Abstract
Background and Purpose
Electrodiagnostic studies can be used to confirm the diagnosis of lumbosacral radiculopathies, but more sensitive diagnostic methods are often needed to measure the ensuing motor neuronal loss and sympathetic failure.
Methods
Twenty-six patients with lumbar radiculopathy and 30 controls were investigated using nerve conduction studies, motor unit number estimation (MUNE), testing of the sympathetic skin response (SSR), quantitative electromyography (QEMG), and magnetic resonance myelography (MRM).
Results
Using QEMG as the gold standard, the sensitivity and specificity of MUNE for the abductor hallucis longus muscle were 71.4% and 70%, respectively. While they were 75% and 68.8%, respectively, when used MRM as gold standard. The sensitivity and specificity of MUNE for the extensor digitorum brevis muscle were 100% and 84.1%, respectively, when the peroneal motor amplitude as the gold standard. The SSR latency was slightly longer in the patients than in the controls.
Conclusions
MUNE is a simple and sensitive test for evaluating autonomic function and for diagnosing lumbosacral radiculopathy in patients. MUNE could be used routinely as a guide for the rehabilitation of patients with radiculopathies. SSR measurements may reveal subtle sympathetic abnormalities in patients with lumbosacral radiculopathy.
Keywords: lumbosacral radiculopathy, nerve conduction study, quantitative electromyography, motor unit number estimation, sympathetic skin response
Introduction
Most lumbosacral radiculopathies are caused by root compression resulting from intervertebral disc degeneration.1 Lumbosacral nerve root compromise is clinically diagnosed by pain in the lower back that radiates into the leg below the buttocks, and may be confirmed with radiologic and electrodiagnostic studies.2-4 Imaging methods such as magnetic resonance imaging demonstrate structural abnormalities from which neurologic sequelae may be inferred. Electrodiagnostic methods can be used to assess the physiologic integrity of the nerve roots.3 Such electrophysiologic procedures include motor and sensory nerve conduction studies (NCSs), late-response studies, somatosensory and motor evoked potentials, nerve root stimulation, and needle electromyography (EMG); needle EMG is currently the single most useful procedure.1
Motor unit number estimation (MUNE) is an electrophysiological method designed to measure axon loss in the peripheral nerves. Since conventional electrophysiologic methods are not sufficiently sensitive measures of axon loss, MUNE may useful method in the diagnosis of diseases whose progression is associated with axon loss.5
The early detection of sympathetic failure using conventional electrodiagnostic procedures has been poorly documented.6,7 The sympathetic skin response (SSR) has been proposed as a reliable and simple test, and is commonly used for the evaluation of sympathetic function in polyneuropathies and dysautonomic disorders.7,8 It measures transient changes in the electrical potential of the skin evoked both spontaneously and by various internal and external stimuli.9
To the best of our knowledge, the use of MUNE for the diagnosis of radiculopathies has not been studied previously, while only a few studies have used SSR for this purpose. The aim of the present study was thus to determine the sensitivity of MUNE for the evaluation of the degree of root involvement in radiculopathies, while simultaneously searching for dysautonomic changes using SSR.
Methods
Subjects
Patients
Patients with clinically diagnosed lumbosacral radiculopathies were examined in this study. Those with a history consistent with lumbar radiculopathy-defined as pain in the lower back that radiates into the leg below the buttocks- were selected. The patient group comprised 12 males and 14 females (n=52 lower limbs) aged 51.6±11.2 years (mean±SD; range, 28-77 years). All of the patients were asked about their peripheral neuropathic symptoms, and examined carefully for such conditions, and all submitted to NCSs, MUNE, and SSR evaluations. Twenty-four of the patients also submitted to quantitative EMG (QEMG), and 24 patients were evaluated by magnetic resonance myelogram (MRM).
Patients with diabetes mellitus, alcoholism, amyloidosis, and other metabolic diseases resulting in neuropathies were excluded, as were those who had undergone lumbar surgery or who suffered from spinal stenosis identified through MRM.
Controls
The control group comprised 30 healthy volunteers (n=60 lower limbs; 16 males and 14 females) aged 40.1±11.0 years (range, 26-69 years) and with normal findings on neurologic examination. None of the subjects in the control group had a history of significant back pain or any previous history of sciatica or neuropathy, and all submitted to NCSs, MUNE, and SSR evaluations.
This study was approved by the ethics committee of Ondokuz Mayıs University, and informed consent to participate was obtained from all of the subjects.
Clinical evaluation
All of the patients were asked to respond to questions about their symptoms of numbness, leg and back pain, burning, tingling, and weakness. One point was assigned for each symptom, and the total number was counted to produce a total symptom score (TSS) for each patient.10
The findings of neurological examinations were also evaluated quantitatively for each of the patients, who were examined to determine the presence of weakness and of sensation and deep-tendon-reflex abnormalities. Both touch and vibration sensation abnormalities were evaluated, and were considered to be present when sensation loss was found or there was reduced sensation in a dermatomal pattern. The patella and Achilles reflexes were examined to establish the presence of a deep tendon reflex abnormality. If a reflex was unobtainable or reduced compared with the contralateral side, it was determined to be abnormal and coded with 1 point. Weakness was assessed via flexion and extension of the hip, knee, ankle, and thumb muscles by manual muscle testing. The Lasègue test was also conducted, and the presence of muscle atrophy was assessed. Each abnormal component was assigned 1 point. The total finding score (TFS) was calculated by assigning 1 point for each abnormal finding.10-12 The patients were questioned regarding the following autonomic symptoms: excessive sweating, feeling cold, and color change of the feet. Again, 1 point was awarded for the presence of each symptom, and summed to provide the sympathetic system score (SSS).9,13-15 The maximum scores for TSS, TFS, and SSS were 6, 14, and 3, respectively.
Neurophysiologic investigation
Nerve conduction studies, QEMG, SSR, and MUNE examinations were performed using Keypoint EMG equipment (Medtronic, Skovlunde, Denmark). All recordings were performed on subjects lying supine in a bed in an air-conditioned and softly lit room that was maintained at a constant temperature of 25℃. Their skin temperature was maintained at 31-32℃.
Nerve conduction studies
All NCSs were performed using standard techniques for supramaximal percutaneous stimulation with a constant-current stimulator and surface electrode recording. Peroneal and tibial motor NCSs (including F-waves and H-reflex)16 and superficial peroneal and sural sensory NCSs were performed.17
Quantitative electromyography
Concentric needle electrodes were used for the QEMG analysis. The vastus lateralis, tibialis anterior, gluteus medius, medial gastrocnemius, and the short head of the biceps femoris muscles were investigated. The L4-5 and S1 paraspinal muscles were occasionally tested to confirm the diagnosis.
The investigations were performed unilaterally on 6 of the patients and bilaterally on the remaining 20. Since the QEMG technique is time consuming and patients have a low tolerance for it, the symptomatic sides of the patients were examined first; QEMG testing was then carried out bilaterally on consenting patients. Motor unit potentials (MUPs) were collected with concentric electrodes and the MUP data were analyzed by multi-MUP analysis.18,19
Sympathetic skin responses
Sympathetic skin response waves were recorded from both feet in each patient via superficial recording electrodes from two channels (impedance, <5 kΩ; frequency range, 0.5-2kHz). The electrical stimulus was applied five times over the sternum at irregular intervals of between 30 and 60 s (stimulus duration, 0.1 ms; intensity, 80-100 mA), and five SSR waves were obtained. The mean latency, peak-to-peak amplitude, and area (including positive and negative phases) of these five waves were calculated.
Motor unit number estimation
Motor unit number estimation was conducted using the incremental technique described by McComas et al.20,21 For the tibial and peroneal nerves, recording and stimulating electrodes were placed as defined in other motor conduction studies.16
Radiological investigation
The MRM findings were analyzed with regard to the grade of nerve root compression (grade 1, contact; grade 3, displacement; grade 5, entrapment), and the association with foraminal stenosis. If there was no contact with the nerve root, the grade was designated as 0. If the herniated disc contacted the nerve root, and the nerve root was in its normal position, it was designated as grade 1. When the herniated disc material displaced the nerve root and this nerve root could be visualized by MRM, it was defined as grade 3. If the nerve root was entrapped between the herniated disc material and the lamina or facet, it was designated as grade 5. When there was an association with the foraminal stenosis, grade 1 was changed to grade 2, and grade 3 was changed to grade 4.
Electrophysiological diagnosis of radiculopathy
Lumbosacral radiculopathies were diagnosed based on the presence of neurogenic involvement in two or more muscles innervated at the same nerve root level but by different peripheral nerves.1 The criteria regarding neurogenic involvement were the presence of spontaneous activity (positive sharp waves, fibrillation potentials, and complex repetitive discharges) and/or changes in MUP morphology (high-amplitude, long-duration, and increased polyphasic MUPs). The changes in MUP morphology were evaluated quantitatively based on the results obtained from the multi-MUP analysis.18,19
The paraspinal muscles were considered as a single muscle for the purposes of the data analysis. If any level of the paraspinal muscles exhibited spontaneous potentials, they were designated as abnormal.22
Statistical methods
MedCalc and SPSS v16.0 computer analysis programs were used for the statistical analyses. NCSs, MUNE, SSR, and height parameters were compared between the patient and control groups using Student's t-test/Mann-Whitney U test. The normal distribution of the data was confirmed using the Kolmogorov-Smirnov test. Student's t-test was used to compare data with a normal distribution. Data that did not conform to a normal distribution were compared using the Mann-Whitney U-test. The chi-square test was used to compare SSSs between the patients and controls.
Correlations between the clinical scores (TSS, TFS, and SSS) and symptoms, MUNE values, compound muscle action potential (CMAP) amplitudes, and the radiological grade of herniation were confirmed as conforming to a normal distribution using the Kolmogorov-Smirnov test, and then investigated respectively using Pearson's correlation coefficient for samples. The correlation between age and the parameters searched in lumbar radiculopathies (MUNE values obtained AHL and EDB; latency, amplitude and area of SSR) were respectively investigated using the Spearman correlation.
Receiver operating characteristics (ROC) analysis was used to assess the abnormal cutoff values of the MUNE and the amplitudes of peroneal and tibial CMAPs. The sensitivity and specificity of MUNE were calculated in patients with a neurophysiologically confirmed diagnosis. In addition, the sensitivity and specificity of MUNE were calculated in patients with MRM abnormalities.
Results
While the study was designed, patient and control group were matched by demographic characteristic including age and sex. There was no difference in demographic characteristics (age and sex) between patient and control group. Unfortunately, some patients could not be included in all of the study. The patient group consisted of 52 lower limbs of 26 patients, 12 male and 14 females with a mean age of 51.7±11.2 years (range, 28-77 years) and the control group consisted of 60 lower limbs of 30 healthy volunteers [16 males and 14 females with a mean age of 40.1±11.0 years (range, 26-69 years)]. Therefore the difference might be due to these changes occurred during the study period. In addition we had searched if there was a correlation between age and the parameters searched in lumbar radiculopathies [MUNE values obtained abductor hallucis longus muscle (AHL) and extensor digitorum brevis muscle (EDB); latency, amplitude and area of SSR] (r=-0.113, -0.274, -0.013, -0.136, -0.184 and p=0.419, 0.052, 0.930, 0.374, 0.227 respectively for AHL and EDB; latency, amplitude and area of SSR). Since we couldn't find any correlation between age and these parameters, adjusted covariant analyses were not done.
Electrophysiological radiculopathy was diagnosed in 21 of the 24 patients examined by EMG. The patients had at least one radiculopathy at L4, L5, or S1, and were affected by different combinations of radiculopathies at these levels. One patient had a bilateral radiculopathy at all three levels, and four patients had a unilateral radiculopathy at all three levels, or at one or two levels contralaterally. Twenty-three patients had L4 radiculopathies, 21 patients had L5 radiculopathies, and 14 patients had S1 radiculopathies.
Twenty one of the 24 patients investigated by MRM had a herniated disc in at least at one of the L3-4, L4-5, or L5-S1 disc spaces, the severity of which ranged from grade 1 to 5. Three patients had a herniated disc at the L3-4, L4-5, and L5-S1 disc spaces. Two patients had a herniated disc at both the L3-4 and L4-5 spaces on their left side, and at the L4-5 spaces on their right side. One patient had a herniated disc at both the L3-4 and L4-5 spaces but only on the left side. Nine patients had radiculopathy only at one level unilaterally, two patients had bilateral radiculopathies at the L4-5 spaces, and another patient had disc abnormalities at the L3-4 level on the right, and at the L5-S1 level on the left side, as revealed by MRM.
The distal motor and F-wave minimum (F-min) latencies of the tibial nerve were longer, and the CMAP amplitudes and conduction velocities (CVs) of both the tibial and peroneal nerves were lower in the patient group than in the control group. The H-wave latency (H-latency) was longer in the patient group than in the control group. These differences were statistically significant. The sensory neurography parameters did not differ significantly between the patient and control groups (Table 1).
Table 1.
NCS results
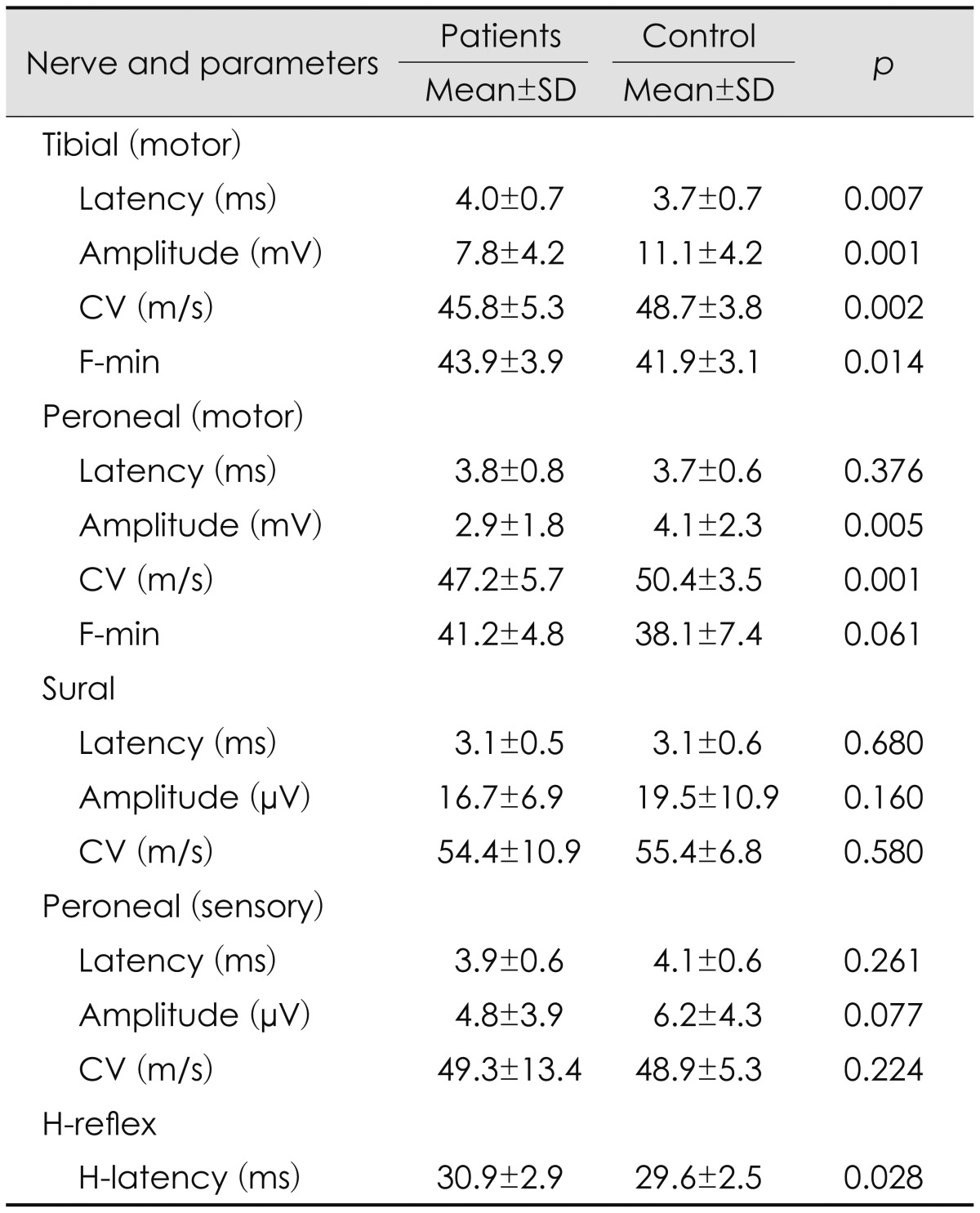
CV: conduction velocity, NCS: nerve conduction study.
The SSR data are presented in Table 2. A comparison of the SSR parameters between the groups did not reveal any significant differences exception for SSR latency, which was slightly longer in the patients than in the healthy subjects. No relationship was observed between SSR parameters and SSS (r=0.073, p=0.686). Comparison of height between the control and patient groups to determine whether there is any correlation between height and SSR parameters revealed no statistically significant difference between the groups: 162.9±10.9 cm (range, 145-182 cm) in the patient group and 164.9±8.6 cm (range, 148-180 cm) among the controls.
Table 2.
Results of SSR studies
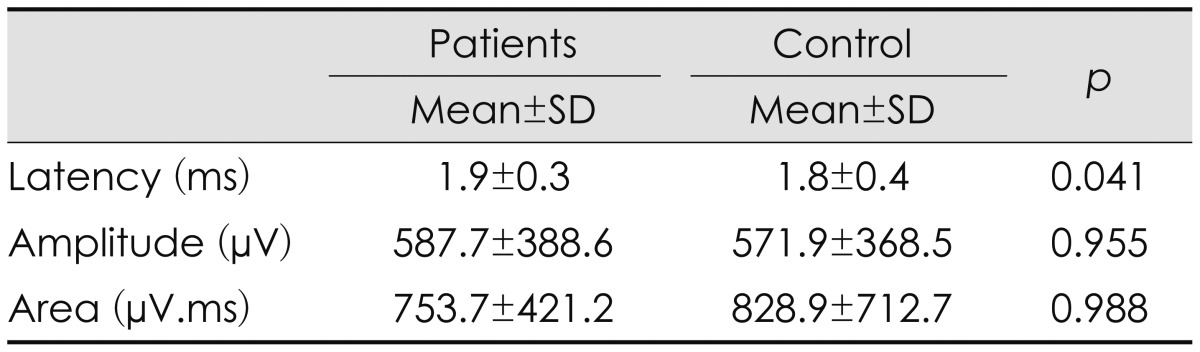
SSR: sympathetic skin response.
The MUNE values of the tibial and peroneal nerves are given in Table 3. The MUNE of the tibial nerve obtained from the AHL and of the peroneal nerve obtained from the EDB was significantly lower in the patient group than in the controls. Table 4 demonstrate the sensitivity, specificity, cut off and area under the ROC curve values of MUNE obtained from EDB and AHL muscles as a new diagnostic tool when used respectively CMAP amplitudes of peroneal and tibial nerves, EMG findings obtained from L5 and S1 innervated muscles, and MRM findings obtained from L4-5 and L5-S1 levels, as the gold standard.
Table 3.
Results of MUNE studies
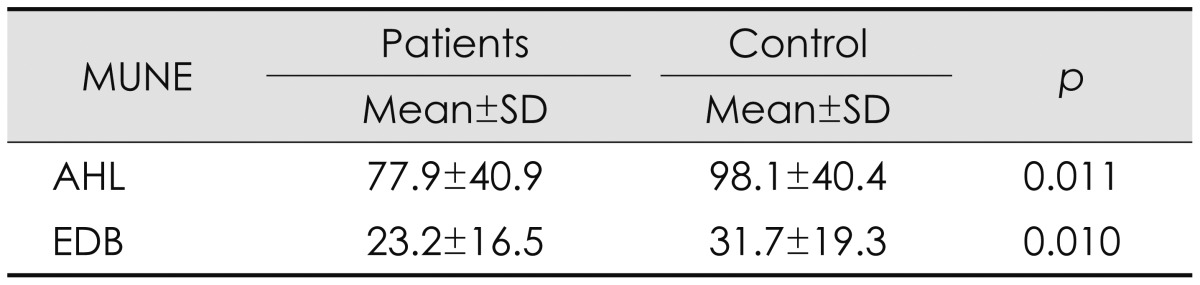
AHL: abductor hallucis longus muscle, EDB: extensor digitorum brevis muscle, MUNE: motor unit number estimation.
Table 4.
MUNE test validity
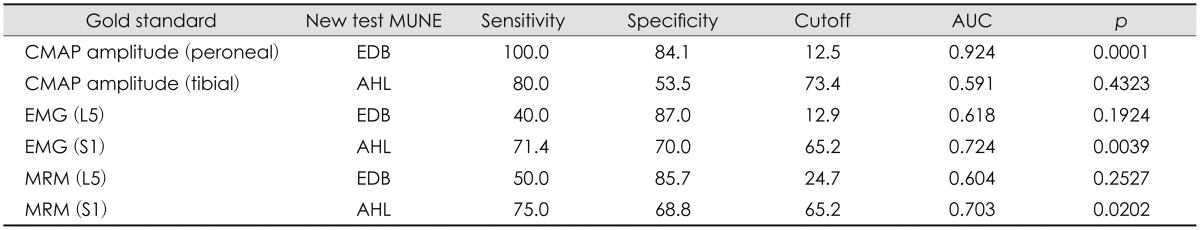
AUC: area under the ROC curve, CMAP: compound muscle action potential, EMG: electromyography, EMG (L5): EMG findings for muscles innervated by the L5 segment, EMG (S1): EMG findings for muscles innervated by the S1 segment, MRM: myelogram, MRM (L5): MRM findings for L4-5 levels, MRM (S1): MRM findings for L5-S1 levels, MUNE: motor unit number estimation.
The sensitivity and specificity MUNE of the AHL were 71.4% and 70%, respectively, when using EMG as the gold standard; corresponding values when using MRM examination as the gold standard were 75% and 68.8%. By comparison, when the peroneal CMAP amplitude was used as the gold standard, the MUNE of the EDB had a sensitivity of 100% and a specificity of 84.1%.
There was a moderate positive correlation between MUNE of the AHL and tibial CMAP amplitude (r=0.463, p=0.001). A stronger positive correlation was also found between MUNE of the EDB and peroneal CMAP amplitude (r=0.636, p=0.001).
There was a moderate negative correlation between the MUNE of the AHL and the grade of MRM findings at the L5-S1 level (r=-0.325, p=0.031) in the patient group. Moreover, a weak negative correlation was found between the MUNE of the AHL and clinical scores (r=-0.208 and -0.216, and p=0.037 and 0.030 for TFS and TSS, respectively).
There was a very strong positive correlation between symptoms and clinical findings (r=0.855, p=0.0001 for both TSS and TFS). There was a weak negative correlation between tibial CMAP amplitude and clinical scores (r=-0.270 and -0.286, and p=0.006 and 0.003 for TFS and TSS, respectively).
Discussion
There are considerable differences in the sensitivities of the various electrodiagnostic procedures that are currently used to detect radiculopathies.1 Motor and sensory NCSs are typically normal in single radiculopathies, for both anatomic and pathophysiologic reasons. The sole parameter in motor conduction studies that may be affected substantially by radiculopathies is the CMAP amplitude. However, this change occurs only if the root lesion causes axonal degeneration.1 In the present study, the CMAP amplitudes of both the tibial and peroneal nerves were lower in the patient group than in the control group, which indicates that root lesions lead to axon loss. In addition, the distal motor and F-min latencies of the tibial nerve were longer, and the tibial and peroneal CV was slower in the patient group than in the control group. These findings may be explained by loss of the fastest-conducting axons.
The H-latency was more prolonged in the patient group than in the control group. This is likely due to the presence of radiculopathy at the S1 level. Since the validity of H-waves was not examined in this study, changes in H-waves relative to the spinal level of the radiculopathy were not investigated.
The MUNEs obtained from the AHL and EDB were significantly lower in the patient group than in the control group. Strong positive correlations were found between the MUNE measurements of the EDB and the peroneal CMAP amplitude; moderate positive correlations were also found between the MUNE measurements of the AHL and tibial CMAP amplitude. These findings are concordant with the presence of axonal loss in our patients. Since the amplitudes of the peroneal and tibial CMAPs are related to the number of axons, the positive correlation between MUNE values and the CMAP amplitude suggests that MUNE could be a direct method for revealing axonal loss.
This is also supported by the finding of negative correlations between tibial CMAP amplitude and clinical scores. The increase in clinical scores, reflecting worse clinical findings, is likely to be associated with the presence of axonal degeneration, leading to persistent symptoms in radiculopathies. By contrast, focal demyelination at the motor root may cause conduction block, which may manifest as a prominent but usually short-lived weakness.1 Small degrees of focal demyelination may result in conduction slowing at the injury point; this is the presumed mechanism underlying the loss of deep tendon reflexes without accompanying clinical weakness or fixed sensory deficit.
The finding of a negative correlation between the MUNE of the AHL and the grade of MRM findings at the L5-S1 level suggests that MUNE is an indicator of axonal loss. This is supported by the finding of a negative correlation between the MUNE of the AHL and clinical scores, and could be explained by greater root compression, as observed through MRM, makes axonal loss more likely.
The sensitivity and specificity of the MUNE measurements of the AHL using EMG as the gold standard (71.4% and 70%, respectively) and using MRM examination as the gold standard (75% and 68.8%, respectively) indicate that MUNE of the AHL could be used for to support diagnoses of radiculopathy.
As a new diagnostic tool, the MUNE measurements of the EDB exhibited a sensitivity of 100% and a specificity of 84.1% when peroneal CMAP amplitude was used as the gold standard; the sensitivity of the MUNE measurements obtained from the EDB was not as high when using EMG or MRM as a gold standard. This difference might be attributable to the anatomy of the EDB, which is a small muscle that can be atrophic in the presence of foot trauma. Since this muscle could have some denervation due to distal traumatic axonal lesion of the peroneal nerve in normal subjects, its sensitivity might not be as high as that of needle EMG or MRM for diagnosing L5 radiculopathy. When the EDB is not as atrophic, MUNE values could be easily obtained from this muscle. Thus, as long as the EDB is not atrophic, the values of peroneal CMAP and MUNE obtained from the EDB will yield more accurate results. The specificity of the MUNE obtained from the EDB should be sufficient to allow a diagnosis in normal subjects when using QEMG and MRM as the gold standard (i.e., 87% and 85.7%, respectively).
All of these findings suggest that MUNE is a useful method for assessing axon count in L5 or S1 radiculopathies, and may therefore be an indicator of the degree of axonal loss and could be used as a guide for the rehabilitation of radiculopathy.
Another finding of the present study was that the SSR latency was slightly longer in the patients than in the healthy subjects. SSRs are considered a simple way of measuring sudomotor activity and are used widely as a rapid and painless method of evaluating sympathetic function,9,23 although there are certain limitations to this viewpoint.24 The high variability of measured SSR amplitudes has resulted in this amplitude not being accepted as a marker for pathology in other SSR studies,24 and the main clinical consideration remains the presence or absence of the response.
Pathophysiologically, the absence of an SSR response indicates a total failure of impulses to propagate along the entire polysynaptic reflex up to the end organs.24 In the present study we were able to obtain SSRs from all of the subjects. Although occasionally it was not possible to yield a single response, in all cases four out of five responses were obtained successfully.
The polysynaptic reflex arc of the SSR involves large myelinated afferent sensory fibers and an efferent pathway formed by sympathetic preganglionic and postganglionic unmyelinated fibers.15,23 Thus, the SSR may be affected not only by efferent autonomic fibers but also by sensorial somatic fibers. However, by applying a suprasternal stimulus, as in this study, the peripheral effects due to the somatic fiber involvement may be ignored.
It is accepted that latency measurements of the SSR are of little value. While the efferent unmyelinated fibers account for most of the latency, it may also be affected by slow conduction in the afferent branch of the reflex arc or central delay in the activation of sympathetic neurons.23
To the best of our knowledge, only a few studies have investigated autonomic involvement in radiculopathies. Although two studies found SSR changes in lumbosacral radiculopathy patients,25,26 another showed that SSR was not significantly altered in L5 and S1 radiculopathies.27 The results of the latter study are probably due to the sympathetic fibers to the leg leaving the upper lumbar (the upper two and sometimes the third lumbar) spinal nerve roots to join the sympathetic trunk.28 Thus, sympathetic vasoconstrictor and sudomotor fibers might not be damaged in pure L5-S1 radiculopathies. However, it is not possible to compare the present results with those of that study due to differences in the electrode placements and in the presence of a dermatomal pattern.
Studies that have revealed SSR abnormalities in radiculopathy were carried out on patients with failed back surgery syndrome (FBSS).25,26 It was found that SSR latency was significantly higher in the 29 patients with FBSS than in the controls. The prolongation of SSR latency was found even in the asymptomatic legs of FBSS patients. The SSR abnormalities found in the asymptomatic leg was attributed to a reduced homeostatic balance that induced stress and affected the central stress response, with sympathetic response changes.25 Another study found that SSR parameters were abnormal in 8 out of 20 patients with FBSS. The application of spinal cord stimulation to relieve the pain improved the SSR parameters (in terms of increased amplitude and shortened latency) in these patients.26 One explanation for the SSR abnormalities in patients with FBSS may be dysfunction of the sympathetic nervous system contributing to the intensity of pain.
Since both the patient group in the present study and other FBSS patient groups included not only patients with L5 and S1 radiculopathies but also patients with upper-level (L3 and L4) radiculopathies, and they could also have had further upper-level (L1, 2, and L3) abnormalities. Thus, another reason for the latency prolongation might be the involvement of the sympathetic fibers to the leg leaving the upper lumbar spinal nerve roots.
The main limitation of this study was that it was performed with the MRM technique implemented as part of a daily routine. Only the L3-S1 roots can be assessed using this MRM technique, making it impossible to state categorically whether some of the patients also had degenerative changes in the upper levels of the spine that could be responsible for the observed SSR abnormalities. However, it can be assumed that some of the patients also had degenerative changes at the upper levels of their spine. In these patients, the sympathetic nerves could be damaged at multiple levels, including the upper lumbar levels.
There was no significant correlation between SSR parameters and the SSS in the present patients. Similarly, there are some reports indicating lack of a correlation between SSR and autonomic symptoms in patients with polyneuropathy or carpal tunnel syndrome.8,9,15,29 One possible explanation for the lack of correlation between SSR and most autonomic symptoms in these different pathological conditions is that measurement of SSRs is sufficiently sensitive to detect subclinical autonomic disturbances.8 These findings suggest that the SSR could reveal subtle sympathetic abnormality presenting as prolongation of latency in patients with a radiculopathy.
The results of this study suggest that MUNE is a simple, fast, and sensitive test for the diagnosis of radiculopathy. MUNE might be useful for determining the degree of axonal loss and prognosis, and thus it could help in the formulation of an appropriate treatment and rehabilitation plan, and for the daily routine in patients with radiculopathy.
Acknowledgements
This study was conducted in the Clinical Neurophysiology Laboratory, Department of Neurology, Ondokuz Mayıs University, Turkey, and was supported financially by Ondokuz Mayıs University (fund no. T-521, code 1901).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Wilbourn AJ, Aminoff MJ American Association of Electrodiagnostic Medicine. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998;21:1612–1631. doi: 10.1002/(sici)1097-4598(199812)21:12<1612::aid-mus2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Nardin RA, Rutkove SB, Raynor EM. Diagnostic accuracy of electrodiagnostic testing in the evaluation of weakness. Muscle Nerve. 2002;26:201–205. doi: 10.1002/mus.10192. [DOI] [PubMed] [Google Scholar]
- 3.Wells MD, Meyer AP, Emley M, Kong X, Sanchez R, Gozani SN. Detection of lumbosacral nerve root compression with a novel composite nerve conduction measurement. Spine (Phila Pa 1976) 2002;27:2811–2819. doi: 10.1097/00007632-200212150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Cho SC, Ferrante MA, Levin KH, Harmon RL, So YT. Utility of electrodiagnostic testing in evaluating patients with lumbosacral radiculopathy: an evidence-based review. Muscle Nerve. 2010;42:276–282. doi: 10.1002/mus.21759. [DOI] [PubMed] [Google Scholar]
- 5.Rashidipour O, Chan KM. Motor unit number estimation in neuromuscular disease. Can J Neurol Sci. 2008;35:153–159. doi: 10.1017/s0317167100008568. [DOI] [PubMed] [Google Scholar]
- 6.Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in patients with polyneuropathy. J Neurol Sci. 1980;47:449–461. doi: 10.1016/0022-510x(80)90099-4. [DOI] [PubMed] [Google Scholar]
- 7.Maselli RA, Jaspan JB, Soliven BC, Green AJ, Spire JP, Arnason BG. Comparison of sympathetic skin response with quantitative sudomotor axon reflex test in diabetic neuropathy. Muscle Nerve. 1989;12:420–423. doi: 10.1002/mus.880120513. [DOI] [PubMed] [Google Scholar]
- 8.Niakan E, Harati Y. Sympathetic skin response in diabetic peripheral neuropathy. Muscle Nerve. 1988;11:261–264. doi: 10.1002/mus.880110311. [DOI] [PubMed] [Google Scholar]
- 9.Shahani BT, Halperin JJ, Boulu P, Cohen J. Sympathetic skin response--a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry. 1984;47:536–542. doi: 10.1136/jnnp.47.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauder TD, Dillingham TR, Andary M, Kumar S, Pezzin LE, Stephens RT, et al. Predicting electrodiagnostic outcome in patients with upper limb symptoms: are the history and physical examination helpful? Arch Phys Med Rehabil. 2000;81:436–441. doi: 10.1053/mr.2000.4426. [DOI] [PubMed] [Google Scholar]
- 11.Dyck PJ. Quantitating severity of neuropathy. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral Neuropathy. 3rd ed. Vol 1. Philadelphia (PA): WB Saunders; 1993. pp. 686–705. [Google Scholar]
- 12.Laaksonen S, Metsärinne K, Voipio-Pulkki LM, Falck B. Neurophysiologic parameters and symptoms in chronic renal failure. Muscle Nerve. 2002;25:884–890. doi: 10.1002/mus.10159. [DOI] [PubMed] [Google Scholar]
- 13.Sener HO, Taşcilar NF, Balaban H, Selçuki D. Sympathetic skin response in carpal tunnel syndrome. Clin Neurophysiol. 2000;111:1395–1399. doi: 10.1016/s1388-2457(00)00333-3. [DOI] [PubMed] [Google Scholar]
- 14.Zyluk A. A new clinical severity scoring system for reflex sympathetic dystrophy of the upper limb. J Hand Surg Br. 2003;28:238–241. doi: 10.1016/s0266-7681(02)00397-2. [DOI] [PubMed] [Google Scholar]
- 15.Bayrak AO, Tilki HE, Coşkun M. Sympathetic skin response and axon count in carpal tunnel syndrome. J Clin Neurophysiol. 2007;24:70–75. doi: 10.1097/01.wnp.0000239107.10424.fa. [DOI] [PubMed] [Google Scholar]
- 16.Stålberg E, Falck B. Clinical motor nerve conduction studies. Meth Clin Neurophysiol. 1993;4:61–80. [Google Scholar]
- 17.Stålberg E, Falck B, Bischoff C. Sensory nerve conduction studies with surface electrodes. Meth Clin Neurophysiol. 1994;5:1–20. [Google Scholar]
- 18.Stålberg E, Bischoff C, Falck B. Outliers, a way to detect abnormality in quantitative EMG. Muscle Nerve. 1994;17:392–399. doi: 10.1002/mus.880170406. [DOI] [PubMed] [Google Scholar]
- 19.Stålberg E, Nandedkar SD, Sanders DB, Falck B. Quantitative motor unit potential analysis. J Clin Neurophysiol. 1996;13:401–422. doi: 10.1097/00004691-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 20.McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121–131. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McComas AJ. Invited review: motor unit estimation: methods, results, and present status. Muscle Nerve. 1991;14:585–597. doi: 10.1002/mus.880140702. [DOI] [PubMed] [Google Scholar]
- 22.Dillingham TR. Electrodiagnostic approach to patients with suspected radiculopathy. Phys Med Rehabil Clin N Am. 2002;13:567–588. doi: 10.1016/s1047-9651(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 23.Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256–270. doi: 10.1007/s10286-003-0107-5. [DOI] [PubMed] [Google Scholar]
- 24.Argyriou AA, Tsolakis I, Papadoulas S, Polychronopoulos P, Gourzis P, Chroni E. Sympathetic skin response in patients with peripheral arterial occlusive disease. Clin Neurophysiol. 2006;117:414–419. doi: 10.1016/j.clinph.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Şahin N, Müslümanoğlu L, Karataş O, Cakmak A, Özcan E, Berker E. Evaluation of sympathetic response in cases with failed back surgery syndrome. Agri. 2009;21:10–15. [PubMed] [Google Scholar]
- 26.de Andrade DC, Bendib B, Hattou M, Keravel Y, Nguyen JP, Lefaucheur JP. Neurophysiological assessment of spinal cord stimulation in failed back surgery syndrome. Pain. 2010;150:485–491. doi: 10.1016/j.pain.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Andary MT, Stolov WC, Nutter PB. Sympathetic skin response in fifth lumbar and first sacral radiculopathies. Electromyogr Clin Neurophysiol. 1993;33:91–99. [PubMed] [Google Scholar]
- 28.Stewart JD. Focal Peripheral Neuropathies. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2000. p. 317. [Google Scholar]
- 29.Park ES, Park CI, Jung KI, Chun S. Comparison of sympathetic skin response and digital infrared thermographic imaging in peripheral neuropathy. Yonsei Med J. 1994;35:429–437. doi: 10.3349/ymj.1994.35.4.429. [DOI] [PubMed] [Google Scholar]


