Abstract
Therapeutic targeting of nuclear receptors (NRs) is presently restricted due to 2 constraints: 1) a limited knowledge of the structural dynamics of intact receptor when complexed to DNA and coregulatory proteins; and 2) the inability to more selectively modulate NR actions at specific organ/gene targets. A major obstacle has been the current lack of understanding about the function and structure of the intrinsically disordered N-terminal domain that contains a major regulatory transcriptional activation function (AF1). Current studies of both mechanism of action and small molecule-selective receptor modulators for clinical uses target the structured pocket of the ligand-binding domain to modulate coregulatory protein interactions with the other activation function AF2. However, these approaches overlook AF1 activity. Recent studies have shown that highly flexible intrinsically disordered regions of transcription factors, including that of the N-terminal domain AF1 of NRs, not only are critical for several aspects of NR action but also can be exploited as drug targets, thereby opening unique opportunities for endocrine-based therapies. In this review article, we discuss the role of structural flexibilities in the allosteric modulation of NR activity and future perspectives for therapeutic interventions.
Nuclear receptors (NRs) act in cell type- and gene-specific manners to regulate numerous physiological and pathological processes including carbohydrate metabolism, lipid metabolism, inflammation, cancer, and cardiovascular disease (1). When viewing receptors as therapeutic targets, the challenge is how to selectively control cell/tissue and target gene specificity in a manner that affects only deleterious actions of NRs in diseased tissues without altering essential normal functions. Small molecule, selective nuclear receptor modulators (SRMs) have been developed. Most notable are tamoxifen and raloxifine, which have sufficient specificity for estrogen receptors (ERs) and tissue-selective antiestrogenic actions to be used effectively in breast cancer therapy (2–4). However, refinement and improvement of SRMs for ER and development of SRMs for other NRs with therapeutic potential have not been fully met. This is due, in part, to our limited understanding of the structural dynamics of NRs, including intra- and intermolecular communications under the influence of various associated coregulatory proteins and posttranslational modifications that contribute to cell/tissue- and target gene-specific actions.
A major obstacle has been complete characterization of the 2 transcriptionally active regions of most NRs: the N-terminal transcriptional activation function (AF)1 and the C-terminal AF2. AF2 resides in the well-ordered ligand-binding domain (LBD), and detailed high-resolution X-ray crystallography structures have revealed how conformational changes in AF2 induced by various ligands can modulate interactions with conserved motifs of coregulatory proteins (5–9). The AF1 is located in the intrinsically disordered (ID) N-terminal domain (NTD) that has hitherto eluded crystallization and high-resolution structure. In the case of steroid hormone receptors (SHRs), a subset of the NR superfamily, the size of the NTD is relatively large (≤500 or more amino acids), and AF1 is often the more active transcriptional activation function. The AF1 of SHRs has also been shown functionally to be a major contributor to cell/tissue- and target gene-specific actions (10–12). It thus is axiomatic that attempts to more precisely control NR selectivity with SRMs and cofactors during differential control of gene expression without understanding the functionally active structural features of the NTD/AF1 will be of limited success. In this article, we review recent developments that provide new insights of how structural flexibility plays an important role in NRs' allosteric regulation leading to the fine tuning of target gene expression and the challenges for drug targeting to more precisely control NRs.
The AF1s Exist in an Intrinsically Disordered (ID) Conformation
In recent years, it has become quite evident that many regulatory proteins possess unstructured ID regions (IDRs). These regions are characterized by a low-complexity amino acid composition that does not enable spontaneous folding into a globular domain. IDRs lack stable secondary and tertiary structure under native conditions but can exist as dynamic ensembles of interconverting conformers capable of undergoing a disorder-to-order transition upon interaction with macromolecules including other proteins or DNA (13, 14). This structural flexibility and process of “coupled folding and binding” appears to have certain advantages for intra- and intermolecular interactions as compared with ordered structural motifs. For example, IDRs have large extended surfaces for potential interaction with a broad range of coregulatory proteins, thereby forming assorted functional conformations. This mechanism provides the opportunity for the same IDR to respond selectively to a variety of input signals (14). In some instances, the same protein-binding partner can mediate either positive or negative cooperativity with varying biological consequences depending on the available ID-binding sites (15). Also, the coupled binding and folding of IDRs within proteins result in high-specificity and low-affinity associations, which are ideal properties for the transient reversible interactions required for regulation of transcriptional signaling (16–19).
Using a variety of biophysical methods, such as circular dichroism spectroscopy, nuclear magnetic resonance, and Fourier transform infrared- and fluorescence emission-spectroscopy, it has been confirmed that NTDs of NRs contain large segments of ID conformations and are deficient in stable secondary structure (20–25). However, ID characteristics have been examined mostly with isolated NR NTDs. Hydrogen-deuterium exchange coupled with mass spectrometry (HDX-MS) has emerged as an important solution-phase biophysical technology that is not limited by the size of the protein and can localize differential conformation dynamics to sequences in the intact protein. Protein regions with stable secondary and tertiary structure undergo slow rates of deuterium exchange, whereas unfolded solvent-exposed regions exhibit rapid exchange. Sequencing of peptides from digests of the intact protein enables assignment of exchange kinetics for specific regions (26, 27). HDX-MS analysis of full-length peroxisome proliferator-activated receptor (PPAR)γ (28) and progesterone receptor (PR) (29) has further revealed that the highly dynamic conformation of NTDs is exhibited in the context of intact NRs, indicating that this property is not abrogated via interdomain interactions. This dynamic unstructured nature of NR NTDs has been problematic in preventing crystallization and high-resolution atomic structures. Importantly, this problem is not unique to NRs. In fact, most activation domains of transcription factors contain IDRs that have been refractory to crystallization (13, 14).
With NRs, both the ligand-dependent AF2 and AF1 are interaction surfaces for coregulatory proteins, including coactivators or corepressors (5, 11, 30). A conserved LXXLL motif of classical coactivators interacts with AF2 through complementary well-structured protein folds of helices 3–5, and 12 of the LBD. In contrast, and consistent with the properties of IDRs, no common structural motif has been defined for coregulators that interact with AF1. It is well known that within the intact NRs, there is a functional synergy between AF1 and AF2 that is an essential component of SHR-mediated target gene regulation (31, 32). This synergy is mediated by interdomain allosteric pathways that clearly involve the conformation flexibility of the NTD but through mechanisms that remain undefined experimentally (33).
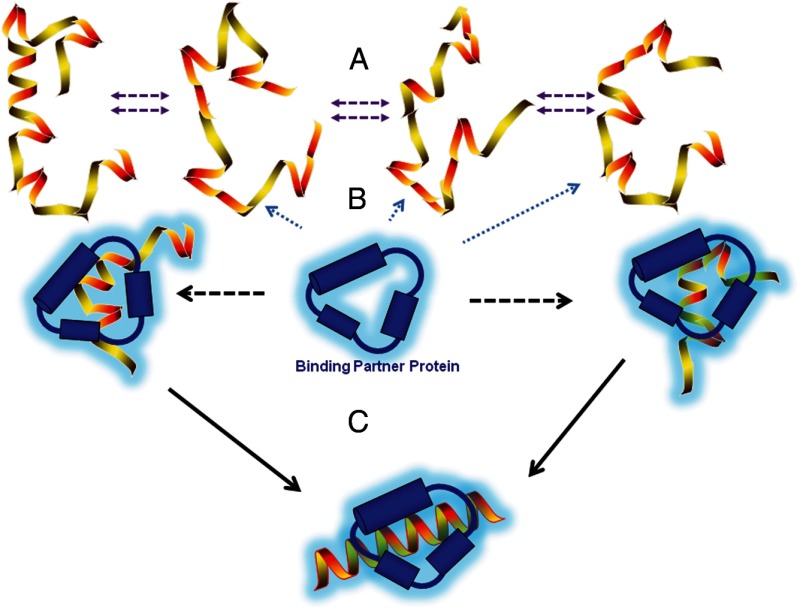
Studies from several laboratories have shown that IDRs of SHR NTDs are capable of undergoing a disorder-to-order transition upon binding specific target molecules (Figure 1). This includes the NTD/AF1 of glucocorticoid (GR), mineralocorticoid (MR), estrogen (ER), androgen (AR), and progesterone receptors (PRs) that undergo an increase in α-helical content and tertiary folding upon interaction with the TATA-binding protein (TBP). Additionally, in studies with GR, MR, and PR, TBP-induced folding of the NTD was observed to be associated with enhancement of NTD/AF1-mediated transcriptional activity (20, 29, 34). The ability of SHRs to respond in a similar manner to the same target protein (TBP), despite the poorly conserved sequence of each NTD at the amino acid level, supports the concept of a common mechanism of action of NTD/AF1 through a coupled folding and binding of IDRs (20, 23, 29, 34, 35). Other unrelated proteins have also been reported to bind and induce folding of the NTD/AF1s of SHRs including transcription factor IIF, CREB-binding protein, receptor-interacting protein 140 and silencing mediator of retinoid and thyroid hormone receptor (16, 20, 24, 25, 32, 36). Interestingly, different regions of the NTD of MR were shown to have the potential to fold into multiple distinct conformations, thus indicating that the large NTDs of SHRs may consist of multiple foldable modules (20).
Figure 1.
A model showing disorder-order transition of ID NTD of NRs. The ID NTDs exist as an ensemble of conformers in equilibrium with each other, which are collectively unstructured. Except for a very small fraction, which may be relatively ordered, all other conformers possess the characteristics of random coil or molten globule-like structures (A). When encountered by a binding partner protein (often a coregulatory protein), the ID NTD undergoes disorder-order transition by first undergoing various partial folded conformations (B) that finally results into functionally folded NTD conformation (C). In the full-length receptor this conformational transition is influenced by several other allosteric regulators (data not shown).
In addition to the LXXLL motifs of the p160 steroid receptor coactivators (SRCs) that bind to AF2 of SHRs, other surfaces have been observed to interact with NTDs including the activation domain 2 in the C terminus (37) of SRC-1 and an amino-terminal portion of transcriptional intermediary factor (TIF)2 (or SRC-2) termed “TIF2.0” (25). Interaction of SRC-1 and activation domain 2 can stimulate NTD/AF1 of AR (37) whereas TIF2.0 modulates corepression functions of GR and PR NTDs and, in intact receptors, may stabilize TIF2 interactions with GR and PR LBDs (38, 39). A potential mechanism by which TBP affects NTD/AF1 activity is to facilitate binding of SRC-1 to NTDs and cooperate with SRC-1 to stimulate NTD-dependent transcriptional activity of GR, MR, and PR. Thus TBP appears to act as an SHR coregulatory protein via reorganizing or stabilizing structures in the NTD for recognition by alternative surfaces of SRC-1 and assembly of coactivator complexes (20, 23, 25, 34, 35). More recently TIF2.0 binding was demonstrated to directly induce folding of the NTD/AF1 of GR. This is the first example that alternate surfaces of SRCs are also capable of directly stabilizing a structural conformation in the NTD/AF1 (25).
There is a strong preference for posttranslational modifications of proteins to occur in IDRs. Consistent with this is that most of the phosphorylation sites of SHRs are located in the NTD (40). Site-specific phosphorylation induces conformational changes in AF1/NTD of GR in a manner that correlates with binding of CREB-binding protein, TBP, and SRC-1 and subsequent stimulation of GR-mediated target gene regulation (41). Thus phosphorylation events may also be tied to protein-induced folding of IDRs of the NTD of SHRs and assembly of coregulatory protein complexes.
Conformational Dynamics and Control of NR Function by Interdomain Allosteric Pathways
Although individual DNA-binding domain (DBD) and LBD structures of the NRs have provided major advances in our understanding of the molecular basis of transcription regulation by NRs, a complete description of structure-function relationships and mechanism of action of NRs necessitates structural studies of the full-length receptor protein. The recent X-ray study of a 2-domain (LBD plus DBD) hepatocyte nuclear factor (HNF)-4α homodimer bound to DNA, and complexed with a coactivator peptide, elegantly revealed an asymmetric convergence zone linking the DBD and LBD and how this interdomain interface can allosterically modulate receptor activity (42). The quaternary structure of HNF-4α also indicates how 2 posttranslational modifications (Arg91 methylation and Ser78 phosphorylation) located in a unique position within the interdomain convergence zone can affect the DNA binding activity of the receptor (42). Interestingly, the NTD of HNF-4α had to be proteolytically removed in order to obtain crystals, indicating the disordered nature of this region. The earlier, ground-breaking X-ray structure of DNA-bound full-length PPARγ/retinoid X receptor (RXR)α heterodimers also revealed extensive domain-domain interactions between LBD and DBD that affect receptor activity but with an arrangement distinct from that of HNF-4α (28). Despite the presence of the NTDs in PPARγ/retinoic acid receptor-α proteins used for crystallography, they were disordered and could not be located in the structure (28). In addition to allosteric communication between LBD and DBD, the NTD may allosterically transmit local unfolding within the structured DBD and LBD through intramolecular interdomain interactions (33, 43). Allosteric coupling between the DBD and NTD, triggered by binding of the DBD to site-specific DNA sequences or to coregulatory proteins, is also known to induce ordered conformation in the NTD/AF1 (40, 43–50). These observations collectively support the notion that NRs display extensive physical and functional interdomain interactions that define their total activity as opposed to each domain functioning entirely independently.
Solution structures of intact NR-RXR heterodimers complexed with direct repeat response element DNA have been analyzed by small-angle X-ray scattering or single-particle cryo-electron microscopy (EM) with 3-dimensional reconstitution and fitting to crystallography structures of isolated LBDs and DBDs to address the spatial localization of individual NR domains (51–53). NRs in solution exhibited a more open conformation without the domain-domain interfaces between LBDs and DBDs observed in the crystal structures of PPARγ/RXRα and HNF-4α homodimers (28, 42). Because crystal packing forces can stabilize protein conformers, the difference between structures in crystals and in solution further illustrates the conformational flexibility of full-length NRs. Interestingly, the short NTD of vitamin D receptor (VDR) in the cryo-EM structure of DNA-bound RXR/VDR was visible and localized near the major groove of DNA adjacent to the VDR-bound DR3 element, suggesting the NTD may have a direct role in modulating DNA binding (53). Thus cryo-EM may be a useful approach by which to examine the structural conformation of the NTD in the intact NR, in particular with the steroid hormone class of NRs with long NTDs. Another approach by which to examine the structural dynamics of intact NRs in solution is by differential HDX-MS. HDX-MS analysis of ER was able to classify SRMs based on their ability to differentially stabilize regions within the LBD independent of AF2 (54). Binding of a VDR/RXR heterodimer to DNA was shown to alter the conformational dynamics and stability of the AF2 coactivator surfaces of the LBD, thus providing direct structural evidence for allosteric interactions between the DBD and LBD (55). HDX-MS was also used to show that the intrinsically disordered activation domain of the PPARγ coactivator-1α/undergoes folding into a more compact structure whereas the LBD of estrogen-related receptor-γ is stabilized upon formation of a PPARγ coactivator-1α/estrogen-related receptor γ complex (56). Thus, differential HDX-MS should be an excellent tool for analysis of effects of protein binding partners on conformational dynamics of NTDs of NR. These complementary approaches to examine NR complexes in solution will be needed to understand the conformational dynamics and integration of multiple domains of the intact receptor required to regulate different activation states.
The Importance of Structural Dynamics for NRs as Drug Targets and Differential Gene Regulators
NRs are well-validated drug targets and are prognostic indicators in a number of pathophysiological conditions. It is generally accepted that NR function is dependent upon ligand-induced conformational changes. Moreover, coactivators and corepressors can bind in some circumstances to either NR-agonist or -antagonist complexes (57). Thus, variations in the expression and/or the recruitment of coregulatory proteins can have an important role for the tissue-specific effects of the SRMs (30, 58, 59). The most widely used small-molecule drugs to target NRs have been designed to bind the structured LBD and block specific binding of natural endogenous ligands or, in the case of SHRs, to block binding sites for steroid hormone agonists. Compared with natural hormone agonists, many SRMs have a bulky side chain that, upon binding the LBD, protrudes from the ligand-binding pocket and thereby hinders the folding of helix-12 into the agonistic orientation. This altered folding leads to suboptimal conformational changes in the LBD, which prevents AF2 from interacting with certain critical coregulatory proteins (8, 30, 58–60). Clinically this phenomenon has extensively been exploited with SRMs that bind to the LBD (9). However, most of these SRMs that bind in the ligand-binding pocket exhibit partial agonist or mixed agonist activities that may not be desirable. Alternatively, it would be clinically desirable to be able to adjust an SRM's activity spectrum so that it has full agonist activity for most target genes except for selected ones for which modulation would have the desired physiological outcome. For example, the amount of residual agonist activity of the antiglucocorticoid dexamethasone mesylate varies with exogenous and endogenous genes in a manner that can be further modulated by changing the cellular concentration of the coactivator TIF2 (61, 62). Also, several peptide probes have been identified that recognize different conformations of SRM-bound SHRs and selectively disrupt receptor-coregulatory protein complexes in a tissue-specific manner (3, 63). Finally, it should be remembered that not all SRMs have bulky substituents and thus would need to act via other interactions and/or mechanisms. Prime examples of this are the antiestrogen genestein, the antiandrogen flutamide, and the antiglucocorticoids progesterone and 11-deoxycortisol. Reduced ligand affinity for SR may account for some effects but cannot explain the antagonist activity of covalent receptor-steroid complexes like those of GR with dexamethasone mesylate (64). Therefore, although it is not yet possible to predict what structural modifications will be useful, it is likely that many useful derivatives have yet to be synthesized.
Based on several in vitro studies, it has been suggested that the tissue-specific residual activity of receptors in the presence of SRMs may mainly be mediated via AF1 and that the relative functional importance of AF1 may be decided by specific SRM-induced conformational changes in either LBD or transmitted allosterically to the NTD (8, 30, 58–60). Interestingly, this does not appear to be true for GRs (39). Due to available LBD/AF2 crystal structures and the disordered nature of NTD/AF1, rational structure-based design of SRMs has been limited by necessity to how compounds modulate coregulatory protein interactions with AF2 (7), thereby neglecting AF1. Similarly, coregulatory protein-induced allosteric effects that alter receptor structure and transcriptional activity are frequently analyzed in the absence of AF1 sequences either with LBD/AF2s linked to heterologous DNA binding domains or with NRs lacking the NTD (65).
For pharmaceutical purposes, it is often difficult to devise strategies to modulate interaction surfaces that are not remodeled by ligand or for orphan NRs that lack known ligands or ligand-binding cavities. It therefore makes sense to investigate the possibility of identifying receptor modulators that act outside of the ligand-binding pocket, which could complement or replace existing SRMs. A number of groups have screened different classes of such molecules with exciting and surprising outcomes, which often correlate with their impact on the conformational dynamics coupled with allosteric regulations of the SHR. For example, the potential for allosteric modulators of SHR function was emphasized by the finding that a small molecule, 3,3′,5-triiodothyroacetic acid, which binds to a hydrophobic surface of the LBD distinct from the ligand-binding pocket, as well as AF2, disrupts AF2-dependent AR transactivation (66). This finding, together with the information from peptide-binding studies, suggests that allosteric regulation could stabilize a specific conformer that reduces the affinity of the second binding site for its ligand. This type of structural dynamics could be explored as novel drug targets to produce differential NR responses (4). Unique conformational screening assays have been developed based on differential interaction of peptides or collections of coregulatory proteins as surrogate probes of allosteric conformational changes in the intact NR in response to ligands. By this approach SRMs have been demonstrated to influence conformational changes in regions of SHRs outside the LBD/ AF2. Furthermore, it has been possible with peptide probes to classify pharmacologic activities of SRMs by differential presentation of protein interaction surfaces of receptors (3, 63, 67, 68). This conformational approach also identified novel small molecules that block AR activity by disrupting unidentified protein interaction surfaces outside of the LBD pocket and failing to promote AR nuclear translocation. These compounds by definition act to allosterically block SHR activity.
An alternative and complimentary approach to differential gene expression involves varying the concentrations of coregulatory proteins. Such simple changes in cofactor concentration can modify not only the total response, but also the EC50 of NR-regulated gene expression. Changes in EC50 are particularly effective in evoking gene-selective effects when the EC50 values of target genes are above and below the concentration of circulating hormone in the organism. Studies of multiple NR-gene targets in the same cell, and of the same target under different cellular conditions, have made it abundantly clear that it is possible to have a continuum of values that can be dialed in as on a rheostat (69–71). Interestingly, some coregulators alter the EC50 without affecting the total amount of induced product (72). Thus, monitoring only those coregulators that influence the total activity will cause one to miss those physiologically relevant species that modify EC50 but not total response. A recently introduced competition assay greatly simplifies the detection and characterization of proteins, cofactors, and pharmaceuticals that alter the EC50 and/or total activity (73–75). This independent regulation of EC50 and total activity (and also SRM or partial agonist activity) has been seen both with endogenous receptor gene targets upon reduction of cellular TIF2 levels with TIF2 small interfering RNA (71) and after removing selected domains of GR and coregulatory proteins (72). Importantly, this last study shows that the contribution of the AF1 domain of GR in the activity of the trimeric GR/TIF2/STAMP complex varies with the composition of the other 2 factors. These results suggest that the influence of the AF1 domain of GR, and probably other NRs, on the total agonist activity, EC50, and SRM activity will not be constant but will be a function of the other cooperating associated factors.
Recent efforts to identify small molecules that act outside of the ligand-binding pocket and block NR activity are mainly focused on their interference with coactivator binding (4). Other small molecules that inhibit NR functions by targeting the receptor degradation also represent a novel class of NR inhibitor with significant clinical potential. It has been known for many years that the ER-antagonist, fulvestrant (ICI 182,780) and related compounds termed selective ER degraders enhance the degradation of ER, possibly by distorting ER structure so that a few hydrophobic amino acids are exposed near the surface, triggering recognition of ER as a misfolded protein and rapid degradation (76–79). More recent studies question whether enhanced degradation of ER alone contributes to antagonist activity of fulvestrant, suggesting that altered conformation of LBD/AF2 is also involved (80). Although fulvestrant is used therapeutically to treat advanced breast cancer, it has certain limitations due mostly to poor oral bioavailabilty and pharmacology properties (81). Another promising selective ER degrader, bazedoxifene, has an improved oral activity profile and ability to inhibit experimental tamoxifen-resistant experimental breast cancer models (82). Interestingly bazedoxifene induces conformational changes in ER distinct from tamoxifen and more comparable to fulvestrant (54, 82). In a recent study, a potent and specific small molecule inhibitor of ERα has been reported that blocks ER-mediated gene expression and estrogen-dependent growth of tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells due, in part, to its ability to enhance proteasome-dependent degradation of ER (83). Unlike fulvestrant, this compound does not compete with estrogens and acts outside of the ligand-binding pocket (83). Several other small-molecule drugs that act outside of the ligand-binding pocket, and target the DNA binding of the receptor, have also been described. For example, harmol hydrochloride and pyrvinium pamoate have been found to inhibit AR activity through interaction with the DBD, apparently by disrupting DNA binding, and by acting at a subsequent step in receptor signaling, possibly disrupting protein-protein interactions (84). In separate studies, a small molecule, theophylline, 8-[(benzylthio)methyl]-(7CI,8CI), was identified that inhibits ERα-dependent DNA binding. Importantly theophylline, 8-[(benzylthio)methyl]-(7CI,8CI) is highly selective for ERα vs other SHRs and inhibits growth of breast cancer cells that are resistant to tamoxifen (85). Identification of these small molecules illustrates the possibilities for developing inhibitors that target domains of SHRs other than the LBD pocket and that can allosterically modulate SHR function.
It is surprising that the ID NTD/AF1 has appeared to be a rather unattractive target for small-molecule inhibitors of receptor function despite 1) the development of several small-molecule inhibitors of receptor that allosterically modulate SHR functions, 2) the critical role that structural flexibility can play in allosteric modulation, and 3) the evidence from both in vitro cell-based and animal studies that the relative contributions of AF1 and AF2 in determining SRM activity is receptor and/or context dependent (86). Recently, several studies have confirmed the tissue-specific role of AF1 in vivo using ER transgenic mice (87–91), highlighting the physiological importance of AF1 for tissue-specific actions of ER and the rationale for designing SRMs and other biologicals that target AF1. The significance of these findings lies in the possibility of therapeutically targeting NTD/AF1 surfaces directly or indirectly, by allosteric modulations, to achieve tissue-restricted effects. The NTD/AF1 of NRs has been considered a difficult drug target because there is no structural 3-dimensional motif for rationale drug design. However, targeting IDRs of transcription factors with small-molecule drugs that can inhibit interactions with protein-binding partners is an emerging field that shows excellent promise (14). Drugs have been defined that interfere with intrinsic disorder-to-order transition induced by a binding protein by either directly interacting with IDRs of the transcription factor (ie, c-myc and EWS-FLI1) and causing misfolding or by targeting a structural motif in the protein binding partner (ie, p53) (14). Recently, a small molecule, a bisphenol A ester derivative (EPI-001) was found to interact with NTD/AF1 of AR and differentially disrupt both AF1-coactivator binding and subsequent AR AF1-regulated target gene expression (92). EP1–001 also blocked androgen-induced proliferation and caused cytoreduction of castration-recurrent prostate cancer in xenografts dependent on androgen receptor for growth and survival without causing toxicity in other tissues (92). EPI-001 appears to bind covalently to IDRs of AF1 in vitro and with endogenous AR in living cells and inhibits the constitutively active AR splice variants lacking the LBD that are suspected to be a cause of resistance in castration-recurrent prostate cancers (93).
Targeting ID proteins by small molecules to block protein-protein interactions is a rapidly evolving field, and the above findings suggest that compounds that bind to NTD/AF1 could be promising small molecules for NR-based therapeutics. A major challenge, though, with developing compounds that target IDRs of NRs is the paucity of information on the physiologically relevant NTD/AF1-interacting coregulatory proteins. Also lacking is a better understanding of the conformation of the functionally active folded state of NTD/AF1 that is induced by a protein-binding partner. Meaningful screens for drugs that block interactions of proteins with IDRs of NTD/AF1s will require identification of the most functionally important coregulatory proteins or protein complexes that interact with the NTD/AF1. Nonetheless, the above results suggest that a multifactorial approach of ligand, coregulator, enzyme, and assorted small-molecule inhibitors (or activators) in an organ-dependent context may provide the additional selectivity needed to target selected genes and thereby reduce the number of undesirable side effects in current endocrine therapies.
Summary and Perspectives
The emerging picture is that in order to access the entire NR-signaling spectrum for the development of novel and potent therapeutic agents, we must determine the structure not only of full-length NRs but also of more than short peptide fragments of associated coregulatory proteins. Elegant studies showing the induction of higher order structures in portions of NRs and coregulators both by small molecules and posttranslational modifications, are especially promising approaches to provide stabilized structures for X-ray analysis and to selectively modulate NR signaling. Unfortunately, no comparable reports yet exist in which all components are full-length proteins. The highly dynamic nature of the intrinsically disordered NTD is not likely to permit high resolution X-ray crystal structures of intact NRs or SHRs unless the NTD can be decorated with a posttranslational modification(s), protein-binding partner, or protein complex that can induce a stable folded conformation. Alternatively, a combination of solution phase approaches such as cryo-EM, small-angle X-ray scattering, and HDX-MS are proving to be of value to gain further insights into how modulation of the structural dynamics of intact SHRs controls receptor function. Based on our current level of understanding of NRs, the combination of allosteric coupling between receptor domains, the cell/tissue-specific concentration of coregulatory proteins, and posttranslational modifications appear to influence the structural dynamics of the NRs in general, and in particular the NTD/AF1 that has so far defied x-ray analysis (Figure 2). Therefore, identifying potential avenues that could modify the structural dynamics of the ID NTD/AF1 domain may provide opportunities for better-targeted endocrine-based therapies. High-throughput screening is well suited to identify potential modulating small molecules. Thus the illuminating reports of structural analyses of even large fragments of NRs should not be viewed as the beginning of the end but rather the end of the beginning of efforts to control NR specificity with greater precision. With new structural information regarding the dynamics and allosteric regulations and identification of physiological coregulatory proteins that regulate NTD/AF1 folding and activity, it should be possible both to design more effective and target-specific SRMs for clinical applications and to better understand the gene-specific activities of NR-complexes.
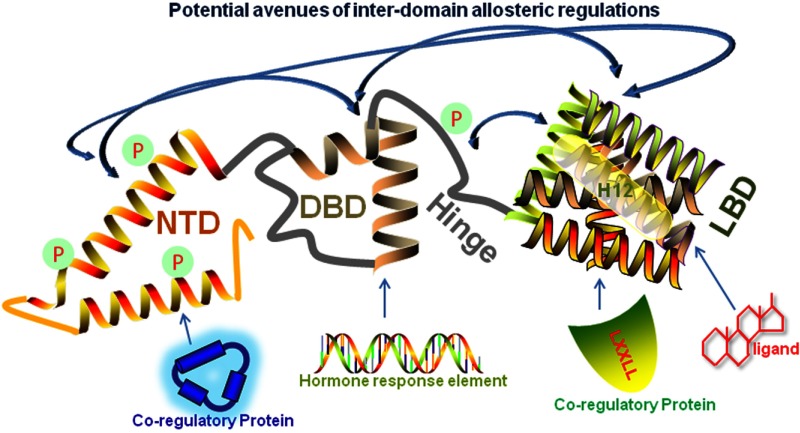
Figure 2.
Various inter- and intramolecular avenues exist for allosteric regulation of NR activity. Shown is a representative folded SHR structure resulting from both posttranslational modifications (“P” inside green circles) and the association (indicated by arrow) of ligand, DNA, and AF1- and AF2-binding proteins (which can be the same molecule interacting at 2 sites). Binding of different molecules in the ligand-binding pocket can pass the signal to the surface of the LBD and dynamically reorient AF2/H12 conformation and other parts of the domain. Signals and consequent conformational changes are then passed (double-ended blue arrows) either sequentially or directly to the hinge, DBD, and/or ID NTD/AF1. In a similar fashion, (hormone response element (HRE)-DBD binding passes signals to influence the structure of NTD/AF1 and/or the AF2 surface. Direct binding of coregulatory proteins, site-specific phosphorylation, and possibly other posttranslational modifications (P), and even ID NTD/AF1-flanking sequences within the NTD can be avenues for allosteric coupling involving ID NTD/AF1 and other receptor domains. The structures/shapes shown for each domain are only representative and are not actual conformations.
Acknowledgments
This work was supported by National Cancer Institute Grant CA046938 (to D.P.E.), National Institutes of Health (NIH) Grant DK049030 (to D.P.E.), and in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases (to S.S.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AF1 and 2
- activation function 1 and 2
- AR
- androgen receptor
- DBD
- DNA-binding domain
- EM
- electron microscopy
- ER
- estrogen receptor
- GR
- glucocorticoid receptor
- HDX-MS
- hydrogen-deuterium exchange coupled with mass spectrometry
- HNF
- hepatocyte nuclear factor
- ID
- intrinsically disordered
- IDR
- ID region
- LBD
- ligand-binding domain
- MR
- mineralocorticoid receptor
- NR
- nuclear receptor
- NTD
- N-terminal domain
- PPAR
- peroxisome proliferator-activated receptor
- PR
- progesterone receptor
- RXR
- retinoid X receptor
- SHR
- steroid hormone receptor
- SRC
- steroid receptor coactivator
- SRM
- selective nuclear receptor modulator
- TBP
- TATA-binding protein
- TIF
- transcriptional intermediary factor
- VDR
- vitamin D receptor.
References
- 1. Kumar R, McEwan IJ. Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocr Rev. 2012;33(2):271–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nilsson S, Koehler KF, Gustafsson JÅ. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov. 2011;10(10):778–792 [DOI] [PubMed] [Google Scholar]
- 3. McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10(6):620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shapiro DJ, Mao C, Cherian MT. Small molecule inhibitors as probes for estrogen and androgen receptor action. J Biol Chem. 2011;286(6):4043–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson AB, O'Malley BW. Steroid receptor coactivators 1, 2, and 3: critical regulators of nuclear receptor activity and steroid receptor modulator (SRM)-based cancer therapy. Mol Cell Endocrinol. 2012;348(2):430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang W, Greene GL, Ravikumar KM, Yang S. Cross-talk between the ligand- and DNA-binding domains of estrogen receptor. Proteins. 2013;81(11):1909–1919 [DOI] [PubMed] [Google Scholar]
- 7. Brzozowski AM, Pike AC, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–758 [DOI] [PubMed] [Google Scholar]
- 8. Wu YL, Yang X, Ren Z, et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell. 2005;18(4):413–424 [DOI] [PubMed] [Google Scholar]
- 9. Nettles KW, Bruning JB, Gil G, et al. Structural plasticity in the oestrogen receptor ligand-binding domain. EMBO Rep. 2007;8(6):563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hollenberg SM, Evans RM. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988;55(5):899–906 [DOI] [PubMed] [Google Scholar]
- 11. Onate SA, Boonyaratanakornkit V, Spencer TE, et al. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem. 1998;273(20):12101–12108 [DOI] [PubMed] [Google Scholar]
- 12. Kitagawa H, Yanagisawa J, Fuse H, et al. Ligand-selective potentiation of rat mineralocorticoid receptor activation function 1 by a CBP-containing histone acetyltransferase complex. Mol Cell Biol. 2002;22(11):3698–3706 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Liu J, Perumal NB, Oldfield CJ, Su EW, Uversky VN, Dunker AK. Intrinsic disorder in transcription factors. Biochemistry. 2006;45(22):6873–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunker AK, Uversky VN. Drugs for 'protein clouds': targeting intrinsically disordered transcription factors. Curr Opin Pharmacol. 2010;10(6):782–788 [DOI] [PubMed] [Google Scholar]
- 15. Ferreon AC, Ferreon JC, Wright PE, Deniz AA. Modulation of allostery by protein intrinsic disorder. Nature. 2013;498(7454):390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demarest SJ, Martinez-Yamout M, Chung J, et al. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature. 2002;415(6871):549–553 [DOI] [PubMed] [Google Scholar]
- 17. Vacic V, Oldfield CJ, Mohan A, et al. Characterization of molecular recognition features, MoRFs, and their binding partners. J Proteome Res. 2007;6(6):2351–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunker AK, Uversky VN. Signal transduction via unstructured protein conduits. Nat Chem Biol. 2008;4(4):229–230 [DOI] [PubMed] [Google Scholar]
- 19. Wright PE, Dyson HJ. Linking folding and binding. Curr Opin Struct Biol. 2009;19(1):31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer K, Kelly SM, Watt K, Price NC, McEwan IJ. Conformation of the mineralocorticoid receptor N-terminal domain: evidence for induced and stable structure. Mol Endocrinol. 2010;24(10):1935–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar R, Lee JC, Bolen DW, Thompson EB. The conformation of the glucocorticoid receptor af1/tau1 domain induced by osmolyte binds co-regulatory proteins. J Biol Chem. 2001;276(21):18146–18152 [DOI] [PubMed] [Google Scholar]
- 22. Bain DL, Franden MA, McManaman JL, Takimoto GS, Horwitz KB. The N-terminal region of human progesterone B-receptors: biophysical and biochemical comparison to A-receptors. J Biol Chem. 2001;276(26):23825–23831 [DOI] [PubMed] [Google Scholar]
- 23. Wärnmark A, Wikström A, Wright AP, Gustafsson JA, Härd T. The N-terminal regions of estrogen receptor α and β are unstructured in vitro and show different TBP binding properties. J Biol Chem. 2001;276(49):45939–45944 [DOI] [PubMed] [Google Scholar]
- 24. Reid J, Kelly SM, Watt K, Price NC, McEwan IJ. Conformational analysis of the androgen receptor amino-terminal domain involved in transactivation. Influence of structure-stabilizing solutes and protein-protein interactions. J Biol Chem. 2002;277(22):20079–20086 [DOI] [PubMed] [Google Scholar]
- 25. Khan SH, Awasthi S, Guo C, et al. Binding of the N-terminal region of coactivator TIF2 to the intrinsically disordered AF1 domain of the glucocorticoid receptor is accompanied by conformational reorganizations. J Biol Chem. 2012;287(53):44546–44560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chalmers MJ, Busby SA, Pascal BD, et al. Probing protein ligand interactions by automated hydrogen/deuterium exchange mass spectrometry. Anal Chem. 2006;78(4):1005–1014 [DOI] [PubMed] [Google Scholar]
- 27. Bruning JB, Chalmers MJ, Prasad S, et al. Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure. 2007;15(10):1258–1271 [DOI] [PubMed] [Google Scholar]
- 28. Chandra V, Huang P, Hamuro Y, et al. Structure of the intact PPAR-γ-RXR-nuclear receptor complex on DNA. Nature. 2008;456(7220):350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar R, Moure CM, Khan SH, et al. Regulation of the structurally dynamic amino-terminal domain of progesterone receptor by protein induced folding. J Biol Chem. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lonard DM, O'Malley BW. Nuclear receptor coregulators: modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8(10):598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen W, Rogatsky I, Garabedian MJ. MED14 and MED1 differentially regulate target-specific gene activation by the glucocorticoid receptor. Mol Endocrinol. 2006;20(3):560–572 [DOI] [PubMed] [Google Scholar]
- 32. Choudhry MA, Ball A, McEwan IJ. The role of the general transcription factor IIF in androgen receptor-dependent transcription. Mol Endocrinol. 2006;20(9):2052–2061 [DOI] [PubMed] [Google Scholar]
- 33. Motlagh HN, Hilser VJ. Agonism/antagonism switching in allosteric ensembles. Proc Natl Acad Sci USA. 2012;109(11):4134–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan SH, Ling J, Kumar R. TBP binding-induced folding of the glucocorticoid receptor AF1 domain facilitates its interaction with steroid receptor coactivator-1. PLoS One. 2011;6(7):e21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar R, Volk DE, Li J, Lee JC, Gorenstein DG, Thompson EB. TATA box binding protein induces structure in the recombinant glucocorticoid receptor AF1 domain. Proc Natl Acad Sci USA. 2004;101(47):16425–16430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar R, Betney R, Li J, Thompson EB, McEwan IJ. Induced alpha-helix structure in AF1 of the androgen receptor upon binding transcription factor TFIIF. Biochemistry. 2004;43(11):3008–3013 [DOI] [PubMed] [Google Scholar]
- 37. Ma H, Hong H, Huang SM, et al. Multiple signal input and output domains of the 160-kilodalton nuclear receptor coactivator proteins. Mol Cell Biol. 1999;19(9):6164–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang D, Simons SS., Jr Corepressor binding to progesterone and glucocorticoid receptors involves the activation function-1 domain and is inhibited by molybdate. Mol Endocrinol. 2005;19(6):1483–1500 [DOI] [PubMed] [Google Scholar]
- 39. Cho S, Blackford JA, Jr, Simons SS., Jr Role of activation function domain-1, DNA binding, and coactivator GRIP1 in the expression of partial agonist activity of glucocorticoid receptor-antagonist complexes. Biochemistry. 2005;44(9):3547–3561 [DOI] [PubMed] [Google Scholar]
- 40. Hill KK, Roemer SC, Churchill ME, Edwards DP. Structural and functional analysis of domains of the progesterone receptor. Mol Cell Endocrinol. 2012;348(2):418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garza AM, Khan SH, Kumar R. Site-specific phosphorylation induces functionally active conformation in the intrinsically disordered N-terminal activation function (AF1) domain of the glucocorticoid receptor. Mol Cell Biol. 2010;30(1):220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chandra V, Huang P, Potluri N, Wu D, Kim Y, Rastinejad F. Multidomain integration in the structure of the HNF-4α nuclear receptor complex. Nature. 2013;495(7441):394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whitford PC. Disorder guides protein function. Proc Natl Acad Sci USA. 2013;110(18):7114–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wardell SE, Kwok SC, Sherman L, Hodges RS, Edwards DP. Regulation of the amino-terminal transcription activation domain of progesterone receptor by a cofactor-induced protein folding mechanism. Mol Cell Biol. 2005;25(20):8792–8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hill KK, Roemer SC, Jones DN, Churchill ME, Edwards DP. A progesterone receptor co-activator (JDP2) mediates activity through interaction with residues in the carboxyl-terminal extension of the DNA binding domain. J Biol Chem. 2009;284(36):24415–24424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garza AS, Khan SH, Moure CM, Edwards DP, Kumar R. Binding-folding induced regulation of AF1 transactivation domain of the glucocorticoid receptor by a cofactor that binds to its DNA binding domain. PLoS One. 2011;6(10):e25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watson LC, Kuchenbecker KM, Schiller BJ, Gross JD, Pufall MA, Yamamoto KR. The glucocorticoid receptor dimer interface allosterically transmits sequence-specific DNA signals. Nat Struct Mol Biol. 2013;20(7):876–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brodie J, McEwan IJ. Intra-domain communication between the N-terminal and DNA-binding domains of the androgen receptor: modulation of androgen response element DNA binding. J Mol Endocrinol. 2005;34(3):603–615 [DOI] [PubMed] [Google Scholar]
- 49. Kumar R, Baskakov IV, Srinivasan G, Bolen DW, Lee JC, Thompson EB. Interdomain signaling in a two-domain fragment of the human glucocorticoid receptor. J Biol Chem. 1999;274(35):24737–24741 [DOI] [PubMed] [Google Scholar]
- 50. Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324(5925):407–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rochel N, Ciesielski F, Godet J, et al. Common architecture of nuclear receptor heterodimers on DNA direct repeat elements with different spacings. Nat Struct Mol Biol. 2011;18(5):564–570 [DOI] [PubMed] [Google Scholar]
- 52. Osz J, Pethoukhov MV, Sirigu S, Svergun DI, Moras D, Rochel N. Solution structures of PPARγ2/RXRα complexes. PPAR Res. 2012;2012:701412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orlov I, Rochel N, Moras D, Klaholz BP. Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J. 2012;31(2):291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dai SY, Chalmers MJ, Bruning J, et al. Prediction of the tissue-specificity of selective estrogen receptor modulators by using a single biochemical method. Proc Natl Acad Sci USA. 2008;105(20):7171–7176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J, Chalmers MJ, Stayrook KR, et al. DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat Struct Mol Biol. 2011;18(5):556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Devarakonda S, Gupta K, Chalmers MJ, et al. Disorder-to-order transition underlies the structural basis for the assembly of a transcriptionally active PGC-1α/ERRγ complex. Proc Natl Acad Sci USA. 2011;108(46):18678–18683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang Q, Blackford JA, Jr, Song LN, Huang Y, Cho S, Simons SS., Jr Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Mol Endocrinol. 2004;18(6):1376–1395 [DOI] [PubMed] [Google Scholar]
- 58. Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9(9):2811–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Simons SS., Jr Glucocorticoid receptor cofactors as therapeutic targets. Curr Opin Pharmacol. 2010;10(6):613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264(5164):1455–1458 [DOI] [PubMed] [Google Scholar]
- 61. Szapary D, Huang Y, Simons SS., Jr Opposing effects of corepressor and coactivators in determining the dose-response curve of agonists, and residual agonist activity of antagonists, for glucocorticoid receptor-regulated gene expression. Mol Endocrinol. 1999;13(12):2108–2121 [DOI] [PubMed] [Google Scholar]
- 62. Lee GS, Simons SS., Jr Ligand binding domain mutations of the glucocorticoid receptor selectively modify the effects with, but not binding of, cofactors. Biochemistry. 2011;50(3):356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Norris JD, Paige LA, Christensen DJ, et al. Peptide antagonists of the human estrogen receptor. Science. 1999;285(5428):744–746 [DOI] [PubMed] [Google Scholar]
- 64. Sistare FD, Hager GL, Simons SS., Jr Mechanism of dexamethasone 21-mesylate antiglucocorticoid action: I. Receptor-antiglucocorticoid complexes do not competitively inhibit receptor-glucocorticoid complex activation of gene transcription in vivo. Mol Endocrinol. 1987;1(9):648–658 [DOI] [PubMed] [Google Scholar]
- 65. Billas I, Moras D. Allosteric controls of nuclear receptor function in the regulation of transcription. J Mol Biol. 2013;425(13):2317–2329 [DOI] [PubMed] [Google Scholar]
- 66. Estébanez-Perpiñá E, Arnold LA, Arnold AA, et al. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc Natl Acad Sci USA. 2007;104(41):16074–16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joseph JD, Wittmann BM, Dwyer MA, et al. Inhibition of prostate cancer cell growth by second-site androgen receptor antagonists. Proc Natl Acad Sci USA. 2009;106(29):12178–12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Norris JD, Joseph JD, Sherk AB, et al. Differential presentation of protein interaction surfaces on the androgen receptor defines the pharmacological actions of bound ligands. Chem Biol. 2009;16(4):452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simons SS, Jr., Chow CC. The road less traveled: new views of steroid receptor action from the path of dose-response curves. Mol Cell Endocrinol. 2012;348(2):373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ong KM, Blackford JA, Jr., Kagan BL, Simons SS, Jr., Chow CC. A theoretical framework for gene induction and experimental comparisons. Proc Natl Acad Sci USA. 2010;107(15):7107–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Luo M, Simons SS., Jr Modulation of glucocorticoid receptor induction properties by cofactors in peripheral blood mononuclear cells. Hum Immunol. 2009;70(10):785–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Awasthi S, Simons SS., Jr Separate regions of glucocorticoid receptor, coactivator TIF2, and comodulator STAMP modify different parameters of glucocorticoid-mediated gene induction. Mol Cell Endocrinol. 2012;355(1):121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dougherty EJ, Guo C, Simons SS, Jr, Chow CC. Deducing the temporal order of cofactor function in ligand-regulated gene transcription: theory and experimental verification. PLoS One. 2012;7(1):e30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Blackford JA, Jr, Guo C, Zhu R, Dougherty EJ, Chow CC, Simons SS., Jr Identification of location and kinetically defined mechanism of cofactors and reporter genes in the cascade of steroid-regulated transactivation. J Biol Chem. 2012;287(49):40982–40995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang Z, Sun Y, Cho YW, Chow CC, Simons SS., Jr PA1 protein, a new competitive decelerator acting at more than one step to impede glucocorticoid receptor-mediated transactivation. J Biol Chem. 2013;288(1):42–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shiau AK, Barstad D, Loria PM, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927–937 [DOI] [PubMed] [Google Scholar]
- 77. Reese JC, Katzenellenbogen BS. Differential DNA-binding abilities of estrogen receptor occupied with two classes of antiestrogens: studies using human estrogen receptor overexpressed in mammalian cells. Nucleic Acids Res. 1991;19(23):6595–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cheng J, Zhang C, Shapiro DJ. A functional serine 118 phosphorylation site in estrogen receptor-α is required for down-regulation of gene expression by 17β-estradiol and 4-hydroxytamoxifen. Endocrinology. 2007;148(10):4634–4641 [DOI] [PubMed] [Google Scholar]
- 79. Johnston SJ, Cheung KL. Fulvestrant—a novel endocrine therapy for breast cancer. Curr Med Chem. 2010;17(10):902–914 [DOI] [PubMed] [Google Scholar]
- 80. Wardell SE, Marks JR, McDonnell DP. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol. 2011;82(2):122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Robertson JF. Fulvestrant (Faslodex)—how to make a good drug better. Oncologist. 2007;12(7):774–784 [DOI] [PubMed] [Google Scholar]
- 82. Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res. 2013;19(9):2420–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kretzer NM, Cherian MT, Mao C, et al. A noncompetitive small molecule inhibitor of estrogen-regulated gene expression and breast cancer cell growth that enhances proteasome-dependent degradation of estrogen receptor α. J Biol Chem. 2010;285(53):41863–41873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jones JO, Bolton EC, Huang Y, et al. Non-competitive androgen receptor inhibition in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106(17):7233–7238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mao C, Patterson NM, Cherian MT, et al. A new small molecule inhibitor of estrogen receptor α binding to estrogen response elements blocks estrogen-dependent growth of cancer cells. J Biol Chem. 2008;283(19):12819–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Arnal JF, Fontaine C, Abot A, et al. Lessons from the dissection of the activation functions (AF-1 and AF-2) of the estrogen receptor α in vivo. Steroids. 2013;78(6):576–582 [DOI] [PubMed] [Google Scholar]
- 87. Arao Y, Hamilton KJ, Goulding EH, Janardhan KS, Eddy EM, Korach KS. Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor α is crucial to maintain male reproductive tract function. Proc Natl Acad Sci USA. 2012;109(51):21140–21145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS. Estrogen receptor α AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA. 2011;108(36):14986–14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Billon-Galés A, Fontaine C, Filipe C, et al. The transactivating function 1 of estrogen receptor α is dispensable for the vasculoprotective actions of 17β-estradiol. Proc Natl Acad Sci USA. 2009;106(6):2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Börjesson AE, Windahl SH, Lagerquist MK, et al. Roles of transactivating functions 1 and 2 of estrogen receptor-α in bone. Proc Natl Acad Sci USA. 2011;108(15):6288–6293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Billon-Galés A, Krust A, Fontaine C, et al. Activation function 2 (AF2) of estrogen receptor-α is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA. 2011;108(32):13311–13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535–546 [DOI] [PubMed] [Google Scholar]
- 93. Myung JK, Banuelos CA, Fernandez JG, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123(7):2948–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]