Abstract
BACKGROUND
Preoperative brain injury is common in neonates with complex congenital heart disease. Increasing evidence suggests a complex interaction of prenatal and postnatal risk factors for development of brain white matter injury called periventricular leukomalacia (PVL) in neonates with complex congenital heart disease. To date, there remains a limited understanding of the risk factors contributing to preoperative PVL in hypoplastic left heart syndrome (HLHS).
METHODS
Neonates with HLHS or HLHS variants from three prospective MRI studies (2003–2010) were selected for this cohort. A preoperative brain MRI was performed the morning of the surgery. Stepwise multilogistic regression of patient characteristics, mode of delivery (cesarean section vs. vaginal), time of diagnosis (prenatal vs. postnatal), HLHS subtypes, brain total maturation score (TMS), time to surgery, individual averaged daily preoperative blood gases and CBC values was used to determine significant associations.
RESULTS
A total of 57 neonates with HLHS were born at 38.7 ± 2.3 weeks, 86% (49/57) had a prenatal diagnosis with 31% (18/57) delivered by cesarean section. HLHS with aortic atresia (AA) was common in this cohort, (41/57, 71%). Preoperative PVL was identified in 19% (11/57). Male patients with aortic atresia (p=0.004) were at higher risk for PVL. Lower total brain maturation score was also identified as a strong predictor for preoperative PVL (p=0.005).
CONCLUSIONS
In neonates with HLHS, non-modifiable patient related factors including male gender with aortic atresia (lack of antegrade blood flow) and lower total brain maturation score placed neonates at the greatest risk for preoperative white matter injury.
BACKGROUND
Advances in the medical and surgical management for children with complex congenital heart disease (CHD) has led to increased survival along with an increased recognition of the associated morbidity. Learning disabilities, attention deficit disorder, speech and motor problems are increasingly recognized among the spectrum of developmental problems in school-aged survivors of heart surgery in infancy.(1–4) It is estimated that 50% of these patients are affected by neurodevelopmental issues that have a significant impact on their academic achievement and quality of life.(5)
Neonates with hypoplastic left heart syndrome (HLHS) and d-transposition of the great arteries (d-TGA) have been the most widely studied. Brain white matter injury (WMI) in the form of periventricular leukomalacia (PVL) has been recognized as the most prevalent brain injury in neonates following surgery for these complex defects.(6) Several studies have demonstrated that in neonatal cohorts with various types of complex CHD, 17 to 40% had evidence of PVL on preoperative MRI (7, 8) while almost 50% had evidence of PVL on postoperative MRI.(9) WMI or PVL has been associated with neurodevelopmental problems and impaired functional outcomes in very low birth weight and preterm neonates. (10) In neonates with various types of complex CHD lesions, lower basal cerebral blood flow CBF (8), brain immaturity (9) and preoperative instrumentation (11) have been identified as risk factors for PVL. It is possible, however that each heart lesion carries its own risk for preoperative PVL based on differences in genetics and underlying cardiac physiology. We have recently published findings suggesting that neonates with d-TGA were at increased risk for preoperative PVL compared to other heart lesions, with a prevalence of PVL of 38% (10/26 patients studied).(12) In that study, analysis of risks for PVL demonstrated that increased time to surgery and lower average daily PaO2 were specific risks for preoperative PVL in neonates with d-TGA. This study also suggested that further investigations should be directed at evaluating CHD lesion specific risk factors for preoperative PVL, rather than looking for factors in a heterogeneous congenital heart disease group. Using retrospective review of a prospectively acquired database on brain injury in CHD, this study investigates the risk factors that contribute to preoperative periventricular leukomalacia (PVL) in neonates with HLHS.
METHODS
Patient Population
Patients with HLHS or HLHS variant (double outlet right ventricle with mitral atresia) born between January 1, 2003 and December 31, 2009 were considered for inclusion in this study. Patients were included from three prospective research protocols studying the incidence of preoperative brain injury using MRI in neonates with complex congenital heart disease.(8, 13) Neonates with multiple forms of complex congenital heart disease were recruited from 2003–2005 while neonates with only d-TGA or HLHS were recruited from 2006–2009. Other than type of CHD, inclusion criteria were identical and included term gestational age (40 ± 4 weeks), an intention to undergo surgical intervention with cardiopulmonary bypass (CPB) with or without deep hypothermic circulatory arrest (DHCA), and medical stability for 24 hours prior to surgery. Infants were excluded if there was a history of birth asphyxia (5-minute Apgar score of ≤ 5 or a cord pH of <7.0), preoperative seizures, signs of end-organ damage, preoperative cardiac arrest, or need for ECMO either preoperatively or postoperatively. Only patients with HLHS (n =57) from the larger cohort of 124 were included in the current study. The Investigational Review Board of The Children's Hospital of Philadelphia approved the study protocol. Informed consent was obtained from the parent or guardian.
Study Protocol
All patients were prepared for surgery following our previously published standard clinical protocols. (8, 13) Briefly, on the morning of surgery, neonates were brought to the operating room by the cardiac anesthesia team to induce anesthesia, perform endotracheal intubation, and secure vascular access. Patients were then transported to the MRI suite. Heart rate, blood pressure, electrocardiogram, SpO2 and end tidal CO2 measurements were monitored throughout transport and during the performance of the MRI.
Brain MRI
All MRI scans were performed on Siemens (Siemens Erlangen, Germany) scanners. Pre-operative brain MRI scans performed before January 1, 2005 (n = 20) were performed on a 1.5T Sonata. MRI scans performed between 2005 and October 2008 (n = 29) were completed on a 3T Trio scanner, while MRIs acquired after this date were performed on a 1.5T Avanto (n = 8). The variation in MR scanners that occurred during the study period was due, in part, to changes in imaging technology that became available. The most recent switch back to a 1.5T scanner was due the recent placement of the 1.5T Avanto on the same floor as the CICU and operating rooms, thus substantially reducing patient transport risk. PVL was diagnosed based on its appearance as hyperintense lesions on T1 sequences. Slice thickness on T1 ranged from 3mm (1.5T Sonata) to 0.9mm (3T Trio) with no gaps between slices regardless of scanner. All neonates underwent preoperative brain MRI scanning immediately prior to surgery for the stage I palliation procedure. MRI study protocols are described in detail previously.(8, 12, 13)
A neuroradiologist (AV), blinded to the patients' clinical history and gestational age, reviewed all brain MRI images for congenital and acquired abnormalities. Focal acquired abnormalities included subdural hemorrhage, choroid plexus hemorrhage, arterial ischemic stroke (AIS) and/or PVL. AIS was defined as a focal area of diffusion restriction in an arterial territory involving cortex. PVL was defined as a punctate periventricular white matter lesions associated with T1 hyperintensity with or without restriction of water diffusion on diffusion-weighted imaging. PVL was graded with a published 4-point scale ranging from none to severe (McQuillen, Licht). Further, PVL lesions were manually segmented using ITK-SNAP (www.itksnap.org), which has excellent intra- and interoperator reliability for measuring regional brain volumes. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability.(14) PVL volumes were expressed in mm3.
Total maturation score (TMS) was available on 37/57 (65%) neonates. TMS is an observational scale developed and validated by Childs. (15) TMS uses standard T1 and T2 MR imaging to grade 4 aspects of brain development: 1. Cortical folding complexity, 2. Myelination progress, 3. Presence and number of migrating glial bands in the frontal white matter, and 4. The presence and location of germinal matrix. For this study, TMS was determined by two investigators (AV and DJL) according to methods published previously.(13) Image quality for earlier MRI scans (2003 to January 2005) made TMS evaluations unreliable and they were not included for this study.
Data Preparation and Analysis
Data Preparation
For each subject, summary measures were computed for all preoperative arterial and venous blood gases and combined readings. For example, a mean daily preoperative blood gas oxygen saturation was computed for each subject using arterial blood gas samples only, while mean daily PCO2 values were based on both venous blood gas and arterial blood gas.
Exploratory Analysis
The analysis plan called for using multiple logistic regression models to predict presence of PVL based on combinations of a list of selected variables including: gender, gestational age, birthweight, head circumference, prenatal versus postnatal diagnosis, subtype of HLHS (aortic atresia (AA) vs aortic stenosis (AS)), mode of delivery (cesarean section versus vaginal delivery), time to surgery, hemoglobin levels, average daily arterial and venous blood gas parameters (pH, PaCO2, PaO2, O2 saturations) and interactions among them. To search for an optimal model over the set of all models we employed both forward and backward selection, applying both AIC and BIC selection criteria. Additional models were fit for a PVL Severity Score (0 = no PVL to 3 = severe PVL, as judged by AV), and PVL volume in cm, from the images.
Results
Patients with HLHS (n=55) or HLHS variants (n=2) were included for the analysis. HLHS variants had double outlet right ventricle with mitral atresia and a diminutive arch. A summary of the demographics is included in Table 1. The three study cohorts were from different time periods with MRIs performed on different scanners and with different magnet strengths; 3T vs 1.5T. However, as table 1 demonstrates there were no statistical differences observed in patient characteristics or prevalence of PVL between the 3 studies. Most patients were prenatally diagnosed 49/57 (86%); the percent prenatally diagnosed increased with study era (Table 1). Cesarean section was the mode of delivery in 18/57 (31%). The mean gestational age at the time of delivery was 38.7 ± 2.3 weeks and the mean birth weight was 3.2 ± 0.6 kg. HLHS with aortic atresia (AA) was common in this cohort (41/57,71%). Preoperative PVL was identified in 11/57 (19%) of the HLHS neonates with a median PVL volume of 115.2 mm2 (range 20.2–647.3). Of the 11 neonates with PVL, 6 neonates had PVL severity scores of 1, 5 neonates had PVL severity scores of 2 and none had a PVL score of 3. HLHS neonates with available TMS (n=37) had a mean TMS score of 9.69 +/−0.95. Small AIS was seen in 4/57 (7%, Figure 1.) and all AIS involved the right hemisphere; one patient had multifocal AIS and there was hemorrhagic transformation in two patients (Figure 1). No large (ie AIS involving one third of an arterial territory or larger) AIS was seen. Because of the small numbers of patients with AIS, no further analysis was performed.
Table I.
Demographics of the entire cohort subdivided by study period.
| Variable | Total cohort (n=57) | CHD cohort (n=20) | DOT cohort (n=29) | DCS cohort (n=8) | p value |
|---|---|---|---|---|---|
|
| |||||
| Prenatal diagnosis | 49 (86%) | 15 (75%) | 26 (90%) | 8 (100%) | 0.16 |
|
| |||||
| Gender (male) | 32 (56%) | 11 (55%) | 18 (62%) | 3 (37%) | 0.34 |
|
| |||||
| Race | |||||
| -White | 43 (75%) | 14 (70%) | 25 (86%) | 4 (50%) | 0.23 |
| -African | 6 (11%) | 3 (15%) | 2 (7%) | 1 (12.5%) | |
| American | 3 (5%) | 2 (10%) | 0 (0%) | 1 (12.5%) | (0.085 cauc. v. non-cauc.) |
| -Hispanic | 5 (9%) | 1 (5%) | 2 (7%) | 2 (25%) | |
| -Other | |||||
|
| |||||
| Gestational Age (wks) | 38.7 ± 2.3 | 38.5 ± 1.3 | 38.7 ± 1.2 | 39 ± 1.1 | 0.65 |
|
| |||||
| Delivery Type (C/s) | 18 (31%) | 7 (35%) | 10 (34%) | 1 (12%) | 0.47 |
|
| |||||
| Birthweight (kg) | 3.2 ± 0.6 | 3.0 ± 0.6 | 3.3 ± 0.5 | 3.3 ± 0.7 | 0.24 |
|
| |||||
| HC (cm) | 34.2 ± 2.2 | 33.9 ± 1.7 | 34.5 ± 1.1 | 34.1 ± 1.4 | 0.28 |
|
| |||||
| HLHS subtype (AA) | 41 (71%) | 13 (65%) | 20 (69%) | 8 (100%) | 0.16 |
|
| |||||
| Total maturation score (TMS) | N/A | N/A | 9.73+/−0.96 | 9.54+/−0.96 | 0.62 |
|
| |||||
| PVL present | 11 (19%) | 4 (20%) | 5 (17%) | 2 (25%) | 0.89 |
|
| |||||
| Age at MRI/Stage I (days) | 3.6 ± 1.9 | 3.4 ± 1.5 | 3.9 ± 2.2 | 3.2 ± 1.4 | 0.63 |
No statistical differences were observed for any of the demographics between groups. Continuous data presented as mean ±SD or % for categorical variables. Significance set at p<0.05.
Figure 1.
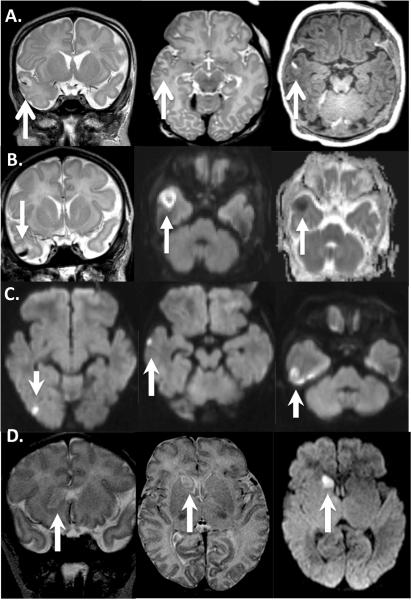
Four HLHS patients found to have stroke (white arrows) on pre-operative brain MRI: Images read left to right. A: coronal and axial T2 demonstrating hyperintense signal in the right temporal lobe, involving the cortex, hemorrhage seen as a hypointensity on T2 and hyperintensity on axial T1 (3rd image on right). B: Similar to A, Coronal T2 (left) shows hemorrhage inside stroke, Diffusion weighted imaging (DWI) showing restriction of water diffusion (center) with Apparent diffusion coefficient (ADC) confirmation (right). C: DWI demonstrating multifocal infarcts in the right temporal lobe. D: Head of the caudate nucleus stroke seen on coronal and axial T2 (left and center) with confirmation of restriction of water diffusion on axial DWI.
Exploratory Analyses
In univariate analyses (Table II), lower total maturation score (TMS) (p=0.005) and Caucasian race (p=0.049) were predictors of the presence of preoperative PVL. In multivariate analyses, the interaction of male gender with aortic atresia was a strong predictor of risk (p = 0.004), although male gender or aortic atresia (AA) alone failed to meet statistical significance as risk factors for PVL in univariate analyses. TMS was not significantly related to either male gender or heart HLHS subtype (p=0.147). PVL occurred in 9/23 Male-AA (39%), 0/9 Male-AS (0%), 1/18 Female-AA (5%) and 1/7 Female-AS (14%).
Table II.
Univariate analyses of demographics, cardiac anatomy and preoperative cardiac ICU variables including blood gases as predictors for periventricular leukomalacia (PVL) in HLHS neonates.
| Variable | Test value: T-test (f for Fisher test) | p Value |
|---|---|---|
|
| ||
| Gender (male) | f | 0.090 |
|
| ||
| Prenatal diagnosis | f | 0.644 |
|
| ||
| Mode of delivery-cesarean section | f | 0.473 |
|
| ||
| Gestational Age | 0.577 | 0.574 |
|
| ||
| Head circumference | −0.604 | 0.554 |
|
| ||
| Birthweight | 0.177 | 0.862 |
|
| ||
| Male-AA vs. Other | f | 0.004* |
|
| ||
| Race (Caucasian vs. Other) | f | 0.049* |
|
| ||
| Total maturation score (TMS) | −3.06 | 0.005* |
|
| ||
| Intubated at admission/preop | f | 0.577 |
|
| ||
| Age at surgery | −0.373 | 0.714 |
|
| ||
| HLHS subtypes AA | f | 0.154 |
|
| ||
| Average daily hemoglobin (Hb) | 1.07 | 0.295 |
|
| ||
| Average daily preop-ABG | ||
| • pH | −0.016 | 0.987 |
| • pCO2 | 1.42 | 0.172 |
| • pO2 | −1.72 | 0.900 |
| • 02 sat | −1.24 | 0.229 |
HLHS neonates with PVL had significantly lower brain maturation score compared to those neonates without PVL (p=0.005). Males with HLHS with aortic atresia (p=0.004) were at an increase risk for PVL. Caucasian race was trending towards significance as well as male gender alone.
Statistical significance set at p<0.05.
Discussion
In this study, 11/57 (19%) neonates with HLHS had PVL on preoperative MRI. Lower brain TMS and the interaction of male gender with AA were identified as independent strong risk factors for preoperative PVL (p=0.005 and p=0.004, respectively). Identification of male gender as a risk factor for PVL in HLHS neonates is consistent with established neonatal outcomes data and recent evidence of gender differences in neurodevelopmental outcomes in CHD. (2, 16) It has been well described that male gender increases the risk for neonatal mortality (17) and recent data provide further evidence of the influence of gender on adverse neurodevelopmental outcomes in both preterm males and term males with congenital heart disease.(2, 16) Animal studies have also demonstrated the influence of male gender on risk for brain injury after cerebral hypoxia-ischemia with gender specific apoptoptic pathways.(18) Males are more susceptible to glutamate mediated excitotoxicity while females may be more at risk for injury via the caspase 3 dependant pathway.
PVL results from hypoxic ischemic injury to susceptible immature pre-myelinating oligodendrocytes.(19) While premature infants are at highest risk for PVL (10), many studies demonstrate that neonates with complex CHD are also at increased risk. (8, 9,12,13) In agreement with this study's findings, a recent study suggested that one of the strongest predictors of both pre- and postoperative PVL (9) was brain maturation as measured with the TMS scale. TMS is an observational scale that was first described in premature neonates (15) and this scale was modified for use in term neonates with CHD.(13) Genetic factors (20) along with perturbations in the utero environment (abnormal placenta) may also contribute to the delayed maturation levels observed. Further understanding of the impact of the in utero environment on CHD brain maturation is required to determine if prenatal interventions may improve the in utero maturation process.
While infants with various forms of complex CHD are at risk for lower than expected brain maturation (as measured by TMS)(13), there have been no reports of differences in TMS among individual cardiac diagnoses, despite the fact that the prevalence of PVL for specific cardiac lesions is quite variable. The variability in the prevalence and risks for PVL injury observed between populations of infants with HLHS and d-TGA suggests that the type of cardiac lesion plays an important role in injury susceptibility. This may occur as a consequence of genetic susceptibility compounded by lesion-specific perturbations in cerebral hemodynamics. (5, 20) The underlying genetic signals that result in congenital heart defects may also contribute to alterations in brain development leading to delayed brain maturation and potential perturbations in the development of neuronal connections. These alterations in neuronal connections and delayed brain maturation along with alterations in cerebral blood flow and oxygenation may lead to an increase risk for preoperative brain injury.
Abnormal circulation in fetuses with complex CHD has been documented to alter brain growth in-utero (21, 22) and brain maturation on both a global (13) and cellular/biochemical level (23) accounting for this vulnerability to injury. Delayed maturation in utero is thought to be secondary to alterations in cerebral blood flow and/or oxygen saturations observed in HLHS fetuses.(24) In HLHS, there is obligate mixing of systemic and pulmonary venous return at the atrial level with aortic atresia and aortic stenosis differing in important ways. When aortic atresia is present in HLHS, complete mixing occurs at the atrial level and supplies the entirety of both the systemic and pulmonary vasculature via the pulmonary artery and the ductus arteriosus. Systemic blood flow via the ductus arteriosus is dependent in large part on the balance between pulmonary and systemic vascular resistance. As pulmonary vascular resistance falls during the transition from fetal (placental) to the neonatal circulation, pulmonary blood flow increases dramatically altering the complex balance between systemic and pulmonary blood flow. To the extent that systemic blood flow decreases, cerebral vascular resistance must also fall and consequently may reach a cerebral autoregulatory threshold. Failure to deliver adequate blood flow, and consequently oxygen and nutrients results in the death of vulnerable pre-myelinating oligodendrocytes.(25) In contrast to patients with AA, in HLHS patients with aortic stenosis there is antegrade blood flow in the ascending aorta from the left heart. This antegrade aortic blood flow derives from pulmonary venous return and in the absence of lung disease is fully oxygenated. To the extent that the left ventricular output in patients with this lesion supplies cervical and cerebral vasculature arteries, the oxygen content of blood perfusing the brain will be substantially higher. These CHD specific perturbations in hemodynamics are also important in-utero (24) and may contribute to alterations in brain development. Neuropathology data of 11 HLHS fetuses who were terminated at 19–22 weeks demonstrated white matter injury suggesting that PVL may be present in utero.(21) Fetal MRI methodology that is currently available makes it difficult to investigate whether brain injury is present prenatally or whether it develops as a result of pertubations in hemodynamics and oxygen saturations during the preoperative period. Development of fetal MRI modalities that allow assessment of brain metabolism and presence of injury will contribute significantly to our understanding of the impact of the in utero environment on risk for PVL.
To the author's knowledge, this is the first study to identify lesion specific risk factors in a HLHS cohort. To date, most of the previous studies have relied on mixed cohorts of infants with different types of CHD. Delayed brain maturation, lower baseline CBF and preoperative procedures are a few of the factors that have been identified as predictors of preoperative PVL in these mixed cohorts.(9, 11, 13) In these populations of neonates with various types of CHD, 20% had PVL on preoperative MRI (7, 8, 26) while 50% had PVL on postoperative MRI.(9) Risk factors for PVL in HLHS were patient specific and included lower brain maturation and male gender with aortic atresia, non-modifiable patient specific related risk factors. As the field focuses on future neuroprotection trials, attention should be directed not only on the preoperative, intraoperative and perioperative period but also on potential gender specific risk pathways (18)along with the in-utero environment with the possibility of influencing brain maturation prenatally.
These data provide evidence of lesion specific risk factors for PVL in neonates with HLHS and as such has important implications for children with other complex congenital heart lesions with the potential to affect preoperative management. Although lower total brain maturity has been identified as an important risk factor, lesion specific risk factors also contribute to preoperative PVL. Identification of both general and CHD specific risk factors is important in further understanding the complex interaction of risks for preoperative PVL. Awareness of patient and lesion specific risk factors is important in designing future neuroprotective trials.
Limitations
There are several limitations to this study. First, the data set for this investigation was created by merging data from three smaller studies, however, inclusion criteria varied only in types of cardiac anatomy targeted for recruitment and magnet strength of the MRI on which the study was accomplished. Thus, none of the major patient or study variables (Table 1) demonstrated any notable differences among studies. Secondly, data on blood gas values were retrospectively collected and therefore some patients did not have arterial blood gas information. Underlying genetic and chromosomal abnormalities were not taken into consideration which may influence underlying brain maturation and development in HLHS (20) along with the presence of preoperative PVL. With our current knowledge of the genetics of CHD the influence of genetics on PVL is not testable at this time. In addition, only 37/57 neonates had TMS scores available for analysis. Finally, type II errors are always possible with smaller cohorts particularly when the outcome variable occurs in a small percentage of these neonates. It is possible that we failed to reach significance for some variables in the univariate analyses due to lack of power.
Conclusion
In neonates with HLHS, patient specific factors, lower TMS and/or male gender with aortic atresia (lack of antegrade blood flow) placed neonates at the greatest risk for preoperative brain injury in the form of PVL.
Acknowledgments
Supported by NINDS (NS052380, NS072338), Dana Foundation, and the June and Steve Wolfson Family Fund for Neurological Research; Dr. Goff is supported by a grant from NHLBI (T-32 HL-0791543)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–53. doi: 10.1016/j.jtcvs.2006.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majnemer A, Limperopoulos C, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C. A new look at outcomes of infants with congenital heart disease. Pediatr Neurol. 2009;40:197–204. doi: 10.1016/j.pediatrneurol.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–67. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 4.Tabbutt S, Nord AS, Jarvik GP, Bernbaum J, Wernovsky G, Gerdes M, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121:476–83. doi: 10.1542/peds.2007-1282. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger DC, Wypij D, duPlessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 6.Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol. 2005;110:563–78. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 7.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–14. [PubMed] [Google Scholar]
- 8.Licht DJ, Wang J, Silvestre DW, Nicolson SC, Montenegro LM, Wernovsky G, et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841–9. doi: 10.1016/j.jtcvs.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–56. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–16. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 11.McQuillen PS, Hamrick SE, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–5. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 12.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–16. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Childs AM, Ramenghi LA, Cornette L, Tanner SF, Arthur RJ, Martinez D, et al. Cerebral maturation in premature infants: quantitative assessment using MR imaging. AJNR Am J Neuroradiol. 2001;22:1577–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Kent AL, Wright IM, Abdel-Latif ME. Mortality and adverse neurologic outcomes are greater in preterm male infants. Pediatrics. 2012;129:124–31. doi: 10.1542/peds.2011-1578. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182–5. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renolleau S, Fau S, Charriaut-Marlangue C. Gender-related differences in apoptotic pathways after neonatal cerebral ischemia. Neuroscientist. 2008;14:46–52. doi: 10.1177/1073858407308889. [DOI] [PubMed] [Google Scholar]
- 19.Volpe JJ. Neurology of the Newborn. 4th ed. WB Saunders; Philadelphia: 2005. [Google Scholar]
- 20.McQuillen PS, Goff DA, Licht DJ. Effects of congenital heart disease on brain development. Prog Pediatr Cardiol. 2010;29:79–85. doi: 10.1016/j.ppedcard.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinton RB, Andelfinger G, Sekar P, Hinton AC, Gendron RL, Michelfelder EC, et al. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res. 2008;64:364–9. doi: 10.1203/PDR.0b013e3181827bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL, Jr., et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 24.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25:32–6. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 25.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–62. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Miller SP, McQuillen PS, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM, et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg. 2004;77:1698–706. doi: 10.1016/j.athoracsur.2003.10.084. [DOI] [PubMed] [Google Scholar]