Abstract
Landmark studies have shown that mutations in kisspeptin and the kisspeptin receptor (Kiss1r) result in reproductive dysfunction in humans and genetically altered mouse models. However, because kisspeptin and its receptor are present in target cells of the central and peripheral reproductive axis, the precise location(s) for the pathogenic signal is unknown. The study described herein shows that the kisspeptin-Kiss1r signaling pathway in the GnRH neuron is singularly critical for both the onset of puberty as well as the attainment of normal reproductive function. In this study, we directly test the hypothesis that kisspeptin neurons regulate GnRH secretion through the activation of Kiss1r on the plasma membrane of GnRH neurons. A GnRH neuron–specific Kiss1r knockout mouse model (GKirKO) was generated, and reproductive development and phenotype were assessed. Both female and male GKirKO mice were infertile, having low serum LH and FSH levels. External abnormalities such as microphallus and decreased anogenital distance associated with failure of preputial gland separation were present in GKirKO males. A delay in pubertal onset and abnormal estrous cyclicity were observed in female GKirKO mice. Taken together, these data provide in vivo evidence that Kiss1r in GnRH neurons is critical for reproductive development and fertility.
During the onset of puberty, a cascading rush of neuroendocrine hormones acting on the hypothalamus stimulates GnRH neurons to secrete GnRH, which leads to pubertal onset and, consequently, gametogenesis and production of sex steroids in the gonads in both males and females. Numerous factors have been implicated in the activation and regulation of this hypothalamo-pituitary-gonadal (HPG) axis. One of the recently emerging players is kisspeptin, which belongs to a family of neuropeptides shown to be powerful stimulators of GnRH synthesis and secretion in mammals (1–11). Kisspeptin neurons localized in the arcuate (ARC) nucleus and preoptic area in humans (12, 13) and in the ARC nucleus and anteroventral periventricular (AVPV) nucleus in rodents (5, 14–18), project a neuronal network interconnected by axons and/or dendrites to induce secretion of GnRH. Present on most GnRH neurons is kisspeptin G protein–coupled receptor (Gpr54/Kiss1r) (6, 15, 19, 20), which binds to kisspeptin and initiates cell depolarization and GnRH secretion (15, 21–23). In humans, the vital role of kisspeptin regulation of the GnRH pathway is underscored in individuals carrying inactivating mutations in KISS1R, who demonstrate normosmic idiopathic hypogonadotrophic hypogonadism (nIHH) (4, 24–29), a disorder that results in failure of sexual maturity and infertility (30, 31). Similarly, in rodents a number of studies have shown that either deletion or loss-of-function mutations in the Kiss1 or Kiss1r gene result in various degrees of hypogonadotrophism, hypogonadism, and infertility (3, 4, 10, 32). In both humans and rodents, treatment using exogenous kisspeptins has been used to induce GnRH secretion and stimulate the reproductive endocrine cascade, demonstrating the potential applications of this group of peptides as a novel therapeutic target (7, 15, 33–35). These findings demonstrate that proper functioning of kisspeptin and its receptor plays a crucial role in the regulation of the reproductive system including gonadotropin secretion, pubertal onset, ovulation, and fertility (4, 36–40).
Despite these advances, only one recent study has explored the effect of kisspeptin signaling directly at the level of the GnRH neuron on mammalian reproductive function (41). In the present study, a mouse model with a deletion of the Kiss1r gene only in the GnRH neuron (GKirKO) is also used to determine the role of kisspeptin signaling directly at the level of the GnRH neuron. We assess the reproductive phenotype of these knockout mice, analyzing key indicators of reproductive competence such as pubertal onset, estrous cyclicity, basal gonadotropin levels, and fertility. The implications of identifying the precise signaling of kisspeptin on the GnRH neuron greatly contribute to our continuously evolving understanding of the mammalian HPG axis and identifies potential targets for pharmacologic treatment of nIHH.
Materials and Methods
Animals
Generation of Kiss1r “floxed” mice to produce a conditional Kiss1r null mutation in GnRH neurons
Floxed Kiss1r mice were designed with LoxP sites flanking exon 2 of the kisspeptin receptor (Kiss1r) in a targeting construct that also included a Frt flanked neomycinr selection cassette. A targeting vector was produced by recombineering and was electroporated into embryonic stem cells. Six positive targeted clones were obtained. The Kiss1r floxed animals were crossed to the GnRH-Cre mice described previously by our laboratory (42–45). Cre recombinase expression in GnRH neurons produced a cell-specific Kiss1r knockout (GKirKO). A similar strategy was used to target the gonadotroph using the common α-subunit gene (46). All of the mice used in these experiments were maintained on a mixed CD1/129SvJ/C57BL6 genetic background, and each genotypic or experimental group was compared with littermate controls carrying either the floxed Kiss1r gene without the Cre gene, the Cre gene without the floxed Kiss1r gene, or neither the Cre gene nor the floxed Kiss1r allele. These littermate controls are referred to as “wild-type (WT)” mice throughout this article. All procedures were performed under standard light and dark cycles with approval of the Johns Hopkins Animal Care and Use Committee. Before all experiments, mice were anesthetized with isoflurane (Penn Veterinary Supply), and blood samples were obtained via mandibular blood samples or ocular blood samples in the case of terminal studies.
Generation of GnRH-specific Kiss1r knockout
Fl-Kiss1r mice (Kiss1rWT/fl) were crossed with GnRH-Cre, Kiss1rWT/fl mice to create a GnRH-specific Kiss1r knockout mouse (GKirKO). After dissection and harvesting of tissue, genomic DNA was isolated using phenol-chloroform extraction and isopropanol precipitation. To determine the presence of the Kiss1r floxed allele, WT allele, and Cre transgene, DNA was subjected to PCR analysis. The genotyping primers designed to detect the presence of the floxed allele, WT allele, or knockout recombination were as follows: P1 sense (located in exon 1), 5′-CTGGTCGGAAACTCATTGGT-3′; and P3 antisense (located in exon 3), 5′-AGAGTGGCACATGTGGCTTG-3′. Primers P1 and P3 were designed to produce a 2096-bp band to indicate the floxed Kiss1r allele and a 1882-bp band to indicate the WT allele in DNA obtained from extrahypothalamic organs (eg, liver, muscle, ovary, testes, pituitary, and tail). These primers were designed to produce a 1120-bp band after excision of the sequence between the LoxP sites (the knockout allele) in DNA extracted from the hypothalamus (a tissue that expresses Cre recombinase). Genotyping primers used to determine the presence of the Cre recombinase gene were Cre sense, 5′-CGACCAAGTGACAGCAATGCT-3′, and Cre antisense, (5′GGTGCTAACCAGCGTTTTCGT-3′, and have been described elsewhere (42, 44).
RT-quantitative PCR (qPCR)
Real-time qPCR was performed to determine the presence and expression levels of Kiss1r mRNA in various tissues and Kiss1 mRNA in the AVPV nucleus and ARC nucleus in ovariectomized (OVX) and intact mice. RNA was isolated from mouse tissues using TRIzol reagent (Invitrogen), according to the protocol provided by the supplier. Then 2 μg of RNA was reverse transcribed using an iScript cDNA kit (Bio-Rad Laboratories). Real-time qPCR was performed in duplicate using the SYBR Green Master Mix (Bio-Rad Laboratories) and the ICycler qPCR machine (Bio-Rad Laboratories). The primers used to amplify Kiss1r were sense primer, 5′-CTGCCACAGACGTCACTTTC-3′, and antisense primer, 5′-ACATACCAGCGGTCCACACT-3′ (47, 48). The primers used to amplify Kiss1 were sense primer, 5′-AGCTGCTGCTTCTCCTCTGT-3′, and antisense primer, 5′-GCATACCGCGATTCCTTTT-3′. 18S RNA was used as an internal control for cDNA input, sense primer, 5′-TGGTTGATCCTGCCAGTAG-3′, and antisense primer, 5′-CGACCAAAGGAACCATAACT-3′. To determine PCR efficiency, a 10-fold serial dilution of cDNA was performed as described previously (49). PCR conditions were optimized to generate >95% PCR efficiency, and only those reactions with between 95% and 105% efficiency were included in subsequent analyses. Relative differences in cDNA concentration between WT and GKirKO mice were then calculated using the comparative threshold cycle (Ct) method (50). In brief, to obtain differences between WT and knockout Kiss1r expression, a ΔCt was calculated: Ct(knockout) − Ct(wild-type). Relative mRNA levels were then calculated using the equation fold difference = 2ΔCt. To obtain the differences in Kiss1 expression in the AVPV nucleus and ARC nucleus, a ΔCt was calculated to normalize for the internal control using the equation: ΔCt = Ct(gene) − Ct(18S). To obtain differences between WT and knockout, ΔΔCt was calculated: ΔCt(knockout) − ΔCt(wild-type). Relative mRNA levels were then calculated using the equation fold difference = 2ΔΔCt.
Pubertal onset and estrous cycle assessment
Prepubertal female mice were examined every day for vaginal opening (VO) through visual examination of the vulva after postnatal day 21. Vaginal smears were collected daily at 10:00 am over a period of 12 days in 2- to 3-month-old mice and examined as stained preparations with a Diff-Quick stain kit (IMEB Inc) to determine the estrous cycle. The stage of the estrous cycle was determined and classified as proestrus, estrus, or metestrus/diestrus based on observed ratios of cornified epithelial, nucleated epithelial, and polymorphonuclear leukocytes as described in Nelson et al (51). The frequencies of the estrous cycles and the days spent in the different phases included in the estrous cycle were compared between the 2 groups (WT and GKirKO mice). After postnatal day 21, preputial separation (PPS) in males was assessed daily. This consisted of attempts to manually retract the prepuce with gentle pressure. PPS is testosterone dependent and thus is an indicator of activation of the reproductive axis in males (52). Puberty in rodents is dependent on weight (53); hence, the weights of GKirKO and control littermates were assessed in prepubertal mice through adulthood.
Hormonal assays
To measure basal levels of serum LH and FSH and serum LH levels after GnRH or kisspeptin stimulation, samples were collected from mice via a mandibular blood sample between 9:00 and 10:00 am to avoid cycle-dependent LH surges that occur in the evening of proestrus. LH and FSH were measured using a Milliplex MAP immunoassay (Mouse Pituitary panel; Millipore) on a Luminex 200IS platform (Luminex Corporation). The GnRH stimulation or kisspeptin stimulation tests were performed on different days, and serum LH was evaluated. Blood was collected 20 minutes after sc injection of 100 ng/kg GnRH (Sigma L7134 LHRH human acetate salt) dissolved in normal saline (54) or 10 minutes after sc injection of 1 nmol of kisspeptin-10 (EMD Biosciences, Inc) dissolved in normal saline (55, 56). Absolute values and fold change in serum LH was assessed. All samples were assayed on 1 plate. A standard curve was generated using 5-fold serial dilutions of the hormone reference provided by Millipore. Low- and high-quality controls were also run on each assay to assess coefficient of variation values. The assay detection limit for LH was 0.012 ng/mL and for FSH was 0.061 ng/mL. The intra-assay coefficient of variation for each assay was between 5% and 9%. All procedures were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee.
Fertility assessment
To determine whether female and male GKirKO mice were fertile, six WT and six GKirKO female mice were housed with either a WT or GKirKO male mouse for 14 consecutive days (2 females and 1 male/cage) and then were separated. Three males of each genotype (only 3 GKirKO males of 15 had preputial separation and could be used for mating) were rotated between the WT or GKirKO females, and each female participated in at least 6 attempts at mating with at least 5 weeks between mating attempts if pregnancy was achieved. At the end of these fertility studies, female and male GKirKO mice were housed continuously with another WT animal of the opposite gender for 4 months.
Anatomy and histology
Ovaries (collected at metestrus or diestrus) and testes were fixed in 10% buffered formalin phosphate (Fisher Scientific) solution and stored at 4°C. Paraffin-embedded organs were sectioned at 7-μm thickness. Ovarian and testicular sections were stained with hematoxylin and eosin, examined with a Zeiss microscope, and photographed with an AxioCamICc1 camera and exported to AxioVision Software. Wet testicular weights were determined in freshly dissected animals.
Evaluation of negative feedback by sex steroids
To evaluate the negative feedback by sex steroids, 2- to 3-month-old female mice were anesthetized with ketamine-xylazine, and an ovariectomy was performed via a dorsal incision. After a 14-day recovery period, blood was collected by heart puncture, and sera were separated by centrifugation at 4000 × g for 15 minutes at 4°C and stored at −80°C until LH and FSH were measured as described above. For Kiss1 mRNA analysis by RT-qPCR in the AVPV nucleus and ARC nucleus, mice were decapitated, and brains were dissected following the protocol described by Quennell et al (57). Morning serum samples from WT control mice were obtained from nonbreeding, postpubertal mice in the metestrus phase, and serum LH and FSH hormone levels were determined and compared with intact GKirKO and OVX WT control and GKirKO mice.
Statistical analysis
Data were analyzed and graphed using the GraphPad Prism 4 program (GraphPad Software, Inc). Values are expressed as means ± SEM. If data satisfied Bartlett tests for equal variances, parametric statistics such as the Student t test were used to determine significance of differences between control and GKirKO mice. A one-way ANOVA with a Bonferroni posttest was used when 3 or more groups were compared. A two-way ANOVA (group vs treatment) with a Bonferroni posttest was used to analyze LH levels in the experiments in Figure 3, F–I, and LH and FSH levels in Figure 6. Significance was assigned for a value of P ≤ .05.
Figure 3.
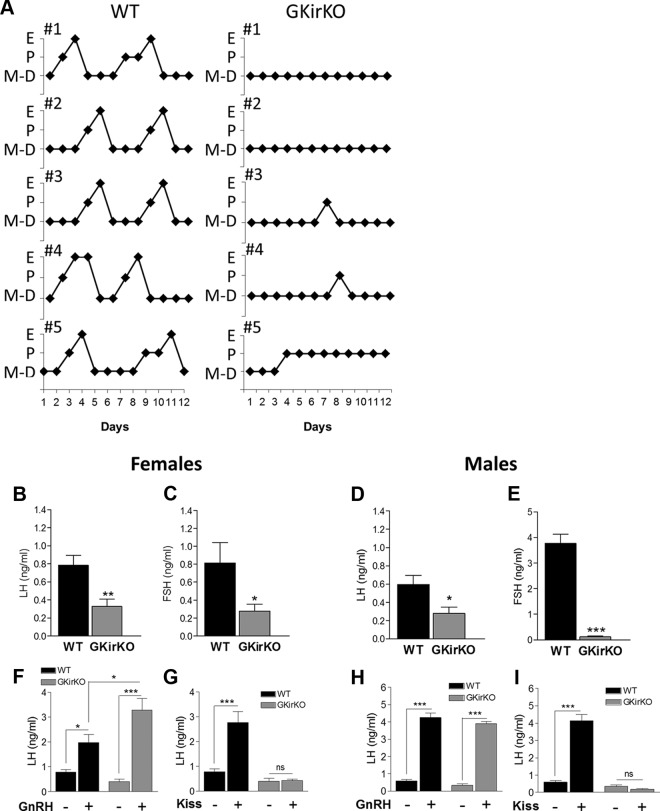
GKirKO mice have an abnormal estrous cycle. A, Graphic representation of the estrous cycle in WT and GKirKO mice determined by vaginal cytology followed for 12 days (n = 5). B–E, Baseline serum LH and FSH levels in female (left, n = 6–8) and male (right, n = 3–10) mice. Assay detection limit = 0.048 ng/mL. F and H, GnRH stimulation test. Evaluation of serum LH levels 20 minutes after injection of GnRH agonist (0.1 ng/g via ip). Increased serum LH levels in mice treated with GnRH agonist (indicated as GnRH +) was observed in both genders of WT and GKirKO (n = 7–9). G and I, Kisspeptin stimulation test. Evaluation of serum LH levels 10 minutes after injection of kisspeptin-10 (1 nmol ip). No LH response to kisspeptin (indicated as Kiss +) was observed in female (n = 7–8) or male (n = 3–10) GKirKO mice. Significant differences compared with saline-treated control groups (indicated as GnRH − or Kiss −): *, P ≤ .05; **, P ≤ .01; ***, P ≤ .001. Two-way ANOVA showed an interaction between genotype and treatment in F (P ≤ .05), G (P ≤ .01), and I (P ≤ .001), an effect of treatment in F (P ≤ .01), G (P ≤ .05), H (P ≤ .001), and I (P ≤ .001) and an effect of genotype in G (P ≤ .001) and I (P ≤ .001).
Figure 6.
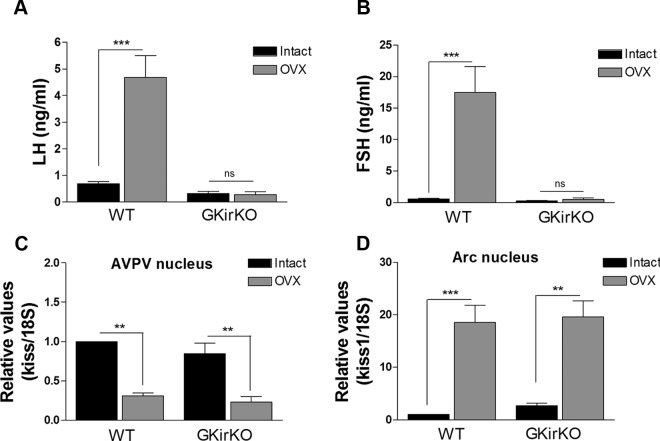
Reduced estradiol negative feedback in GKirKO mice. A and B, Serum LH and FSH levels in intact WT control mice in metestrus and intact GKirKO mice (noncycling) compared with OVX WT control mice and OVX GKirKO mice, respectively (n = 4–11). C and D, Kiss1 mRNA in AVPV nucleus and ARC nucleus of intact WT control mice in metestrus and intact GKirKO mice compared with OVX WT control mice and OVX GKirKO mice, respectively (n = 4–5). Significant differences compared with intact WT and GKirKO control groups: *, P ≤ .05; **, P ≤ .01; ***, P ≤ .001. Two-way ANOVA showed an interaction between genotype and treatment in A (P ≤ .001) and B (P ≤ .001), an effect of treatment in A, B, C, and D (P ≤ .001) and an effect of genotype in A and B (P ≤ .001).
Results
Generation of GnRH neuron–specific Kiss1r knockout mice
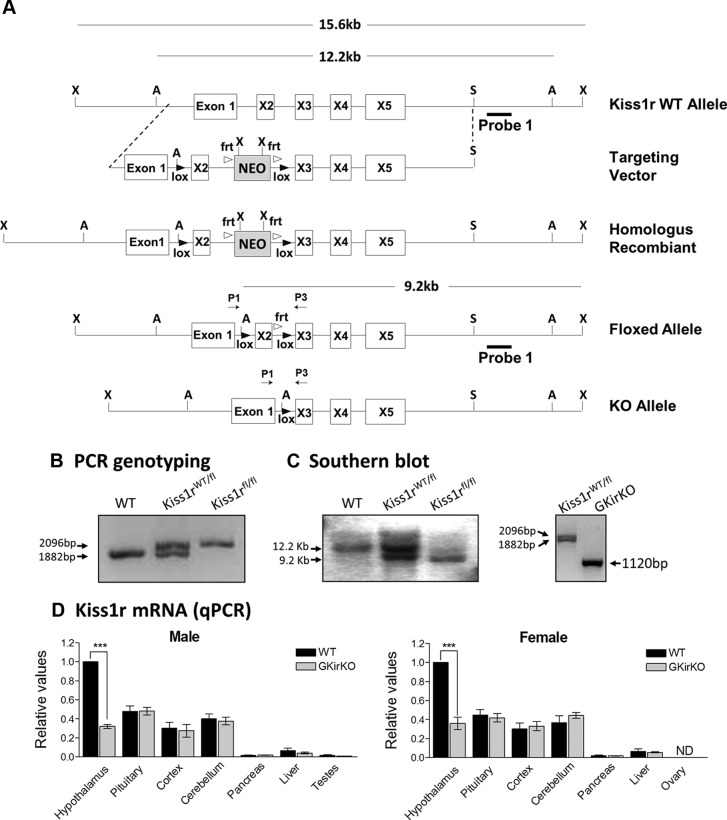
GKirKO mice were generated by breeding homozygous floxed Kiss1r (Kiss1rfl/fl) mice with mice expressing Cre recombinase (Cre+/−) under the control of the GnRH promoter to target the hypothalamic GnRH neurons. Kiss1rWT/fl/Cre+/− mice were mated with Kiss1rfl/fl mice to obtain the GKirKO (Kiss1rfl/fl/Cre+/−) mice. Upon expression of the Cre recombinase enzyme, exon 2 of the Kiss1r is excised, resulting in loss of function of the Kiss1r. Mice were genotyped using a PCR strategy schematized in Figure 1A. The PCR product (using primers P1 and P3) indicates the homozygous floxed-Kiss1r alleles (2096 bp) and WT alleles (1882 bp); both bands are present in the heterozygous floxed-Kiss1r mouse (Figure 1B). The appropriate chromosomal recombination was identified in the GKirKO mice by Southern blot analysis. DNA was digested with the Asp718I enzyme and hybridized with probe 1 to detect WT (12.2 kb), homozygous (9.2 kb), and heterozygous (both bands) alleles (Figure 1, A and C). GKirKO mice were born with the expected Mendelian frequency and were of normal size and weight.
Figure 1.
Development of GnRH-specific Kiss1r knockout mouse. A, Schematic diagrams of constructs used to generate GKirKO mice. Mice bearing LoxP sites flanking exon 2 of the Kiss1r were crossed with transgenic mice expressing Cre recombinase specifically in GnRH neurons. Primers used in PCR genotyping are labeled P1 (exon 1) and P3 (exon 3). X, XbaI; A, Asp718I; S, SpeI restriction enzyme sites. B, Genotyping by PCR analysis of the genomic DNA produced a band migrating at 2096 bp in the mice bearing a floxed allele and a band at 1882 bp in WT mice. These primers were designed to produce a 1120-bp band after excision of the sequence between the LoxP sites in DNA extracted from the hypothalamus (that is, tissue that express Cre recombinase). C, Southern blot analysis was performed by digesting the DNA with Asp718I and detected a band of 9.2 kb in the floxed allele and 12.2 kb in the WT allele with probe 1. D, qPCR analysis of Kiss1r mRNA extracted from male and female mouse tissues (P ≤ .001, n = 3). WT, wild-type; Kiss1rWT/fl, heterozygous floxed; Kiss1rfl/fl, homozygous floxed; ND, not detected.
Hypothalamic and tissue Kiss1r mRNA expression in GKirKO mice
As reported previously, mice bearing the GnRH Cre+/− transgene express Cre recombinase specifically in GnRH neurons (42–44). To document that Cre recombinase expression in GnRH neurons produced a cell-specific Kiss1r knockout, an RT reaction followed by qPCR analysis of RNA extracted from tissues of male and female mice was performed. RT-qPCR demonstrated a reduction in Kiss1r mRNA by 68% and 64% in the hypothalamus of male and female GKirKO mice, respectively, compared with that in WT mice. In contrast, no change in Kiss1r mRNA was observed in other tissues including the pituitary gland, cortex, cerebellum, liver, or gonads (P ≤ .001, n = 3) (Figure 1D).
External morphologic abnormalities of GKirKO mice
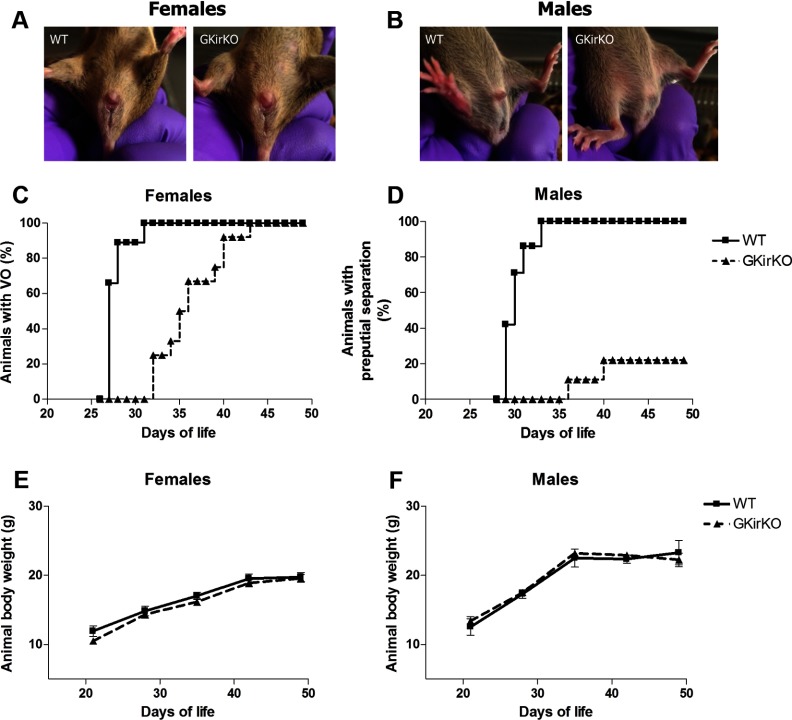
External anatomic abnormalities were not observed in GKirKO females compared with that of littermates (Figure 2A). In contrast, external anatomic abnormalities were observed in GKirKO males compared with those of littermates (Figure 2B). GKirKO males exhibited microphallus and a decreased anogenital distance, which are androgen-dependent processes (androgen exposure dependent) (Figure 2B).
Figure 2.
External anatomical abnormalities in GKirKO mice and puberty assessment. A, External anatomic abnormalities were not observed in GKirKO female mice. B, GKirKO males exhibited microphallus and decreased anogenital distance. Delayed puberty in GKirKO mice. C, Graphic representation of the time course of the day of VO in females (n = 5–12). D, Time course of the day of PPS in males (n = 7–15). E, Body weight change over time in female mice (n = 4–7). F, Body weight change over time in male mice (n = 9).
Pubertal onset is delayed in GKirKO mice
To assess the reproductive phenotype of the GKirKO mice, we assessed multiple parameters. Pubertal onset in GKirKO mice was assessed in females by VO and in males by PPS. VO was significantly delayed by approximately 9 days in GKirKO mice (postnatal day 36 ± 0.62, P ≤ .001, n = 12) (Figure 2C) compared with that in WT mice (postnatal day 27 ± 0.20, n = 5) (Figure 2C). This could not be attributable to differences in body weight, because there was no difference in weight between WT and GKirKO animals (n = 9) (Figure 2E). In 20% of GKirKO males, a delay of 7 to 11 days in PPS was observed (postnatal day 39 ± 0.89, P ≤ .001, n = 15) (Figure 2D) compared with that in WT mice (postnatal day 30.14 ± 0.55, n = 7) (Figure 2D). The remaining 80% failed to exhibit preputial gland separation. This was again not related to differences in the body weight (n = 4–7) (Figure 2F). Littermate controls carrying either the floxed Kiss1r gene without the Cre gene, the Cre gene without the floxed Kiss1r gene, or neither the Cre gene nor the floxed Kiss1r allele had normal VO and PPS.
GKirKO female mice have abnormal estrous cycles
To characterize further the reproductive phenotype exhibited by GKirKO females, estrous cyclicity was evaluated. All animals were housed according to their genotype. Vaginal smears were collected daily over a period of 12 days in 2- to 3-month-old mice and examined under the microscope to determine the stage of the estrous cycle. Control mice had an average cycle length of 5.67 ± 0.33 days (n = 6) (Figure 3A). In contrast, GKirKO mice showed an absence of normal estrous cycling, making the calculation of cycle length not possible (n = 6) (Figure 3A). GKirKO mice were found in metestrus/diestrus 83% of the time, with occasional evidence of proestrus (4%), which was a significantly different pattern than that observed in control animals (Figure 3A). In addition, 1 GKirKO female mouse was found in proestrus 67% of the time. Hence, GKirKO mice demonstrated an extensively abnormal pattern of estrous cyclicity.
GKirKO mice have low basal serum LH and FSH levels
Morning serum samples were obtained from nonbreeding, postpubertal mice and serum LH and FSH hormone levels were determined. Serum LH values in GKirKO mice were significantly lower in both sexes compared with those in WT mice (GKirKO female: 0.33 ± 0.08 ng/mL, P ≤ .01, n = 8; GKirKO male: 0.28 ± 0.07 ng/mL, P ≤ .01, n = 10 and WT female: 0.79 ± 0.1 ng/mL, n = 6; WT male: 0.59 ± 0.01 ng/mL, n = 6) (Figure 3, B and D) as were the serum FSH levels in both GKirKO females (0.28 ± 0.07 ng/mL, P ≤ .05, n = 11) and GKirKO males (0.12 ± 0.02 ng/mL, P ≤ .001, n = 7) compared with those in both WT females (0.81 ± 0.23 ng/mL, n = 8) and males (3.77 ± 0.36 ng/mL, n = 6) (Figure 3, C and E).
Gonadotrope function is preserved in GKirKO mice with increased GnRH responsiveness in female GKirKO mice
To determine the status of gonadotroph function in GKirKO mice, GnRH-induced LH release from the pituitary was evaluated by performing GnRH stimulation testing. Preserved gonadotroph function was observed in both sexes of GKirKO mice. Serum LH values in GKirKO and WT mice were significantly increased after GnRH compared with the baseline levels of the same gender and genotype (GKirKO female: 3.29 ± 0.45 ng/mL, P ≤ .001, n = 9; GKirKO male: 3.9 ± 0.13 ng/mL, P ≤ .001, n = 3 and WT female: 1.97 ± 0.32 ng/mL, n = 7; WT male: 4.26 ± 0.27 ng/mL, n = 6) (Figure 3, F and H). Interestingly, an approximately 2-fold increased responsiveness to exogenous GnRH was observed in female GKirKO mice when changes in serum LH levels in response to GnRH were compared between WT and GKirKO mice (Figure 3F), possibly due to the lack of negative feedback by estrogen in the GKirKO female mice. In contrast, this increased responsiveness to exogenous GnRH observed in female GKirKO mice was not detected in male GKirKO mice because increased serum LH levels in response to exogenous GnRH were not different between WT and knockout mice (Figure 3H).
GKirKO mice do not respond to exogenous kisspeptin stimulation
A kisspeptin stimulation test was also performed, and serum gonadotropin levels were evaluated. Serum LH values in WT mice were significantly increased after kisspeptin compared with baseline serum LH levels of the same gender and genotype (kisspeptin-treated WT female: 2.78 ± 0.42 ng/mL, P ≤ .001, n = 10; kisspeptin-treated WT male: 4.15 ± 0.34 ng/mL, P ≤ .001, n = 6) (Figure 3, G and I) and control mice (WT female: 0.79 ± 0.1 ng/mL, n = 6; WT male: 0.78 ± 0.09 ng/mL, n = 3) (Figure 3, G and I). In contrast, and as expected, serum LH values in GKirKO mice remained unchanged after kisspeptin compared with baseline serum LH levels (kisspeptin-treated GKirKO female: 0.42 ± 0.05 ng/mL, n = 7; kisspeptin-treated GKirKO male: 0.18 ± 0.04 ng/mL, n = 3) (Figure 3, G and I) and control GKirKO mice (saline GKirKO female: 0.40 ± 0.11 ng/mL, n = 8; saline GKirKO male: 0.34 ± 0.08 ng/mL, n = 10) (Figure 3, G and I).
Dramatically impaired fertility in GKirKO mice
A fertility assessment was performed using the paradigm shown in a matrix format in Figure 4. WT and GKirKO female mice were placed in a cage (3 cages for each genotype, 2 females/cage) with either a WT or GKirKO male mouse. Six cycles of pairings were evaluated, and females were observed for evidence of pregnancy. When paired with WT females, WT males succeeded in impregnating their mates in 95% of the intents (Figure 4A), which delivered normal-sized litters. The same WT females were mated with GKirKO males (only 3 GKirKO males of 15 had PPS and could be used for mating), and no pregnancies were observed, indicating infertility in GKirKO males in many cases (Figure 4B). Following the same paradigm, when paired with GKirKO females, WT males failed to succeeded in impregnating their mates in 100% of the intents (Figure 4C), indicating infertility of adult GKirKO females. As expected, no successful matings were observed when both female and male GKirKO mice were mated (Figure 4C). These results prove that deletion of Kiss1r in GnRH neurons severely compromises the reproductive success in GKirKO mice.
Figure 4.
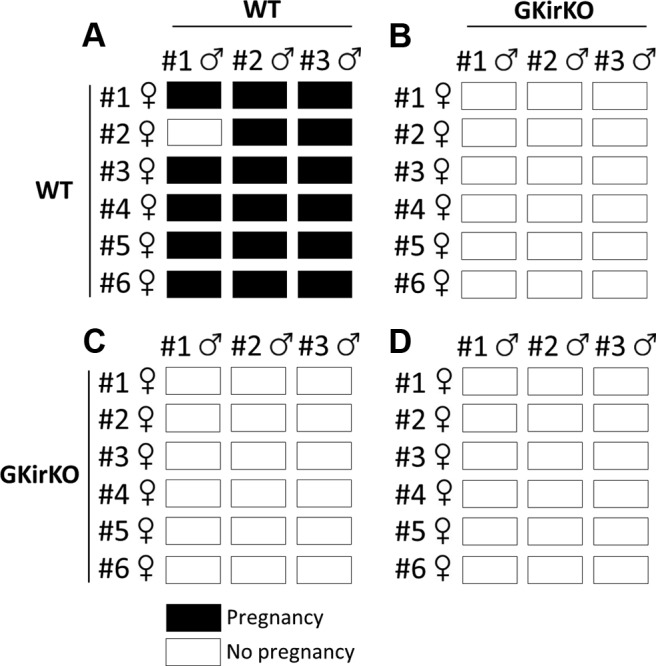
Matrix of the representative breeding study. Six WT and GKirKO female mice were paired with 3 WT and GKirKO males for 14 days and then returned to their own cages for 3 weeks to allow for birth of pups, indicating a successful pairing. Each row represents 1 individual female, and each box represents one of her pairings. Black boxes represent a successful pregnancy and white boxes a lack of pregnancy noted.
Reproductive tract abnormalities of GKirKO mice
A decrease in uterine size and an approximately 70% reduction in ovarian weight (WT control mice: 4.63 ± 0.85 mg vs GKirKO mice: 1.32 ± 0.65, P ≤ .05, n = 4) (Figure 5A) were observed in the GKirKO mice. Ovaries of WT female mice displayed all stages of follicular development with normal corpora lutea (Figure 5C). However, oogenesis was disrupted in GKirKO female mice with the presence of primary follicles, preantral follicles, and antral follicles but no evidence of corpora lutea formation (Figure 5C). In addition, there was an approximately 70% reduction in testicular weight (42.8 ± 3.5 mg, P ≤ .001, n = 5) in GKirKO males compared with that of WT mice (122.6 ± 3.9 mg, n = 3). Seminiferous tubules of WT mice showed all stages of spermatogenesis with numerous luminal sperm (Figure 5D). A large number of sperm were also observed in the epididymis of WT mice (Figure 5D). In contrast, the male GKirKO mice appeared to have decreased numbers of sperm in the seminiferous tubules and epididymis (Figure 5D).
Figure 5.
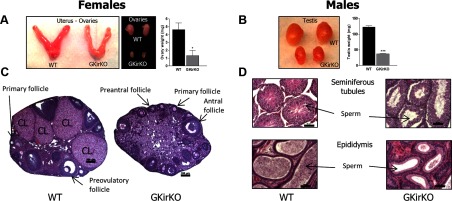
Gross gonadal anatomy and gonadal histology of GKirKO mice. A, Small ovaries (size and weight) and uterus found in GKirKO mice compared with WT. B, Smaller size and weight of the testes in GKirKO male mice compared with WT mice. C, Representative sections from a WT ovary (left image) showing follicles at all stages of development, including primary, preantral, antral, preovulatory follicles as well as corpora lutea (CL); GKirKO mice (right image), in contrast, do not contain follicles past the antral stage and have no corpora lutea formation. Scale bars correspond to 100 μm. The most representative microphotographs were chosen. D, Representative sections of testes. Seminiferous tubules from a WT mouse (left, top image) show all stages of spermatogenesis with numerous sperm. In contrast, seminiferous tubules from GKirKO mice (right, top image) lack spermatogenesis. Representative sections of the epididymis: the lumen from a WT mouse is filled with sperm (left, bottom image), whereas that of GKirKO mice have fewer sperm (right, bottom image). Scale bars represent 50 μm.
Reduced estrogen negative feedback in the GKirKO female mice
Serum LH levels in OVX WT control mice were significantly higher than those in intact WT control mice in metestrus (OVX WT female: 4.68 ± 0.82 ng/mL, n = 4 vs intact WT female: 0.70 ± 0.07 ng/mL, n = 5, P ≤ .001) (Figure 6A). No increase in serum LH was observed in OVX GKirKO mice (OVX GKirKO female: 0.29 ± 0.11 ng/mL, n = 4 vs intact GKirKO female: 0.33 ± 0.08 ng/mL, n = 8) (Figure 6A). In addition, serum FSH values in OVX WT control mice were also significantly higher than those in intact WT control mice in metestrus (OVX WT female: 17.5 ± 4.04 ng/mL, n = 4 vs intact WT female: 0.60 ± 0.09 ng/mL, n = 5, P ≤ .001) (Figure 6B). No significant increase in serum FSH levels was observed in OVX GKirKO mice (OVX GKirKO female: 0.52 ± 0.24 ng/mL, n = 4 vs intact GKirKO female: 0.28 ± 0.07 ng/mL, n = 11) (Figure 6B).
Kiss1 mRNA was evaluated in the AVPV nucleus and ARC nucleus of intact WT control mice in metestrus and in intact (noncycling) or OVX GKirKO mice. No significant differences in Kiss1 mRNA levels in the AVPV nucleus (documented to be increased by estradiol) of intact GKirKO mice was observed compared with that in intact WT control mice in metestrus (n = 4–5, P ≥ .05) (Figure 6C). A reduction of approximately 70 to 80% in Kiss1 mRNA in the AVPV nucleus in the OVX WT control and OVX GKirKO mice was observed compared with that in intact WT or GKirKO mice (n = 4–5, P ≤ .01) (Figure 6C). In contrast, Kiss1 mRNA in the ARC nucleus (documented to be down-regulated by estradiol) was higher in OVX WT and OVX GKirKO mice than in intact WT (n = 4–5, P ≥ .001) (Figure 6D) and GKirKO (n = 4–5, P ≥ .01) (Figure 6D) mice. Thus, a reduced, although not complete, estradiol negative feedback effect was observed in GKirKO mice.
Discussion
Our studies using a novel kisspeptin receptor knockout mouse model carrying a Kiss1r null mutation only in GnRH neurons (GKirKO mouse) provide evidence that kisspeptin signaling in hypothalamic GnRH neurons is critically responsible for the proper functioning of the reproductive HPG axis. Disruption of the kisspeptin signal solely in the GnRH neuron results in infertility due to hypogonadotropic hypogonadism.
Previous studies performed in global Kiss1r knockout mice demonstrated that the Kiss1r is vital for normal pubertal onset as well as proper reproductive function (3, 4, 10) (Table 1). Because kisspeptin receptors are found in many reproductive and nonreproductive tissues, until the publication of Kirilov et al in 2013 (41) and our present study, we could only speculate on the target tissue(s) in the reproductive HPG axis that relayed the critical kisspeptin signal for normal reproductive function. Although elegant and highly informative studies examining the broad reproductive phenotype of the Kiss1 or Kiss1r knockout mice have been published, global knockout studies were unable to document the independent role of kisspeptin signaling in hypothalamic GnRH neurons. Although several studies have documented the presence of the Kiss1r on GnRH neurons and GnRH responsiveness to kisspeptin using cell model systems (47, 48), the effects on mammalian reproductive function remained in question. To further address this issue, a new approach was needed to provide direct evidence for the role of kisspeptin-Kiss1r signaling in GnRH neurons in vivo. Kirilov et al (41) recently generated GnRH neuron–specific Kiss1r-deleted mice, which presented failure to go through puberty, reduced gonadal size, and infertility. Using a similar strategy, a LoxP/Cre-recombinase mouse model with a targeted deletion of kisspeptin receptor only in GnRH neurons, our laboratory was able to investigate kisspeptin signaling directly at the level of these neurons and determine its crucial role for the successful functioning of the reproductive HPG axis. In addition, Padilla et al (58), using a specific application of the LoxP/Cre technology, raised interesting concerns about the reliability of this technology using Pomc as a Cre driver. Experiments that focused on proopiomelanocortin (POMC) expression during development demonstrated that POMC was transiently expressed in a wide range of neurons before becoming localized in the more mature POMC neurons. Thus, brief Pomc promoter activity during development would probably be sufficient to drive Cre expression, resulting in brain locations having an undesired recombination (58, 59). In another study, promoter transgenics targeting GnRH neurons demonstrated multiple GnRH-expressing cell populations of different embryologic origin that presented in a transient manner during development in mouse brain (60). Low levels of Xgal-expressing cells (marking GnRH neurons) were detected in the intermediate division of the lateral septum, bed nucleus of the stria terminalis, and tectum. Because no cells in the bed nucleus of the stria terminalis and very few in the lateral septum express endogenous Kiss1r, it is possible that Kiss1r will be deleted from a very small population of lateral septum cells as well as from essentially all GnRH neurons (41).
Table 1.
Summary of Transgenic Lines
| Phenotype/Name | Global Knockouts |
GnRH-Specific Knockouts |
|||
|---|---|---|---|---|---|
| GPR54tm1SPR | GPR54tm1PTL | GPR54tm1HPC | GnRH-Cre+/−-GPR54f/f | GKirKO | |
| Strategy (deletion) | Part of exon 2 | Part of exon 1 and 2 | Exon 2 | Exon 2 | Exon 2 |
| Vaginal opening, d | NR | 100 | 37 | No pubertal onset | 36 |
| Preputial separation, % | NR | NR | None | None | 20 |
| Estrous cycling | NR | No | NR | No | No |
| LH (F) ng/mL (vs WT) | NR | 0.38 ± 0.12 (↔) | 0.4 ± 0.2 (↔) | AVNR (↔) | 0.33 ± 0.08 (↓) |
| LH (M) ng/mL (vs WT) | NR | 0.18 ± 0.10 (↔) | 0.1 ± 0.0 (↓) | 0.13 ± 0.03 (↔) | 0.28 ± 0.07 (↓) |
| FSH (F) ng/mL (vs WT) | NR | 6.8 ± 1.8 (↓) | 3.3 ± 0.6 (↓) | AVNR (↓) | 0.32 ± 0.10 (↓) |
| FSH (M) ng/mL (vs WT) | NR | 2.0 ± 0.7 (↓) | 1.5 ± 0.2 (↓) | 0.25 ± 0.01 (↓) | 0.12 ± 0.02 (↓) |
| Ovary (vs WT), % | Smaller | 18 | 41 (hemiblock) | 10 | 30 |
| Uterus (vs WT) | Smaller | Smaller | NR | Threadlike | Smaller |
| Penis development | Microphallus | Microphallus | Microphallus | NR | Microphallus |
| Testes (weight vs WT), % | 10 | 28 | 17 | 10 | |
| Reference | Funes et al, 2003 (3) | Seminara et al, 2003 (4) | Lapatto et al, 2007 (10) | Kirilov et al, 2013 (41) | Novaira et al (this study) |
Abbreviations: AVNR, absolute value not reported; F, female; GKirKO, GnRH kisspeptin receptor knockout; HPC, Harvard Partners Center; M, male; NR, not reported; PTL, Paradigm Therapeutics; SPR, Schering Plough Research; tm, targeted mutation; (↔), unchanged; (↓), decreased. The inherent variability of LH and FSH assays makes direct comparisons challenging.
Previous global knockout studies have shown that Kiss1r knockout mice display a drastically altered hormone signature (3, 4, 8, 10). Concurrent with the results of Lapatto et al (10), who similarly deleted exon 2 in the Kiss1r (although globally), deletion or disruption of the kisspeptin receptor leads to dramatically decreased serum levels of gonadotropins in both males and females compared with that in the WT control group. Our results agree with the previous findings, but localize the impairment solely to the Kiss1r signaling pathway in hypothalamic GnRH neurons. Kirilov et al (41) surprisingly found no changes in serum LH levels in either males or females in contrast to our model. The difference in the genetic background strains of the mice used in the studies could explain the differences in basal serum LH levels. All of the mice used in our experiments were maintained on a mixed CD1/129SvJ/C57BL6 genetic background, and the studies performed by Kirilov et al were in mice on a backcrossed C57BL/6 background.
A GnRH stimulation test was performed to determine the status of gonadotroph function in GKirKO mice. Similar to the findings of Lapatto et al (10), we observed that exogenous GnRH administered to GKirKO mice was able to increase serum LH levels. Thus, the remarkably dramatic phenotype displayed by the GKirKO mice was attributable only to the deletion of the Kiss1r in the GnRH neuron. In addition, a kisspeptin stimulation test performed in male and female GKirKO mice demonstrated, not surprisingly, that administration of kisspeptin did not stimulate an increase in gonadotropin levels in GKirKO mice. Hence, a direct effect of kisspeptin, on the gonadotroph, even acting as a compensatory mechanism, is unlikely. Similarly, to provide an in vivo functional assay of the ability of the GnRH neuronal network to respond to kisspeptin, Kirilov et al (41) reported a significant 7-fold increase in serum LH levels after a 100-nmol kisspeptin ip injection in control male mice with no effect in the mutant mice.
To determine the role of reduced estrogen negative feedback on the neuroendocrine axis in GKirKO female mice, oophorectomies were performed in GKirKO and WT mice. Metestrus was chosen as the comparator, because it is the most characteristic of a noncycling hormonal profile. GKirKO mice did not respond with a characteristic increase in serum LH or FSH levels as seen in the oophorectomized WT mice. Both the GKirKO and WT mice experienced a similar decrease in Kiss1 expression in the AVPV nucleus and an increase in the ARC nucleus after oophorectomy. These data suggest a defect in estradiol negative feedback on gonadotropin secretion, although not completely manifest in the regulation of kisspeptin in this model.
From previous studies, we learned that kisspeptin plays a direct role in the development and proper functioning of the reproductive organs in both males and females (5, 10, 55). Several studies have shown that deletions or inactivating mutations in the Kiss1r or deletion of the ligand kisspeptin result in various degrees of hypogonadotrophic hypogonadism as well as incomplete or dysfunctional development of secondary sex organs (3, 4, 6, 8, 10, 29, 55, 61). In agreement with these previous findings, the GKirKO mice displayed distinct evidence for hypogonadism. A common feature of the global mutant mice is hypotropic development of the female reproductive tract (3, 4, 10); Seminara et al (4) and Funes et al (3) described the uteri of the Kiss1r-null mutant female mice as being “threadlike.” The GKirKO female mice had only a slight decrease in uterine size and an approximately 70% reduction in ovarian weight, suggesting a role for extrahypothalamic kisspeptin signaling (62, 63). In contrast to the GKirKO mice and similar to the global Kiss1r-null mutant mice (3, 4), Kirilov et al (41) reported a dramatic reduction (>90%) in ovarian weight and uterine size that mimics the threadlike uterus observed in the global Kiss1r null mutant. The dramatic nature of this finding is not understood.
In addition, oogenesis was disrupted in GKirKO female mice with the presence of primary follicles, preantral follicles, and antral follicles but no evidence of corpora lutea formation, indicating infertility in these knockout mouse models.
GKirKO males had a microphallus and a significant decrease in anogenital distance, which was also reported by Lapatto et al (10), Seminara et al (4), and Funes et al (3) in global knockout mouse models. The size of the phallus was not reported in the GnRH neuron–specific Kiss1r knockout (41). In addition, the knockout males had a dramatic reduction in testicular size compared with that of the control mice. Furthermore, similar to the GnRH neuron–specific Kiss1r knockout and the global Kiss1r knockout mouse models (3, 4, 10, 41), the male GKirKO mice exhibited a defect in spermatogenesis, with the epididymis of the GKirKO mice having fewer sperm present in the lumen. These findings argue that disruption of Kiss1r signaling in GnRH neurons is the etiology of the profoundly abnormal reproductive phenotypes.
In addition, Popa et al (64) hypothesized that kisspeptin signaling is safeguarded by production of kisspeptin in excess of what is required to support reproductive function. To test this hypothesis the authors examined the reproductive phenotype of mice with a 95% reduction in Kiss1 transcript levels. Reproductive function in males was normal, whereas females were subfertile. In GKirKO mice, an approximate reduction in hypothalamic Kiss1r expression levels by 70% was observed. Male and female GKirKO mice had phenotypic and biochemical evidence of a disrupted reproductive axis, indicating that disruption of the kisspeptin signal in GnRH neurons is critical for reproductive development and fertility.
Consistent with previous observations from hypogonadotrophic hypogonadism in mice and nIHH in human patients, deletion or loss-of-function mutations in the Kiss1r results in various delays in pubertal onset (4, 10, 24, 26). Interestingly, in GKirKO males, PPS either never occurred or was drastically delayed. Of the 15 mutant male mice, 12 had complete failure of preputial gland separation and the remaining 3 had a delay of around 9 days compared with that in the WT controls. In GKirKO females, VO was also delayed by about 9 days compared with that in the WT controls. However, all female mutant mice did eventually have VO, despite having abnormal estrous cyclicity, with most of them in constant diestrus.
In contrast with the global Kiss1r-null mutant (3, 4, 10) and the GKirKO model, the GnRH neuron–selective deletion of Kiss1r reported by Kirilov et al (41) resulted in a failure to go through puberty indicated by the absence of VO. This finding is consistent with the more severe anatomic phenotype seen in the GnRH neuron–selective deletion of the Kiss1r model and similarly remains unexplained.
Seminara et al (4) suggested 3 possible mechanisms that may allow abnormalities in Kiss1r knockout to cause pubertal delay: (1) Kiss1r knockout perturbs GnRH neuronal migration during development; (2) Kiss1r modulates the activity of GnRH at the level of the pituitary; and (3) Kiss1r regulates the activity of GnRH at the level of the hypothalamus. Findings from Messager et al (6) in the global Kiss1r knockout and Kirilov et al (41) in the GnRH neuron–specific Kiss1r knockout negate the first possibility, demonstrating that GnRH neurons are found in appropriate anatomical locations in the hypothalamus of Kiss1r knockout mice and also show morphology and projections to the median eminence similar to those in WT mice. The second possibility, that Kiss1r modulates the activity of GnRH at the level of the pituitary, remained tantalizing because gonadotrophs were shown to express Kiss1r. However, KISS1R-deficient humans and mice bearing Kiss1r loss-of-function mutations do not have diminished gonadotroph sensitivity to GnRH (4). Our study further demonstrates normal responsiveness of the pituitary in the GKirKO mice. Our research and the very important findings reported by Kirilov et al (41) affirm the third possibility posed by Seminara et al (4), that the abnormal reproductive phenotypes associated with deletion of the Kiss1r in mice are directly caused by disruption in kisspeptin signaling localized in GnRH neurons. These results extend this hypothesis to document that the sole critical signal for normal mammalian reproduction occurs through kisspeptin signaling at the level of the GnRH neuron.
In conclusion, our mouse model bearing a Kiss1r knockout in GnRH neurons (GKirKO) demonstrates that the hypogondotrophic hypogonadism is attributable solely to disrupted kisspeptin signaling in the hypothalamic GnRH neurons. This work provides increased clarity to our understanding of the mammalian HPG axis and points to the GnRH neuron as the singular target for consideration in the treatment of hypothalamic reproductive abnormalities in humans.
Acknowledgments
We thank Dr Fredric Wondisford, Dr Sara DiVall, and Dr Sheng Wu for the critical support of this work and Ikeoluwa Adeshina for technical assistance.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH) Grant R01 HD370246 and through cooperative agreement [Partnership U01HD066432] as part of the Cooperative Partnerships to Promote Workforce Diversity in the Reproductive Sciences Program), and the National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Baltimore Diabetes Research and Training Center (Grant P60DK079637).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- actuate
- AVPV
- anteroventral periventricular
- HPG
- hypothalamo-pituitary-gonadal
- Kiss1r
- kisspeptin receptor
- nIHH
- normosmic idiopathic hypogonadotrophic hypogonadism
- OVX
- ovariectomized
- POMC
- proopiomelanocortin
- PPS
- preputial separation
- qPCR
- quantitative PCR
- VO
- vaginal opening
- WT
- wild-type.
References
- 1. Nelson SB, Lawson MA, Kelley CG, Mellon PL. Neuron-specific expression of the rat gonadotropin-releasing hormone gene is conferred by interactions of a defined promoter element with the enhancer in GT1–7 cells. Mol Endocrinol. 2000;14:1509–1522 [DOI] [PubMed] [Google Scholar]
- 2. Kelley CG, Givens ML, Rave-Harel N, Nelson SB, Anderson S, Mellon PL. Neuron-restricted expression of the rat gonadotropin-releasing hormone gene is conferred by a cell-specific protein complex that binds repeated CAATT elements. Mol Endocrinol. 2002;16:2413–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 4. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 5. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 6. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 8. Dungan HM, Gottsch ML, Zeng H, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 10. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936 [DOI] [PubMed] [Google Scholar]
- 11. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 13. Hrabovszky E, Ciofi P, Vida B, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998 [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 15. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith JT, Clarke IJ. Kisspeptin expression in the brain: catalyst for the initiation of puberty. Rev Endocr Metab Disord. 2007;8:1–9 [DOI] [PubMed] [Google Scholar]
- 17. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682 [DOI] [PubMed] [Google Scholar]
- 18. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152:2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–3618 [DOI] [PubMed] [Google Scholar]
- 20. Irwig MS, Fraley GS, Smith JT, et al. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272 [DOI] [PubMed] [Google Scholar]
- 21. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149:3598–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lanfranco F, Gromoll J, von Eckardstein S, Herding EM, Nieschlag E, Simoni M. Role of sequence variations of the GnRH receptor and G protein-coupled receptor 54 gene in male idiopathic hypogonadotropic hypogonadism. Eur J Endocrinol. 2005;153:845–852 [DOI] [PubMed] [Google Scholar]
- 26. Semple RK, Achermann JC, Ellery J, et al. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–1855 [DOI] [PubMed] [Google Scholar]
- 27. Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–1144 [DOI] [PubMed] [Google Scholar]
- 28. Teles MG, Trarbach EB, Noel SD, et al. A novel homozygous splice acceptor site mutation of KISS1R in two siblings with normosmic isolated hypogonadotropic hypogonadism. Eur J Endocrinol. 2010;163:29–34 [DOI] [PubMed] [Google Scholar]
- 29. Nimri R, Lebenthal Y, Lazar L, et al. A novel loss-of-function mutation in GPR54/KISS1R leads to hypogonadotropic hypogonadism in a highly consanguineous family. J Clin Endocrinol Metab. 2011;96:E536–E545 [DOI] [PubMed] [Google Scholar]
- 30. Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539 [DOI] [PubMed] [Google Scholar]
- 31. Bianco SD, Kaiser UB. Molecular biology of the kisspeptin receptor: signaling, function, and mutations. Adv Exp Med Biol. 2013;784:133–158 [DOI] [PubMed] [Google Scholar]
- 32. Seminara SB. Kisspeptin in reproduction. Semin Reprod Med. 2007;25:337–343 [DOI] [PubMed] [Google Scholar]
- 33. Jayasena CN, Dhillo WS. Kisspeptin offers a novel therapeutic target in reproduction. Curr Opin Investig Drugs. 2009;10:311–318 [PubMed] [Google Scholar]
- 34. Jayasena CN, Nijher GM, Chaudhri OB, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab. 2009;94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 35. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97:E1458–E1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navarro VM, Fernández-Fernández R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides 2009;30:57–66 [DOI] [PubMed] [Google Scholar]
- 38. Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69 [DOI] [PubMed] [Google Scholar]
- 39. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316 [DOI] [PubMed] [Google Scholar]
- 41. Kirilov M, Clarkson J, Liu X, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. [DOI] [PubMed] [Google Scholar]
- 42. Wolfe A, Divall S, Singh SP, et al. Temporal and spatial regulation of CRE recombinase expression in gonadotrophin-releasing hormone neurones in the mouse. J Neuroendocrinol. 2008;20:909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Divall SA, Williams TR, Carver SE, et al. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest. 2010;120:2900–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu S, Divall S, Hoffman GE, Le WW, Wagner KU, Wolfe A. Jak2 is necessary for neuroendocrine control of female reproduction. J Neurosci. 2011;31:184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Diaczok D, DiVall S, Matsuo I, Wondisford FE, Wolfe AM, Radovick S. Deletion of Otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism. Mol Endocrinol. 2011;25:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh SP, Wolfe A, Ng Y, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor α (ESR1). Biol Reprod. 2009;81:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacobi JS, Martin C, Nava G, Jeziorski MC, Clapp C, Martínez de la Escalera G. 17-β-Estradiol directly regulates the expression of adrenergic receptors and kisspeptin/GPR54 system in GT1–7 GnRH neurons. Neuroendocrinology. 2007;86:260–269 [DOI] [PubMed] [Google Scholar]
- 48. Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. BioTechniques. 2005;39:75–85 [DOI] [PubMed] [Google Scholar]
- 50. Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR–a perspective. J Mol Endocrinol. 2005;34:597–601 [DOI] [PubMed] [Google Scholar]
- 51. Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339 [DOI] [PubMed] [Google Scholar]
- 52. Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303 [DOI] [PubMed] [Google Scholar]
- 53. Frisch RE, Hegsted DM, Yoshinaga K. Body weight and food intake at early estrus of rats on a high-fat diet. Proc Natl Acad Sci USA. 1975;72:4172–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brothers KJ, Wu S, DiVall SA, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. d'Anglemont de Tassigny X, Fagg LA, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Novaira HJ, Fadoju D, Diaczok D, Radovick S. Genetic mechanisms mediating kisspeptin regulation of GnRH gene expression. J Neurosci. 2012;32:17391–17400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morrison CD, Münzberg H. Capricious Cre: the devil is in the details. Endocrinology. 2012;153:1005–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. J Neurosci. 1999;19:5955–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635 [DOI] [PubMed] [Google Scholar]
- 62. Roa J, Vigo E, García-Galiano D, et al. Desensitization of gonadotropin responses to kisspeptin in the female rat: analyses of LH and FSH secretion at different developmental and metabolic states. Am J Physiol Endocrinol Metab. 2008;294:E1088–E1096 [DOI] [PubMed] [Google Scholar]
- 63. Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Popa SM, Moriyama RM, Caligioni CS, et al. Redundancy in Kiss1 expression safeguards reproduction in the mouse. Endocrinology. 2013;154:2784–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]