Abstract
This Progress Report reviews recent advances in the utility of extracellular matrix (ECM)-mimic biomaterials in presenting and delivering therapeutic cells to promote tissue healing. This overview gives a brief introduction of different cell types being used in regenerative medicine and tissue engineering while addressing critical issues that must be overcome before cell-based approaches can be routinely employed in the clinic. A selection of 5 commonly used cell-associated, biomaterial platforms (collagen, hyaluronic acid, fibrin, alginate, and poly(ethylene glycol)) are reviewed for treatment of a number of acute injury or diseases with emphasis on animal models and clinical trials. This article concludes with current challenges and future perspectives regarding foreign body host response to biomaterials and immunological reactions to allogeneic or xenogeneic cells, vascularization and angiogenesis, matching mechanical strength and anisotropy of native tissues, as well as other non-technical issues regarding the clinical translation of biomatrix/cell-based therapies.
Keywords: cell-based therapy, biomaterials, cell presentation, extracellular matrix, foreign body response
1. Introduction
Cell-based regenerative medicine is increasingly being considered for treatment of acute injuries, degenerative diseases as well as congenital, metabolic, and immunological disorders as an alternative to full organ or tissue transplantation as well as drug, growth factor, or gene therapies.[1-7] The development of Food and Drug Administration (FDA)-approved cells that meet the set guidelines of high purity, potency, safety, and manufacturing quality for highly processed biologicals has yet to be fully realized.[8,9] There also lacks clear standards required for commercialization of many regenerative medicine products as compared to more well-defined devices or drugs.[8,9] Cell-based therapy often requires invasive isolation, cell purification, and expansion using optimized delivery platforms with high seeding density that may require millions or billions of cells per treatment to repair extensively damaged tissue such as after myocardial infarction.[10] Risks associated with fetal bovine serum supplemented culture and potential bacterial, viral, endotoxin, or bovine spongiform encephalopathy contamination must also be carefully evaluated.[11] Characterization of cell phenotype, function, and mode of action must also be appropriately addressed before cell-based treatments can be clinically translated.[11] Relevant pre-clinical studies and large-scale multicenter human clinical trials must address the specific disease or injury for intervention with careful consideration of the administration method, dosing, timing, duration, and end-points to assess for the safety and efficacy of cell-based therapies.[12]
Early regenerative therapies involved surgical harvestation of autologous differentiated cells such as chondrocytes and ex vivo expansion for final administration into the critical defect to restore tissue function.[13,14] Such methods were time-consuming and problematic as specific cell types de-differentiated in 2D culture and adopted a spindle-shaped morphology similar to fibroblasts, and had limited clonogenicity for in vitro expansion.[15] Allogeneic differentiated cells from cadavers have also been considered for β islet-cell or hepatocyte replacement therapy; however, transplant rejection and continued usage of immunosuppressive drugs have limited the long-term success of such therapies in many patients.[16,17] Although transgenic cells from pig that lack expression of 1, 3-α-galactose has been successfully developed to evade hyperacute rejection, delayed-onset rejection still occurs with increased antibody production against the xenogeneic cells along with coagulation cascade, macrophage, natural killer cell, and T cell activation.[18,19] Mesenchymal stromal/stem cells (MSCs) and progenitor cells have also been derived from a number of tissue sources for rapid expansion and differentiation into a number of cell types with appropriate growth factor induction for replacement of injured tissues.[20,21] Allogeneic MSCs have also been extensively used for treatment of acute injuries and immunological disorders due to their high secretion of immunomodulatory cytokines and growth factors without the need for strict human leukocyte antigen matching normally required for traditional organ or tissue graft transplantation.[22-24] MSCs’ transdifferentiation capacity into some types of epithelial tissues remains poorly understood and may not be appropriate for replacement of specialized ectoderm- or endoderm-derived tissues.[25] Although to a lesser degree than differentiated cells, MSCs are still limited in their expansion capacity and senesce over time and MSCs’ therapeutic potential can also vary greatly depending on the tissue source, culture conditions, age, or disease state of the patient.[26-28] Embryonic stem cells (ESCs) have been successfully differentiated into multiple cell types derived from endodermal, mesodermal, and ectodermal lineages however have been limited in their application in some countries due to ethical dilemmas.[29,30] Moreover, inability to completely differentiate the entire ESC population to the desired cell type is a major safety concern as undifferentiated ESCs can cause teratoma or teratocarcinoma formation and immune reactions in patients.[31] Amniotic epithelial cells harvested from placental tissue after normal term pregnancies can be differentiated in vitro into ectodermal-, mesodermal-, and endodermal-derived lineages although to varying degrees compared to ESCs but have not been shown to form teratomas in vivo.[32,33] Umbilical cord stem cells (UCs) have intermediate properties of both ESCs and MSCs with intermediate clonogenicity, differentiation capacity into ectodermal- and mesodermal-derived lineages, and do not form teratomas after in vivo administration.[34] UCs also can be harvested after birth, cryopreserved and banked for later culture and administration for both future autologous or allogeneic therapeutic applications.[35] Methods generating induced pluripotent stem cells (iPS) that do not involve ESC nuclear transfer (ie. plasmid or viral transfection, transcription factor, or small molecule induction) eliminate major ethical controversy associated with ESCs and allow for autologous cells to be subsequently differentiated and used for patient-specific cell regenerative therapies that avoid adverse immune reactions.[36] iPS generation efficiency however remains low and can vary greatly depending on which iPS creation method is employed and the specific cell type that is utilized.[37] As iPS cells are reverted to an ESC-like state, possible teratoma, teratocarcinoma, or tumor formation due to continued oncogene induction (Klf4 and c-MYC), or undesirable side effects after transcription factor transgene expression using lentiviral or retroviral constructs are significant barriers that prevent clinical translation.[38] Genetically modified cells transfected with viral or non-viral carriers for enhanced growth factor (VEGF, PDGF) or anti-apoptosis (bcl2 or Akt) expression have also been considered for regenerative medicine.[39] The transfection efficiency and long-term stability as well as potential risks associated with immunological reactions, oncogene activation, or aberrant cell signaling have all hindered full clinical translation of genetically modified cells.[39] In short, there are numerous cell sources that are viable candidates for cell-based therapies. Although each has its own unique advantages and disadvantages, an effective delivery and presentation of these cells is one shared application requirement for all of these potentially therapeutic cell types.
2. Scaffold-free Cell Delivery
Despite these on-going challenges, cell therapies remain a viable and very active research direction particularly in tackling complex degenerative diseases and wounds which are not amenable to single agent therapies. Direct injection of therapeutic cells has been used in several clinical trials due to the ease in administration, easy storage, and mimimal invasiveness to the patient involving intra-tissue or systemic administration.[40] Intra-tissue delivery of autologous chondrocytes for knee cartilage repair or MSCs for invertebral disc regeneration have shown functional improvement after administration despite relatively low engraftment efficiency.[41,42] Intra-myocardial injection or NOGA® XP Cardiac Navigation System catheter guidance for administration of skeletal myoblasts or MSCs have shown modest gains in cardiac output however carry significant risks such as heart hypertrophy, calcification, cardiac puncture, or life-threatening arrhythmias or embolisms.[43-46] Systemic administration methods have primarily utilized MSCs due to evidence suggesting that MSCs’ possess a homing capability to injured or ischemic tissue for direct engraftment.[47,48] These results were later challenged by MSC tracking studies using more sensitive detection methods that indicated the majority of MSCs become entrapped in the lung and have a short residence time in the body and severely limited engraftment efficiency.[47-49] Genetically modified MSCs made to over-express pro-survival proteins or cell surface markers that enhance homing to sites of injury have also been used to improve survival and engraftment efficiency in animal models; but additional regulatory hurdles must be addressed before using such cells in a clinical setting.[50,51] Cell sheets have also been utilized for regenerative medicine using poly(N-isopropylacrylamide) thermo-responsive surfaces that can grow confluent cell layers using normal culture conditions (37°C).[52] A decrease in temperature below 32°C induces hydrophilic swelling of the polymer forming a hydration layer on the culture surface to form a cell sheet; thus, allowing the retention of cell surface receptors, cell-cell interactions, and cell-ECM interactions that would otherwise be compromised by trypsin digestion.[53] Despite these advantages, cell sheets are limited in their application due to their overall fragility, required surgical intervention, long culture time, and inability to produce thick layers to occupy three-dimensional tissue defects.[54] Injectable microtissues or spheroids (between 100 – 500 μm in diameter) that mimic functional tissue units are highly adhesive, pro-angiogenic, and less prone to wash out after injection also have been developed but require further investigation to assess their therapeutic potential in humans.[55,56] Microtissues or spheroids can be applied as building blocks for larger constructs using 3-D bioprinting; however, this technology remains largely in its infancy and primarily consists of proof-of-concept in vitro investigations (Figure 1).[55,56]
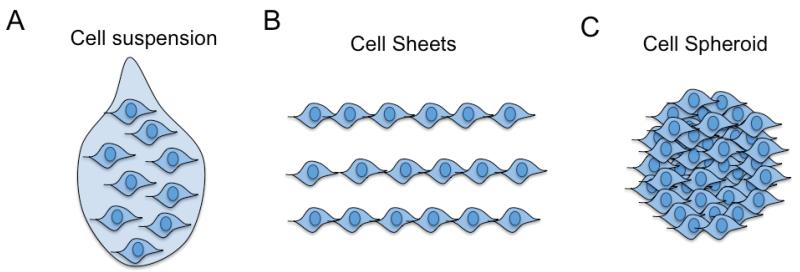
Figure 1.
Schematic of scaffold-free, cell-based therapies. A) Cell suspension for systemic or direct injection of trypsin-harvested cells; B) Cell sheets for surgical implantation of 2D cell layers; C) Spheroids for direct injection or surgical implantation of 3D cell clusters.
3. Tissue Engineered Constructs for Enhanced Cell Presentation and Improved Function
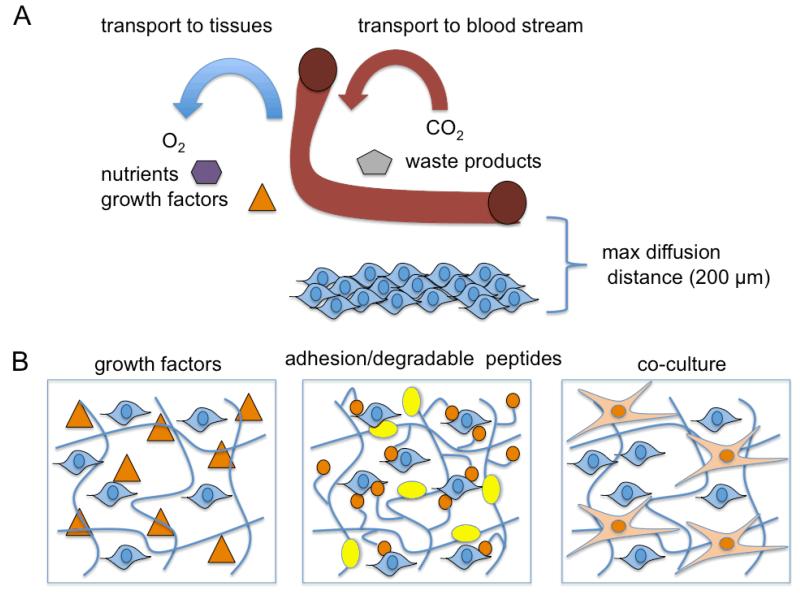
Inability to control cell delivery, retention, and engraftment using cell suspension injections motivated developments in three-dimensional scaffolds, foams, sponges, and electrospun nanofibers that could be fabricated into different sizes, structures, geometries, and topographies with tailored biodegradability for customized design of tissue engineered constructs.[57] The efficiency of cell colonization of various constructs is highly dependent on the overall porosity, pore size, and pore interconnectivity of the material.[58] Spinner flask and convective flow bioreactors can achieve a more uniform cell distribution during initial cell seeding of various scaffolds but a necrotic core within the interior of the construct can still occur when implanted in vivo due to inadequate mass transport of nutrients.[59] Injectable, highly porous microcarriers have also been designed that significantly improve cell colonization efficiency and mass transport.[60] Engineered constructs also require patient-specific customization for the geometry and size of the tissue defect, require extended culture times to establish cell colonization of the biomaterial, and surgical intervention for implantation of the cell-device all of which can be prohibitively expensive for clinical application.[61] Decellularized extracellular matrices have also been successfully used and seeded with high viability for anastomosed vascularized structures; however, there are still significant limitations concerning potential pathogenicity using animal sourced tissues and animal-to-human tissue length scale mismatch as well as overall lack of standardization and reproducibility.[62-64] Cells also initially interact with fabricated scaffolds or decellularized ECMs as they would on a two-dimensional surface, which may significantly differ from the three-dimensional physiological behavior.[65] The scaffolds degradation profile must also be carefully matched to cell remodeling and cell-ECM deposition otherwise necrosis and an undesirable foreign body response can occur or cell-secreted proteins could potentially diffuse out of the three-dimensional scaffolds respectively.[65]
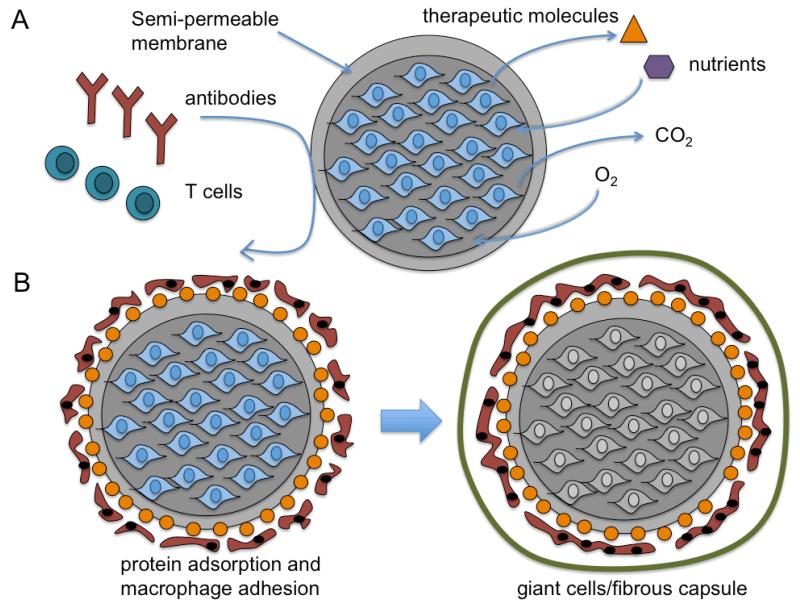
Hydrogels are water-swellable, three-dimensional crosslinked networks commonly used in tissue engineering that can be injected for simultaneous cell encapsulation and in situ polymerization that conform to specific tissue void geometries and adhere to adjacent tissues.[66] Thermal-, physical-, ionic-, or enzymatic-crosslinked gels composed of collagen/gelatin, alginate, or fibrin were previously utilized for cell encapsulation as they are FDA-approved base materials with favorable biocompatibility. Nonetheless, these biomatrices often demonstrated significant batch-to-batch variability in gelation time, network pore size, chemical functionality, and dissolution or degradation once injected in vivo.[67] Hydrogels containing both synthetic and biological macromolecules can be crosslinked as interpenetrating (covalent) or semi-interpenetrating polymeric networks (physical or ionic) to obtain better control over the hydrogels physiochemical properties over unmodified biodegradable polymers.[68] Alternatively, synthetic hydrogels that incorporate cell adhesive- or proteolysis susceptible-peptides can lend inert hydrogels additional biofunctionalization to support the viability of anchorage-dependent cells and facilitate active remodeling of the matrix similar to ECM turnover that normally occurs in vivo.[69] Single component systems that undergo stimuli-sensitive (pH, temperature) sol-gel transitions or photopolymerization and dual component systems that undergo stereocomplexation or self-crosslinking of two pre-polymer solutions are commonly used for injection of in situ forming cell-hydrogel networks.[70] The crosslinking agent, the weight concentration of synthetic or natural polymers, the molecular weight of the macromolecules, and the crosslinking density can all impact the overall mechanical strength, swelling, degradation, transport, and pore size of the polymerized gels.[71] Un-reacted monomers and crosslinkers or free-radical generation during polymerization may negatively impact cell viability.[71] The mechanical and biochemical properties of the encapsulating biomatrix could also influence cell adhesion, morphology, apoptosis, proliferation, differentiation, and migration and also lead to variable secretion of growth factors, cytokines, or ECM proteins.[72] Needle gauge, viscosity limitations of the gel applicator, maximum shear force cells can tolerate during injection, and practical gelation times that avoid cell leakage, blood flow blockage, and tissue necrosis are all important considerations relevant for application that need to be addressed for in situ forming hydrogel-cell delivery methods.[73] Although higher degrees of crosslinking confer greater mechanical integrity and durability some hydrogel systems remain unsuitable for specific load bearing tissue engineering applications.[74] Injectable microcapsules fabricated as hydrogel-core/shells, liquid-core/shells, cell-core/shells have also been considered for regenerative medicine due to their spherical shape and small size (100 - 500 μm in diameter), which are less susceptible to mechanical disruption compared to larger constructs and provide more efficient surface-to-volume mass transport of oxygen, nutrients, metabolic wastes, and therapeutic proteins.[75-77] Controlling the molecular weight cutoff (MWCO) by varying the porosity of the semi-permeable membrane can also prevent immune cell infiltration or immunoglobulin entry into the microcapsule that would otherwise damage encapsulated cells without accompanying drug immunosuppression (Figure 2).[78]
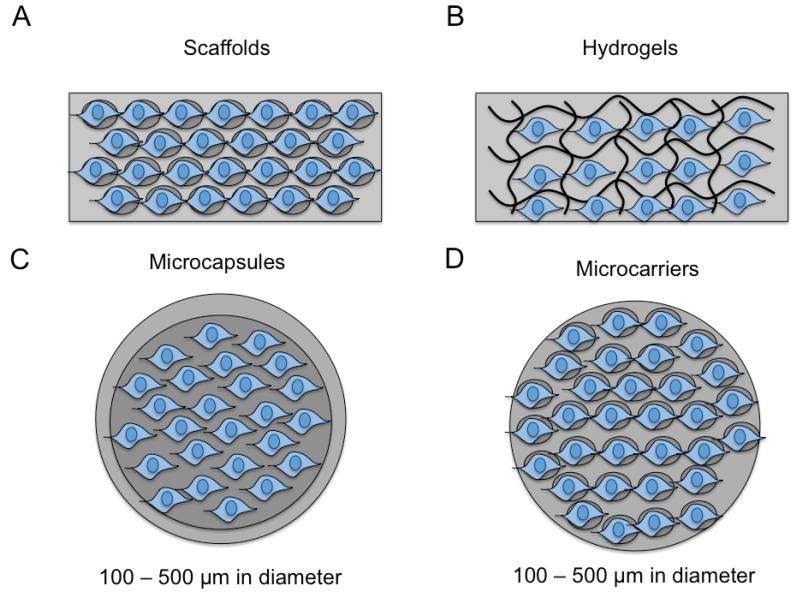
Figure 2.
Schematic of biomaterial-centered, cell-based therapies. A) Scaffolds (macroscale) with porous structure seeded with cells prior to implantation; B) Hydrogels (macroscale) with crosslinked structure (chemical, ionic, physical) that gels in vitro or in situ containing encapsulated cells ; C) Microcapsules consisting of hydrogel-core/shells, liquid-core/shells, cell-core/shells that contain cells surrounded by a semi-permeable membrane that can be injected into specific tissue sites; D) Microcarriers (similar to scaffolds) with a porous structure seeded will cells prior to injection.
Scaffolds, hydrogels, and microcapsules comprised of either natural biopolymers, synthetic, or composite systems will be further discussed in this review as they are versatile platforms that allow for more controlled delivery and presentation of therapeutic cells.
4. Cell-Containing-Biomatrices for Treatment of Acute Injuries or Disease
4.1. Collagen-based Materials and Selected Case Studies
Collagen is a fibrous protein primarily secreted by fibroblasts and is the most abundant biomacromolecule in the human body that comprises 25% of the total dry weight.[79] Collagen macromolecules are composed of three α chains with each chain consisting of greater than a thousand amino acids with the repeating sequence Gly-X-Y (X and Y positions mostly proline and 4-hydroxyproline) that confer tight packing to form a triple helix structure.[80] Specific proline and lysine residues on pro-α chains are modified by hydroxylation and glycosylation and pro-α chains then self-assemble into pro-collagen. In the extracellular space, propeptides are cleaved allowing telopeptide-mediated auto-polymerization that induces tropocollagen self-assembly of 10 – 300 nm sized fibrils (stabilized by intra- and inter-molecular crosslinking) and allows further agglomeration into 0.5 – 3 μm collagen fibers (Figure 3).[81] These crosslinked collagen fibrils form strong networks that provide the supporting connective structure needed for skin, tendons, and bones among other tissues.[82] Type I collagen is the most abundant collagen protein of the 29 collagen types (90% of total collagen in human body) and is one of the most commonly used biopolymer in tissue engineering due to its high conservation amongst different species, high biocompatibility, and low incidence of humoral immunity (after telopeptide removal) or allergic reaction to purified collagen exposure from xenogenic sources.[83,84] Collagen-based biomaterials can be degraded by collagenases, gelatinases, and matrix metalloprotienases (MMPs), which vary in their ability to bind collagen macromolecules, unwind the three α chains, and cleave each strand of the triple helix.[85] Cell receptors glycoprotein VI, integrins, and discoidin receptors (DDR1 and DDR2) also recognize oligopeptide sequences in collagen such as the GPO (Gly-Pro-Hyp), GFO (Gly-Phe-Hyp), and RGD (Arg-Gly-Asp) motifs, which can influence cell adhesion, migration, and differentiation.[86] Decellularization and collagen extraction from cadavers or xenogenic sources involves a combination of solubilization techniques including treatment in acidic or alkali conditions, neutral salts, or proteolytic solutions, which can dissociate the organization of the collagen fibrils and partially denature or cleave telopeptides decreasing the overall mechanical stability of the collagen biomatrices.[87] Collagen undergoes sol-gel transition after pH neutralization at 37°C forming hydrogels and can additionally be freeze dried to create sponges or foams with tailored porosity and chemical crosslinking can also be utilized to improve the stability of collagen scaffolds.[88] Retention of cell-derived antigens such as 1, 3-α-galactose or xenogenic DNA as well potential viral, prion, or endotoxin contamination during purification have motivated the use of human- or recombinant-derived collagen; however, have not been cost-effective when compared to animal sources.[89-91] Sterilization of collagen-based materials usually involves peracetic acid treatment, ethanol immersion, or antibiotic incubation as well as other methods such as autoclaving or ethylene oxide treatment as well as γ-ray, β-ray, and electron beam-irradiation.[92,93] All of which can cause additional collagen degradation and alter overall mechanical strength.[92,93] Additional acid or alkali treatment in combination with high temperature incubation (45°C) induces a helix-to-coil transition for soluble conversion and additional degradation of collagen into either type A (isoelectric point at pH ~ 8 – 9) or type B gelatin (isoelectric point at pH ~ 4 – 5).[94] Gelatin is a denatured version of collagen that acts as a random coil in solution and has a wide molecular weight distribution after chemical processing.[95]
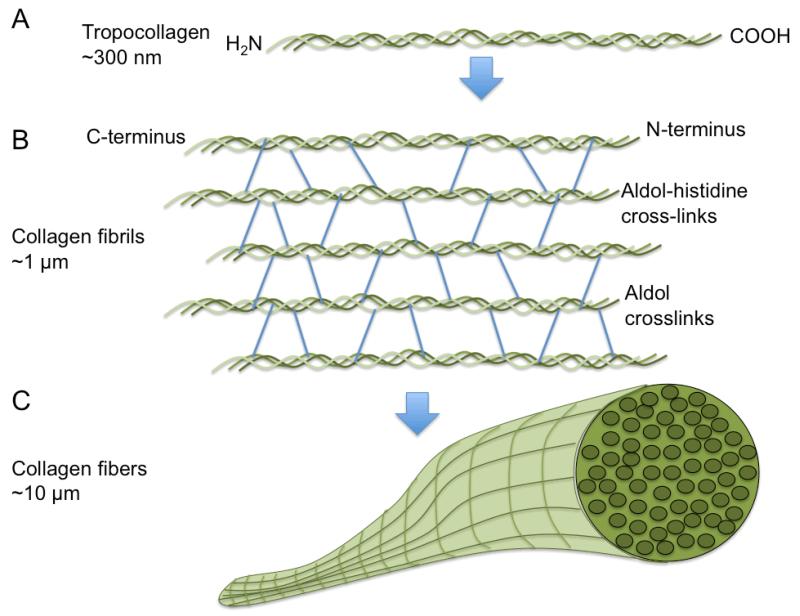
Figure 3.
Schematic of collagen organization. A) Tropocollagen organization of triple helical polypeptides that consists of a repeating Gly-X-Y (where X is often proline and Y is often hydroxyproline) amino acid sequence to form a right handed coiled-coil ; B) Collagen helices self-assemble into collagen fibrils via aldol-histidine and aldol crosslinking; C: Collagen fibrils further organize into via end-to-end self-assembly into collagen fibers.
4.1.1. Collagen-based Biomatrices for Cutaneous Wound Repair
Collagen sponges and gels have been utilized due to the absorbant property to accommodate wound exudates, adherence to wound beds, minimized heat loss, shielding of wound tissue from potential mechanical pertubation, and protective barrier function that resists bacterial infiltration and infection in minimizing bioburden.[88] Type I collagen (0.66 mg/mL) has been used as an encapsulating matrix for allogeneic neonatal dermal fibroblasts with the collagen hydrogel surface seeded with allogeneic keratinocytes in transwell co-cultures during manufacturing (Apligraf®, Organogenesis, Canton, MA).[96] After collagen contraction and keratinocyte confluency is attained, the culture system is exposed to the air-liquid interface to induce stratification and cornification of the keratinocyte layer to recapitulate the dense bi-layer structure of native skin (epidermis and dermis).[96] This human skin equivalent (HSE) is an FDA-approved product that is applied directly as a fenestrated dressing to treat diabetic foot and venous leg ulcers that remain refractory to more conventional wound therapies.[97] Apligraf® treatment for diabetic foot or venous ulcers usually results in faster wound closure and better patient outcome as neonatal-derived cells secrete numerous growth factors that stimulate adjacent tissues within the wound bed that are previously senescent and non-healing.[98] Apligraf® has been shown to be immunologically tolerated by patients as collagen amino acid sequences are highly conserved amongst different species and keratinocyte and fibroblasts do not express human leukocyte antigen II or co-stimulatory molecules that would otherwise activate T cells and lead to hyperacute inflammation and graft rejection.[98] Stratagraft® (Stratatech Corporation, Madison, WI) has similarly been developed as a HSE containing type I collagen encapsulated neonatal fibroblasts and a stratified epidermis consisting of Near-diploid Immortalized KeratinocyteS (NIKS®, Stratatech Corporation).[99] This HSE has been tested in a phase I/II clinical trial as a temporary dressing when autologous skin grafting is not immediately possible due to patient instability or lack of available skin tissue after third-degree full-thickness burns.[100] Stratagraft® provided a temporary barrier and secreted high concentrations of human β-defensins-3 that prevented wound infection with similar safety profiles to cadaver allografts (the current standard of care), which are limited by tissue availability and sufficient quality for wound bed preparation prior to autologous skin grafting.[100] Although HSEs have shown large clinical success, drawbacks to their usage include short shelf life (~5 days), delicate handling, potential for disease transmission, and high cost ($28 per cm2) that are significant barriers to application in burns or skin wounds covering a large total body surface area.[101] Despite type I collagen ability to neutralize proteases present in the wound bed, Apligraf® and Stratagraft® display poor mechanical properties, undesirable contraction, and weak integration with the surrounding wound bed, which could potentially be improved by increasing the collagen concentration.[102]
4.1.2. Collagen-based Biomatrices for Articular Cartilage Repair
Full-thickness cartilage injuries (lesions of 5-mm diameter) are difficult to heal due to the low regenerative capacity of chondrocytes within articular cartilage and often lead to osteoarthritis that later in life may require total joint replacement.[102] Microfracture techniques stimulate cartilage repair using marrow stimulation and has successfully been employed in young patients with small defects.[103] Fibrocartilage produced from this technique however is inferior in its ability to resist shear and compressive forces compared to hyaline cartilage and can even precipitate further joint cartilage deterioration with a 23% failure rate after 5 years.[103] Similarly, arthofibrosis and hypertrophy of the periosteum are frequent complications that result after autologous chondrocyte injection into the periosteal flap.[103] In a phase I clinical trial, Neocart® (Histogenic Corporation, Waltham, MA) was utilized for the treatment of full-thickness cartilage injuries (grade III) of the femoral condyle with defects (range: 1.2 – 3.0 cm2) associated with trauma (n = 3), focal osteoarthritis (n = 3), prior ACL injury (n = 2), and osteonecrosis (n = 1).[103] Neocart® consists of a three-dimensional, honey-comb type I collagen scaffold seeded with autologous chondrocytes (derived from a non-weight bearing portion of the femoral condyle or femoral notch of the ipsilateral knee) cultured in a bioreactor under hydrostatic pressure that produces a viable, tissue-like construct rich in proteoglycans and glycosaminoglycans (after 67 ± 18 days).[103] The implant bed was surgically debrided and Neocart® was sized to the defect and fixed with CT3 (Histiogenics), an adhesive consisting of collagen and poly(ethylene glycol), that was spread above and below the Neocart® implant.[103] Patients were immobile for 6 weeks and were clinically evaluated at 6, 12, and 24 months following implantation using the Visual Analog Scale (VAS) pain score at and the International Knee Documentation Committee (IKDC) score.[103] Articular cartilage was also assessed using a clinical 1.5-T MRI unit (Signa HD Excite or HDx, General Electric Healthcare, Milwaukee, WI) following 12 and 24 months NeoCart® post-surgical implantation.[103] The Neocart® repair cartilage was compared to the surrounding cartilage (hypointense, isointense, or hyperintense) measured using a standardized region of interest (ROI) in the center of the repair both within the deep and superficial sections of the repaired cartilage tissue as well as the adjacent and opposite articular cartilage surfaces.[103] The VAS pain score decreased from 3.3 ± 2.8 at baseline to 0.9 ± 1.5 at 12 months and remained lower than the baseline (p < 0.05) at 24 months; the average range of motion improved from 128° ± 10° at baseline to 136° ± 7° at 24 months; knee function by IKDC evaluation improved from 57 ± 25 at baseline to 76 ± 17 at 24 months.[103] All 8 patients demonstrated improvement in at least 2 of 3 key clinical efficacy measurements (VAS pain, IKDC score, or range of motion) at 12 and 24 month post-surgical implantation.[103] MRIs of 6 of the 8 patients showed good to complete (67% - 100%) defect fill at 24 months with no associated hypertrophy or overgrowth of the NeoCart® patch.[103] Quantitative T2 mapping stratification characterized by shorter relaxation times in the basilar sections and longer times in the superficial components can be correlated to the organization of the collagen fibrils that is similar to physiological hyaline cartilage.[103] At 12 month post-implantation, 25% of patients showed partial T2 stratification and further increased to 50% by 24 months indicating the NeoCart® implant stimulates cartilage regeneration similar to hyaline cartilage.[103] The 4 patients that did not show T2 improvement after NeoCart® treatment had previously undergone previous debridement surgery and showed signs of early osteoarthritis, which correlated with previous studies indicating that patients who undergone multiple knee operations often have less promising clinical outcomes and a longer time delay before returning to physical activity.[103] Neocart® implantation clinical assessment and MRI outcome overall showed considerable improvement especially considering the variable age (range: 25 – 46) and BMI (range: 20 – 38) as well as chronic joint pathophysiology (duration longer than 2 years in 6 patients).[103] Improvement in defect filling and overall knee function could be attributed to the supportive role of the collagen scaffold and prior bioreactor culture of the implant to develop a more tissue-like structure that was better integrated into the adjacent hyaline cartilage architecture as compared to autologous chondroctye injection alone or microfracture techniques.[103] Patients with more severe knee injury (multiple large defects), higher BMI, or advanced age however did not show higher functional improvement indicating that the Neocart® implant does not demonstrate favorable integration with host tissues when the extent of knee damage is more severe, higher loads are placed on the knee joint (due to obesity), or the regenerative potential is lower in the patient (due to age or pathophysiology of the knee joint cartilage tissue).[103]
4.1.3. Collagen-based Biomatrices for Osteochondral Repair
Although collagen contains both Asp-Gly-Glu-Ala/Arg-Gly-Asp (DGEA/RGD) cell-adhesive moieties that support MSC viability and osteoblast differentiation in vitro, unmodified collagen is mechanically weak and undergoes rapid biodegradation making it unsuitable for healing critical defects in weight-bearing bones.[104] Collagen can be further crosslinked to retard in vivo degradation or combined with α-hydroxy acid polymers (poly-lactic acid, poly-lactic-coglycolic acid) and ceramic components (hydroxyapatite, tricalcium phosphate) to mimic the mechanical properties and bone minerals present in native bone tissue.[105] The repair of large osteochondral defects is a complex problem that requires healing of mechanical distinct regions composed primarily of cartilage, calcified cartilage, and bone tissue that requires appropriate union to adjacent host tissues.[105] Three different scaffold layers were synthesized to mimic the morphological and mineral gradients present in newly formed bone; the upper layer (mimicking the outer cartilaginous layer) composed of 10% hyaluronic acid and 90% collagen, the intermediate zone (mimicking tidemark bone) composed of 40% hydroxyapatite : 60% collagen, and the lower zone (mimicking subchondral bone) composed of 70% hydroxyapatite and 30% collagen.[105] The cartilaginous upper layer was formed by precipitating 100g of 1 wt% type I collagen in acid suspension after NaOH neutralization (0.1M, pH 5.5) with 0.1 wt% of hyaluronic acid added prior to gel formation (2.0 mm thick).[105] The intermediate bony layer (tidemark region) was synthesized by mixing 100g of H3PO4 (0.040 M) solution with 100g of 1 wt% collagen solution dropped in a basic solution containing 0.491 g of Ca(OH)2, which nucleates during collagen gel self-assembly to yield a mineralized composite (40% hydoxyapatite, 60% collagen, 1.5 mm thick).[105] Similarly, the lower bony layer (subchrondral bone) was synthesized by mixing 244 mL of H3PO4 (0.040 M) with 70g of 1 wt% collagen gel dropped into a basic solution containing 1.203g Ca(OH)2 to form a mineralized composite (70% hydroxyapatite, 30% collagen, 2.5 mm thick).[105] Each layer underwent 1,4 butadienediol diglycidyl ether (BDDGE, 2.5 mM) crosslinking for 48 hours and were knitted together to avoid potential delamination at the layered scaffold interfaces.[105] Additional freeze drying and heat ramping (1°C/min) from 25°C to −25°C and from −25°C to 25°C for 50 minutes under vacuum (P = 0.20 mbar) was employed to create anisotropic pores (250-450 μm).[105] Human chondrocytes (isolated from the lateral condyles of cadaver knee joints) were statically seeded (1.6x107 cells/scaffold) on the cartilaginous or subchondral bone layers and cultured for 2 weeks in chondrogeneic media.[105] Chondrocytes cultured on the cartilaginous outer layer displayed a chondrocyte morphology and positively stained for Safranin-O while cells in the subchondral bone layer appeared morphologically similar to fibroblasts and did not stain positively for Safranin-O.[105] Sheep bone marrow mesenchymal stromal cells were statically seeded (2.0x106 cells/scaffold) onto the tri-layer composite scaffold and then subcutaneously implanted into immuno-deficient mice (CD-1 nu/nu) for ectopic bone formation.[105] After 8 weeks, scaffold associated BM-MSCs generated a well-organized bone tissue in the subchondral region and loose connective tissue in the cartilagineous layer.[105] Osteochondral biomimetic scaffold (Fin-Ceramica Faenza S.p.A., Faenza, Italy) treatment groups (osteochondral injury left untreated; scaffold without chondrocytes; or scaffold seeded with autologous chondrocytes) were pressed fitted into experimentally induced osteochondral lesions in sheep and then assessed for bone and cartilage healing after 6 months.[106] Both the scaffold alone and chondrocyte-seeded scaffolds demonstrated good integration with the surrounding bone and favorable healing whereas the untreated control group was characterized by lytic holes containing amorphous fibrous tissue and lack of bone growth in the subchondral bone layer.[106] Likewise, both the scaffold and chondrocyte-seeded scaffold demonstrated the formation of hyaline-like cartilage tissue with columnar chondrocyte arrangement and high proteoglycan content as well as organized subchondral trabecular bone structure that was well integrated with the adjacent bone tissues.[106] Type I collagen was stained from the cartilaginous layer down to subchondral bone boundary layer while the subchondral bone layer displayed distinct regions that stained positive for type II collagen suggestive of hypertrophic chondrocyte morphology and active bone matrix formation and remodeling for both the scaffold only condition and the scaffold-chondrocyte seeded treatment group.[106] Conversely, the untreated group with the osteochondral lesion showed dispersed type II collagen and positive type I collagen staining throughout the entire joint space that was indicative of fibrotic scarring.[106] Similar osteochondral healing observed for both treatment groups (scaffold only and scaffold-chondrocyte) demonstrates potential endogenous mesenchymal precursor cell recruitment from the subchondral bone layer and synovial-derived chondrocytes contributing to osteogenic and chondrogenic regeneration of the osteochondral defect.[106] The biomimetic scaffold provided a crosslinked tri-layered structure of graded collagen/HA wt% and mechanical strength similar to physiological osteochondral tissue and conferred favorable integration with adjacent joint tissues and lack of fibrotic scarring, necrosis, or foreign body giant cell formation that could negatively impact overall joint function.[106]
4.1.4. Collagen-based Biomatrices for Nerve Repair
Wounds of the peripheral nervous system caused by transection, crush, or gap injuries can vary greatly in healing depending on the length of the gap, localization, extent of injury, patient’s age, time of surgery, vascular microenvironment, or type of nerve (sensory, motor, or autonomic neurons).[107] A collagen-hyaluronic acid matrix that consisted of 1% collagen and 1% hyaluronic acid was injected into a collagen conduit (Institute of Medical Equipment, Academy of Military Sciences, China), crosslinked with 1-ethyl-3-dimethylamino carboiimide, and later embedded with neural stem cells (NSCs, derived from Sprague-Dawley rats), which had previously been shown to enhance NSC differentiation into neurons, astrocytes, and oligodendrocytes.[107] NSCs (4x106 cells/scaffold) were injected into the collagen-hyaluronic matrix conduit and supplemented with 1 mL Neurotrophin-3 (NT-3) in DMEM/F12 media for two to three days.[107] Treatment subjects (rabbits) were grouped as normal control, bilateral facial nerve transected without reconstruction, lateral nerve transected with implantation of collagen-hyaluronic acid scaffold, lateral nerve transected with implantation of NSC and collagen-hyaluronic scaffold, and lateral nerve transected with implanted NSC and collagen-hyaluronic scaffold supplemented with NT-3.[107] Approximately 2 mm of the facial nerve were surgically removed to create a peripheral nerve gap defect (enlarged to 5 mm after contraction) that was treated with a 7 mm collagen-hyaluronic acid conduit.[107] Rabbit behavior (signs and extent of muscular atrophy of lips, blink reflex, and ear motion) was monitored before and after 12 weeks post-surgical treatment and electromyography readings to assess neuromuscular function were taken at 1, 4, 8, and 12 weeks after peripheral nerve transection.[107] Rabbits treated with NSC-embedded, NT-3 supplemented collagen-hyaluronic matrices displayed slight blink reflex and ear movement but no ear erection and muscular atrophy of the upper lip was evident.[107] Injured rabbits treated with the collagen-hyaluronic scaffold, NSC and collagen-hyaluronic acid scaffold, or NT-3 supplemented collagen-hyaluronic scaffold demonstrated atrophied upper lip muscles, torpid blink reflex, and ear palsy.[107] By 12 weeks, electromyography thresholds for NSC-embedded, NT-3 supplemented collagen-hyaluronic scaffolds demonstrated comparable thresholds to normal controls (4.11 mA vs. 3.41 mA) indicating enhancement of nerve fiber regeneration.[107] Histological examination of the peripheral facial nerve for the NSC-embedded NT-3-supplemented collagen-hyaluronic acid composite showed attenuated nerve degeneration and were comparable in mean number, area, and circumference to normal controls that were more organized in rabbit nerves than other treatment groups.[107] After 12 weeks, there was also no evidence of macrophage infiltration for the NSC-embedded, NT-3 supplemented collagen-hyaluronic acid and NSC and collagen-hyaluronic acid treatment groups suggesting that xenogenic NSCs, which lack detectable levels of MHC I or II, were immunologically tolerated and did not induce excessive inflammation.[107] The combination of NSCs, NT-3, and the collagen-hyaluronic acid scaffold attenuated the extent of nerve degeneration by providing therapeutic cells and survival factors in a nerve microenvironment highly limited in its capacity for autoregeneration while providing a scaffold that encouraged neural cell adhesion, trafficking, and axonal sprouting from nerve stumps.[107] The peripheral nerve treated with the NSC-embedded, NT-3 supplemented collagen-hyaluronic acid scaffolds partially regenerated damaged peripheral nerves; however, complete return of neuromuscular function was not evident and may be attributed to observed hypertrophy, swelling, and some persistent degeneration of myelin sheath.[107] Although the collagen-hyaluronic acid conduit appeared to facilitate NSC anchorage and trafficking for peripheral nerve defects, mismatched mechanical properties may have differentiated NSCs into glial cells or oligodendricytes rather than neural cells or partially contributed to myelin sheath dysfunction after injury that did not encourage more functional integration of the two peripheral nerve stumps contributing to a lack of return in neuromuscular function.[107]
4.1.5. Collagen-based Biomatrices for Cardiac Repair
Cardiac ECM is primarily composed of type I and type II collagen (with small amounts of elastin, laminin, and fibronectin) that provide the necessary stiffness and resistance to deformation necessary for repeated heart contractions.[108] Following myocardial infarction and ischemic heart disease, the cardiac ECM undergoes pathological remodeling and loss of type I collagen (down from 80% to 40%) that contributes to left-ventricular (LV) wall thinning.[108] The lack of improvement in LV function after stem cell injection may be due to washout and anoikis of administered cells and has motivated the use of cell-seeded scaffolds that enhance cell localization and viability for long-term improvement of cardiac function.[108] In a non-randomized, phase I clinical trail consisting of patients presenting chronic ischemia after (LV) myocardial infarction (LV ejection fraction 35% or less), treatment groups were either treated with an intramyocardial injection of bone marrow cells (BMCs) or an implanted collagen cardiac patch containing seeded BMCs.[108] CE Mark collagen (Pangen 2, Urgo Laboratory, Chenove, France) utilized in this investigation consisted of a biodegradable biomatrix (size: 5 × 7 × 0.6 cm) of lyophilized type I collagen with matrix pores sizes ranging from 50 to 100 μm.[108] BMCs (250 ± 28 million cells) were seeded on the collagen biomatrix with a uniform distribution within matrix pores after 10 minutes of continuous shaking (120g, Orbital Shaker).[108] The BMC-scaffolds were directly applied to the infarct and peri-infarct regions of the heart that was secured to the epicardium with 6 PDS sutures (6-0) and covered by an additional non-cellularized matrix while patients in the other cohort received an single administration of BMCs (250 ± 28 million cells) within and principally around the infarct (12 ± 3 injection points) using 25G × 40 mm ophthalmic needle after undergoing off-pump coronary artery bypass graft (OP-CABG) surgery.[108] In this feasibility/safety study, no mortality or adverse events occurred and malignant cardiac arrhythmias or ventricular tachycardias could not be detected.[108] LV ejection fraction improved but were not statistically different for the two treatment groups at 10 ± 3.5 months follow-up.[108] The LV end-diastolic volume, deceleration time, and scar area thickness improved (142.4 ± 24.5 mL to 112.9 ± 27.3 mL, 162 ± 7 ms to 198 ± 9 ms, 6 ± 1.4 mm to 9 ± 1.1 mm) for the BMC-collagen biomatrix treated patients compared to patients with only BMC administration (138.9 ± 36.1 mL to 148.7 ± 41 mL, 159 ± 5 ms to 167 ± 8 ms, 5 ± 1.5 mm to 6 ± 0.8 mm) at long-term endpoints.[108] Long-term improved cardiac function observed for the combined BMC-collagen therapy following OP-CABG surgery demonstrated improved LV function may be due to temporary replacement of lost type I collagen ECM or a girding effect that decreased LV wall strain in addition to the therapeutic benefit of BMC administration, which may act to prevent further myoblast apoptosis and fibrotic scarring as well as enhance angiogenesis, increase cardiac stem cell recruitment, or transdifferentiate for direct engraftment into cardiac tissue.[108] Despite observed improvements in cardiac function, a mismatch of mechanical elasticity under repetitive contraction characteristic of cardiac tissue could be improved upon for better tissue integration using a more tailored collagen matrix that minimizes fibrotic scarring and dysfunctional LV wall thinning along with a higher seeding density for greater replacement of damaged cardiomyocytes localized within the infract zone.[108]
4.2. Fibrin-based Biomatrices and Selected Case Studies
Fibrin clots form as a consequence of vascular injury, initiating the coagulation cascade that induces serine protease thrombin cleavage of soluble fibrinogen to generate peptides that cluster together to form an insoluble fibrin network.[109] Platelets also contribute to clot stabilization and crosslink to the nascent mesh via transglutaminase Factor XIII.[110] Fibrin glue is a commercially available product (Tissel VH®, Baxter Deerfield, IL) used as a biological adhesive during surgical interventions that mimic the final step in the coagulation cascade where fibrinogen (20 – 40× more concentrated than normal human sera) is cleaved by thrombin into monomers that form the fibrin clot.[111] Fibrin clot adhesive strength, rate of gelation, network structure, permeability, and fiber diameter are influenced by the concentration and relative ratio of fibrinogen to thrombin as well as to the local pH and osmolarity.[109] Fibrin clots undergo fibrinolysis when tissue plasminogen activator converts plasminogen to plasmin which actively degrades clots into soluble fibrin degradation products and D-dimers that enable patency re-establishment (Figure 4).[112]
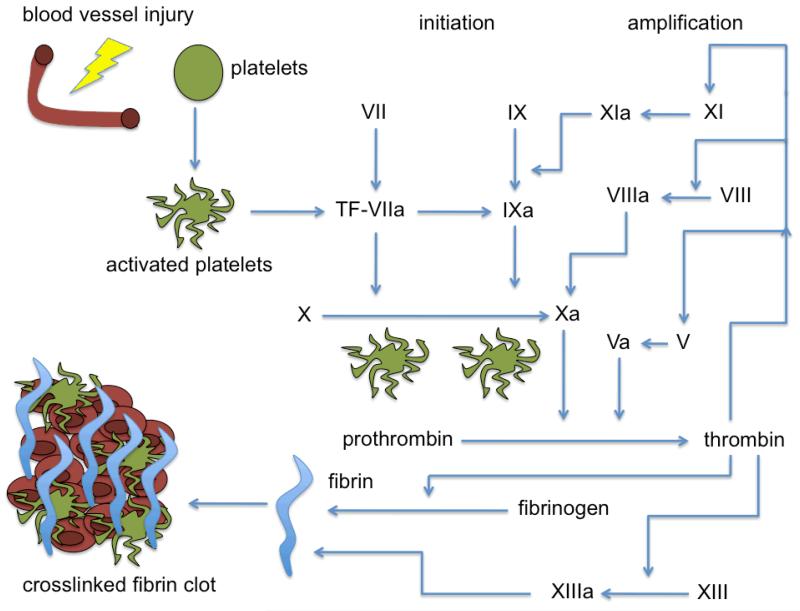
Figure 4.
Schematic of the coagulation cascade for fibrin clot generation. Blood vessel injury leads to platelet activation involving several mediators lead to thrombin conversion of fibrinogen to fibrin. Fibrin polymerization along with platelet aggregation leads to blot clot stabilization and hemostasis.
4.2.1. Fibrin-based Biomatrices for Intervertebral Disc Repair
Lower back pain can be caused by pathophysiological degeneration in the intervertebral disc that leads to decreased cell density within the nucleus pulposus as well as lower synthesis and greater proteolytic turnover of disc-associated proteoglycans.[113] Moreover, the breakdown or loss of anti-angiogenic/neurogenic factors (associated with nerve fibers) produced within the invertebral disc may contribute to chronic lower back pain.[112] In a swine model of intervertebral disc repair, allogeneic juvenile chondrocytes were isolated from condyles of the distal femur and MSCs were isolated from the bone marrow.[113] A horizontal incision (1 cm) was made and the nucleus pulposus (NP) was removed using SpineJet™ MicroResector (HydroCision, Inc.) and was subsequently treated with either fibrin alone or allogeneic cells (MSCs or juvenile chondrocytes) encapsulated in fibrin.[113] Bovine thrombin (1000 IU/mL, Thrombin-JMI, King Pharmaceutical) containing the cell suspension (7 – 10 million cells) was combined with allogeneic fibrinogen (collected from whole blood) using a FibriJet® applicator (Micromedics) and directly injected into the void space (0.5 – 0.75 ml) left after NP removal.[113] After 3, 6, and 12 months post-surgery, viable allogeneic juvenile chondrocytes could be observed using Y-chromosome fluorescent in situ hybridization (FISH) whereas MSCs could not be observed at any of the euthanasia endpoints.[113] Greater sulfated-glycosaminoglycan (S-GAG) content and more pronounced collagen II expression rather than collagen I secretion (indicative of scar tissue) was observed for the allogeneic juvenile chondrocytes-fibrin group over the fibrin only or the MSC-fibrin treatment groups indicating the overall suitability of juvenile allogeneic chondrocyte-fibrin administration for disc repair in an ischemic microenvironment.[113] The elevated S-GAG content observed for the intervertebral discs for the combined juvenile chondrocyte-fibrin treatment is indicative of high GAG content that is better able to resist compressive forces and than fibrotic scar tissue formed with other treatments, which showed less than desirable tissue integration.[113]
4.2.2. Fibrin-based Biomatrices for Cardiac Repair
In a swine model of myocardial infarction (MI), the left anterior descending coronary artery was ligated for 60 minutes and then reperfused resulting in 10% left ventricular (LV) mass damage in surviving Yorkshire mini-pigs.[114] Swine were either left untreated, treated with the fibrin patch alone, or treated with a fibrin patch containing embedded endothelial cells (ECs, 0.5x106 cells/mL) and smooth muscle cells (SMCs, 0.5x106 cells/mL) derived from human ESCs genetically modified to express green fluorescent protein (GFP) and luciferase (Luc).[114] The fibrin gel polymerized in 1 min after mixing fibrinogen (100 mg/ml) and thrombin (~400 IU/ml) and adhered to the left ventricle as a cardiac patch.[114] Pigs also received a daily immunosuppressive drug regimen of cyclosporine A (30 mg/kg) to avoid graft rejection of xenotransplanted cells.[114] Treatment of MI with fibrin embedded ECs and SMCs demonstrated significant engraftment (Luc expression) and more capillaries were observed in both infarct and peri-infarct regions as shown by von Willebrand Factor (vWF)/GFP+ positive cells after 4 weeks post-surgical intervention.[114] The fibrin encapsulated EC/SMC treatment group demonstrated improved cardiac function as observed by attenuated left ventricle hypertrophy, decreased infarct size, reduced LV thinning in the infarct and border zones (by 4 weeks) as well as an improved ejection fraction by 7 days.[114] Improved cell retention using fibrin cardiac patches during repetitive contractions shown by cell tracking studies contributed to less LV dysfunction most likely through rescue of native cardiomyocytes from apoptosis/necrosis after undergoing significant oxidative stress in an ischemic microenvironment.[114] The fibrin patch also remains tightly adhered to the infracted area and was well integrated with the cardiac tissue as observed by attenuated fibrosis and a lack of cardiac LV wall bulging at the border region between the cardiac patch and the peri-infarct zone.[114] Fibrin patch-based cell delivery requires use of a double-barrel syringe and open-heart surgery (sternotomy or thoracotomy) for application in cardiac therapy. Unfortunately, this method is not compatible with minimally invasive catheter injection delivery, which requires multiple washes with saline between injections and could lead to adverse fibrin particle accumulation in the blood stream.[115]
4.2.3. Fibrin-based Biomatrices for Cutaneous Wound Repair
Fibrin sealant (Baxter Healthcare, Glendale, CA) containing 5 mg/mL fibrinogen and 25 U/mL thrombin embedded with bone marrow-derived MSCs (2x106 cells/cm2) was directly applied (3 applications at least 1 week apart) to acute cutaneous full-thickness or chronic wounds (greater than 1 year) in humans using a spray system with an inert CO2 carrier (2.5 - 5.0 psi, held 1 - 3 cm away from wound bed).[116] MSCs in this formulation were shown to be capable of migrating out of the fibrin biomatrix using GFP+ expression tracking in mice however did not persist long-term despite wound healing acceleration.[116] Significant pain alleviation was noted early during the treatment and complete re-epithelialization occurred by 7 weeks for fibrin-MSC spray treated acute wounds while healing was still accomplished in chronic wounds that were previously refractory to conventional and even advanced wound therapies.[116] Wound biopsies taken 8 days after application of the fibrin-MSCs showed higher deposition of elastin that is more indicative of scarless healing, which was not observed for fibrin only treated wounds.[116] Similarly, patients underwent liposuction for isolation of adipose tissue-derived MSCs (ASCs) which were expanded and later embedded in fibrin sealant (1x107 cells/mL) that was directly applied for complex perianal fistula treatment during a phase II clinical trial.[117] ASC-fibrin treatment of complex fistulas-in-ano was safe and demonstrated 4x higher greater probability in healing with less recurrence (after 1 year post-intervention) than subjects treated with fibrin alone.[117] For patients where healing was incomplete (not completely re-epithelialized), less suppuration and improved appearance was observed with an improved quality of life compared to fibrin alone.[117] Fibrin served as an effective hemostatic wound dressing that provided temporary barrier function against infection and could be easily remodeled or cleared by infiltrating keratinocytes and fibroblasts of adjacent dermal and epidermal tissues.[117] Poor integration due to the presence of an abscess or perianal sepsis was still observed for some patients who received the ASC-fibrin treatment indicating the fibrin matrix could be improved by providing a more mechanically robust ECM barrier (more crosslinked structure) that resists early proteolysis/degradation that would otherwise to incomplete wound closure and infection.[117]
4.2.4. Fibrin-based Biomatrices for Articular Cartilage Repair
Fibrin is a natural biopolymer involved in wound healing processes and has self-adhesive properties that can improve implant fixation and provide a supportive microenvironment for chondrocytes as compared to clinical interventions such as autologous chondrocyte injection or microfracture.[118] Biocart™II (CartiMate™, ProChon, Isreal) was implanted into the knee of 8 patients for a prospective clinical trial to ascertain whether articular cartilage defects could be repaired using clinical assessment and MRI.[118] BioCart™II consists of human plasminogen-free fibrinogen and thrombin with small amounts of non-crosslinked hyaluronic acid that can absorb seeded autologous chondrocytes (0.5 × 106 cells/cm2) and allow for a homogeneous cell distribution.[118] Additionally, the BioCart™II biomatrix is impregnated with a fibroblast growth factor-2 (FGF-2) variant to enhance chondrocyte proliferation and chondrogenic potential to produce hyaline-like repair tissue (3 - 4 days prior to surgery) in patients with deep cartilage defects.[118] Patients were selected if they were between 19 – 40 years of age with regular joint alignment, no signs of osteoarthritis, had intact ligaments and menisci, a single lesion with sizes between 1 - 4 cm2, lesion depth below 5 mm, and complied with an extensive rehabilitation regimen.[118] After 3 to 6 weeks following autologous chondrocyte harvestation, the Biocart™II graft was cut, press-fitted to the exact defect size, and fixed with fibrin glue.[118] A hinged knee brace was used with continuous passive motion within a range of 0 - 30° until full flexion was achieved after 4 weeks of rehabilitation.[118] The baseline Lysholm score (56.38 ± 14.40) improved after 6 months (82.75 ± 6.45) and after 12 months (84.50 ± 6.02), which was similarly observed using the IKDC clinical assessment.[117] MRIs taken using the 3T MR unit (Magnetom Trio, Siemens Erlangen, Germany) using the MOCART clinical evaluation system (determines the degree of defect repair, the surface and structure of the implant, the condition of the subchondral lamina and bone, the signal intensity of the implant and the presence of adhesions or joint effusions, and the interface between the implant, cartilage, and bone).[118] MRI evaluation of BioCart™II treated knees did not demonstrate hypertrophy, delamination, or displacement and had 75% to 100% filling of the cartilage defect, showed favorable stability and integration to adjacent cartilage tissue after 1 year, and generally met the criteria for hyaline cartilage tissue, with only minor effusion for some patients that resolved without further medical intervention.[118] The fibrin-hyaluranon construct supported a high density chondrocyte population that could retain their chondrocyte phenotype using an FGF-2 variant over a brief culture time for subsequent implantation and repair of an articular cartilage defect that stimulated functional improvement in knee joint tissues.[118]
4.3. Hyaluronic Acid-based Biomatrices and Selected Case Studies
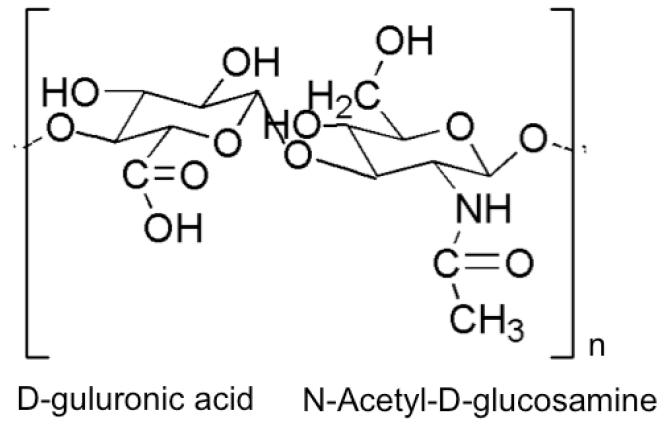
Hyaluronan (hyaluronic acid, HA) is a linear, non-sulfated glycosaminoglycan (2 - 25 μm in length) of repeating disaccharides [(1→4)-glcA-β-(1→3)-glcNAc-β] of D-glucuronic acid and N-acetylglucosamine that forms a twisting ribbon structure stabilized by extensive hydrogen bonding (Figure 5).[119] HA molecules are negatively charged and absorb large quantities of water that enhance lubricity and support compressive loads in different tissues and joints.[120] Chondrocytes secrete HA using membrane-bound HA synthases HAS1, HAS2, and HAS3, which produce HA of molecular weight 2x105 – 2×106 Da (HAS1 and HAS2) and 1×105 - 1×106 Da (HAS3).[121] HA is degraded by hyaluronidases into small oligosaccharides while b-D-glucuronidase and β-N-acetyl-hexoaminidase further degrades oligosaccharides by removing non-reducing terminal sugars.[122] HA binding to CD44 and the receptor for HA-mediated motility (RHAMM) is implicated in critical signal transduction cascades that influence cell detachment, mitosis, migration, and inflammation involved in embryonic development, tissue organization, wound healing, and angiogenesis, which can vary greatly depending on the molecular weight of HA.[123] HA can be extracted from human cadaver tissues (skin, vitreous humor, umbilical cord, or synovial fluid) or animal sources (rooster comb).[124] HA can also be easily and controllably produced using large-scale microbial (Streptococci) fermentation without the risk of animal-derived pathogen contamination.[124] HA can be chemically modified (esterification, acrylation), reacted with poly(ethylene glycol)-based synthetic polymers, or grafted to RGD peptides to facilitate more controlled gelation, transport, degradation, and cell adhesion of the encapsulated cells in HA-based materials.[125]
Figure 5.
Basic structure of hyaluronic acid, which requires additional crosslinking or additional modification and reaction with poly(ethylene glycol) to form stable hydrogel structures.
4.3.1. Hyaluronic Acid-based Biomatrices for Vocal Cord Repair
Vocal cord injury often leads to persistent dysphonia and treatments are often ineffective due to the limited ability to match the biomechanical properties of the vocal cord lamina propria.[126] HA can be appropriately crosslinked to match the biomechanical properties of the vocal cord provided by glycosaminoglycan chains and aid in wound healing processes however requires the addition of gelatin to promote cell adhesion of encapsulated therapeutic cells.[126] A synthetic ECM (Extracel®, Glycosan, Salt Lake City, UT) composed of hyaluranon crosslinked with gelatin-DTPH [3,3′-dithiolbis(propanoic hydrazide)] in a poly(ethylene glycol) diacrylate solution using a 1:4 ratio, with a 5% (w/v) gelatin concentration was studied in a pre-clinical model of vocal cord repair.[126] MSCs from eGFP expressing Balb/c mice were encapsulated in Extracel® and injected into vocal folds that had undergone post-surgical scarring using a 27-gauge needle.[126] Rats that received an Extracel®-MSC injection retained greater mouse MSCs at 30 days (53% area ± 28%) compared to MSC only injection (4.5 % area ± 3%) and displayed enhanced cellularity and maintenance of the normal vocal fold architecture demonstrating the combined influence of Extracel® and the MSC treatment for vocal cord repair.[126] MSC-Extracel® injections also induced greater gene expression of pro-collagen III, fibronectin, TGF-β1, hyaluronidase (HYAL3), and HAS3 while inhibiting smooth muscle actin expression and myofibroblast differentiation indicating favorable healing/remodeling of the vocal fold lamina propria with less associated scarring and loss of biomechanical properties required for phonation.[126] The high hyaluronic acid content in Extracel® made for a softer material (lower elastic moduli), which was associated with an improved mucosal wave and decreased tissue fibrosis.[127] The high concentration of hyaluronic acid was also associated with scarless healing and minimized inflammation resulting in better integration and repair of the vocal fold lamina propria required for proper phonation.[127]
4.3.2. Hyaluronic Acid-based Biomatrices for Bone Repair
Repair of critical bone defects using grafts (autologous, allogenic, or xenogenic) is complicated by the limited supply, potential for disease transmission, or rejection whereas implants (ceramic, polymeric, or metallic) are cost prohibitive, do not favorably integrate with adjacent host tissues, and carry significant risks associated with surgical implantation.[128] The carboxyl groups ofHA (170,000 Da, 0.25 mmol, Lifecore Biomedical Co, Chaska, MN) were reacted with adipic acid dihydrazide (2.2g, 12.5 mmol) mediated by 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (024g, 1.25 mmol) and 1-hydroxybenzotriazole hydrate (0.17g, 1.25 mmol) for 8 hours.[128] HA-ADH was subsequently dialyzed (MWCO of 14,000 Da, SpectraPro, Rancho Dominguez, CA) against 100 nM NaCl for 2.5 days and then in distilled water for 1 day.[128] N-acryloxysuccinimide (0.5g, 3 mmol, Polyscience, Inc. Warrington, PA) was then reacted for 12 hours and the HA-ADH-NHS was again dialyzed against 100 nM NaCl and then distilled water as before and lyophilized for 3 days to obtain a solid arcylated HA (HA-Ac).[128] Gel formation was induced at 37°C via a Michael-type reaction between HA-Ac and PEG-tetra thiols (PEG-SH4, 10,000 Da, Sun Bio Inc., Orinda, CA) at equal molar ratio of acryl and thiol groups (5% wt/vol of HA and PEG-SH4) dissolved in triethanolamine-buffered solution (TEA; 0.3, pH 8).[128] Human bone marrow-derived MSCs (1x106 cells/construct) encapsulated in HA-PEG hydrogels demonstrated a rounded morphology and 72% viability (after 48 hours). This is most likely due to the poor cell-adhesive properties of PEG and hyaluronic acid.[128] When bone morphogenic protein-2 (BMP-2, 500 ng/construct) was incorporated into the construct cell viability increased to 81%.[128] In a rat calvarial bone defect model, incorporation of hMSCs and BMP-2 in HA-PEG hydrogels was compared to control, HA-PEG hydrogel, hMSCs and HA-PEG hydrogel, and BMP-2 and HA-PEG hydrogel.[128] HA-PEG hydrogels with encapsulated hMSCs and BMP-2 demonstrated a synergistic effect with thicker and denser bone formation and displayed a large hematopeotic stromal area with abundant cells and a more mature calcified inorganic bone matrix over HA-PEG and hMSC or HA-PEG with BMP-2 treatments after 4 weeks post-surgical implantation.[128] The HA-PEG hydrogel with BMP-2 and hMSCs also stained positively for osteocalcin throughout the regenerated bone matrix, and demonstrated greater angiogenesis as assessed by VEGF, CD31, and vWF expression (especially within the newly formed hematopoietic stomal region).[128] CD31 also co stained with human-specific Nucleolin expression indicating that encapsulated hMSCs differentiated into endothelial cells within the mesenchymal tissues.[128] Likewise, the treatment group that received the HA-PEG hydrogel containing both BMP-2 and hMSCs no longer showed the presence of hydrogel fragments (indicative of complete hydrogel degradation) by 4 weeks and did not interfere with new bone formation (lack of fibrotic scar tissue) due to the high amount of osteoblast infiltration/calcification and formation of new hematopoetic stromal tissues.[128]
4.3.3. Hyaluronic Acid-based Biomatrices for Cartilage Repair
A 3-year clinical study was executed to determine the safety and efficacy of Hyalograft® C, a tissue engineered construct consisting of autologous chondrocytes and Hyaff-11 (hyaluranon reacted with benzyl alcohol for complete esterification), to repair articular cartilage in patients with chronic defects at the joint surface.[129] Hyalograft® C was seeded (2 weeks of prior chondrocyte culture) and directly molded into the defect and sealed with fibrin glue (Tissucol, Baxter, Vienna) and patients remained non-weight bearing for 4 weeks and progressed to full-weight bearing in 10 - 12 weeks.[129] Clinical evaluations (Lysholm score and International Knee Documentation Committee (IDCK) forms) were performed at 6, 12, 24, and 36 months with patients grouped depending on their age (less than 30 years of age, greater than 30 years of age) or whether they had a single lesion or multiple defects.[129] Patients under 30 years of age with only one lesion demonstrated functional improvement at 1 year that was statistically significant and continued to improve over time.[129] Patients with multiple lesions however did not show functional improvement and patients greater than 30 years of age with only one lesion showed only modest improvement that did not reach statistical significance.[129] A 2 - 7 year follow-up study similarly concluded that Hyalograft® C can provide a favorable clinical outcome with good tissue integration for young, active patients with only single cartilage knee defects that still have normal knee alignment and stable joints however should not be recommended for patients with osteoarthritis or as a salvage treatment to avoid total knee replacement.[130] This is most likely due to the limited regenerative capacity of hyaline articular cartilage, the advanced severity of the injury, as well as the age- or disease-influenced therapeutic potential of patient-derived chondrocytes.[130]
4.4. Alginate-based Biomatrices and Selected Case Studies
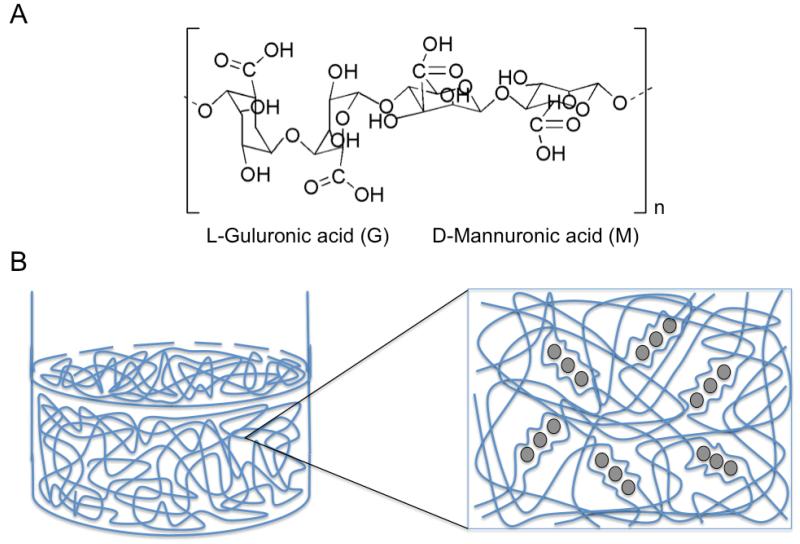
Alginates are natural block copolymers derived from marine brown algae (Laminaria hyperborean, Ascophyllum nodosum, Macrocystis pyrifera) that consist of different regions of repeating β-D-mannuronic acid monomers (M-blocks), α-L-guluronic acid (G-blocks), and sections of interspersed M and G units (that vary greatly depending on the species).[131] Alginates undergo reversible gelation upon exposure to divalent cations such as Ca2+, Sr2+, or Ba2+ that form ionic inter-chain bridges between G-blocks of adjacent alginate chains to form spherical microcapsules.[132] Greater glucoronic acid content develop into stiffer, more porous alginate microcapsules that maintain their mechanical integrity for longer time periods whereas greater mannuronic acid blocks create softer, less porous microcapsules that degrade more quickly over time.[133] Increasing the molecular weight of alginate can also improve the mechanical stability of alginate.[134] Highly viscous alginates induce large shear forces on cells during mixing or injection into the body, which can negatively impact cell viability.[134] Alginate microcapsules are favorable constructs for cell encapsulation as they have a high surface/volume ratio, favorable mass transport, and are less immunogeneic due to their spherical geometry, small size, and smooth topography.[134] Negatively charged alginate droplets are often coated with positively charged poly-L-lysine that control the MWCO of the microcapsule membrane and are then extruded into millimolar concentrations of calcium or barium ions to form alginate beads.[135] The alginate-PLL beads are additionally incubated in alginate (alginate-PLL-alginate, APA) to mask the presence of PLL, which is mildly immunogeneic and can contribute to fibrosis around the microcapsule.[136-139] The PLL layer may also extrude to the outer shell layer however and additional studies have indicated that APA capsules still elicit some complement activation as well as IL-1 and TNF-α production by macrophages.[136-139] Although alginate alone is a relatively abundant material with favorable biocompatibility properties, alginate can easily become contaminated with heavy metals, endotoxins, proteins, or polyphenols during extraction and purification leading to undesirable foreign body responses or cause life-threatening accumulation of toxic organic compounds.[137,140] The development of medical-grade alginate after aqueous alkali treatment containing very low contaminants with reasonable cost-effectiveness and reproducibility for large industrial scale-up are still being actively investigated.[140] Oxidation of alginate with sodium periodate can also cleave the carbon-carbon bond of the cis-diol group in uronate residues allowing for greater in vivo degradation and improved renal clearance.[141] Alginate microcapsules with improved biofunctionalization have also been designed by chemically coupling alginate backbones to RGD, DGEA, or (Tyr-Ile-Gly-Ser-Arg) YIGSR using water-soluble carbodiimide chemistry (Figure 6).[142]
Figure 6.
Alginate polymer overview. A) Basic structure of alginate that form block co-polymers repeating G and M monomers; B) Addition of divalent cations such as calcium or barium ions (grey circles) in aqueous solution causes an exchange of sodium ions from guluronic acids (G) units. The divalent cations cause the guluronic (G) units to undergo a conformational change (stiff egg-box structure) that permits the stacking of G units between adjacent alginate chains, which induces a sol-gel transition for hydrogel or microcapsule formation. The concentration of the sodium, alginate wt%, and number of G units in the alginate structure dictate the overall stability and viscoelastic properties of the alginate gels.
4.4.1. Alginate-based Biomatrices for Pancreatic β-Islet Therapy
Clinical-grade protamine sulfate (PS) and Ba2+ was used to induce alginate microsphere gelation with an electrostatic bead generator (flow rate 0.1 ml/min) to produce a more mechanically stable hydrogel (Ba2+/APSA gelled microcapsules) than more commonly used Ca2+/APA gelled microcapsules, which were evaluated for mechanical integrity by applying osmotic pressure, bead agitation (shear force), and time stability tests.[143] Pancreatic β-islet cells encapsulated in alginate microcapsules (20 mM Ba2+, 0.05% PS, 0.15% LVM alginate, diameter 441 ± 5 μm) demonstrated good viability (>80%) while retaining long-term islet structure (>30 days) and C-reactive peptide secretion (>24 days) in vitro.[143] APSA microcapsules could easily be observed for up to 15 months in vitro or after subcutaneous injection in C57B1/6 mice due to the retention of APSA-associated Ba2+ ions, which had sufficient radiopacity for x-ray/micro computer tomography (μCT) imaging without the use of exogenous contrast agents.[143] Allogeneic human pancreatic β-islets encapsulated in Ba2+/alginate microcapsules were intra-abdominally injected into 4 patients with type I diabetes mellitus, with a co-administration of mild anti-inflammatory (Atorvastatin, 20 mg) and anti-oxidant agents (vitamin A, vitamin B6, vitamin E) after islet transplantation.[144] C-reactive peptide expression was detected in the urine (range: 0.11 – 1.79 nmol/l) while blood glucose levels (36 ± 8%) and insulin requirements (22 ± 3%) were noticeably reduced on the 1st day after encapsulated-islet transplantation.[144] Pancreatic β-islets stopped secreting C-peptide between 1 - 4 weeks after injection however one subject that received multiple encapsulated islet injections continued to show detectable C-reactive peptide expression 2.5 years after the third infusion.[144] Patients developed cytotoxic antibodies to glutamic acid decarboxylase (remained detectable 1.1 – 2.5 years after initial infusion) but not to islet cell auto-antigen and biopsies of the Ba2+/alginate encapsulated pancreatic β-islets demonstrated a necrotic islet core surrounded by fibrous tissue and small clusters of histiocytes.[144] Rapid loss of function of the human pancreatic β-islets encapsulated in Ba2+/alginate microcapsules could be attributed to ischemic necrosis (lack of perfusion at administration site), inflammation and fibrosis induction as initiated by fibrinogen adherence to the microcapsule surface, or pancreatic β-islet allo-antigen shedding and subsequent activation of host immune cells for cytotoxic, inflammatory cytokine release lower than the MWCO (250 kDa) pore size of the alginate microspheres.[144]
4.4.2. Alginate-based Biomatrices for Articular Cartilage Repair
Cartipatch® grafts consists of a mixture of alginate and agarose that gels at physiological temperature and can be directly press-fitted into an articular cartilage defect without using additional sutures or membranes.[144] The Cartipatch® graft also has favorable compressive strength (74 ± 8 kPa), can encapsulate a high density of chondrocytes per hydrogel (10 to 20 million cells), maintain the chondrocyte phenotype, and retain a homogeneous cell distribution within the construct.[145] In a prospective phase 2 clinical trial, Cartipatch® grafts were implanted in 7 patients with traumatic injury and 13 patients with osteochondritis dissecans with defect sizes ranging from 1 to 1.5 cm2 and an average age of 29.8 years.[145] Chondrocyte harvest arthroscopy was performed as previously described and defect size was estimated for subsequent Cartipatch® implantation of 18 mm, 14 mm, or 10 mm diameter hydrogels (width: 4 mm) with 1 to 5 constructs transplanted.[145] After surgery, patients received heparin doses for 1 month to prevent clotting and knees were restricted by a brace locked at 10° degrees of flexion, which allowed for 0° to 90° of passive mobilization.[145] Between 1 and 2 months post-surgery, knees were allowed mobilization between 0° to 140° with progressive weight bearing followed after 10 weeks.[145] Clinical IKDC values improved from an average of 36.6 (n = 20) to 75.5 (n = 17) after 1 year and to 83.7 (n = 6) after 2 years.[145] Biopsies taken after 2 years post-Cartipatch® implantation, demonstrated high chondrocyte viability and collagen II staining similar to hyaline cartilage and displayed favorable collagen-bone integration.[145] Likewise, collagen I staining was only observed in the superficial layers and chondrocyte organization was almost identical to native cartilage with a similar column orientation in the deeper zone whereas a linear organization was observed in the superficial zone.[145]
4.4.3. Alginate-based Biomatrices for Bone Repair
Cooperative interactions (both physical and biochemical) between vascular endothelial cells and osteoblasts/osteoprogenitor cells have previously been implicated in the extent of bone regeneration and could be employed as a co-cultured tissue engineering strategy to enhance bone regeneration.[146] Alginate microspheres were utilized to investigate whether the addition of endothelial cells could enhance the osteogenic potential of osteoprogenitor cells in vitro and in vivo for a critical bone defect in mice.[146] Low molecular weight alginate underwent γ-irradiation and sodium periodate oxidation to improve degradation and (Gly)4-Arg-Gly-Asp-Ser-Pro (G4RGDSP, Commonwealth Biotechnologies) peptides were covalently grafted to alginate chains (16.7 mg of peptide per gram of alginate) using aqueous carbodiimide chemistry.[146] Unmodified purified alginate LF 20/40 sodium alginate (FMC Biopolymers) with high guluronic acid content (25% high MW) was crosslinked to RGD-alginate (75% low MW).[146] Human osteoprogenitor cells (HOPs) and human umbilical vein endothelial cells (HUVECs) were encapsulated alone or in co-culture (1:2 ratio of HOPs to HUVECs) after homogenization with sterile 2% (w/v) RGD-alginate in 0.9% NaCl solution (20x106 cells/mL) using a dual-syringe system and electrostatic bead generator (Nisco), which was dropped (3 cm height) into isotonic 0.1 M CaCl2 solution under constant stirring to facilitate microsphere polymerization (10 minute duration).[146] Cell-alginate microspheres (200 – 500 μm) in spinner flasks were cultured for 24 hrs at 37°C and delivered to bone perforations (0.9 mm in diameter) created using an electric drill at the metaphyseal site.[146] Nude mice were grouped to receive microspheres without cells, microspheres only with HOPs, or microspheres with co-cultured HOPs and HUVECs sacrificed after 3 or 6 weeks post-implantation.[146] Co-immobilization of HOPs and HUVECs in alginate hydrogels in vitro contributed to persistent vWF expression (after 3 weeks of culture) as well as greater alkaline phosphatase (at 8 days) and osteocalcin (after 22 days) expression as compared to alginate-HOPs.[146] Similarly, in vitro co-immobilization of HOPs and HUVECs resulted in greater mineralization (von Kossa staining) and greater VEGF gene expression (after 15 and 22 days).[146] X-ray/μCT images of the bone defect containing co-immobilized HOPs/HUVECs demonstrated significantly greater mineralization within the alginate microspheres at 3 weeks than the alginate-HOPs implant.[146] By 6 weeks post-implantation, the quantified mineralized fraction (assessed by von Kossa staining) was significantly greater for the alginate-HOPs/HUVECs treatment group as compared to aliginate-HOPs treatment group.[146] The combination of dynamic culture conditions, RGD modification of alginate chains, and inclusion of HUVECs into the HOPs culture system contributed to enhanced osteoprogenitor function with greater mineralization and angiogenesis that resulted in a greater extent of bone regeneration and integration with adjacent bone tissues observed in vivo without accompanying scar formation.[146]
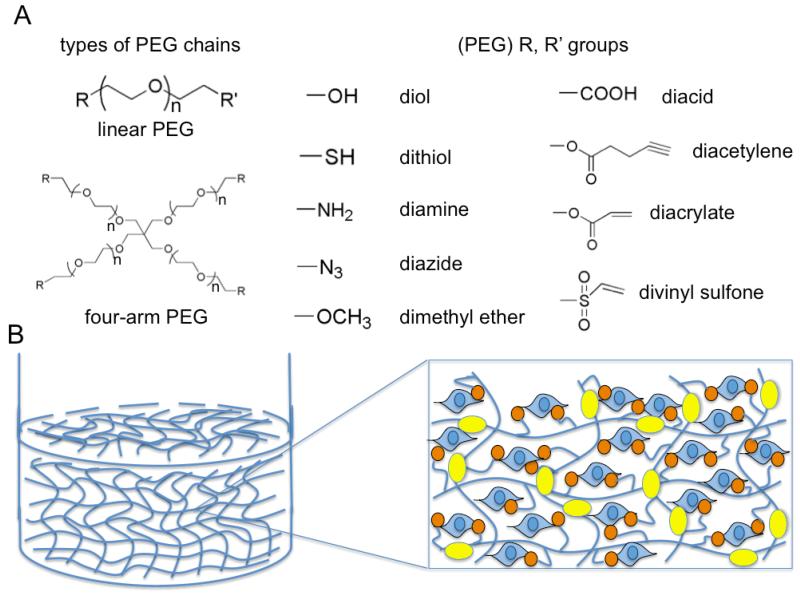
4.5. Poly(ethylene glycol) (PEG)-based Biomatrices and Selected Case Studies
Poly(ethylene glycol) (PEG) hydrogels have been extensively used in tissue engineering as they provide swellable, three-dimensional environments that allow for passive diffusion of nutrients, resist protein adsorption, have good biocompatibility and are non-immunogenic, have tailorable mechanical properties, and are platforms that allow for more control of scaffold architecture and chemical properties.[147] PEG has a linear or branched structure (multiarm or star) whose functional groups can easily be modified to contain hydroxyl, methyloxyl, carboxyl, amine, thiol, azide, vinyl sulfone, acetylene, or acrylate end groups.[148] PEG can be crosslinked by photopolymerization, free-radical polymerization, and specific chemical reactions such as condensation, Michael-type addition, click chemistry, chemical ligation, and enzymatic reactions all of which have variable cytotoxicity for encapsulated cells during polymerization.[149] Additional modifications to PEG hydrogels have been devised for cell encapsulation as PEG chains are difficult to degrade in vivo, biologically inert, and non-adhesive to anchorage-dependent cells.[150] Incorporation of large macromolecules (covalent PEGylation or physical incorporation) such as collagen, fibrin, and hyaluranon have previously been used to improve the biofunctionality of PEG hydrogels however issues with denaturation, degradation, batch-to-batch inconsistency, cost-effectiveness, scale-up, and sterilization, as well as possible bacterial, endotoxin, viral, or prion contamination similarly exist for PEG-protein or PEG-proteoglycan composite materials.[151] Synthetic polymers such as poly(lactic acid) and poly(glycolic acid) as ABA triblock copolymers have also been incorporated into PEG hydrogels to increase hydrolysis and enhance surface and/or bulk degradation however remodeling of the hydrogel is not cell-mediated and degradation by-products can decrease the local pH and adversely impact host tissues.[150] Cell surface receptors such as integrins, syndecans, CD44, and selectins interact to make stable cell-ECM interactions with adjacent proteins such as collagen, laminin, fibronectin, and elastin which dictate cell morphology and behavior.[152-154] Native proteins can be derivatized into less complex peptides and incorporated into PEG hydrogels at the crosslinks or as pendant molecules on adjacent PEG chains to encourage cell adhesion of anchorage dependent cells and enable active remodeling of the PEG hydrogel via sequences that are enzymatically degradable.[149] Fibronectin- (RGD, KQAGDV, REDV, PHSRN), laminin-(YIGSR, LGTIPG, IKVAV, LRGDN, and IKLLI), collagen- (DGEA, GFOGER) and elastin- (VAPG) derived peptides have been covalently attached to PEG hydrogels to enhance cell adhesion.[149] RGD, which is one of the most commonly utilized cell-adhesive peptides, has also been further engineered to have spacer amino acids and a cyclic structure as pendant molecules on PEG chains to re-capitulate the characteristic loop structure of the cell adhesive domain in fibronectin.[155,156] These modifications led to increased affinity for the integrin receptor ανβ3 and enhanced the biological activity (240×) when compared to linear RGD peptides incorporated into PEG hydrogels.[157] Enzyme sensitive sequences such GPQG↓IAGQ (collagen-derived), GPQG↓IWGQ, APG↓L, and L↓GPA (peptide library-derived) have been utilized for MMP-mediated degradation, YK↓NRD, and VR↓N (fibrin-derived) for plasmin-mediated degradation, as well as AAPV↓RGGG, and AAAAAAA (elastin-derived) for elastase-mediated degradation of PEG hydrogels that relies on the proteolytic activity of endogenous human enzymes (↓ indicates cleavage site) (Figure 7).[149,158-160]
Figure 7.
Poly(ethylene glycol) (PEG) synthetic polymer overview. A) Types of PEG chains and example functional groups that can be conjugated to the ends of PEG chains; B) Bioactive modification of PEG for maintenance of viability and function (via cell attachment of anchorage-dependent cells) using cell adhesive peptides derived from adhesive protein domains and enzyme-degradable peptide sequences that permit cell or host-mediated remodeling of the hydrogel network. Cell adhesive peptides (orange circles with linkers) can be incorporated into the hydrogel network by post-grafting cell-adhesive moieties to a polymerized hydrogel network or by co-polymerization with acrylated- or thiolated-peptides using Michael-type reactions or acrylate-acrylate or thiol-acrylate photopolymerization. Enzyme-sensitive peptide sequences (yellow ovals) can also be conjugated to acrylated-PEG effectively inserting a degradable moiety between two PEG monoarcylate chains or thiolated enzyme-degradable peptides can be incorporated at crosslink junctions using multi-arm PEG during photopolymerization.
4.5.1. PEG-based Biomatrices for Cardiac Repair
Benzotriazole carbonate functionalized PEG (BTC-PEG-BTC, 3400 Da, Nektar, San Carlos, CA) was reacted to the amino groups of fibrinogen (10:1 molar ratio) to form carbamate linkages that effectively PEGylated fibrinogen.[161] BTC-PEG-BTC was reacted to fibrinogen (20 mg/mL) in tris buffered saline (pH 7.6 for 20 min) and subsequently reacted with an equal volume of thrombin (200 U/mL in 40 mM CaCl2, Sigma) for a 10 mg/mL: 100 U/mL fibrinogen to thrombin ratio.[161] PEGylated fibrin patches supported encapsulated porcine MSC viability (50,000 MSCs per patch) better than non-PEGylated fibrin patches (5-20 mg/mL fibrinogen, 2-200 U/mL thrombin).[161] PEGylated fibrin-MSCs expressed greater vascular endothelial growth factor (VEGF), formed tube-like structures reminiscent of endothelial vascular networks, and positively stained for endothelial cell markers CD31 and vWF.[161] In a follow-up in vivo study, murine hepatocyte growth factor (HGF, 16 ng/μL in TBS) was reacted with PEGylated fibrinogen for 1 hour, which underwent gelation after adding thrombin (20 U/mL in 40 mM CaCl2) for a final concentration of 2 ng/μL HGF, 10 mg/mL fibrinogen, and 10 U/mL thrombin.[162] Murine MSCs transfected with a LacZ expression vector were injected (0.5x106 cells) into both infracted and periscar regions of the myocardium after left coronary artery ligation that caused 30% infarction of the left ventricle.[162] Greater Murine MSCs (LacZ+) could be detected in the infarct zone and periscar region for the PEGylated-fibrin-HGF/MSC treatment as compared to direct MSC injection 4 weeks after treatment of MI.[162] The PEGylated-HGF/MSC treatment enhanced angiogenesis (vWF staining), attenuated the extent of apoptosis (assessed via TUNEL staining) particularly in the border zone of the infarct, and significantly reduced the fibrotic scar area (15.9 ± 1.6%) as compared to the saline injected MI control (29.3 ± 1.7%).[162] The PEGylated-fibrin-HGF/MSC treatment group also reduced LV dilation (decreased aneurysm incidence) and maintained a greater ejection fraction at 4 weeks compared to other treatment groups post-MI.[162] This platform enhanced MSC viability and engraftment with greater rescue from apoptosis of injured cardiomyocytes and less fibrosis within the infarct zone possibly via HGF signaling or enhanced biomimicry of the myocardial ECM and mechanical reinforcement using the PEGylated-fibrin patch thereby reducing LV wall thinning that normally contributes to LV dysfunction and aneurysm formation.[162] Despite short-term gains in LV function, improvements in cardiac patch technology are still needed to create a high cell density scaffold of similar thickness to physiological myocardium and prevent non-uniformity in LV wall stress when supported by cardiac patches, which can contribute to abnormalities in cardiac function due to poor matching of mechanical properties under cyclic stress to healthy cardiac tissue.[163] Moreover, cardiac patches require highly invasive open heart surgery and may potentially cause life-threatening arrhythymias that must be investigated further before human clinical translation.[163]
5. Challenges and Future Directions
A plethora of biomatrix/cell-based constructs continue to be developed however the most successful therapies that have reached target clinical endpoints have primarily been for skin, cartilage, or corneal repair that can tolerate greater diffusion distances (> 200 μm) for oxygen diffusion and transport.[164] Implantation or injection of biomatrix/cell constructs that lack sufficient vascularization often results in tissue necrosis of seeded or encapsulated cells within the center of the construct.[164] Pre-vascularized networks using bioreactor systems have been developed that consist of scaffolds colonized by endothelial cells (ECs), smooth muscle cells (SMCs), or fibroblasts in vitro that can later be implanted into ischemic tissues to form stable anastomosis with neighboring vascular networks.[164] However, growth of new vessels is a time consuming process and requires microsurgical connection to host blood vessel architecture for achieving sufficient perfusion.[164] Biomatrices or scaffolds containing therapeutic cells have also incorporated exogenous growth factors (ie. VEGF, PDGF, angiopoeitin-1) using pre-encapsulated microspheres or covalent attachment to supporting scaffolds to achieve slow-release devices that encourage EC and SMC recruitment in vivo.[165] Optimal dosing, timing, and presentation of single growth factors or a combination of growth factors for promoting angiogenesis and blood vessel stabilization in specific tissues has not been definitively established and thus remains to be further elucidated by trial-and-error with significant biovariations and in vitro-in vivo disconnects.[165] Genetically modified cells designed to overexpress pro-angiogeneic growth factors can also enhance vascularization within scaffolds to a greater extent than non-transfected cells.[165] Controlled incorporation of cell-adhesive moieties and proteolysis-susceptible peptides have also been used as strategy to encourage EC and SMC infiltration for improved neovascularization (Figure 8).[165]
Figure 8.
Vascularization overview and solutions to improve vascularization for cell-encapsulating tissue engineered constructs. A) Transport of oxygen, nutrients, and growth factors into tissues and transport of carbon dioxide and waste products into the blood stream. Maximum diffusion distance in most tissues is 200 μm and is significant limitation with most macro-scale scaffolds or polymers for most medical applications that lack pre-vascularization or are not anastomosed to an existing vascular bed. B) Inclusion of growth factors, adhesion peptides (orange circles) and enzyme-degradable peptide sequences (yellow oval), or co-culturing with endothelial cells can all improve the vascularization of specific constructs when implanted in vivo.
Clinical examples of scaffolds or biomatrices containing therapeutic cells have largely been restricted to relatively simple designs (type I scaffold or hydrogel) that are biocompatible and supportive of cell proliferation, migration, and differentiation.[166] These constructs are limited in application due to cell-mediated contraction of the material, batch-to-batch inconsistency, insufficient mechanical properties, and uncontrolled degradation.[166] Despite advances in the development of cell-scaffolds/biomatrices with appropriate stiffness and elasticity, graft failure in vivo often occurs for tissue sites subjected to high magnitude and repetitive stress.[167] Multiple parameters in designing engineered scaffolds must be considered such as strength, viscoelastic behavior, anisotropic mechanical and physiological properties, which often have a gradient of such properties for specific tissues.[167] Computer-aided design and rapid prototyping has been applied to create polyglycolic acid and polylactic acid scaffolds with controlled porosity and pore interconnectivity as well as graded mechanical properties that approximate the complex, multiphasic properties of load-bearing tissue.[167] These tissue engineered constructs require additional biofunctionalization with biological polymers or adhesion peptides to facilitate the biochemical signaling required for the long-term survival of anchorage-dependent cells and are often not suitable for current rapid fabrication technologies such as 3-D printing.[168] Incorporation of appropriate growth factors and proteolysis-sensitive peptides that encourage cell proliferation and facilitate active remodeling of the surrounding biomatrix microenvironment are also needed for favorable integration and regeneration of host tissues.[167,168] Bioreactors that subject cells to dynamic compression, cyclic stretch, hydrostatic pressure, or pulsatile fluid flow can also align cells and cell-secreted proteins along a particular axis that better models physiological function than static culture systems.[167] A number of these strategies may require utilization for replacing damaged tissues and controlling cell fate and function while promoting favorable integration with adjacent host tissues.[168] Scaling up to industrial production levels for these complex manufacturing processes remains a technical and an economic hurdle.[57]
Host foreign body response to the presence of implanted scaffolds, biomatrices, and microcapsules is highly dependent on the severity of injury and on the size, degradability, wear debris, surface properties (charge, hydrophilicity/hydrophobicity), presence of antigenic epitopes, and topography of the biomaterial.[169] Implantation of cell-biomaterials to host tissues induces an immediate adsorption of blood proteins and interstitial fluids whose concentration, composition, and presentation vary greatly (in a time-dependent manner) and influence the downstream events such as the coagulation cascade, complement activation (primarily classical and alternative activation), and the adhesion of infiltrating immune cells.[169] Acute injuries before or after implantation of cell-biomatrix constructs also induce the release endogenous danger signals that can attract circulating PMNs or monocyte/macrophages (also tissue-specific macrophages).[169] PMNs and monocyte/macrophages that form stable integrin adhesions to proteins coating the biomaterial surface release a number of proteolytic enzymes and reactive oxygen species that can act to degrade the biomaterial in addition to secreted inflammatory cytokines that could be potentially cytotoxic to delivered cells or host tissues and serve to attract additional phagocytes to the implant surface.[169] Monocyte/macrophages that remain associated with the implant surface can continue to undergo foreign body giant cell (FBGC) formation on non-resolving biomaterials that cause further oxidation, enzymatic degradation, and decreased pH at the implant surface.[170] Degradation products and wear debris can potentially be cytotoxic to scaffold- or biomatrix-associated cells and neighboring tissues as well as induce further inflammation and eventual graft failure due to bulk degradation or poor integration with adjacent tissues.[170] FBGCs interact with neighboring fibroblasts and endothelial cells via the release of pro-fibrotic factors that enhance matrix remodeling and scar formation surrounding the implant site.[170] As discussed previously, the source of therapeutically beneficial cells can also influence foreign body response and adaptive immunity depending greatly on the host’s tolerance to differences and the amount of major histocompatibility complex I and II, co-stimulatory molecule expression, or secreted antigenic molecule expression from allogeneic, xenogeneic, or genetically modified cells.[171] For example, immunoisolation of encapsulated allogeneic or xenogeneic cells (pancreatic β cells) often lack returning therapeutic efficacy long-term due to both the persistence of the microcapsule and long-term transplanted cell antigen shedding that contributes to the continued activation of innate and adaptive innate cells and fibrous encapsulation leading to poor transport and eventual necrosis of cells within the interior of the construct (Figure 9).[171] Low-dose immunosuppressive or anti-inflammatory drug regimens can attenuate immune cell activation to the cell-microcapsule however may leave the recipient susceptible to infection albeit more likely to a lesser degree than receiving full-organ transplantation.[171]
Figure 9.
Microcapsules and foreign body reaction. A) Schematic of alginate microcapsules containing cells that secrete therapeutic molecules with necessary transport that block large antibodies and T cells due to the MWCO of the semi-permeable membrane effectively evading immune recognition; B: Blood protein adsorption to the capsule surface can facilitate macrophage adhesion, which leads to downstream foreign body giant cell formation and fibrous encapsulation with eventual necrosis of the microencapsulated cells.
The long-term function of infiltrating monocyte/macrophages as a result of natural foreign body reaction is a critical issue that needs to be further understood as it can determine the ultimate success or failure of cell-scaffolds for wound repair in different tissue systems.[172-174] The extent of monocyte/macrophages adhesion and detachment via integrin-matrix interactions can also influence macrophage apoptosis and necrosis, where apoptosis is favorable for healing while necrosis leads to a more pro-inflammatory response that can act as a barrier to wound resolution.[172-174] Likewise, the composition, concentration, and the timing of secreted macrophage products such as tumor necrosis factor-α (TNF-α), interleukins (IL-1β, IL-12), ROS, chemotactic, and proteolytic enzymes can contribute to a deleterious inflammatory response that can lead to an adverse foreign body reaction to the cell-containing biomatrix.[172-174] Conversely, macrophage-secreted growth factors and immunomodulatory cytokines (IL-6 and IL-10) that can promote healing and the integration of the cell-containing biomatrix with adjacent tissues can improve wound resolution.[172-174] Recently, MSCs have been shown to secrete a number immunomodulatory factors that attenuated excessive PMN- and macrophage-mediated tissue damage after acute injury, which increased the number of surviving subjects, attenuated loss of tissue function, and improved wound resolution.[23] Biomatrix encapsulated MSCs also programmed adherent monocyte/macrophages in co-culture studies that produced a unique cytokine profile characterized as TNF-αlow, IL-6high, IL-10high, and IL-12low suggested to be favorable for wound resolution.[175,176] Adherent monocyte/macrophages and the encapsulating biomatrix also influenced MSC multidifferentiation potential into adipocytes, chondrocytes, and osteocytes.[176] Taken together, these critical studies highlight the importance of three-way regulation involving host inflammatory cells, MSCs, and the cell-delivery biomatrix.[176] Preliminary work in our laboratory using a MSC-encapsulated gelatin/poly(ethylene glycol) biomatrix polymerized via Michael-type addition was applied to full-thickness cutaneous wounds in rats showed accelerated wound closure and re-epithelialization by 7 days post-treatment as compared to gelatin/poly(ethylene glycol) and sham wound groups. Ongoing work will seek to determine how the combinatorial MSC-biomatrix influenced macrophage polarization (M1: classically activated/pro-inflammatory versus M2: alternatively activated/anti-inflammatory) based on cell surface marker expression and how such activation correlates with the extent wound resolution observed in vivo.
The road from discovery to development of FDA-approved cell-biomatrix products is challenging and involves multiple critical paths: basic research, invention and patent application, product development, verification and validation, scale-up, manufacturing, pre-clinical and clinical testing, regulatory review, and final commercialization, post-market monitoring, and life-cycle management.[177] Cell-biomatrices are combination products (biologic/device) that are often put under additional regulatory scrutiny than traditional drugs or devices.[177] Cell-biomatrices are subject to similar good manufacturing practices that may be difficult to address or could be prohibitively expensive due to issues with scale-up and equipment limitations.[177] Effectively managing potential viral, bacterial, or endotoxin contamination are also critical issues that must be addressed with cell-biomatrix products as they could negatively impact the safety of the patient.[177] Partnerships are increasingly being formed between industry (cost-effective, risk-adverse) and academia (hypothesis-driven) that involve extensive interaction amongst applied engineers, basic research scientists, physicians, and entrepreneurs to solve clinical issues that have not adequately been addressed by current treatment modalities and could potentially benefit from combinatorial cell-biomatrix interventions.[177]
Acknowledgements
Research was supported impart by the University of Wisconsin-Madison School of Pharmacy and the NIH grant EB6613.
Biographies

David Antonio Cantu obtained his Bachelor’s degree (2009) in Biomedical Engineering from the University of Texas at Austin, Austin, Texas and his Master’s degree (2012) in Pharmaceutical Sciences from the University of Wisconsin-Madison, Madison, Wisconsin. He is currently working towards his Ph.D. in the Department of Biomedical Engineering at the University of Wisconsin-Madison. His thesis work is focused on the development of gelatin/poly(ethylene glycol) biomatrices for encapsulation of MSCs to favorably modulate macrophage-mediated inflammatory events and promote cutaneous wound healing.

W. John Kao is a Chair Professor of the Division of Pharmaceutical Sciences, School of Pharmacy, Department of Surgery, School of Medicine and Public Health, and Department of Biomedical Engineering, College of Engineering (Vice Chair) at the University of Wisconsin-Madison. Dr. Kao received his Bachelor’s degree in Biomedical Engineering from Johns Hopkins University, Baltimore, Maryland. He completed his Master’s degree in Biomedical Engineering and also earned his Ph.D. in Macromolecular Sciences at Case Western Reserve University, Cleveland, Ohio. Currently, his group is developing biomaterials that deliver pharmaceuticals, modulate the immune system, and encapsulate therapeutic cells for favorable promotion of wound healing.
References
- [1].Grim SA, Clark NM. Nature. 2011;90:333. doi: 10.1038/clpt.2011.90. [DOI] [PubMed] [Google Scholar]
- [2].Daley GQ, Scadden DT. Cell. 2008;132:544. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- [3].Lechler RI, Sykes M, Thomson AW, Turka LA. Nature. 2005;11:605. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- [4].Ziche M, Donnini S, Morbidelli L. Curr. Drug Targ. 2004;5:485. doi: 10.2174/1389450043345371. [DOI] [PubMed] [Google Scholar]
- [5].Anitua E, Sanchez M, Orive G, Andia I. TRENDS Pharma. Sci. 2007;29:37. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- [6].Scannell JW, Blanckley A, Boldon H, Warrington B. Nature Rev. Drug Disc. 2012;11:191. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- [7].Qasim W, Gaspar HB, Thrasher AJ. Gene Ther. 2009;16:1285. doi: 10.1038/gt.2009.127. [DOI] [PubMed] [Google Scholar]
- [8].Halme DG, Kessler DA. New Eng. J. Med. 2006;355:1730. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- [9].Messenger MP, Tomlins PE. Adv. Mater. 2011;23:H10. doi: 10.1002/adma.201100254. [DOI] [PubMed] [Google Scholar]
- [10].Robey TE, Saiget MK, Reinecke H, Murry CE. J. Mol. Cell Cardiol. 2008;45:567. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rayment EA, Williams DJ. Stem Cell Trans. Clin. Res. 2010;28:996. doi: 10.1002/stem.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fink DW. Science. 2009;324:1662. doi: 10.1126/science.1173712. [DOI] [PubMed] [Google Scholar]
- [13].Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. New Eng. J. Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- [14].Fodor WL. Rep. Bio. Endocrin. 2003;1:102. [Google Scholar]
- [15].Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vécsei V, Schlegel J. Osteoarth. Cart. 2002;10:62. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- [16].Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M, Ellis E, Kraemer M, Nocken F, Fleig W, Manns M, Strom SC, Hengstler JG. J. Hepat. 2006;45:144. doi: 10.1016/j.jhep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [17].Halban PA, German MS, Kahn SE, Weir GC. J. Clin. Endocrinol. Metab. 2010;95:1034. doi: 10.1210/jc.2009-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yamada K, Griesemer A, Okumi M. Transplant. Rev. 2005;19:164. [Google Scholar]
- [19].Ekser B, Ezzelarabg M, Hara H, Windt DJVD, Wijkstrom M, Bottino R, Trucco M, Cooper DKC. Lancet. 2012;379:672. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- [20].Barry FP, Murphy JM. Int. J. Biochem. Cell Biol. 2004;36:568. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [21].Tuan RS, Boland G, Tuli R. Arth. Res. Ther. 2003;5:32. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uccelli A, Moretta L, Pistoia V. Nature Rev. 2008;8:726. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- [23].Prockop DJ, Oh JY. Mol. Ther. 2011;20:14. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, Shi Y. Stem Cells Trans. Med. 2011;1:51. doi: 10.5966/sctm.2011-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Phinney DG, Prockop DJ. Stem Cells. 2007;25:2896. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- [26].Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, Oner FC, Bruijn JDD, Blitterswijk CAV. Tissue Eng. 2002;8:911. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- [27].Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Stem Cells. 2004;22:675. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- [28].Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Stem Cells. 2006;24:1294. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- [29].Robertson JA. Nature Rev. 2001;2:74. doi: 10.1038/35047594. [DOI] [PubMed] [Google Scholar]
- [30].Hyun I. J. Clin. Invest. 2010;120:71. doi: 10.1172/JCI40435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mimeault M, Hauke R, Batra SK. Clin. Pharma. Ther. 2007;82:252. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- [32].Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Bio. Reprod. 2007;77:577. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- [33].Can A, Karahuseyinoglu S. Stem Cells. 2007;25:2886. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- [34].Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring H-J, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Stem Cells. 2008;26:300. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- [35].Sullivan MJ. Nature Rev. 2008;8:554. [Google Scholar]
- [36].Amabile G, Meissner A. Trends Mol. Med. 2009;15:59. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- [37].Kiskinis E, Eggan K. J. Clin. Inv. 2010;120:51. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun N, Longaker MT, Wu JC. Cell Cycle. 2010;9:880. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. Burns. 2009;35:171. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kelm JM, Fussenegger M. Adv. Drug Del. Rev. 2010;62:753. doi: 10.1016/j.addr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- [41].Grande DA, Pitman MI, Peterson L, Menche D, Klein M. J. Orthop. Res. 1989;7:208. doi: 10.1002/jor.1100070208. [DOI] [PubMed] [Google Scholar]
- [42].Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Spine. 2005;30:2379. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- [43].Perin EC, Lopez J. Nature Clin. Pract. Cardiovasc. Med. 2006;3:S110. doi: 10.1038/ncpcardio0447. [DOI] [PubMed] [Google Scholar]
- [44].Menashe P. Mol. Ther. 2009;17:758. [Google Scholar]
- [45].Tongers J, Losordo DW, Landmesser U. Eur. Heart J. 2011;32:1197. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rutanen J, Rissanen TT, Markkanen JE, Gruchala M, Silvennoinen P, Kivela A, Hedman A, Hedman M, Heikura T, Orden MR, Stacker SA, Achen MG, Hartikainen J, Yla-Herttuala S. Circ. 2004;109:1029. doi: 10.1161/01.CIR.0000115519.03688.A2. [DOI] [PubMed] [Google Scholar]
- [47].Karp JM, Teo GSL. Cell Stem Cell. 2009;4:206. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [48].Parekkadan B, Milwid JM. Ann. Rev. Biomed. Eng. 2010;12:87. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Stem Cell Dev. 2009;18:683. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haider H, Jiang S, Idris NM, Ashraf M. Circ. Res. 2008;103:1300. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- [51].Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. J. Mol. Cell Cardiol. 2008;44:281. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yang J, Yamato M, Kohno C, Nishitmoto A, Sekine H, Fukai F, Okano T. Biomaterials. 2005;26:6415. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- [53].Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. J. Biomed. Mater. Res. 1999;45:355. doi: 10.1002/(sici)1097-4636(19990615)45:4<355::aid-jbm10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- [54].Hamdi H, Furuta A, Bellamy V, Bel A, Puymirat E, Peyrard S, Agbulut O, Menasche P. Ann. Thorac. Surg. 2009;87:1196. doi: 10.1016/j.athoracsur.2008.12.074. [DOI] [PubMed] [Google Scholar]
- [55].Kelm JM, Djonov V, Ittner LM, Fluri D, Born W, Hoerstrup SP. Fussenegger. Tissue Eng. 2006;12:2151. doi: 10.1089/ten.2006.12.2151. [DOI] [PubMed] [Google Scholar]
- [56].Kelm JM, Lorber V, Snedeker JG, Schmidt D, Broggini-Tenzer A, Weisstanner M, Odermatt B, Mol A, Zund G, Hoerstrup SP. J. Biotech. 2010;148:46. doi: 10.1016/j.jbiotec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- [57].Archer R, Williams DJ. Nature Biotech. 2005;23:1353. doi: 10.1038/nbt1105-1353. [DOI] [PubMed] [Google Scholar]
- [58].Cheah CM, CK Chua, Leong KF, Chua SW. Int. J. Adv. Manufact. Tech. 2003;21:291. [Google Scholar]
- [59].Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. Biotech. Bioeng. 2003;82:403. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- [60].Chung HJ, Kim K, Kim TG, Park TG. Tissue Eng. Part A. 2008;14:607. doi: 10.1089/tea.2007.0263. [DOI] [PubMed] [Google Scholar]
- [61].Prestwich GD. J. Cell Biochem. 2007;101:1370. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- [62].Brown DL, Meagher PJ, Knight KR, Keramidaris E, Romeo-Meeuw R, Penington AJ, Morrison WA. Cell Transplant. 2006;15:319. [PubMed] [Google Scholar]
- [63].Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Nature Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- [64].Perez-Castillejos R. Mater. Today. 2010;13:32. [Google Scholar]
- [65].Lareu RR, Subramhanya KH, Peng Y, Benny P, Chen C, Wang Z, Rajagopalan R, Raghunath M. FEBS Let. 2007;581:2709. doi: 10.1016/j.febslet.2007.05.020. [DOI] [PubMed] [Google Scholar]
- [66].Nicodemus GD, Bryant SJ. Tissue Eng. Part B. 2008;14:149. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee KY, Mooney DJ. Chem. Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- [68].Fu Y, Xu K, Zheng X, Giacomin AJ, Mix AW, Kao WJ. Biomaterials. 2012;33:48. doi: 10.1016/j.biomaterials.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Benton JA, Fairbanks BD, Anseth KS. Biomaterials. 2009;30:6593. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chung HJ, Park TG. Nanotech. Today. 2009;4:429. [Google Scholar]
- [71].Seliktar D. Science. 2012;336:1124. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- [72].Place ES, Evans ND, Stevens MM. Nature Mater. 2009;8:457. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- [73].Wang F, Li Z, Khan M, Tamama K, Kuppusamy P, Wagner WR, Sen CK, Guan J. Acta Biomaterialia. 2010;6:1978. doi: 10.1016/j.actbio.2009.12.011. [DOI] [PubMed] [Google Scholar]
- [74].Kretlow JD, Klouda L, Mikos AG. Adv. Drug Del. Rev. 2007;59:263. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [75].Murua A, de Castro M, Orive G, Hernandez RM, Pedraz JL. Biomacromolecules. 2007;8:3302. doi: 10.1021/bm070194b. [DOI] [PubMed] [Google Scholar]
- [76].Jonston APR, Cortez C, Angelatos AS, Caruso F. Curr. Opin. Coll. Inter. Sci. 2006;11:203. [Google Scholar]
- [77].Vos PD, Andersson A, Tam SK, Faas MM, Halle JP. Immunol. Endocrin. Metabol. Agents Med. Chem. 2006;6:139. [Google Scholar]
- [78].Uludag H, Vos PD, Tresco PA. Adv. Drug Deliv. Rev. 2000;42:29. doi: 10.1016/s0169-409x(00)00053-3. [DOI] [PubMed] [Google Scholar]
- [79].Alberts B, Johnson A, Lewis J, Raff M, Roberts K. Molecular Biology of the Cell. Garland Science; New York, NY, USA: 2002. [Google Scholar]
- [80].Rest MVD, Garrone R. FASEB J. 1991;5:2814. [PubMed] [Google Scholar]
- [81].Prockop DJ, Kivirikko KI. Ann. Rev. Biochem. 1995;64:403. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- [82].Bateman JF, Lamande SR, Ramshaw JAM, Comper WD. Extracellular matrix. Molecular components and interactions. Hardwood Academic Publishers; Amsterdam, Netherlands: 1996. [Google Scholar]
- [83].Charriere G, Bejot M, Schnitzler L, Ville G, Hartmann DJ. J. Am. Acad. Derma. 1989;21:1203. doi: 10.1016/s0190-9622(89)70330-3. [DOI] [PubMed] [Google Scholar]
- [84].Eaglestein WH, Alvarez OM, Auletta M, Leffel D, Rogers GS, Zitelli JA, Norris JE, Thomas I, Irondo M, Fewkes J, Hardin-young J, Duff RG, Sabolinski ML. Dermatol. Surg. 1999;25:195. doi: 10.1046/j.1524-4725.1999.08186.x. [DOI] [PubMed] [Google Scholar]
- [85].Lauer-Fields JL, Fields GB. Bio. Chem. 2002;383:1095. doi: 10.1515/BC.2002.118. [DOI] [PubMed] [Google Scholar]
- [86].Parenteau-Bariel R, Gauvin R, Berthod F. Materials. 2010;3:1863. [Google Scholar]
- [87].Gilbert TW, Sellaro TL, Badylak SF. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [88].Lee CH, Singla A, Lee Y. Int. J. Pharma. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- [89].Badylak SF, Gilbert TW. Sem. Immunol. 2008;20:109. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lynn AK, Yannas IV, Bonfield W. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004;71B:343. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- [91].Friess W. Eur. J. Pharma. Biopharma. 1998;45:113. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- [92].Doillon CJ, Drouin R, Cote MF, Dallaire N, Pague JF, Laroche G. J. Biomed. Mater. Res. 1997;37:212. doi: 10.1002/(sici)1097-4636(199711)37:2<212::aid-jbm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [93].Weigand C, Abel M, Ruth P, Wilhelms T, Schulze D, Norgauer J, Hipler UC. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009;90:710. doi: 10.1002/jbm.b.31338. [DOI] [PubMed] [Google Scholar]
- [94].Gomez-Guillen MC, Gimenez B, Lopez-Caballero ME, Montero MP. Food Hydro. 2001;25:1813. [Google Scholar]
- [95].Ross-Murphy SB. Polymer. 1992;33:2622. [Google Scholar]
- [96].Zaulyanov L, Kirsner RS. Clin. Intervent. Aging. 2007;2:93. doi: 10.2147/ciia.2007.2.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Veves A, Falanga V, Armstrong DG, Sabolinski ML. Diab. Care. 2001;24:290. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- [98].Falanga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, Jensen J, Sabolinski M, Hardin-Young J. Arch. Derma. 1998;134:293. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- [99].Centanni JM, Straseki JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, Schurr MJ, Foster KV, Faucher LD, Caruso DM, Comer AR, Allen-Hoffman BL. Ann. Surg. 2011;253:1. doi: 10.1097/SLA.0b013e318210f3bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Schurr MJ, Foster KN, Centanni JM, Comer AR, Wicks A, Gibson AL, Thomas-Virnig CL, Schlosser SJ, Faucher LD, Lokuta MA, Allen-Hoffman BL. J. Trauma. 2009;66:866. doi: 10.1097/TA.0b013e31819849d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Shevchenko RV, James SL, James SE. J. Roy. Soc. Inter. 2010;7:229. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Helary C, Abed A, Mosser G, Louedec L, Meddahi-Pelle A, Giraud-Guille MM. J. Tissue Eng. Reg. Med. 2011;5:248. doi: 10.1002/term.326. [DOI] [PubMed] [Google Scholar]
- [103].Crawford DC, Heveran CM, Cannon WD, Foo LF, Potter HG. Am. J. Sports Med. 2009;37:1334. doi: 10.1177/0363546509333011. [DOI] [PubMed] [Google Scholar]
- [104].Cai YZ, Wang LL, Cai HX, Qi YY, Zou XH, Ouyang HW. J. Biomed. Mater. Res. Part A. 2010;95A:49. doi: 10.1002/jbm.a.32816. [DOI] [PubMed] [Google Scholar]
- [105].Tampieri A, Sandri M, Landi E, Pressato D, Francioli S, Quarto R, Martin I. Biomaterials. 2008;29:3539. doi: 10.1016/j.biomaterials.2008.05.008. [DOI] [PubMed] [Google Scholar]
- [106].Kon E, Delcogliano M, Filardo G, Fini M, Giavaresi G, Francioli S, Martin I, Pressato D, Arcangeli E, Quarto R, Sandi M, Marcacci M. J. Ortho. Res. 2010;28:116. doi: 10.1002/jor.20958. [DOI] [PubMed] [Google Scholar]
- [107].Zhang H, Wei YT, Tsang KS, Sun CR, Li J, Huang H, Cui FZ, An YH. J. Trans. Med. 2008;6:67. doi: 10.1186/1479-5876-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chachgues JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O, Carpentier A. Ann. Thorac. Surg. 2008;85:901. doi: 10.1016/j.athoracsur.2007.10.052. [DOI] [PubMed] [Google Scholar]
- [109].Wolberg AS. Blood Rev. 2007;21:131. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [110].Rowe SL, Lee S, Stegemann JP. Acta Biomaterialia. 2007;3:59. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Aper T, Schmidt A, Duchrow M, Bruch H-P. Eur. J. Vasc. Endovasc. Surg. 2007;33:33. doi: 10.1016/j.ejvs.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [112].Lee KW, Lip GYK. JAMA Int. Med. 2003;163:2368. doi: 10.1001/archinte.163.19.2368. [DOI] [PubMed] [Google Scholar]
- [113].Acosta FL, Metz L, Adkisson HD, Liu J, Carruthers-Liebenberg E, Milliman C, Maloney M, Lotz JC. Tissue Eng. Part A. 2011;17:3045. doi: 10.1089/ten.tea.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Xiong Q, Hill KL, Suntharalingam P, Mansoor A, Wang X, Jameel MN, Zhang P, Swingen C, Kaufman DS, Zhang J. Stem Cells. 2011;29:367. doi: 10.1002/stem.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Singelyn JM, Christman KL. J. Cardiovasc. Trans. Res. 2010;3:478. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Tissue Eng. 2007;13:1299. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- [117].Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Dis. Colon Rectum. 2009;52:79. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- [118].Nehrer S, Chiari C, Domayer S, Barkay H, Yayon A. Clin. Ortho. Rel. Res. 2008;466:1849. doi: 10.1007/s11999-008-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Laurent TC, Laurent UBG, Fraser JRE. Ann. Rheum. Dis. 1995;54:429. doi: 10.1136/ard.54.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Liao YH, Jones SA, Forbes B, Martin GP, Brown MB. Drug Deliv. 2005;12:327. doi: 10.1080/10717540590952555. [DOI] [PubMed] [Google Scholar]
- [121].Schoenfelder M, Einspanier R. Bio. Reprod. 2003;6:269. doi: 10.1095/biolreprod.102.011577. [DOI] [PubMed] [Google Scholar]
- [122].Necas J, Bartosikova L, Brauner P, Kolar J. Veterin. 2008;53:397. [Google Scholar]
- [123].Allison DD, Grande-Allen KJ. Tissue Eng. 2006;12:2131. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- [124].Malafaya PB, Silva GA, Reis RL. Adv. Drug Del. Rev. 2007;59:207. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- [125].Kim IL, Mauck RL, Burdick JA. Biomaterials. 2011;32:8771. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Johnson BHQ, Fox R, Chen X, Thibeault S. Laryngoscope. 2010;120:537. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Gaston J, Thibeault SL. Biomatter. 2013;3:1. doi: 10.4161/biom.23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K. Biomaterials. 2007;28:1830. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- [129].Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Eur. J. Radio. 2006;57:3. doi: 10.1016/j.ejrad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- [130].Nehrer S, Dorotka R, Domayer S, Stelzeneder D, Kotz R. Am. J. Sports Med. 2009;37:81. doi: 10.1177/0363546509350704. [DOI] [PubMed] [Google Scholar]
- [131].Geoge TM, Abraham TE. J. Control. Rel. 2006;114:1. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- [132].Stevens MM, Qanadilo HF, Langer R, Shastri VP. Biomaterials. 2004;25:887. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- [133].Drury JL, Dennis RG, Mooney DJ. Biomaterials. 2004;25:3187. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- [134].Kong HJ, Smith MK, Mooney DJ. Biomaterials. 2003;24:4023. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- [135].Dufrane D, Goebbels RM, Saliez A, Guiot Y, Gianello P. Transplant. 2006;81:1345. doi: 10.1097/01.tp.0000208610.75997.20. [DOI] [PubMed] [Google Scholar]
- [136].Hunkeler D. Trends Poly. Sci. 1999;5:286. [Google Scholar]
- [137].Pueyo ME, Darquy S, Capron F, Reach G. J. Biomater. Sci. Poly. Ed. 1993;5:197. doi: 10.1163/156856293x00294. [DOI] [PubMed] [Google Scholar]
- [138].Strand BL, Ryan L, Veld PI, Kulaeng B, Rokstad AM, Skjak-Braek G, Espevik T. Cell Transplant. 2001;10:263. doi: 10.3727/000000001783986800. [DOI] [PubMed] [Google Scholar]
- [139].Tam SK, de Haan BJ, Faas MM, Halle JP, Yahia L, de Vos P. J. Biomed. Mater. Res. Part A. 2009;89A:609. doi: 10.1002/jbm.a.32002. [DOI] [PubMed] [Google Scholar]
- [140].Darquy S, Pueyo ME, Capron F, Reach G. Artif. Organs. 1994;18:898. doi: 10.1111/j.1525-1594.1994.tb03341.x. [DOI] [PubMed] [Google Scholar]
- [141].Lee KY, Mooney DJ. Prog. Poly. Sci. 2012;37:106. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Zimmermann H, Zimmermann D, Reuss R, Feilen J, Manz b, Katsen A. J. Mater. Sci. 2005;16:491. doi: 10.1007/s10856-005-0523-2. [DOI] [PubMed] [Google Scholar]
- [143].Arifin DR, Manek S, Call E, Arepally A, Bulte JWM. Biomaterials. 2012;33:4681. doi: 10.1016/j.biomaterials.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V, Philips R. Diab. Care. 2009;32:1887. doi: 10.2337/dc09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Selmi TAS, Neyrt PH, Verdonk PCM, Barnuin L. Tech. Knee Surg. 2007;6:253. [Google Scholar]
- [146].Grellier M, Granja PL, Fricain J-C, Bidarra SJ, Renard M, Bareille R, Bourget C, Amedee J, Barbosa MA. Biomaterials. 2009;30:3271. doi: 10.1016/j.biomaterials.2009.02.033. [DOI] [PubMed] [Google Scholar]
- [147].Hoffman AS. Adv. Drug Deliv. Rev. 2002;43:3. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- [148].Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. J. Control Rel. 1999;62:81. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- [149].Zhu J. Biomaterials. 2010;31:4639. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nuttelman CR, Tripodi MC, Anseth KS. Matrix Bio. 2005;24:208. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [151].Massia SP, Hubbell JA. Cytotech. 1992;10:189. doi: 10.1007/BF00146670. [DOI] [PubMed] [Google Scholar]
- [152].Cohen M, Joester D, Geiger B, Addadi L. ChemBioChem. 2004;5:1393. doi: 10.1002/cbic.200400162. [DOI] [PubMed] [Google Scholar]
- [153].Henricks PAJ, Nijkamp FP. Eur. J. Pharma. 1998;344:1. doi: 10.1016/s0014-2999(98)00036-3. [DOI] [PubMed] [Google Scholar]
- [154].Woods A, Couchman JR. TRENDs Cell Bio. 1998;8:189. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- [155].Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [156].Leahy DJ, Aukhil I, Erickson HP. Cell. 1996;84:155. doi: 10.1016/s0092-8674(00)81002-8. [DOI] [PubMed] [Google Scholar]
- [157].Zhu J, Tang C, Kottke-Marchant K, Marchant RE. Bioconj. Chem. 2009;20:333. doi: 10.1021/bc800441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Mann BK, West JL. J. Biomed. Mater. Res. 2002;60:86. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- [159].West JL, Hubbell JA. Macromolecules. 1999;32:241. [Google Scholar]
- [160].Aimetti AA, Tibbitt MW, Anseth KS. Biomacromolecules. 2009;10:1484. doi: 10.1021/bm9000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Zhang G, Wang X, Wang Z, Zhang J, Suggs LJ. Tissue Eng. 2006;12:9. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- [162].Zhang G, Hu Q, Bruanlin EA, Suggs LJ, Zhang J. Tissue Eng. Part A. 2008;14:1025. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- [163].Rane AA, Chirstman KL. J. Am. Coll. Cardiol. 2011;58:2615. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- [164].Lovett M, Lee K, Edwards A, Kaplan DL. Tissue Eng. Part B. 2009;15:353. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Novosel EC, Kleinhans C, Kluger PJ. Adv. Drug Del. Rev. 2011;63:300. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- [166].Xu K, Fu Y, Chung W, Zheng X, Cui Y, Hsu IC, Kao WJ. Acta Biomaterialia. 2012;8:2504. doi: 10.1016/j.actbio.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Leong KF, Chua sCK, Sudarmadji N, Yeong WY. J. Mech. Behav. Biomed. Mater. 2008;1:140. doi: 10.1016/j.jmbbm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [168].Freed LE, Guilak F, Guo E, Gray ML, Tranquillo R, Holmes JW, Radisic M, Sefton MV, Kaplan D, Vunjak-Novakovic G. Tissue Eng. 2006;12:3286. doi: 10.1089/ten.2006.12.3285. [DOI] [PubMed] [Google Scholar]
- [169].Franz S, Rammelt S, Scharnweber D, Simon JC. Biomaterials. 2011;32:6692. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- [170].Anderson JM, Rodriguez, Chang DT. Sem. Immunol. 2008;20:86. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Schmidt JJ, Rowley J, Kong HJ. J. Biomed. Mater. Res. Part A. 2008;87:1113. doi: 10.1002/jbm.a.32287. [DOI] [PubMed] [Google Scholar]
- [172].Waldeck H, Wang X, Joyce E, Kao WJ. Biomaterials. 2012;33:29. doi: 10.1016/j.biomaterials.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Chung AS, Waldeck H, Schmidt DR, Kao WJ. J. Biomater. Mat. Res. 2009;91:742. doi: 10.1002/jbm.a.32259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Schmidt DR, Kao WJ. J. Biomater. Mater. Res. 2007;83:617. doi: 10.1002/jbm.a.31270. [DOI] [PubMed] [Google Scholar]
- [175].Jaehyup Kim, Hematti P. Exp. Hemat. 2009;37:1445. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Cantu D, Hematti P, Kao WJ. Stem Cell Trans. Med. 2012;10:740. doi: 10.5966/sctm.2012-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kleinbeck K, Anderson E, Ogle M, Burmania J, Kao WJ. Biointerfaces. 2012;7:12. doi: 10.1007/s13758-011-0012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]