Abstract
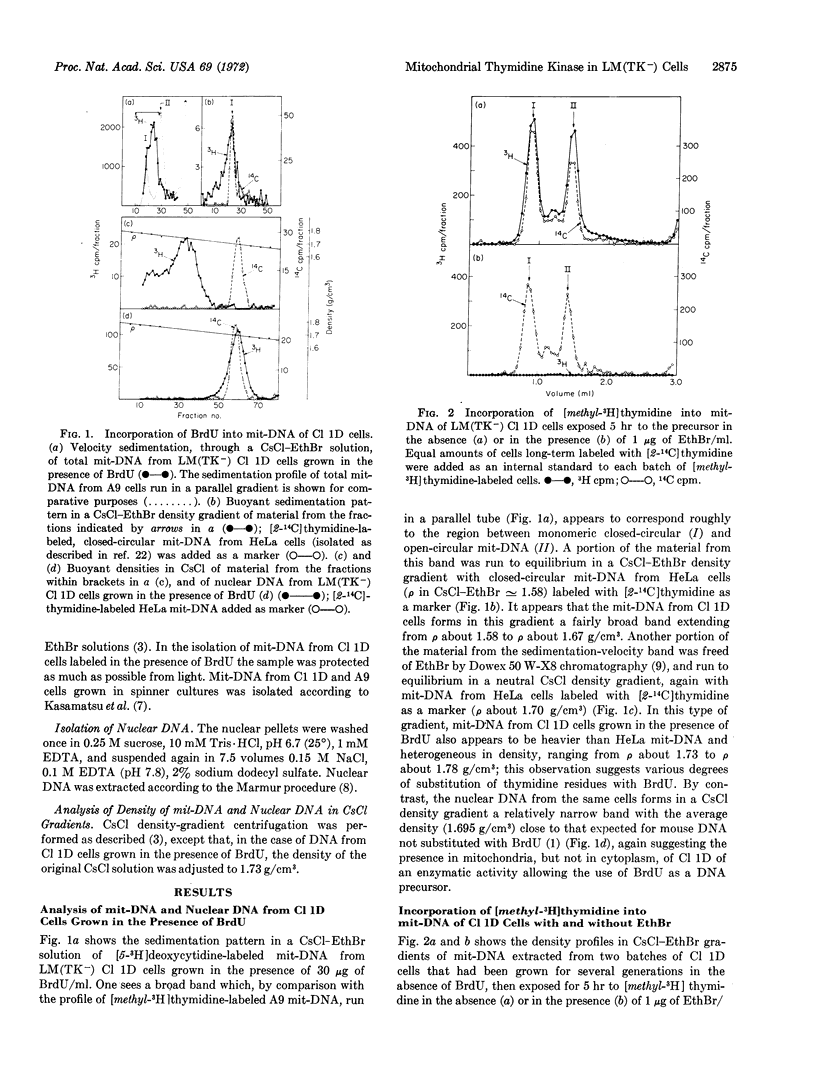
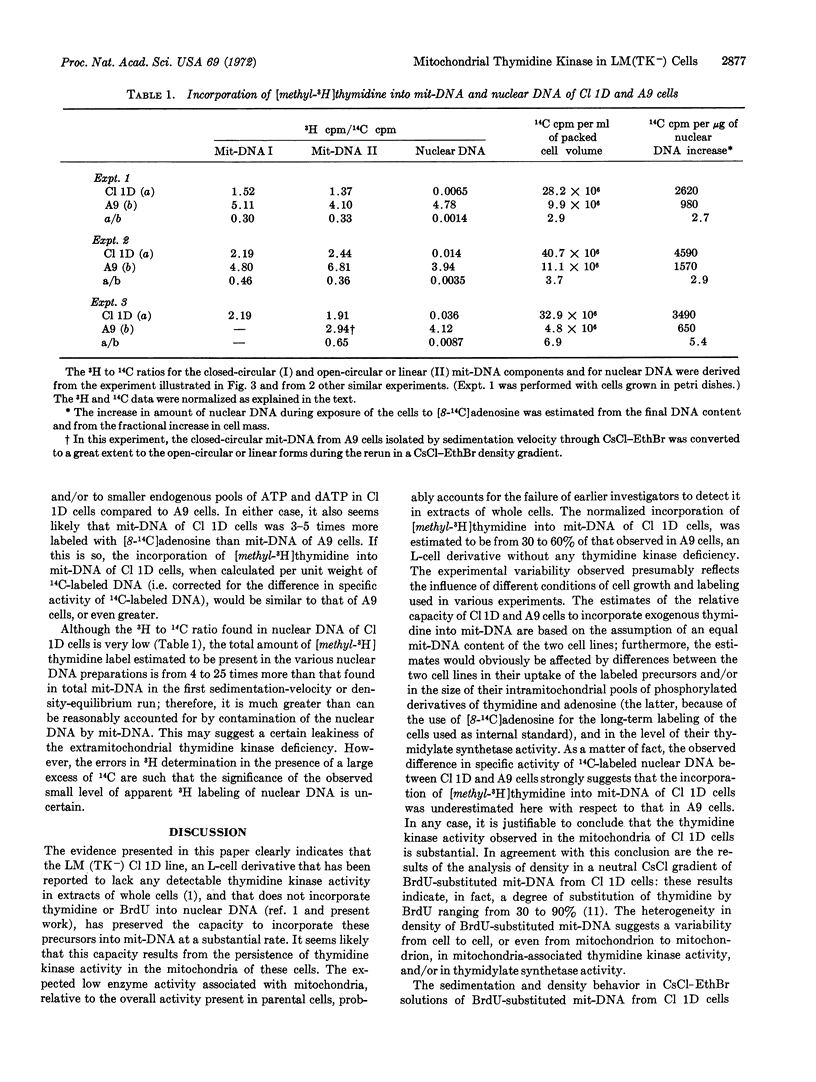
The cell line LM(TK-) Cl 1D, a derivative of mouse L fibroblasts deficient in thymidine kinase (EC 2.7.1.21) that shows very little thymidine kinase activity in extracts of whole cells as compared to the parental line, and that does not incorporate thymidine or 5-bromodeoxyuridine into nuclear DNA, has maintained the capacity to incorporate these precursors into mitochondrial DNA at a substantial rate. The amount of [methyl-3H]thymidine incorporated into mitochondrial DNA of LM(TK-) Cl 1D cells after long-term labeling has been conservatively estimated in different experiments to be between 30 and 60% of that incorporated into mitochondrial DNA or nuclear DNA of strain A9, an L-cell derivative without any thymidine-kinase dificiency; by contrast, the incorporation of thymidine into nuclear DNA of Cl 1D cells is less than 1% of that in A9 cells. These results strongly suggest that the loss of thymidine kinase activity in the extramitochondrial compartment of LM(TK-) Cl 1D cells has not been accompanied by the loss of the mitochondria-associated enzyme activity, pointing to a different genetic or epigenetic control of the extramitochondrial and mitochondrial enzymes.
Keywords: 5-bromodeoxyuridine, ethidium bromide, mitochondrial DNA, density gradient centrifugation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi B., Attardi G. Fate of mitochondrial DNA in human-mouse somatic cell hybrids (density gradient centrifugation-ethidium bromide-karyotype). Proc Natl Acad Sci U S A. 1972 Jan;69(1):129–133. doi: 10.1073/pnas.69.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley R. L., Vitols E. The control of nucleotide biosynthesis. Annu Rev Biochem. 1968;37:201–224. doi: 10.1146/annurev.bi.37.070168.001221. [DOI] [PubMed] [Google Scholar]
- Brown I. H., Vinograd J. Sedimentation velocity properties of catenated DNA molecules from HeLa cell mitochondria. Biopolymers. 1971 Oct;10(10):2015–2028. doi: 10.1002/bip.360101017. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Teplitz R. L. Intracellular mosaicism (nuclear - -mitochondrial + ) for thymidine kinase in mouse L cells. J Cell Sci. 1972 Mar;10(2):487–493. doi: 10.1242/jcs.10.2.487. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. EFFECT OF HALOGENATED PYRIMIDINES AND THYMIDINE ON GROWTH OF L-CELLS AND A SUBLINE LACKING THYMIDINE KINASE. Exp Cell Res. 1964 Jan;33:19–28. doi: 10.1016/s0014-4827(64)81006-5. [DOI] [PubMed] [Google Scholar]
- Haut W. F., Taylor J. H. Studies of bromouracil deoxyriboside substitution in DNA of bean roots (Vicia faba). J Mol Biol. 1967 Jun 28;26(3):389–401. doi: 10.1016/0022-2836(67)90311-7. [DOI] [PubMed] [Google Scholar]
- Humphrey R. M., Hsu T. C. Further studies on biological properties of mammalian cell lines resistant to 5-bromodeoxyuridine. Tex Rep Biol Med. 1965 Jun;23(Suppl):321–336. [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KRISS J. P., REVESZ L. Quantitative studies of incorporation of exogenous thymidine and 5-bromodeoxyuridine into deoxyribonucleic acid of mammalian cells in vitro. Cancer Res. 1961 Oct;21:1141–1147. [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTOW L., DARNELL J. E., Jr A simplified procedure for purification of large amounts of poliovirus: characterization and amino acid analysis of Type 1 poliovirus. J Biol Chem. 1960 Jan;235:70–73. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Masui H., Garren L. D. On the mechanism of action of adrenocorticotropic hormone. The stimulation of thymidine kinase activity with altered properties and changed subcellular distribution. J Biol Chem. 1971 Sep 10;246(17):5407–5413. [PubMed] [Google Scholar]
- Matsuya Y., Green H. Somatic cell hybrid between the established human line D98 (presumptive HeLa) and 3T3. Science. 1969 Feb 14;163(3868):697–698. doi: 10.1126/science.163.3868.697. [DOI] [PubMed] [Google Scholar]
- Nass S. Incorporation of 33Pi into mitochondrial and nuclear DNA in regenerating liver. Biochim Biophys Acta. 1967 Aug 22;145(1):60–67. doi: 10.1016/0005-2787(67)90654-5. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G. Electron microscopic visualization of mitochondrial RNA-DNA hybrids. J Mol Biol. 1971 Jan 28;55(2):267–270. doi: 10.1016/0022-2836(71)90196-3. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Jordan J. M., Vinograd J. In vivo effects of intercalating drugs on the superhelix density of mitochondrial DNA isolated from human and mouse cells in culture. J Mol Biol. 1971 Jul 28;59(2):255–272. doi: 10.1016/0022-2836(71)90050-7. [DOI] [PubMed] [Google Scholar]



