Abstract
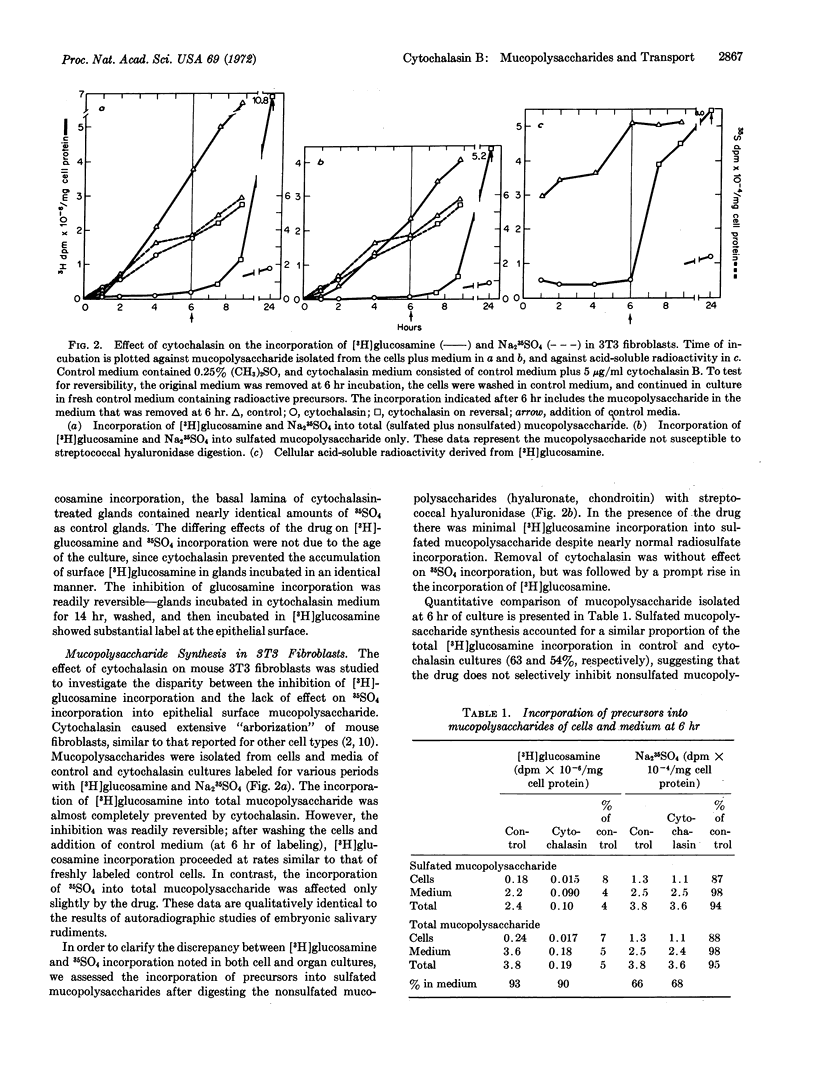
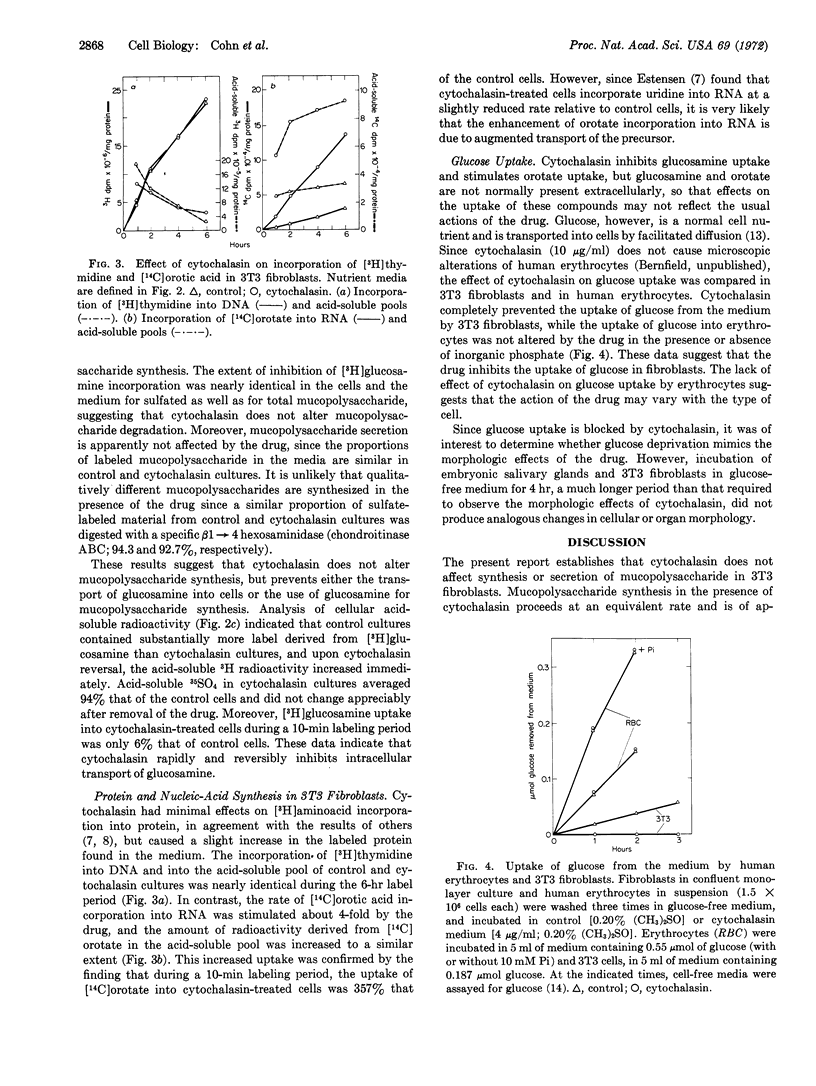
Synthesis and secretion of mucopolysaccharide in mouse 3T3 fibroblasts and in embryonic submandibular glands are unaffected by amounts of cytochalasin B that alter the morphology of these cells and tissues. The drug markedly and reversibly inhibits incorporation of [3H]glucosamine into mucopolysaccharide by preventing cellular uptake of the precursor, but does not affect incorporation of radiosulfate. Cytochalasin does not alter DNA, RNA, or protein synthesis, but stimulates the uptake of orotic acid and markedly inhibits the uptake of glucose. These selective effects on the transport of small molecules suggest that the primary action of the drug may be on cell membranes. Since processes unrelated to microfilament disruption may be altered by cytochalasin, great caution must be exercised in interpreting studies with the drug.
Keywords: glucose transport; DNA, RNA, and protein synthesis; morphogenesis; microfilaments; plasma membrane
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernfield M. R., Banerjee S. D. Acid mucopolysaccharide (glycosaminoglycan) at the epithelial-mesenchymal interface of mouse embryo salivary glands. J Cell Biol. 1972 Mar;52(3):664–673. doi: 10.1083/jcb.52.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M. R., Banerjee S. D., Cohn R. H. Dependence of salivary epithelial morphology and branching morphogenesis upon acid mucopolysaccharide-protein (proteoglycan) at the epithelial surface. J Cell Biol. 1972 Mar;52(3):674–689. doi: 10.1083/jcb.52.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluemink J. G. Cytokinesis and cytochalasin-induced furrow regression in the first-cleavage zygote of Xenopus laevis. Z Zellforsch Mikrosk Anat. 1971;121(1):102–126. doi: 10.1007/BF00330921. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Ebstensen R. D., Plagemann P. G. Cytochalasin B: inhibition of glucose and glucosamine transport. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1430–1434. doi: 10.1073/pnas.69.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D. Cytochalasin B. I. Effect on cytokinesis of Novikoff hepatoma cells. Proc Soc Exp Biol Med. 1971 Apr;136(4):1256–1260. doi: 10.3181/00379727-136-35470. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The effects of cytochalasin B on the microfilaments of baby hamster kidney (BHK-21) cells. J Cell Biol. 1972 Feb;52(2):246–254. doi: 10.1083/jcb.52.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F., Springer A. Cytochalasin A and B. Inhibition of sugar uptake in cultured cells. J Biol Chem. 1972 May 10;247(9):2964–2966. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Sanger J. W., Holtzer H. Cytochalasin B: effects on cell morphology, cell adhesion, and mucopolysaccharide synthesis (cultured cells-contractile microfilaments-glycoproteins-embryonic cells-sorting-out). Proc Natl Acad Sci U S A. 1972 Jan;69(1):253–257. doi: 10.1073/pnas.69.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. Cytokinesis: filaments in the cleavage furrow. Exp Cell Res. 1968 Oct;53(1):272–276. doi: 10.1016/0014-4827(68)90373-x. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Wessells N. K. An analysis of salivary gland morphogenesis: role of cytoplasmic microfilaments and microtubules. Dev Biol. 1972 Jan;27(1):38–54. doi: 10.1016/0012-1606(72)90111-x. [DOI] [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wrenn J. T., Wessells N. K. Cytochalasin B: effects upon microfilaments involved in morphogenesis of estrogen-induced glands of oviduct. Proc Natl Acad Sci U S A. 1970 Jul;66(3):904–908. doi: 10.1073/pnas.66.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Axon growth: roles of microfilaments and microtubules. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]



