Abstract
The results of a recently completed Phase III clinical trial suggest that concurrent chemoradiotherapy followed by tecemotide provides superior benefits to Stage IIIa and IIIb non-small cell lung carcinoma patients as compared with sequential chemoradiotherapy followed by tecemotide. These clinical observations will be dissected in a transgenic model of lung cancer that we have recently established (hMUC1.Tg C57BL/6 mice).
Keywords: anticancer vaccines, L-BLP25, mucin 1, non-small cell lung carcinoma
Introduction
Tecemotide (formerly known as L-BLP25 or Stimuvax®) is an active immunotherapeutic agent targeting a cell surface glycoprotein, mucin 1 (MUC1). MUC1 is aberrantly expressed (often in an under- or unglycosylated form) by various epithelial cancers, including non-small cell lung carcinoma (NSCLC), breast carcinoma, and prostate cancer.1 In malignant cells, underglycosylated MUC1 is more efficiently processed into peptides and loaded onto MHC molecules than its normally glycosylated counterpart.2 This yields a novel and large epitope repertoire bound to MHC molecules and presented on the surface of neoplastic cells that can be recognized by MUC1-specific cytotoxic T lymphocytes (CTLs). The uptake of tecemotide by antigen-presenting cells (APCs) leads to MUC1 presentation on MHC class I and class II molecules, thus eliciting a TH1 immune response that results in the induction of MUC1-specific CTLs and interferon γ (IFNγ) secretion.3
Tecemotide is currently under development as a therapeutic intervention against a variety of cancers. As of July 2013, Merck KGaA had initiated 14 clinical trials based on this agent: eight for the immunotherapy of NSCLC, two for the immunotherapy of prostate cancer, one for immunomonitoring multiple myeloma, one for immunomonitoring rectal cancer, one safety follow-up study, and one enrolling breast carcinoma patients. In a Phase I clinical trial, tecemotide was shown to be safe and well tolerated among advanced NSCLC patients.4 In this setting, no tumor-specific antibodies or antitumor responses were observed even though 5 out of 12 patients developed CTLs. In a randomized Phase IIb trial testing tecemotide in advanced NSCLC patients, increased survival was reported in a subgroup of patients with loco-regional Stage III disease.5 Moreover, some advanced prostate cancer patients have been reported to manifest stable or decreased prostate-specific antigen (PSA) levels and/or a prolongation of PSA doubling time in response to tecemotide.6
Recently, the results of the global Phase III START clinical trial testing tecemotide in patients with Stage III NSCLC has become available. While the primary objective of overall survival (OS) prolongation in the target population was not met, a clinically meaningful increase in OS was observed among a pre-defined subgroup of patients previously treated with concurrent chemoradiotherapy (CRT).7 In contrast, patients that had previously been treated with sequential CRT obtained no clinical benefits from tecemotide. Tecemotide was generally well tolerated, with no safety concerns identified and no emergent evidence of immunological adverse events. The biological rationale for such a difference in the response to tecemotide of NSCLC patients previously receiving concurrent as opposed to sequential CRT remains unclear. Preclinical animal models may help in the identification of the causal factors underlying these observations.
Development of Preclinical Animal Models
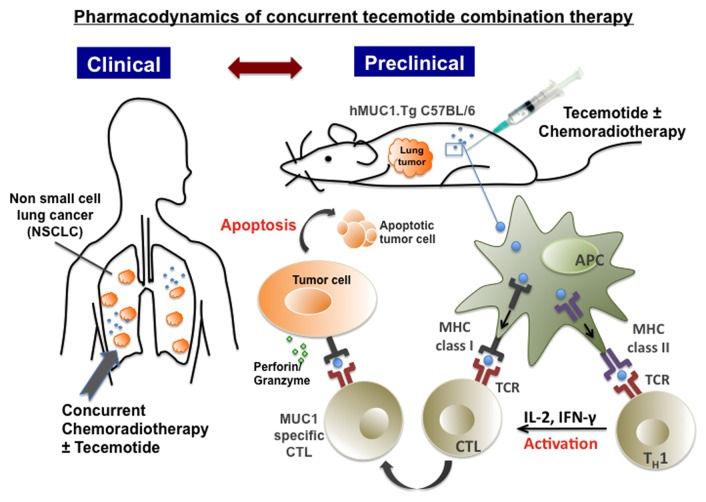
Well-designed preclinical studies can lead to successful clinical trials, and an immunocompetent animal model that accurately reproduces the microenvironment of human neoplasms would surely increase the translational potential of this approach.8 In order to accurately evaluate the effects of tecemotide in a preclinical setting, we have established a model of carcinogen-induced lung cancer in C57BL/6 mice expressing human MUC1 (hMUC1.Tg C57BL/6 mice).9 By administering 10 weekly doses of urethane (0.75 mg/g), 100% of hMUC1.Tg mice develop lung adenomas of variable size and phenotype.9 These neoplastic foci actually resemble human atypical adenomatous foci and bronchioloalveolar carcinomas. In the course of carcinogenesis, no TH1 or TH2 cytokine responses can be detected in these animals, but the levels of pro-inflammatory cytokines increase with distinctive kinetics. Conversely, 24 h after the second of 2 cycles of tecemotide, the levels of various TH1 cytokines including interleukin (IL)-2, IL-12 and IFNγ rise significantly, while those of TH2 and pro-inflammatory cytokines are elevated to a lesser extent. In addition, the administration of a second cycle of tecemotide in the course of tumor progression leads to a significant reduction in the number of tumor foci. These observations suggest that the hMUC1.Tg mouse model may be useful for determining the most efficient combinatorial immunotherapy to be tested in clinical trials (Fig. 1).
Figure 1. Pharmacodynamics of tecemotide-based combination therapy. Recent results from the global Phase III START clinical trial point to a schedule-dependent activity of tecemotide upon combination with chemoradiotherapy. These observations will be used to define efficient combination therapies (for evaluation in future clinical trials) in a carcinogen-driven model of lung cancer established in immunocompetent C57BL/6 mice expressing human mucin 1 (MUC1).
Future Directions
NCSLC is minimally responsive to conventional treatments and represents a difficult target for immunotherapy. However, in view of the results of the Phase III START trial, it appears premature to think that targeting a single tumor-associated antigen (TAA) is an invalid approach against NSCLC.10 In this context, it is essential to monitor the immune response of cancer patients receiving immunotherapy over time and identify parameters that correlate with survival. For instance, it may be worthwhile to investigate an indicator of antigen-specific immune responses, such as the circulating levels of IFNγ, 24–48 h post-treatment, to ensure that a given patient is at least exhibiting an immunological response.
In most cases, conducting elaborate animal studies that translate to human trials are prohibitive in terms of costs, especially in the absence of an industrial/academic alliance. Further, it is common to encounter “Yes, but…” critiques throughout all stages of drug development. For example, a recent commentary entitled “Victories and Deceptions in Tumor Immunology: Stimuvax®,”10 Kroemer et al. considered the fact that tecemotide failed to increase overall survival in the Phase III START trial as a deception. The results of this study are not considered a failure anymore, but rather a guide to understanding the interaction between antigen-specific immunotherapy and prior CRT. In the START trial, subset analyses suggested that concurrent CRT followed by tecemotide therapy is clinically superior to sequential CRT followed by tecemotide. These clinical observations offer an opportunity for the preclinical assessment of the mode of action of tecemotide and the identification of the most appropriate tecemotide-based combinatorial immunotherapy in a variety of tumor models. Our future preclinical studies in mouse tumor models will be based on these data. Specifically, we will study in detail administration schedules (concurrent vs. sequential) and investigate the effects of various combinations of chemotherapeutic agents with radiotherapy on the immune response to tecemotide.
Citation: Kao C, Wurz G, Schroder A, Wolf M, DeGregorio M. Clarifying the pharmacodynamics of concurrent Tecemotide (L-BLP25) combination therapy. OncoImmunology 2013; 2:e26285; 10.4161/onci.26285
Disclosure of Potential Conflicts of Interest
CJK and GTW declare no competing interests. MWD is the Principal Investigator of a grant from Merck KGaA. AS, and MW are employees of Merck KGaA.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/26285
References
- 1.Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, Dahiya R, Kim YS. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–51. [PubMed] [Google Scholar]
- 2.Hiltbold EM, Alter MD, Ciborowski P, Finn OJ. Presentation of MUC1 tumor antigen by class I MHC and CTL function correlate with the glycosylation state of the protein taken Up by dendritic cells. Cell Immunol. 1999;194:143–9. doi: 10.1006/cimm.1999.1512. [DOI] [PubMed] [Google Scholar]
- 3.Mehta NR, Wurz GT, Burich RA, Greenberg BE, Griffey S, Gutierrez A, Bell KE, McCall JL, Wolf M, DeGregorio M. L-BLP25 vaccine plus letrozole induces a TH1 immune response and has additive antitumor activity in MUC1-expressing mammary tumors in mice. Clin Cancer Res. 2012;18:2861–71. doi: 10.1158/1078-0432.CCR-12-0168. [DOI] [PubMed] [Google Scholar]
- 4.Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, Kehoe M, MacLean G, Longenecker M. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer. 2001;3:49–57, discussion 58. doi: 10.3816/CLC.2001.n.018. [DOI] [PubMed] [Google Scholar]
- 5.Butts C, Maksymiuk A, Goss G, Soulières D, Marshall E, Cormier Y, Ellis PM, Price A, Sawhney R, Beier F, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137:1337–42. doi: 10.1007/s00432-011-1003-3. [DOI] [PubMed] [Google Scholar]
- 6.North SA, Graham K, Bodnar D, Venner P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J Urol. 2006;176:91–5. doi: 10.1016/S0022-5347(06)00494-0. [DOI] [PubMed] [Google Scholar]
- 7.Butts CA, Socinski MA, Mitchell P, Thatcher N, Havel L, Krzakowski MJ, et al. START: A phase III study of L-BLP25 cancer immunotherapy for unresectable stage III non-small cell lung cancer. 2013 ASCO annual meeting: J Clin Oncol, 2013. [Google Scholar]
- 8.Degregorio M, Degregorio M, Wurz GT, Wurz GT, Gutierrez A, Gutierrez A, Wolf M. L-BLP25 vaccine plus letrozole for breast cancer: Is translation possible? Oncoimmunology. 2012;1:1422–4. doi: 10.4161/onci.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurz GT, Gutierrez AM, Greenberg BE, Vang DP, Griffey SM, Kao CJ, Wolf M, Degregorio MW. Antitumor effects of L-BLP25 Antigen-Specific tumor immunotherapy in a novel human MUC1 transgenic lung cancer mouse model. J Transl Med. 2013;11:64. doi: 10.1186/1479-5876-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroemer G, Zitvogel L, Galluzzi L. Victories and deceptions in tumor immunology: Stimuvax(®) Oncoimmunology. 2013;2:e23687. doi: 10.4161/onci.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]