Abstract
Treatment of chronic hepatitis B virus (HBV) infection with interferon and viral reverse transcriptase inhibitor regimens results in poor viral clearance, loss of response, and emergence of drug-resistant mutant virus strains. These problems continue to drive the development of new therapeutic approaches to combat HBV. Here, we engineered a bispecific antibody using two monoclonal antibodies cloned from hepatitis B surface antigen (HBsAg)-specific memory B cells from recombinant HBsAg-vaccinated healthy volunteers. Next, we evaluated its efficacy in neutralizing HBV in HepaRG cells. This bispecific antibody, denoted as C4D2-BsAb, had superior HBV-neutralizing activity compared with the combination of both parental monoclonal antibodies, possibly through steric hindrance or induction of HBsAg conformational changes. Moreover, C4D2-BsAb has superior endocytotic characteristics into hepatocytes, which inhibits the secretion of HBsAg. These results suggest that the anti-HBsAg bispecific antibody may be an effective treatment method against HBV infection.
Keywords: hepatitis B virus, bispecific antibody, neutralizing activity, steric hindrance, conformational change, endocytosis
Introduction
Hepatitis B virus (HBV) infection is a worldwide public health problem.1 It causes chronic liver disease, which may lead to cirrhosis, liver failure, and hepatocellular carcinoma.2 The therapeutic approach for HBV is based on interferon and viral reverse transcriptase inhibitor regimens, but long-term treatment using existing methods results in poor viral clearance, loss of response, and emergence of drug-resistant mutants virus strains. These problems continue to drive the development of new therapeutic agents to combat HBV. Administration of hepatitis B immunoglobulin (HBIG) prepared from human serum has provided passive immunization against infection with HBV and useful ways to prevent infection with HBV.3,4 The currently available HBIG, however, is not an ideal source because of low specificity and the potential for contamination with infectious agents. Currently, monoclonal antibodies (mAbs) are popular candidates for providing new therapeutic tools against HBV and other virus due to their long half-life, low toxicity, as well as high affinity and specificity.5-8
In this study, artificial hepatitis B surface antigen (HBsAg) was used to purify HBsAg-specific memory B cells from the peripheral blood mononuclear cells (PBMCs) in recombinant HBsAg-vaccinated healthy volunteers. Immunoglobulin light and heavy chains were cloned from these single memory B cell and were used to construct mAbs. We mapped epitopes of these mAbs on HBsAg and determined their neutralizing activities against HBV infection. Next, the two mAbs with the best neutralizing activities, C4G4, and D2H2, were combined and shown to have synergistic effects in vitro. Therefore, we developed bispecific antibody C4D2-BsAb using C4G4 and D2H2. Surprisingly, C4D2-BsAb showed superior neutralizing activity against HBV infection compared with the combination of the two parental mAbs. The possible mechanisms by which this bispecific antibody exerts stronger activity were also elucidated. Moreover, C4D2-BsAb has superior endocytotic characteristics into hepatocytes, which inhibits the secretion of HBsAg into culture supernatants.
Results
HBsAg-specific mAbs cloned grom HBsAg-specific memory B cells
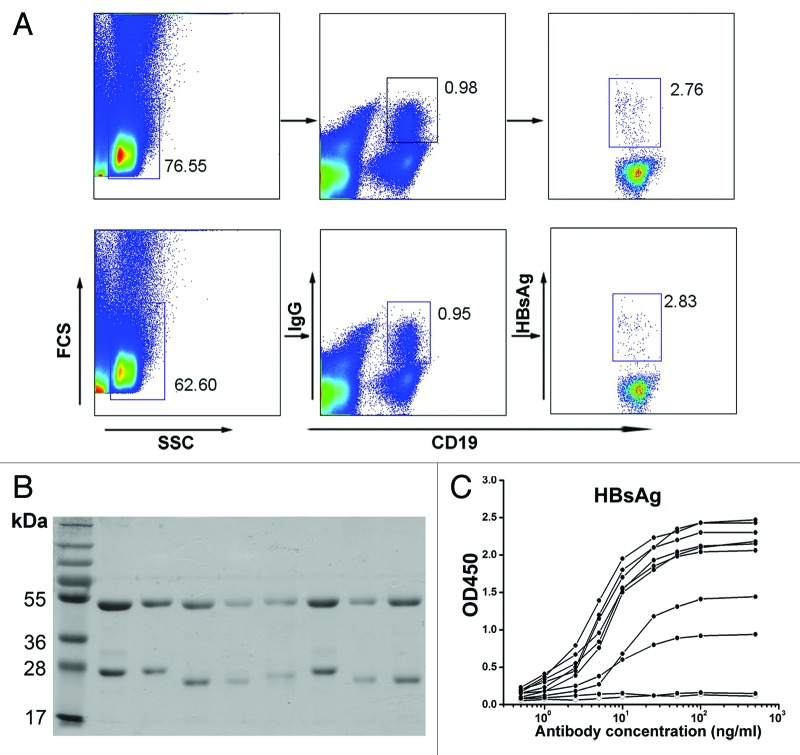
Antigen-specific IgG+ B cells make up a small percentage of the circulating B-cell pool.8 Artificial HBsAg was expressed with a tagged amino acid sequence that allows biotin labeling.9 We used a recently described method of antigen-specific memory B-cell sorting obtained from the PBMCs of healthy volunteers who received a recombinant hepatitis B vaccine, along with single cell PCR to amplify antibody light and heavy chain variable region genes from the cDNA of individual B cells.10,11 Among CD19+IgG+ B cells of the HBsAg-vaccinated healthy volunteers, we found a distinct population of cells that bound biotinylated HBsAg (Fig. 1A). Single antigen-specific memory B cells (HBsAg+CD19+IgG+) were sorted into each well of 96-well PCR plates and then subjected to single cell PCR. The matching light and heavy chain variable region genes were cloned into corresponding antibody expression vectors that reconstituted the light and heavy chain constant regions, and the full IgG1 mAbs were expressed in 293F cells. Under reducing conditions, each mAb yielded two protein bands with molecular masses of 50 kDa (heavy chain) and 25 kDa (light chain) (Fig. 1B). In addition, viral antigen reactivity analysis by ELISA showed that most of these mAbs bound to recombinant HBsAg with high affinity (Fig. 1C).
Figure 1. HBsAg-specific monoclonal antibodies cloned from HBsAg-specific memory B cells. (A) Flow cytometry plots of PBMCs of healthy volunteers received recombinant hepatitis B vaccine with anti-CD19, anti-IgG and biotin-HBsAg. (B) Purified mAbs run on a 10% SDS-PAGE under reducing conditions. (C) HBsAg-binding ELISA for mAbs from CD19+IgG+HBsAg+ cells.
Characterization of anti-HBsAg antibodies
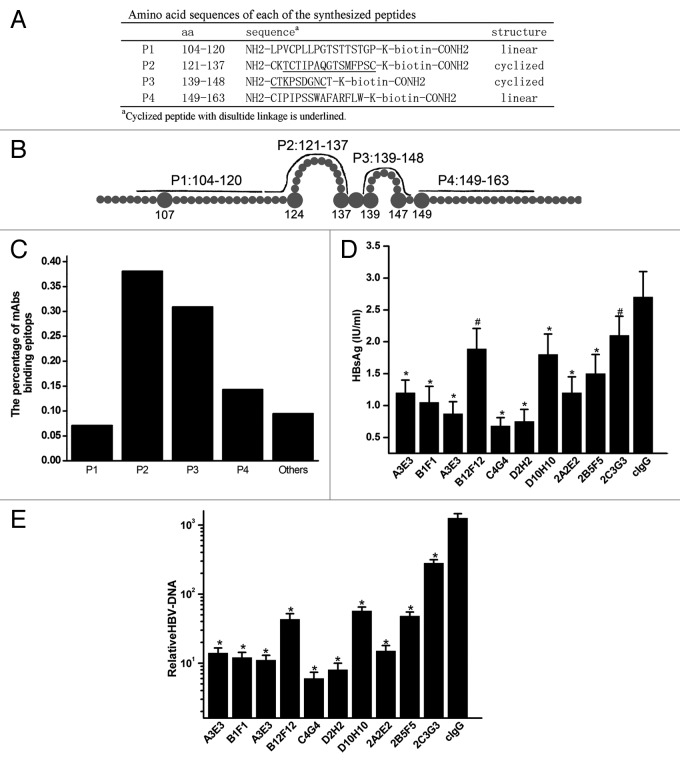
The epitopes for anti-HBsAg mAbs were mapped using a collection of synthesized peptides covering the extracellular region of HBsAg: P1 (aa: 104–120), P2 (aa: 121–137), P3 (aa: 139–148), and P4 (aa: 149–163) (Fig. 2A and B).12 We synthesized peptides P1 and P4 with a linear structure in addition to P2 and P3 with the appropriate conformational structures corresponding to the extracellular loop domain of HBsAg. As summarized in Figure 2C and Table 1, 7.1% (3/42) of these mAbs bound to peptides P1, 38.1% (16/42) bound to peptides P2, 31.0% (13/42) bound to peptides P3, 9.5% (4/42) bound to peptides P4, and 14.3% (6/42) did not bind to any of the four synthesized peptides. The affinities of these mAbs for HBsAg ranged from 1 × 10−7 to 1 × 10−9 M by ELISA (Table 1). To determine HBV-neutralizing activity of these anti-HBsAg mAbs, we measured their ability to inhibit infection of HepaRG cells by HBV. The HepaRG cell line has been established as the HBV infection model in vitro.13,14 We pretreated HBV with anti-HBsAg mAbs before infection of HepaRG cells, and quantified HBsAg in both the supernatant and intracellular HBV-DNA copies after 7 d in culture. As shown in Figure 2D and E, the majority of the mAbs significantly inhibited HBsAg expression in the supernatant and intracellular HBV-DNA copies, exceeding levels observed in the control human IgG (cIgG). These results suggest that these antibodies bind to four synthesized peptides covering the extracellular region of HBsAg and are responsible for their HBV-neutralizing activities in the circulating pool.
Figure 2. Characterization of anti-HBsAg antibodies. (A) Amino acid sequences of the synthesized peptides. The bold lines represent lined (P1 and P4) and cyclized synthesized peptides (P2 and P3) on conformational structure of the extracellular domain of HBsAg. (B) Schematic representation of synthesized peptides covering the extracellular domain of HBsAg. (C) Proportion of mAbs binding to synthesized peptides by ELISA. (D) Quantification of HBsAg levels in the supernatant of HepaRG cells and HBV-DNA in the cells at 7 d after infection with HBV pretreated with antibodies. (E) Quantification of HBV-DNA in HepaRG cells at 7 d after infection with HBV pretreated with mAbs. Results were shown as mean ± SD of three independent experiments. *P < 0.05; #P > 0.05.
Table 1. Characterization of anti-HBsAg antibodies.
| Antibody | Affinity KD(M) a | Epitopeb | Neutralization activityc | Antibody | Affinity KD(M) a | Epitopeb | Neutralization activityc |
|---|---|---|---|---|---|---|---|
| A3E3 | 5.67×10−8 | 3 | ++ | D12H12 | 2.04×10−7 | 2 | ++ |
| A5E5 | 3.75×10−9 | 4 | -/+ | 2A1E1 | 2.39×10−9 | 4 | -/+ |
| A6E6 | 3.24×10−8 | ND | + | 2A2E2 | 3.72×10−8 | 3 | ++ |
| A7E7 | 6.64×10−9 | 1 | -/+ | 2A9E9 | 4.35×10−8 | ND | -/+ |
| A10E10 | 2.12×10−8 | 2 | + | 2B4F4 | 1.71×10−9 | 2 | ++ |
| A11E11 | 5.63×10−8 | 1 | -/+ | 2B5F5 | 6.63×10−8 | 2 | + |
| B1F1 | 2.95×10−7 | 2 | ++ | 2B6F6 | 5.11×10−8 | 3 | ++ |
| B6F6 | 4.82×10−8 | 2 | ND | 2B10F10 | 8.35×10−9 | 4 | ++ |
| B7F7 | 6.55×10−9 | 3 | -/+ | 2B11F11 | 6.28×10−8 | ND | -/+ |
| B9F9 | 2.86×10−9 | 4 | ++ | 2C2G2 | 1.77×10−7 | 2 | + |
| B12F12 | 1.33×10−8 | 3 | + | 2C3G3 | 3.66×10−8 | ND | -/+ |
| C1G1 | 6.74×10−7 | 3 | ND | 2C5G5 | 3.28×10−9 | 2 | + |
| C2G2 | 1.85×10−9 | 2 | ++ | 2C6G6 | 1.11×10−8 | 3 | ++ |
| C4G4 | 3.66×10−9 | 2 | ++ | 2C7G7 | 8.6×10−8 | 3 | + |
| C8G8 | 3.03×10−7 | 2 | -/+ | 2C8G8 | 8.28×10−7 | 3 | ++ |
| C9G9 | 3.81×10−8 | ND | + | 2D1H1 | 5.04×10−8 | 1 | ++ |
| D2H2 | 5.82×10−9 | 3 | ++ | 2D4H4 | 3.48×10−9 | 2 | ++ |
| D3H3 | 4.14×10−8 | 2 | ND | 2D5H5 | 2.73×10−7 | 2 | ND |
| D5H5 | 1.88×10−9 | 2 | ++ | 2D8H8 | 2.12×10−8 | 3 | ++ |
| D6H6 | 4.86×10−7 | 3 | ++ | 2D9H9 | 5.29×10−8 | 3 | + |
| D10H10 | 1.58×10−8 | 2 | + | C4D2-BsAb | 3.88×10−9 | 2,3 | ++ |
| D11H11 | 8.92×10−9 | ND | -/+ | D2C4-BsAb | 4.95×10−9 | 2,3 |
a Affinities were analyzed with ELISA. bEpitopes were analyzed by ELISA using synthesized peptides (P1, amino acids 104–120; P2, amino acids 121–137; P3, amino acids 139–148; P4, amino acids 149–163) covering extracellular domain of HBsAg. cHBV-neutralization activity : ++, more than 60% inhibition; +, 40%–60% inhibition; –/+, 20-40% inhibition at day 7 post-infection from that with control IgG. ND, not determined.
Synergistic effect of a combination of two HBsAg-specific mAbs
Previous studies have indicated that a combination of two anti-HBsAg mAbs could have a synergistic neutralizing effect on HBV infection.15,16 Of all the anti-HBsAg mAbs we obtained, C4G4 and D2H2 showed the best neutralizing activity in our study. C4G4 and D2H2 bound to peptides P2 and P3, respectively (Table 1). We determined the HBV-neutralizing effects of the combination of C4G4 and D2H2 in HepaRG cells. Our results indicated that these two mAbs synergistically neutralized HBV infection (Fig. 3A and B).
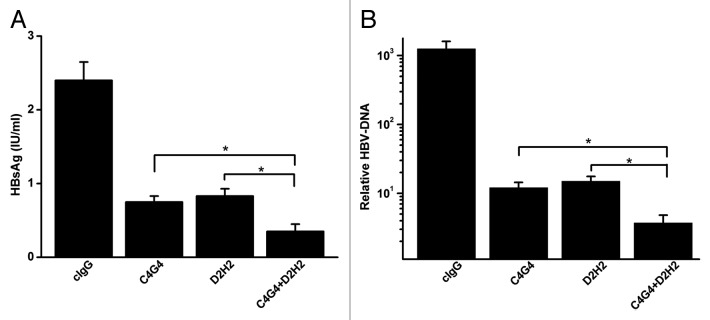
Figure 3. Synergistic effects of two mAbs on HBV-neutralizing activity. (A) Quantification of HBsAg levels in the supernatant of HepaRG cells at 7 d after infection with HBV pretreated with C4G4, D2H2 and C4G4 plus D2H2. (B). Quantification of HBV-DNA in HepaRG cells at 7 d after infection with HBV pretreated with C4G4, D2H2 and C4G4 plus D2H2. Results were shown as mean ± SD of three independent experiments. *P < 0.05.
Characterization of bispecific anti-HBsAg antibodies
We engineered two bispecific antibodies, C4D2-BsAb and D2C4-BsAb, using C4G4 and D2H2 (Fig. 4A). C4D2-BsAb is in VC4G4-VD2H2-constant orientation and D2C4-BsAb is in VD2H2-VC4G4-constant orientation. Under reducing conditions, the bispecific antibodies yielded two bands with molecular masses of ~65 kDa (heavy chain) and 35 kDa (light chain), while a monospecific IgG1 antibody showed two bands with molecular mass of ~50 kDa (heavy chain) and 25 kDa (light chain) (Fig. 4B). The binding of C4D2-BsAb and D2C4-BsAb to synthesized peptides P2 and P3 was assessed by ELISA. As shown in Figure 4C, C4D2-BsAb showed a similar P2-binding activity as C4G4, while the P2-binding activity of D2C4-BsAb was much lower than that of C4G4. Both of the bispecific antibodies displayed P3 binding activity comparable to that of D2H2 (Fig. 4C). The HBsAg-binding affinities of C4D2-BsAb and D2C4-BsAb were also measured and compared. The results indicated that the binding affinity of D2C4-BsAb to HBsAg was slightly lower than that of C4D2-BsAb (Fig. 4D). The data suggest that C4D2-BsAb retained the antigen-binding activity of both parental mAbs. Therefore, we chose C4D2-BsAb for further investigation.
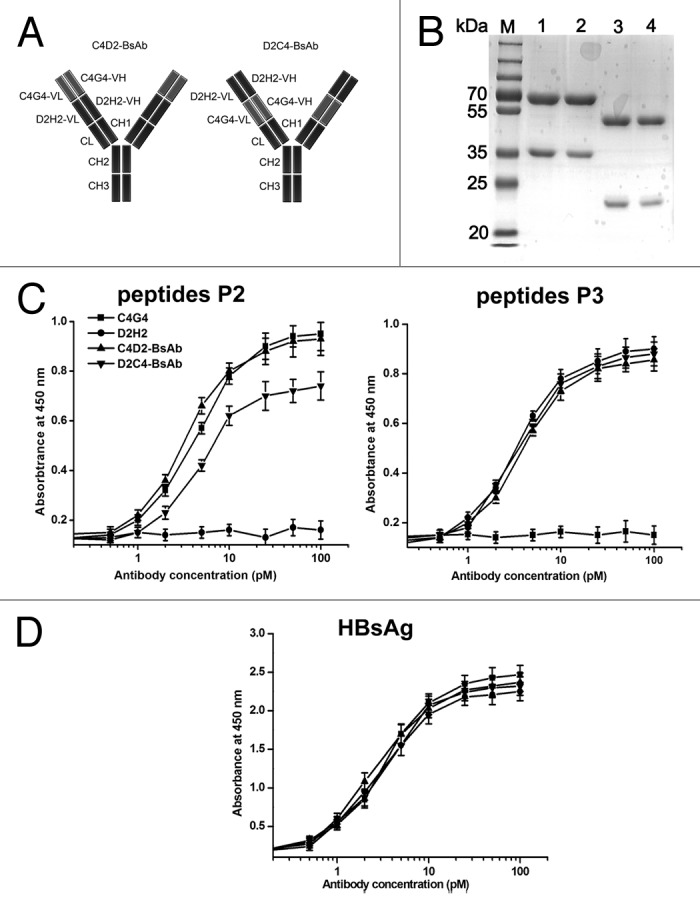
Figure 4. Schematic representation and characterization of bispecific antibodies. (A) Schematic representation of the structures of C4D2-BsAb and D2C4-BsAb. (B) SDS-PAGE analysis of purified antibodies under reducing conditions. Lane M, protein markers; lane 1, C4D2-BsAb; lane 2, D2C4-BsAb; lane 3, C4G4; lane 4, D2H2. (C and D) HBsAg-binding activity of bispecific antibodies. Increasing concentrations of C4G4, D2H2, C4D2-BsAb and D2C4-BsAb were added to 96-well plates coated with HBsAg or synthesized peptides P2 or P3. After 2 h incubation, the plates were washed and bound antibodies were detected with HRP-conjugated goat anti-human IgG. Results were shown as mean ± SD of three independent experiments.
HBV-neutralizing activities of C4D2-BsAb
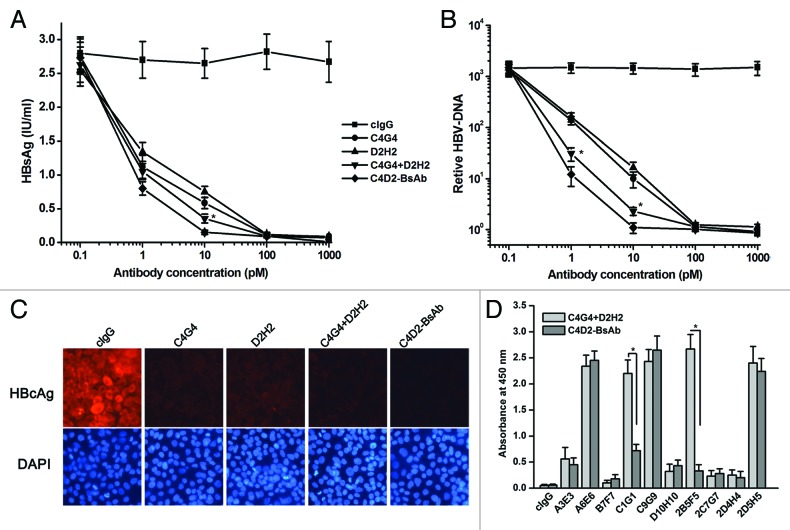
We evaluated HBV-neutralizing activities of C4D2-BsAb in HepaRG cells. The levels of HBsAg in the cells culture supernatants and HBV-DNA replication in the cells from different treatment groups were compared. The results clearly indicated that the levels of HBsAg in cell culture supernatants and HBV-DNA copies in the cells were markedly reduced by treatment with C4G4, D2H2, C4G4 plus D2H2, or C4D2-BsAb (Fig. 5A and B). We also found that the HBV-neutralizing activities of these anti-HBsAg antibodies were dose-dependent (Fig. 5A and B). It is particularly noteworthy that C4D2-BsAb appeared to be significantly more effective than C4G4 plus D2H2 at the dose of 10 pM (Fig. 5A and B). In addition, we evaluated HBV-neutralizing activities of C4D2-BsAb in HepaRG cells using immunofluorescence staining. As shown in Figure 5C, we observed a greater marked decrease in hepatitis B core antigen (HBcAg) with C4D2-BsAb compared with the combination of C4G4 plus D2H2. These observations indicated that C4D2-BsAb, which is directed to two different epitopes of HBsAg, shows a superior neutralizing effect on HBV infection. To investigate why C4D2-BsAb has a stronger HBV-neutralizing activity than that of C4G4 plus D2H2, we examined the binding of a panel of anti-HBsAg mAbs (A3E3, A6E6, B7F7, C1G1, C9G9, D10H10, 2B5F5, 2C7G7, 2D4H4, and 2D5H5) to HBsAg in the presence of C4D2-BsAb, or C4G4 plus D2H2. As shown as in Figure 5D, we found that the binding of C1G1 and 2B5F5 to HBsAg was markedly reduced when HBsAg was preincubated with C4D2-BsAb, but not with C4G4 plus D2H2. The data suggest that C4D2-BsAb might affect the binding of C1G1 and 2B5F5 through steric hindrance or inducing HBsAg conformational changes, which might also be responsible for the difference in HBsAg-neutralizing activity between C4D2-BsAb and C4G4 plus D2H2.
Figure 5. HBV-neutralizing activity of the bispecific antibodies. (A) Quantification of HBsAg levels at 7 d after infection with HBV pretreated with different dose of C4G4, D2H2, C4G4 plus D2H2 and C4D2-BsAb. (B) Quantification of relative HBV-DNA copies at 7 d after infection with HBV pretreated with different dose of C4G4, D2H2, C4G4 plus D2H2 and C4D2-BsAb. (C) Immunohistochemisty staining with anti-HBcAg antibody in HepaRG cells at 7 d after infection with HBV pretreated with C4G4, D2H2, C4G4 plus D2H2 and C4D2-BsAb. (D) The binding of C1G1 and 2B5F5 to HBsAg was markedly reduced when HBsAg was preincubated with C4D2-BsAb but not with C4G4 plus D2H2. C4G4 plus D2H2, or C4D2-BsAb was first incubated with biotin-HBsAg. The mixtures were then transferred to the plates coated with anti-HBsAg antibodies A3E3, A6E6, B7F7, C1G1, C9G9, D10H10, 2B5F5, 2C7G7, 2D4H4 and 2D5H5. After 2 h incubation, the plates were washed and biotin-HBsAg bound were detected with HRP-strepavidin conjugated. Results were shown as mean ± SD of three independent experiments. *p < 0.05.
Endocytosis of C4D2-BsAb into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions
Previous studies have shown that anti-HBsAg antibodies were internalized into hepatocyte-derived cell lines and inhibited secretion of HBsAg and virions from these cells in vitro.15,17 Moreover, the combination of two anti-HBsAg antibodies showed a greater HBsAg retention within hepatocyte-derived cells than did either antibody alone. Here, we investigated whether the anti-HBsAg bispecific antibody C4D2-BsAb could be internalized in hepatocytes and interfere with secretion of HBsAg and virions from the infected-HBV cells. We used PLC/PRF/5 cells, which provide the stable production of HBsAg.17 PLC/PRF/5 cells were treated with C4G4, D2H2, C4G4 plus D2H2, C4D2-BsAb, and cIgG, respectively. After the first 24 h, the cells were washed and fresh medium was added. During the second 24 h, the cell culture supernatants were collected at hour 3, 6, 12, and 24, and the HBsAg levels in cell culture supernatants were measured. The levels of HBsAg in cell culture supernatants from different treatment groups were compared. The results shown in Figure 6A clearly indicated that the level of HBsAg in cell culture supernatants was significantly reduced by treatment with C4G4, D2H2, C4G4 plus D2H2 and C4D2-BsAb (p < 0.05). However, either C4G4 alone or D2H2 alone appeared to be much less effective than C4G4 plus D2H2 at inhibiting HBsAg release (p < 0.05). There was no statistically significant difference in HBsAg release between C4G4 plus D2H2 and C4D2-BsAb treatment groups (p > 0.05). Moreover, western blotting was used to analyze the antibody-treated cells after the first 24 h (Fig. 6B). The results indicated a marked intracellular accumulation of HBsAg in the cells treated with anti-HBsAg antibodies, but not in control cells. C4G4 plus D2H2 and C4D2-BsAb, however, had a greater effect on HBsAg retention within the cells than did either C4G4 or D2H2 alone. In addition, both cIgG (nonspecific for HBsAg) and anti-HBsAg antibodies were uptaked into the cells with different density (Fig. 6B).
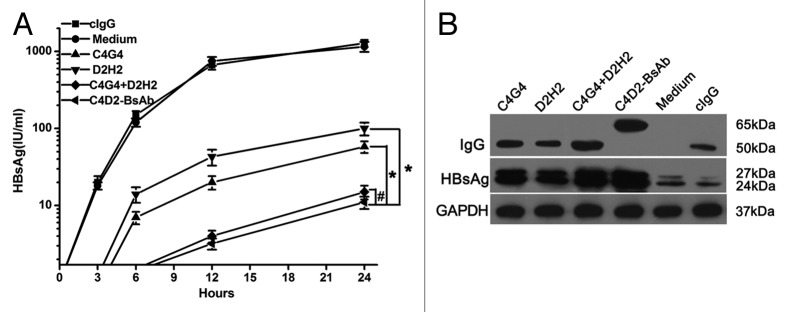
Figure 6. C4D2-BsAb inhibited HBsAg release from PLC/PRF/5 cells in vitro. (A) Quantitation of HBsAg levels in the supernatants of PLC/PRF/5 cells cultured with C4G4, D2H2, C4G4 plus D2H2, C4D2-BsAb and control human IgG during the second 24 h. (B) western blotting analysis of cytoplasmic extracts from PLC/PRF/5 cells cultured with these antibodies at the first 24 h. Results were shown as mean ± SD of three independent experiments. *P < 0.05; #P > 0.05.
Discussion
As targeted therapies, mAbs have been widely used to treat human diseases; however, targeting only one antigen usually not sufficiently effective. Because of this phenomenon, combinations of two mAbs have already been studied.18,19 However, development of two separate mAbs for clinical use as combination therapy is limited due to regulatory hurdles and cost. Use of genetic engineering to convert two mAbs into an IgG-like bispecific format would greatly facilitate the clinical development of antibodies capable of targeting two antigens.20 As a consequence, antibody formats that can bind two different targets simultaneously have been described.21
Previous studies have indicated that a combination of two anti-HBsAg antibodies could have a synergistic neutralizing effect on HBV infection in vitro.15 Eren et al. developed two human mAbs directed against different epitopes on HBsAg.22 A single administration of the mAbs mixture into HBV chronic carrier chimpanzees resulted in an immediate reduction in HBsAg levels followed by recurrence to initial levels within few days.22 Heijtink et al. also reported that two anti-HBsAg mAbs may complement each other due to their different mechanisms of inactivation in a chronic hepatitis B virus carrier chimpanzee.23 Moreover, Galun et al. reported that HBV-ABXTL, a combination of two anti-HBsAg antibodies, reduces serum viral titers and HBsAg levels in a Phase 1 clinical study.16 Thus, the use of a combination of antibodies with HBV-neutralizing activity may represent an effective strategy to treat chronic hepatitis B virus infection.
In our study, we showed that C4G4 and D2H2, the two mAbs with the best neutralizing activity, synergistically neutralized HBV infection. In contrast to oligoclonal formats, bispecific antibodies offer functionalities and targeting mechanisms unattainable by a combination of two antibodies, including increased functional affinity through avidity,24 receptor activation,25 delivery of toxins,26 tissue-specific targeting,27 and immunodiagnostics.28 Therefore, we constructed the bispecific antibody C4D2-BsAb from C4G4 and D2H2. Our results suggest that C4D2-BsAb is directed simultaneously against two different epitopes on HBsAg. It is particularly noteworthy that C4D2-BsAb has significantly more potent HBV-neutralizing activity in comparison to its parental combinations. Further analysis indicated that the binding of two anti-HBsAg antibodies, C1G1 and 2B5F5, to HBsAg was markedly reduced when HBsAg was preincubated with C4D2-BsAb, but not with C4G4 plus D2H2. C1G1 and 2B5F5 did not compete with either C4G4 or D2H2 for binding to HBsAg. Previous studies indicated that particular antibodies could induce conformational change in neighboring sites of the antigen, thus enhancing or inhibiting their binding to receptors or to additional antibodies.29-31 Lu et al. engineered a bispecific antibody using two anti-vascular endothelial growth factor receptor (VEGFR)2 single chain antibodies (scFvs).32 Neither of the two scFvs blocks VEGFR-2 from binding to VEGF or has any effect on VEGF-induced receptor activation,32 but, surprisingly, the bispecific antibody effectively blocks VEGFR-2/VEGF interactions.32 Taken together, Lu et al.’s and our results suggest that a bispecific antibody directed against two different epitopes with the same antigen might exert unique effects through steric hindrance or by causing major conformational changes of the antigen.
In summary, we cloned a panel of mAbs from the PBMCs of healthy volunteers who received a recombinant hepatitis B vaccine and developed a bispecific antibody, C4D2-BsAb, using two anti-HBsAg mAbs with the best HBV-neutralizing activity. The most unexpected finding of the present study is that C4D2-BsAb showed superior HBV-neutralizing activity over the combination of its parental mAbs. Our results suggest that C4D2-BsAb has the potential to be a promising candidate for HBV infection treatment.
Materials and Methods
Ethics statements
Healthy volunteer specimens were collected in accordance with the Second Military Medical University and healthy volunteer written informed consent was obtained.
Cell lines and virus
Human hepatocellular carcinoma cell lines PLC/PRF/5, Huh7 and Hep3B, human intestinal epithelial cell line HT29 and human cervical epithelial cell line HeLa were purchased from ATCC. HepG2.2.15 (HBV positive, supporting full HBV replication) was obtained from the Institute of Translational Hepatology, Beijing 302 Hospital. HepRG cell line was purchased from Biopredic International. HBV was prepared from freshly collected HepG2.2.15 culture supernatants by precipitating viral particles in the presence of 10% polyethylene glycol 8000 (PEG8000, Sigma). The Sediment was resuspended in PBS containing 25% fetal calf serum (FCS) (Hyclone). The HBV was stored at -80 °C.
Single memory B-cell sorting and PCR amplification
Biotinylated HBsAg was generated as previously described.9 Briefly, the plasmid constructs for HBsAg were modified by adding sequence encoding the Avi-tag signal for biotinylation (LNDIFEAQKIEWHE) to the carboxylic terminus of the gene. The modified genes were then subcloned into the pFUSE-hIgG1-Fc2 expression vector (Invivogen). The proteins were biotinylated using biotin ligase Bir A (Avidity) at 30þC for 30 min. The HBsAg-specific memory B cells were identified as CD19+IgG+HBsAg+ cells from PBMCs of healthy volunteers who received a recombinant hepatitis B vaccine (Bimmugen, adr, genotype C). The CD19+IgG+HBsAg+ single B cells were sorted into 96-well PCR plates (BioRad) containing 5 μL/well cell lysis solution.9-11 Plates were immediately kept at -80 °C. The frozen plates with single B-cell RNA were thawed on ice, and the reverse transcription (RT) reaction was performed by adding 150 ng random hexamer primer (Promega), 0.5 μL 10mM dNTP-mix (10mM each nucleotide; Promega), 0.5% v/v Igepal CA-630 (Sigma), 1 μl 0.1 M DTT (Invitrogen), 10U RNAsin® (Promega), 1.5μL of 5 × first strand buffer (Invitrogen), 100U SuperScriptIII (Invitrogen) into each well.11,33,34 The thermocycle program for the RT reaction was performed at 42 °C for 10 min, 25 °C for 10 min, 50 °C for 60 min and 94 °C for 5 min. The cDNA was stored at -20 °C. IgH, Igλ and Igκ variable region genes were amplified independently by nested PCR starting from 3 μL of cDNA as template. All PCRs were performed in 96-well PCR plates in a total volume of 50 μL containing 25 μL of GoTaq® GreenMaster Mix (Promega) for first round PCR and second round PCR, 25 μM each primer or primer mix.34 Each round of PCR was performed for 50 cycles of 94 °C for 30 s, 58 °C for IgH and Igκ or 60 °C for Igλ for 30 s, and 72 °C for 1 min. The positive second round PCR products were sequenced with reverse PCR primers to determine the respective V and J gene. The V gene and J gene-specific primers containing restriction sites were used in another round of amplification using the 1st PCR product as template. All PCR products were cloned into the corresponding Igγ1, Igκ, and Igλ expression vectors (pcDNA3.1[+] based).
Construction of bispecific antibodies
The bispecific antibodies were constructed using the previously described method.21,35 Briefly, the light chain variable region (VL) and heavy chain variable region (VH) of a mAb were genetically fused to the 5′ terminus of the light chain and heavy chain of another mAb through a long linker, respectively. The linkers between the two variable regions in both the light chain (the linker sequence is TVAAPSVFIFPP) and the heavy chain (the linker sequence is ASTKGPSVFPLAP) were selected from the N termini of human Cκ and CH1 sequences, respectively.
Expression and purification of antibodies
Antibodies were expressed and purified using the previously reported method.11 Briefly, appropriate light and heavy expression vectors were cotransfected into 293F cells (Invitrogen) in serum-free freestyle medium (Invitrogen). Culture supernatants were harvested when cells viability dropped to 50–60%. Finally, antibodies were purified by Protein A affinity chromatography (GE Healthcare) from the serum-free culture supernatants. The purified antibodies were analyzed on 12% SDS-PAGE under reducing conditions, followed by Coomassie brilliant blue staining.
Antibody affinity measurement
Different anti-HBsAg antibodies were incubated with varying concentrations of HBsAg for 1 h. The concentration of free antibody was then determined by ELISA using immobilized HBsAg and was used to calculate affinity (KD) as previously described.36
Analysis of HBV-neutralizing activity
We investigated the HBV-neutralizing activity of antibodies in HepaRG cells using a method similar to the method described by Gripon et al.13 Briefly, the HepaRG cells were cultured in Williams E medium (Invitrogen) supplemented with 10% FCS (Hyclone), 100 U/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen), 5 μg/mL insulin (Invitrogen), 5 × 10−7 M hydrocortisone hemisuccinate (Sigma) for 2 weeks. Then, the differentiated cells were maintained in medium supplemented with 2% dimethylsulfoxide (DMSO) (Sigma) for 2 more weeks. We preincubated 106 copies of HBV and 1 μg of each mAb for 1 h at room temperature before infection and then added them to HepaRG cells in 1 ml of culture medium with 4% PEG8000 (Sigma). After 24 h, cells were washed 3 times and maintained in fresh medium. On day 2, 4, and 7 after infection, DNA was isolated from whole cell lysates and HBV-DNA copies were quantified using a commercial HBV-DNA PCR-Fluorescence Quantitation kit (KHB). We then quantified HBsAg in the culture supernatant using a commercial chemiluminescent immunoassay kit (China Diagnostics). We used HBIG (Hualan Bio) and human IgG1 (cIgG) as control.
Antibody binding activity assays
ELISA plates were previous coated with goat anti-biotin antibody (Sigma), blocked with 10% fat-free milk, and incubated with synthesized peptides at 200 ng/mL in 10% fat-free milk at 37 °C for 2 h. Different concentrations of anti-HBsAg antibodies were then added to ELISA plates. After 2 h incubation, the plates were washed and HRP-conjugated goat anti-human IgG (KPL) was added and further incubated for 1 h. Finally, 3,3′,5,5′-tetramethylbenzidine was added as a substrate for HRP and the absorbance was measured at 450 nm.
Western blotting
Cells lysate concentration were determined by BCA assay (Pierce), resolved on 10% SDS-PAGE gel, and transferred onto PVDF membranes (Millipore). The membranes were blocked with 10% fat-free milk, probed with mouse anti-human HBsAg IgG (Santa Cruze Biotechnology), and goat anti-human IgG (Abcam). Protein bands were visualized with ECL (Pierce).
Immunofluorescence
Cells were fixed in 4% paraformaldehyde (Sigma) and followed by with 0.25% Triton X-100 at room temperature. Cells were blocked with 1% BSA and stained with mouse anti-human HBcAg mAbs (Santa Cruz Biotechnology) and followed by incubation with goat anti-mouse Alexa Fluor 546-conjugated IgG in dark. They were incubated with 1% DAPI (Sigma) and were examined using OLYMPUS inverted fluorescence microscope (IX51).
Kinetics of HBsAg secretion from PLC/PRF/5 cells pretreated with Anti-HBsAg antibody
PLC/PRF/5 cells were pretreated with 0.5 mg/ml of C4G4, D2H2, C4G4 plus D2H2, C4D2-BsAb or cIgG. After 24 h, the supernatants in all wells were replaced with fresh medium. The cells were kept in culture for a further 24 h. During the second 24 period, an aliquot of the supernatants were collected and the HBsAg levels were quantitated using a commercial chemiluminescent immunoassay kit (China Diagnostics).
Statistical analysis
Data were analyzed using the Student unpaired t-test to identify significant differences unless otherwise indicated. Differences were considered significant at a P value of < 0.05.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China, National Key Project for Infectious Diseases (2012ZX10002012–009), Ministry of Science and Technology of China (973 and 863 program projects), Shanghai Commission of Science and Technology (Key Laboratory and Projects), and Shanghai Leading Academic Discipline Project (B905). We thank Xueling Wu for providing the method for cloning monoclonal antibodies from human peripheral blood. We also thank Liangliang Wu and Linxiong Wang for technical assistance.
Glossary
Abbreviations:
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HBIG
immunoglobulin
- mAb
monoclonal antibody
- PBMCs
peripheral blood mononuclear cells
- IgG
immunoglobulin G
- cIgG
control human IgG
- HBcAg
hepatitis B core antigen
- VEGFR2
vascular endothelial growth factor receptor 2
- scFvs
single chain antibodies
- FCS
fetal calf serum
- RT
reverse transcription
- VL
light chain variable region
- VH
heavy chain variable region
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26390
References
- 1.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Wands JR. Prevention of hepatocellular carcinoma. N Engl J Med. 2004;351:1567–70. doi: 10.1056/NEJMe048237. [DOI] [PubMed] [Google Scholar]
- 3.EASL Jury EASL International Consensus Conference on Hepatitis B. 13-14 September, 2002: Geneva, Switzerland. Consensus statement (short version) J Hepatol. 2003;38:533–40. doi: 10.1016/s0168-8278(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 4.Alter MJ. Epidemiology and prevention of hepatitis B. Semin Liver Dis. 2003;23:39–46. doi: 10.1055/s-2003-37583. [DOI] [PubMed] [Google Scholar]
- 5.Luk JM, Wong KF. Monoclonal antibodies as targeting and therapeutic agents: prospects for liver transplantation, hepatitis and hepatocellular carcinoma. Clin Exp Pharmacol Physiol. 2006;33:482–8. doi: 10.1111/j.1440-1681.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- 6.Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, Wu YM, Chang MH, Lee PH. High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr. 2009;48:203–8. doi: 10.1097/MPG.0b013e3181819ad4. [DOI] [PubMed] [Google Scholar]
- 7.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–60. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 8.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, Cutrell E, Seaman MS, Mascola JR, Wyatt RT, Wardemann H, et al. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343:65–7. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, Kinoshita K, Kadowaki S, Takahashi K, Sugiyama T, Kishi H, et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat Med. 2009;15:1088–92. doi: 10.1038/nm.1966. [DOI] [PubMed] [Google Scholar]
- 13.Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, Guyomard C, Lucas J, Trepo C, Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99:15655–60. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucifora J, Durantel D, Testoni B, Hantz O, Levrero M, Zoulim F. Control of hepatitis B virus replication by innate response of HepaRG cells. Hepatology. 2010;51:63–72. doi: 10.1002/hep.23230. [DOI] [PubMed] [Google Scholar]
- 15.Galun E, Eren R, Safadi R, Ashour Y, Terrault N, Keeffe EB, Matot E, Mizrachi S, Terkieltaub D, Zohar M, et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hepatology. 2002;35:673–9. doi: 10.1053/jhep.2002.31867. [DOI] [PubMed] [Google Scholar]
- 16.Neumann AU, Phillips S, Levine I, Ijaz S, Dahari H, Eren R, Dagan S, Naoumov NV. Novel mechanism of antibodies to hepatitis B virus in blocking viral particle release from cells. Hepatology. 2010;52:875–85. doi: 10.1002/hep.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schilling R, Ijaz S, Davidoff M, Lee JY, Locarnini S, Williams R, Naoumov NV. Endocytosis of hepatitis B immune globulin into hepatocytes inhibits the secretion of hepatitis B virus surface antigen and virions. J Virol. 2003;77:8882–92. doi: 10.1128/JVI.77.16.8882-8892.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tvorogov D, Anisimov A, Zheng W, Leppänen VM, Tammela T, Laurinavicius S, Holnthoner W, Heloterä H, Holopainen T, Jeltsch M, et al. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell. 2010;18:630–40. doi: 10.1016/j.ccr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20:460–70. doi: 10.1016/j.coi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25:1290–7. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 22.Eren R, Ilan E, Nussbaum O, Lubin I, Terkieltaub D, Arazi Y, Ben-Moshe O, Kitchinzky A, Berr S, Gopher J, et al. Preclinical evaluation of two human anti-hepatitis B virus (HBV) monoclonal antibodies in the HBV-trimera mouse model and in HBV chronic carrier chimpanzees. Hepatology. 2000;32:588–96. doi: 10.1053/jhep.2000.9632. [DOI] [PubMed] [Google Scholar]
- 23.Heijtink R, Paulij W, van Bergen P, van Roosmalen M, Rohm D, Eichentopf B, Muchmore E, de Man R, Osterhaus A. In vivo activity of a mixture of two human monoclonal antibodies (anti-HBs) in a chronic hepatitis B virus carrier chimpanzee. J Gen Virol. 1999;80:1529–35. doi: 10.1099/0022-1317-80-6-1529. [DOI] [PubMed] [Google Scholar]
- 24.Cheong HS, Chang JS, Park JM, Byun SM. Affinity enhancement of bispecific antibody against two different epitopes in the same antigen. Biochem Biophys Res Commun. 1990;173:795–800. doi: 10.1016/S0006-291X(05)80857-5. [DOI] [PubMed] [Google Scholar]
- 25.Madrenas J, Chau LA, Teft WA, Wu PW, Jussif J, Kasaian M, Carreno BM, Ling V. Conversion of CTLA-4 from inhibitor to activator of T cells with a bispecific tandem single-chain Fv ligand. J Immunol. 2004;172:5948–56. doi: 10.4049/jimmunol.172.10.5948. [DOI] [PubMed] [Google Scholar]
- 26.Bonardi MA, French RR, Amlot P, Gromo G, Modena D, Glennie MJ. Delivery of saporin to human B-cell lymphoma using bispecific antibody: targeting via CD22 but not CD19, CD37, or immunoglobulin results in efficient killing. Cancer Res. 1993;53:3015–21. [PubMed] [Google Scholar]
- 27.Vasu C, Gorla SR, Prabhakar BS, Holterman MJ. Targeted engagement of CTLA-4 prevents autoimmune thyroiditis. Int Immunol. 2003;15:641–54. doi: 10.1093/intimm/dxg061. [DOI] [PubMed] [Google Scholar]
- 28.Khaw BA, Rammohan R, Abu-Taha A. Bispecific enzyme-linked signal-enhanced immunoassay with subattomole sensitivity. Assay Drug Dev Technol. 2005;3:319–27. doi: 10.1089/adt.2005.3.319. [DOI] [PubMed] [Google Scholar]
- 29.Roguin LP, Retegui LA. Monoclonal antibodies inducing conformational changes on the antigen molecule. Scand J Immunol. 2003;58:387–94. doi: 10.1046/j.1365-3083.2003.01320.x. [DOI] [PubMed] [Google Scholar]
- 30.Davies DR, Sheriff S, Padlan EA. Antibody-antigen complexes. J Biol Chem. 1988;263:10541–4. [PubMed] [Google Scholar]
- 31.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol. 2012;86:1563–76. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu D, Kotanides H, Jimenez X, Zhou Q, Persaud K, Bohlen P, Witte L, Zhu Z. Acquired antagonistic activity of a bispecific diabody directed against two different epitopes on vascular endothelial growth factor receptor 2. J Immunol Methods. 1999;230:159–71. doi: 10.1016/S0022-1759(99)00135-0. [DOI] [PubMed] [Google Scholar]
- 33.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 34.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–24. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C, Ying H, Bose S, Miller R, Medina L, Santora L, Ghayur T. Molecular construction and optimization of anti-human IL-1alpha/beta dual variable domain immunoglobulin (DVD-Ig) molecules. MAbs. 2009;1:339–47. doi: 10.4161/mabs.1.4.8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]