Abstract
Vascular endothelial growth factor receptor 3 (VEGFR-3) is a receptor for the vascular endothelial growth factor C and D (VEGF-C and D) and plays a critical role in the development of embryonic vascular system and regulation of tumor lymphangiogenesis. In this report, we generated a novel panel of 17 monoclonal antibodies (mAbs) against human VEGFR-3 and determined their ability to inhibit the proliferation of human erythroleukemia (HEL) cells and angiogenesis of chick embryo chorioallantoic membrane (CAM). Among these mAbs, BDD073 was demonstrated to inhibit the interaction of soluble VEGFR-3 with VEGF-D and the proliferation of HEL cells. Furthermore, in chick embryo CAM angiogenesis experiments, the angiogenesis induced by recombinant glutathione-S-transferase-VEGF-D was decreased in the presence of antibody BDD073. These data suggest that this novel neutralizing antibody against human VEGFR-3 could be a tool for the investigations into the biology of VEGFR-3, and potentially a reagent for blocking VEGF-D-induced angiogenesis and lymphogenesis.
Keywords: VEGFR-3, VEGF-D, antibody, angiogenesis
Introduction
Tumor metastasis is a major cause of death in cancer patients. Tumor cells disseminate through three major routes, i.e., direct invasion of surrounding tissues, and dissemination via the blood vascular system (hematogenous metastasis) or through the lymphatic system (lymphatic metastasis).1 During these processes, vascular endothelial growth factor (VEGF) proteins and their receptors (VEGFRs) play important roles. There are five known members of the VEGF family, VEGF A, B, C, D, and placenta growth factor (PIGF). These factors interact with three membrane receptors, VEGFR-1 (Flt1), VEGFR-2 (KDR), and VEGFR-3 (Flt4), which belong to the subfamily of receptor protein tyrosine kinase.1
VEGFR-3, a receptor for VEGF-C and D, plays a critical role in the development of the embryonic vascular system. In the adult, VEGFR-3 expression is lost from vascular endothelial cells, but is retained in lymphatic endothelial cells.2,3 It was therefore suggested that in the adult, VEGF-C and D interactions with VEGFR-3 are limited to the maintenance of lymphatic endothelial cells or lymphangiogenesis.2,4 More recent work, however, indicates that VEGFR-3 may also be involved in angiogenesis and lymphangiogenesis in tumors.5,6 It was reported that the expression of VEGF-D was elevated in gastric cancer and associated with lymph node metastasis. Moreover, the high expression of VEGF-D and VEGFR-3 was closely related with poor prognosis in gastric cancer.7 In addition, Wakisaka et al. demonstrated that VEGF-C/VEGFR-3 signal was involved in the tumor-induced lymphangiogenesis, which resulted in the metastasis of nasopharyngeal carcinoma.8 The importance of VEGF-D/VEGFR-3 was further strengthened by recent results showing that VEGF-D/VEGFR-2/VEGFR-3 promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium.9 Taken together, these studies reveal that VEGFR-3 mediated signaling pathway is of paramount importance in the regulation of lymphangiogenesis during cancer development. On the other hand, recent studies have demonstrated that neutralizing antibodies to VEGF-D decreased the number of lymphatic metastases in the VEGF-D-producing tumors in experimental animal models.10 Moreover, other molecules targeting the VEGF-C/VEGF-D/VEGFR-3 signal pathway could also restrict tumor growth or cancer metastasis.11-13 Hence, antagonists directed to VEGF and its VEGFRs have been used as therapeutic agents and powerful experimental tools.14
In this study, we generated and characterized a novel monoclonal antibody (mAb) BDD073 that inhibited VEGF-D-mediated VEGFR-3 activation and partially suppressed glutathione-S-transferase (GST)-VEGF-D-induced angiogenesis in a chick embryo CAM model. These results suggest that BDD073 can be used as a powerful neutralizing antibody for VEGFR-3 signal studies on therapeutic angiogenesis and lymphangiogenesis.
Results
Characterization of recombinant GST-VEGF-D
Because screening of the antagonist antibodies against VEGFR-3 requires the soluble ligand, we first expressed the fusion protein of GST-VEGF-D in E. coli and characterized its biological activity. GST-VEGF-D was recognized by antibodies to VEGF-D and GST (Fig. 1A). Subsequently, we measured the binding activities of soluble GST-VEGF-D to VEGFR-3/Fc by ELISA assay. The results showed that the soluble GST-VEGF-D could interact with VEGFR-3/Fc and this interaction could be inhibited by pre-incubation of GST-VEGF-D (Fig. 1B). This assay suggested that the interaction system of GSF-VEGF-D and VEGFR-3/Fc could be used for screening the neutralizing antibodies to VEGFR-3.
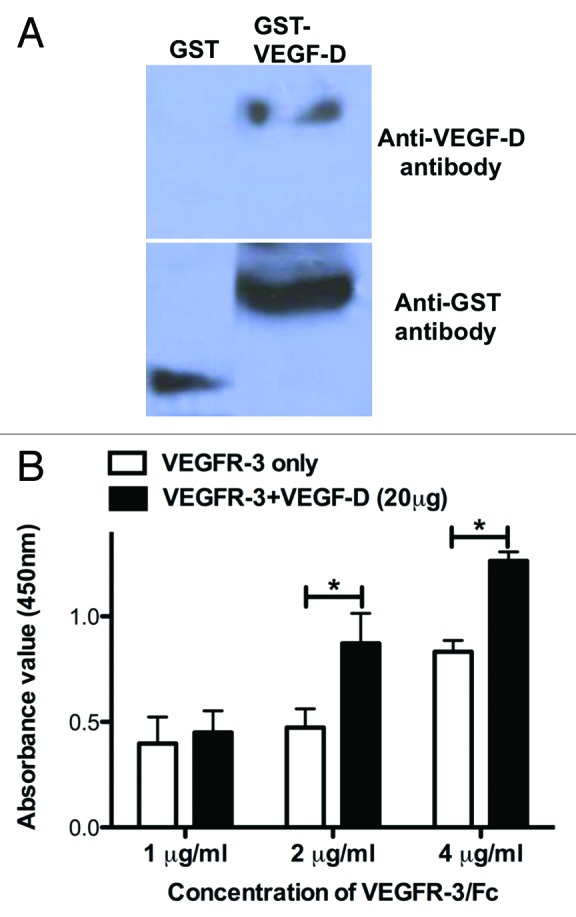
Figure 1. Characterization of GST-VEGF-D. (A) western blot analysis of GST-VEGF-D expression in E. coli. (B) In vitro interaction of GST-VEGF-D and VEGFR-3/Fc. VEGFR3/Fc or VEGF-D proteins were added to 96-well microtiter plates coated with GST-VEGF-D and incubated at 37 °C for 1 h, respectively. The plates were washed for three times and the standard ELISA protocol was followed to detect the binding activity of GST-VEGF-D to VEGFR3/Fc. Results were shown as the means ± SEM of three wells.
Panning and functional characteristics of BDD073
To obtain mAbs that recognize the extracellular domain of VEGFR-3, we used VEGFR-3/Fc fusion protein that contained the full-length (Ig domains 1–7) extracellular region of human VEGFR-3 for immunization. After immunization with VEGFR-3/Fc, mice were sacrificed and the splenocytes from each mouse were fused to myeloma cells. Individual hybridomas were panned and 17 were positive for VEGFR-3, but not for human IgG. To further screen the antagonist antibodies to VEGFR-3, VEGFR-3/Fc-VEGF-D interaction system established above was used. Our results showed that antibodies BDD073 and BBE022 had the highest inhibitory activity (Fig. 2A); however, the clone of BBE022 lost the reactivity to VEGFR-3/Fc during the subcultures. To further confirm the neutralizing activity of BDD073, the binding activities of BAD045 (control antibody) and BDD073 at different concentrations to VEGFR-3 and GST-VEGF-D were evaluated. The results showed that BDD073 could inhibit the binding of VEGFR-3/Fc to immobilized GST-VEGF-D in a dose-dependent manner, indicating that the effect of BDD073 was specific. (Fig. 2B).
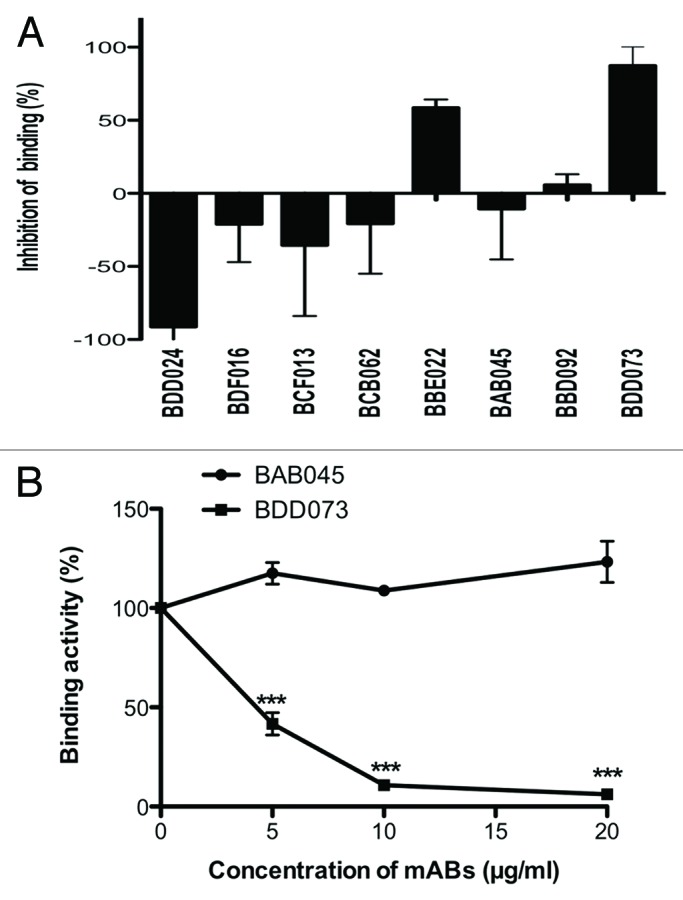
Figure 2. Screening and characterization of anti-VEGFR-3 monoclonal antibodies. (A) Inhibition of VEGFR-3/Fc binding to GST-VEGF-D by the mAbs. BBE022 and BDD073 had the inhibitory activities on VEGFR-3/Fc and GST-VEGF-D interaction. Results are shown as the means ± SEM of three wells. (B) Neutralizing activities of BDD073 to the binding activity of VEGFR-3 to GST-VEGF-D in a dose dependent manner. Various antibodies were mixed with 50 ng of VEGFR3/Fc, incubated at 37 °C for 1 h and transferred to 96-well microtiter plates coated with GST-VEGF-D, after an additional 1 h, the plate was washed three times and the standard ELISA protocol was followed to detect the bounded VEGFR3/Fc molecules. BAD045 antibody was used as control. Results are shown as the means ± SEM of three wells.
mAb BDD073 significantly inhibits GST-VEGFD-induced proliferation
The specificity of BDD073 was further confirmed by fluorescence-activated cell sorting (FACS) analysis. As shown in Figure 3A, localization of VEGFR-3 on the plasma membrane of human erythroleukemia (HEL) cells was detected by FACS analysis. In our previous study, the cell viability of HEL cells could be stimulated by GST-VEGF-D in a dose-dependent manner;15 therefore, we used this system to further validate the neutralizing effects of BDD073 on VEGFR-3 in HEL cells. MTS assay was used to detect the inhibitory effects of BDD073 on GST-VEGF-D induced-proliferation in HEL cells. As shown in Figure 3B, BDD073 antibody exhibited a dose-dependent inhibitory effect on VEGF-D-induced proliferation in HEL cells. In addition, it has been reported that VEGF-D could stimulate cell growth in angiogenesis.16 To further evaluate the effects of BDD073, we determined the inhibitory capability in human umbilical vein endothelial (HUVEC) cells by MTS assay. The results showed that BDD073 significantly decreased the cell viability of HUVEC cells that were induced by recombinant VEGF-D (Fig. 3C).
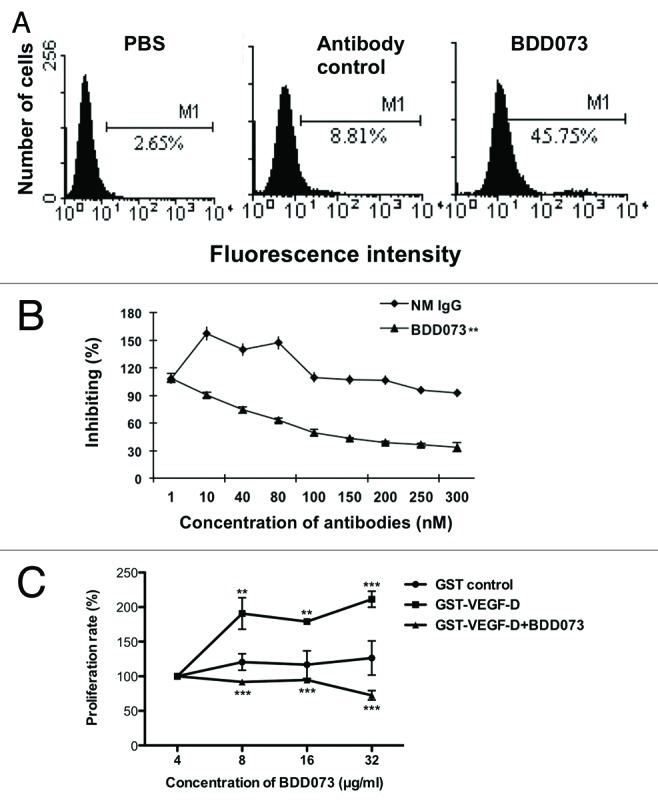
Figure 3. Effects of BDD073 on cell viability of HEL cells. (A) Representative charts showing BDD073 could recognize the VEGFR-3 on the plasma membrane of HEL cells by FACS. (B) Dose-dependent inhibition of GST-VEGFD-induced HEL cell viability was observed by treatment of BDD073. Statistical analysis of cell viability of HEL cells. Values represent the mean ± SEM (**, p < 0.01). (C) GST-VEGFD-induced proliferation of HUVEC cells was inhibited by BDD073. Values represent the mean+SEM (**P < 0.01; ***P < 0.001).
mAb BDD073 partially suppresses GST-VEGF-D induced angiogenesis
The chick CAM is an extra-embryonic membrane that serves as a gas exchange surface. Because of a dense network of lymphatics accompanying the arteries and veins, the CAM has been broadly used to investigate the angiogenetic and lymphatic development and tumor angiogenesis.17,18 In the present study, we used the chick CAM model to determine the inhibitory effects of BDD073 on VEGF-D-induced angiogenesis. Our results demonstrated that 20 μg/ml GST-VEGF-D dramatically induced angiogenesis, as illustrated by the significant increase of microvessels in the GST-VEGF-D-treated CAM. In the presence of BDD073, however, CAM angiogenesis induced by GST-VEGF-D was partially inhibited by the antibody compared with the control antibody-induced angiogenesis, suggesting the neutralizing activity of BDD073 to VEGFR-3 (Fig. 4).
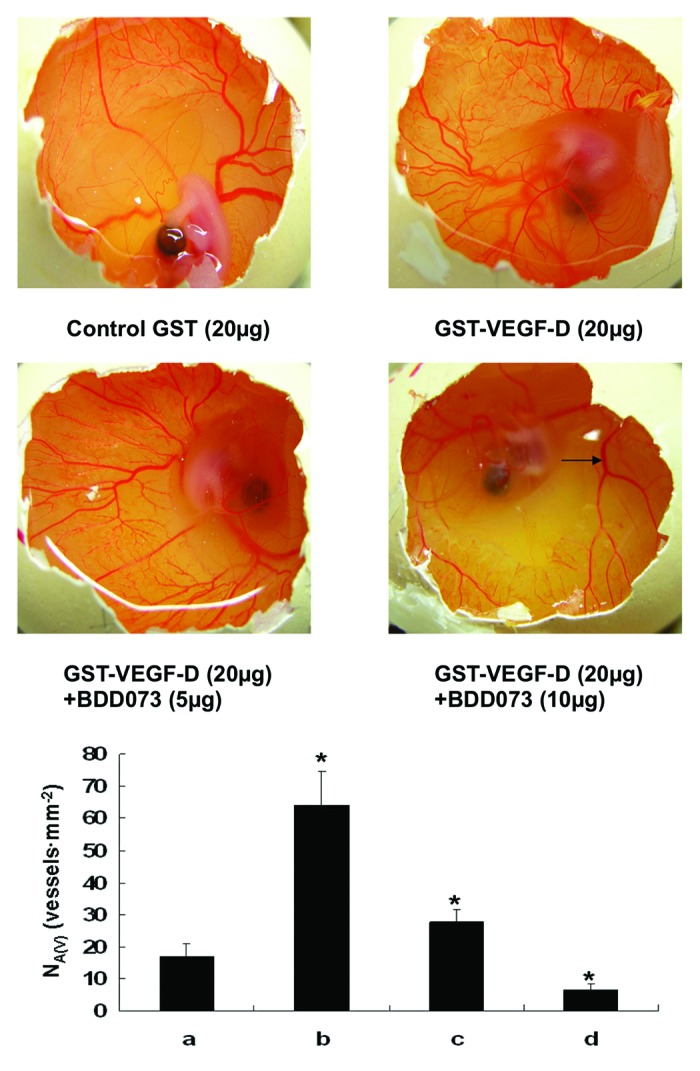
Figure 4. Angiogenesis inhibition of the chick CAM induced by BDD073 (n = 8). The development of micro-vessels was induced by 20 µg GST-VEGF-D. Note that the number of micro vessels was reduced by the presence of BDD073 in a dose-dependent manner. It suggested that BDD073 have neutralizing activities in the chick CAM (*, P < 0.05). a. Control GST (20µg), b. GST-VEGF-D (20 µg), c. GST-VEGF-D (20 µg) + BDD073 (5 µg), d. GST-VEGF-D (20 µg) + BDD073 (10 µg).
Discussion
Although VEGFR-3 plays a critical role in the development of embryonic vascular system, its expression is restricted in endothelial cells of lymphatic vessels and specialized fenestrated capillaries postnatally.19 Consistently, unlike VEGFR-1 and VEGFR-2, VEGFR-3 becomes a lymphatic marker due to its specificity and role in lymphangiogenesis and lymphatic metastasis. On the other hand, given the key importance of VEGF and its receptor in angiogenesis and lymphangiogenesis, VEGF, and VEGF receptor antagonists have been expected to eliminate the tumor vasculature and suppress metastasis.14 Indeed, anti-VEGF bevacizumab20,21 and the second-generation multi-targeted receptor tyrosine kinase inhibitors sunitinib22,23 and sorafenib24,25 have been shown to prolong the life of cancer patients. In this report, we generated the specific neutralizing antibody BDD073 to VEGFR-3, which could recognize the VEGFR-3 expressed on HEL cells (Fig. S1) and the vascular endothelial both in the fetal heart and kidney (Fig. S2).
In ELISA assay, BDD073 showed high binding activity to VEGFR-3. Furthermore, BDD073 suppressed GST-VEGF-D-induced HEL and HUVEC cell viability and CAM angiogenesis. Taken together, these results suggested that BDD073 could be a potential VEGFR-3 antagonist mAb after being humanized.
VEGF-C and VEGF-D are two well-characterized ligands for VEGFR-3 that have the highest affinity for VEGFR-3 and VEGFR-2 when they are fully processed.26 In our experiments, we used the fusion GST-VEGF-D (mature form of VEGF-D) as a stimulator to investigate the inhibitory effects of BDD073 on HEL cell viability. The results indicated that BDD073 could significantly, but not completely, inhibit VEGF-D-enhanced cell viability in HEL cells. It should be noted that the mature form of VEGF-D binds to both VEGFR-3 and VEGFR-2, which is expressed on the surface of HEL cells as well.27 Thus, the observed partial inhibitory effect on VEGF-D-induced cell proliferation by BDD073 could be explained by the possibility that BDD073 specifically blocked the interaction of VEGF-D with VEGFR-3, but not VEGFR-2.
Although both VEGF-C and VEGF-D bind to and activate VEGFR-2 besides VEGFR-3, VEGF-D promotes tumor angiogenesis whereas VEGF-C essentially does not. This difference may be partly explained by the fact that the 31 kDa form of VEGF-C preferentially binds to VEGFR-3, whereas the fully processed 21 kDa form can barely be detected.28 Hence, fully processed VEGF-D was used to activate the endothelial cells proliferation and angiogenesis in the chick embryo CAM model. In the present study, cell viability of HUVEC cells induced with GST-VEGF-D was obviously inhibited by BDD073, suggesting its potential neutralizing capacity. Consistent with the cell growth, similar results were achieved in the experiments of chick CAM angiogenesis: angiogenesis induced by VEGF-D was partially inhibited by neutralizing anti-VEGFR-3 antibody (BDD073).
In this study, we produced a panel of mAbs that target VEGFR-3 and one of them (BDD073) was shown to exhibit neutralizing effects. Functional studies demonstrated that BDD073 antibody could antagonize the binding activity between the VEGFR-3 and GST-VEGF-D, and inhibit the cell viability of HEL and HUVEC cells. Furthermore, BDD073 has inhibitory effects on VEGF-D induced angiogenesis in a chick embryo CAM model. These results suggest that BDD073 may be a useful research tool for investigating the mechanisms of VEGFR-3 signaling. Humanization of the antibody may provide a therapeutic molecular agent for the treatment of cancer metastases.
Materials and Methods
Animals
Six-to-eight week male Balb/c mice were used in this study. All experiments were performed under a license from the guiding principles of China National law for animal usage in Medical Research.
Reagents
Soluble recombinant human VEGFR3/Fc (R&D Systems, 349-F4–050) and anti-human VEGF-D antibody (R&D Systems, MAB2861) were purchased from R&D Systems. pGEX-5x-1 plasmid (GE healthcare, 28-9545-53) and GST-affinity chromatography media (GE healthcare, 17-5132-01) were used for recombinant protein purification.
Cell culture
HEL cells were purchased from the Academy of Chinese Medical Science and cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin-Streptomycin and maintained in a 5% CO2 incubator at 37 °C. HUVEC cells were cultured in F12K medium with 10% FBS, 6 U/ml heparin and 30 μg/ml endothelial cell growth supplement.
Expression of GST-VEGF-D fusion protein
cDNA encoding the mature form of VEGF-D was prepared by polymerase chain reaction and cloned into the EcoR I and Not I restriction sites of plasmid pGEX-5x-1. The GST-VEGF-D fusion protein was expressed in transformed E.coli BL21-DE3 and purified using GST affinity chromatography as described previously.15 The GST-alone vector protein was used as control.
Western blot
Total lysates of protein (10 μg per lane) at different groups were subjected to SDS-PAGE and transferred onto nitrocellulose membranes (Pall life science, 66485). The transferred membrane was blocked by Tris-buffered saline with Tween-20 (TBST) plus 5% fat-free milk. The membrane was then washed 3 times with TBST and incubated with different clones of anti-VEGFR-3 antibodies in TBST plus 1.5% fat-free milk at 4 °C for overnight. The membrane was subsequently washed with TBST and incubated for 1 h with peroxidase-conjugated secondary antibody. The membrane was washed 3 times with TBST and then detected by enhanced chemiluminescence (Amersham, RPN2232).
Generation of antibodies to human VEGFR-3
Four Balb/C mice were immunized with VEGFR-3/Fc protein. Splenocytes from two of the mice demonstrating the highest antibody titer in an enzyme linked immunosorbent assay (ELISA) of VEGFR-3/Fc were fused to mouse myeloma cells (SP2/0) following standard procedures as previously described.29 Ten days after the fusion, the supernatants were harvested and screened for antibody production by ELISA. The supernatants of selected hybridoma clones were purified by protein G Sepharose 4 Fast Flow column (GE healthcare, 17-0618-01).
ELISA-based on VEGFR-3/VEGF-D interaction
For screening of anti-VEGFR-3 clones, 1 μg recombinant VEGFR-3/Fc was coated into the 96-well plates. These pre-coated wells were blocked with 1% bovine serum albumin (BSA) in PBS with 0.1% Tween 20 and incubated with 100 μl of supernatant from different clones of anti-VEGFR-3 for 1 h at room temperature (RT). Bound mAb was then detected with goat anti-mouse IgG coupled with horseradish peroxidase (HRP).
To examine GST-VEGF-D binding activity with VEGFR-3, microtiter plates were coated with 4 μg GST-VEGF-D in 100 mM carbonate buffer, pH 9.5, then blocked with 1% BSA in PBS with 0.1% Tween 20. Recombinant VEGFR-3/Fc with different concentration (1, 2, or 4 μg/ml) was incubated with 20 μg/ml GST-VEGF-D for 1 h at RT. The mixture of pre-incubated VEGFR-3/Fc/GST-VEGF-D was transferred into GST-VEGF-D-coated microtiter plates and incubated for another 1 h at 37 °C. Mouse-anti-VEGFR-3 antibody (R&D Systems, MAB3491) was used to bind VEGFR-3/Fc and absorbance was detected with anti-mouse secondary antibody coupled with HRP. The assays were quantified by reading the absorbance at 405 nm in a multi-well plate reader.
Competitive VEGF-D blocking assay
Various VEGFR-3 antibodies purified from different hybridoma clones were incubated with 50 ng of VEGFR-3/Fc at 37 °C for 1 h. The mixtures were then transferred into 96-well microtiter plate coated with GST-VEGF-D and incubated for 1 h. After that, the standard ELISA protocol described before was followed to examine the binding of VEGFR-3/Fc.
FACS analysis
HEL cells were fixed with 2% formaldehyde for 10 min, washed twice with PBS and then incubated with 10 μg/ml BDD073 in PBS plus 1% BSA for 1 h on ice. The cells were subsequently washed three times with PBS and secondary anti-mouse IgG FITC conjugated antibody was added. After 1 h at 4 °C, the cells were washed with PBS and the analysis was performed by flow cytometer (Becton–Dickinson) using Cell Quest Software.
Cell viability assay by MTS analysis
Cell viability was analyzed by CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega Corporation, G5421). HEL cells or HUVEC cells (1 × 104 per well) were seeded into a 96-well culture plate with growth medium (RPMI-1640 medium with 10% FBS). Cells were treated with either GST-VEGF-D or GST in the presence or absence of different concentrations of BDD073 for 72 h. Treatment with non-specific antibody was taken as control. The optical density (OD) was measured by Tecan Sunrise plate reader and analyzed with Prism 5.0.
Chick chorioallantoic membrane (CAM) angiogenesis assay
The CAM angiogenesis assay was performed as previously described.15,30 Briefly, oosperm eggs purchased from the Chinese Academy of Agricultural Sciences’ Institute of Animal Sciences, were used for the experiments. The eggs were incubated for three days at 39 °C with 70% of humidity, and then a round window was cut on the top of each egg. Twenty micrograms per milliliter of GST-VEGF-D or GST control were added in the presence or absence of the different concentration of BDD073. Seventy-two hours later, the CAM was excised. Angiogenesis was quantified by counting the density of blood vessel using MetaMorph Microscopy Automation and Image Analysis Software (Molecular Devices, LLC) and expressed as vessels/mm2.
Statistical analysis
All morphometric data were collected blindly. Statistics for comparison between two measurements was analyzed by unpaired or paired 2-tailed Student t-test. One way or two-way ANOVA was used for analysis involving more than two measurements. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
The work was supported in parts by National Natural Science Foundation of China (No. 31100841), National Basic Research Program of China (2014CB745200), Shenzhen City Science and Technology Project Medicine and Health (No. 201202035), Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (No. LYM11112), Li Ka Shing Institute of Health Sciences, and the Focused Investment Scheme of the Chinese University of Hong Kong.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/mabs/article/26239.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/26239
References
- 1.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–34. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 2.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–37. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 3.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–70. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–5. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 5.Valtola R, Salven P, Heikkilä P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–90. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korpelainen EI, Alitalo K. Signaling angiogenesis and lymphangiogenesis. Curr Opin Cell Biol. 1998;10:159–64. doi: 10.1016/S0955-0674(98)80137-3. [DOI] [PubMed] [Google Scholar]
- 7.Choi JH, Oh YH, Park YW, Baik HK, Lee YY, Kim IS. Correlation of vascular endothelial growth factor-D expression and VEGFR-3-positive vessel density with lymph node metastasis in gastric carcinoma. J Korean Med Sci. 2008;23:592–7. doi: 10.3346/jkms.2008.23.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakisaka N, Hirota K, Kondo S, Sawada-Kitamura S, Endo K, Murono S, Yoshizaki T. Induction of lymphangiogenesis through vascular endothelial growth factor-C/vascular endothelial growth factor receptor 3 axis and its correlation with lymph node metastasis in nasopharyngeal carcinoma. Oral Oncol. 2012;48:703–8. doi: 10.1016/j.oraloncology.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–95. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–25. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–9. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 13.Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, et al. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–9. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Bohlen P, Witte L. Clinical development of angiogenesis inhibitors to vascular endothelial growth factor and its receptors as cancer therapeutics. Curr Cancer Drug Targets. 2002;2:135–56. doi: 10.2174/1568009023333881. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Ding XY, Gao Y, Liu XL, Gao JE, Sun QH. [Recombinant human VEGF-D induces the angiogenesis of the chick embryo chorioallantoic membrane] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:364–8. [PubMed] [Google Scholar]
- 16.Papiewska-Pajak I, Boncela J, Przygodzka P, Cierniewski CS. Autocrine effects of VEGF-D on endothelial cells after transduction with AD-VEGF-D(DeltaNDeltaC) Exp Cell Res. 2010;316:907–14. doi: 10.1016/j.yexcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Ribatti D. Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis. Int Rev Cell Mol Biol. 2008;270:181–224. doi: 10.1016/S1937-6448(08)01405-6. [DOI] [PubMed] [Google Scholar]
- 18.Tufan AC, Satiroglu-Tufan NL. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr Cancer Drug Targets. 2005;5:249–66. doi: 10.2174/1568009054064624. [DOI] [PubMed] [Google Scholar]
- 19.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–9. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 21.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 22.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 25.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. TARGET Study Group Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 26.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–83. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 27.Schuch G, Machluf M, Bartsch G, Jr., Nomi M, Richard H, Atala A, Soker S. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002;100:4622–8. doi: 10.1182/blood.V100.13.4622. [DOI] [PubMed] [Google Scholar]
- 28.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Gao Y, Ju Y, Yang J, Wu Q, Zhang J, Du X, Wang Z, Song Y, Li H, et al. Proteomics-based generation and characterization of monoclonal antibodies against human liver mitochondrial proteins. Proteomics. 2006;6:427–37. doi: 10.1002/pmic.200500409. [DOI] [PubMed] [Google Scholar]
- 30.Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM. Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Proc Natl Acad Sci U S A. 2000;97:12227–32. doi: 10.1073/pnas.220399597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


