The differential role of atRA in promoting CD4+- and CD8+-induced Tregs; a caution for a protocol in developing Treg therapy in clinic.
Keywords: TGF-β, all-trans retinoic acid, regulatory T cells, Foxp3, Autoimmunity, GVHD
Abstract
It is known that ATRA promotes the development of TGF-β-induced CD4+Foxp3+ iTregs, which play a vital role in the prevention of autoimmune diseases; however, the role of ATRA in facilitating the differentiation and function of CD8+Foxp3+ iTregs remains elusive. Using a head-to-head comparison, we found that ATRA promoted expression of Foxp3 and development of CD4+ iTregs, but it did not promote Foxp3 expression on CD8+ cells. Using a standard in vitro assay, we demonstrated that CD8+ iTregs induced by TGF-β and ATRA were not superior to CD8+ iTregs induced by TGF-β alone. In cGVHD, in a typical lupus syndrome model where DBA2 spleen cells were transferred to DBA2xC57BL/6 F1 mice, we observed that both CD8+ iTregs induced by TGF-β and ATRA and those induced by TGF-β alone had similar therapeutic effects. ATRA did not boost but, conversely, impaired the differentiation and function of human CD8+ iTregs. CD8+ cells expressed the ATRA receptor RAR and responded to ATRA, similar to CD4+ cells. We have identified the differential role of ATRA in promoting Foxp3+ Tregs in CD4+ and CD8+ cell populations. These results will help to determine a protocol for developing different Treg cell populations and may provide novel insights into clinical cell therapy for patients with autoimmune diseases and those needing organ transplantation.
Introduction
Foxp3+ Tregs are crucial in establishing and maintaining self-tolerance and therefore are considered to be therapeutic for autoimmune diseases [1]. Foxp3+ Tregs consist of nTregs and iTregs, and both Treg populations may have different developmental mechanisms and functional characteristics [2–4]. Although CD4+ cells have been predominately studied, results in recent studies have demonstrated that CD8+ Tregs may play an important role in the prevention of autoimmunity and regulation of immune tolerance [5–7].
ATRA, a vitamin A derivative, has evident effects on immune homeostasis and is particularly crucial in embryonic morphogenesis, vision, reproduction, cell differentiation, and growth [8]. It has been reported to control and modulate autoimmune diseases [9]. For example, vitamin A and its derivatives can markedly alleviate the symptoms of disease syndromes in several models of autoimmune diseases, including inflammatory bowel disease, rheumatoid arthritis, type 1 diabetes, and experimental encephalomyelitis [10–13]. Accordingly, a deficiency in vitamin A is associated with the exacerbation of experimental colitis [14].
It is likely that ATRA suppresses autoimmune diseases through its inhibitory effect on the differentiation and function of T effector cells. It has been reported that ATRA down-regulates Th1 and -17 cell development [15, 16] and that both Th1 and -17 cells are involved in initiating many autoimmune diseases [17]. In addition, ATRA possibly stifles autoimmunity through up-regulating CD4+Foxp3+ Treg development first and then indirectly inhibiting the ongoing autoimmune disease [18–20]. ATRA can be produced from many sources. DCs—particularly CD103+ DCs—may be a major source of ATRA that can promote the induction of Foxp3+ Tregs [21, 22].
We and others have recently reported that ATRA promotes murine CD4+Foxp3+ Tregs induced by TGF-β from conventional CD4+Foxp3− cells [18, 19]. ATRA also improves the stability of nTregs in inflammatory conditions [23]. More important, ATRA is crucial for the successful induction of human iTregs by TGF-β [24]. However, although most related studies so far have focused on CD4+Foxp3+ Treg subsets, the role of ATRA in regulating the differentiation of the CD8+Foxp3+ subset is less understood.
In reporting our head-to-head comparison study, we provide evidence that, although ATRA significantly promotes CD4+Foxp3+ Treg development and function in mice and humans, it does not similarly boost the differentiation and function of CD8+Foxp3+ iTregs. On the contrary, it interferes with the development and function of human CD8+ iTregs induced by TGF-β alone. This study indicates the complexity of immune systems and the immune tolerance induction procedure. The knowledge gained will help in devising appropriate protocols for the development of Treg populations for the treatment of patients with autoimmune diseases and those needing organ transplantation.
MATERIALS AND METHODS
Mice
Male or female DBA/2 (D2, H-2d), C57BL/6 CD45.1 (B6, H-2b), female NOD/SCID/IL2R common γ chain−/− (NOG), and female (DBA/2xC57BL/6) F1 (B6D2F1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6 Foxp3-GFP knockin mice were generously provided by Dr. Talil Chatilla (University of California Los Angeles). The animals were used for experiments at 8–12 weeks of age. All were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of the University of Southern California Committee for the Use and Care of Animals.
Flow cytometry
The following FITC-, PE-, Cyc-, or APC-conjugated mouse antibodies were used for flow cytometry: from Biolegend (San Diego, CA, USA): PerCP/Cy5.5 or PE-CD103 (2E7), PE or PerCP-CD8 (53-6.7), PE-CD25 (PC61), PE-PD-1 (RMP1-30), PE-GITR (YGITR 765), PE-TNFRII/p75 (TR75-89), PE-CD62L (MEL-14), APC-PD-L1 (10F.9G2), PE-CCR-9 (9B1), and Alexa Fluor 488-Foxp3 (150D); and from eBioscience (San Diego, CA, USA): PE-CTLA-4 (UC10.4B9), and APC-CD44 (IM7). Each subset was stained with mAbs to the markers indicated above and analyzed on a flow cytometer (FACSCalibur, with Cell Quest Software; BD Biosciences, Franklin Lakes,NJ, USA). For intracellular staining, such as Foxp3 and CTLA-4, the cells were stained with surface antigen CD4 or CD8 and further fixed and permeabilized for intracellular staining. Histograms were prepared with FlowJo software (Treestar, Inc., Ashland, OR, USA).
Cell preparation
Enriched T cells were isolated from spleen cells in C57BL/6 (B6) mice or B6 Foxp3-GFP knockin mice by collecting nylon–wool column nonadherent cells [25]. Various T-cell populations were acquired by negative selection via the auto-MACS method. Briefly, enriched T cells were first stained with PE-conjugated anti-CD8a, -CD25, -CD11b, and -B220 mAbs and then washed and combined with anti-PE microbeads (Miltenyi Biotec, Auburn, CA, USA). After they passed through the MACS separation columns, the negative exports were collected as CD4+CD25− cells. Subsequently, naive CD4+CD25−CD62L+ T cells were positively selected from the enriched CD4+CD25− T-cell fraction by the anti-CD62L antibody and microbeads. Naive CD8+CD25− cells were similarly selected, except that total T cells were primed with PE-conjugated anti-CD4 antibody instead of anti-CD8a antibody. Without anti-CD4 and -CD8 antibodies, CD3+CD25− cells were obtained to serve as responder T cells. Cell purity was more than 96%.
APCs were prepared from non-T cells isolated from splenocytes, by passing them through a nylon–wool column. The adherent cells (non-T cells) were harvested and irradiated (30 cGy) to serve as APCs.
The generation of mouse CD4+ or CD8+ iTregs ex vivo
For the induction of CD4+ and CD8+ Tregs, naive CD4+CD25− CD62L+ and naive CD8+CD25− CD62L+ T cells were isolated from the spleens of B6 mice or B6 Foxp3-GFP knockin mice by using a naive CD4+ or CD8+ T cell negative isolation kit (Miltenyi Biotec) as described above. Naive CD4+CD25− cells were cultured in 96-well plates and stimulated with anti-CD3/28 microbeads (the ratio of beads to cell was 1:5), rhIL-2 (50 U/ml; R&D Systems, Minneapolis, MN, USA), with (CD4TGFβ) or without rhTGF-β (2 ng/ml; Humanzyme, Chicago, IL) (CD4Med) and with or without ATRA (0.5 μM; Sigma-Aldrich, St. Louis, MO), for 3 days. RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 10 mM HEPES (Invitrogen-Life Technologies, Gaithersburg, MD, USA), and 10% heat-inactivated FCS (HyClone Laboratories, Logan, UT, USA) was used for all cultures. When the cultures were terminated, the cells were harvested, and the beads were removed for secondary culturing. Naive CD8+ cells were stimulated with plate-bound mouse anti-CD3 (2 μg/ml; Biolegend), soluble mouse anti-CD28 (2 μg/ml; Biolegend), and rhIL-2 (50 U/ml; R&D Systems, Minneapolis, MN, USA), with (CD8TGFβ) or without rhTGF-β (2–20 ng/ml; Humanzyme) (CD8Med) and with or without ATRA (0.5 μM; Sigma-Aldrich), for 3 days. The expression of Foxp3 was determined by flow cytometry.
Generation of human iTregs ex vivo
PBMCs were prepared from the heparinized venous blood of healthy adult volunteers by Ficoll-Hypaque density gradient centrifugation. All protocols that involved human blood donors were approved by the Institutional Review Board at the University of Southern California. T cells were prepared by negative selection to a purity of >95% [24]. CD4+CD45RA+ or CD8+CD45RA+ cells were isolated from the CD4+ or CD8+CD25− cells by negative selection, in an approach similar to that used to isolate the mouse cells, except that the antibodies used (described above) were activated with anti-human CD3/CD28 beads 1:10 (1 bead:10 cells) for 5 days in AIM-V serum-free medium (Life Technologies, Carlsbad, CA, USA) containing HEPES buffer (10 mM), sodium pyruvate (1 mM), glutamine, nonessential amino acids, and penicillin and streptomycin. This complete medium was supplemented with IL-2 (50–100 U/ml), with or without TGF-β1 (5 ng/ml) and ATRA (100 nM). Depending on their density, the cells were split, and the culture medium with the corresponding additives was replaced every 3 days.
In vitro suppressive assay
Freshly isolated T cells (responder cells) labeled with CFSE were stimulated with anti-CD3 mAb (0.025 μg/mL) and irradiated APCs (30 Gy, 1:1 ratio) for 3 days, with or without iTregs generated as described above. (The ratio of Treg:T cell was 1:4, or in graded ratios.) T-cell proliferation was determined by the CFSE dilution rate after 3 days of culture.
Real-time PCR
Total RNA was extracted with the RNeasy mini kit (Qiagen, Valencia, CA, USA). cDNA was generated with the Omniscript RT kit (Qiagen). RARα mRNA expression was quantified with ABsolute SYBR Green ROX mix (Thermo Scientific, Waltham, MA, USA). The samples were run in triplicate, and the relative expression of RARα mRNA was determined by normalizing the expression of each target to HPRT.
Induction and assessment of cGVHD with a lupus-like syndrome
cGVHD with a lupus-like syndrome was induced in B6D2F1 mice by injecting 80 × 106 DBA/2 spleen cells through the tail vein, as described previously [26]. Other groups received this number of DBA/2 cells plus 5 × 106 CD4/CD8med, CD4/CD8TGFβ, or CD4/CD8TGFβ+ATRA. Before transfer and weekly thereafter, blood was collected, and serum IgG and anti-dsDNA autoantibodies were measured by ELISA. All samples tested for anti-dsDNA antibodies were processed at the same time. Serum was diluted 1:400 or 1:800 for anti-dsDNA and 1:40,000 for IgG measurement. Each mouse was placed on sterile plastic packaging and massaged in the bladder area until it started urinating. Proteinuria was assayed by the colorimetric method with Albustix reagent strips (Bayer, Elkhart, IN, USA). The levels of proteinuria are judged by the color of the sticks when compared to a standard control provided by the manufacturer (Bayer).
Xeno-GVHD model
Blood samples collected from healthy donors who provided informed written consent were used for the isolation of hPBMCs by Ficoll-Hypaque separation. The PBMCs were resuspended in 200 μl of PBS. NOD/SCID mice received total body irradiation with a single dose of 350 cGy from a linear accelerator and were infused with (20×106) hPBMCs via the tail vein. Survival and weight loss were monitored at least 3 times per week, as previously described [26]. When weight loss was >20% and the mice showed either limited mobility or disruption of general appearance, they were humanely euthanized for ethical reasons. The mice were diagnosed with GVHD on the basis of a minimum 10% weight loss, appearance of ruffled fur, and limited mobility [27].
Statistics
Data are expressed as the mean ± sem, unless otherwise indicated. The data were analyzed by using Student's t test for comparison between 2 groups or ANOVA for comparison among multiple groups, as appropriate. Differences were considered statistically significant at P < 0.05.
RESULTS
ATRA promoted Foxp3 expression in CD4+ but not CD8+ cells treated with TGF-β
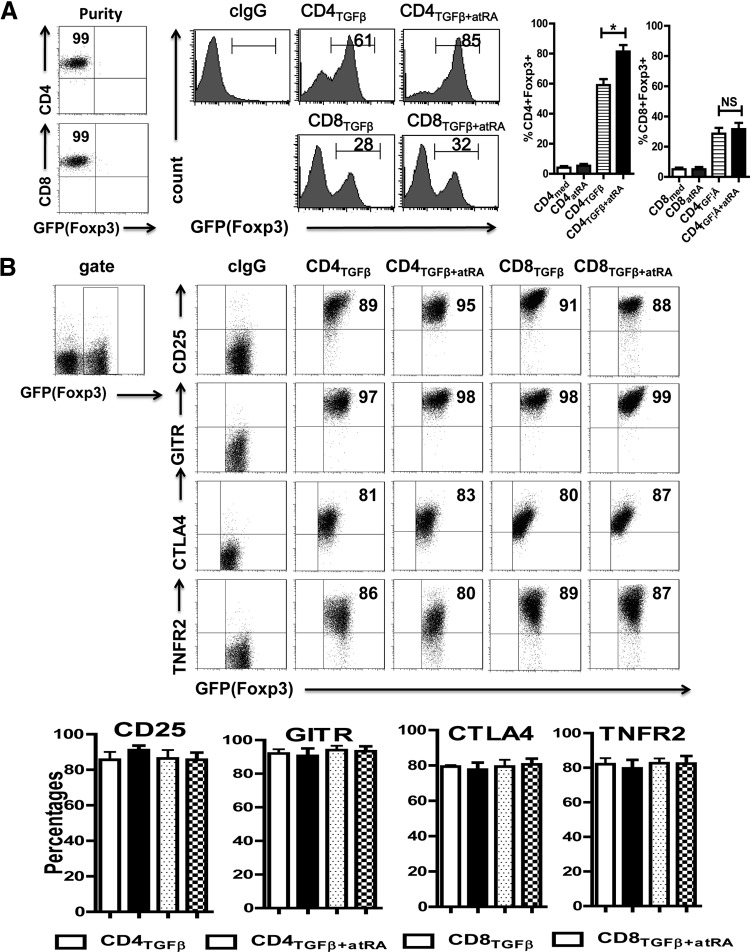
Like naive CD4+CD25− cells, naive CD8+CD25− cells isolated from spleen activated with TCR with TGF-β began to express Foxp3, although the level of Foxp3 expression in the CD8+ cells was much lower than that of the TGF-β-treated CD4+ cells (Fig. 1A). In line with previous reports [6], the addition of ATRA to CD4+ cell cultures containing TGF-β significantly increased the proportions of CD4+CD25+Foxp3+ cells induced from naive CD4+CD25−Foxp3− cells (or GFP− cells in Foxp3-GFP knockin mice). However, the addition of ATRA did not significantly increase Foxp3 expression on the TGF-β-primed CD8+ cells (Fig. 1A). That the starting populations of isolated naive CD4+CD5− and CD8+CD25− cells hardly expressed Foxp3 and that TCR stimulation alone or TCR with ATRA did not result in Foxp3 induction in the CD4+ and CD8+ cell populations suggests that TGF-β or the TGF-β signaling pathway is crucial for Foxp3 induction [28]. In addition, the total Foxp3 protein level and the number of Foxp3+ cells increased significantly in the CD4+ cells but not in the CD8+ cells treated with the combination of ATRA and TGF-β. The increases were more than in those treated with TGF-β alone (Supplemental Fig. S1), implying that ATRA does not promote Foxp3 differentiation of CD8+ cells. ATRA also significantly decreased the number of Foxp3− cells in the CD4+ but not in the CD8+ population (Supplemental Fig. S1), indicating that ATRA selectively promotes CD4+Foxp3+ cell conversion. After the CD4+Foxp3+ cells had been induced, the addition of ATRA maintained but did not expand the number of Foxp3+ cells [18]. It is likely that ATRA mostly affects the differentiation rather than the expansion of Foxp3+ cells. Moreover, ATRA enhanced Foxp3 mRNA expression on the TGF-β-primed CD4+ cells but not on the TGF-β-primed CD8+ cells (Supplemental Fig. S2), providing further evidence that ATRA promotes Foxp3+CD4+ cell differentiation. The inability of ATRA to boost Foxp3 expression in the CD8+ cells cannot be corrected by TCR strength (anti-CD3 antibody concentrations), the doses of IL-2 or TGF-β, or culture periods (data not shown).
Figure 1. ATRA increased the percentages of Foxp3 expression on TGF-β-primed CD4+, but not on CD8+ cells.
(A) CD8+CD62L+CD25−Foxp3−(GFP−) and CD4+CD62L+CD25−Foxp3−(GFP−) cells isolated from C57BL/6 Foxp3gfp reporter mice were stimulated with immobilized anti-CD3 (1 μg/ml), soluble anti-CD28 (1 μg/ml), IL-2 (100 U/ml), or TGF-β (2 ng/ml), with (CD4TGFβ+ATRA or CD8TGFβ+ATRA) or without ATRA (50 nM) (CD4TGFβ or CD8TGFβ) for 3 days. Foxp3 (GFP) expression was examined by flow cytometry. Left: typical FACS histograms. Right: summary of data showing the frequency of Foxp3+ cells from TGF-β-primed CD4+ or CD8+ cells. *P < 0.05, NS. (B) The expression levels of regulatory T-cell associated markers including CD25, GITR, CTLA-4, and TNFR2 on CD4TGFβ, CD8TGFβ, CD4TGFβ+ATRA, or CD8TGFβ+ATRA cells were analyzed by flow cytometry. The graph data indicate the mean ± sem of 3 separate experiments showing the frequency of the indicated markers gated on the CD4 or CD8 cell populations.
We also examined other phenotypic features related to Treg differentiation besides Foxp3. The TGF-β-primed CD4+ cells expressed high levels of CD25, GITR, CTLA-4, and TNFR2, but the addition of ATRA did not alter their expression. Similarly, the TGF-β-treated CD8+ cells expressed these Treg-related markers in levels similar to those in the TGF-β-treated CD4+ cells. In addition, ATRA did not change the expression of these Treg-related makers in the TGF-β-treated CD8+ cells (Fig. 1B).
Inability of ATRA to promote Foxp3 induction in TGF-β-primed CD8+ was not due to nonresponse of CD8+ cells to ATRA
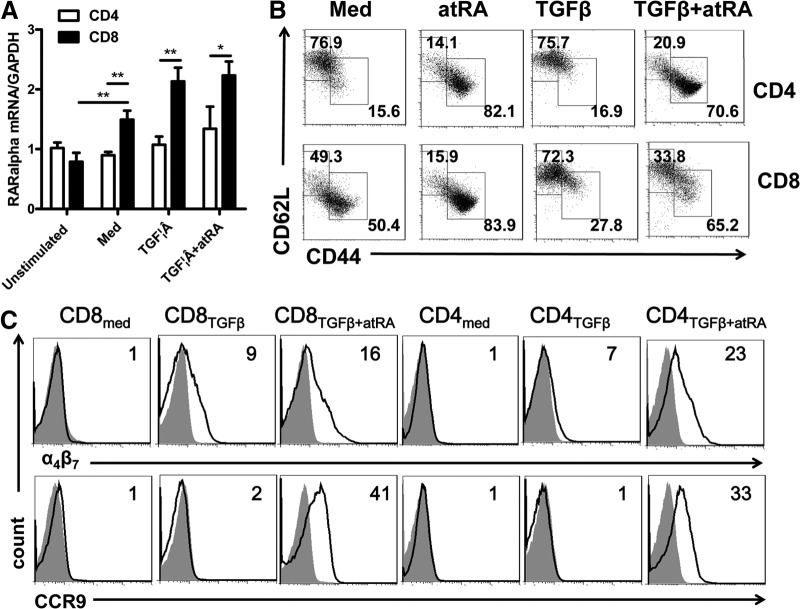
To determine whether the differential response of CD8+ cells to ATRA is responsible for the low Foxp3 induction by ATRA in TGF-β-activated CD8+ cells, we first studied the levels of ATRA receptor expressed on the CD8+ cells. A previous study revealed that ATRA mainly binds RARα, one of the RARs that are expressed on T cells [29]. Using RT-PCR, we observed that the levels of RARα mRNA in the naive CD8+ cells was similar to that on the naive CD4+ cells, and the levels of RARα mRNA in the CD8+ subsets were slightly but significantly increased after they were stimulated with TCR in the presence of ATRA, TGF-β, or both, compared with CD4+ subsets that had experienced similar stimulations or unstimulated CD8+ cells (Fig. 2A). In Western blot analysis, we also observed that the protein levels of RARα were similar in the unstimulated and stimulated CD4+ and CD8+ cells (Supplemental Fig. S3). Furthermore, ATRA decreased CD62L and increased CD44 expression on both the CD8+ and CD4+ cells, implying that ATRA similarly induces the maturation of CD8+ and CD4+ cells (Fig. 2B). In addition, the impact of ATRA on the CD8+ cells was further supported by the data showing that ATRA significantly enhanced the expression of α4β7 and CCR-9 on both the CD8+ and CD4+ cells (Fig. 2C). Both α4β7 and CCR-9 are gut-homing markers, and ATRA enhances gut immunity through induction of α4β7+ and CCR-9+ T cells [30]. These results strongly suggest that CD8+ cells respond to ATRA the same as CD4+ cells. The low ATRA-mediated Foxp3 promotion may be due to an intrinsic defect in CD8+ cells.
Figure 2. CD8+ cells had the same response to ATRA as did CD4+ cells.
(A) The expression of ATRA RARα mRNA was determined by quantitative RT-PCR on naive or activated CD4+ and CD8+ cells. These cells were activated with TCR and IL-2, with or without TGF-β and with or without ATRA. Data are the mean ± se of 3 separate experiments. (B) CD4TGFβ, CD8TGFβ, CD4TGFβ+ATRA, or CD8TGFβ+ATRA cells were induced as in Fig. 1A, and the expression of CD44 and CD62L gated on CD4 or CD8 was analyzed by flow cytometry. Dot plots are representative of 3 independent experiments. (C) The expression of α4β7 and CCR-9 on CD4med, CD8med, CD4TGFβ, CD8TGFβ, CD4TGFβ+ATRA, or CD8TGFβ+ATRA cells was analyzed by flow cytometry. Data are representative of 3 independent experiments showing staining (black) plus isotype control (gray).
Combination of ATRA and TGF-β induced CD4+ but not CD8+ cells to develop immune suppressive qualities in vitro and in vivo
Previous reports have revealed that TGF-β-primed CD4+ cells are suppressor cells and that addition of ATRA to TGF-β improves the development and function of these CD4+ iTregs [16, 28, 31]. To determine whether ATRA also enhances the suppressive activities of TGF-β-primed CD8+ cells, we developed a standard in vitro suppressive assay [32]. CD3+ T cells depleted of CD25+ cells were used as responder T cells. These responder T cells were labeled with CFSE and stimulated with anti-CD3 in the presence of APCs, with or without various subpopulations of CD4+ and CD8+ iTregs.
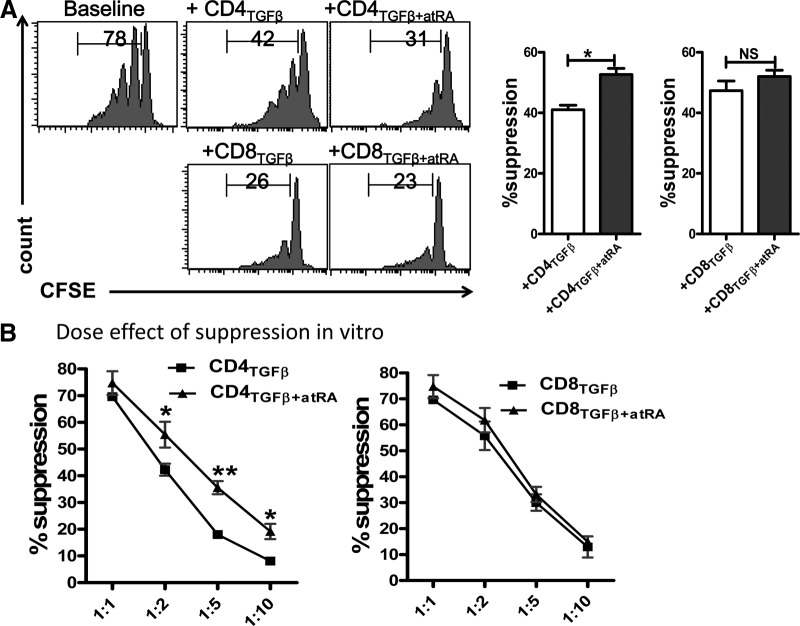
As shown in Fig. 3A, the addition of TGF-β-primed CD4+ cells to responder T cells at a ratio of 1:4 significantly suppressed the CD8+ responder T cell proliferation in vitro. We gated the CD8+ cells in the responder T cells, to exclude the contamination of TGF-β-primed CD4+ cells. Similarly, when we looked at the suppressive activity of the TGF-β-primed CD8+ cells, gated the CD4+ cells from the responder T cells. As expected, CD4+ cells primed with ATRA and TGF-β had a greater suppressive activity than did those primed with TGF-β alone (Fig. 3A). This effect was more evident when the ratios of the Tregs to the responder T cells were reduced (Fig. 3B). Although Foxp3 expression in the TGF-β-primed CD8+ cells was significantly lower than in their CD4+ cell counterparts, the suppressive activity of these cells was not compromised (Fig. 3A). Nonetheless, the suppressive activity of the ATRA/TGF-β-primed CD8+ cells was similar to that of the TGF-β-primed CD8+ cells (Fig. 3). In addition, we observed that the suppressive ability of the CD4+ cells primed with TGF-β/ATRA against IFN-γ production by responder T cells was superior to that of the CD4+ cells primed with TGF-β alone. This effect was not observed in the CD8+ cells (Supplemental Fig. S4). Thus, ATRA promoted the development of function of CD4+Foxp3+ iTregs, but not of CD8+Foxp3+ iTregs.
Figure 3. ATRA enhanced the suppressive activity of CD4+ iTregs, but not of CD8+ iTregs, in vitro.
(A) CD4TGFβ, CD8TGFβ, CD4TGFβ+ATRA, or CD8TGFβ+ATRA cells generated as in Fig. 1A were added to cultures of soluble anti-CD3 (0.025 μg/ml), irradiated APCs (1:1), and CFSE-labeled responder T cells for 3 days. Suppressive activity by each cell population was demonstrated and compared by percentages of CFSE-diluted cells. A histogram representative of 3 separate experiments in 1:2 ratios of Treg-to-responder T cells is shown. (B) The same culture as in (A), but the graded ratio of Tregs was added to the culture. The graph shows the mean ± sem of results in 3 independent experiments. *P < 0.05. **P < 0.01.
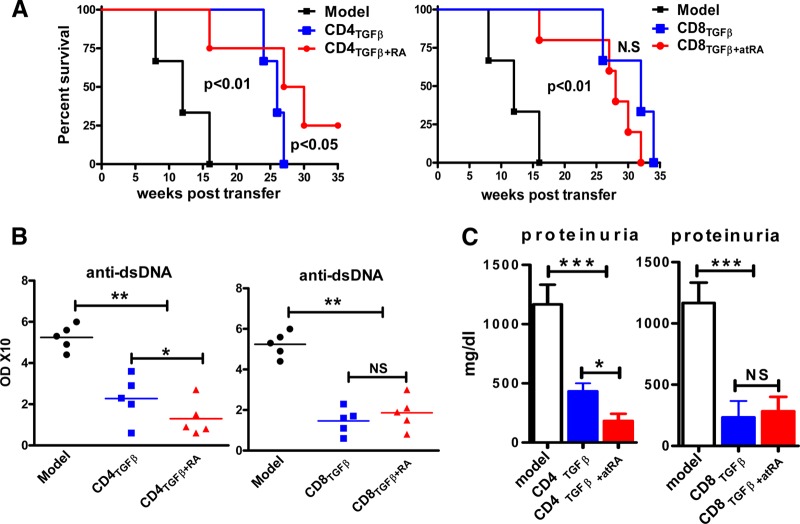
We then used an in vivo animal model to validate whether the results gained in the in vitro experiments would have an effect on autoimmune diseases in vivo. To compare the therapeutic effects of different CD4+ and CD8+ iTreg populations, generated as described above, we used a rapid-read cGVHD in a lupus-like syndrome [26]. Previous reports have demonstrated that the transfer of parental DBA/2 splenocytes to DBA/2xC57BL/6 F1 mice induces polyclonal B cell activation and anti-dsDNA autoantibodies in 1–2 weeks, proteinuria in 8–12 weeks, and death at approximately 16 weeks after cell transfer [26, 33]. We demonstrated that the cotransfer of 5 × 106 TGF-β-primed CD4+ cells with 80 × 106 pathogenic spleen cells significantly prolonged survival (Fig. 4A), prevented anti-dsDNA production in the sera (Fig. 4B), and suppressed proteinuria (Fig. 4C) in the mice with cGVHD. As expected, the levels of IgG, anti-dsDNA, and proteinuria were markedly lower in the mice with cGVHD treated with ATRA/TGF-β-primed CD4+ cells than in the mice treated with TGF-β-primed CD4+ cells (Fig. 4). Compared to TGF-β-primed CD4+ cells, the effect of ATRA/TGF-β-primed CD4+ cells on the survival of the mice with cGVHD was even better. Although all the cGVHD mice receiving a single co-injection of TGF-β-primed CD4+ cells died within 27 weeks, more than 75% of the mice receiving CD4+ cells treated with both ATRA and TGF-β were alive at that time point.
Figure 4. ATRA promoted the development of function of TGF-β-induced CD4+ iTregs, but not of CD8[supb]+ iTregs, in vivo.
CD4TGFβ, CD8TGFβ, CD4TGFβ+ATRA, or CD8TGFβ+ATRA cells were induced as in Fig. 1A. Freshly isolated splenocytes (80 × 106) from DBA/2 mice or together with 5 × 106 of various CD4+ or CD8+ cell populations, as indicated, were intravenously transferred into C57BL/6xDBA/2 F1 mice. PBS was used for the negative control and marked as the model group. Each group had 6 mice, and the experiment was repeated twice. (A) Survival was monitored weekly. A Kaplan-Meier survival curve is shown. (B) The levels of anti-dsDNA IgG antibody in sera were measured by ELISA 4 weeks after cell transfer. (C) The proteinuria levels were examined 8 weeks after cell transfer. Data are combined from 2 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Similarly, co-injection of TGF-β-primed CD8+ cells and pathogenic splenocytes in the mice with cGVHD significantly prolonged survival, prevented anti-dsDNA production and IgG elevation, and suppressed proteinuria production. Although Foxp3 expression was lower in the TGF-β-primed CD8+ cells than in the TGF-β-primed CD4+ cells, the suppressive activity was not compromised and was even stronger (Fig. 4A). It is likely that TGF-β induced a novel CD8+Foxp3−CD103+ Treg cell subset [34]. Nonetheless, the addition of ATRA to TGF-β no longer increased the functional activities of the CD8+ iTregs in vivo (Fig. 4). Taken together, these results indicate that the addition of ATRA to TGF-β strengthens the differentiation and function of CD4+ iTregs and provides a better approach to the treatment of autoimmune and other diseases. This additive does not promote the differentiation and development of CD8+ iTregs.
The different roles of ATRA in the induction of CD4+ and CD8+ iTregs in humans
We further used human cells to determine whether the differential effects of ATRA on mouse CD8+Foxp3+ and CD4+Foxp3+ iTregs are generally shared in different species. The addition of ATRA to human cells enhanced Foxp3 expression on the TGF-β-primed CD4+ but not on the TGF-β-primed CD8+ cells (Fig. 5A). The addition of ATRA to the human cells increased the maturation of both the CD4+ and CD8+ cells (Fig. 5B), indicating that human CD8+ cells respond to ATRA in a manner similar to CD4+ cells.
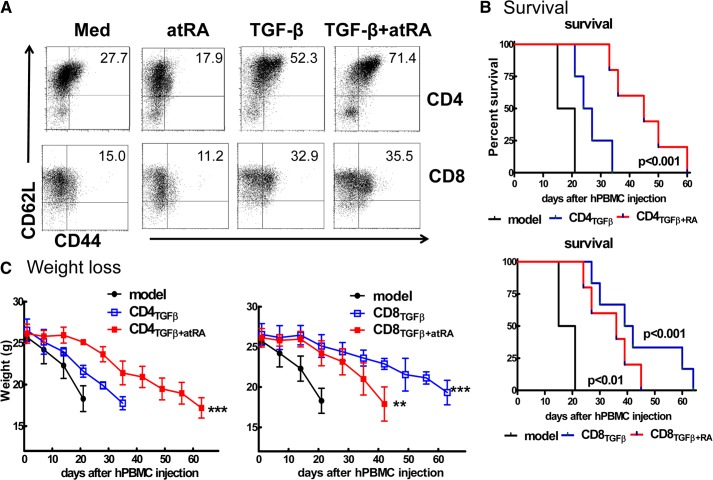
Figure 5. ATRA promoted the function of TGF-β-induced human CD4+ iTregs, but not CD8+ iTregs, in a humanized xeno-GVHD model.
(A) Human CD4+CD45RA+ or CD8+CD45RA+ cells isolated from peripheral PBMCs were stimulated with anti-human CD3/CD28 beads 1:10 (1 bead:10 cells) with IL-2 (50–100 U/ml), with or without TGF-β1 (5 ng/ml) and with or without ATRA (100 nM) for 5 days. Foxp3 expression was determined by flow cytometry. Histogram data are representative of 3 independent experiments. (B) Kaplan-Meier survival estimates of NOD/SCID mice that received 20 × 106 CD25− hPBMCs or plus 5 × 106 CD4TGFβ, CD8TGFβ, CD4TGFβ+ATRA, or CD8TGFβ+ATRA cells. (D) Body weight of xeno-GVHD model mice treated with or without conditioning cells, as described in (A). Data were collected from 3 independent experiments. *P < 0.05, **P < 0.01.
As has been reported, human polyclonally stimulated CD4+ cells primed with TGF-β do not acquire marked suppressive activity [35, 36]. By contrast, the addition of ATRA to CD4+ cells primed with TGF-β induces potent suppressive activity [24]. We therefore used a previously established mouse model to examine in vivo effects of human iTregs [27]. Lightly irradiated NOD SCID common γ chain−/− (NOG) mice injected with 20 × 106 CD25-depleted hPBMCs rapidly lost weight and survived for approximately 3 weeks. The addition of 5 × 106 TGF-β-primed CD4+ cells slightly but nonsignificantly prolonged survival or alleviated weight loss (Fig. 5C). However, co-injection of 5 × 106 of ATRA/TGF-β primed CD4+ cells significantly prolonged the survival and maintained the weight of the mice with xeno-GVHD. Of note, the adoptive transfer of the CD8+ cells primed with TGF-β resulted in an ideal protective effect against the demise of the mice with xeno-GVHD. The addition of ATRA unexpectedly did not enhance the functionality of the TGF-β primed CD8+ iTregs; in fact, it decreased their functionality (Fig. 5C).
DISCUSSION
We and others have previously demonstrated that ATRA promotes the differentiation and function of TGF-β-induced CD4+Foxp3+ cells [16, 18]. It seems that ATRA mainly affects CD4+ but not CD8+ cells. It promotes Foxp3 expression on CD4+ cells and enhances the suppressive activities of TGF-β-primed CD4+ cells in vitro and in vivo, but in our study, the combination of ATRA and TGF-β neither improved Foxp3 expression nor boosted the suppressive activity of CD8+ cells.
We conducted various experiments to exclude the possibility that CD8+ cells respond less to TGF-β and ATRA. In using various strengths of TCR stimulation and various concentrations of IL-2 and TGF-β, as well as a time dynamic, we observed that the maximum levels of Foxp3 expressed in TGF-β-primed CD8+ cells were about 35%, which is significantly lower than in TGF-β-primed CD4+ cells. Naive CD8+ cells expressed TβRI and -II and phosphorylated Smad3 after the stimulation of TGF-β, which is similar to naive CD4+ cells [34]. Thus, the intrinsic difference between CD8+ and CD4+ cells may be responsible for the TGF-β-mediated differential Foxp3 induction in the two T-cell populations.
Although ATRA did not enhance Foxp3 expression on CD8+ cells, these cells expressed the ATRA receptor RARα. We now provide evidence that CD8+ cells, regardless of resting or activated status, express levels of RARα mRNA and protein that are at least similar to those of their CD4+ counterparts. Thus, it is unlikely that the lack of RARα in CD8+ cells limited their response to ATRA. Naive CD8+ cells gradually lost the naive phenotypic feature and assumed a memory phenotype after the stimulation. TGF-β treatment helped to maintain the naive phenotype on both CD4+ and CD8+ cells, providing additional evidence that CD8+ cells do respond to TGF-β [37, 38]. However, with the addition of ATRA to the cultures, most CD8+ cells assumed a memory phenotype, even though these cells were stimulated with TGF-β, implying that CD8+ and CD4+ cells respond similarly to ATRA. Studies have demonstrated that ATRA accelerates the maturation of naive CD4+ and CD8+ cells to become effector/memory T cells [24, 37]. It has been reported that α4β7 and CCR-9, 2 gut-homing molecules, are markedly upregulated in T cells after treatment with ATRA [30], and our findings showed that ATRA enhanced the expression of α4β7 and CCR-9 in both TGF-β-primed CD4+ and CD8+ cells. Moreover, we extended this observation from mouse cells to human cells, and the results strongly suggest that CD8+ cells normally recognize and respond to ATRA, although it does not promote Foxp3 induction in these cells.
It is notable that Foxp3 expression was much lower in the CD8+ iTregs than in the CD4+ iTregs; however, the lower level of Foxp3 expression did not compromise the suppressive activity of the CD8+ iTregs. In fact, the CD8+ iTregs induced by TGF-β were even more suppressive than the CD4+ iTregs, particularly in human cells. It is likely that Foxp3 is not crucial for CD8+ iTreg induction, although this transcription factor is crucial for CD4+ Treg development and function [39]. We recently identified that CD8+CD103+Foxp3− cells represent newly identified CD8+ iTregs [34].
It is very interesting that CD8+ iTregs displayed a potent therapeutic effect on xeno-GVHD in a humanized animal model. Conversely, the effect of CD4+ cells treated with TGF-β was mild, consistent with a previous report claiming that TGF-β-primed CD4+ cells are not suppressor cells [35]. However, the addition of ATRA to TGF-β-primed CD4+ cells induced human CD4+ cells to become iTregs, further confirming reports that the combination of ATRA and TGF-β induces CD4+ cells to become Tregs in humans [24, 40]. We did not anticipate that the addition of ATRA would fail to enhance the suppressive activity of CD8+ cells. It decreased the suppressive ability of TGF-β-primed CD8+ cells in xeno-GVHD, raising a caution regarding the development of CD8+ iTregs using ATRA.
Tregs are crucial for the prevention of autoimmune diseases and the maintenance of immune tolerance. These cells consist of thymus-derived nTregs and those that can be induced in vitro and in vivo. Among induced Treg populations, although most studies have focused on CD4+ Tregs, the CD4+ Treg counterpart, CD8+ Tregs have gained attention as an interesting regulator [41–43].
It is possible that CD8+ and CD4+ Tregs play different roles. Although both types of Treg suppress immune responses and could be therapeutic for many autoimmune diseases, the studies have found that CD8+ Tregs exist predominately in immune-privileged sites or organs, particularly in the eye [6]. In addition, the treatment related to the induction of immune tolerance in transplantation and autoimmune diseases is usually accompanied by the increased CD8+ rather than CD4+ Treg cell frequency [44–47].
In summary, we found that ATRA displays a completely differential role in promoting the phenotypic and functional development of TGF-β-induced CD4+ and CD8+ Foxp3+ iTregs. Although ATRA promotes Foxp3 expression and boosts the functional capacity of CD4+ iTregs, it does not similarly promote the development of CD8+ iTregs, particularly in human cells. In fact, the addition of ATRA to TGF-β interferes with the development of human CD8+ iTregs induced with TGF-β. This study demonstrates the complexity of the immune system and its regulation and raises an important caution regarding developing a protocol to induce Treg subsets and their clinical therapy in autoimmune diseases and the prevention of rejection in organ transplantation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the U.S. National Institutes of Health (AR059103 and AI084359), The American College of Rheumatology (ACR) Within Our Reach Fund, the Arthritis Foundation, the Wright Foundation, the Science and Technology Committee Project of Shanghai Pudong new area (PKJ2009-Y41), the National Natural Science Foundation of China (81274161 and 81001307), the Zhejiang Provincial Natural Science Foundation of China (Y2090918), the Health Bureau of Zhejiang Province (2012RCA046), and the Hangzhou Science and Technology Bureau (20080333Q28).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- cGVHD
- chronic GVHD
- Cyc
- cyclin
- DC
- dendritic cells
- GITR
- glucocorticoid-induced TNFR-related protein
- GVHD
- graft-verse-host disease
- hPBMC
- human PBMC
- iTreg
- induced Treg
- nTreg
- natural Treg
- PBMC
- peripheral blood mononuclear cell
- RAR
- retinoic acid receptor
- TβR
- TGF-β receptor
- Treg
- regulatory T cell
AUTHORSHIP
S.G.Z. and J.M conceived and designed the experiments. J.M., Y.L., J.G., J.L., J.W., and Y.S performed the experiments. J.M., D.D.B., B.R., Z.L., and S.G.Z. analyzed the data. S.G.Z. wrote the paper.
DISCLOSURES
The authors declare no financial or commercial conflicts of interest.
REFERENCES
- 1. Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 [DOI] [PubMed] [Google Scholar]
- 2. Horwitz D. A., Zheng S. G., Gray J. D. (2008) Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 29, 429–435 [DOI] [PubMed] [Google Scholar]
- 3. Lan Q., Fan H., Quesniaux V., Ryffel B., Liu Z., Zheng S. G. (2012) Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J. Mol. Cell Biol. 4, 22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou X., Kong N., Zou H., Brand D., Li X., Liu Z., Zheng S. G. (2011) Therapeutic potential of TGF-beta-induced CD4(+) Foxp3(+) regulatory T cells in autoimmune diseases. Autoimmunity 44, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cosmi L., Liotta F., Lazzeri E., Francalanci M., Angeli R., Mazzinghi B., Santarlasci V., Manetti R., Vanini V., Romagnani P., Maggi E., Romagnani S., Annunziato F. (2003) Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood 102, 4107–4114 [DOI] [PubMed] [Google Scholar]
- 6. Sharafieh R., Lemire Y., Powell S., O'Rourke J., Cone R. E. (2011) Immune amplification of murine CD8 suppressor T cells induced via an immune-privileged site: quantifying suppressor T cells functionally. PloS One 6, e22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siegmund K., Ruckert B., Ouaked N., Burgler S., Speiser A., Akdis C. A., Schmidt-Weber C. B. (2009) Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J. Immunol. 182, 2124–2130 [DOI] [PubMed] [Google Scholar]
- 8. Giguere V. (1994) Retinoic acid receptors and cellular retinoid binding proteins: complex interplay in retinoid signaling. Endocr. Rev. 15, 61–79 [DOI] [PubMed] [Google Scholar]
- 9. Winoto A., Littman D. R. (2002) Nuclear hormone receptors in T lymphocytes. Cell 109(Suppl) S57–S66 [DOI] [PubMed] [Google Scholar]
- 10. Osanai M., Nishikiori N., Murata M., Chiba H., Kojima T., Sawada N. (2007) Cellular retinoic acid bioavailability determines epithelial integrity: role of retinoic acid receptor alpha agonists in colitis. Mol. Pharmacol. 71, 250–258 [DOI] [PubMed] [Google Scholar]
- 11. Miyagawa N., Homma T., Kagechika H., Shudo K., Nagai H. (2003) Effect of synthetic retinoid, TAC-101, on experimental autoimmune disease. Pharmacology 67, 21–31 [DOI] [PubMed] [Google Scholar]
- 12. Zunino S. J., Storms D. H., Stephensen C. B. (2007) Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in nonobese diabetic mice. J. Nutr. 137, 1216–1221 [DOI] [PubMed] [Google Scholar]
- 13. Racke M. K., Burnett D., Pak S. H., Albert P. S., Cannella B., Raine C. S., McFarlin D. E., Scott D. E. (1995) Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J. Immunol. 154, 450–458 [PubMed] [Google Scholar]
- 14. Reifen R., Nur T., Ghebermeskel K., Zaiger G., Urizky R., Pines M. (2002) Vitamin A deficiency exacerbates inflammation in a rat model of colitis through activation of nuclear factor-kappaB and collagen formation. J. Nutr. 132, 2743–2747 [DOI] [PubMed] [Google Scholar]
- 15. Iwata M., Eshima Y., Kagechika H. (2003) Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int. Immunol. 15, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 16. Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 [DOI] [PubMed] [Google Scholar]
- 17. Chen M., Su W., Lin X., Guo Z., Wang J., Zhang Q., Brand D., Ryffel B., Huang J., Liu Z., He X., Le A. D., Zheng S. G. (2013) Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheumatism 65, 1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu L., Ma J., Li Z., Lan Q., Chen M., Liu Y., Xia Z., Wang J., Han Y., Shi W., Quesniaux V., Ryffel B., Brand D., Li B., Liu Z., Zheng S. G. (2011) All-trans retinoic acid promotes TGF-beta-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PloS One 6, e24590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao S., Jin H., Korn T., Liu S. M., Oukka M., Lim B., Kuchroo V. K. (2008) Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 181, 2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Y. H., Lee W. H., Ortiz S., Lee M. H., Qin H. J., Liu C. P. (2009) All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes 58, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coombes J. L., Siddiqui K. R., Arancibia-Carcamo C. V., Hall J., Sun C. M., Belkaid Y., Powrie F. (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Housley W. J., O'Conor C. A., Nichols F., Puddington L., Lingenheld E. G., Zhu L., Clark R. B. (2009) PPARγ regulates retinoic acid-mediated DC induction of Tregs. J. Leukoc. Biol. 86, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X., Kong N., Wang J., Fan H., Zou H., Horwitz D., Brand D., Liu Z., Zheng S. G. (2010) Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J. Immunol. 185, 2675–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lu L., Zhou X., Wang J., Zheng S. G., Horwitz D. A. (2010) Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PloS One 5, e15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng S. G., Wang J. H., Stohl W., Kim K. S., Gray J. D., Horwitz D. A. (2006) TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol. 176, 3321–3329 [DOI] [PubMed] [Google Scholar]
- 26. Zheng S. G., Wang J. H., Koss M. N., Quismorio F., Jr, Gray J. D., Horwitz D. A. (2004) CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 172, 1531–1539 [DOI] [PubMed] [Google Scholar]
- 27. Van Rijn R. S., Simonetti E. R., Hagenbeek A., Hogenes M. C., de Weger R. A., Canninga-van Dijk M. R., Weijer K., Spits H., Storm G., van Bloois L., Rijkers G., Martens A. C., Ebeling S. B. (2003) A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood 102, 2522–2531 [DOI] [PubMed] [Google Scholar]
- 28. Zheng S. G., Wang J. H., Gray J. D., Soucier H., Horwitz D. A. (2004) Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 172, 5213–5221 [DOI] [PubMed] [Google Scholar]
- 29. Zhou X., Wang W., Yang Y. (2008) The expression of retinoic acid receptors in thymus of young children and the effect of all-transretinoic acid on the development of T cells in thymus. J. Clin. Immunol. 28, 85–91 [DOI] [PubMed] [Google Scholar]
- 30. Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S. Y. (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 [DOI] [PubMed] [Google Scholar]
- 31. Zheng S. G., Gray J. D., Ohtsuka K., Yamagiwa S., Horwitz D. A. (2002) Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 169, 4183–4189 [DOI] [PubMed] [Google Scholar]
- 32. Zhou X., Wang J., Shi W., Brand D. D., Liu Z., Fan H., Zheng S. G. (2010) Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J. Mol. Cell Biol. 2, 164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rolink A. G., Radaszkiewicz T., Pals S. T., van der Meer W. G., Gleichmann E. (1982) Allosuppressor and allohelper T cells in acute and chronic graft-vs-host disease: I, alloreactive suppressor cells rather than killer T cells appear to be the decisive effector cells in lethal graft-vs.-host disease. J. Exp. Med. 155, 1501–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y., Lan Q., Lu L., Chen M., Wang J., Fan H., Shen Y., Ryffel B., Brand D., Quismorio F., Liu Z., Horwitz D., Xu A., Zheng S. G. (2013) Phenotypic and functional characteristic of a newly identified CD8+Foxp3−CD103+ regulatory T cells. J. Mol. Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tran D. Q., Ramsey H., Shevach E. M. (2007) Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110, 2983–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tran D. Q. (2012) TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J. Mol. Cell Biol. 4, 29–37 [DOI] [PubMed] [Google Scholar]
- 37. Tan X., Sande J. L., Pufnock J. S., Blattman J. N., Greenberg P. D. (2011) Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J. Virol. 85, 8316–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pino-Lagos K., Benson M. J., Noelle R. J. (2008) Retinoic acid in the immune system. Ann. N. Y. Acad. Sci. 1143, 170–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hori S., Nomura T., Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 40. Wang J., Huizinga T. W., Toes R. E. (2009) De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J. Immunol. 183, 4119–4126 [DOI] [PubMed] [Google Scholar]
- 41. Kapp J. A., Bucy R. P. (2008) CD8+ suppressor T cells resurrected. Hum. Immunol. 69, 715–720 [DOI] [PubMed] [Google Scholar]
- 42. Kim H. J., Verbinnen B., Tang X., Lu L., Cantor H. (2010) Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 467, 328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng J., Liu Y., Liu Y., Liu M., Xiang Z., Lam K. T., Lewis D. B., Lau Y. L., Tu W. (2013) Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Sci. Transl. Med 5, 168ra9. [DOI] [PubMed] [Google Scholar]
- 44. Ciubotariu R., Vasilescu R., Ho E., Cinti P., Cancedda C., Poli L., Late M., Liu Z., Berloco P., Cortesini R., Suciu-Foca Cortesini N. (2001) Detection of T suppressor cells in patients with organ allografts. Hum. Immunol. 62, 15–20 [DOI] [PubMed] [Google Scholar]
- 45. Brand D. D., Myers L. K., Whittington K. B., Latham K. A., Stuart J. M., Kang A. H., Rosloniec E. F. (2002) Detection of early changes in autoimmune T cell phenotype and function following intravenous administration of type II collagen in a TCR-transgenic model. J. Immunol. 168, 490–498 [DOI] [PubMed] [Google Scholar]
- 46. Hahn B. H., Singh R. P., La Cava A., Ebling F. M. (2005) Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting. CD8+ T cell suppressors. J. Immunol. 175, 7728–7737 [DOI] [PubMed] [Google Scholar]
- 47. Kang H. K., Michaels M. A., Berner B. R., Datta S. K. (2005) Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J. Immunol. 174, 3247–3255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.