Abstract
The delivery of adult skeletal muscle stem cells, called satellite cells, to several injured muscles via the circulation would be useful, however, an improved understanding of cell fate and biodistribution following their delivery is important for this goal to be achieved. The objective of this study was to evaluate the ability of systemically delivered satellite cells to home to injured skeletal muscle using single-photon emission computed tomography (SPECT) imaging of 111In-labeled satellite cells. Satellite cells labeled with 111In-oxine and green fluorescent protein (GFP) were injected intravenously after bupivicaine-induced injury to the tibialis anterior muscle. Animals were imaged with a high-resolution SPECT system called FastSPECT II for up to 7 days after transplantation. In vivo FastSPECT II imaging demonstrated a three to five-fold greater number of transplanted satellite cells in bupivicaine-injured muscle as compared to un-injured muscle after transplantation; a finding that was verified through autoradiograph analysis and quantification of GFP expression. Satellite cells also accumulated in other organs including the lung, liver, and spleen, as determined by biodistribution measurements. These data support the ability of satellite cells to home to injured muscle and support the use of SPECT and autoradiograph imaging techniques to track systemically transplanted 111In labeled satellite cells in vivo, and suggest their homing may be improved by reducing their entrapment in filter organs.
Keywords: satellite cell, skeletal muscle, SPECT, 111In
1. Introduction
Satellite cells are resident adult stem cells that contribute to hypertrophy and repair in adult skeletal muscle. Based on the contention that satellite cells are the cell type largely responsible for normal skeletal muscle regeneration, it is plausible to suggest they are a tool to improve muscle regeneration when a depletion or challenge to the myogenic pool exists; eg, Duchenne muscular dystrophy (Blau et al. 1983; Schultz and Jaryszak 1985; Wright 1985; Skuk and Tremblay 2003; Mouly et al. 2005) and aging (Chakravarthy et al. 2000; Lees et al. 2006; Day et al. 2010). It is intuitive that the injection of satellite cells in proximity to the area of damage would result in the most effective treatment. Although endogenous satellite cells located several millimeters away from a site of injury are stimulated to proliferate and later migrate toward the site of injury (Schultz et al. 1985), the migratory capabilities of myogenic cells delivered intramuscularly is limited (Ito et al. 1998). Increasing the number of injections and injections sites partially addresses this complication (Skuk et al. 2007), but this technique is still insufficient to deliver enough stem cells to all of the affected regions equally. In many cases, areas of skeletal muscle needing repair may be overlooked or may not be readily accessible. Therefore, others have focused on alternate routes for the delivery of skeletal muscle stem cells, including intra-arterial, extracorporeal, and intravenous delivery (Neumeyer et al. 1992; Torrente et al. 1999; Torrente et al. 2001; Peault et al. 2007), intravenous delivery being the least invasive of these procedures that supports cell engraftment in skeletal muscle (Ferrari et al. 1998; Bachrach et al. 2004; Dezawa et al. 2005). However, the extent to which the systemic delivery of satellite cells is limited by the tendency to reside in organs, including the lungs, liver, and spleen, as described for other stem cells (Gao et al. 2001) has yet to be fully characterized.
Although cell labeling and immunostaining of tissue sections are commonly used to characterize satellite cell survival and migration, these experiments are difficult to quantify, often require muscle explants and animal sacrifice to determine results, and are limited by the large number of animals required and inter-animal variability. To improve our understanding of cell survival and migration of transplanted cells in vivo it would be advantageous to have the capability of using other sensitive quantification techniques and imaging modalities. Noninvasive in vivo imaging techniques, including magnetic resonance imaging (MRI) of nanosized superparamagnetic iron oxide (SPIO) labeling (Cahill et al. 2004), nuclear imaging of radio-labeled cells (Brenner et al. 2004), and optical imaging of cells labeled with fluorescent or bioluminescent dyes (Lin et al. 2007; Rosen et al. 2007; Sacco et al. 2008), have been used in recent years for studying satellite cells and other skeletal muscle stem cells. The successful development of high-resolution small-animal SPECT systems provides a powerful new means for studying transplanted satellite cell homing using small animals. A stationary small-animal SPECT imager called FastSPECT II, which was constructed completely at the University of Arizona, provides advantages over single-detector SPECT systems, including improved sensitivity from acquiring all views at once and the ability to collect and reconstruct four-dimensional data from non-periodic processes (Furenlid LR 2004). This unique high-resolution SPECT system has been used successfully in cardiac imaging studies and stem cell tracking with small-animal models (Liu et al. 2002; Liu et al. 2004). Based on these reports, nuclear imaging with SPECT seems well suited for tracking and mapping the biodistribution and cell fate of transplanted satellite cells in small-animal models, however, the use of 111In-oxine for radio-labeling of satellite cells has yet to be performed. Therefore, the goals of the study were to determine the ability of satellite cells to home to injured skeletal muscle and to determine the usefulness of 111In-oxine labeling and SPECT imaging for in vivo quantification of satellite cell homing when delivered systemically. Previously, most studies of satellite cells were delivered to skeletal muscle with intramuscular injections. For comparison, our first experiment was designed to validate the feasibility of radiolabeling satellite cell with 111In-oxine and capability of imaging transplanted satellite cells via intramuscular injection with FastSPECT II. In the second experiment, satellite cells were first labeled with GFP, followed by 111In-oxine labeling. The dual-labeled cells were intravenously administered into animals with skeletal muscle injury induced by bupivicaine injections twenty-four hours earlier. GFP labeling of cells allowed for a verification of SPECT imaging and provided support to the observation of satellite cells homing to the injured muscle. In the third experiment, satellite cells were labeled with quantum dots to support the observations of satellite cells homing to injured muscle and to provide information regarding the regenerative potential of the satellite cells following intravenous delivery.
2. Materials and Methods
2.1 Satellite Cell Isolation and Tissue Culture
Primary satellite cells were isolated from the gastrocnemius, soleus, and plantaris muscles as previously described (Allen et al. 1997). Briefly, muscles were excised, trimmed of excess connective tissue, hand-minced with sterile scissors, and digested for 1 hour at 37°C with 1.25 mg/ml pronase (Sigma, St. Louis, MO). Cells were then separated from muscle fiber fragments and tissue debris by differential centrifugation, pre-plated in 10% fetal bovine serum/Dulbecco’s modified Eagle’s medium (FBS/DMEM) for 2 hours, and then seeded in growth media (GM) composed of 20% FBS/DMEM with 1% antibiotic antimycotic (ABAM) (Invitrogen, Grand Island, NY) and 0.05% gentamicin (Invitrogen, Grand Island, NY) on tissue culture-treated plates coated with 20 μl/cm2 poly-lysine (0.1 mg/ml in distilled water) and a 10-μg/ml solution of fibronectin in sterile phosphate buffered saline (PBS).
2.2 Transfection
First passage satellite cells were transfected with pEGFP-C1 plasmid vector (BD Biosciences Clontech: GenBank Accession #:U55763) using FuGENE 6 (Roche, Indianapolis, Indiana) at a 1:3 ratio of deoxyribonucleic acid (DNA) (mg): transfection reagent (ml). Briefly, 12 mg of plasmid DNA diluted in a solution containing 1164 ml of Opti-MEM (Invitrogen, Grand Island, NY) and 36 ml of FuGENE 6 complex was added to each 15-cm dish. The cells were incubated for 6 hours at 37°C; then the media was replaced with growth media (20% FBS/DMEM with antibiotics). The pEGFP transfected cells were then radio-labeled with 111In-oxine as described below.
2.3 111In-oxine Labeling of Satellite Cells
Three days after GFP transfection satellite cells were trypsinized, washed with PBS, and resuspended in 1 ml of serum-free media (DMEM) at a concentration of 1 × 106 cells/ml. The cells were then incubated with 111In-oxine (GE Healthcare, Phoenix, AZ) at 100 μCi/million cells for 20 minutes at room temperature. To remove the unbound label, the cells were centrifuged at 400 g for 7 minutes (800 rpm for 7 minutes), washed twice with PBS, and the supernatant was removed (Brenner et al. 2004). Labeling efficiency of satellite cells ranged from 70% to 90%, as measured on a CRC-15W dose calibrator/well counter (Capintec, Ramsey, NJ). Cell viability based on trypan blue staining after cell replating was determined to be 97%, and there was no effect on the ability of satellite cells to differentiate into muscle when exposed to differentiation media (data not shown). Once cells were washed, cells were resuspended in DMEM for satellite cell transplantation as described below.
2.4 Quantum Dot Labeling of Satellite Cells
Passage two satellite cells were labeled with Qtracker 705 (Invitrogen, Grand Island, NY) for 3 hours at a concentration of 2 nM to 15 nM in antibiotic free growth media. After 3 hours, the media were changed to normal growth media (20% FBS/DMEM) with antibiotics. Twenty-four hours after labeling, cells were trypsinized with TrypLE Select (Invitrogen, Grand Island, NY) and resuspended in 100 ul of DMEM for intravenous injections.
2.5 Animal Preparations and Skeletal Muscle Injury
Adult male Fisher 344 rats (National Institutes of Health Aging Colony maintained by Harlan Sprague Dawley Laboratories) were used for the transplantation studies. They were housed at 21°C, maintained on a 12:12-h light-dark cycle, and allowed free access to food and water. All animal care and use was conducted according to National Research Council guidelines and approved and supervised by the University of Arizona Institutional Animal Care and Use Committee.
Twenty four hours prior to satellite cell transplantation as described below, the tibialis anterior (TA) muscle was injured in three Fisher rats by injecting 0.2 ml of 0.75% bupivacaine hydrochloric acid (HCl) (Sensorcaine, AstraZeneca), a local anesthetic that causes acute muscle fiber necrosis and massive cellular infiltration of macrophages, followed by rapid regeneration of the muscle fiber (Benoit and Belt 1970); but it does not permanently damage satellite cells (Hall-Craggs 1980), the vasculature (Grim et al. 1988), basal lamina, or endomysial tubes (Hall-Craggs 1974). The TA muscle was selected because it is a superficial muscle that is injured and imaged easily with gamma ray and other optical imaging modalities. After experiments, the animals were sacrificed by intraperitoneal injection of ketamine (80 mg•kg 1), xylazine (10 mg•kg 1), and acepromazine (4 mg•kg 1), followed by exsanguination.
2.6 111In-oxine labeled Satellite Cell Transplantation
Animals were anesthetized with 1.0–2.0 % isoflurane. Intramuscular cell transplantation in two healthy rats without muscle injury was carried out by direct injection of 40 μL of 111In-oxine labeled satellite cells (6.0–10 x 105 cells) (60–100 μCi, 2.22–3.70 MBq) into the muscle using a syringe with 26-gauge needle. The cells in DMEM were delivered by inserting the needle from the proximal and then the distal ends of the muscle parallel to the long axis. Radioactivity was measured before and after injections in the syringes.
Three anesthetized Fisher rats with left TA injury received intravenous transplantation of 111In-labeled satellite cells. The cells (2 x 106 cells/100 μl, 200 μCi) were injected into the tail vein of each rat and chased with 100 μl of DMEM to assure proper dispensing of cells. After cell transplantation, the rats were allowed to recover, move freely, and have free access to food and water.
2.7 In vivo High-resolution SPECT Imaging and Analysis
Under anesthesia with 1% to 2% isoflurane, animals that received 111In-oxine-labeled satellite cells were imaged with a stationary high-resolution SPECT system, FastSPECT II, which was built in the Center for Gamma-Ray Imaging of the University of Arizona. FastSPECT II consists of 16 modular scintillation cameras. Each camera has a scintillation crystal of sodium iodide doped with thalium [NaI (Tl)] and a 3 × 3 array of 1.5-inch-diameter photomultiplier tube (PMT). The spatial resolution of the system is approximately 1 mm, and the system sensitivity is 10 cpc/mCi (Liu et al. 2007). The field of view of FastSPECT II is 40 mm, but for this study, the focus was the region around the legs. Animals were transported into the system aperture using a translational stage and positioned so that both the right and left TA muscles were in the center of the field of view.
High-resolution SPECT images were acquired at 2–3, 24, 72, 144, and 168 hours post-administration of 111In-oxine-labeled satellite cells in the intramuscular and intravenous injection groups. Intramuscular injections were used to demonstrate the feasibility of satellite cell labeling and estimation of cell location over time. Acquisitions were obtained for 5 minutes from a total of 16 projections to generate a data set for tomographic reconstruction. To enhance signal intensity for better visualization and region-of-interest (ROI) establishment, images at 168 hours post-injection were acquired for 10 minutes. The physical half-life of 111In allowed for the monitoring of cell distribution for at least 7 days. Serial tomographic transaxial, coronal, and sagittal slices with one-pixel thickness (0.5 mm) were generated by using 50 iterations of the ordered-subset expectation maximization (OS-EM) algorithm. The reconstructed coronal slices were selected and quantitatively analyzed by creating Ellipsoid or 3D Isocontour region of interest (ROI) using AMIDE (A Medical Imaging Data Examiner) 0.9.1 software. The ROI was created and centered on the right or left TA muscle areas with or without injury using previous scans of rats with fiduciary markers. The radioactive counts over the ROIs were applied to generate regional time-activity curves from 2–3 to 168 hours post intramuscular or intravenous 111In-labeled cell injection, which were corrected for ROI size (pixels), radioactive decay, and acquisition time. Care was taken to draw ROIs on both sides consistently. To avoid effects from variations of injected dose, individual TAs were normalized at each time point for initial peak counts at 3 hours post-injection.
2.8 Biodistribution measurement and Autoradiography
On day 7 after cell transplantation and last imaging of intravenously injected rats, they were euthanized and major organs, injured muscles, and remote control muscles were collected, weighed, and radioactivity measured using a CRC-15W dose calibrator/well counter (Capintec, Ramsey, NJ). Radioactivity in the tissue was corrected for 111In decay and normalized to the tissue weight and injected dose (%ID/g). Spatial distribution of radioactivity in the TA and extensor digitorum longus (EDL) muscles was visualized using autoradiograph imaging. The muscles were removed and exposed to Fuji Film phosphor imaging plates for 15 to 30 minutes. Images were acquired from the plates using a Fuji Film BAS5000 Bio-Imaging Analysis System (Stamford, CT).
2.9 Immunostaining of Tissue Sections
Selected muscle samples from intravenously injected rats were embedded in optimal cutting temperature (OCT) tissue-freezing media (Tissue Tex, Inc.) using 2-methylbutane precooled in liquid nitrogen 7 days after injection. TA muscles were cryo-sectioned at 10-um thickness and stained for dystrophin, MyoD, Pax7, and 4′,6-diamidino-2-phenylindole (DAPI). Antibodies used in staining include rabbit anti-dystrophin (Santa Cruz Biotechnology, Inc., Dallas, TX; 1: 200) with a secondary Alexa Fluor 488 donkey anti-rabbit (Invitrogen, Grand Island, NY; 1:700), anti-MyoD with a secondary Alexa fluor 488 (Molecular Probes; 1: 1000), mouse anti-Pax7 with a secondary Alexa fluor 488 (Molecular Probes; 1:1000), and a glycerol DAPI stain (Santa Cruz) was used to cover slip slides for visualization of nuclei. For muscles injected with 111In oxine and GFP-labeled cells, tissues were embedded in OCT tissue freezing media (Tissue Tex, Inc.) using 2-methylbutane precooled in liquid nitrogen. TA muscles were cryosectioned at 10-μm thickness, and a glycerol DAPI stain (Santa Cruz Biotechnology, Inc., Dallas, TX) was used to cover slip slides for visualization of nuclei, and GFP-labeled cells.
2.10 GFP RT-PCR Assay
TA and EDL muscles from animals injected intravenously were excised, frozen in liquid nitrogen, and stored in a −80°C freezer for later DNA extraction. Tissue samples were ground into fine powder in mortar and pestle with liquid nitrogen and digested using the DNEasy kit (Qiagen Inc., Valencia, CA). For real-time PCR measurements, DNA samples were loaded at 5 ng/ml using SYBR green (BioRad Laboratories, Hercules, CA) and readings acquired using the Bio-Rad IQ5 icycler. The pEGFP primers included Forward: GAC GTA AAC GGC CAC AAG TT, and Reverse: AAG TCG TGC TGC TTC ATG TG. The annealing temperature used for these experiments was 63°C.
2.11 Statistical Analyses
The results are presented as mean intensity normalized to the control leg. All quantitative data are expressed as mean ± standard error of the mean (SEM). Statistical comparisons of variables were assessed using ANOVA or a paired-t-test, and p < 0.05 was considered significant.
3. Results
3.1 FastSPECT II imaging of 111In-oxine-labelled cells into uninjured muscle
Approximately 6–10 x 105 cells were injected intramuscularly into uninjured muscles in each rat. As shown in Fig. 1a, the FastSPECT II images acquired for 5–10 minutes showed sufficient radioactive intensity on the injected sites to define the individual legs for comparison. The in vivo focal distributions of 111In radioactivity could be well visualized on tomographic images from 2 to 168 hours post-injection. The radioactive distribution in the control left leg was invisible and at the level of soft-tissue background. The time-activity curves of right TA muscle versus the control side (left TA) measured by ROI analysis over time were shown in Fig. 1b. After corrected for the 111In decay and acquisition time, a significant difference was observed at each time point from 2 hours to 168 hours between the left and right leg. The percentage of initial radioactivities at 2 hours post-injection on the right TA muscle that persisted for 7 days was 9.97±1.46%. Assuming that most radioactivity remains associated with satellite cells, the diminishing activity reflects diminishing cell numbers and is consistent with low survival rates of cultured satellite cells injected intramuscularly (Skuk and Tremblay 2003).
Fig 1.
a Representative FastSPECT II Images (coronal view) of legs in a healthy rat received intramuscularly transplanted 111In-oxine labeled satellite cells in right TA muscle. The legs of the rat were imaged using FastSPECT II over 168 hours. Images were taken with 5-min acquisition times. Time-activity curves of legs were generated by ROI analysis and plotted below in panel (b), which indicate locally administered radioactivity in the healthy muscle decreases with time. Based on this in vivo radioactive data, 5–10% of transplanted cells were estimated to survive out to 168 hours.
3.2 FastSPECT II Imaging of systemically delivered 111In-oxine-labeled satellite cells in injured sites
To determine whether satellite cells can be transplanted systemically (intravenously) and migrate to sites of injury, the injured and control muscles in anesthetized rats were imaged by FastSPECT II for 15–30 minutes at 3, 24, 72, 144, and 168 hours post transplantation of 111In-oxine-labeled satellite cells. Twenty four hours after bupivicaine-induced muscle injury and three hours after cell injection, the radioactive distribution in both injured and control muscle was barely detectable and at the level of soft-tissue background. The in vivo focal distributions of 111In radioactivity in the injured legs, which were identifiable on multiple transverse, coronal, and sagittal slices, could be visualized on tomographic images after 24 hours of cell transplantation (Fig. 2a). The radioactivities in the control legs remained at low background level up to 168 hours post-injection. Quantitative results of computerized ROI analysis showed that the injection of 111In-oxine-labeled satellite cells resulted in very little radioactive accumulation in the injured leg 3 hours after intravenous injection of satellite cells, but radioactivity increased after 24 hours and up to a peak at 72 hours post transplantation, after which the radioactivity diminished slightly (Fig. 2). After radioactive distributions detected by FastSPECT II imaging were compensated for differences in injected dose as well as 111In decay over time, the results of imaging measurements in the injured sites were significantly different from that in the control areas at 72, 144, and 168 hours (p < 0.05). Seven days after injury there was an ~ 26% reduction in the wet weight of the TA muscle from the injured leg as compared to the non-injured leg (p < 0.05), but no difference between the EDL muscles between injured and control legs. This suggests the injury was significant but the therapeutic transfer of satellite cells was insufficient to maintain muscle mass at that time point.
Fig 2.
a Representative FastSPECT II Images (coronal view) of legs in a rat with tibialis anterior (TA) injury received intravenously transplanted 111In-oxine labeled satellite cells. Twenty-four hours after bupivacaine-induced injury to the TA muscle, 2 × 106 satellite cells were injected into the tail vein (0 hours), and muscles were imaged with FastSPECT II imaging at 3, 24, 72, 144, and 168 hours after injection. Identical radioactive accumulation could be visualized on images collected at 3 to 168 hours post-injection. b Quantification of radioactivity in TA muscle following systemic delivery of 111In-oxine labeled satellite cells. Radioactivity levels were corrected for 111In decay and acquisition time. At 72, 144, and 168 hours after systemic injection of satellite cells, the radioactivity in injured TA was significantly higher than the control leg (n = 3, p < 0.05). (*) indicates significantly different from the control non-injured leg. Values are mean ± standard error of the mean (SEM).
3.3 Biodistribution and Spatial Distribution of Systemically Delivered 111In-oxine-Labeled Satellite Cells
The results of biodistribution measurement 7 days after intravenous cell injection and FastSPECT II imaging in the rats with muscle injury are shown in Figure 3a. High levels of radioactivity were found in the lung, liver, spleen, and kidneys. Radioactivities in the injured muscles (TA, EDL, gastrocnemius, and soleus) were significantly higher than that in control muscles (Fig. 3a inset; p < 0.05). Muscle mass was not different between control and injured EDL muscles; however, muscle weight was significantly less in the injured TA (Fig. 3b). Autoradiography was also performed to determine spatial distribution of radioactivity and to confirm the FastSPECT II imaging results in the injected TA muscle and underlying EDL. Intense signal was observed in the injured TA and EDL muscles but only slight background signal from the non-injured TA and EDL muscles (Fig. 4).
Fig 3.
a Biodistribution of radioactivity following intravenous (IV) injection of 111In-oxine labeled satellite cells. Bupivicaine was injected intramuscularly for injury. Seven days after IV injection and after the completion of FastSPECT II imaging, some muscles and organs were explanted and their level of radioactivity determined. In addition to an increase in radioactivity of muscles of the injured leg (inset), there was an increase in radioactivity in organs such as the lung, liver, spleen, and kidneys. Data were computed as radioactivity normalized to mass of tissue and presented as mean ± SEM, n = 3 per organ. b Wet weight of tibialis anterior (TA) and extensor digitorum longus (EDL) muscles seven days after intravenous (IV) injection of 111In-oxine labeled satellite cells. Values are mean ± SEM, n = 3.
Fig 4.

Autoradiography images of bupivicaine-damaged tibialis anterior (TA) and extensor digitorum longus (EDL) muscles (left) versus uninjured TA and EDL muscles (right). After imaging with FastSPECT II for seven days, the TA and EDL muscles were explanted and autoradiography images were taken. The right side of each panel is an image of the left TA muscle with the TA muscle at the top and the EDL muscle below. Images a, b, and c support time-based imaging results shown in Fig. 2 and indicate the presence of transplanted satellite cells to the injured muscle seven days after intravenous injection. These images are from the same rats from which data were collected as described in Figure 2, where injury was induced in the TA muscle 24 hours prior to intravenous delivery of 111In- oxine labeled satellite cells.
3.4 Systemically Delivered Satellite Cells Labeled with 111In-Oxine and eGFP Migrate to Injury Sites eGFP verification
Satellite cells co-labeled with 111In- oxine and eGFP were injected systemically as previously described. Since the cells were transfected with the pEGFP vector during culture, fluorescent imaging as well as real-time PCR techniques were used to validate the existence of transplanted satellite cells within the injured TA muscles at 7 days post-transplantation. The visualization of eGFP-labeled cells indicate that co-labeled cells reside within the injured muscle (Fig. 5a), an observation that was confirmed quantitatively with eGFP gene expression (Fig. 5b). The difference in GFP in injured muscles was similar to that determined with FASTSPECT imaging (Fig. 2). These data support the contention that the intense radioactivity in the left TA muscles (injured side) is associated with the transplanted satellite cells.
Fig 5.
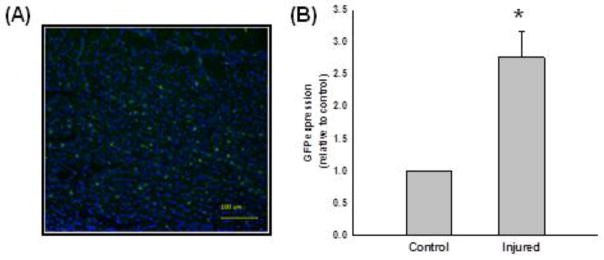
Twenty-four hours after bupivicaine-induced injury to the TA muscle, 2 × 106 satellite cells transfected with a GFP expression plasmid and labeled with 111In-oxine were injected intravenously, and muscles were imaged with FastSPECT II imaging at 2–3, 24, 72, 144, and 168 hours after injury. Seven days after transplantation and after the completion of in-vivo FastSPECT II imaging, TA muscles were explanted. a GFP was imaged histologically to confirm the localization of satellite cells within injured TA muscle (n = 1). The DAPI nuclear stain (blue) was used to co-stain section with GFP labeled cells (green). These data support the contention that 111In-oxine labeled cells injected systemically migrated to, and were localized within, the injured muscle. b mRNA levels of TA muscles were measured with RT-PCR. The yellow scale bar represents 100 μm.
3.5 Systemically Delivered Satellite Cells Labeled with Quantum Dots Migrate to Injury Sites
To provide additional confirmation that systemic administration of cultured satellite cells results in migration to sites of muscle injury, cells were labeled with quantum dots and injected intravenously 24 hours after bupivacaine-induced injury. Satellite cells labeled with quantum dots (red) were found in regenerating skeletal muscle 6 days after injection (Figs. 6a–c). Satellite cells labeled with quantum dots and expressing MyoD were also observed in (Figs. 6d–e) providing evidence that these cells remain myogenic. Although limited, some transplanted cells still express Pax7 a marker for satellite cells (Fig. 6f).
Fig 6.
Satellite cells were labeled with quantum dots (QD705 nm) and injected intravenously 24 hours after bupivacaine-induced muscle injury of left TA muscle. Seven days after injury (6 days post-transplantation), the left TA muscle was explanted, cryosectioned, and visualized for the presence of quantum dot labeled cells and stained for myogenic markers. Panels a and b are sections from rat TA muscle stained for dystrophin (green), DAPI nuclear stain (blue), and QD705 labeled satellite cell (red). Panel c is a magnification of the outlined image in panel image b. Panel d is an image of TA muscle stained for MyoD (green) and QD705 satellite cells (red), e image of TA muscle stained for MyoD (green), DAPI nuclear stain (blue), and QD705 labeled satellite cells (red), f image of TA muscle stained for Pax7 (green), DAPI nuclear stain (blue), and QD705 labeled satellite cells (red). Yellow scale bar is 100 μM. QD labeled satellite cells migrate to sites of injury and stain positive for MyoD (d), contribute to regeneration (e), and stain positive for Pax7 (f).
4. Discussion
To address the issue of whether 111In-oxine labeling and SPECT imaging can be used for in vivo quantification of satellite cell homing when delivered systemically, and whether satellite cells migrate to injured tissue after intravenous injections, three different approaches were used in these experiments, each providing unique advantages that provide information about cell survival, migration, and phenotype. Radiolabeling cells with 111In oxine has the advantage of quantification in real time and with high sensitivity for approximately 1 week. As demonstrated, labeled cells can be imaged using SPECT techniques and can provide quantifiable measurements based on these images. It was important, however, to verify that the radiolabel remained associated with the cells that were originally injected, which was accomplished by transfecting cultured cells with a plasmid expressing GFP prior to labeling with 111In-oxine, which provided a sensitive, quantifiable marker of cell survival with a low number of cells. The third tracking approach involved pre-loading cultured satellite cells with fluorescent quantum dots, followed by immunolocalization of labeled cells in frozen sections of muscle at the end of the experiment. In order to study the ability of systemically delivered satellite cells to home to injured tissue bupivacaine injections were used to generate muscle damage which provided a stimulus for migration and homing. Bupivacaine is a known cytotoxin that causes damage to muscle fibers without damaging the satellite cell population (Hall-Craggs 1974), and injury to skeletal muscle has been shown to improve satellite cell engraftment (Torrente et al. 1999). Using this common model of muscle injury, we sought to determine the usefulness of 111In oxine labeling for tracking cells. The highly sensitive nature of radioisotopes combined with imaging capabilities have provided the means to determine migration that may have been overlooked in the past using standard techniques of histological assessment of sectioned tissue. By utilizing the non-invasive high-resolution SPECT imaging, long-term studies of transplanted satellite cells were possible without sacrificing numerous rats. As shown in Fig. 2, there was a significant increase in the number of 111In-oxine labeled cells in the injured leg, as compared to the non-injured control leg after intravenous injection. Autoradiography of the muscles verified the FastSPECT II analysis (Fig. 4). Interestingly, we observed a similar increase in GFP gene expression (Fig. 5) in the injured leg, which is consistent with FastSPECT II imaging data (Fig. 2); this provides verification of the 111In-oxine labeling and FastSPECT II imaging data. In addition, analysis of GFP expression suggests an egress from the vasculature to the injured TA muscle and not to the control non-injured side, similar to that found with 111In-oxine. Although in the current study the persistence of cells in the lungs was not measured over time, it is interesting to compare our findings with those of Huang et al. (Huang et al. 2010) where the arrival of embryonic stem cell-derived endothelial cells in the injured muscle was coincident with a decrease in the number of cells localized in the lungs. A plausible speculation is that the increased cell localization in the injured muscle over time in the current study was a result of cells migrating from the lungs or other clearance organs.
The biodistribution of the 111In-oxine labeled cells indicates that high levels of activity were measured in the lung, liver, spleen, and kidneys (Fig. 2a), similar to that previously described (Gao et al. 2001; Hou et al. 2005). Since the EDL muscle lies directly below the TA muscle, it is likely that the bupivacaine leaked into the EDL muscle causing muscle damage or that the damage in the TA muscle caused compensation by the EDL muscle resulting in an increased need for repair and satellite cell recruitment. Subsequent experiments using QD705-loaded cells verified that the data generated by 111In-oxine labeling was consistent with the migration of labeled satellite cells to the injury site (Fig. 6). Furthermore, the detection of MyoD and, to a lesser extent Pax7, in QD705-loaded cells demonstrated that injected cells had maintained their myogenic potential. Collectively, these data support the idea that systemically injected satellite cells migrate to the site of injury and that 111In-oxine labeling and SPECT imaging can be used to track this process.
Systemic delivery of satellite cells and other stem cells promises to be a valuable technique, but it has not been easy to study. To date, most of the current knowledge of cell migration has been elucidated through in-vitro migration assays in which lack the tissue complexity derived from contributing factors from immune cells, extracellular matrix interactions, three-dimensional nature of cell migration, and fiber-to-fiber migration. A compelling demonstration of the utility of 111In-oxine labeling and SPECT tracking is the observation that transplanted satellite cells do not go directly to the site of injury after tail vein injections but arrive there over a 3-day time frame. These results raise questions as to where the cells go during this time and what mechanisms are involved in homing to an injury site. A limitation to the current study was that cell proliferation and cell viability in vivo was not measured; therefore, it is not possible to ascertain the extent to which the cells localized to a specific area are representative of the entire, original population delivered. Nonetheless, from a therapeutic perspective the current study supports the idea that satellite cells can be delivered systemically to injured muscle, regardless of changes in cell kinetics. Cells injected into both injured and non-injured animals were found in the lungs, kidney, and spleen; however, in the injured rats, more cells migrated to the site of injury. The 111In-oxine/SPECT tracing technique lends itself to experiments that can address this question, and this tracking technique may facilitate studies designed to improve targeted migration from the bloodstream to the site of injury after intravenous injection.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants HL-007955 (JE), NIBIB P41-EB002035, and the Muscular Dystrophy Association 3685 (RA).
References
- Allen RE, Temm-Grove CJ, Sheehan SM, Rice G. Skeletal muscle satellite cell cultures. Methods Cell Biol. 1997;52:155–176. doi: 10.1016/s0091-679x(08)60378-7. [DOI] [PubMed] [Google Scholar]
- Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A. 2004;101(10):3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit PW, Belt WD. Destruction and regeneration of skeletal muscle after treatment with a local anaesthetic, bupivacaine (Marcaine) J Anat. 1970;107(Pt 3):547–556. [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Webster C, Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 1983;80(15):4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U, Heeschen C, Kampen WU, Zeiher AM, Dimmeler S, Henze E. 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med. 2004;45(3):512–518. [PubMed] [Google Scholar]
- Cahill KS, Germain S, Byrne BJ, Walter GA. Non-invasive analysis of myoblast transplants in rodent cardiac muscle. Int J Cardiovasc Imaging. 2004;20(6):593–598. doi: 10.1007/s10554-004-3902-8. [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol. 2000;89(4):1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340(2):330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279(5356):1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Furenlid LRWD, Chen YC, Kim H, Pietraski PJ, Crawford MJ, Barrett HH. FastSPECT II: a second-generation high-resolution dynamic SPECT imager. IEEE Trans Nucl Sci. 2004;51:631–635. doi: 10.1109/TNS.2004.830975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Grim M, Rerabkova L, Carlson BM. A test for muscle lesions and their regeneration following intramuscular drug application. Toxicol Pathol. 1988;16(4):432–442. doi: 10.1177/019262338801600403. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs EC. Rapid degeneration and regeneration of a whole skeletal muscle following treatment with bupivacaine (Marcain) Exp Neurol. 1974;43(2):349–358. doi: 10.1016/0014-4886(74)90176-9. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs EC. Survival of satellite cells following exposure to the local anesthetic bupivacaine (Marcaine) Cell Tissue Res. 1980;209(1):131–135. doi: 10.1007/BF00219929. [DOI] [PubMed] [Google Scholar]
- Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(9 Suppl):I150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30(5):984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Hallauer PL, Hastings KE, Tremblay JP. Prior culture with concanavalin A increases intramuscular migration of transplanted myoblast. Muscle Nerve. 1998;21(3):291–297. doi: 10.1002/(sici)1097-4598(199803)21:3<291::aid-mus2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290(2):C609–615. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- Lin S, Xie X, Patel MR, Yang YH, Li Z, Cao F, Gheysens O, Zhang Y, Gambhir SS, Rao JH, Wu JC. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007;7:67. doi: 10.1186/1472-6750-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Barrett HH, Stevenson GD, Kastis GA, Bettan M, Furenlid LR, Wilson DW, Pak KY. High-resolution imaging with (99m)Tc-glucarate for assessing myocardial injury in rat heart models exposed to different durations of ischemia with reperfusion. J Nucl Med. 2004;45(7):1251–1259. [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kastis GA, Stevenson GD, Barrett HH, Furenlid LR, Kupinski MA, Patton DD, Wilson DW. Quantitative analysis of acute myocardial infarct in rat hearts with ischemia-reperfusion using a high-resolution stationary SPECT system. J Nucl Med. 2002;43(7):933–939. [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhao M, Zhu X, Furenlid LR, Chen YC, Barrett HH. In vivo dynamic imaging of myocardial cell death using 99mTc-labeled C2A domain of synaptotagmin I in a rat model of ischemia and reperfusion. Nucl Med Biol. 2007;34(8):907–915. doi: 10.1016/j.nucmedbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, Furling D, Gidaro T, Jacquemin V, Mamchaoui K, Negroni E, Perie S, Renault V, Silva-Barbosa SD, Butler-Browne GS. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand. 2005;184(1):3–15. doi: 10.1111/j.1365-201X.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- Neumeyer AM, DiGregorio DM, Brown RH., Jr Arterial delivery of myoblasts to skeletal muscle. Neurology. 1992;42(12):2258–2262. doi: 10.1212/wnl.42.12.2258. [DOI] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, Robichaud KJ, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25(8):2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456(7221):502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL. Effects of skeletal muscle regeneration on the proliferation potential of satellite cells. Mech Ageing Dev. 1985;30(1):63–72. doi: 10.1016/0047-6374(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8(3):217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P, Hogrel JY, Paradis M, Bouchard JP, Sylvain M, Lachance JG, Tremblay JP. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17(1):38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Skuk D, Tremblay JP. Myoblast transplantation: the current status of a potential therapeutic tool for myopathies. J Muscle Res Cell Motil. 2003;24(4–6):285–300. [PubMed] [Google Scholar]
- Torrente Y, D’Angelo MG, Del Bo R, DeLiso A, Casati R, Benti R, Corti S, Comi GP, Gerundini P, Anichini A, Scarlato G, Bresolin N. Extracorporeal circulation as a new experimental pathway for myoblast implantation in mdx mice. Cell Transplant. 1999;8(3):247–258. doi: 10.1177/096368979900800305. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El Fahime M, D’Angelo MG, Caron NJ, Constantin G, Paulin D, Scarlato G, Bresolin N. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152(2):335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE. Myoblast senescence in muscular dystrophy. Exp Cell Res. 1985;157(2):343–354. doi: 10.1016/0014-4827(85)90119-3. [DOI] [PubMed] [Google Scholar]






