Abstract
Chronic inflammation increases the risk of developing several malignancies, including gastric cancer. A better understanding of how inflammation promotes gastric oncogenesis is therefore urgently needed. We have recently developed and characterized a mouse model that will be useful to elucidate the molecular and cellular circuitries bridging inflammation and gastric cancer.
Keywords: gastric cancer, autoimmune gastritis, inflammation, mouse models, gastric epithelial cells
Gastric cancer is the fourth most common and second leading cause of cancer-related deaths worldwide.1 Most of these deaths are due to adenocarcinomas, which originate from gastric epithelial cells. Chronic gastric inflammation, which is often provoked by Helicobacter pylori persistent infection, increases the risk of developing of gastric cancer, at least in part by altering the growth and differentiation of the gastric epithelium. However, only a small fraction of H. pylori-infected individuals develops gastric cancer. Evidence indicates that genetic variation in both H. pylori and the host alter the likelihood of developing gastric cancer. The genetic factors of H. pylori-infected individuals that are associated with an increased propensity of developing gastric cancer include polymorphisms in genes coding for regulators of the immune response. Thus, a better understanding of how the host immune response influences gastric oncogenesis may favor the development of new strategies to reduce the number of gastric cancer-related deaths.
Autoimmune gastritis also provokes a chronic state of gastric inflammation. Recent studies have demonstrated that individuals with severe autoimmune gastritis exhibit an increased risk of developing multiple neoplasms, including gastric cancer.2,3 To understand how to suppress chronic inflammation in the gastric mucosa, we have been developing a murine model of autoimmune gastritis (TxA23 mice).4-6 In a recent issue of Cancer Research, we have reported that the autoimmune disease affecting TxA23 mice mimics many aspects of the corresponding human condition.7 Importantly, we have demonstrated that the inflammatory state associated with autoimmune gastritis in mice provoked many of the pathological and molecular alterations that accompany the development of gastric cancer in humans. For example, the pathogenesis of autoimmune gastritis in TxA23 mice progresses through a series of steps that includes: chronic inflammation, loss of parietal cells (oxyntic atrophy), mucous neck cell hyperplasia, metaplasia, and eventually severe dysplasia including gastric intraepithelial neoplasms (Fig. 1). This sequence of events is very similar to the cascade of pathological changes that occur in the course of gastric oncogenesis in humans, which is often referred to as “Correa pathway”8. Moreover, the gastric neoplasms that eventually affect TxA23 mice share many molecular features with their human counterparts, including the overexpression of gastric cancer biomarkers, the development of spasmolytic polypeptide-expressing metaplasia (SPEM), and elevated levels of signal transducer and activator of transcription 3 (STAT3) phosphorylation. Finally, as TxA23 mice age, their pathological condition progressed to include severe dysplasia and gastric intraepithelial neoplasms.
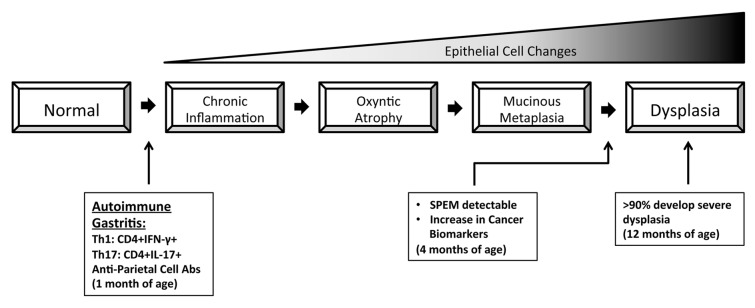
Figure 1. Inflammation-induced gastric carcinogenesis in TxA23 mice mimics several aspects of the corresponding human condition. In TxA23 mice, the source of the chronic inflammatory state that alters the normal gastric epithelium is represented by the self-reactive CD4+ T cells that cause autoimmune gastritis. TxA23 mice not only develop oxyntic atrophy and spasmolytic polypeptide-expressing metaplasia (SPEM), but also express several biomarkers that have been associated with human gastric cancer. Ultimately, by 12 months of age, TxA23 mice exhibit severe dysplasia and gastric intraepithelial neoplasms.
This new mouse model demonstrates that chronic inflammation, even in the absence of H. pylori infection, can drive gastric oncogenesis. Our data support recent findings demonstrating that individuals with severe autoimmune gastritis have an increased risk of developing gastric cancer. Moreover, this mouse model will be useful to identify not only novel host factors that influence gastric oncogenesis, but also new therapeutic interventions that may reduce the risk of developing gastric cancer as they suppress chronic inflammation. We are currently crossing TxA23 mice with animals lacking various cytokine-coding genes, in order to investigate the impact of these soluble mediators on the severity of autoimmune gastritis and the progression of gastric cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25911
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Landgren AM, Landgren O, Gridley G, Dores GM, Linet MS, Morton LM. Autoimmune disease and subsequent risk of developing alimentary tract cancers among 4.5 million US male veterans. Cancer. 2011;117:1163–71. doi: 10.1002/cncr.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Autoimmune disease and subsequent digestive tract cancer by histology. Ann Oncol. 2012;23:927–33. doi: 10.1093/annonc/mdr333. [DOI] [PubMed] [Google Scholar]
- 4.McHugh RS, Shevach EM, Margulies DH, Natarajan K. A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur J Immunol. 2001;31:2094–103. doi: 10.1002/1521-4141(200107)31:7<2094::AID-IMMU2094>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 5.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–93. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TL, Sullivan NL, Ebel M, Teague RM, DiPaolo RJ. Antigen-specific TGF-β-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol. 2011;187:1745–53. doi: 10.4049/jimmunol.1004112. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TL, Khurana SS, Bellone CJ, Capoccia BJ, Sagartz JE, Kesman RA, Jr., Mills JC, DiPaolo RJ. Autoimmune gastritis mediated by CD4+ T cells promotes the development of gastric cancer. Cancer Res. 2013;73:2117–26. doi: 10.1158/0008-5472.CAN-12-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. [PubMed] [Google Scholar]


