Abstract
Antibodies of human IgA isotype are critical components of the mucosal immune system, but little is known about their immunotherapeutic potential. Compared with IgG antibodies, IgA molecules carry a C-terminal tail piece extension of 18 amino acids with a free cysteine at position 471. This cysteine is required for the formation of dimeric IgA antibodies, but may impair molecular characteristics of monomeric IgA antibodies as therapeutic reagents. Thus, we generated and characterized a d471-mutated antibody against the epidermal growth factor receptor (EGFR) and compared it to its respective IgA2 m(1) wild type antibody. Both wild type and mutated IgA antibodies demonstrated similar EGFR binding and were similarly efficient in inhibiting EGF binding and in blocking EGF-mediated cell proliferation. Recruitment of Fc-mediated effector functions like antibody-dependent cell-mediated cytotoxicity by monocytes, macrophages or PMN was similar, but the d471-mutated IgA exhibited different biochemical properties compared with wild type antibody. As expected, mutated IgA did not form stable dimers in the presence of human joining (J)-chain, but we also observed reduced levels of dimeric aggregates in the absence of J-chain. Furthermore, glycoprofiling revealed different glycosylation patterns for both antibodies, including considerably less mannosylation of d471-mutated antibodies. Overall, our results demonstrate that the deletion of the C-terminal cysteine of IgA2 did not affect the investigated effector functions compared with wild type antibody, but it improved biochemical properties of an IgA2 m(1) antibody against EGFR, and may thereby assist in exploring the immunotherapeutic potential of recombinant IgA antibodies.
Keywords: IgA, Fc receptor, immunotherapy, cytotoxicity, epidermal growth factor receptor
Introduction
Human IgG1 is the most prevalent isotype of therapeutic antibodies approved or in clinical trials.1 Thus, the majority of our clinical knowledge has been obtained with IgG1 antibodies, with less contributed by evaluations of IgG2 and IgG4. This preferential use of IgG antibodies is likely caused by well-established production and purification technologies and knowledge about the relevant safety issues assessable for regulatory agencies.2 Another benefit of their use includes an elongated serum half-life caused by the interaction of IgG antibodies with the neonatal Fc receptor (FcRn) in vivo.3 Furthermore, IgG1 antibodies effectively recruit NK cells, macrophages and complement for tumor cell killing.4,5 There is also an increasing amount of data from animal and clinical trials that underlies the importance of fragment crystallizable (Fc)-mediated effector mechanisms of monoclonal antibodies.6-8 Since therapies with monoclonal antibodies are not curative in most patients and are accompanied by unwanted side effects, a number of strategies have been investigated to enhance and optimize Fc-mediated effector mechanisms, for example protein- or glycoengineering the IgG1-Fc domain9,10 or switching to alternative isotypes, like IgG2.11 Although IgG2 antibodies effectively recruit myeloid effector cells for ADCC, the cytotoxic potential of monocytes, macrophages and granulocytes in particular are recruited and activated by antibodies of the human IgA isotype.12-14 To investigate the immunotherapeutic activity of IgA antibodies, we established production and purification protocols similar to those used for IgG1 antibodies.12,15 Furthermore, it has been demonstrated that IgA antibodies are as effective in activating fragment antigen binding (Fab)-mediated effect mechanisms like growth inhibition, blocking ligands and induction of apoptosis as IgG, but are particularly effective in recruiting myeloid effector cells by inducing degranulation, “oxidative burst,” and ADCC.11,12,15,16 Thus, antibodies of IgA isotype represent an interesting and potent isotype for potential therapeutic monoclonal antibodies.
Among the different classes of immunoglobulins, IgA is the most abundantly produced antibody isotype in humans.17 Although IgG1 and IgA antibodies share a similar structural composition of light and heavy chains, some structural details are unique for IgA.18 For example, IgA antibodies are more heavily and heterogeneously glycosylated than IgG with glycans contributing significantly to the molecular mass.17 Additionally, the CH2/CH3 interface in IgA is critical for binding to the myeloid IgA receptor (FcαRI, CD89), and does not contain a FcRn binding site. Two different isotypes of IgA, named IgA1 and IgA2, can be distinguished and different IgA2 allotypes are found in Caucasian (IgA2 m[1]) or African and Asian (IgA2 m[2]) populations.17,19 Compared with IgA2, IgA1 antibodies are characterized by an extended hinge region with an additional sequence of 16 amino acids and up to six O-glycosylation sites.20,21 This elongated hinge region is more susceptible to bacterial proteases, possibly causing stability issues in vivo, in particular at bacterially-colonized sites. Furthermore, aberrantly O-glycosylated IgA1 antibodies are critically involved in the development of IgA nephropathy, a common glomerulonephritis often leading to renal failure. This O-glycosylation is usually diverse and difficult to control during biomolecule production, which limits regulatory and safety experience.2 IgA2 antibodies lack this elongated hinge region. Importantly, IgA2 antibodies have proved to be more effective than IgA1 in preclinical models.
These points comprise the major reasons why we selected the IgA2 isotype to develop a therapeutic monoclonal antibody.22,23 The differently constituted hinge and chain linkage might influence the Fab arm orientation which may explain the increased efficiency of IgA2 antibodies to recruit granulocytes.13,24 Whereas the heavy and light chain disulfide bonds in IgA2 m(2) are similar to those in IgA1 and IgG1, the Caucasian IgA2 m(1) allotype possesses a unique heavy and light chain linkage, with disulfide bonds formed between the light and heavy chain homodimers, respectively, but not between the heavy and light chains.17 Usual heavy-light chain pairing of IgA2 m(1) can be established by exchanging proline at position 231 against arginine, which is found in the IgA2 m(2) allotype at this position.16,25
All IgA antibodies contain an elongation of 18 amino acids at the C-terminus, called the tail piece, which plays a critical role in the formation of dimeric IgA (dIgA) antibodies and in antibody assembly and secretion.25,26 In dIgA, two monomeric IgA (mIgA) antibodies are linked covalently by the Cys-471 to the joining (J)-chain, which enables the interaction with the secretory component (SC) of polymeric immunoglobulin receptor (pIgR).27,28 This receptor transports dIgA onto serosal surfaces thereby releasing secretory IgA (SIgA) consisting of dIgA covalently linked to SC.29,30 Thus, Cys-471 located within the tail piece plays a key role in the formation of mucosal antibodies, which in turn builds a first line of defense in mucosal immunology.30,31
The tail piece, however, does not seem to be required for interaction with the most prominent and intensively characterized IgA Fc receptor, FcαRI, because residues responsible for this interaction have been identified exclusively in the Cα2-Cα3 interface and deletion of the entire tail piece did not affect binding of human IgA1 to the receptor.32-37 Binding of IgA to FcαRI mediates effector functions, such as phagocytosis, oxidative burst, cytokine release, antigen presentation and ADCC.34 FcαRI is expressed in monocytes/macrophages, granulocytes, subsets of dendritic cells and Kupffer cells and binds both mIgA and dIgA antibodies with intermediate affinity; however, mIgA seems to dissociate from FcαRI more rapidly than dIgA.37 The main functions of mIgA and FcαRI appear to consist of neutralizing pathogens that pass through the mucosal barrier, thus providing a second line of natural defense.38
The importance of the tail piece for the assembly and function of dIgA is well-understood, but its relevance for mIgA is still unclear.39 Considering monomeric IgA as a therapeutic reagent, the free Cys-471 represents an exposed free sulfhydryl group, a reactive residue that could impair the structure, stability and biological function of this molecule.40 Free thiol groups may catalyze degradation of proteins by chemical or physical reactions, e.g., in reducing their thermal stability.41,42 Additionally, free cysteines enable the formation of non-native intermolecular disulphide bonds.43 Indeed, IgA appears to form complexes with human serum albumin, α1 anti-trypsin, α1-microglobulin and C3d, which may involve the penultimate cysteine.44-47 Both a lower thermal stability and the risk of aberrant linkage probably enhance the risk for immunogenic responses in vivo.48,49 Thus, deletion of the C-terminal cysteine may enhance stability, reduce formation of non-native aggregates and change intracellular routes. In the present study, we investigated the effect of a Cys-471 deletion on the productivity, assembly, glycosylation and functionality of an IgA2 min(1) antibody directed against the epidermal growth factor receptor (EGFR), a relevant target in tumor immunotherapy.50
Results
Biochemistry and glycosylation of 225-IgA2-d471
Antibody-containing supernatants from CHO-K1 cell cultures were harvested weekly. IgA concentrations in supernatants were analyzed by Sandwich-ELISA and specific production rates (SPR) were calculated to estimate the productivity at cellular levels. For both wild type and d471-mutated IgA2 m(1) antibodies, similar SPRs were calculated and similar amounts of antibodies were produced (Fig. 1A). Affinity-purified antibodies were loaded onto size exclusion columns to separate monomeric antibodies from aggregates. In the case of the 225-IgA2-d471, less spontaneous dimeric antibodies were detected than for wild type IgA2 (Fig. 1B). The relative amount of dIgA compared with mIgA was determined by calculating the areas under the curves resulting in 25.5 ± 2.2% and 16.6 ± 1.6% for wild type and mutated IgA2, respectively. Purity of antibody preparations was demonstrated by denaturing SDS-PAGE followed by silver staining (Fig. 1C1). Both, 225-IgA2-wt and –d471 displayed complete monomeric antibodies (170 kDa) and dissociated molecules consisting of homodimers of heavy (100 kDa) and light (50 kDa) chains. Separating antibodies under native conditions confirmed high purity and both antibody preparations to consist of fully assembled monomeric antibodies (Fig. 1C2). Purified antibodies were then separated by SDS-PAGE, transferred onto PVDF-membranes. Staining for light (50 kDa, Fig. 1C3) and heavy chains (100 + 150 kDa, Fig. 1C4) using human κ- and α-chain specific secondary antibodies confirmed the expected molecular masses for both IgA2 variants. Since both antibodies belong to the IgA2 m(1) allotype, light chains are covalently linked to each other but not to heavy chains. Thus, both antibodies dissociated under non-reducing denaturing conditions. Next, glycosylation of wild type and mutated IgA2 was analyzed by glycoprofiling. We released the N-glycans with PNGaseF from IgA immobilized in a gel-block. The released glycans were fluorescently-labeled with 2-amino benzamide (2AB), then separated and identified by hydrophilic interaction liquid chromatography (HILIC) chromatography in combination with exoglycosidase sequencing (Table 1). The N-glycans identified for 225-IgA2-d471 were generally of lower size (mono-antennary N-glycans with exposed GlcNAc) and more intensively fucosylated and terminally galactosylated than wt IgA2, which on the other hand contained more mono- and disialylated structures. Furthermore, higher amounts of Man5 structures were detected for wild type than for mutated IgA2.
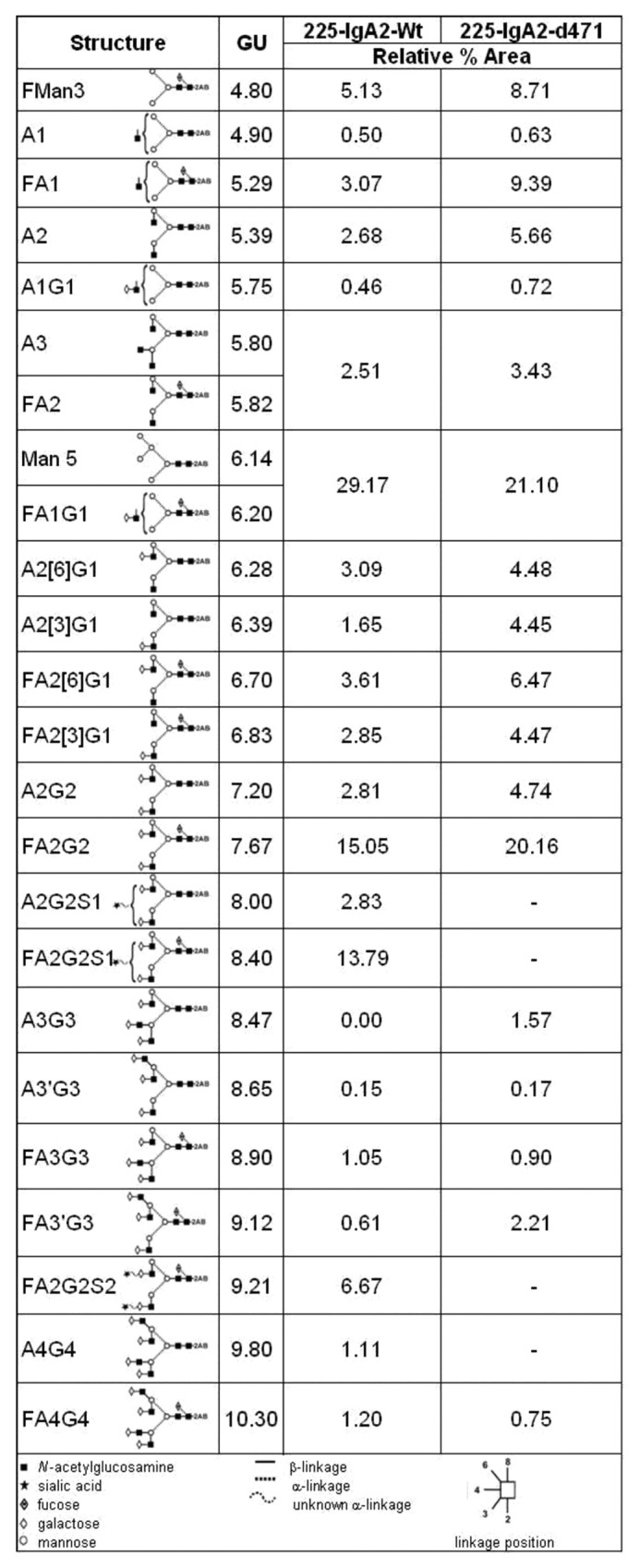
Figure 1. Biochemical characterization of 225-IgA2-d471 and 225-IgA2-wt antibody. (A) Antibody content in supernatants was analyzed by ELISA, and specific production rates were calculated. (B) Affinity-purified antibodies were separated by size exclusion to isolate monomeric antibodies. (C1) Purity of antibodies was analyzed by denaturing SDS-PAGE stained with silver nitrate. (C2) Formation of non-native aggregates was analyzed using native-PAGE stained with Coomassie. Proteins were transferred onto PVDF membranes and probed using polyclonal antibodies against κ- (C3) or α-chain (C4). Lanes: (1) control IgA2, (2) 225-IgA2-wt, 3) 225-IgA2-d471. (D) 225-IgA2-wt and –d471 producing cells were transfected with a pIRESpuro3 plasmid encoding human J-chain to allow the formation of stable dimeric IgA. Proteins were transferred onto PVDF membranes and stained using polyclonal antibodies against human α-chain. Lanes: (1) control IgA2, (2) purified monomeric 225-IgA2-wt, (3) purified monomeric 225-IgA2-d471, (4) 225-IgA2-wt containing SUP from CHO cells transfected with human J chain, (5) 225-IgA2-d471 containing SUP from CHO cells transfected with human J chain, (6) purified dimeric 225-IgA2-wt. (E) The composition of freshly prepared (E1), two (E2) and four (E3) year old preparations of 225-IgA2-wt (lane 1) and 225-IgA2-d471 (lane 2) was analyzed by analytical size exclusion chromatography on a Superdex200 10 × 300 column. (E4) Relative areas under the curve (rAUC) of monomeric (FM, elution volumes 10‒14 ml) and polymeric (FP, elution volumes 6.5–10 ml) IgA were calculated. (F) Thermal stability was analyzed by incubating antibodies at denaturing temperatures and measuring maintenance of functionality in51chromium release assays using A431 as targets and freshly isolated human PMN as effector cells. Results are presented as “mean ± SEM” of “relative specific lysis [%]” of three independent experiments.
Table 1. Summary of relative proportions of major N-glycans identified for 225-IgA2-wt and –d471.
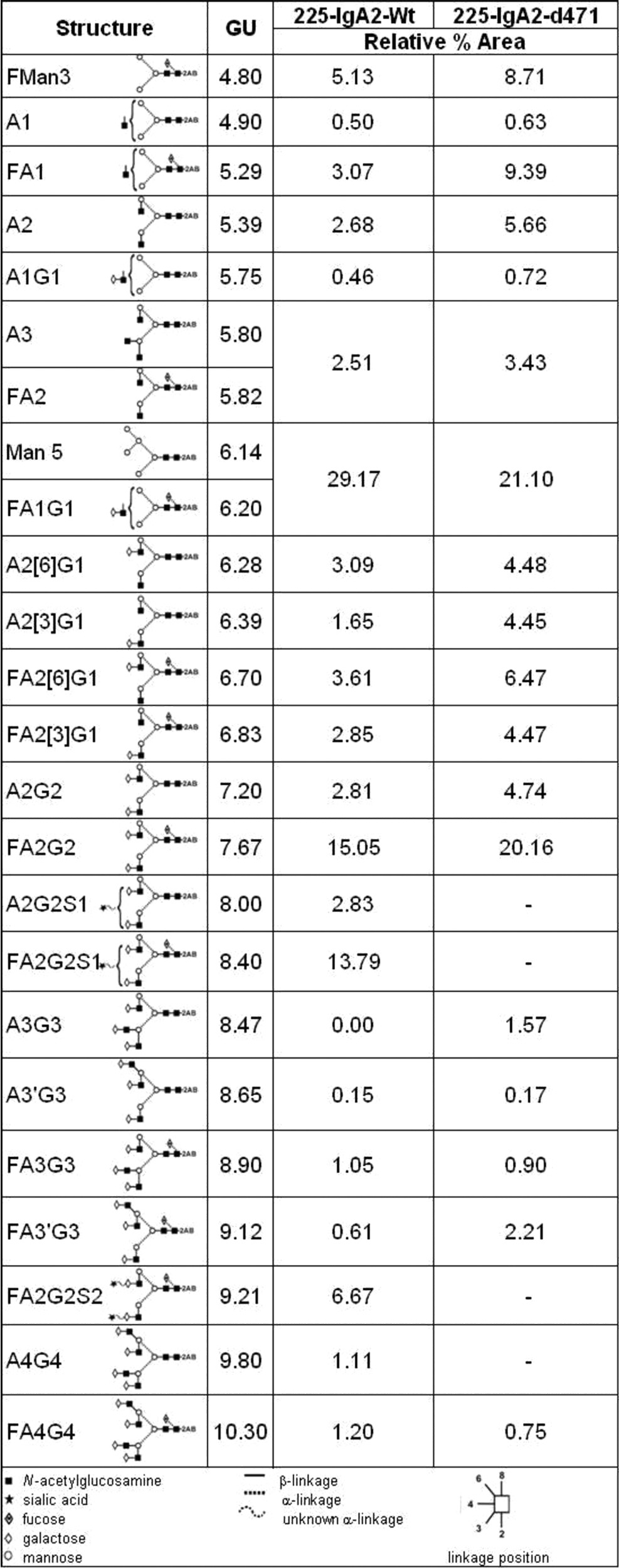
Data were obtained from the percentage areas following exoglycosidase digestion and HILIC-UPLC analysis. Structures are annotated by oxford nomenclature and a schematic illustration. Glucose unit (GU) values are related to the specific retention time on gel filtration. Data are presented as relative area under curve (%)
Since the C-terminal cysteine 471 is essential for the dimerization of IgA antibodies, we tested if stable dimeric IgA antibodies were produced when IgA2 producing CHO-K1 cells were additionally transfected with a J-chain encoding plasmid (Fig. 1D). Supernatants of J-chain transfected cells were separated under non-reducing conditions on SDS-PAGE and human α-chain was detected by western blot. For wild type 225-IgA2, heavy chains were detected at 100, 150 and 320 kDa, representing heavy chain homodimers, monomeric and dimeric antibodies, respectively. However, for 225-IgA2-d471 no polymeric molecules were detected. Long-term stability and composition of preparations were then evaluated by analytical size-exclusion chromatography of newly produced, two or four year old preparations of wild type and d471-mutated 225-IgA2 (Fig. 1E). In all preparations, one major peak at 10 ml presenting monomeric antibodies and only marginal amounts of polymeric antibodies were detected. Relative areas under the curves were calculated for monomeric and polymeric fractions. Results from these experiments revealed that the preparations of the mutated 225-IgA2-d471 contained significantly less polymeric aggregates than the preparations of the wild type 225-IgA2. Next, both wild type and mutated IgA2 were incubated at different denaturing temperatures to analyze their thermal stability. Maintenance of functionality was tested in51chromium release assays using PMN as effector and A431 as targets cells, respectively. Both IgA2 variants and IgG1 were similarly susceptible to denaturing temperatures (Fig. 1F).
Fab-mediated effector functions of EGFR-specific IgA2 antibodies
We compared then both molecules concerning functional aspects. First, binding of wild type and mutated IgA2 to EGFR-expressing A431 cells was analyzed in indirect immunofluorescence analyses (Fig. 2A). Both antibodies displayed similar avidities in these experiments. Next, the capability to block binding of the ligand EGF was investigated by incubating A431 cells with FITC-labeled EGF and increasing concentrations of wild type and mutated IgA2 (Fig. 2B). Both specific antibodies prevented binding of EGF with similar efficacy. Consequently, growth of EGFR-expressing DiFi colon carcinoma cells was inhibited at similar concentrations by both antibodies (Fig. 2C).
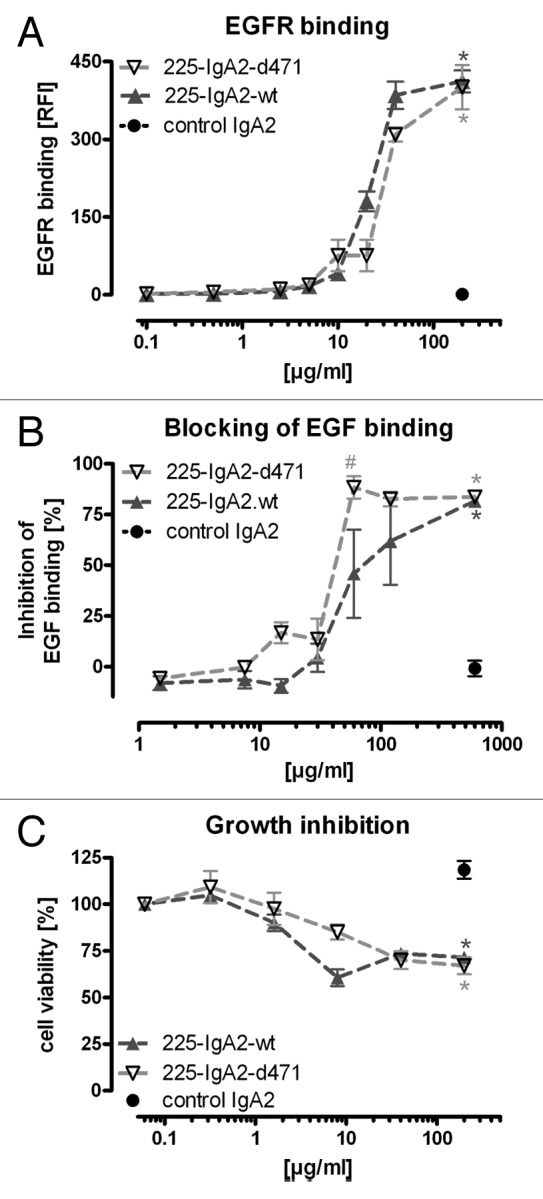
Figure 2. Fab-mediated effector functions were not impaired by the d471 mutation. (A) Binding of 225-IgA2-wt and –d471 to EGFR-expressing A431 cells was analyzed by indirect immunofluorescence analyses using anti-human kappa light chain directed secondary antibody. (B) Blocking of EGF binding was detected by FACS analyses treating A431 cells simultaneously with FITC-labeled EGF and excess of specific or nonspecific antibodies. (C) DiFi colon carcinoma cells were incubated with IgA2 antibodies for 72 h before viability was measured by MTS assay. Results are shown as “mean ± SEM” of “EGFR binding [RFI]” (A), “inhibition of EGF binding [%]” (B) and “cell viability [%]” (C) of five different experiments. Significant differences (p ≤ 0.001) between EGFR-specific and non-specific (control IgA2) antibodies are indicated by *, between mutant and wild type IgA2 by #.
FcαRI binding and myeloid effector cell recruitment
To investigate the effect of the tail piece mutation on Fc-mediated effector mechanisms, binding of both 225-IgA2-wt and –d471 to FcαRI/FcRγ-coexpressing BHK-21 cells was analyzed by flow cytometry (Fig. 3A). Results from these experiments demonstrated that the mutated antibody binding to FcαRI was not significantly different from its wild type counterpart. The capability of both antibodies to mediate ADCC was compared in51chromium release assay using various antibody concentrations and whole blood as a source of effector cells. In these assays, the mutated IgA2 was similarly effective in Fc-mediated tumor cell killing to wild type IgA2 (Fig. 3B). Next, we investigated the capability of both IgA2 antibodies in ADCC assays using increasing volumes of whole blood or E:T cell ratios. Both IgA2 antibodies were similarly effective both in whole blood assays and in ADCC experiments employing PMN, monocytes and macrophages (EC50 values for PMN, monocytes and macrophage ADCC were 27.3 ± 2.1, 8.1 ± 1.1 and 8.4 ± 2.6 effector cells/target cells for 225-IgA2-wt and 28.6 ± 5.8, 10.3 ± 3.3 and 10.7 ± 3.5 effector cells/target cells for 225-IgA2-d471, Fig. 3C-F).
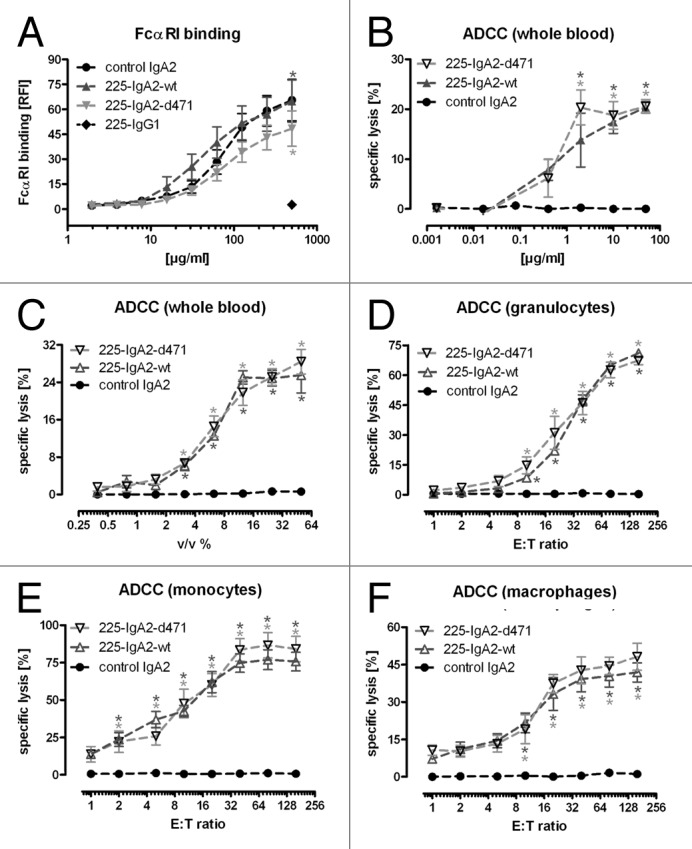
Figure 3. FcαRI-binding and recruitment of myeloid effector cells. In (A), binding of 225-IgA2-wt and –d471 to BHK-21 cells co-transfected with human FcαRI and FcRγ was measured by indirect immunofluorescence using FITC-labeled anti-human kappa light chain directed secondary antibody. Efficiency of IgA2 antibodies in mediating ADCC against A431 targets cells was analyzed in51chromium release assays using whole blood and increasing antibody concentrations (B). Next, increasing volumes of whole blood (C) and increasing ratios of isolated granulocytes (D), monocytes (E) and monocyte-derived macrophages (F) were used as effectors cells. Results are shown as “mean ± SEM” of “FcαRI binding [RFI %]” (A) or “specific lysis [%]” (B–F) of at least five independent experiments. Significant differences between EGFR-specific and control antibodies (control IgG1 in [A], control IgA2 in [B‒F]) are indicated by * (P ≤ 0.001).
Glycosylation of different antibody batches
To investigate whether the observed differences in glycosylation of wild type vs. mutated IgA2 were caused by the d471 mutation or by the individual transfectomas, we produced both 225-IgA2-wt and -d471 from three different, independently obtained transfectomas (named single clones 1–3), respectively. The corresponding antibodies were loaded in lanes 2–4 and 6–8, respectively (Fig. 4). For comparison, the first of each of the 225-IgA2-wt and-d471 transfectomas were grown in bioreactors, which were loaded in lanes 1 and 5. Glycosylation was analyzed by lectin blots employing R. communis agglutinin I (detection of terminal galactose; Fig. 4A1), A. aurantia lectin (α(1,6)-linked fucose; Fig. 4A2), ConA (α-linked mannose; Fig. 4A3), G. nivalis lectin (α(1,3)-linked mannose; Fig. 4A4), G. simplicifolia lectin (N-acetylglucosamine; Fig. 4A5) and S. nigra lectin (terminal sialic acid; Fig. 4A6). As expected, there was some variation in glycosylation across the IgA2 antibody batches. However, the densitometric evaluation of the lectin blots revealed that N-glycans of mutated IgA2 antibodies contained consistently less mannose, especially α(1,3)-linked mannose, and more N-acetylglucosamine (GlcNAc) than wild type IgA2 antibodies (Fig. 4A8). Next, we investigated if the different glycosylation affected the functionality of the antibodies regarding the recruitment of PMN or monocytes for ADCC. Interestingly, the capacity to induce ADCC was not affected by these observed variations in the glycosylation patterns, neither for wild type nor for the tail piece mutated IgA2 antibodies (Fig. 5).
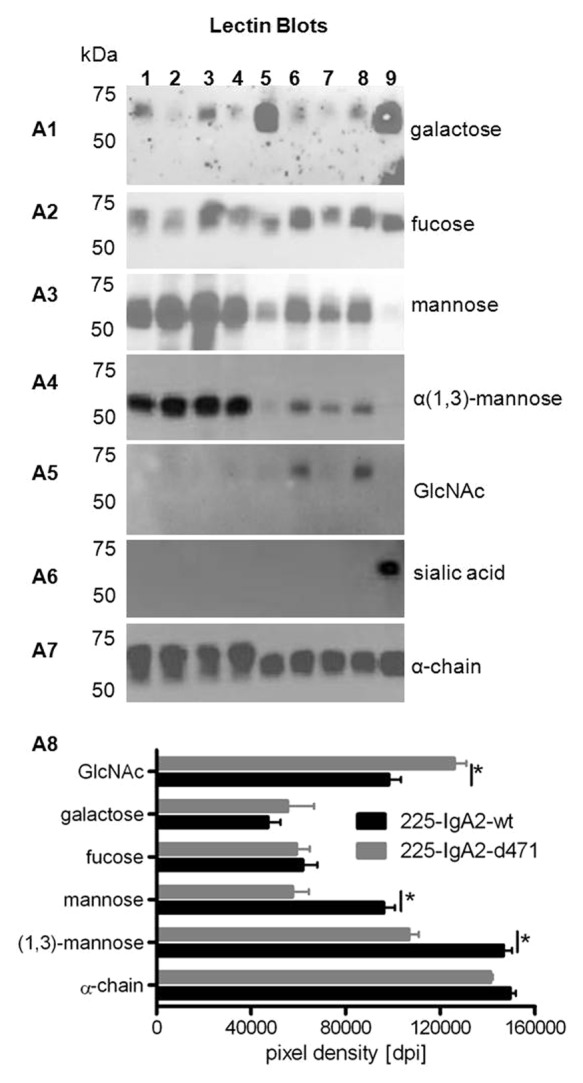
Figure 4. Glycosylation of wild type and d471-mutated IgA2 antibodies. Single clones producing 225-IgA2-wt and –d471 were cultured in tissue culture flasks (TCF), and glycosylation of affinity-purified antibodies was analyzed using lectin blots. Different sugar moieties were detected using biotinylated lectins specific for terminal galactosylation (A1), α(1,6)-linked fucosylation (A2), α-linked mannosylation (A3), α(1,3)-linked mannosylation (A4), N-acetylglucosamine (A5), terminal sialic acid (A6), and human α-chain as loading control (A7). Lanes: 1. 225-IgA2-wt (CL1000 produced), 2. 225-IgA2-wt clone 1, 3. 225-IgA2-wt clone 2, 4. 225-IgA2-wt clone 3, 5. 225-IgA2-d471 (CL1000 produced), 6. 225-IgA2-d471 clone 1, 7. 225-IgA2-wt clone 2, 8. 225-IgA2-wt clone 3, 9. control IgA2. (A8) To evaluate whether that glycosylation of wild type and mutant IgA2 antibodies was different, lectin blots were analyzed densitometrically using ImageJ software. Results are presented as “pixel density (dots per inch [dpi])” and significant differences between wild type and mutant IgA2 glycosylation are indicated by * (P ≤ 0.001).
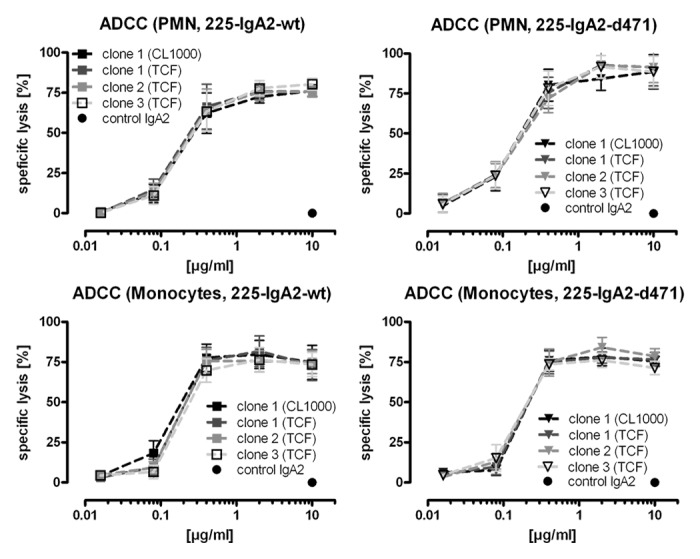
Figure 5. Recruitment of myeloid effector cells by differently glycosylated IgA2 antibodies. The capacity of differently glycosylated wild type and d471-mutated IgA2 antibodies to mediate ADCC of human A431 tumor cells was analyzed in51chromium release assays using isolated granulocytes or monocytes as effector cells. Results are shown as “mean ± SEM” of “specific lysis [%]” of at least three independent experiments.
Discussion
All IgA isotypes and allotypes share a penultimate Cys-471 at their C-terminus, which is of central importance for the formation of stable native dimeric IgA antibodies.27 Structural studies of dimeric IgA have confirmed that tail piece cysteines are covalently linked to J-chain.51-54 In the absence of J-chain, however, tail piece cysteines may react with other free sulfhydryl groups, especially since free thiol groups are reactive functional groups that are capable of forming alternate, unusual or additional disulfide bonds.55 Thus, we hypothesized that deletion of the penultimate cysteine 471 might lead to a monomeric IgA antibody with increased stability and decreased unspecific reactivity due to a reduced number of reactive side chains. The results presented above confirmed that deletion of cysteine 471 prevents the formation of J-chain-stabilized dimeric IgA antibodies and are in keeping with earlier reports that the mutation of cysteine 471 to Ser and the deletion of the entire tail piece prevented dimer formation.26 Furthermore, the amount of non-covalently linked IgA aggregates was also found to be reduced. Importantly, productivity of CHO cells transfected with vectors for mutated or wild type IgA antibodies was observed to be similar, although the tail piece of IgA antibodies is thought to confer some intracellular retention.28 To enhance production of recombinant IgA antibodies, the complete deletion of the tail piece may need to be considered. An accelerated release of IgA antibodies by CHO cells may, however, alter the composition of their glycosylation, which in turn may affect pharmacological properties, especially in vivo.56,57
Both antibodies investigated in this study are of the IgA2 m(1) allotype. Antibodies of this allotype typically have four N-glycosylation sites at the positions 166, 263, 337, and 459.17 Glycosylation analyses revealed that native human IgA antibodies are mainly terminally sialylated. Recombinant IgA antibodies produced in CHO cells, however, have been shown to display different glycosylation patterns and presented a higher degree of terminal galactosylation.58 Our results confirmed these data because both wild type and d471-mutated IgA2 antibodies were heavily terminally galactosylated, but less or not sialylated, respectively. It has been shown previously that deletions of the C-terminal peptide in IgA and IgM can result in altered processing of oligosaccharides.33,59 Therefore the d471-mutation may have influenced the glycosylation by altering the intracellular routing and retention of IgA antibodies. In addition, high production rates and specific culture conditions (higher cell mortality at higher cell densities) may also result in the release of incompletely posttranslational processed IgA antibodies. Thus, it is not surprising that the analyses of the glycosylation of wild type and mutated IgA2 antibodies revealed that the glycosylation differed between individual transfectomas and between production systems. Nevertheless, the d471 mutation appeared to influence the level of mannosylation, which was lower than in the wild type as measured by both detailed N-glycoprofiling (which revealed a lower content of Man5 structures) and the lectin blots [which showed lower levels of mannose—especially α(1,3)-linked mannose].
Regardless of their different glycosylation, the wild type and mutated IgA2 antibodies produced by different transfectomas were similarly effective in granulocyte- and monocyte-dependent ADCC. Thus, the results of our present work suggest that the tail piece cysteine and the specific glycosylation of the α-chain were not critical for FcαRI interactions in agreement with previous reports.32,33 These results are validated by previous observations that differences in the fucoslytion of IgG1 antibodies affected their capacity to mediate ADCC.60 However, the glycosylation of IgA antibodies has been proposed to be critical for their serum half-life.56,57 Due to the low levels (or absence) of sialylation that has been previously reported for these antibodies, we would expect increased clearance of the mutated IgA compared with the wild type antibody by the asialo-glycoprotein receptor (ASGPR).56,57 It would therefore be logical to optimise the glycosylation of the recombinant IgA antibodies to have high levels of sialylated glycans before further in vivo evaluations.
In summary, the deletion of the C-terminal cysteine of an IgA2 m(1) antibody prevented the formation of J-chain-stabilized and aggregated dimeric antibodies. Importantly, Fab- or Fc-mediated effector functions against EGFR expressing tumor cells were not affected by this mutation or by changes in the glycosylation of the α-chain. However, the absent sialylation of the mutant IgA2 antibody may limit some of its benefits because the antibody would be expected to be cleared more rapidly from the circulation. Thus, we would suggest a combination of the d471- with the previously described P221R-mutation16 together with efforts to optimize antibody glycosylation to finally develop an optimized IgA antibody for potential clinical development.
Material and Methods
Experiments were approved by the Ethical Committee of the Christian-Albrechts-University (Kiel, Germany) in accordance with the Declaration of Helsinki.
Cell lines
The human epidermoid carcinoma cell line A431 (German Collection of Microorganisms and Cell Cultures) and BHK-21 cells co-transfected with FcαRI (CD89) and FcR γ-chain12 were cultured in RPMI 1640 containing 10% heat-inactivated fetal calf serum (FCS) (media and FCS from Life Technologies), 100 U/ml penicillin and 100 µg/ml streptomycin (both from PAA). The human colon carcinoma cell line DiFi (European Collection of Cell Culture, ECACC) was maintained in the Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin). BHK transfectants were positively selected by addition of 1 mg/ml geneticin (PAA) and 20 µM methotrexate (Sigma).
225-IgA production, purification and gel electrophoresis
Purified human myeloma IgA2 m(1) antibody was used as control IgA2 (Meridian Life Science). Monomeric wild type 225-IgA2 m(1), named 225-IgA2-wt, was produced from the variable regions of the 225 antibody as previously described.12 D471-mutated IgA2 m(1) antibody (further named 225-IgA2-d471) was generated by converting the codon for Cys-471 into a stop codon by using overlap extension PCR. The translated protein lacks the C-terminal cysteine 471 and tyrosine 472. The mutation of the heavy chain was confirmed by sequencing. The 225-IgA2-d471 heavy chain sequence was subcloned into the pEE14.4 glutamine synthetase-expression system and transfected into CHO-K1 cells (Lonza Biologics). Transfectomas were grown in disposable CELLine CL 1000 bioreactors (Sartorius) under serum-free suspension culture conditions. Alternatively, for comparison of individual antibody batches expressed by different transfectomas, transfected CHO-K1 cells were cultured in hybridoma 175 cm2 tissue culture flasks (GreinerBio). Supernatants were collected twice a week for three months reseeding cells each time. For control experiments, 225-IgG1 (cetuximab) was purchased from Merck. Recombinant IgA2 antibodies were affinity-purified using Capture Select Fab Kappa chromatography medium (Capture Select) and a prepacked Superdex 200 26 × 600 column (GE Healthcare).12 For analytical gel filtration, antibodies were loaded onto a Superdex200 10 × 300 column. All purification steps were run on an ÄKTAprime liquid chromatography system (GE Healthcare). UV absorbance at 280 nm, pH and conductivity of the effluent stream were continuously recorded and analyzed using Unicorn 4.11 software (GE Healthcare). Determination of antibody concentrations, gel electrophoresis, Western and lectin blotting were done as described earlier.12,16 Profiles of N-glycosylation were performed as previously described.21,61
Flow cytometry and growth inhibition
Binding to EGFR, FcαRI and blocking of EGF binding were analyzed by flow cytometry as described before.12 All samples were analyzed on a Coulter EPICS XL-MCL flow cytometer (Beckman Coulter), collecting 1 × 104 events for each experimental value. Data were analyzed using XL-System II V3.0 software (Beckman Coulter). Relative fluorescence intensities were calculated as the ratio of mean linear fluorescence intensity of relevant to irrelevant isotype-matched antibodies. Growth inhibition of DiFi colon carcinoma cells was analyzed using the 3-(3,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt (MTS) assay (Promega). Cells were seeded in triplicates at a density of 4 × 104 cells per well and treated with serial dilutions of EGFR or control antibodies. After 72 h, MTS substrate was added, and absorption at 490 nm was measured after two hours. Viable cell mass in the presence of control antibody served as reference (100% cell growth) to calculate growth inhibition by EGFR antibodies according to the equation: absorption (EGFR antibody) / absorption (control antibody) × 100.
Preparation of effector cells
Citrate-anticoagulated blood from healthy volunteers was layered over a discontinuous Percoll (Biochrom) gradient consisting of 70% and 63% Percoll. After centrifugation, MNC were collected from the plasma/Percoll interface and PMN (mainly neutrophilic granulocytes) from the interface between the two Percoll layers. Monocytes were isolated from MNC by CD14 positive selection using magnetic beads (Miltenyi Biotec). To generate macrophages, monocytes were seeded into tissue culture flasks in RPMI1640 supplemented with 10% FCS, 1% PenStrep, 1% non-essential amino acids (Life Technologies), 0.1% sodium pyruvate (Sigma) containing 25 ng/ml human recombinant macrophage-colony stimulating factor (M-SCF), 10 ng/ml IL-1β (both Peprotech) and 1% (v/v) human sera for seven days.62 Adherent macrophages were harvested by scraping.
Antibody dependent cell-mediated cytotoxicity (ADCC) assays
ADCC was measured using a 51chromium release assay.12 Briefly, whole blood or effector cells and antibodies were added to round-bottom microtiter plates (Wallac, Turku, Finnland). Assays were started by adding effector (E) and target (T) cells at an E:T ratio of 80:1 (40:1 in the case of macrophages) unless otherwise indicated. After incubation at 37°C (3 h for whole blood and PMN assays, 16 h for monocytes/macrophages), aliquots of the supernatants were transferred into 96-well plates containing a scintillation mixture (OptiPhase Scintillator Supermix, PerkinElmer, Waltham, USA). 51Cr release was measured in cpm using a scintillation counter (MetaBase TriLux, PerkinElmer). Percentage of cellular cytotoxicity was calculated using the formula: percent specific lysis = (experimental cpm - basal cpm) / (maximal cpm - basal cpm) × 100, with maximal 51Cr release determined by adding Triton X-100 (Merck, 1% final concentration) to target cells, and basal release as measured in the absence of sensitizing antibodies and effector cells. Relative specific lysis [%] was calculated in relation to the untreated sample defined as 100%. Antibody-independent cytotoxicity (effectors without target antibodies) or effector-independent (target antibodies without effectors) was not observed.
Data processing and statistical analyses
Data are displayed graphically and statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software). Group data are reported as mean ± SEM. Significance was determined by two-way Anova repeated measures test with the Bonferroni post hoc correction. EC50 values were calculated from dose-response curves, reported as means ± SEM and compared by paired Students-t-test to calculate significant differences between data groups. Significance was accepted when P-values were ≤ 0.05.
Acknowledgments
We gratefully acknowledge the excellent technical assistance from Christyn Wildgrube and Yasmin Brodtmann, as well as financial support from the German Research Organization (Lo 1853/1–1) intramural grants and the Wilhelm Sander Foundation (2009.098.1 and 2).
Disclosure of Potential Conflicts of Interest
Louise Royle and Li Phing Liew are employees of Ludger Ltd.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26396
References
- 1.Reichert JM. Antibody-based therapeutics to watch in 2011. MAbs. 2011;3:76–99. doi: 10.4161/mabs.3.1.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–8. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 3.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–66. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 4.Brüggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–61. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dechant M, Vidarsson G, Stockmeyer B, Repp R, Glennie MJ, Gramatzki M, van De Winkel JG, Valerius T. Chimeric IgA antibodies against HLA class II effectively trigger lymphoma cell killing. Blood. 2002;100:4574–80. doi: 10.1182/blood-2002-03-0687. [DOI] [PubMed] [Google Scholar]
- 6.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, Lamy A, Penault-Llorca F, Frébourg T, Michel P, et al. Impact of FcγRIIa-FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 7.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 9.Peipp M, Dechant M, Valerius T. Effector mechanisms of therapeutic antibodies against ErbB receptors. Curr Opin Immunol. 2008;20:436–43. doi: 10.1016/j.coi.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Schlaeth M, Berger S, Derer S, Klausz K, Lohse S, Dechant M, Lazar GA, Schneider-Merck T, Peipp M, Valerius T. Fc-engineered EGF-R antibodies mediate improved antibody-dependent cellular cytotoxicity (ADCC) against KRAS-mutated tumor cells. Cancer Sci. 2010;101:1080–8. doi: 10.1111/j.1349-7006.2010.01505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PH, Derer S, Beyer T, Lohse S, Bleeker WK, Peipp M, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol. 2010;184:512–20. doi: 10.4049/jimmunol.0900847. [DOI] [PubMed] [Google Scholar]
- 12.Beyer T, Lohse S, Berger S, Peipp M, Valerius T, Dechant M. Serum-free production and purification of chimeric IgA antibodies. J Immunol Methods. 2009;346:26–37. doi: 10.1016/j.jim.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Dechant M, Beyer T, Schneider-Merck T, Weisner W, Peipp M, van de Winkel JG, Valerius T. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J Immunol. 2007;179:2936–43. doi: 10.4049/jimmunol.179.5.2936. [DOI] [PubMed] [Google Scholar]
- 14.Huls G, Heijnen IA, Cuomo E, van der Linden J, Boel E, van de Winkel JG, Logtenberg T. Antitumor immune effector mechanisms recruited by phage display-derived fully human IgG1 and IgA1 monoclonal antibodies. Cancer Res. 1999;59:5778–84. [PubMed] [Google Scholar]
- 15.Lohse S, Derer S, Beyer T, Klausz K, Peipp M, Leusen JH, van de Winkel JG, Dechant M, Valerius T. Recombinant dimeric IgA antibodies against the epidermal growth factor receptor mediate effective tumor cell killing. J Immunol. 2011;186:3770–8. doi: 10.4049/jimmunol.1003082. [DOI] [PubMed] [Google Scholar]
- 16.Lohse S, Brunke C, Derer S, Peipp M, Boross P, Kellner C, Beyer T, Dechant M, van der Winkel JG, Leusen JH, et al. Characterization of a mutated IgA2 antibody of the m(1) allotype against the epidermal growth factor receptor for the recruitment of monocytes and macrophages. J Biol Chem. 2012;287:25139–50. doi: 10.1074/jbc.M112.353060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590–7. doi: 10.1038/mi.2011.39. [DOI] [PubMed] [Google Scholar]
- 18.Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol. 2004;4:89–99. doi: 10.1038/nri1266. [DOI] [PubMed] [Google Scholar]
- 19.Wang AC, Fudenberg HH. IgA and evolution of immunoglobulins. J Immunogenet. 1973;1:3–31. [Google Scholar]
- 20.Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwan RM, Wilson IA, Daha MR, Dwek RA, Rudd PM. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278:20140–53. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 22.Oortwijn BD, Roos A, Royle L, van Gijlswijk-Janssen DJ, Faber-Krol MC, Eijgenraam JW, Dwek RA, Daha MR, Rudd PM, van Kooten C. Differential glycosylation of polymeric and monomeric IgA: a possible role in glomerular inflammation in IgA nephropathy. J Am Soc Nephrol. 2006;17:3529–39. doi: 10.1681/ASN.2006040388. [DOI] [PubMed] [Google Scholar]
- 23.Senior BW, Woof JM. The influences of hinge length and composition on the susceptibility of human IgA to cleavage by diverse bacterial IgA1 proteases. J Immunol. 2005;174:7792–9. doi: 10.4049/jimmunol.174.12.7792. [DOI] [PubMed] [Google Scholar]
- 24.Furtado PB, Whitty PW, Robertson A, Eaton JT, Almogren A, Kerr MA, Woof JM, Perkins SJ. Solution structure determination of monomeric human IgA2 by X-ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1. J Mol Biol. 2004;338:921–41. doi: 10.1016/j.jmb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Chintalacharuvu KR, Yu LJ, Bhola N, Kobayashi K, Fernandez CZ, Morrison SL. Cysteine residues required for the attachment of the light chain in human IgA2. J Immunol. 2002;169:5072–7. doi: 10.4049/jimmunol.169.9.5072. [DOI] [PubMed] [Google Scholar]
- 26.Atkin JD, Pleass RJ, Owens RJ, Woof JM. Mutagenesis of the human IgA1 heavy chain tailpiece that prevents dimer assembly. J Immunol. 1996;157:156–9. [PubMed] [Google Scholar]
- 27.Johansen FE, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol. 2001;167:5185–92. doi: 10.4049/jimmunol.167.9.5185. [DOI] [PubMed] [Google Scholar]
- 28.Sørensen V, Rasmussen IB, Sundvold V, Michaelsen TE, Sandlie I. Structural requirements for incorporation of J chain into human IgM and IgA. Int Immunol. 2000;12:19–27. doi: 10.1093/intimm/12.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MJ, Pleass RJ, Batten MR, Atkin JD, Woof JM. Structural requirements for the interaction of human IgA with the human polymeric Ig receptor. J Immunol. 2005;175:6694–701. doi: 10.4049/jimmunol.175.10.6694. [DOI] [PubMed] [Google Scholar]
- 30.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 31.Phalipon A, Corthésy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–8. doi: 10.1016/S1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 32.Gomes MM, Wall SB, Takahashi K, Novak J, Renfrow MB, Herr AB. Analysis of IgA1 N-glycosylation and its contribution to FcalphaRI binding. Biochemistry. 2008;47:11285–99. doi: 10.1021/bi801185b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattu TS, Pleass RJ, Willis AC, Kilian M, Wormald MR, Lellouch AC, Rudd PM, Woof JM, Dwek RA. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1998;273:2260–72. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro RC, Van De Winkel JGJ. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. doi: 10.1146/annurev.immunol.21.120601.141011. [DOI] [PubMed] [Google Scholar]
- 35.Wines BD, Hogarth PM. IgA receptors in health and disease. Tissue Antigens. 2006;68:103–14. doi: 10.1111/j.1399-0039.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 36.Pleass RJ, Dunlop JI, Anderson CM, Woof JM. Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human fcα receptor (FcαR) CD89. J Biol Chem. 1999;27:23508–14. doi: 10.1074/jbc.274.33.23508. [DOI] [PubMed] [Google Scholar]
- 37.Oortwijn BD, Roos A, van der Boog PJ, Klar-Mohamad N, van Remoortere A, Deelder AM, Daha MR, van Kooten C. Monomeric and polymeric IgA show a similar association with the myeloid FcalphaRI/CD89. Mol Immunol. 2007;44:966–73. doi: 10.1016/j.molimm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 38.van Egmond M, van Garderen E, van Spriel AB, Damen CA, van Amersfoort ES, van Zandbergen G, van Hattum J, Kuiper J, van de Winkel JG. FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–5. doi: 10.1038/76261. [DOI] [PubMed] [Google Scholar]
- 39.Asano M, Ogura Y, Takenouchi-Ohkubo N, Chihaya H, Chung-Hsing W, Ishikawa K, Kobayashi K, Vaerman JP, Moro I. Endoplasmic reticulum resident, immunoglobulin joining chain, can be secreted by perturbation of the calcium concentration in the endoplasmic reticulum. DNA Cell Biol. 2004;23:403–11. doi: 10.1089/1044549041474779. [DOI] [PubMed] [Google Scholar]
- 40.Banks DD, Gadgil HS, Pipes GD, Bondarenko PV, Hobbs V, Scavezze JL, Kim J, Jiang XR, Mukku V, Dillon TM. Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm Sci. 2008;97:775–90. doi: 10.1002/jps.21014. [DOI] [PubMed] [Google Scholar]
- 41.Lacy ER, Baker M, Brigham-Burke M. Free sulfhydryl measurement as an indicator of antibody stability. Anal Biochem. 2008;382:66–8. doi: 10.1016/j.ab.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi MV, Laurence JS, Siahaan TJ. The role of thiols and disulfides on protein stability. Curr Protein Pept Sci. 2009;10:614–25. doi: 10.2174/138920309789630534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brych SR, Gokarn YR, Hultgen H, Stevenson RJ, Rajan R, Matsumura M. Characterization of antibody aggregation: role of buried, unpaired cysteines in particle formation. J Pharm Sci. 2010;99:764–81. doi: 10.1002/jps.21868. [DOI] [PubMed] [Google Scholar]
- 44.Vaerman JP, Hagiwara K, Kobayashi K, Rits M. Complexes of albumin and α 1-antitrypsin with Fc-fragment of IgA monomer are disulfide-bound to penultimate C-terminal cysteine in the C α 3-domain. Immunol Lett. 1987;15:67–72. doi: 10.1016/0165-2478(87)90078-2. [DOI] [PubMed] [Google Scholar]
- 45.Calero M, Escribano J, Grubb A, Méndez E. Location of a novel type of interpolypeptide chain linkage in the human protein HC-IgA complex (HC-IgA) and identification of a heterogeneous chromophore associated with the complex. J Biol Chem. 1994;269:384–9. [PubMed] [Google Scholar]
- 46.Scott LJ, Evans EL, Dawes PT, Russell GI, Mattey DL. Comparison of IgA-alpha1-antitrypsin levels in rheumatoid arthritis and seronegative oligoarthritis: complex formation is not associated with inflammation per se. Br J Rheumatol. 1998;37:398–404. doi: 10.1093/rheumatology/37.4.398. [DOI] [PubMed] [Google Scholar]
- 47.Doi T, Kanatsu K, Sekita K, Yoshida H, Nagai H, Hamashima Y. Detection of IgA class circulating immune complexes bound to anti-C3d antibody in patients with IgA nephropathy. J Immunol Methods. 1984;69:95–104. doi: 10.1016/0022-1759(84)90281-3. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs. 2012;4:17–23. doi: 10.4161/mabs.4.1.18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MF. A role for protein misfolding in immunogenicity of biopharmaceuticals. J Biol Chem. 2007;282:2229–36. doi: 10.1074/jbc.M605984200. [DOI] [PubMed] [Google Scholar]
- 50.Peipp M, Dechant M, Valerius T. Effector mechanisms of therapeutic antibodies against ErbB receptors. Curr Opin Immunol. 2008;20:436–43. doi: 10.1016/j.coi.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Bastian A, Kratzin H, Fallgren-Gebauer E, Eckart K, Hilschmann N. Intra- and inter-chain disulfide bridges of J chain in human S-IgA. Adv Exp Med Biol. 1995;371A:581–3. doi: 10.1007/978-1-4615-1941-6_122. [DOI] [PubMed] [Google Scholar]
- 52.Della Corte E, Parkhouse RME. Biosynthesis of immunoglobulin A (IgA) and immunoglobulin M (IgM). Requirement for J chain and a disulphide-exchanging enzyme for polymerization. Biochem J. 1973;136:597–606. doi: 10.1042/bj1360597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Pardo A, Lamm ME, Plaut AG, Frangione B. J chain is covalently bound to both monomer subunits in human secretory IgA. J Biol Chem. 1981;256:11734–8. [PubMed] [Google Scholar]
- 54.Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J Biol Chem. 2009;284:5077–87. doi: 10.1074/jbc.M807529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prahl JW, Abel CA, Grey HM. Carboxy-terminal structure of the chain of human IgA myeloma proteins. Biochemistry. 1971;10:1808–12. doi: 10.1021/bi00786a012. [DOI] [PubMed] [Google Scholar]
- 56.Boross P, Lohse S, Nederend M, Jansen JH, van Tetering G, Dechant M, Peipp M, Royle L, Liew LP, Boon L, et al. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med. 2013;5:1213–26. doi: 10.1002/emmm.201201929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med. 2000;191:2171–82. doi: 10.1084/jem.191.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoo EM, Yu LJ, Wims LA, Goldberg D, Morrison SL. Differences in N-glycan structures found on recombinant IgA1 and IgA2 produced in murine myeloma and CHO cell lines. MAbs. 2010;2:320–34. doi: 10.4161/mabs.2.3.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finley EM, Rebbe NF, Hickman S. The effect of peptide deletions on the glycosylation of murine immunoglobulin M heavy chains. Arch Biochem Biophys. 1990;279:395–401. doi: 10.1016/0003-9861(90)90507-U. [DOI] [PubMed] [Google Scholar]
- 60.Peipp M, Lammerts van Bueren JJ, Schneider-Merck T, Bleeker WW, Dechant M, Beyer T, Repp R, van Berkel PH, Vink T, van de Winkel JG, et al. Antibody fucosylation differentially impacts cytotoxicity mediated by NK and PMN effector cells. Blood. 2008;112:2390–9. doi: 10.1182/blood-2008-03-144600. [DOI] [PubMed] [Google Scholar]
- 61.Royle L, Campbell MP, Radcliffe CM, White DM, Harvey DJ, Abrahams JL, Kim YG, Henry GW, Shadick NA, Weinblatt ME, et al. HPLC-based analysis of serum N-glycans on a 96-well plate platform with dedicated database software. Anal Biochem. 2008;376:1–12. doi: 10.1016/j.ab.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 62.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M, Golay J. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]


