Abstract
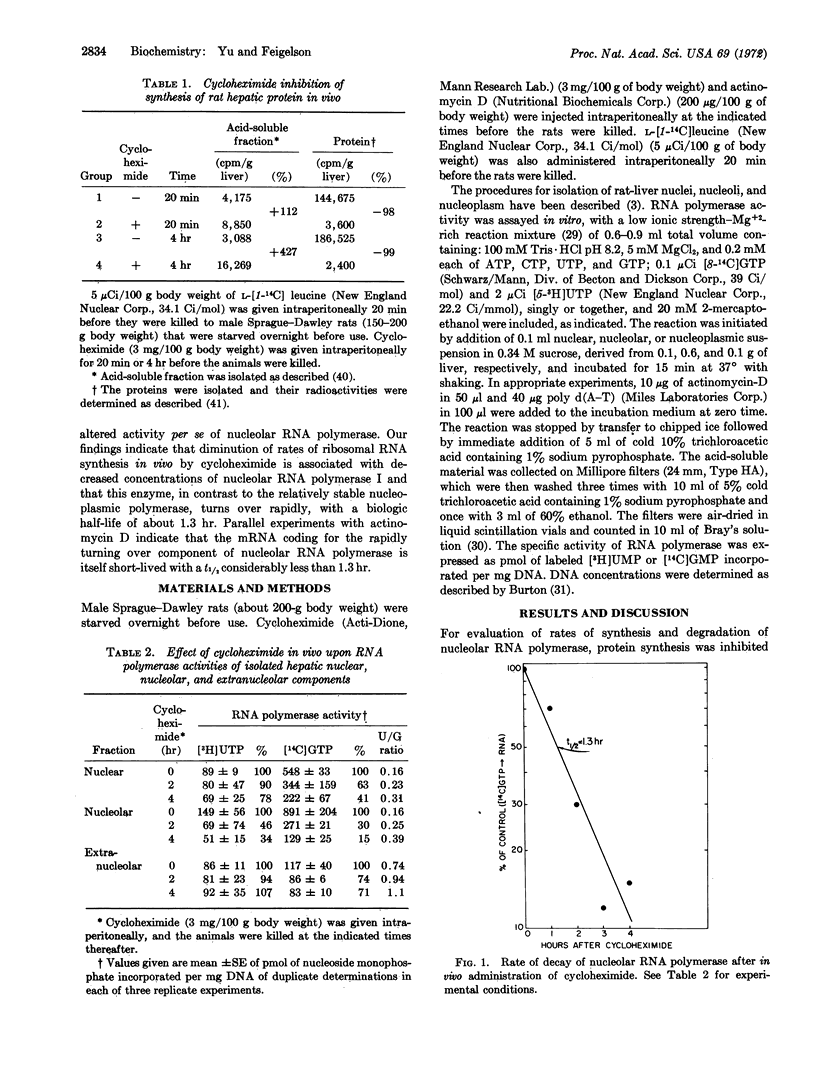
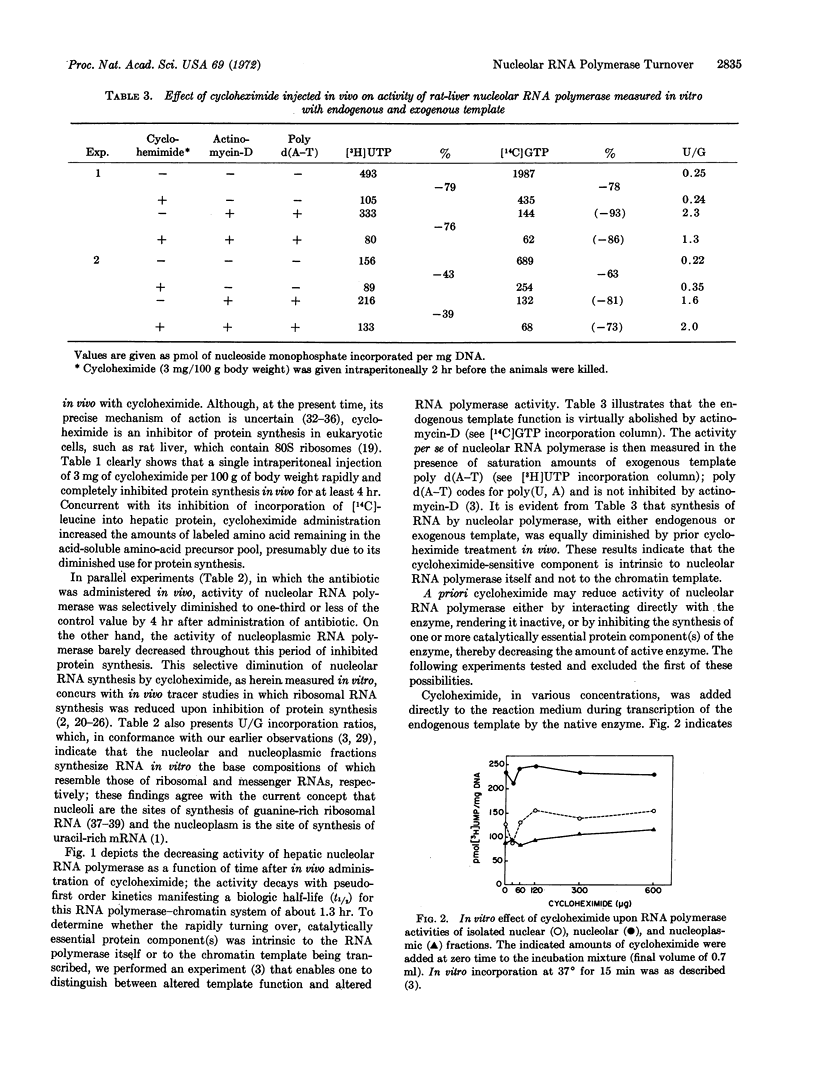
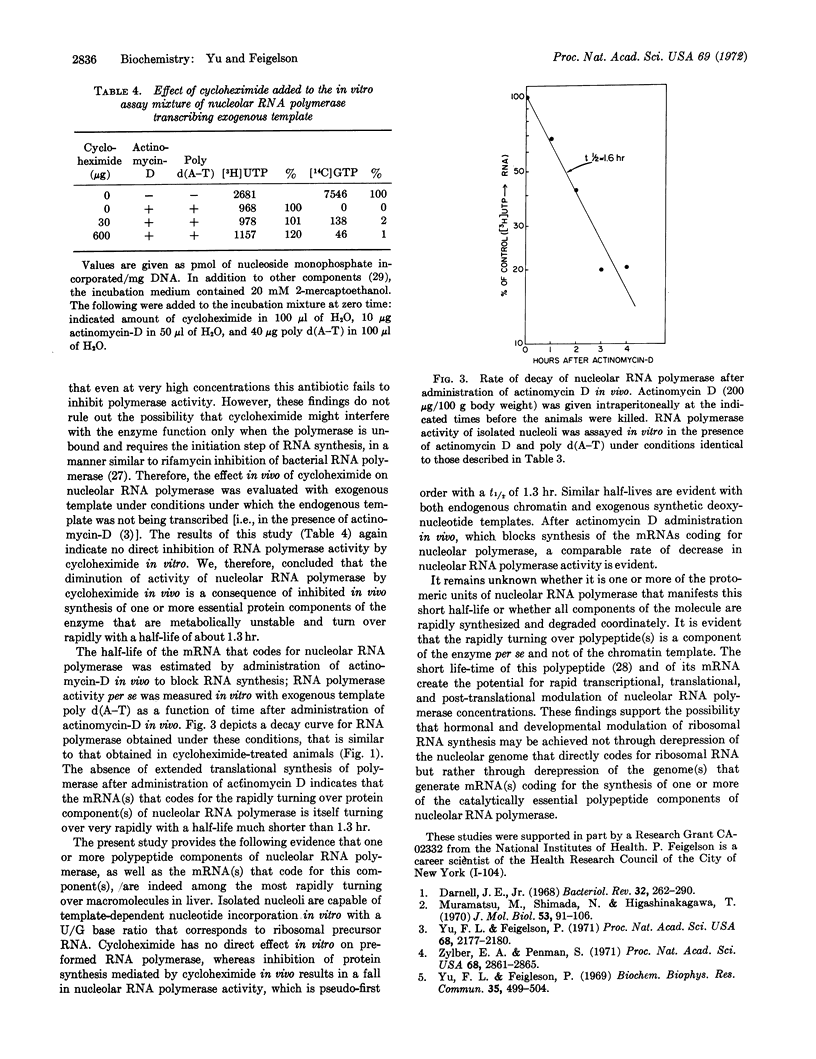
Turnover rates of the components of systems for RNA synthesis of rat-liver nucleus, nucleolus, and nucleoplasm were investigated. Cycloheximide administered in vivo selectively diminished nucleolar RNA synthesis in vitro. In contrast to the relatively stable nucleoplasmic RNA polymerase, nucleolar RNA polymerase (polymerase I) from rat liver decays rapidly upon cycloheximide administration, following pseudo-first order kinetics with a half-life of about 1.3 hr. Cycloheximide elicits this effect not through direct interaction with nucleolar RNA polymerase itself, nor by alteration of template function, but rather by inhibition of de novo synthesis of one or more of the protein components of nucleolar RNA polymerase. Similarly, when actinomycin-D was administered in vivo to inhibit RNA synthesis, the rate of decay of nucleolar RNA polymerase, assayed in the presence of exogenous poly d(A-T) template, was similar to that observed after cycloheximide administration. Thus, the messenger RNA(s) that codes for one or more of the catalytically essential polypeptide components of this enzyme turn over very rapidly with a half-life considerably shorter than 1.3 hr. The rapidity of turnover of both the enzyme protein and its messenger RNA(s) renders nucleolar RNA polymerase highly responsive to altered transcriptional, translational, or post-translational modulation. The marked differences in turnover rates of nucleolar and nucleoplasmic RNA polymerases indicate that at least certain of the protomeric components of nucleolar RNA polymerase I are distinct from those of nucleoplasmic RNA polymerases II and III.
Keywords: cycloheximide, actinomycin D, polymerase I
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN D. D., GURDON J. B. ABSENCE OF RIBOSOMAL RNA SYNTHESIS IN THE ANUCLEOLATE MUTANT OF XENOPUS LAEVIS. Proc Natl Acad Sci U S A. 1964 Jan;51:139–146. doi: 10.1073/pnas.51.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliga B. S., Pronczuk A. W., Munro H. N. Mechanism of cycloheximide inhibition of protein synthesis in a cell-free system prepared from rat liver. J Biol Chem. 1969 Aug 25;244(16):4480–4489. [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEIGELSON P., FEIGELSON M. Studies on the mechanism of regulation by cortisone of the metabolism of liver purine and ribonucleic acid. J Biol Chem. 1963 Mar;238:1073–1077. [PubMed] [Google Scholar]
- FIALA E. S., DAVIS F. F. PREFERENTIAL INHIBITION OF SYNTHESIS AND METHYLATION OF RIBOSOMAL RNA IN NEUROSPORA CRASSA BY ACTIDIONE. Biochem Biophys Res Commun. 1965 Jan 4;18:115–118. doi: 10.1016/0006-291x(65)90892-2. [DOI] [PubMed] [Google Scholar]
- FUKUHARA H. RNA SYNTHESIS OF YEAST IN THE PRESENCE OF CYCLOHEXIMIDE. Biochem Biophys Res Commun. 1965 Jan 18;18:297–301. doi: 10.1016/0006-291x(65)90757-6. [DOI] [PubMed] [Google Scholar]
- Franze-Fernández M. T., Pogo A. O. Regulation of the nucleolar DNA-dependent RNA polymerase by amino acids in Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3040–3044. doi: 10.1073/pnas.68.12.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Adamson S. D., Herbert E. Effects of cycloheximide on polyribosome function in reticulocytes. J Mol Biol. 1967 Jul 14;27(1):57–72. doi: 10.1016/0022-2836(67)90351-8. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. EFFECTS OF PUROMYCIN ON RNA SYNTHESIS IN MAMMALIAN CELLS. Proc Natl Acad Sci U S A. 1963 Sep;50:436–446. doi: 10.1073/pnas.50.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J., Feigelson P. Turnover of protein and RNA of liver ribosomal components in normal and cortisol-treated rats. Biochim Biophys Acta. 1970 Jan 21;199(1):214–223. doi: 10.1016/0005-2787(70)90710-0. [DOI] [PubMed] [Google Scholar]
- Higashi K., Matsuhisa T., Kitao A., Sakamoto Y. Selective suppression of nucleolar RNA metabolism in the absence of protein synthesis. Biochim Biophys Acta. 1968 Sep 24;166(2):388–393. doi: 10.1016/0005-2787(68)90226-8. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of actidione and other antifungal agents on nucleic acid and protein synthesis in Saccharomyces carlsbergensis. J Gen Microbiol. 1958 Dec;19(3):497–506. doi: 10.1099/00221287-19-3-497. [DOI] [PubMed] [Google Scholar]
- Kedinger C., Gniazdowski M., Mandel J. L., Jr, Gissinger F., Chambon P. Alpha-amanitin: a specific inhibitor of one of two DNA-pendent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970 Jan 6;38(1):165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Vaughan M. H., Warner J. R., Darnell J. E. Effects of valine deprivation on ribosome formation in HeLa cells. J Mol Biol. 1969 Oct 28;45(2):265–275. doi: 10.1016/0022-2836(69)90104-1. [DOI] [PubMed] [Google Scholar]
- McKeehan W., Hardesty B. The mechanism of cycloheximide inhibition of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1969 Aug 15;36(4):625–630. doi: 10.1016/0006-291x(69)90351-9. [DOI] [PubMed] [Google Scholar]
- Miyahara E., Abe H., Yamana K. Differential inhibition of 28S-18S RNA and 5S RNA synthesis in the cycloheximide-treated Xenopus laevis embryonic cells. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1070–1075. doi: 10.1016/0006-291x(70)90903-4. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Shimada N., Higashinakagawa T. Effect of cycloheximide on the nucleolar RNA synthesis in rat liver. J Mol Biol. 1970 Oct 14;53(1):91–106. doi: 10.1016/0022-2836(70)90047-1. [DOI] [PubMed] [Google Scholar]
- Obrig T. G., Culp W. J., McKeehan W. L., Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem. 1971 Jan 10;246(1):174–181. [PubMed] [Google Scholar]
- Perry R. P. THE CELLULAR SITES OF SYNTHESIS OF RIBOSOMAL AND 4S RNA. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmi S., Liang H., Sarma D. S., Kisilevsky R., Farber E. Cycloheximide, an inhibitor of peptide chain termination or release in liver in vivo and in vitro. Biochem Biophys Res Commun. 1971 Jan 22;42(2):259–265. doi: 10.1016/0006-291x(71)90096-9. [DOI] [PubMed] [Google Scholar]
- Shields R., Korner A. Regulation of mammalian ribosome synthesis by amino acids. Biochim Biophys Acta. 1970 Apr 15;204(2):521–530. doi: 10.1016/0005-2787(70)90172-3. [DOI] [PubMed] [Google Scholar]
- TSUKADA K., LIEBERMAN I. LIVER NUCLEAR RIBONUCLEIC ACID POLYMERASE FORMED AFTER PARTIAL HEPATECTOMY. J Biol Chem. 1965 Apr;240:1731–1736. [PubMed] [Google Scholar]
- Wanka F., Schrauwen P. J. Selective inhibition by cycloheximide of ribosomal RNA synthesis in chlorella. Biochim Biophys Acta. 1971 Dec 16;254(2):237–240. doi: 10.1016/0005-2787(71)90832-x. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M., Penman M., Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969 Apr;41(1):177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Cortisone stimulation of nucleolar RNA polymerase activity. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2177–2180. doi: 10.1073/pnas.68.9.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Effects of cortisone and actinomycin-D upon pyrimidine nucleotide and RNA metabolism in rat liver. Arch Biochem Biophys. 1969 Jan;129(1):152–157. doi: 10.1016/0003-9861(69)90161-1. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Paper disc estimation of radioactive RNA: studies on the presence and elimination of metabolically generated artifacts from labeled purine and pyrimidine precursors. Anal Biochem. 1971 Feb;39(2):319–321. doi: 10.1016/0003-2697(71)90420-9. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. Studies on the role of ammonium sulfate in nuclear transcription in vitro. Biochim Biophys Acta. 1972 Jun 22;272(1):119–123. doi: 10.1016/0005-2787(72)90039-1. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. The sequential stimulation of uracil-rich and guanine-rich RNA species during cortisone induction of hepatic enzymes. Biochem Biophys Res Commun. 1969 May 22;35(4):499–504. doi: 10.1016/0006-291x(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet S. R. Ribonucleic acid synthesis in yeast. The effect of cycloheximide on the synthesis of ribonucleic acid in Saccharomyces carlsbergensis. Biochem J. 1966 Jun;99(3):566–581. doi: 10.1042/bj0990566. [DOI] [PMC free article] [PubMed] [Google Scholar]


