Abstract
Vedolizumab (VDZ) is a humanized monoclonal antibody in development for the treatment of inflammatory bowel disease. VDZ binds to the α4β7 integrin complex and inhibits its binding to mucosal addressin cell adhesion molecule-1 (MAdCAM-1), thus preventing lymphocyte extravasation to gut mucosal tissues. To understand whether VDZ has additional effects that may affect its overall safety as a therapeutic molecule, we examined other potential actions of VDZ. In vitro assays with human peripheral blood lymphocytes demonstrated that VDZ fails to elicit cytotoxicity, lymphocyte activation, and cytokine production from memory T lymphocytes and does not interfere with the suppressive ability of regulatory T cells. Furthermore, we demonstrated that VDZ induces internalization of α4β7 and that the integrin is rapidly re-expressed and fully functional after VDZ withdrawal. These studies provide insight into the mechanisms underlying the observed safety profile of VDZ in clinical trials.
Keywords: in vitro, inflammatory bowel disease, integrin, lymphocyte binding, receptor internalization, safety profile, vedolizumab
Introduction
Vedolizumab (VDZ) is a humanized immunoglobin (Ig)G1 monoclonal antibody (mAb) currently in development for the treatment of ulcerative colitis and Crohn’s disease. It acts by targeting the α4β7 integrin and inhibiting its ability to bind mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressed on the gastrointestinal endothelium,1 thus inhibiting T-cell migration into the gut. The safety and efficacy of VDZ [previous humanized versions were known as LDP-02, MLN02, and MLN0002; for simplicity, all versions of Millennium’s investigational humanized antibodies with the epitopic specificity of the murine version of VDZ (ACT-1) mAb2 will be referred to herein as “vedolizumab”] have been studied in Phase 23-5 and Phase 36-9 trials. In clinical studies, VDZ was not associated with depletion of lymphocyte subsets and did not cause lymphocytosis.3-5 Complete blood counts in normal volunteers and patients with inflammatory bowel disease have not shown increases in circulating lymphocytes, monocytes, eosinophils, or basophils after VDZ administration.3-5
During the construction of VDZ, point mutations were made to the Fc receptor (FcR) γ binding motif (ELLGGP), exchanging Leu239 and Gly241 with Ala to reduce binding to the FcR.10 The observed clinical pharmacology and safety profiles are consistent with both Fc modifications, but it is nonetheless important to confirm that VDZ has no function other than that of inhibiting α4β7 binding to its ligand.
Antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are potential consequences associated with IgG1 binding to FcR and complement components, respectively. Under normal circumstances, these processes aid in the removal of infectious organisms and infected or damaged cells; however, in the case of VDZ, these properties could potentially lead to depletion of α4β7-expressing cells or alterations in cellular function. Lysis of target cells, particularly memory T lymphocytes, can lead to a prolonged absence of cellular activity, which may be disadvantageous if adverse events occur in response to a therapeutic. Additionally, it has been previously demonstrated that some integrins are internalized after binding to ligands or antibodies.11,12 Theoretically, VDZ-α4β7 may also be internalized, which could lead to various biological consequences such as cytokine release and cellular activation. Therefore, the objective of this study was to characterize the effect of VDZ on peripheral blood cells by examining Fc effector functions, cellular activation, and internalization of the α4β7 integrin complex.
Results
Vedolizumab binding to leukocyte α4β7 integrin does not elicit Fc-mediated functions
CDC and ADCC are common Fc-mediated, cytotoxic mechanisms of action for therapeutic IgG1 mAbs, exemplified by the anti-CD3 OKT3 and the anti-CD20 rituximab, respectively.13,14 VDZ was engineered to contain 2 amino acid changes (Leu239 and Gly241 to Ala) in the FcR binding region of the heavy chain to eliminate these binding sites. We compared potential CDC activity of VDZ in human peripheral blood mononuclear cells (PBMCs) to that of OKT3 in vitro. No CDC was observed in the presence of VDZ or IgG1 isotype control at a concentration as high as 10 µg/mL (Fig. 1A), a concentration that was approximately 20-fold greater than that needed to saturate binding of VDZ to human whole blood cells. In contrast, OKT3 induced CDC in PBMCs in a dose-dependent manner, with maximal lysis occurring at 10 µg/mL. These results suggest that the in vivo activity of VDZ does not involve CDC.
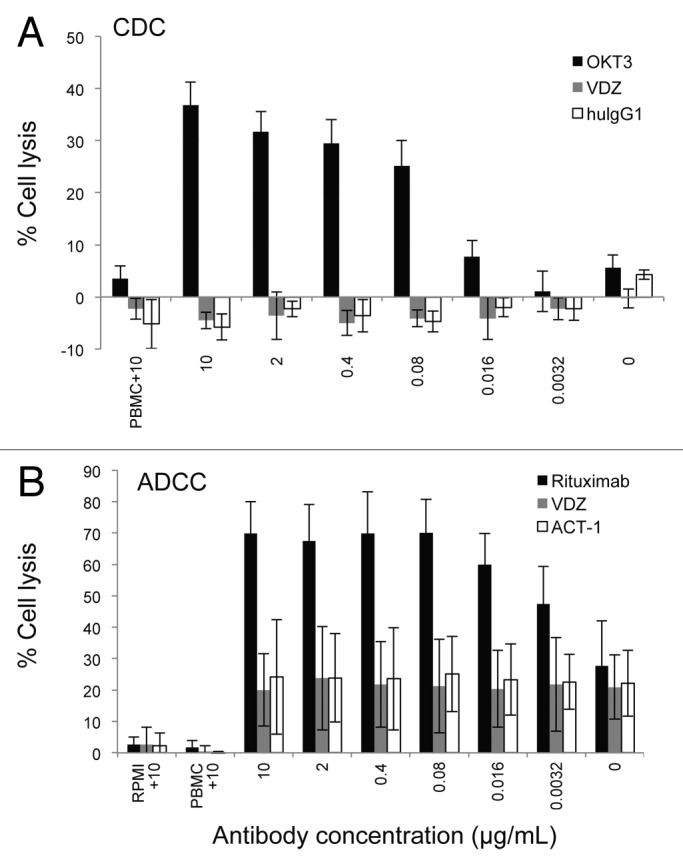
Figure 1. VDZ does not affect CDC or ADCC. (A) PBMCs were incubated with increasing concentrations of VDZ, OKT3, or human IgG1 in the presence of rabbit complement. Spontaneous lysis of the cells in the presence of 10 µg/mL of each antibody in the absence of complement is shown (n = 4). (B) CD20+ α4β7+ RPMI8866 cells were incubated with increasing concentrations of either VDZ, rituximab (anti-CD20), or ACT-1 (the murine precursor of VDZ) in the presence of PBMCs. Spontaneous lysis of the cells in the presence of 10 µg/mL of each antibody in the absence of either effectors (PBMCs) or targets (RPMI8866) is shown (n = 3). Results are representative of 3 experiments.
We also compared potential ADCC activity of VDZ with that of anti-CD20 rituximab in RPMI8866 cells, which stably express high levels of the α4β7 integrin and CD20 (data not shown). Relative to the cells treated with IgG1 isotype control, there was no ADCC activity observed in the presence of VDZ at a concentration as high as 10 µg/mL (Fig. 1B), a concentration that was approximately 100-fold greater than that needed to saturate binding of VDZ to RPMI8866. In contrast, rituximab induced ADCC in RPMI8866 cells in a dose-dependent manner, with maximal lysis occurring at 0.08 µg/mL. These results suggest that VDZ does not induce ADCC activity.
Vedolizumab binding to leukocyte α4β7 integrin does not elicit agonist activity
It is important to understand whether the binding and internalization of VDZ leads to cellular activation and cytokine release, given that antibody-induced cytokine release can have significant clinical effects.15 We therefore examined the ability of VDZ to activate T lymphocytes and induce cytokine production. VDZ binding to human whole blood does not affect expression of the T-lymphocyte early activation marker CD69 or late activation marker CD25 (Fig. 2). In contrast, stimulation with lipopolysaccharide (LPS) or phytohemagglutinin (PHA) did induce upregulation of CD69 and CD25 on T lymphocytes.

Figure 2. VDZ does not induce the expression of the activation markers CD25 or CD69 in T cells. Peripheral blood was incubated in the presence of PBS (control), LPS + PHA, or VDZ and examined at 5.5 h and 25 h for expression of either (A) CD25 or (B) CD69 by flow cytometry (n = 4). Results are representative of 1 of 3 experiments.
To examine whether VDZ induces cytokine release, PBMCs were incubated with VDZ, LPS, or phosphate-buffered saline (PBS) for 5.5 h or 24 h, and cultures were examined for the presence of cytokines. VDZ binding to leukocytes did not elicit cytokine production, including interferon-γ (IFN-γ), tumor necrosis factor (TNF), or interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-12 (p70), and IL-17 (Table 1). In contrast, LPS elicited early cytokine responses that persisted and accumulated over 24 h.
Table 1. In vitro cytokine production (ng/mL)*.
| Time post treatment, h | Treatment | IFN-γ | IL-1β | IL-2 | IL-4 | IL-6 | IL-8 | IL-12 (p70) | IL-17 | TNF |
|---|---|---|---|---|---|---|---|---|---|---|
| 5.5 | PBS | 2.2 ± 3.5 | 0.2 ± 2.0 | 1.8 ± 2.0 | 1.0 ± 0.4 | 7.0 ± 1.8 | 30 ± 50 | 0.8 ± 0.3 | 6.9 ± 6.0 | 5.5 ± 6.0 |
| VDZ | 3.0 ± 5.0 | 0.2 ± 2.3 | 1.9 ± 2.3 | 1.4 ± 1.1 | 9.0 ± 8.2 | 29 ± 36 | 1.1 ± 0.9 | 7.4 ± 9.2 | 7.2 ± 9.7 | |
| LPS | 39 ± 13 | 118 ± 10 | 19 ± 10 | 9.2 ± 3.2 | 25517 ± 9614 | 5755 ± 1903 | 1.9 ± 1.3 | 56 ± 31 | 660 ± 211 | |
| 24 | PBS | 2.8 ± 4.4 | 0.2 ± 2.6 | 3.9 ± 2.6 | 1.0 ± 0.3 | 112 ± 191 | 422 ± 685.4 | 1.1 ± 0.8 | 27 ± 34 | 15 ± 14 |
| VDZ | 3.6 ± 6.2 | 0.2 ± 3.3 | 3.6 ± 3.3 | 0.9 ± 0.4 | 77 ± 122 | 126 ± 124 | 1.4 ± 1.4 | 31 ± 37 | 22 ± 25 | |
| LPS | 4345 ± 2963 | 3403 ± 30 | 50 ± 30 | 17 ± 1.8 | 49258 ± 9606 | 2292 ± 3632 | 4.0 ± 2.9 | 96 ± 83 | 1408 ± 517 |
LPS, lipopolysaccharide; IL, interleukin; PBS, phosphate-buffered saline; TNF, tumor necrosis factor; VDZ, vedolizumab; IFN, interferon. *n, 4
Effect of vedolizumab binding on regulatory T cells
Regulatory T (Treg) cells play a crucial role in maintaining mucosal immune homeostasis, suggesting that Treg cell dysfunction could be a contributing factor in the pathogenesis of human inflammatory bowel disease. For this reason, the effect of VDZ on the suppressive activity of human Treg cells was investigated. To determine if any fraction of the α4+β7+ cells in the periphery were Treg cells, PBMCs from healthy human volunteers were stained with fluorescently labeled anti-α4 and anti-β7 antibodies and analyzed by flow cytometry. Treg cells (as defined by gating on the CD4+ CD45RO+ α4+β7+ and subsequent gating on FoxP3) constituted approximately 13% of the total β7+ CD4+ cell population residing in peripheral blood (Fig. 3A).
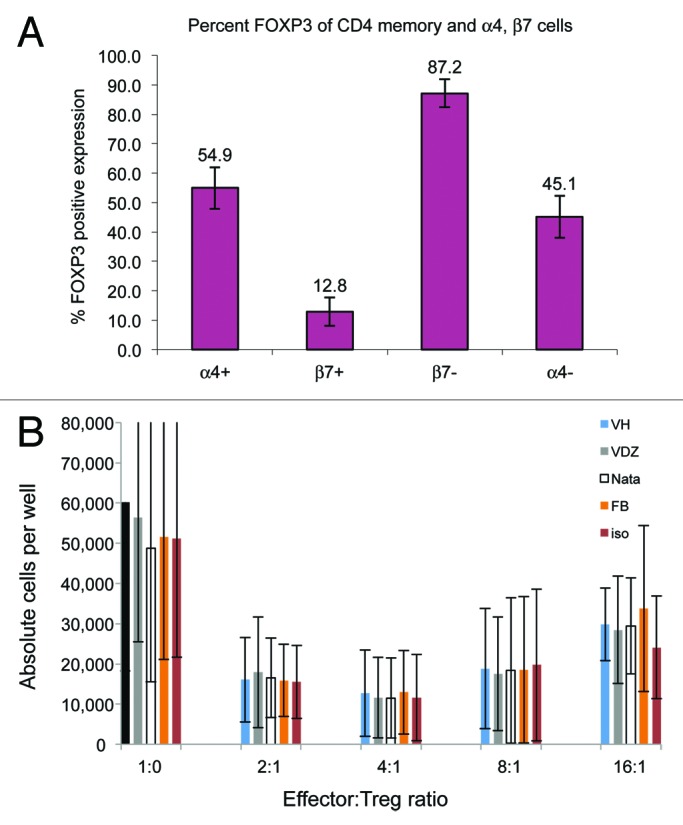
Figure 3. VDZ does not affect suppressor activity of α4+β7+ Treg cells. (A) FoxP3+ Treg cells were examined for the expression of α4 and β7 by flow cytometry, demonstrating the FoxP3+ Treg population constitutes only approximately 13% of the CD4+ CD45RO β7+ memory population. Results are representative of one of 3 experiments. (B) Suppressor activity of purified Treg cells was assessed after pre-incubation with vehicle, VDZ, natalizumab, FIB504, or isotype control, demonstrating a lack of effect of VDZ on suppressor cells. Results are from 3 donors.
The presence of Treg cells in the α4+β7+ population suggests a potential for VDZ to perturb the regulatory balance in the gastrointestinal mucosa. To address this, 3 different α4β7 antagonists (VDZ, natalizumab, and the anti-β7 mAb FIB504) were examined for their ability to affect the suppressive activity of this Treg cell subpopulation in vitro. There was no consistent effect of VDZ, natalizumab, or FIB504 on the suppressive activity of highly purified human α4+β7+ CD4+ CD25+ CD127low FoxP3+ Treg cells compared with that of vehicle or an isotype control antibody. These data indicate that VDZ does not affect the suppressive activity of human Treg cells (Fig. 3B).
The α4β7-vedolizumab complex is internalized by CD4+ memory lymphocytes
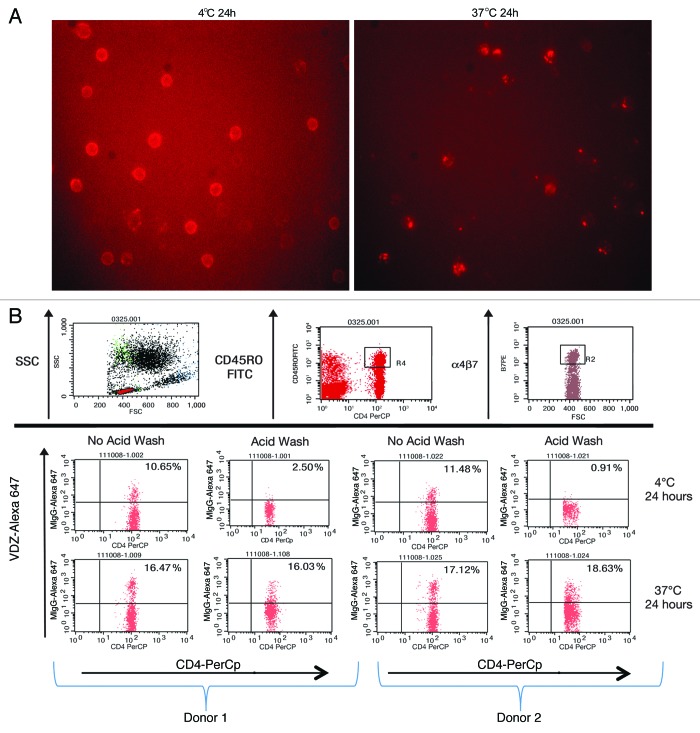
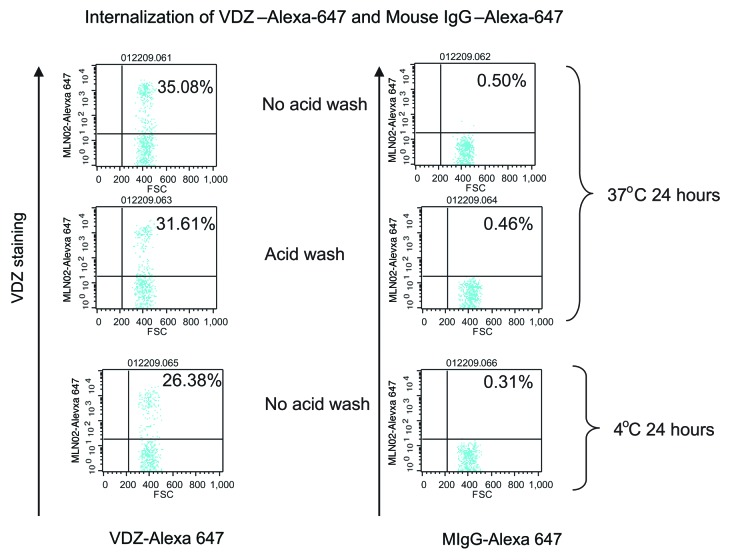
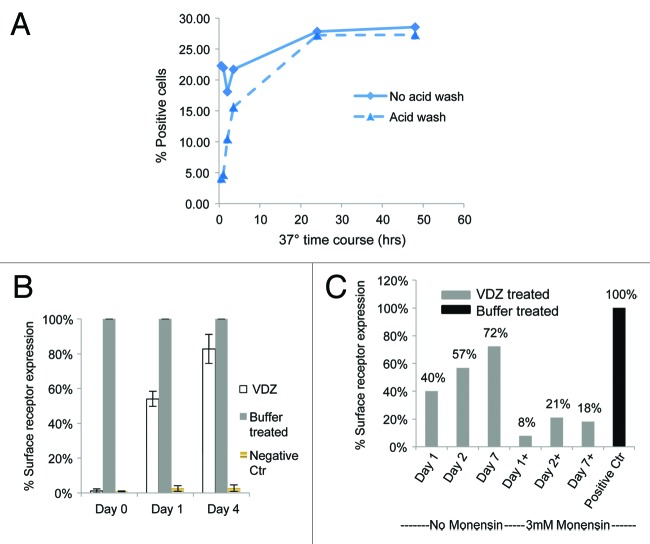
It has been previously demonstrated that antibody binding to integrins induces internalization of the integrin-antibody complex.12 VDZ binds specifically to the α4+β7 integrin with high affinity; it does not bind to other integrin heterodimers containing α4 or β7 chains.1 We therefore investigated whether the VDZ-α4β7 surface complex is internalized. α4β7+ cells were incubated at 37 °C with fluorescently labeled VDZ and examined using microscopy, revealing the classic punctate staining pattern indicative of internalization into endosomes.16,17 In contrast, the staining pattern after incubation at 4 °C indicated membrane staining without collection into endosomes (Fig. 4A). To verify the internalization of the complexes, an acid-stripping flow cytometric assay was used. Alexa 647-labeled VDZ was incubated with CD4+ CD45RO+ memory lymphocytes at 37 °C for 24 h, then washed to remove surface-bound antibody. Alexa 647-positive staining after the wash indicated the receptors were internalized by CD4+ CD45RO+ memory lymphocytes (Fig. 4B). In contrast, cells incubated at 4 °C showed no staining after acid wash. The internalization was target specific and was not a result of nonspecific pinocytosis of labeled antibody, as mouse IgG–Alexa 647 was not internalized by CD4+ memory lymphocytes (Fig. 5). A time course for internalization showed that within 3.5 h, 50% of α4β7 receptors were internalized, and within 24 h, 100% of the receptors were internalized (Fig. 6A).
Figure 4. The α4β7 integrin is internalized after VDZ binding. (A) Peripheral blood T lymphocytes were stained with VDZ–Alexa 647 and visualized by fluorescence microscopy. An intracellular punctate staining pattern is typical of internalization into endosomes, whereas a ringed pattern indicates surface staining. (B) PBMC from 2 representative donors were stained with VDZ-Alexa 647, incubated, and then subjected to acid wash to remove extracellular antibody. For the flow cytometery, cells were gated first on the lymphocyte side scatter (SSC) gate, followed by gating on the CD4+ CD45RO high memory T-cell population. The CD4+ CD45RO high population was subsequently examined for binding to labeled VDZ.
Figure 5. Internalization of Alexa-647 labeled antibodies occurred only with VDZ and not with mouse immunoglobulin (mIgG), indicating the internalization is specific for VDZ. Cells were incubated with either Alexa-647 labeled mIgG control or VDZ for 24 h at 37 °C. Cells were gated initially on forward scatter (FCS)/SSC followed by CD4 and CD45RO to obtain the CD4+ CD45RO+ memory T cells.
Figure 6. α4β7 expression is restored after removal of VDZ in a Golgi-dependent manner. Whole blood was incubated with VDZ, washed, and cultured in the presence or absence of the Golgi inhibitor monensin, and α4β7 expression was assessed by flow cytometry. (A) Time course of receptor internalization. After 24 h, VDZ internalization was reached a plateau. (B) α4β7 expression returned after 1 d and is nearly complete after 4 d of culture in the absence of VDZ. A negative control of fluorescent labeled mouse Ig was included to show percentage of cells with fluorescently background staining. (C) The re-expression of α4β7 was inhibited by co-incubation with monensin.
Basal levels of α4β7 integrin expression on CD4+ memory lymphocytes are restored
A question that remained was whether the α4β7 heterodimer returns to the cell surface after removal of VDZ. To address this, cells that had been treated with VDZ and had internalized the VDZ-α4β7 complex were washed extensively and incubated at 37 °C for 4 d. Re-expression of the α4β7 integrin on the cell surface was monitored on days 0, 1, and 4 by flow cytometry. On average, 54% (n = 3) of the basal level of α4β7 surface expression was restored within 24 h and 83% (n = 3) was restored within 96 h, relative to cells that had not been pretreated with VDZ (Fig. 6B). Treatment with monensin indicated that the recovery of receptors was dependent on intracellular transport through the Golgi (Fig. 6C).18
Newly expressed α4β7 receptors bind to MAdCAM-1
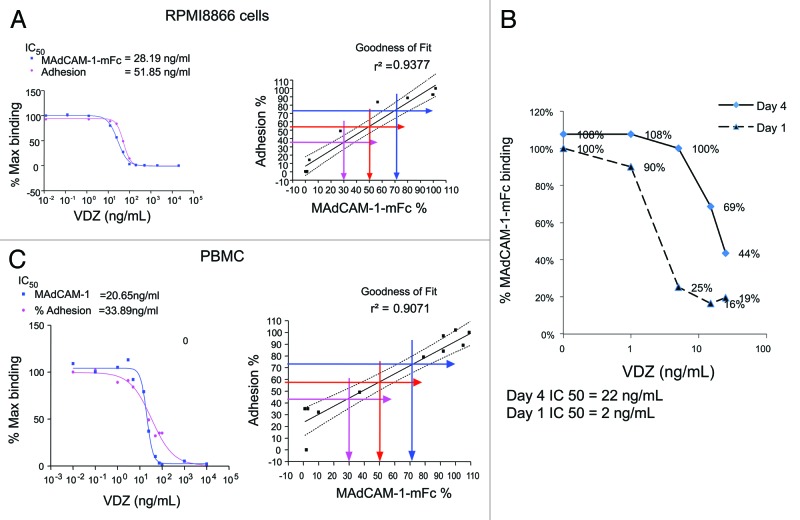
The function of α4β7 integrin is to facilitate adherence of α4β7-expressing cells to surface-bound MAdCAM-1. Because of the processing of the cells through the VDZ treatment, α4β7 internalization, and α4β7 re-expression, however, the adherence assay was too variable to demonstrate functional binding of MAdCAM-1 to the newly expressed receptor. Therefore, it was important to correlate the ability of newly expressed α4β7 to bind MAdCAM-1. To accomplish this, both RPMI8866 and purified CD4+ CD45RO+ memory T cells were tested for adherence and MADCAM-1 binding in the presence of VDZ. The 50% inhibitory concentration (IC50) for each cell type and the goodness of fit were compared between the 2 assays. IC50 values between MAdCAM-1-mFc binding and adhesion assays were within 2-fold in RPMI8866 cells (binding: 28.19 ng/mL; adhesion: 51.85 ng/mL; Fig. 7A) and in CD4+ memory T lymphocytes from PBMCs (binding: 20.65 ng/mL; adhesion: 33.89 ng/mL; Fig. 7A). Furthermore, MAdCAM-1 binding and adhesion were well correlated in both cell types (RPMI8866 cells, r2 = 0.9377; CD4+ memory T lymphocytes, r2 = 0.9071; Fig. 7A).
Figure 7. The re-expressed α4β7 integrin is functional. (A) The binding of soluble MAdCAM-1-mFc to RPMI8866 cells and PBMCs (gated on the CD4+ CD45RO+ population) strongly correlated with adherence of the cells to plate-bound MAdCAM-1-mFc, demonstrating the use of soluble MAdCAM-1 as a surrogate for functional adhesion. (B) MAdCAM-1-mFc was incubated with whole blood 1 and 4 d after initiation of the recovery of the α4β7 complex in the presence of increasing concentrations of VDZ, demonstrating the ability of the newly expressed receptor to bind MAdCAM-1-mFc.
Since binding to soluble MAdCAM-1-mFc was comparable to adhesion, the functional capacity of newly expressed α4β7was examined using soluble MAdCAM-1-mFc binding to the newly expressed cell surface α4β7. The amount of MAdCAM-1 bound to α4β7 receptors increased from day 1 to day 4, as demonstrated by an increase in the amount of VDZ required to inhibit MADCAM-1-mFc binding to the newly expressed integrin complex (IC50: 2 ng/mL and 22 ng/mL for day 1 and day 4, respectively; Fig. 7B). These results are consistent with the reappearance of α4β7 on the surface of the cells and their functional binding to soluble MAdCAM-1. Taken together, these results demonstrate that the α4β7 that is re-expressed after VDZ-α4β7 complex internalization is functional.
Discussion
mAbs are an important part of the physician’s armamentarium for treatment of many autoimmune and oncologic diseases. The safety and pharmacology of these drugs is dependent on both the primary and secondary effector properties of each antibody,19 so it is important to fully characterize the effector properties of the antibody during development. VDZ is a humanized IgG1 antibody in development for the treatment of ulcerative colitis and Crohn disease.4,5 To date, VDZ has been shown to be tolerable and to provide benefit in Phase 2 and Phase 3 clinical trials.3-9 The safety and pharmacology of therapeutic antibodies is dependent on both the primary and secondary effector properties of each antibody,19 and it is thus important to fully characterize these properties.
To investigate activities that could lead to potential safety concerns, we investigated if VDZ is stimulatory or has Fc-mediated activity. Here, we demonstrated that VDZ does not induce CDC or ADCC activity. Additionally, VDZ does not activate T lymphocytes or stimulate the production of cytokines from PBMCs, including the pro-inflammatory cytokines IL-1β, IL-6, and TNF. These data are consistent with results observed in clinical trials. In early Phase 1 studies, there were no observed changes in serum levels of TNF, IL-2, IL-1, or IFN-γ in patients dosed with VDZ (unpublished results). Along with clinical data in the literature,3-9 the results reported here suggest that the induction of cytokines after VDZ treatment is unlikely.
Treg cells are of particular interest because they are postulated to provide inhibitory activity that may decrease inflammation in ulcerative colitis and Crohn’s disease patients.20 There was no consistent effect of VDZ or 2 other α4β7 antagonists on the suppressive activity of highly purified human α4β7+ CD4+ CD25+ CD127low FoxP3+ Treg cells (Fig. 3B), indicating that VDZ does not affect the suppressive activity of human Treg cells in the interstitial matrix of stroma.
We demonstrated that VDZ-α4β7 complexes are rapidly and completely internalized within 24 h of VDZ exposure. This effect is reversible in that, after removal of VDZ, cells will replace functional α4β7 on their surface within 24–48 h. These results suggest that the effect of VDZ binding to cells is that of inhibiting the binding of α4β7+ cells to ligands, particularly MAdCAM-1, and does not include Fc-mediated effects. These data indicate that inhibition of α4β7 function by VDZ in vivo includes a direct interference with MAdCAM-1 binding and decreased surface expression of α4β7 through internalization.
Notably, cells that have internalized the complexes are still viable and able to re-express functional α4β7 after removal of VDZ. These data suggest that the immune system has the potential to return to its previous state upon VDZ withdrawal, which could restore protective activity, as well as pathogenic inflammation. Taken together, these results provide molecular and cellular bases to explain key aspects of VDZ’s tolerability profile in clinical trials to date.
Materials and Methods
Reagents
MAdCAM-1-mFc fusion protein, VDZ, and Alexa 647–labeled VDZ were available in-house. Cy5 goat anti-mouse IgG (H+L) (Cat # 115-175-062) was purchased from Jackson ImmunoResearch. CD4-PerCP (Cat # 550631), CD45RO-FITC (Cat # 555492), and mouse IgG1-Alexa 647 (Cat # 557732) were purchased from BD Biosciences. Sucrose (Cat # S7903), monensin (Cat # M5273), and bovine serum albumin (BSA) fraction V (Cat # A3294) were obtained from Sigma-Aldrich. Fetal bovine serum (Cat # SH30071.02) was obtained from Thermo Fisher Scientific. Dulbecco’s PBS without Ca2+and Mg2+ (Cat # 14190), RPMI-1640 (Cat # 22400), DMEM high glucose-no phosphates medium (Cat #11971), penicillin/streptomycin (Cat # 15070), and L-glutamine (Cat # 25030) were obtained from Invitrogen. Red blood cell (RBC) lysis buffer (Cat # 555899) was obtained from BD Biosciences. Alamar Blue® (Cat # 00–100) was from Trek Diagnostic System. The α4β7-stably expressing human B-cell lymphoma cell line RPMI8866 was a kind gift from Dr David Erle (San Francisco, CA). Culture medium for RPMI8866 cells consisted of RPMI-1640 medium supplemented with 1% penicillin/streptomycin and 1% L-glutamine and 10% US-defined fetal bovine serum. The mouse mAb ACT-1 used was an in-house reagent.
Cytotoxicity assays
Briefly, CDC was measured by incubating VDZ or control antibodies with α4β7−expressing target cells in the presence of rabbit complement at 37 °C. OKT3 (anti-CD3) and normal human IgG1 were used as positive and negative controls, respectively. Spontaneous release was demonstrated in the absence of added complement (PBMC-10) but in the presence of the maximum concentration of antibodies. To measure ADCC, α4β7−expressing target cells were incubated with VDZ or control antibodies, followed by incubation with PBMCs. The anti-CD20 antibody rituximab was used as a positive control, and the murine ACT-1 parental antibody for VDZ as a negative control. Spontaneous release was determined by incubation of both target (RPMI) and effector cells (PBMCs) individually in the presence of the maximum concentration of antibodies. Cellular toxicity for both the CDC and ADCC assays was measured using the colorimetric CytoTox 96 assay (Promega Cat # TB163), which quantitatively measures lactate dehydrogenase, a stable cytosolic enzyme that is released upon cell lysis. Visible wavelength absorbance data were collected using a standard 96-well plate reader. Assays were performed per the manufacturer’s instructions.
Isolation of peripheral blood mononuclear cells and CD4+ memory T lymphocytes
Human whole blood was collected in sodium heparin tubes and mixed 1:1 with Dulbecco’s PBS without Ca2+and Mg2+ and layered onto Ficoll-Hypaque solution (GE Healthcare, Cat # 17-1440-02). The mononuclear cells at the plasma-Ficoll interface were collected based on the manufacturer’s instruction manual. CD4+ memory T lymphocytes were purified using immunomagnetic bead depletion based on the manufacturer’s instructions (Miltenyi Biotech, Cat # 130-091-893).
Internalization of α4β7 receptors on CD4+ memory T lymphocytes
Purified CD4+ memory T lymphocytes or PBMCs were incubated with VDZ-Alexa 647 or mouse IgG–Alexa 647 at 4 °C or 37 °C for 24 h. Internalization was inhibited with 0.45 M sucrose in control samples. Cells were then washed with an acidic solution (0.5 M NaCl and 0.2 M acetic acid) to remove the external fluorescence. The intensity of VDZ-Alexa 647 on CD4+ memory T lymphocytes was measured using flow cytometry.
Cytokine production and cellular activation
Blood was collected in heparin tubes, diluted 1:5 with assay buffer, distributed into endotoxin-free tubes, and stimulated with 5 μg/mL LPS or PHA in the presence or absence of 400 μg/mL VDZ. Production of the following cytokines was measured by enzyme-linked immunosorbent assay (ELISA): IFNγ, TNF, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-12 (p70), and IL-17 by Pierce Biotechnology (Thermo Fisher). Cells were examined for the presence of activation markers CD25 (BD Cat # 555434) and CD69 (BD Cat # 555560) using flow cytometry.
Time course for α4β7 re-expression
Whole blood was incubated with 500 ng/mL VDZ at 37 °C for 24 h and extensively washed to remove unbound antibody. The samples were subsequently incubated at 37 °C with or without 3 μM monensin. α4β7 expression was measured on days 1, 4, and 7 by staining with VDZ-Alexa 647 or mouse IgG-Alexa 647 and visualized by flow cytometry after RBC lysis.
α4β7 binding ability
Whole blood was incubated with 1–1000 ng/mL VDZ at 37 °C for 24 h, washed thoroughly, and cultured. On day 1 and day 4, cells were stained with MadCAM-1-mFc and Cy5 anti-mouse antibody. Mouse serum was added to absorb free Cy5 anti-mouse antibody before staining with anti-CD45RO and anti-CD4 and RBCs lysis. Staining was visualized by flow cytometry.
To assess IC50, whole blood cells that had internalized and then re-expressed receptors were incubated with 3 μg/mL MAdCAM-1-mFc and 4 mM MnCl2 in the presence or absence of VDZ, washed, then stained with 10 μg/mL Cy5 anti-mouse. For lymphocyte samples, mouse serum was added to absorb free Cy5 anti-mouse antibody prior to staining with anti-CD45RO and anti-CD4. For RPMI8866 cells (0.2 × 106 cells/sample), 1 mM MnCl2 was included in the assay buffer.
α4β7 adhesion ability
Ninety-six-well ELISA plates were coated with 3 μg/mL MAdCAM-1-mFc at 4 °C overnight, washed, and blocked with 0.5% BSA in PBS at 37 °C for 1 h. Purified CD4+ memory T lymphocytes or RPMI8866 cells (0.2 × 106/well) were pre-incubated with VDZ and added to the plate, incubated at 37 °C for 1 h, and washed. The bound CD4+ memory T lymphocytes were detached using 5 mM EDTA/PBS and were analyzed by flow cytometry. The bound RPMI8866 cells were incubated with 20% Alamar Blue at 37 °C for 3 h and were read at 530 nm/590 nm on a fluorescence plate reader (Molecular Devices). The percentage of bound cells vs. antibody concentration was plotted using GraphPad Prism Version 4; IC50 values were determined using nonlinear regression curve fits.
Flow cytometry
Samples were analyzed with a FACSCalibur™ instrument and CellQuest™ Pro software (BD Biosciences). 2000 events were collected in the CD4+ memory lymphocyte gate, as determined by FSC vs. SSC and CD4+/CD45RO+ staining. The percentage of α4β7-positive cells was examined in the CD4+ memory lymphocyte gate. Alexa 647-labeled mIgG was used as a negative control.
All procedures utilizing human blood were collected under policies approved by an institutional review board. All samples were deidentified.
Acknowledgments
The authors would like to acknowledge the scientific contributions of Dulce Soler and Hughes Bernard to this manuscript. Funding for these studies was provided by Takeda Cambridge US, formerly Millennium Pharmaceuticals Inc.
Glossary
Abbreviations:
- ACT-1
murine version of VDZ
- ADCC
antibody-dependent cytotoxicity
- BSA
bovine serum albumin
- CDC
complement-dependent cytotoxicity
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- FcR
Fc receptor
- FSC
forward scatter
- IC
inhibitory concentration
- Ig
immunoglobulin
- IL
interleukin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PHA
phytohemagglutinin
- RBC
red blood cell
- SSC
side scatter
- TNF
tumor necrosis factor
- Treg
regulatory T (cells)
- VDZ
vedolizumab
Disclosure of Potential Conflicts of Interest
Wyant T and Yang L are employees of Takeda Cambridge US. Fedyk E is an employee of Takeda Pharmaceuticals International.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26392
References
- 1.Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–75. doi: 10.1124/jpet.109.153973. http://jpet.aspetjournals.org/content/330/3/864.long [DOI] [PubMed] [Google Scholar]
- 2.Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984;133:1857–62. http://www.jimmunol.org/content/133/4/1857.long [PubMed] [Google Scholar]
- 3.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Cohen A, Bitton A, Baker J, Dubé R, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–7. doi: 10.1016/j.cgh.2008.06.007. http://www.sciencedirect.com/science/article/pii/S1542356508006174 [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507. doi: 10.1056/NEJMoa042982. http://www.nejm.org/doi/full/10.1056/NEJMoa042982 [DOI] [PubMed] [Google Scholar]
- 5.Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, Ponich T, Fox I, Feagan BG. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18:1470–9. doi: 10.1002/ibd.21896. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Rutgeerts P, Sands BE, Sandborn W, Colombel J, Hanauer S, et al. Induction therapy for ulcerative colitis: results of GEMINI I, a randomized, placebo-controlled, double-blind, multicenter phase 3 trial. Gastroenterology. 2012;142(Suppl 1):S160–1. http://download.journals.elsevierhealth.com/pdfs/journals/0016-5085/PIIS0016508512606076.pdf [abstract] [Google Scholar]
- 7.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, et al. GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 8.Hanauer SB, Colombel JF, Feagan BG, Rutgeerts P, Sandborn W, Sands B, et al. Vedolizumab maintenance therapy for Crohn's disease: Results of GEMINI II, a randomized, placebo-controlled, double-blind, multicenter phase 3 trial. Am J Gastroenterol. 2012;107(Suppl 1):S620–1. http://www.nature.com/ajg/journal/v107/n1s/pdf/ajg2012275a.pdf [abstract] [Google Scholar]
- 9.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, et al. GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 10.Chappel MS, Isenman DE, Everett M, Xu YY, Dorrington KJ, Klein MH. Identification of the Fc gamma receptor class I binding site in human IgG through the use of recombinant IgG1/IgG2 hybrid and point-mutated antibodies. Proc Natl Acad Sci U S A. 1991;88:9036–40. doi: 10.1073/pnas.88.20.9036. http://www.pnas.org/content/88/20/9036.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leone DR, Giza K, Gill A, Dolinski BM, Yang W, Perper S, Scott DM, Lee WC, Cornebise M, Wortham K, et al. An assessment of the mechanistic differences between two integrin alpha 4 beta 1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2003;305:1150–62. doi: 10.1124/jpet.102.047332. http://jpet.aspetjournals.org/content/305/3/1150.long [DOI] [PubMed] [Google Scholar]
- 12.Ricard I, Payet MD, Dupuis G. VCAM-1 is internalized by a clathrin-related pathway in human endothelial cells but its alpha 4 beta 1 integrin counter-receptor remains associated with the plasma membrane in human T lymphocytes. Eur J Immunol. 1998;28:1708–18. doi: 10.1002/(SICI)1521-4141(199805)28:05<1708::AID-IMMU1708>3.0.CO;2-Y. http://onlinelibrary.wiley.com/doi/10.1002/(SICI)1521-4141(199805)28:05%3C1708:AID-IMMU1708%3E3.0.CO;2-Y/abstract;jsessionid=890C87BCE07EBD63A04EC79127C1CEDA.d04t04 [DOI] [PubMed] [Google Scholar]
- 13.Golay J, Gramigna R, Facchinetti V, Capello D, Gaidano G, Introna M. Acquired immunodeficiency syndrome-associated lymphomas are efficiently lysed through complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity by rituximab. Br J Haematol. 2002;119:923–9. doi: 10.1046/j.1365-2141.2002.03935.x. [DOI] [PubMed] [Google Scholar]
- 14.Raasveld MH, Bemelman FJ, Schellekens PT, van Diepen FN, van Dongen A, van Royen EA, Hack CE, ten Berge IJ. Complement activation during OKT3 treatment: a possible explanation for respiratory side effects. Kidney Int. 1993;43:1140–9. doi: 10.1038/ki.1993.160. [DOI] [PubMed] [Google Scholar]
- 15.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. http://www.nejm.org/doi/full/10.1056/NEJMoa063842 [DOI] [PubMed] [Google Scholar]
- 16.Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 1989;8:1341–8. doi: 10.1002/j.1460-2075.1989.tb03514.x. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC400960/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memmo LM, McKeown-Longo P. The alphavbeta5 integrin functions as an endocytic receptor for vitronectin. J Cell Sci. 1998;111:425–33. doi: 10.1242/jcs.111.4.425. http://jcs.biologists.org/content/111/4/425.long [DOI] [PubMed] [Google Scholar]
- 18.Kaiser J, Stockert RJ, Wolkoff AW. Effect of monensin on receptor recycling during continuous endocytosis of asialoorosomucoid. Exp Cell Res. 1988;174:472–80. doi: 10.1016/0014-4827(88)90316-3. [DOI] [PubMed] [Google Scholar]
- 19.Liu XY, Pop LM, Vitetta ES. Engineering therapeutic monoclonal antibodies. Immunol Rev. 2008;222:9–27. doi: 10.1111/j.1600-065X.2008.00601.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1600-065X.2008.00601.x/pdf [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Liu XP, Zhao ZB, Chen JH, Yu CG. Expression of CD4+ forkhead box P3 (FOXP3)+ regulatory T cells in inflammatory bowel disease. J Dig Dis. 2011;12:286–94. doi: 10.1111/j.1751-2980.2011.00505.x. [DOI] [PubMed] [Google Scholar]