Abstract
A drawback of targeting soluble antigens such as cytokines or toxins with long-lived antibodies is that such antibodies can prolong the half-life of the target antigen by a “buffering” effect. This has motivated the design of antibodies that bind to target with higher affinity at near neutral pH relative to acidic endosomal pH (~pH 6.0). Such antibodies are expected to release antigen within endosomes following uptake into cells, whereas antibody will be recycled and exocytosed in FcRn-expressing cells. To understand how the pH dependence of antibody-antigen interactions affects intracellular trafficking, we generated three antibodies that bind IL-6 with different pH dependencies in the range pH 6.0–7.4. The behavior of antigen in the presence of these antibodies has been characterized using a combination of fixed and live cell fluorescence microscopy. As the affinity of the antibody:IL-6 interaction at pH 6.0 decreases, an increasing amount of antigen dissociates from FcRn-bound antibody in early and late endosomes, and then enters lysosomes. Segregation of antibody and FcRn from endosomes in tubulovesicular transport carriers (TCs) into the recycling pathway can also be observed in live cells, and the extent of IL-6 association with TCs correlates with increasing affinity of the antibody:IL-6 interaction at acidic pH. These analyses result in an understanding, in spatiotemporal terms, of the effect of pH dependence of antibody-antigen interactions on subcellular trafficking and inform the design of antibodies with optimized binding properties for antigen elimination.
Keywords: antigen buffering, antigen-antibody trafficking, pH-dependent
Introduction
Developments in antibody engineering have led to a rapid expansion in the use of antibodies in the clinic.1 Antibodies with high affinity and specificity for an almost unlimited number of targets can be isolated using different display technologies.2-4 More recently, considerable effort has been directed toward producing next generation antibodies that are improved over their parent molecules in one or more respects. For example, Fc engineering can be used to enhance ADCC5,6 or increase in vivo persistence7-9 by modifying binding to FcγRs or FcRn, respectively. The next-generation antibody category can also be extended to encompass the modification of V regions with the goal of altering antigen interactions.10-12
Antibodies can be used to target both membrane receptors and soluble molecules such as inflammatory cytokines;1 however, a concern with the targeting of soluble molecules is that the binding of antibody to antigen can prolong the in vivo persistence of the antigen.13-17 This “antibody buffering” effect is due to the recycling or transcytosis of the antibody-antigen complexes by FcRn through the endosomal pathway in cells.13,18-20 Consequently, there is interest in developing antibodies that can both neutralize their soluble target in the extracellular environment and release bound antigen into a degradative pathway following uptake into cells.11,12,21
Interleukin-6 (IL-6) is a secreted 26-kDa pleiotropic, pro-inflammatory cytokine produced by multiple cell types.22 IL-6 acts on different target cells by forming a tight complex (KD ~1 nM) with either soluble or membrane-bound IL-6 receptor (IL-6R). In addition to being involved in numerous autoimmune and chronic inflammatory diseases, this cytokine is associated with the development and progression of lymphoid malignancies (e.g., multiple myeloma and lymphoma) and prostate, ovarian, and renal cell carcinomas. These disorders are frequently accompanied by a dramatic elevation of IL-6,23,24 motivating the development of effective therapeutic antibodies for the blockade of IL-6 or IL-6R.25 Although the delivery of anti-IL-6R antibodies such as tocilizumab is a potential route for blocking the effect of IL-6, the use of antibodies of this class has resulted in adverse side effects.26 Thus, substantial efforts have been directed toward developing therapeutic antibodies against IL-6.27,28
Despite displaying clinical efficacy by reducing tumor size and neutralizing acute phase responses, anti-IL-6 antibodies can result in increased levels of IL-6 through antibody-mediated extension of half-life.15,29-33 In addition, antibody buffering effects can promote the transport of IL-6 from local sites of inflammation to the circulation and an autocrine-induced increase in IL-6 levels.13,14,16,30,34 Consequently, high molar excesses of anti-IL-6 antibodies are necessary to achieve clinical efficacy.31 An alternative approach is to use combinations of antibodies to generate immune complexes with IL-6 that are cleared rapidly, but this complicates both production and clinical trial design. These disadvantages motivate the design of a novel class of antibodies that bind to antigens such as cytokines in the extracellular environment while releasing target into acidic (~pH 6.0), endosomal compartments following entry into cells.11,12 The use of such antibodies is predicted to result in the dissociation of antigen and delivery to lysosomes, whereas antibody will be recycled by FcRn for re-use. Antibodies of this class are expected to have superior properties in terms of antigen neutralization and clearance relative to their counterparts that bind in a pH-independent mode.11,12 To date, however, how the pH dependence of an antibody-antigen interaction affects the intracellular trafficking of antigen is unexplored, and the current study seeks to address this need for data. An understanding of the timing and extent of dissociation of antigen within cells, and how this is affected by changes in pH dependence, would inform the engineering of pH-dependent antibodies for improved efficacy. Further, in vitro cell assays could provide a valuable and highly sensitive system prior to pharmacokinetic analyses.
In the study presented here, phage display was combined with histidine scanning to generate anti-IL-6 neutralizing antibodies with a range of pH dependencies for binding to antigen.11,12,35,36 The dynamics of the intracellular trafficking of these antibodies and antigen (IL-6) were investigated using live cell imaging in FcRn-expressing cells. Our analyses show that as the pH dependence for IL-6 binding increases, the recycling of IL-6 mediated by antibodies is reduced concomitant with an increase in dissociation in the compartments that recycle IgG, namely endosomes and late endosomes. This in vitro system gives valuable insight into the behavior of pH-dependent antibodies and their target antigens within cells, and provides a useful model for predicting in vivo behavior.
Results
Isolation and characterization of anti-IL-6 antibodies with different binding properties
The goal of this study was to generate and characterize, at the level of intracellular trafficking, anti-IL-6 neutralizing antibodies with different pH dependencies for antigen binding. We therefore first used phage display to isolate a panel of single-chain variable fragments (scFvs) specific for IL-6. These scFvs were analyzed for binding to IL-6 at pH 6.0 and 7.4 using surface plasmon resonance. One scFv (0218) had an approximately 3-fold lower affinity for binding to IL-6 at pH 6.0 relative to pH 7.4 (Table 1). To further increase the pH dependence of 0218, the CDR3 residues of both VH and VL domain genes were systematically replaced with histidines and the resulting antibodies expressed as full-length, human IgG1 molecules (Fig. S1). One mutated antibody, VH4, that had negligible binding to IL-6 at pH 6.0, and a slightly reduced affinity (relative to 0218) at pH 7.4 was identified (Table 1; Fig. S2). This mutant has an aspartic acid to histidine substitution at the fourth residue of the heavy chain CDR3 (Fig. S3). Of a total of 22 histidine mutants (Fig. S1), two heavy chain mutants (VH3 and VH6) and four light chain mutants (VL1, VL4, VL5, and VL9) retained binding to IL-6 at neutral pH but did not display increased pH dependence in binding to IL-6. The remainder of the heavy chain mutants and three light chain mutants (VL3, VL7, and VL8) had substantially decreased binding affinities for IL-6 at neutral pH and therefore were not analyzed further. Although VH14, VL2, and VL6 exhibited increased pH dependence relative to their parent wild type antibody (0218), their binding affinities for IL-6 at neutral pH were lower than that of VH4. VH4 was therefore chosen for subsequent analyses. In addition, 0222 is an IL-6 antibody with similar binding properties for IL-6 at pH 7.4 relative to 0218, but the affinity does not change in the pH range 6.0–7.4 (Fig. S2; Table 1). This antibody was used for comparisons with 0218 and VH4. The sequences of the VH and VL domain genes of the antibodies (0218, 0222, and VH4) used in this study are presented in the SI (Fig. S3). Further, these three antibodies partially neutralize binding of IL-6 to the IL-6 receptor (data not shown).
Table 1. Equilibrium dissociation constants of the interactions between IL-6 and anti-IL-6 antibodies or single chain Fv (scFv) at pH 6.0 and 7.4.
| Antibody or scFv | Dissociation Constant (KD, nM)1 |
|
|---|---|---|
| pH 7.4 | pH 6.0 | |
| scFv 0218 | 23.4 | 64.7 |
| IgG 0218 | 17.9 | 39.5 |
| IgG VH4 | 36.4 | N.D2 |
| IgG 0222 | 15.2 | 17.6 |
1 KDs were determined with antibodies or scFv immobilized on sensor chips and IL-6 as analyte. 2N.D: Not determined, since affinity of the interaction is too low to estimate dissociation constant.
Reduced binding affinity at acidic pH results in increased dissociation from antibody in endosomal compartments
We next assessed how the binding properties of the antibodies for IL-6 affect the intracellular trafficking behavior of antigen in cells that express the Fc receptor, FcRn. Human endothelial cells (HMEC-1) were transiently co-transfected with FcRn-GFP and β2-microglobulin.18 Throughout these studies, a mutated form of human FcRn with analogous binding properties to those of mouse FcRn was used to allow us to correlate our imaging data with studies in mice.37 Consistent with our earlier studies, FcRn-GFP can be detected on the limiting membrane of early and late endosomes in these cells.18 The relatively large size of the endosomes (1–2 μm in diameter) allows a distinction to be made between IgG and antigen that is colocalized with FcRn on the limiting membrane of endosomes and antigen and/or antibody that has dissociated from FcRn into the endosomal vacuole. Further, FcRn and any bound cargo have been shown in our earlier studies to recycle from both early (sorting) endosomes and late endosomes.18,38
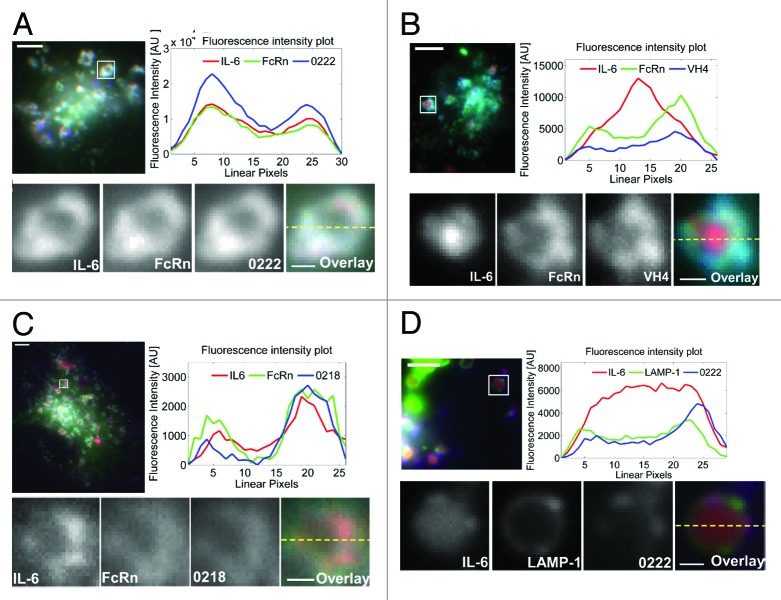
Transfected cells were pulsed at pH 7.4 with a pre-incubated equimolar ratio (1:1) of IL-6 and anti-IL-6 antibodies at 37 °C for one hour to allow uptake of antibody:IL-6 complexes. The mutated FcRn used in the current studies has a low affinity for human IgG1 at neutral pH, which is also observed for human IgG1-mouse FcRn interactions and could result in uptake of antibody:IL-6 complexes by both fluid phase and receptor-mediated pathways.39 Pulsed cells were subsequently washed, fixed and imaged. As expected, the three antibodies, 0218, VH4 and 0222 share the same human IgG1-derived Fc region and remain associated with FcRn on the limiting membrane of endosomes in the transfected cells (Fig. 1). Importantly, the behavior of IL-6 was markedly affected by the antigen binding properties of the antibodies. For cells treated with 0222 in complex with IL-6, the antigen remains extensively colocalized with antibody and FcRn on the limiting membrane of the majority (93%, n = 384 endosomes from 100 cells) of endosomal compartments (Fig. 1A). By contrast, incubation of cells with the highly pH-dependent antibody, VH4, and IL-6 results in poor colocalization of antibody and antigen in endosomes, with detectable levels of IL-6 in the vacuole of 81% (n = 256 endosomes from 104 cells) of endosomes (Fig. 1B). In the presence of 0218, which has an approximately 2-fold lower affinity for IL-6 at pH 6.0 relative to pH 7.4 (Table 1), IL-6 retains association with FcRn/0218 on the limiting membrane of the majority of endosomes (75%, n = 320 endosomes from 97 cells) (Fig. 1C), demonstrating that the endosomal colocalization of IL-6 with 0218 is not as extensive as for 0222.
Figure 1. The pH dependence of antibody:IL-6 interactions affects the endosomal trafficking of IL-6 in FcRn-expressing HMEC-1 cells. (A‒C) HMEC-1 cells were co-transfected with FcRn–GFP and β2 m or with (D) LAMP-1–GFP, FcRn–Stop and β2 m. Transfected cells were pulsed with an equimolar ratio of IL-6 (labeled with Alexa-555) and Atto-647 labeled antibodies 0222 (A and D), VH4 (B), and 0218 (C) for 60 m, fixed and imaged. Images of representative cells are shown with GFP, Atto-647 and Alexa-555 pseudo-colored in green, blue and red, respectively. In each of (A‒D) the endosome in the inset is cropped, expanded and presented as black and white images for the labeled proteins. In addition, the black and white images are shown as an overlay with the proteins represented by their pseudo-colors. Background-subtracted fluorescence intensities along the dotted lines in the overlays are shown in the fluorescence intensity plots. The scale bars for the complete cell images and the cropped endosomes represent 4 and 0.5 µm, respectively.
To exclude the possibility that the accumulation of IL-6 in the vacuole of endosomes when pulsed in the presence of VH4 is not due to fluid phase uptake of IL-6, we analyzed the behavior of IL-6 in the presence of an antibody (VH9) that does not bind detectably to IL-6 (Fig. S4). In HMEC-1 cells pulsed with medium containing either IL-6 alone or IL-6 and VH9, FcRn+ endosomes have no detectable IL-6 on the limiting membrane and very low levels of IL-6 in the endosomal vacuole relative to cells treated with VH4:IL-6 complexes (Fig. S4). Thus, under the conditions of these experiments, IL-6 accumulates at higher levels in cells when in complex with antibody (at pH ~7.4) relative to unbound IL-6.
We further analyzed the intracellular site of IL-6 dissociation from 0218 and 0222 by transfecting cells with LAMP-1 tagged with GFP to distinguish late endosomes (intermediate levels of LAMP-1-GFP) from early endosomes (LAMP-1-negative) and lysosomes (higher levels of LAMP-1 relative to late endosomes).40 Analyses of fixed cells demonstrated that IL-6 dissociates from 0222 or 0218 in a subset of late endosomes (~26% for 0222, n = 31 endosomes from 24 cells; ~62% for 0218, n = 47 endosomes from 22 cells) (Fig. 1D; Fig. S5A). By contrast, there is no detectable IL-6 dissociation from 0222 or 0218 in LAMP-1-negative endosomes, i.e., early or sorting endosomes (Fig. S5B).
IL-6 is inefficiently sorted into tubulovesicular recycling compartments by the highly pH-dependent antibody, VH4
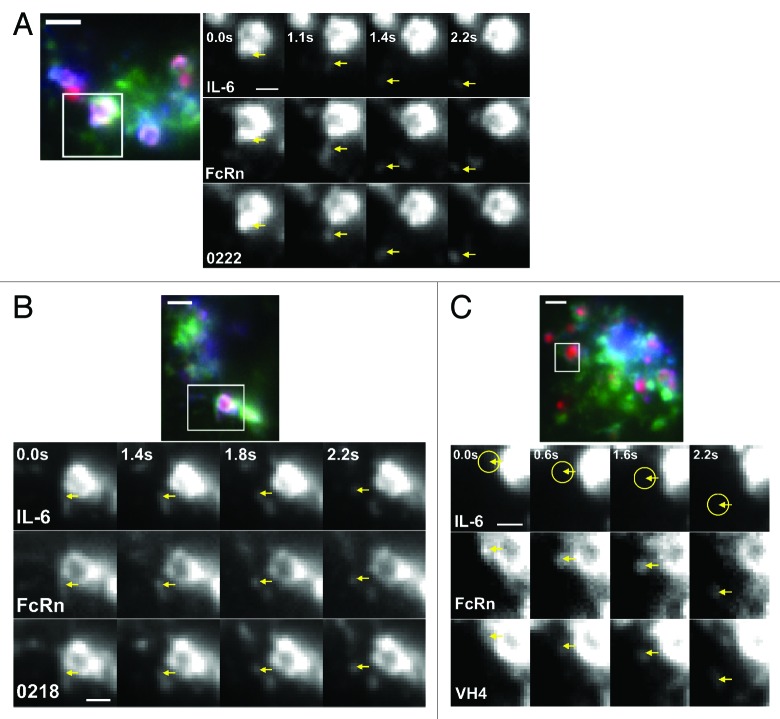
In earlier studies, we used live cell imaging to demonstrate that FcRn and human IgG1 segregate from sorting early and late endosomes in tubulovesicular transport carriers (TCs) that subsequently exocytose at the plasma membrane.18,41,42 A similar approach was used here to directly assess the frequency with which IL-6 leaves endosomes in TCs when cells are incubated with antibody:IL-6 complexes comprising antibodies with different pH dependencies.
Cells were pulsed with antigen-antibody complexes, washed and imaged during the chase period at 37 °C. Consistent with the fixed cell data, for 0222 and 0218, IL-6 is associated with the antibodies on the limiting membrane of the early endosomes. TCs that segregate from these endosomes contain FcRn, IgG and IL-6. Figure 2A and B, and Videos 1 and 2 show typical “leaving” events that were observed for these two antibodies. In addition, IL-6 is present in the vacuole of a low percentage (~5–10% for 0222 and ~20–30% for 0218) of endosomes, which, based on our fixed cell data, are most likely late endosomes (Fig. 1D). By marked contrast with the behavior of 0222 and 0218, VH4 and FcRn consistently leave endosomes in TCs without detectable levels of IL-6 (Fig. 2C; Vid. 3). This is congruent with our fixed cell data in which the majority of IL-6 remains in the vacuole of the endosomal compartments in the presence of VH4 (Fig. 1B).
Figure 2. Endosomal sorting of IL-6 into recycling transport carriers (TCs) is affected by the pH dependence of antibody:IL-6 interactions in FcRn-expressing HMEC-1 cells. HMEC-1 cells were co-transfected with FcRn–GFP and β2 m. The transfected cells were pulsed with an equimolar ratio of 0222 (A), 0218 (B) or VH4 (C) (labeled with Atto-647) and IL-6 (labeled with Alexa-555) for 60 m, and then washed and imaged live at 37 °C. An image of a representative cell with GFP, Atto-647 and Alexa-555 pseudo-colored in green, blue and red, respectively is shown for cells treated with antibody:IL-6 complexes. Individual movie frames (from Vid. 1‒3) of sorting events involving the cropped endosomes (boxed regions) are presented. The individual frames show the event of interest (marked by an arrow) at various time points, where the first time point of 0.0 s is arbitrarily set and corresponds to the first frame of each corresponding supplementary movie. The endosomal sorting event is shown separately in grayscale for the proteins IL-6 (top row), FcRn (middle row) and 0222, 0218 or VH4 (bottom row) and the region of interest is indicated by arrows at the same position in each frame. For panel (C), yellow circles indicate the absence of detectable levels of IL-6 in the TC. The scale bars for the complete cell images and the cropped endosomes represent 4 and 1 µm, respectively.
We also analyzed leaving events from endosomes in which IL-6 was in the endosomal vacuole in the presence of 0222 (Fig. 1D). Based on the data shown in Figure 1D, these compartments are late endosomes, and we have previously shown that FcRn can segregate from endosomes into the recycling pathway at the late endosomal stage.38 Despite the presence of IL-6 in the vacuole of the (late) endosome, antibody and FcRn can segregate in TCs in association with IL-6 (Fig. 3; Vid. 4), indicating partial dissociation of IL-6 from 0222. For cells pulsed with 0222:IL-6 complexes, a total of 25 events were observed where IL-6 remains associated with antibody in FcRn+ TCs that leave endosomes. In addition, IL-6 dissociation from 0222 was observed in four endosomes from which 0222+ TCs segregated, and, consistent with partial dissociation, IL-6 was associated with 0222 and FcRn for three of these leaving events. For 0218, a total of 15 events were detected where IL-6 remains associated with 0218 and leaves endosomes in FcRn+ TCs. Further, of six segregating 0218+ TCs from endosomes in which vacuolar IL-6 was observed, two TCs had detectable levels of IL-6. For VH4, 20 events involving VH4+ TCs leaving from endosomes were analyzed, all of which had detectable levels of FcRn but no IL-6.
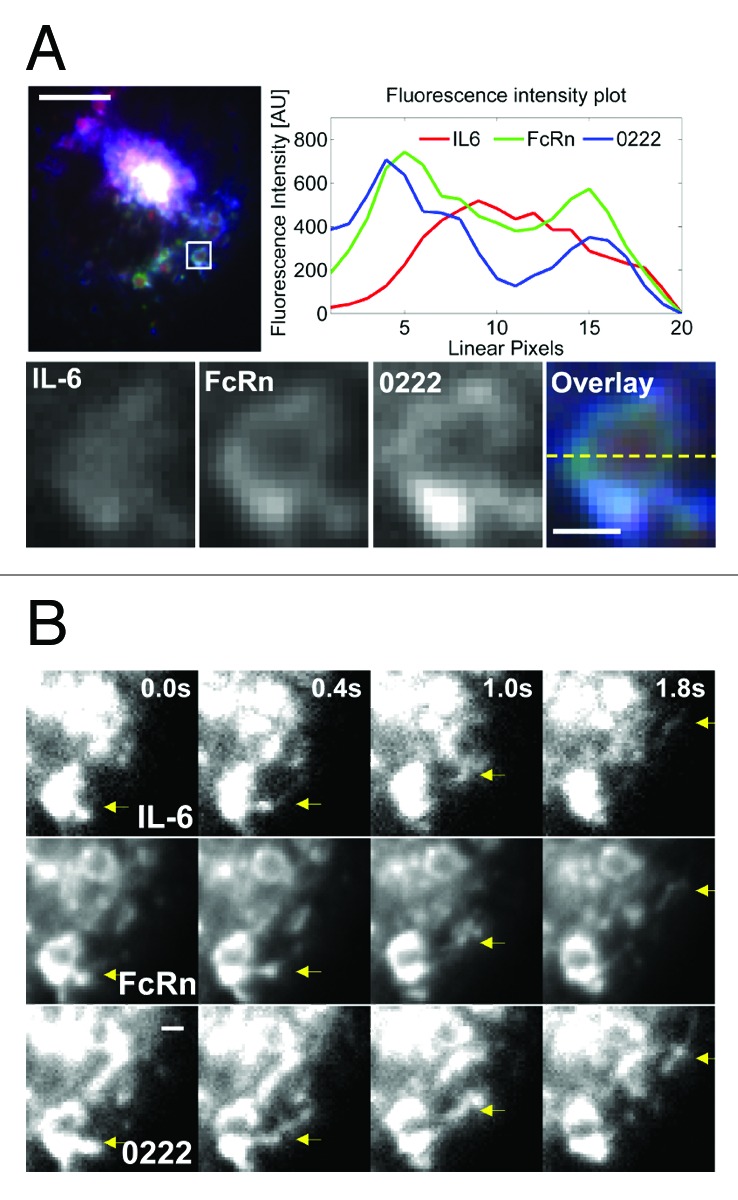
Figure 3. Partial dissociation of IL-6 in the presence of 0222 can occur within endosomes in FcRn-expressing HMEC-1 cells. HMEC-1 cells were co-transfected with FcRn–GFP and β2 m. Transfected cells were pulsed with an equimolar ratio of 0222 (labeled with Atto-647) and IL-6 (labeled with Alexa-555) for 60 m. Cells were washed and imaged live at 37 °C. (A) An image of a representative cell is shown with GFP, Atto-647 and Alexa-555 pseudo-colored in green, blue and red, respectively. The endosome in the inset is cropped, expanded and presented as black and white images for all three proteins (IL-6, FcRn and 0222). In addition, the black and white images are shown in an overlay with the proteins represented by their pseudo-colors. Background-subtracted fluorescence intensities along the dotted line in the overlay are shown in the fluorescence intensity plot, and the fluorescence intensity signals of 0222 and IL-6 are multiplied seven times to match the FcRn fluorescence intensity. The scale bar for the whole cell image represents 4 µm, and the scale bar for the cropped endosome represents 0.5 µm. (B) Individual movie frames (from Vid. 4) of a sorting event (marked by an arrow) involving the endosome in the inset (A) are shown. The frames capture the event at time points 0.0 s, 0.4 s, 1.0 s, and 1.8 s, where the first time point of 0.0 s is arbitrarily set and corresponds to the first frame of Video 4. The sorting event is shown separately in grayscale for the proteins IL-6 (top row), FcRn (middle row) and 0222 (bottom row), and the region of interest is indicated by arrows at the same position in each frame. Scale bar = 1 µm.
We also investigated the destination of IL-6 following dissociation from VH4 within cells. FcRn-GFP transfected HMEC-1 cells were prepulse-chased with fluorescently labeled dextran to label lysosomes.43 During the chase phase, cells were pulsed with VH4:IL-6 complexes and chased for two hours. Cells were then fixed and imaged. Under these conditions, IL-6 within cells is present in dextran-positive lysosomes (Fig. 4).
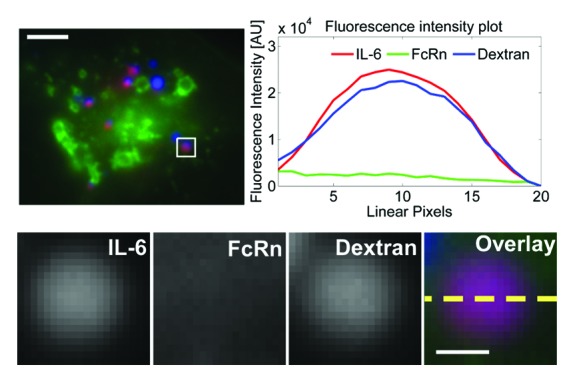
Figure 4. IL-6 enters lysosomes following dissociation in endosomes. HMEC-1 cells were co-transfected with FcRn–GFP and β2 m. Transfected cells were pulsed with dextran (labeled with Atto-647) for 90 m and chased for four hours. During the dextran chase period, the cells were pulsed with an equimolar ratio of VH4 (unlabeled) and IL-6 (labeled with Alexa-555) for 60 m and chased for two hours. The cells were then fixed and imaged. An image of a representative cell is shown with GFP, Atto-647 and Alexa-555 pseudo-colored in green, blue and red, respectively. The lysosome in the inset is cropped, expanded and presented as black and white images for IL-6, FcRn and dextran. In addition, the black and white images are shown as an overlay with the proteins and dextran represented by their pseudo-colors. Background-subtracted fluorescence intensities along the dotted line in the overlay are shown in the fluorescence intensity plot. The scale bars for the complete cell images and the cropped endosomes represent 4 and 0.5 µm, respectively.
Effects of the antibodies on the behavior of IL-6 in mice
The effects of the three antibodies (0218, 0222 and VH4) on the pharmacokinetics of IL-6 were analyzed in female CD-1 mice. This analysis demonstrated that 0218 or 0222 significantly increases the in vivo half-life of IL-6 (Fig. 5). By contrast with the effects of 0218 and 0222, the half-lives of IL-6 alone and IL-6 in the presence of VH4 are not significantly different.
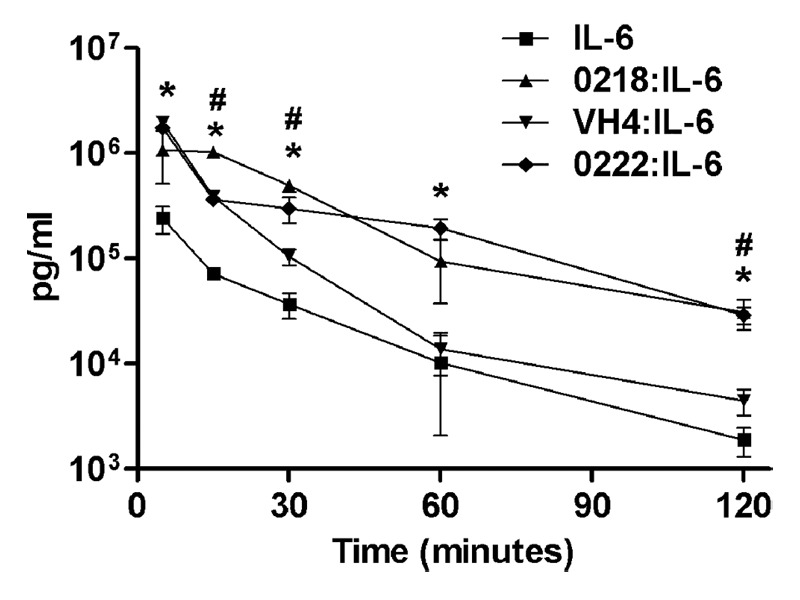
Figure 5. Serum persistence in mice of IL-6 injected in complex with anti-IL-6 antibodies. Female CD-1 mice were injected with human IL-6 pre-incubated with 0222, 0218, or VH4, and uncomplexed human IL-6. Concentrations of human IL-6 in serum samples taken at the indicated time points were determined by ELISA, and mean values for each mouse group are presented. Error bars indicate standard errors. The symbols * and # indicate statistically significant differences between IL-6 levels injected in uncomplexed form compared with IL-6 injected in complex with 0222 and 0218, respectively. Differences between IL-6 and IL-6 pre-incubated with VH4 were not significant at 30, 60, and 120 min.
Proposed binding interaction between IL-6 and antibodies
ELISA and BIAcore analyses indicate that the antibodies 0218/VH4 and 0222 bind to the same or overlapping region of IL-6 (Fig. S6). Interaction models of IL-6 with the Fv fragments of 0218 and VH4 were generated by homology modeling and docking to provide a molecular explanation for the changes in binding interactions between IL-6 and 0218 or VH4 at near neutral and acidic pH. The model with the top-ranking score (Fig. S7) suggests that helices A and D of IL-6 interact with CDRL1, L2, H2, and H3 of the 0218 Fv. The replacement of the fourth residue in the VH domain of CDR3 with histidine to generate VH4 resulted in binding of an ordered water molecule that hydrogen bonds with R40 or K171 of IL-6 at pH 7.4. Consistent with the change in affinity of the VH4:IL-6 interaction as the pH is lowered, the water-mediated hydrogen bonds are not affected by changing histidine to aspartic acid (residue in 0218) at pH 7.4, but are abolished by histidine protonation at pH 6.0.
Discussion
The use of antibodies to target inflammatory cytokines for the treatment of disease represents an attractive therapeutic strategy,1 but, in some cases, antibodies can prolong the persistence of the targeted antigen through a buffering effect.13,14 This has motivated the development of antibodies that bind to targets such as PCSK9 and (soluble) IL-6R in the extracellular environment and subsequently release antigen following entry into acidic, endosomal compartments in cells (refs. 11, 12 and this study), therefore reducing the dose of antibody necessary to reduce serum antigen levels. Further, in the case of pH-dependent IL-6R-specific antibodies, interaction of antibody with membrane-bound IL-6R at near neutral pH followed by internalization and release in acidic endosomes can contribute to antibody recycling and reuse.11 Here, we used fluorescence microscopy, combined with live cell imaging, to analyze the spatiotemporal behavior of antibody-antigen complexes with different binding properties in cells.
To counteract the effects of antigen half-life extension through antibody buffering, antibodies that bind to the inflammatory cytokine IL-6 with different pH dependencies in the pH range 6.0–7.4 were generated.11,12 Using a combination of phage display and protein engineering, three antibodies were isolated that show either no change in binding in the range pH 6.0–7.4 (0222), ~2-fold decreased binding at pH 6.0 relative to pH 7.4 (0218) and essentially negligible binding at pH 6.0 (VH4, derived from 0218). The strategy taken was to identify an antibody (0218) that showed moderate (~2-fold) reduction in binding at pH 6.0 relative to pH 7.4 and systematically introduce histidines at each possible position of the heavy and light chain CDR3s. Using this approach, an antibody (VH4) in which the fourth residue of heavy chain CDR3 was substituted by histidine was isolated. VH4 has a slight reduction in affinity at pH 7.4 with greatly reduced binding at pH 6.0. Although several other mutants generated by this histidine scan showed increases in pH-dependent binding to IL-6, their affinities for antigen at near neutral pH were greatly reduced. Nevertheless, in combination with analyses of others, this study demonstrates that histidine scanning can frequently result in “pH-dependent” antibodies that retain relatively high affinities at near neutral pH.11,12
We have previously shown that FcRn-GFP transfected endothelial cells have a clear distribution of FcRn in early/sorting and late endosomes of 1–2 μm in diameter.18 FcRn can segregate from these compartments in tubulovesicular transport carriers (TCs) and recycle to the cell surface, together with bound cargo.41 This cellular system is therefore well-suited for the analysis of the intracellular trafficking of antigens such as IL-6 in the presence of antibodies that bind with different pH dependencies.
Treatment of FcRn-GFP transfected cells with antibody-antigen complexes containing the three different antibodies demonstrated a clear increase in antigen levels in the vacuole of endosomes and late endosomes as the pH dependence for antigen binding increased. Further, in the presence of VH4, IL-6 accumulates in lysosomes. The segregation of FcRn-IgG complexes from endosomes in recycling TCs in live cells was also investigated. For the pH-independent antibody, 0222, IL-6 was almost invariably associated with FcRn and IgG in TCs. By contrast, IL-6 was never detected in VH4+/FcRn+ TCs, whereas it was usually associated with 0218+ FcRn+ TCs. In some cases, partial dissociation in endosomes for both 0222:IL-6 and 0218:IL-6 complexes occurs, although this was more marked for the pH-dependent antibody 0218. Consequently, whether IL-6 is sorted into recycling TCs or destined for lysosomal degradation is highly dependent on the pH sensitivity of the antibody-antigen interaction.
The presence of IL-6 in the vacuole for a significant proportion of endosomes following treatment of cells with 0218:IL-6, and to a lesser extent 0222:IL-6, complexes prompted us to analyze the nature of these endosomes further. Essentially all endosomes with vacuolar IL-6 in the presence of 0218 or 0222 were late endosomes. We have shown previously that FcRn can dissociate from both early and late endosomes in TCs.18,38 This has relevance to the design of engineered antibodies because it extends the pH range to which recycling antibodies are exposed following entry into cells.
To correlate the subcellular imaging data with in vivo behavior, the serum persistence of injected IL-6 in association with 0218, 0222 and VH4 was analyzed in mice. Consistent with the intracellular trafficking behavior, the half-lives of IL-6 injected with 0218 or 0222 are longer than that of IL-6 complexed with VH4. However, although subcellular trafficking analyses indicate that IL-6 dissociates more frequently from 0218 than 0222 in early/late endosomes, the half-lives of IL-6 in complex with 0218 or 0222 are not significantly different. This may relate to the sensitivity of the in vivo assay, which is limited by the rapid clearance of IL-6, and the relatively few data points that can be acquired from studies of this nature in mice. Thus, we expect that our in vitro imaging assay will provide a highly sensitive and quantitative tool to analyze how pH dependence affects antigen behavior.
Our studies also have relevance to the presentation of antigen by MHC class II molecules when immunogenic molecules such as toxins are being targeted for antibody-mediated neutralization or clearance. Antigen loading onto MHC class II molecules can occur in both endosomal recycling compartments and late endosomes/lysosomes, and the site of antigen loading can affect the conformer of peptide-MHC class II complex that is displayed at the cell surface.44-47 In addition, antibody binding to antigen has been shown in other studies to “protect” epitopes from presentation by MHC class II proteins.48-51 Antibodies that bind with pH independence are therefore expected to have two effects on MHC class II-mediated presentation: first, they will recycle antigen away from degradative compartments through FcRn-mediated bridging.52 Second, they may protect specific epitopes from proteolytic processing and presentation.53 By contrast, pH-dependent antibodies with low affinity at pH ~6.0 will result in antigen dissociation in early endosomes followed by antigen entry into late endosomes and/or lysosomes and are not expected to show any protective effects. Consequently, these differences are expected to result in variations in the epitopes that are presented, at both the level of the peptide sequence and the conformer of peptide-MHC complex that is formed.45 Although here we only consider the targeting of single epitope antigens with monoclonal antibodies, polyclonal mixes of antibodies that form multimeric immune complexes are expected to deliver antigens to late endosomes and/or lysosomes in antigen presenting cells independently of their pH dependence for antigen binding.54,55
In summary, subcellular trafficking analyses of antigen in the presence of antibodies with a range of pH dependencies can be used to inform the dynamic behavior of targeted antigen within cells. Decreased binding of antibody to antigen at acidic, endosomal pH results in reduced recycling of antibody/antigen complexes in tubulovesicular TCs and increased lysosomal trafficking of antigen for degradation, thus decreasing the serum level of targeted antigen. Further, the combination of highly pH-dependent binders with half-life extension platforms or antigen-sweeping approaches could lead to further improvements in therapeutic efficacy.8,21,56
Materials and Methods
Selection and production of anti-IL-6 antibodies and binding analyses
Detailed methods for the isolation of anti-IL-6 scFvs, their reformatting as complete IgG1 molecules, histidine scanning and binding analyses using surface plasmon resonance (SPR) are provided in the Supplemental Materials and Methods.
Cells, plasmids and transfections
HMEC-1 cells were co-transfected with expression plasmids encoding a mutated variant of FcRn with C-terminally linked GFP and human β2-microglobulin (β2 m) using previously described methods.18 The mutated variant of human FcRn (Leu137Glu combined with replacement of residues 79–89 of human FcRn with the corresponding sequence of mouse FcRn37) used for all the imaging experiments has similar binding properties to those of mouse FcRn.
Microscopy image acquisition and analysis
Sample preparation of transfected HMEC-1 cells for fixed and live cell microscopy was performed as described previously.18,38,57 Details of the microscopy configurations, data acquisition and processing are provided in the Supplemental Materials and Methods.
Pharmacokinetic analyses
Pharmacokinetic analyses were performed using 6–8 week old female CD-1 mice obtained from Harlan Laboratories using methods described in the Supplemental Materials and Methods.
Molecular modeling and docking
The homology model structure of the Fv fragment of the 0218 was built using Discovery Studio 3.1 suite (DS 3.1) (Accelrys). ZDOCK in DS 3.1 was used to dock IL-6 onto the refined model of the 0218 Fv. Details of modeling and docking are described in the Supplemental Materials and Methods.
Supplementary Material
Acknowledgments
We thank our colleague Melanie Medcalf and members of the IgG team for help with full length antibody expression.
Disclosure of Potential Conflicts of Interest
This study was supported by MedImmune (http://www.medimmune.com), which is a wholly owned subsidiary of AstraZeneca (http://www.astrazeneca.com). The authors who are MedImmune employees may own AstraZeneca stock or stock options.
Author Contributions
Devanaboyina SC, Lynch S, Ober RJ, Ram S, Zhong H, Jermutus Lutz, Wu H, Webster C, Ward ES, and Gao C designed research; Devanaboyina SC, Lynch S, Kim Dongyoung, Puig-Canto A, Breen S, Kasturirangan S, Fowler S, and Peng L performed research; Devanaboyina SC, Lynch S, Ober RJ, Ram S, Ward ES, Breen S, Zhong H, Webster C, and Gao C analyzed data; Devanaboyina SC and Ward ES wrote the paper.
Supplemental Materials
Supplemental materials can be found here: www.landesbioscience.com/journals/mabs/article/26389.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26389
References
- 1.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–16. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 2.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–55. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 3.Gai SA, Wittrup KD. Yeast surface display for protein engineering and characterization. Curr Opin Struct Biol. 2007;17:467–73. doi: 10.1016/j.sbi.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao CS, Mao SL, Kaufmann G, Wirsching P, Lerner RA, Janda KD. A method for the generation of combinatorial antibody libraries using pIX phage display. Proc Natl Acad Sci U S A. 2002;99:12612–6. doi: 10.1073/pnas.192467999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer’s perspective. Drug Discov Today. 2007;12:898–910. doi: 10.1016/j.drudis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Stavenhagen JB, Gorlatov S, Tuaillon N, Rankin CT, Li H, Burke S, Huang L, Johnson S, Koenig S, Bonvini E. Enhancing the potency of therapeutic monoclonal antibodies via Fc optimization. Adv Enzyme Regul. 2008;48:152–64. doi: 10.1016/j.advenzreg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, Ward ES. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15:637–40. doi: 10.1038/nbt0797-637. [DOI] [PubMed] [Google Scholar]
- 8.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–24. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 9.Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–56. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol. 2008;317:103–23. doi: 10.1007/978-3-540-72146-8_4. [DOI] [PubMed] [Google Scholar]
- 11.Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, Moriyama C, Watanabe T, Takubo R, Doi Y, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 2010;28:1203–7. doi: 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- 12.Chaparro-Riggers J, Liang H, DeVay RM, Bai L, Sutton JE, Chen W, Geng T, Lindquist K, Casas MG, Boustany LM, et al. Increasing serum half-life and extending cholesterol lowering in vivo by engineering antibody with pH-sensitive binding to PCSK9. J Biol Chem. 2012;287:11090–7. doi: 10.1074/jbc.M111.319764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–6. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Hear CE, Foote J. Antibody buffering of a ligand in vivo. Proc Natl Acad Sci U S A. 2005;102:40–4. doi: 10.1073/pnas.0405797102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–44. [PubMed] [Google Scholar]
- 16.Rehlaender BN, Cho MJ. Antibodies as carrier proteins. Pharm Res. 1998;15:1652–6. doi: 10.1023/A:1011936007457. [DOI] [PubMed] [Google Scholar]
- 17.Phelan JD, Orekov T, Finkelman FD. Cutting edge: mechanism of enhancement of in vivo cytokine effects by anti-cytokine monoclonal antibodies. J Immunol. 2008;180:44–8. doi: 10.4049/jimmunol.180.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172:2021–9. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 19.Ward ES, Ober RJ. Chapter 4: Multitasking by exploitation of intracellular transport functions: the many faces of FcRn. Adv Immunol. 2009;103:77–115. doi: 10.1016/S0065-2776(09)03004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 21.Igawa T, Maeda A, Haraya K, Tachibana T, Iwayanagi Y, Mimoto F, Higuchi Y, Ishii S, Tamba S, Hironiwa N, et al. Engineered monoclonal antibody with novel antigen-sweeping activity in vivo. PLoS One. 2013;8:e63236. doi: 10.1371/journal.pone.0063236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 23.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–62. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu ZY, Brailly H, Rossi JF, Wijdenes J, Bataille R, Klein B. Overall interleukin-6 production exceeds 7 mg/day in multiple myeloma complicated by sepsis. Cytokine. 1993;5:578–82. doi: 10.1016/S1043-4666(05)80007-9. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Itoh Y, Yokomizo C, Nishimura T, Niimi T, Umemura A, Fujii H, Okanoue T, Yoshikawa T. Blockade of IL-6 signaling exacerbates liver injury and suppresses antiapoptotic gene expression in methionine choline-deficient diet-fed db/db mice. Lab Invest. 2011;91:609–18. doi: 10.1038/labinvest.2011.2. [DOI] [PubMed] [Google Scholar]
- 27.van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, Sokol L, Crawford J, Cornfeld M, Qi M, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28:3701–8. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- 28.Xu ZH, Bouman-Thio E, Comisar C, Frederick B, Van Hartingsveldt B, Marini JC, Davis HM, Zhou H. Pharmacokinetics, pharmacodynamics and safety of a human anti-IL-6 monoclonal antibody (sirukumab) in healthy subjects in a first-in-human study. Br J Clin Pharmacol. 2011;72:270–81. doi: 10.1111/j.1365-2125.2011.03964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heremans H, Dillen C, Put W, Van Damme J, Billiau A. Protective effect of anti-interleukin (IL)-6 antibody against endotoxin, associated with paradoxically increased IL-6 levels. Eur J Immunol. 1992;22:2395–401. doi: 10.1002/eji.1830220932. [DOI] [PubMed] [Google Scholar]
- 30.May LT, Neta R, Moldawer LL, Kenney JS, Patel K, Sehgal PB. Antibodies chaperone circulating IL-6. Paradoxical effects of anti-IL-6 “neutralizing” antibodies in vivo. J Immunol. 1993;151:3225–36. [PubMed] [Google Scholar]
- 31.van Zaanen HC, Lokhorst HM, Aarden LA, Rensink HJ, Warnaar SO, van der Lelie J, van Oers MH. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998;102:783–90. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 32.Martens E, Dillen C, Put W, Heremans H, van Damme J, Billiau A. Increased circulating interleukin-6 (IL-6) activity in endotoxin-challenged mice pretreated with anti-IL-6 antibody is due to IL-6 accumulated in antigen-antibody complexes. Eur J Immunol. 1993;23:2026–9. doi: 10.1002/eji.1830230846. [DOI] [PubMed] [Google Scholar]
- 33.May LT, Patel K, García D, Ndubuisi MI, Ferrone S, Mittelman A, Mackiewicz A, Sehgal PB. Sustained high levels of circulating chaperoned interleukin-6 after active specific cancer immunotherapy. Blood. 1994;84:1887–95. [PubMed] [Google Scholar]
- 34.Frassanito MA, Cusmai A, Iodice G, Dammacco F. Autocrine interleukin-6 production and highly malignant multiple myeloma: relation with resistance to drug-induced apoptosis. Blood. 2001;97:483–9. doi: 10.1182/blood.V97.2.483. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar CA, Lowenhaupt K, Horan T, Boone TC, Tidor B, Lauffenburger DA. Rational cytokine design for increased lifetime and enhanced potency using pH-activated “histidine switching”. Nat Biotechnol. 2002;20:908–13. doi: 10.1038/nbt725. [DOI] [PubMed] [Google Scholar]
- 36.Murtaugh ML, Fanning SW, Sharma TM, Terry AM, Horn JR. A combinatorial histidine scanning library approach to engineer highly pH-dependent protein switches. Protein Sci. 2011;20:1619–31. doi: 10.1002/pro.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Mateos F, Ober RJ, Ward ES. Conferring the binding properties of the mouse MHC class I-related receptor, FcRn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J Mol Biol. 2005;345:1071–81. doi: 10.1016/j.jmb.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Gan Z, Ram S, Vaccaro C, Ober RJ, Ward ES. Analyses of the recycling receptor, FcRn, in live cells reveal novel pathways for lysosomal delivery. Traffic. 2009;10:600–14. doi: 10.1111/j.1600-0854.2009.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Johnson JE, Ghetie V, Ober RJ, Ward ES. Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J Mol Biol. 2003;332:901–13. doi: 10.1016/S0022-2836(03)00952-5. [DOI] [PubMed] [Google Scholar]
- 40.Goldenthal KL, Hedman K, Chen JW, August JT, Vihko P, Pastan I, Willingham MC. Pre-lysosomal divergence of alpha 2-macroglobulin and transferrin: a kinetic study using a monoclonal antibody against a lysosomal membrane glycoprotein (LAMP-1) J Histochem Cytochem. 1988;36:391–400. doi: 10.1177/36.4.2450119. [DOI] [PubMed] [Google Scholar]
- 41.Ober RJ, Martinez C, Lai X, Zhou J, Ward ES. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci U S A. 2004;101:11076–81. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, Ober RJ, Ward ES. Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc Natl Acad Sci U S A. 2007;104:5889–94. doi: 10.1073/pnas.0700337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferris AL, Brown JC, Park RD, Storrie B. Chinese hamster ovary cell lysosomes rapidly exchange contents. J Cell Biol. 1987;105:2703–12. doi: 10.1083/jcb.105.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pu Z, Lovitch SB, Bikoff EK, Unanue ER. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity 2004; 20:467-76. [DOI] [PubMed] [Google Scholar]
- 45.Lovitch SB, Unanue ER. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev. 2005;207:293–313. doi: 10.1111/j.0105-2896.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang JC, Han M, Minguela A, Pastor S, Qadri A, Ward ES. T cell recognition of distinct peptide:I-Au conformers in murine experimental autoimmune encephalomyelitis. J Immunol. 2003;171:2467–77. doi: 10.4049/jimmunol.171.5.2467. [DOI] [PubMed] [Google Scholar]
- 47.Sercarz EE, Maverakis E. Mhc-guided processing: binding of large antigen fragments. Nat Rev Immunol. 2003;3:621–9. doi: 10.1038/nri1149. [DOI] [PubMed] [Google Scholar]
- 48.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–63. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watts C, Antoniou A, Manoury B, Hewitt EW, Mckay LM, Grayson L, Fairweather NF, Emsley P, Isaacs N, Simitsek PD. Modulation by epitope-specific antibodies of class II MHC-restricted presentation of the tetanus toxin antigen. Immunol Rev. 1998;164:11–6. doi: 10.1111/j.1600-065X.1998.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 50.Ozaki S, Berzofsky JA. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987;138:4133–42. [PubMed] [Google Scholar]
- 51.Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med. 1993;178:1459–63. doi: 10.1084/jem.178.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mi WT, Wanjie S, Lo ST, Gan Z, Pickl-Herk B, Ober RJ, Ward ES. Targeting the neonatal Fc receptor for antigen delivery using engineered Fc fragments. J Immunol. 2008;181:7550–61. doi: 10.4049/jimmunol.181.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manca F, Fenoglio D, Kunkl A, Cambiaggi C, Sasso M, Celada F. Differential activation of T cell clones stimulated by macrophages exposed to antigen complexed with monoclonal antibodies. A possible influence of paratope specificity on the mode of antigen processing. J Immunol. 1988;140:2893–8. [PubMed] [Google Scholar]
- 54.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 55.Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci U S A. 2008;105:9337–42. doi: 10.1073/pnas.0801717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci U S A. 2006;103:18709–14. doi: 10.1073/pnas.0606304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ward ES, Martinez C, Vaccaro C, Zhou J, Tang Q, Ober RJ. From sorting endosomes to exocytosis: association of Rab4 and Rab11 GTPases with the Fc receptor, FcRn, during recycling. Mol Biol Cell. 2005;16:2028–38. doi: 10.1091/mbc.E04-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.