxicities and treatment related mortality rates. A more targeted form of TBI delivery is therefore needed to allow for dose escalation with acceptable toxicity and to allow for the potential to improve outcomes. Rapid advances in the delivery of external beam radiotherapy using intensity modulated radiation therapy coupled with image guided radiation therapy now allow the radiation oncologist to “sculpt” dose to the unique shape of each patient’s tumor. These advances are being translated to large field applications.
Recently, we reported on the concept of using helical tomotherapy to deliver a more targeted, conformal form of TBI (7–9). Dosimetry studies demonstrated lower organ doses and predicted for reduced toxicities. This report details the observed acute toxicities and initial clinical experience of patients undergoing HCT using this approach. The potential advantages and challenges of this approach are discussed.
MATERIALS AND METHODS
Patient characteristics
The first 21 patients treated from June, 2005 to June, 2007 with targeted TBI using HT on two separate studies are the subjects of this report. Both trials defined acute toxicities as those occurring in the first 30 days after targeted TBI using the NCI Common Toxicity Criteria (CTC) version 3, and were approved by the City of Hope Cancer Center IRB.
Phase I tandem autologous HCT trial of melphalan and targeted TBI in multiple myeloma (MM)
Patients with stage I–III disease by Durie staging system (10) and who were less than 18 months from diagnosis were eligible for this trial. Previous radiotherapy was allowed if delivered to ≤ 20% of the bone marrow containing areas and ≤ 20 Gy conventionally fractionated. Patients first underwent mobilization with cyclophosphamide (1.5 g/m2 and filgrastim 10 ug/kg/d) followed by apheresis to collect a minimum of 4 × 106 CD34+ cells/kg. This was followed by melphalan at 100 mg/m2/d for two days, followed by reinfusion of one half of collected peripheral stem cells.
A minimum of 6 weeks later, targeted TBI using HT was administered using methods described below. The gross target volume (GTV) was defined as skeletal bone and we have referred to this technique as total marrow irradiation (TMI). As part of the phase I portion of this study, TMI dose was escalated in cohorts of 3–6 patients per standard phase I trial design. Five dose levels were defined for this trial: 10, 12, 14, 16, 18, and 20 Gy delivered at 2 Gy QD to BID (minimum 6 hours between fractions) over 5 days, followed by peripheral stem cell infusion. Dose-limiting toxicity was defined as grade 3 or 4 non-hematologic toxicity (except fatigue) or grade 4 leukopenia or thrombocytopenia greater than 28 days duration.
Maintenance dexamethasone (40 mg/d × 4 days every 28 days) and thalidomide (50–200 mg/d) were started a minimum of 30 days after TMI. Follow-up included serum protein electrophoresis every 3 months, and bone x-rays and bone marrow biopsies at 3, 6 and 12 months post TMI and yearly thereafter. Complete response was defined as absence of serum and urinary M-protein and no more than 5% plasma cells on bone marrow; very good partial response as ≥ 90% reduction in bone marrow plasma cells and blood M-protein levels; partial response as ≥ 50% reduction in blood and bone marrow findings; and stable disease as < 25 % reduction in blood and bone marrow findings for a minimum of 3 months duration. Progression was defined as > 25 % increase in M-protein, > 25% increase of bone marrow plasma cells, or new bone lesions.
Pilot allogeneic HCT trial with targeted TBI combined with the reduced intensity chemotherapy regimen of fludarabine and melphalan
Patients with advanced hematologic malignancies (acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), non-Hodgkin’s lymphoma (NHL), multiple myeloma (MM)) defined as either high risk remission, less than 10% blasts in marrow, or ≥ 50% reduction of marrow blasts after induction therapy were eligible. Previous radiotherapy was allowed if delivered to ≤ 20% of the bone marrow containing areas and ≤ 20 Gy conventionally fractionated. Patients older than age 50 or with co-morbidities who may have benefited from HCT, but who were not eligible for standard full intensity, myeloablative HCT conditioning regimens, were eligible to enter this study.
Targeted TBI was delivered from Days −7 to − 4 at 1.5 Gy BID (minimum 6 hours between fractions) for a total 12 Gy, combined with fludarabine (25 mg/m2/d × 5 on days −7 to −3) and melphalan at 140 mg/m2 day −2. Graft versus host disease (GVHD) prophylaxis consisting of tacrolimus and sirolimus was started on day −1. On Day 0, collected peripheral blood stem cells from HLA-matched related or matched unrelated donors was infused. For all patients the GTV was defined as skeletal bone, major lymph node chains, and spleen. Brain and testes were also included in the GTV for patients with ALL. We have referred to this technique as total marrow and lymphoid irradiation (TMLI). For the first 100 days after discharge, patients were followed at least weekly with complete differential blood counts and comprehensive metabolic panel. Bone marrow biopsy was performed at approximately 100 days after stem cell infusion.
In both trials, standard anti-emetic regimens were used and palifermin was not administered.
Radiotherapy technique
Details of the technique have been previously published (8). All patients were initially scanned for treatment planning purposes on a large bore (85 cm) CT simulator with 60 cm field of view (Philips Medical System, Eindhoven, The Netherlands). CT scans in 4 mm slices were obtained during shallow breathing, inspiration and expiration to account for changes in position during respiration of the ribs, lungs, kidneys, spleen and liver. A full body vac-lok bag (CIVCO Medical Systems, Kalona, IA) and thermoplastic mask over the head and neck were used as immobilization devices.
Target and avoidance structures were contoured on an Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). Avoidance structures contoured included lungs, heart, kidneys, liver, esophagus, oral cavity, parotid glands, thyroid gland, eyes, lens, brain, stomach, small bowel, rectum, bladder, and testes. GTV for each trial is described above. The mandible and maxillary bones were excluded from the GTV in an effort to minimize oral cavity dose and mucositis. DICOM-RT images were then transferred to the Hi-Art tomotherapy treatment planning system (Tomotherapy, Inc. Madison, WI). Plans were designed such that a minimum of 85% of the GTV received the prescribed dose (8).
Our current procedure involves initial laser alignment of the patient in a vac-lok bag and thermoplastic mask. The mask is then removed and a megavoltage CT (MVCT) scan is performed with fusion to the planning CT. The necessary couch shifts are then made, followed by initiation of treatment. A slice thickness of 2.5 mm, pitch of 0.3–0.45, and a modulation factor of 2.5–3.0 were used for treatment, resulting in a beam-on time of approximately 50 minutes.
The current treatment table on the HT unit has a maximum travel length of approximately 150 cm. We chose to treat the lower extremities on a conventional C-arm linear accelerator through standard AP-PA fields given the lack of sensitive organs in this area in this adult population. At the time of treatment planning, a radio-opaque marker was placed in the thigh region to define the lower border (50% isodose line) of the HT plan. AP-PA fields were gapped to this border at midplane.
RESULTS
Twenty-one patients were treated with either TMI or TMLI. There were no delays in treatment delivery due to toxicities and all patients completed the planned dose and schedule with the exception of one patient on the autologous HCT myeloma trial, who received 12 Gy instead of the planned 14 Gy due to machine down time. Most patients were treated without mask immobilization in place due to initial concerns of vomiting during treatment. However to date, only 1 patient experienced nausea during the therapy that required a brief treatment interruption.
Phase I tandem autologous HCT trial of melphalan and TMI in MM
Thirteen MM patients received TMI per protocol with 3 patients at each dose level of 10, 12, 14, and 16 Gy, with the exception of a 4th patient added to the 14 Gy level for reasons stated above. Median age was 54 years (range 42–66). Seven were male and 6 female. Median time from diagnosis to receiving melphalan was 8 months (range 4–13), and median number of prior chemotherapy regimens was 2 (range 1–4). No patients received prior radiotherapy. The median number of days between melphalan and TMI was 79 days (range 4–125).
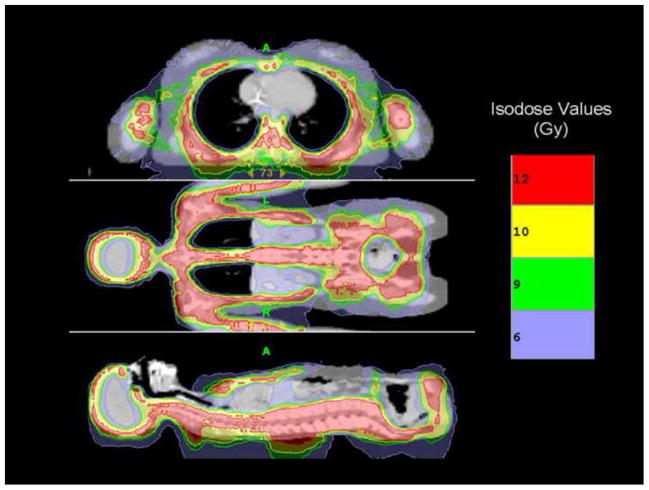
Figure 1 displays a color wash of the first patient treated to 10 Gy. Figure 2 displays DVHs for major critical organs for the same patient, demonstrating substantial dose sparing relative to the GTV. Figure 3 shows the median (D50) doses averaged for each dose level for all major organs. As expected, with escalation of the prescribed GTV dose, median doses for all organs increased, but still remained below that seen for standard TBI. At our institution standard TBI is delivered through AP-PA fields to 12 or 13.2 Gy with 50% lung transmission blocks and electron boost of 6 Gy to the underlying rib cage, resulting in a median lung dose of approximately 9 Gy (7).
Figure 1.
Color wash demonstrating dose distribution of the first patient treated with targeted TBI using Tomotherapy. The target structure is skeletal bone. Reprinted with permission from Wong JYC, Liu A, Schultheiss T et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: An alternative to standard total body irradiation. Biol Blood Marrow Transplant 2006; 12: 306–315 with permission from American Society for Blood and Marrow Transplantation.
Figure 2.
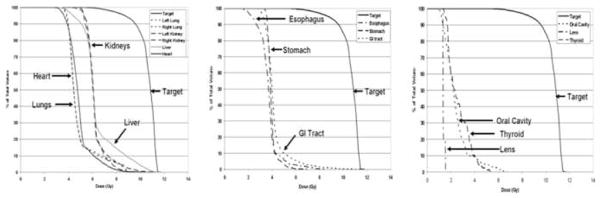
Major organ and target (GTV) DVH curves of first MM patient treated with TMI.
Figure 3.
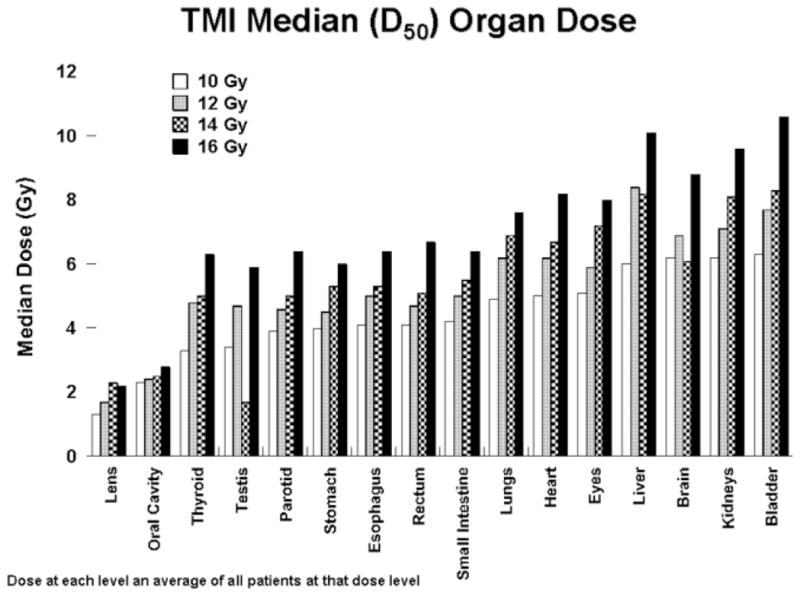
Median (D50) organ doses for each dose level.
The relative degree of organ dose sparing seen with each patient was similar despite differences in patient size and body habitus. Table 1 shows the average and range of D50 organ doses for all 13 patients with organ doses normalized to a GTV dose of 12 Gy for comparison purposes. The D50 doses to lens and oral cavity were < 25% of the prescribed GTV dose; to lungs, heart, GI tract and thyroid approximately 35–50%; and kidneys, liver, brain, bladder, and eyes approximately 50–65%.
Table 1.
D50 organ doses (Gy) for 13 MM patients treated with TMI (doses normalized assuming a GTV dose of 12 Gy) and for 8 patients treated with TMLI to 12 Gy.
| Organ | TMI Mean D50 (n = 13) |
TMI Range D50 |
TMLI Mean D50 (n = 8) |
TMLI Range D50 |
|---|---|---|---|---|
| Lens | 1.7 | 1.4 – 3.1 | 2.5 | 1.3 – 3.0 |
| Oral Cavity | 2.3 | 1.6 – 2.9 | 3.0 | 2.8 – 3.2 |
| Thyroid | 4.4 | 2.8 – 5.5 | 5.2 | 4.6 – 6.4 |
| Parotids | 4.6 | 3.7 – 5.0 | 5.3 | 4.4 – 5.6 |
| Stomach | 4.6 | 4.0 – 5.2 | 4.5 | 4.0 – 5.0 |
| Rectum | 4.7 | 4.2 – 5.5 | 4.8 | 4.3 – 5.6 |
| Esophagus | 4.8 | 4.3 – 5.6 | 4.9 | 4.4 – 5.4 |
| Small Intestine | 4.8 | 4.5 – 5.3 | 4.8 | 4.6 – 5.2 |
| Lungs | 5.9 | 5.3 – 6.7 | 5.7 | 4.9 – 6.8 |
| Eyes | 6.0 | 5.3 – 6.5 | 6.2 | 5.8 – 6.9 |
| Heart | 6.0 | 5.6 – 6.6 | 6.2 | 6.0 – 6.8 |
| Brain | 6.4 | 4.7 – 8.8 | 5.2 | 4.1 – 6.2 |
| Testes | 6.5 | 5.8 – 7.3 | 6.6 | 5.0 – 9.5 |
| Kidneys | 7.2 | 6.7 – 7.7 | 6.7 | 6.0 – 7.4 |
| Bladder | 7.5 | 6.8 – 8.4 | 7.5 | 6.8 – 8.2 |
| Liver | 7.5 | 6.6 – 9.7 | 9.2 | 7.8 – 11.2 |
The observed acute toxicities appeared to be consistent with the reduced organ doses predicted from treatment plans (Table 2). No grade 4 toxicities were observed. Grade 3 toxicities were infrequent with two patients reporting fatigue and one anorexia. All patients reported grade 1–2 nausea, but with 6 experiencing no vomiting, 8 no diarrhea, 9 no mucositis, and 12 no erythema. All patients successfully engrafted with a median time to independence from platelet transfusions of 8 days (range 6–11) and a median time to absolute neutrophil count of ≥ 1000/ul of 10 days (range 9–12).
Table 2.
Acute toxicities in 13 MM patients treated with TMI.
| Age (years) | 54 | 42 | 49 | 54 | 66 | 53 | 56 | 54 | 61 | 52 | 60 | 49 | 62 |
| Gender | F | M | M | F | M | M | F | M | F | F | M | F | M |
|
| |||||||||||||
| TMI Dose (Gy) | 10 | 10 | 10 | 12 | 12 | 12 | 12* | 14 | 14 | 14 | 16 | 16 | 16 |
|
| |||||||||||||
| Nausea | 2@ | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 |
| Vomiting | 1 | 2 | 1 | 1 | 2 | 2 | 1 | ||||||
| Anorexia | 3 | ||||||||||||
| Fatigue | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | ||||
| Erythema | 2 | ||||||||||||
| Diarrhea | 1 | 1 | 1 | 1 | 2 | ||||||||
| Esophagitis | 1 | ||||||||||||
| Oral Mucositis | 2 | 2 | 2 | 1 | |||||||||
12 Gy delivered over 7 days
Toxicities graded using NCI Common Toxicity Criteria version 3
With a median follow-up of 13 months (range 5–25), 5 patients remain in complete remission (the longest 25 months), 1 in very good partial remission, 4 in partial remission, and 2 with stable disease. One patient who received 10 Gy progressed at 9 months and died of disease at 13 months. Eight remain on maintenance thalidomide and decadron. Although follow-up is limited, radiation pneumonitis was not observed.
Pilot allogeneic HCT trial with TMLI combined with the reduced intensity chemotherapy regimen of fludarabine and melphalan
Eight patients have been treated on this trial, 5 with a diagnosis of AML, 1 ALL, 1 MM, and 1 NHL Two were in first remission, 1 in first relapse, 4 were induction failures and 1 had progressive disease. Median age was 52 years (range 24–62). Three were 3 male and 5 female. Four received hematopoietic stem cells from an HLA identical sibling donor and 4 from a matched unrelated donor. No patient had received previous radiotherapy.
Median organ doses with TMLI were comparable to those seen with TMI on the MM trial (Table 1), with the exception of slightly higher doses to parotids and thyroid due lymph nodes included in the GTV. Acute toxicities were primarily grade 2 to 3. Grade 3 nausea, vomiting, mucositis, and diarrhea occurred in 6, 2, 6, and 3 patients, respectively. No grade 4 non-hematologic acute toxicities were observed. All patients engrafted successfully with median days to absolute neutrophil count ≥ 1000/ul of 15 days (range 11–21) and platelet count ≥ 20,000/ul of 15 days (range 10–18). With a median follow-up of 4.6 months (range 1.6–12.0) 7 of 8 are alive and remain in remission, the longest out 12 months. To date 3 patients have had bone marrow biopsies at day 100 demonstrating no evidence of disease and 100% donor chimerism. One patient died of influenza type A pulmonary infection. Acute GVHD occurred in 6 patients.
DISCUSSION
TBI plays an important role in patients undergoing HCT to eradicate malignant cells and, in patients undergoing allogeneic HCT, as a means of immunosuppression to prevent rejection of donor hematopoietic cells. TBI has distinct advantages over chemotherapy. Unlike chemotherapy, delivery of radiation therapy to target sites is not dependent on blood supply, not influenced by inter-patient variability of drug absorption, metabolism, biodistribution, or clearance kinetics, and can reach potential sanctuary sites, such as testes and brain. Some randomized studies have demonstrated an advantage in relapse rate, disease free survival, and overall survival with TBI-containing over non-TBI containing high dose conditioning regimens, particularly in acute leukemias (1–4).
Several groups have attempted to escalate TBI doses in an effort to improve outcomes. The group from Fred Hutchinson Cancer Center carried out two randomized trials comparing cytoxan combined with 12 Gy at 2 Gy/day or 15.75 Gy at 2.25 Gy/day. In a trial of 116 patients with CML in chronic phase, the higher dose resulted in a significantly lower relapse rate (0% versus 25% p = 0.008), but higher treatment related mortality rate (24% 12 Gy and 34% 15.75 Gy, p = 0.13), and as a result no significant change in overall survival (5). In a separate trial of 71 patients with AML in first remission, relapse rate was also decreased with the higher dose (14% versus 39% p = 0.06), but these gains were offset by an increase in nonrelapse mortality (38% versus 19%, p = 0.05), resulting in no difference in overall survival between the two arms (6). Therefore escalation of TBI dose has been challenging. Gains in disease control are associated with an increase in regimen related toxicities and nonrelapse mortality, resulting in no improvement in overall survival. New, more targeted strategies are clearly needed to allow further dose escalation without associated increase in short and long term side effects.
Biologically guided systemic radiotherapy, using radiolabeled antibodies (11) or bone seeking radiopharmaceuticals such as 166Ho-DOTMP (12), are currently undergoing evaluation as part of HCT conditioning regimens. A limitation with these approaches is the inability to completely control the radiation dose to tumor or normal organ for a given patient. Dose distribution is dictated by multiple factors, such as tumor size, tumor burden, antigen expression, tumor microenvironment, clearance kinetics, and in vivo stability of the agent. These factors can lead to low, unpredictable doses to a given tumor or higher than expected doses to critical organs. Tumor doses using biologically guided systems have in general been less than that achievable by external beam radiotherapy (13).
Recent technological advances in the delivery of external beam radiotherapy now allow for the delivery of a more targeted form of TBI. Helical tomotherapy (HT) (commercially available as the Tomotherapy HiArt System®) integrates CT image-guided radiotherapy and intensity modulated radiation therapy in a single device. Specifically, a 6 MV linear accelerator is mounted on a CT ring gantry and rotates around the patient as the patient translates through the ring. The field is shaped by a binary MLC. The maximum target size possible is approximately 60 cm in width by approximately 160 cm in length. HT therefore allows for the delivery of highly conforming dose distributions to large complex target shapes while simultaneously reducing dose to critical normal organs (14) making it suited for the delivery of conformal targeted TBI.
Results from the two trials reported demonstrate the clinical utility of using HT to deliver targeted TBI. Median organ doses were approximately 15–65% of the prescribed GTV dose. With dose escalation to 16 Gy, median organ doses still remained below that for standard TBI to 12 Gy, which predicted for reduced acute toxicities compared to TBI. Dose escalation continues on this phase I trial.
Acute toxicities observed on the tandem autologous transplant MM trial were modest and compare favorably to results from a phase I tandem autologous HCT trial of similar design reported by Zaucha et al. (15). In this study patients with multiple myeloma or breast cancers or sarcomas with primarily bone metastases were first treated with busulfan, melphalan, and thiotepa followed a median of 105 days later by modified TBI to 12, 13.5 or 15 Gy using 5% transmission blocks over the lungs and liver. Grade 1–2 mucositis was seen in 100% and diarrhea in 34% of patients using the Bearman toxicity scale (16), which is comparable to grade 2–3 toxicity by NCI CTC. Death from pulmonary toxicity was observed in 3 of 17 patients treated at 15 Gy.
Acute toxicities observed with the combination of TMLI, fludarabine and melphalan were also acceptable, with grade 2–3 and no grade 4 toxicities observed. This compares favorably to a trial by Petropoulos et al. (17) who evaluated 9 Gy TBI (3 Gy QD) combined with fludarabine (120 mg/m2) and melphalan (140 mg/m2), which although tolerated in a pediatric population, was not tolerated in adults due to mucositis, forcing study closure.
Interstitial pneumonitis is an associated toxicity with TBI. Lung D80, D50 and D10 doses are shown in Table 3 for the 13 MM patients on the Phase I MM dose escalation trial. For comparison, average lung D80, D50 and D10 values are shown for standard TBI plans to 12 Gy, which were generated using previously published methods (8) and utilized planning CT data sets from the first 3 patients. Further dose escalation above 16 Gy may be associated with pneumonitis risks comparable to 12 Gy TBI. Although the D50 for 16 Gy TMI is less than that for 12 Gy TBI, D80 values are comparable. We are currently evaluating methods to further reduce lung dose on the Tomotherapy Hi-Art system as we continue to dose escalate.
Table 3.
Lung D80, D50 and D10 for 13 MM patients on the TMI dose escalation trial. Values are an average for patients at each dose level. Lung D80, D50 and D10 for standard TBI 12 Gy are shown for comparison.
| TBI 12 Gy | TMI 10 Gy | TMI 12 Gy | TMI 14 Gy | TMI 16 Gy | |
|---|---|---|---|---|---|
| D80 | 7.0 | 4.5 | 5.6 | 6.4 | 6.9 |
| D50 | 9.4 | 4.9 | 6.2 | 6.9 | 7.6 |
| D10 | 12.3 | 6.8 | 8.4 | 9.4 | 10.9 |
Several questions arise using this approach. For example, does organ sparing also spare cancer cells or reduce the immunosuppression needed for allogeneic HCT? Organ sparing appears at this time to not be an adverse factor, but this will eventually have to be substantiated through clinical trials. However, it is important to note that most avoidance structures received approximately 35–65% of the target dose and similar to a shrinking field concept, areas with lower tumor burden may require lower dose than those areas with the higher tumor burden, such as bone marrow. In addition, if a dose escalation strategy is pursued the maximum tolerated dose will likely to be at a level where most major organs receive a dose comparable to standard TBI. The end result would be a dose distribution similar to standard TBI with concomitant boost to areas of greatest tumor burden. Chemotherapy, which is often part of the conditioning regimen, adds to the therapeutic effect in avoidance areas. Finally, to date there is no evidence of compromised immunosuppression with all patients having successfully engrafted and 100% donor chimerism documented in all 3 patients who have had follow-up day 100 bone marrow biopsies.
The dose-rate used with this technique (approximately 400 cGy/min) is higher than that used with standard TBI (approximately 5–30 cGy/hr) which may have greater effects on normal tissues. However, the available data show that higher dose-rates may not be as significant a factor. We have seen no evidence of graft failure at this dose-rate. The reduced acute toxicities observed suggest that reduction of dose and volume of organ receiving a given dose probably outweigh any dose-rate effects. Dose-rate effects appear to be clinically relevant only if less than 5 – 10 cGy/hr (18) (19). Finally, dose-rate effects are probably outweighed by other factors such as fractionation, which was used in this trial. Sampath et al. (20) in a multivariate logistic regression analysis of TBI in 1090 patients from 20 published studies found an inverse correlation with interstitial pneumonitis incidence and fractionation, but no dose-rate effect. A preclinical study by Tarbell et al. (21) reported a higher radiation related mortality rate in mice receiving single fraction TBI at high dose-rate (80 cGy/minute) compared to single fraction TBI at low dose-rate (5 cGy/min). Dose-rate effects were not seen with BID or TID fractionation schedules, suggesting that fractionation was a greater factor in normal organ sparing than dose-rate effects.
TMI and TMLI using HT, with its potential to reduce short and long term toxicities compared to conventional TBI approaches, has the potential to improve outcomes and to broaden the scope of how TBI is used. Regimens utilizing standard TBI are difficult to administer to older patients or those with co-morbidities, with many centers excluding patients over 50 years of age from these regimens. Reduced intensity chemotherapy alone regimens, such as fludarabine/melphalan, are better tolerated but may not provide the needed tumoricidal activity in patients with more aggressive high-risk disease. For this reason, groups have attempted to combine TBI with these reduced intensity regimens, which has been challenging due to associated toxicities (17). Results from this study demonstrate the feasibility of combining TMLI with an established reduced intensity chemotherapy regimen, with patients over 60 years of age tolerating the regimen.
These results also demonstrate the feasibility of combining 12 Gy targeted TMLI with high dose melphalan (140 mg/m2). A previous randomized Phase III trial demonstrated no advantage and increased toxicity with 8 Gy TBI (2 Gy QD) and melphalan (140 mg/m2) compared to melphalan alone (200 mg/m2) in patients with MM (22). Using TMLI now provides an opportunity to re-explore the combination of melphalan and TBI at higher doses with acceptable toxicities.
Attempts to decrease relapse rates and improve outcomes with TMI or TMLI containing regimens through dose escalation may now be possible and should be explored in clinical trials. Although the maximum tolerated doses of targeted TBI with HT remains to be defined for each clinical regimen and setting, even modest increases in TBI doses to approximately 15.75 Gy have resulted in statistically significant decreases in relapse rates in AML (6). Selective conformal boost doses to areas of greater tumor burden with simultaneous TMI or conformal dose avoidance to areas previously irradiated may also be possible with this approach.
In summary, TMI/TMLI using HT is feasible and demonstrates promise as a way to deliver TBI with reduced toxicity, without compromising dose to areas of greatest tumor burden. Reduced doses to major normal organs were predicted from treatment plans. The acute toxicities observed are consistent with these dose predictions and compare favorably to those seen with standard TBI regimens. Initial results are encouraging and warrant further evaluation in clinical trials as a method to dose escalate with acceptable toxicity and to offer TBI containing regimens to patients unable to tolerate standard approaches. Further evaluation is also needed to better characterize long term toxicities and to assess the impact this new approach will have on disease control.
Acknowledgments
Supported by Grant NCI 43904
Footnotes
Presented at the 49th Annual Meeting of the American Society of Therapeutic Radiology and Oncology (ASTRO), Los Angeles, October 28–November 1, 2007.
Conflict of Interest: Jeffrey Y.C. Wong, M.D. has previously received honoraria from TomoTherapy, Inc. for speaking engagements.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blaise D, Maraninchi D, Michallet M, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97:3669–3671. doi: 10.1182/blood.v97.11.3669. [DOI] [PubMed] [Google Scholar]
- 2.Dusenbery KE, Daniels KA, McClure JS, et al. Randomized comparison of cyclophosphamide-total body irradiation versus busulfan-cyclophosphamide conditioning in autologous bone marrow transplantation for acute myeloid leukemia. Int J Radiation Oncology Biol Phys. 1995;31:119–128. doi: 10.1016/0360-3016(94)00335-i. [DOI] [PubMed] [Google Scholar]
- 3.Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: A report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–2730. [PubMed] [Google Scholar]
- 4.Bunin N, Aplenc R, Kamani N, et al. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32:543–548. doi: 10.1038/sj.bmt.1704198. [DOI] [PubMed] [Google Scholar]
- 5.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: A randomized trial of two irradiation regimens. Blood. 1991;77:1660–1665. [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Appelbaum FR, et al. Long-term follow-up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998;92:1455–1456. [PubMed] [Google Scholar]
- 7.Wong JYC, Liu A, Schultheiss T, et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: An alternative to standard total body irradiation. Biol Blood Marrow Transplant. 2006;12:306–315. doi: 10.1016/j.bbmt.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Schultheiss TE, Wong J, Liu A, et al. Image-guided total marrow and total lymphatic irradiation using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1259–1267. doi: 10.1016/j.ijrobp.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 9.Schultheiss T, Liu A, Wong J, et al. Total marrow and total lymphatic irradiation with helical tomotherapy [Abstract] Medical Physics. 2004;31:1845. [Google Scholar]
- 10.Durie BGM, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Pagel JM, Matthews DC, Appelbaum FR, et al. The use of radioimmunoconjugates in stem cell transplantation. Bone Marrow Transplant. 2002;29:807–816. doi: 10.1038/sj.bmt.1703524. [DOI] [PubMed] [Google Scholar]
- 12.Giralt S, Bensinger W, Goodman M, et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102:2684–2691. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
- 13.Wong JYC. Systemic targeted radionuclide therapy: Potential new areas. Int J Radiat Oncol Biol Phys. 2006;66:S74–S82. doi: 10.1016/j.ijrobp.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Seminars in Radiation Oncology. 1999;9:108–117. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- 15.Zaucha RE, Buckner DC, Barnett T, et al. Modified total body irradiation as a planned second high-dose therapy with stem cell infusion for patients with bone-based malignancies. Int J Radiat Oncol Biol Phys. 2006;64:227–234. doi: 10.1016/j.ijrobp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Bearman S, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. Journal of Clinical Oncology. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 17.Petropoulos D, Worth LL, Mullen CA, et al. Total body irradiation, fludarabine, melphalan, and allogeneic hematopoietic stem cell transplantation for advanced pediatric hematologic malignancies. Bone Marrow Transplant. 2006;37:463–467. doi: 10.1038/sj.bmt.1705278. [DOI] [PubMed] [Google Scholar]
- 18.Weiner R, Bortin M, Gale RP, et al. Interstitial pneumonitis after bone marrow transplantation. Ann Intern Med. 1986;104:168–175. doi: 10.7326/0003-4819-104-2-168. [DOI] [PubMed] [Google Scholar]
- 19.Travis EL, Peters LJ, McNeil J, et al. Effect of dose-rate on total body irradiation: Lethality and pathologic findings. Radiotherapy and Oncology. 1985;4:341–351. doi: 10.1016/s0167-8140(85)80122-5. [DOI] [PubMed] [Google Scholar]
- 20.Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis following bone marrow transplant. Int J Radiat Oncol Biol Physics. 2005;63:876–884. doi: 10.1016/j.ijrobp.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 21.Tarbell NJ, Amato DA, Down JD, et al. Fractionation and dose rate effects in mice: a model for bone marrow transplantation in man. Int J Radiat Oncol Biol Phys. 1987;13:1065–1069. doi: 10.1016/0360-3016(87)90046-0. [DOI] [PubMed] [Google Scholar]
- 22.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]