Abstract
PURPOSE
Targeted interventions to reduce the risk and increase the early detection of melanoma have the potential to save lives. We aimed to assess the effect of such an intervention on patient prevention behavior.
METHODS
We conducted a pilot clustered randomized controlled trial, comparing a targeted screening and education intervention with a conventional information-based campaign in 20 private surgeries in western France. In the intervention group, 10 general practitioners identified patients at elevated risk for melanoma with a validated assessment tool, the Self-Assessment Melanoma Risk Score (SAMScore), examined their skin, and counseled them using information leaflets. In the control group, 10 general practitioners displayed a poster and the leaflets in their waiting room and examined patients’ skin at their own discretion. The main outcome measures were sunbathing and skin self-examinations among patients at elevated risk, assessed 5 months later with a questionnaire.
RESULTS
Analyses were based on 173 patients. Compared with control patients, intervention patients were more likely to remember the campaign (81.4% vs 50.0%, P = .0001) and to correctly identify their elevated risk of melanoma (71.1% vs 42.1%, P = .001). Furthermore, intervention patients had higher levels of prevention behaviors: they were less likely to sunbathe in the summer (24.7% vs 40.8%, P = .048) and more likely to have performed skin self-examinations in the past year (52.6% vs 36.8%, P = .029). The intervention was not associated with any clear adverse effects, although there were trends whereby intervention patients were more likely to worry about melanoma and to consult their general practitioner again about the disease.
CONCLUSIONS
The combination of use of the SAMScore and general practitioner examination and counseling during consultations is an efficient way to promote patient behaviors that may reduce melanoma risk. Extending the duration of follow-up and demonstrating an impact on morbidity and mortality remain major issues for further research.
Keywords: melanoma; prevention; health behavior; behavior change; randomized trial; screening, primary care; practice-based research
INTRODUCTION
Among all cancers, melanoma is the one for which incidence has increased the most worldwide during the last 20 years.1 The prognosis of this malignancy is better with early diagnosis.2 Guidelines highlight the role of general practitioners in prevention,3 including primary prevention by counseling on sun exposure4,5 and secondary prevention by regularly performing a total skin examination and encouraging self-examination.3,6 Self-examination has been shown to improve the probability of early diagnosis7 and may reduce melanoma-related mortality by 63%.8 In more than one-half of cases of melanoma, patients may be the first to identify the lesion7,9; however, less than 25% of patients perform skin self-examination.10
There have been many advertising campaigns worldwide on melanoma prevention, but their effectiveness in modifying patient behavior is difficult to measure. They have been shown to improve patient knowledge, but not enough to change relevant behaviors.11–13 It is well known, for example, that awareness of the risks associated with tanning beds does not prevent the use of such equipment.14
Various factors promoting behavior changes have been described from a theoretical perspective in the literature.15 Patients’ perception of their own risk may be a decisive factor in changing their behavior.16 Giving patients personalized information about whether or not they are at elevated risk for melanoma could affect their perception of the risk. The Self-Assessment Melanoma Risk Score (SAMScore) provides such information (Figure 1) and has been validated by our team.17,18 It lists 7 risk factors for a given patient and also expresses overall risk in dichotomous format so that a patient is either at elevated risk or not for melanoma. The development of the score and criteria used to define elevated risk have been described previously.17,18
Figure 1.
Questionnaire used for the Self-Assessment of Melanoma Risk Score (SAMScore).18
Note: According to the SAMScore, a patient is considered at elevated risk for melanoma if at least 1 of these 3 criteria is verified: (1) The patients has at least 3 risk factors among the following 7 risk factors: phototype I or II, freckling tendency, more than 20 melanocytic nevi on the arms, severe sunburn during childhood or teenage years, life in a country at low latitude, a history of previous melanoma, and a history of melanoma in a first-degree relative, (2) The patient is younger than 60 years of age and has more than 20 melanocytic nevi on the arms, (3) The patient is aged 60 years or older and has a freckling tendency.
Other authors have reported that identifying existing lesions may have an impact on patients’ sun exposure behavior.19,20 In addition, Swedish investigators have recently shown a greater impact of general practitioners providing counseling during a personalized consultation as compared with only patient receipt of written information.21
In this study, we aimed to compare the effect of a targeted screening and education strategy using the SAMScore on patient prevention behavior with that of a conventional prevention campaign based on mass communication. Prevention behaviors of patients at elevated risk for melanoma were assessed 5 months later with a telephone survey.
METHODS
Study Design
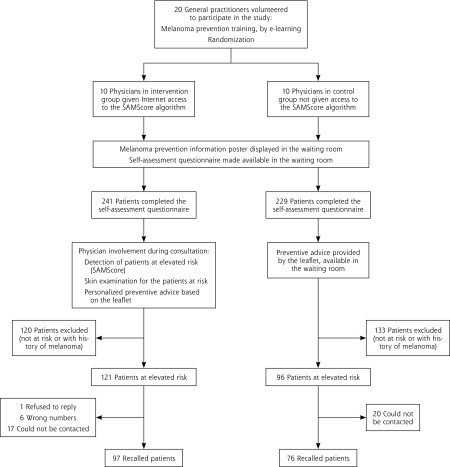
The study was a pilot clustered randomized controlled trial, the first part of the COPARIME Project (Cohort of Patients at Risk for Melanoma, ClinicalTrials.gov NCT01610531). A total of 20 general practitioner volunteers were randomized evenly into 2 parallel groups: an intervention group and a control group (Figure 2).
Figure 2.
Flow diagram of the trial.
Setting and Target Population
The study took place in general practitioner surgeries on the French West Coast (Loire-Atlantique and Vendée geographic areas); no more than 1 physician volunteered to participate in each surgery. Patients who consulted the surgeries were recruited during a 2-week period in the spring of 2011 so that their sun exposure behavior during the subsequent summer could be evaluated. The French West Coast is a temperate geographic area (latitude 47° 13′ 0″ N); during the 3 summer months in 2011, this area received 606 hours of sunshine, with temperatures ranging from 22°C to 32°C in the afternoon.
The target population was patients at elevated risk for melanoma as determined from the SAMScore, indicating that their relative risk of developing a melanoma was estimated to be 11 times higher than that of the general population.17,18 Patients with a personal melanoma history were excluded because a specialized follow-up is immediately recommended in this situation.
Intervention and Control Procedures
Before starting the study, all of the general practitioners had to view an e-learning module on melanoma screening to update their knowledge and skin examination practices. They all personally received the same documents necessary for study participation: a poster to be displayed in the waiting room, information leaflets on melanoma produced by the French National Cancer Institute, and printed SAMScore questionnaires listing 7 risk factors for melanoma.
Between April 11 and 23, 2011, all patients consulting in the practices were asked to complete the SAMScore questionnaires in the waiting room, regardless of why they were seeking consultation.18 Patients received oral and written information about the study and had to provide their written informed consent to participate.
In the intervention group, general practitioners accessed a SAMScore risk calculator on a server using an individual password. During the consultation, the general practitioner entered each patient’s responses to the 7 questions (phototype, freckling tendency, number of moles, residence in a country with strong sunshine, severe sunburn during infancy, personal history of melanoma, and family history of melanoma). The calculator integrated the risk factors using the SAMScore algorithm and expressed the risk in dichotomous format: either at elevated risk or not for melanoma (a demonstration is available at www.dmg-nantes.fr/coparime/). For all patients identified as having elevated risk, general practitioners performed a total skin examination, counseled the patient, and gave the patient the information leaflet detailing primary and secondary prevention measures.
In the control group, general practitioners undertook a conventional public health campaign that entailed displaying the documents described above in their waiting room, but they did not have access to the calculator to interpret the risk factors, and therefore did not have access to the patient’s dichotomous risk status. In this group, general practitioners did not receive specific instructions to perform skin examinations; thus, they were free to perform them at their own discretion as in any conventional screening campaign.
All patients found to have a suspicious lesion were referred to a dermatologist.
Data Collection
Five months after enrollment was completed, patients having an elevated risk of melanoma were identified from their answers to the 7 items of the SAMScore from the questionnaires completed in the waiting room. These patients were surveyed by telephone using another questionnaire that probed their knowledge and prevention behavior, described below. Those with a personal history of melanoma were excluded.
Outcome Measures
The telephone questionnaire had 1 part that collected information on sociodemographic data (age, sex, educational level, occupation) and 4 parts that collected information on outcomes. The theory of planned behavior22 has been used in the literature to investigate prevention behavior for other cancers. We therefore designed our questionnaire to assess patients’ memory of the campaign, knowledge of their elevated risk of melanoma, perceived effectiveness of the prevention campaign, perceived ability to perform skin self-examination, and primary and secondary prevention behaviors (Supplemental Appendix). Items were generated based on the recommendations of the World Health Organization,20 on a questionnaire adopted by the US National Cancer Institute and the Emory Prevention Research Center,23 and on a review of the literature.
General practitioners pretested the questionnaire, favoring closed-ended responses to encourage comprehension. The questionnaire could be completed in about 10 minutes.
Statistical Analysis
We analyzed data using the SAS Statistical Package 9.2 (SAS Institute Inc) and its GLIMMIX procedure to take into account the clustering effect; general practitioners were considered as a random effect, whereas age, sex, and education level were considered as fixed cofactors. We performed 2-tailed analyses and set the significance threshold at .05. We used intention-to-treat analyses, allowing us to bypass the degree of compliance with total skin examination and to better consider the real-life situation. No stratification was planned. Results were analyzed with and without adjustment for other cofactors.
Ethical Approval
We obtained approval for the study from the human subject ethics committee of Tours (protocol no. 2011-R2-BRD 10/11-N).
RESULTS
Patient Characteristics
Using the SAMScore, we identified 121 and 96 patients at elevated risk for melanoma in the intervention group and control group, respectively. A total of 97 (80%) and 76 (79%) of these patients responded to the telephone survey (Figure 2).
The mean age was 43.6 ± 17.1 years and 42.8 ± 14.6 years in the intervention and control group, respectively. In both groups, 76% of patients were women. The groups did not differ significantly with respect to the highest level of education attained (P = .10).
Outcomes
Five months after enrollment, compared with patients in the control group, those in the intervention group were more likely to remember the poster displayed in the waiting rooms (81.4% vs 50.0%, P = .0001) and to have consulted the information leaflets (63.9% vs 31.6%, P = .0004) (Table 1).
Table 1.
Patient Memory of the Melanoma Education Campaign
| Measure | Intervention Group (n = 97) | Control Group (n = 76) | Covariance Cluster Effect Estimates | Covariance SD | Unadjusted P Value | Adjusted P Valuea |
|---|---|---|---|---|---|---|
| Remembered the poster in the waiting room, No. (%) [95% CI] | 79 (81.4) [72.3–88.6] |
38 (50.0) [38.3–61.7] |
−0.003 | 0.027 | .0001 | .0001 |
| Consulted the leaflets in the waiting room, No. (%) [95% CI] | 62 (63.9) [53.5–73.4] |
24 (31.6) [21.4–43.3] |
−0.016 | 0.029 | .0004 | .0005 |
Multivariate model adjusted for age, sex, and education level.
There were nonsignificant trends whereby a greater proportion of patients in the intervention group worried about developing melanoma (28.9% vs 18.4%, P = .16) and consulted their general practitioner again to discuss the disease (15.5% vs 9.2%, P =.23).
Compared with their peers in the control group, patients in the intervention group were more likely to correctly know that they had an elevated risk of melanoma (71.1% vs 42.1%, P = .001) (Table 2). After adjustment for age, sex and education level, knowledge of the risk factors was significantly higher in the intervention group for 4 items. When we developed a knowledge score based on the sum of the responses, the mean score was greater in the intervention group (4.71 ± 1.33 vs 3.79 ± 1.50, P = .007).
Table 2.
Patient Knowledge of Personal Risk of Melanoma and of Melanoma Risk Factors
| Measure | Intervention Group (n = 97) | Control Group (n = 76) | Covariance Cluster Effect Estimates | Covariance SD | Unadjusted P Value | Adjusted P Valuea |
|---|---|---|---|---|---|---|
| Correctly knew they had an elevated risk of melanoma, No. (%) [95% CI] | 69 (71.1) [61.1–79.9] |
32 (42.1) [30.9–54.0] |
−0.010 | 0.038 | .001 | .002 |
| Correctly identified melanoma risk factors, No. (%) [95% CI] | ||||||
| Having >20 moles on the 2 arms | 76 (78.4) [68.8–86.1] |
48 (63.2) [51.3–73.9] |
0.002 | 0.045 | .045 | .085 |
| Having freckles | 62 (63.9) [53.5–73.4] |
34 (44.7) [33.3–56.6] |
−0.016 | 0.033 | .017 | .038 |
| Having phototype 1 or 2 | 90 (92.8) [85.7–97.1] |
55 (72.4) [60.9–82.0] |
−0.003 | 0.039 | .003 | .005 |
| Having been sunburned during childhood | 90 (92.8) [85.7–97.1] |
61 (80.3) [69.5–88.5] |
−0.030 | 0.025 | .011 | .053 |
| Having lived for more than a year in a country with strong sunshine | 77 (79.4) [70.0–86.9] |
58 (76.3) [65.2–85.3] |
0.011 | 0.048 | .65 | .97 |
| Having a family history of melanoma | 62 (63.9) [53.5–73.4] |
32 (42.1) [30.9–54.0] |
−0.006 | 0.034 | .01 | .006 |
| Overall knowledge score,b mean (SD) | 4.71 (1.33) | 3.79 (1.50) | 1.47 e−08c | 0.0001 | .007 | .002 |
Multivariate model adjusted for age, sex, and education level.
Possible scores ranged from 0 to 6, with higher scores indicating greater knowledge.
Mixed model.
The proportion of patients who reported having sunbathed during the summer was significantly lower in the intervention than in the control group (24.7% vs 40.8%, P = .048) (Table 3). Patients in the intervention group were significantly more likely to have performed a skin self-examination during the past 12 months (52.6% vs 36.8%, P = .029), and they were also more likely to report being able to detect a change in a mole (70.1% vs 54.0%, P = .023).
Table 3.
Patient Report of Primary and Secondary Melanoma Prevention Behaviors
| Behavior | Intervention Group (n = 97) |
Control Group (n = 76) |
Covariance Cluster Effect Estimates | Covariance SD | Unadjusted P Value | Adjusted P Valuea |
|---|---|---|---|---|---|---|
| Sunbathed in past year, No. (%) [95% CI] | 24 (24.7) [16.5–34.5] |
31 (40.8) [29.7–52.7] |
0.020 | 0.043 | .048 | .040 |
| Took protective actions during the most recent exposure, No. (%) [95% CI] | 65 (67.0) [56.7–76.2] |
42 (55.3) [43.4–66.7] |
−0.065 | 0.018 | .079 | .060 |
| Had a session in tanning bed, No. (%) [95% CI] | 10 (10.3) [5.1–18.1] |
5 (6.6) [2.2–14.7] |
−0.057 | NA | .21 | .069 |
| Sustained a sunburn in the past summer, No. (%) [95% CI] | 26 (26.8) [18.3–36.8] |
23 (30.3) [20.3–41.9] |
0.018 | 0.044 | .65 | .42 |
| Performed a skin self-examination in the past 12 months, No. (%) [95% CI] | 51 (52.6) [42.2–62.8] |
28 (36.8) [26.1–48.7] |
−0.036 | 0.025 | .029 | .020 |
| Used a mirror or person to assist with skin self-examination, No. (%) [95% CI] | 73 (75.3) [65.5–83.5] |
52 (68.4) [56.7–78.6] |
0.057 | 0.12 | .51 | .47 |
| Took a photograph for skin self-examination, No. (%) [95% CI] | 8 (8.3) [3.6–15.6] |
5 (6.6) [2.2–14.7] |
−0.017 | NA | .51 | .62 |
| Reported feeling able to detect a change in a mole, No. (%) [95% CI] | 68 (70.1) [60.0–79.0] |
41 (54.0) [42.1–65.5] |
0.057 | 0.064 | .023 | .14 |
NA = not applicable.
Multivariate model adjusted for age, sex, and education level.
The 2 groups were statistically indistinguishable in terms of other measures of prevention behavior. After adjustment for age, sex, and education level, perceived efficacy in detecting lesions was no longer significantly different between groups (P = .14).
DISCUSSION
Key Findings
Our study suggests that a multifaceted general practitioner–mediated intervention—that is, identifying patients at risk, performing skin examinations, and giving advice and printed information on prevention—had a greater impact on patients than a conventional information-based campaign in terms of changing behaviors in ways that may decrease melanoma risk and increase early detection. Five months later, the patients in the intervention group better remembered the information provided. Their knowledge of melanoma risk factors was significantly greater, and for 2 main prevention outcomes, they were significantly more likely to have favorable behaviors. Improved knowledge following a prevention intervention has already been described24–27 but is not always associated with behavioral changes.28,29 In this study, the higher rates of behavioral changes after intervention in terms of sunbathing and performing skin self-examinations are therefore particularly relevant.
At the beginning of the consultation, intervention physicians accessed the Web calculator to assess patients’ risk status. Informing patients about their risk status, based on the SAMScore, might raise awareness of their vulnerability. Some authors have reported that patients may underestimate their personal risk of developing melanoma,30 and a better personal perception of their risk would improve prevention behavior.31
Skin examination by the general practitioner provided an opportunity for individualized counseling allowing patient education, based on visual feedback.19 The practitioners could give tailored advice on primary and secondary prevention, and adapt and personalize their counseling to the patient depending on the results of the clinical examination. Linking the counseling to the objective identification of lesions is an efficient and powerful strategy,31–34 as general practitioners can perform 2 tasks simultaneously and possibly have greater influence than if they had simply given out written information.21 Additionally, the practitioners can show patients which moles should be monitored and how to do it, improving patients’ sense of self-efficacy. Another study has shown that a feeling of taking effective action apparently reinforces adherence in performing skin self-examinations.32
Our general practitioner–mediated intervention appeared generally safe and acceptable to patients. It is noteworthy, however, that the intervention patients tended to be more likely to worry about developing melanoma and to consult their practitioner again to discuss the disease. Further study will therefore be important to assess the long-term impact of the intervention on patients’ psychological well-being and health care use.
Strengths and Limitations
Our results are especially noteworthy because our design—a randomized controlled trial comparing a new strategy with a conventional (gold standard) strategy—leads to a higher level of evidence than do other studies based on before-and-after testing.24–27 Another strength of this applied research was that it was grounded in primary care and used a brief intervention requiring few resources, so that its implementation in everyday practice would be possible. The clinical intervention and protocol for handling patients during the office visit were designed to be compatible with the pace of primary care consultations.
At the same time, this pilot study had limitations. Patients gave responses on self-administrated questionnaires. Despite displayed posters and information given by the receptionist, some patients did not participate in the study, leading to a selection bias. Women were overrepresented in both groups (76%), as has been previously described among people who are interested in cancer prevention.35,36 The mean age of patients (43 years) was also in concordance with those reported in the literature.37 Further research will be necessary to characterize the selection and participation bias associated with this targeted screening procedure.
In addition, this was a short-term study, and we did not collect data on longer-term disease outcomes such as the incidence of melanoma, the stage at diagnosis, and melanoma-related mortality. Follow-up of the study population will be important for assessing an impact on these outcomes. Cost and cost-effectiveness analyses would also be informative.
Some general practitioners might have neglected to follow the intervention protocol if they were too busy. Competing demands are a universal issue when it comes to implementing a new procedure. Our inability to ensure compliance might have affected our findings, even though we tried to avoid such bias using an intent-to-treat statistical analysis.
We collected data with a questionnaire, and responses were therefore self-reports. Biases related to self-reported data and social desirability are an inevitable limitation. Moreover, self-reports would produce valid measures of exposure to ultraviolet radiation.38,39 In contrast, the use of objective measures (observations, skin reflectance, personal dosimetry)40 would have probably led to major changes in patient prevention behaviors in both groups, so that assessment of patient spontaneous behaviors would have been impossible.
Finally, we did not compare the 2 groups at baseline to avoid any influence of pretesting on the control group that may have improved their knowledge, which would have made it more difficult to demonstrate an impact of the COPARIME intervention.
Implications
This study shows that a targeted screening and education strategy based on the SAMScore has a favorable impact on patient behavior. The first part of this strategy, the SAMScore, provides dichotomous information that probably affects risk perception in patients at elevated risk. Simply identifying risk factors (even multiple) would not be a relevant message in practice for the patient, whereas the unambiguous, black-and-white risk status communicated on completion of the SAMScore would be more understandable. The second part of the strategy, the total skin examination of patients identified as having an elevated risk, leads to focused general practitioner counseling on the objective assessment of the lesions. The success of such a targeted screening strategy may therefore also rely on the selection of concerned patients, allowing general practitioners to focus their attention, energy, and time on the education of at-risk populations, with greater efficiency.
Extending the duration of follow-up in this study and demonstrating an impact on morbidity and mortality remain major issues for further research. Furthermore, assessing the impact of targeted screening and education as promoted by policy makers would require ongoing use of the intervention and repeated assessments.
Acknowledgments
The authors wish to thank the 20 general practitioners of Loire-Atlantique and Vendée for their participation in patient recruitment.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study was funded and supported by the French National Institute of Cancer) (http://www.e-cancer.fr).
Role of the sponsor: The sponsor (French National Institute of Cancer) had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Trial registry: ClinicalTrials.gov, http://clinicaltrials.gov. Registration number: NCT01610531.
Previous presentations: Rat C, Quereux G, Senand R, Dreno B, Nguyen JM. COPARIME: design and first results. European General Practice Research Network Meeting. Ljubljana, Slovenia; May 10–13, 2012.
Rat C, Quereux G, Riviere C, et al. Protection solaire et auto-examen cutané: impact d’un dépistage ciblé sur des patients à haut risque de mélanome consultant en médecine générale. 6ème congrès français de la médecine générale. Nice, France; June 21–23, 2012.
Rat C, Quereux G, Riviere C, et al. Impact of a targeted screening on melanoma prevention behaviour. A randomised controlled trial. 42nd Annual European Society for Dermatological Research Meeting. Venice, Italy; September 19–22, 2012.
Supplementary materials: Available at www.AnnFamMed.org/content/12/1/21/suppl/DC1/
References
- 1.GLOBOCAN 2008. Cancer incidence, mortality and prevalence. http://globocan.iarc.fr Accessed January 11, 2012
- 2.Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54(3):131–149 [DOI] [PubMed] [Google Scholar]
- 3.French National Authority for Health. Early-diagnosis strategy for melanoma. [In French.] 2006. [Accessed January 11, 2012]. http://www.has-sante.fr.
- 4.Lin JS, Eder M, Weinmann S, et al. Behavioral Counseling to Prevent Skin Cancer: Systematic Evidence Review to Update the 2003 U.S. Preventive Services Task Force Recommendation. Rockville, MD: Agency for Healthcare Research and Quality, 2011 [PubMed] [Google Scholar]
- 5.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29(3):257–263 [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Health Diagnosis and treatment of early melanoma. NIH Consensus conference. JAMA. 1992;268:1314–1319 [DOI] [PubMed] [Google Scholar]
- 7.Carli P, De Giorgi V, Palli D, et al. Dermatologist detection and skin self-examination are associated with thinner melanomas: results from a survey of the Italian Multidisciplinary Group on Melanoma. Arch Dermatol. 2003;139(5):607–612 [DOI] [PubMed] [Google Scholar]
- 8.Berwick M, Begg CB, Fine JA, Roush GC, Barnhill RL. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88(1):17–23 [DOI] [PubMed] [Google Scholar]
- 9.Hamidi R, Peng D, Cockburn M. Efficacy of skin self-examination for the early detection of melanoma. Int J Dermatol. 2010;49(2):126–134 [DOI] [PubMed] [Google Scholar]
- 10.Pollitt RA, Geller AC, Brooks DR, et al. Efficacy of skin self-examination practices for early melanoma detection. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3018–3023 [DOI] [PubMed] [Google Scholar]
- 11.Kiekbusch S, Hannich HJ, Isacsson A, et al. Impact of a cancer education multimedia device on public knowledge, attitudes, and behaviors: a controlled intervention study in Southern Sweden. J Cancer Educ. 2000;15(4):232–236 [DOI] [PubMed] [Google Scholar]
- 12.Crane LA, Schneider LS, Yohn JJ, Morelli JG, Plomer KD. “Block the sun, not the fun”: evaluation of a skin cancer prevention program for child care centers. Am J Prev Med. 1999;17(1):31–37 [DOI] [PubMed] [Google Scholar]
- 13.Geller AC, Rutsch L, Kenausis K, Selzer P, Zhang Z. Can an hour or two of protection education keep the sunburn away? Evaluation of the Environmental Protection Agency’s Sunwise School Program. Environ Health. 2003;2(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight JM, Kirincich AN, Farmer ER, Hood AF. Awareness of the risks of tanning lamps does not influence behavior among college students. Arch Dermatol. 2002;138(10):1311–1315 [DOI] [PubMed] [Google Scholar]
- 15.Becker MH, Maiman LA. Sociobehavioral determinants of compliance with health and medical care recommendations. Med Care. 1975;13(1):10–24 [DOI] [PubMed] [Google Scholar]
- 16.Leventhal H, Kelly K, Leventhal EA. Population risk, actual risk, perceived risk, and cancer control: a discussion. J Natl Cancer Inst Monogr. 1999;(25):81–85 [DOI] [PubMed] [Google Scholar]
- 17.Quéreux G, Moyse D, Lequeux Y, et al. Development of an individual score for melanoma risk. Eur J Cancer Prev. 2011;20(3):217–224 [DOI] [PubMed] [Google Scholar]
- 18.Quéreux G, Nguyen JM, Cary M, Jumbou O, Lequeux Y, Dréno B. Validation of the Self-Assessment of Melanoma Risk Score for a melanoma-targeted screening. Eur J Cancer Prev. 2012;21(6):588–595 [DOI] [PubMed] [Google Scholar]
- 19.Hollands GJ, Hankins M, Marteau TM. Visual feedback of individuals’ medical imaging results for changing health behaviour. Cochrane Database Syst Rev. 2010;20:CD007434. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization global Solar UV Index: A Practical Guide. Geneva, Switzerland: World Health Organization; 2002:1–18 [Google Scholar]
- 21.Falk M, Magnusson H. Sun protection advice mediated by the general practitioner: an effective way to achieve long-term change of behaviour and attitudes related to sun exposure? Scand J Prim Health Care. 2011;29(3):135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajzen I. The theory of planned behavior. Org Behav Hum Decis Proc. 1991;50:179–211 [Google Scholar]
- 23.Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144(2):217–222 [DOI] [PubMed] [Google Scholar]
- 24.Broadwater C, Heins J, Hoelscher C, Mangone A, Rozanas C. Skin and colon cancer media campaigns in Utah. Prev Chronic Dis. 2004; 1(4):A18. [PMC free article] [PubMed] [Google Scholar]
- 25.Glanz K, Maddock JE, Lew RA, Murakami-Akatsuka L. A randomized trial of the Hawaii Sun Smart program’s impact on outdoor recreation staff. J Am Acad Dermatol. 2001;44(6):973–978 [DOI] [PubMed] [Google Scholar]
- 26.Miller DR, Geller AC, Wood MC, Lew RA, Koh HK. The Falmouth Safe Skin Project: evaluation of a community program to promote sun protection in youth. Health Educ Behav. 1999;26(3):369–384 [DOI] [PubMed] [Google Scholar]
- 27.Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66(1):e9–e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quéreux G, Nguyen JM, Volteau C, Dréno B. Prospective trial on a school-based skin cancer prevention project. Eur J Cancer Prev. 2009;18(2):133–144 [DOI] [PubMed] [Google Scholar]
- 29.Milne E, Jacoby P, Giles-Corti B, Cross D, Johnston R, English DR. The impact of the Kidskin Sun Protection intervention on summer sun tan and reported sun exposure: was it sustained? Prev Med. 2006;42(1):14–20 [DOI] [PubMed] [Google Scholar]
- 30.Bränström R, Kristjansson S, Ullén H. Risk perception, optimistic bias, and readiness to change sun related behaviour. Eur J Public Health. 2006;16(5):492–497 [DOI] [PubMed] [Google Scholar]
- 31.Kim SE, Pérez-Stable EJ, Wong S, et al. Association between cancer risk perception and screening behavior among diverse women. Arch Intern Med. 2008;168(7):728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hay JL, Oliveria SA, Dusza SW, et al. Psychosocial mediators of a nurse intervention to increase skin self-examination in patients at high risk for melanoma. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1212–1216 [DOI] [PubMed] [Google Scholar]
- 33.Mahler HI, Kulik JA, Gerrard M, Gibbons FX. Long-term effects of appearance-based interventions on sun protection behaviors. Health Psychol. 2007;26(3):350–360 [DOI] [PubMed] [Google Scholar]
- 34.Emmons KM, Geller AC, Puleo E, et al. Skin cancer education and early detection at the beach: a randomized trial of dermatologist examination and biometric feedback. J Am Acad Dermatol. 2011; 64(2):282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sach TH, Whynes DK. Men and women: beliefs about cancer and about screening. BMC Public Health. 2009;9:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Göbl CS, Ortag F, Bozkurt L, Smeikal A. Health behaviour and attitude towards screening examinations in an Austrian urban and rural population: gender aspects—screening and sex. Wien Med Wochenschr. 2011;161(5–6):143–148 [DOI] [PubMed] [Google Scholar]
- 37.Reen B, Coppa K, Smith DP. Skin cancer in general practice: impact of an early detection campaign. Aust Fam Physician. 2007;36(7):574–576 [PubMed] [Google Scholar]
- 38.Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144(2):217–222 [DOI] [PubMed] [Google Scholar]
- 39.Glanz K, Gies P, O’Riordan DL, et al. Validity of self-reported solar UVR exposure compared with objectively measured UVR exposure. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3005–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glanz K, Mayer JA. Reducing ultraviolet radiation exposure to prevent skin cancer methodology and measurement. Am J Prev Med. 2005;29(2):131–142 [DOI] [PubMed] [Google Scholar]