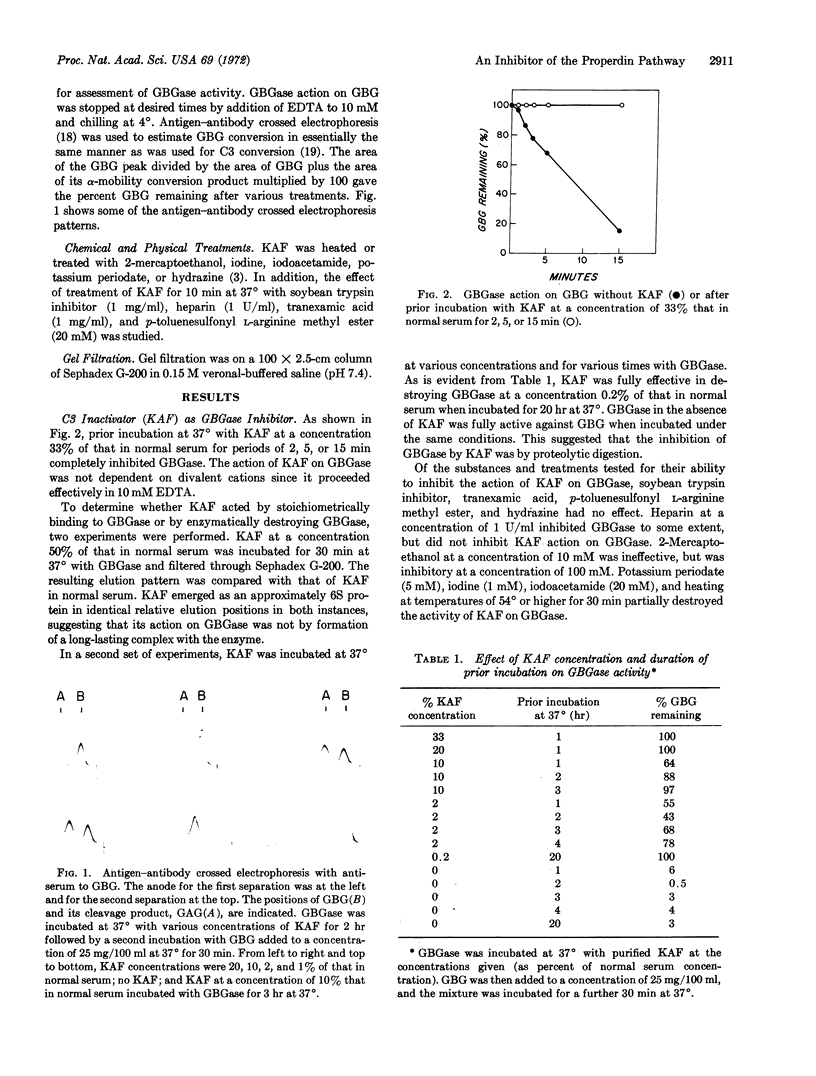
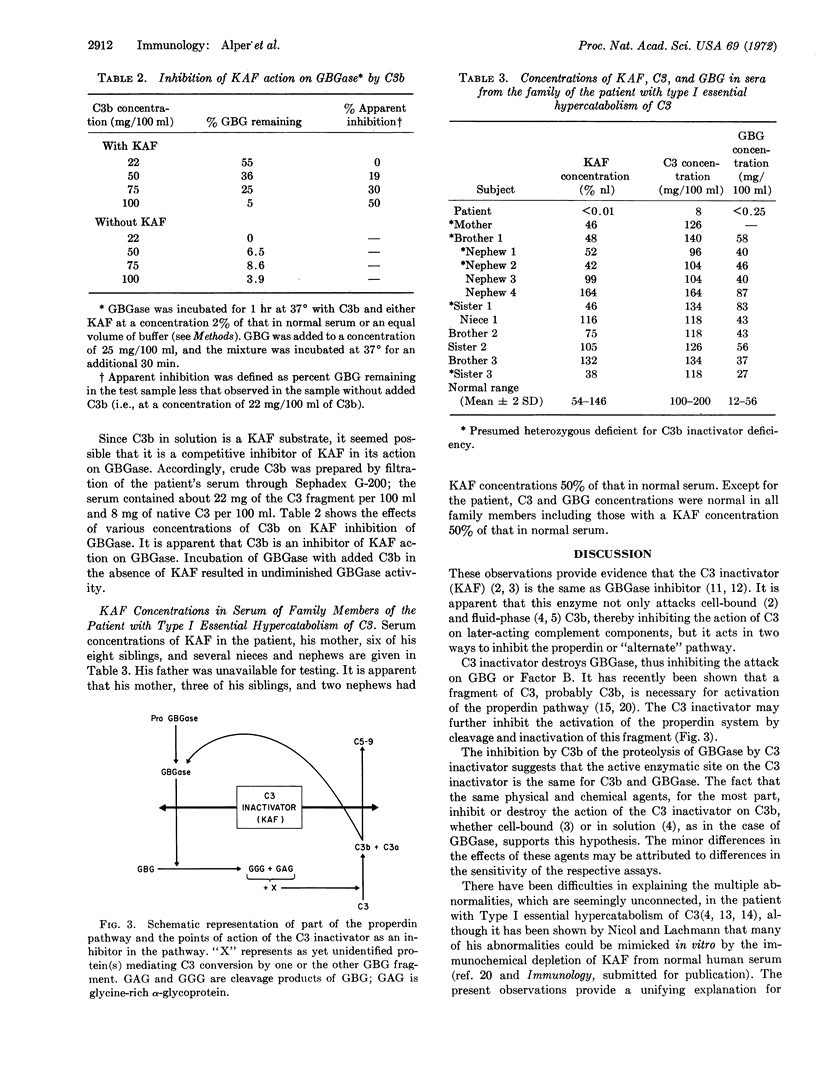
Abstract
Evidence has been obtained that a single protein, known to modulate classical complement activation, also acts as an inhibitor in the properdin or alternate complement pathway. A highly purified inactivator of the third component of complement (C3) from human serum inhibited the proteolysis of Factor B in the properdin system (glycine-rich β-glycoprotein) by glycine-rich β-glycoproteinase. The inhibition was by the enzymatic destruction of glycine-rich β-glycoproteinase activity. The major fragment of C3, C3b, which is the only known substrate of the C3 inactivator, blocked the destruction of glycine-rich β-glycoproteinase by the C3 inactivator. Thus, in its inhibition of the porperdin pathway, the C3 inactivator destroys both the active form of glycine-rich β-glycoproteinase and a protein involved in the conversion of the zymogen form of this enzyme (proglycine-rich β-glycoproteinase) to its active form. The increased susceptibility to infections in a patient homozygous for deficiency of the C3 inactivator demonstrates the biologic significance of this protein.
Keywords: glycine-rich β-glycoprotein, C3b, KAF, conglutinogen-activating factor, crossed electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Alper C. A., Lachmann P. J., Rosen F. S., Jandl J. H. Deficiency of C3 inactivator in man. J Immunol. 1971 Jul;107(1):19–27. [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Increased susceptibility to infection associated with abnormalities of complement-mediated functions and of the third component of complement (C3). N Engl J Med. 1970 Feb 12;282(7):350–354. doi: 10.1056/nejm197002122820701. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970 Nov;49(11):1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Genetic aspects of the complement system. Adv Immunol. 1971;14:251–290. doi: 10.1016/s0065-2776(08)60286-2. [DOI] [PubMed] [Google Scholar]
- BLUM L., PILLEMER L., LEPOW I. H. The properdin system and immunity. XIII. Assay and properties of a heat-labile serum factor (factor B) in the properdin system. Z Immun exp ther. 1959 Oct-Nov;118:349–357. [PubMed] [Google Scholar]
- Boenisch T., Alper C. A. Isolation and properties of a glycine-rich beta-glycoprotein of human serum. Biochim Biophys Acta. 1970 Dec 22;221(3):529–535. doi: 10.1016/0005-2795(70)90224-2. [DOI] [PubMed] [Google Scholar]
- Goodkofsky I., Lepow I. H. Functional relationship of factor B in the properdin system to C3 proactivator of human serum. J Immunol. 1971 Oct;107(4):1200–1204. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENSKY J., LEVY L. R., LEPOW I. H. Partial purification of a serum inhibitor of C'1-esterase. J Biol Chem. 1961 Jun;236:1674–1679. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]




