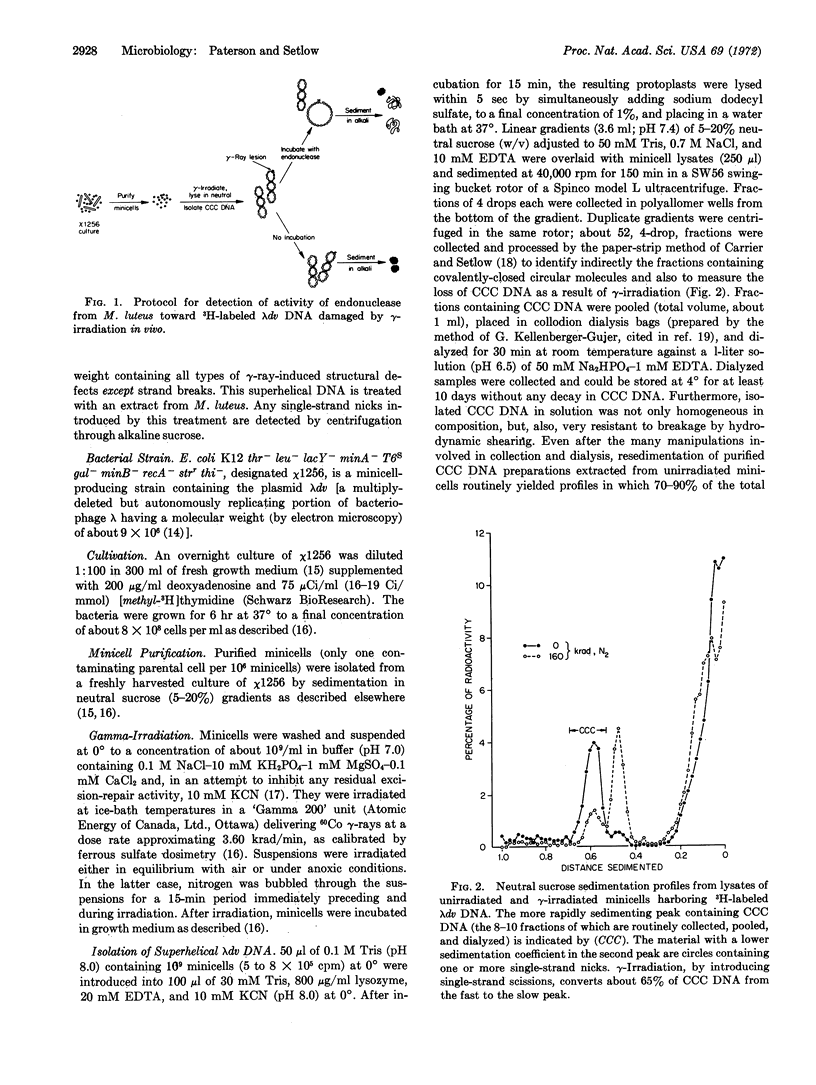
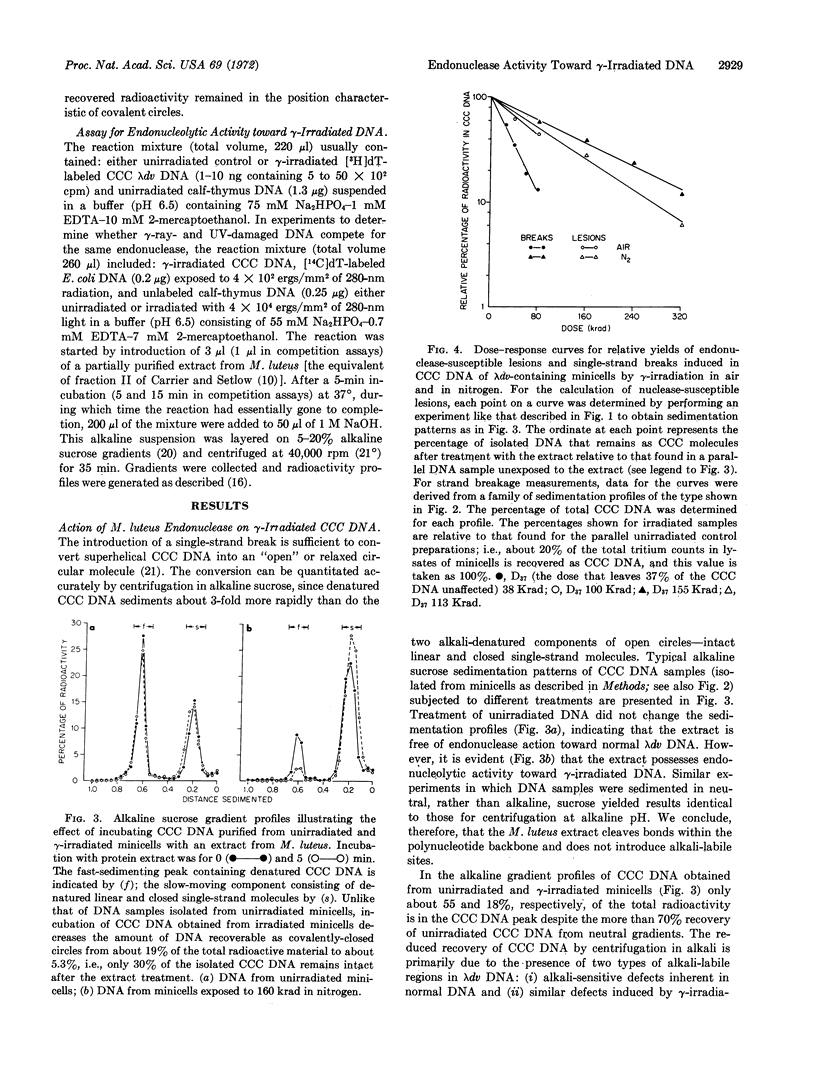
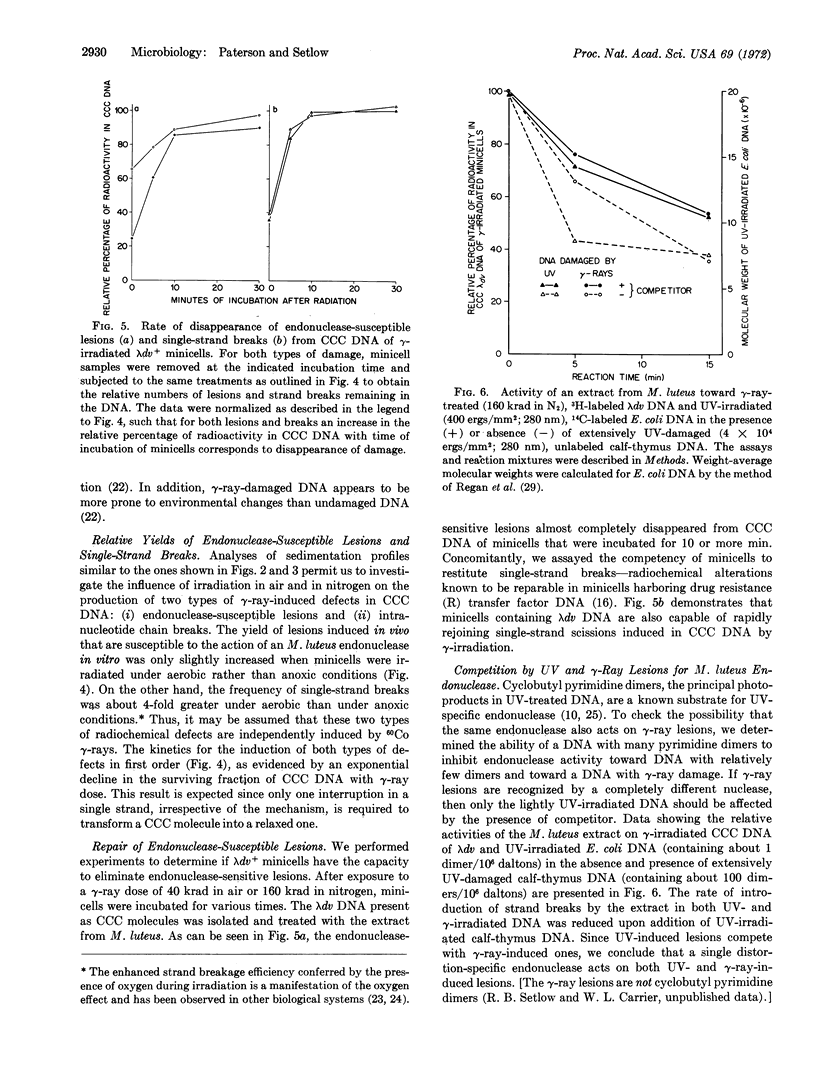
Abstract
A partially purified extract from Micrococcus luteus contains endonucleolytic activity toward ultraviolet (UV)-irradiated deoxyribonucleic acid (DNA). We found that the same extract also acts on superhelical, covalently-closed circular λdv DNA isolated from γ-irradiated minicells of E. coli. The introduction of nicks in isolated covalently-closed circular DNA by an endonuclease in the extract results in relaxed circles, and these two circular DNA species are easily distinguishable by their sedimentation properties in alkaline sucrose. The frequency with which the endonuclease-susceptible lesions are produced in superhelical DNA is only marginally enhanced when 60Co γ-rays are administered to an aerobic rather than an anoxic minicell suspension. The ratio of endonuclease-sensitive defects to single-strand scissions, induced by γ-irradiation in air, is about 1:3. The nuclease-sensitive lesions disappear from γ-irradiated minicells during incubation after radiation presumably as a consequence of excision repair. Since the addition of UV-irradiated calf-thymus DNA depresses the ability of the M. luteus extract to attack not only UV-damaged E. coli DNA (a known substrate for the so-called UV-specific endonuclease that catalyzes the initial single-strand incision adjacent to the structural defect) but, also λdv DNA injured by γ-rays, we conclude that physicochemical alterations induced by both types of radiation are recognized by one and the same endonuclease.
Keywords: covalently-closed circular DNA, excision repair, strand-rejoining repair, sucrose gradient analysis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T. A characteristic of the lethal effect of ionizing radiation on "Hcr-" bacterial strains. Mutat Res. 1967 Feb;4(1):15–20. doi: 10.1016/0027-5107(67)90105-4. [DOI] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R. P., Tepper M. X-ray-induced single-strand breaks and joining of broken strands in superinfecting lambda DNA in Escherichia coli lysogenic for lambda. Virology. 1968 Feb;34(2):344–351. doi: 10.1016/0042-6822(68)90245-6. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- Grossman L., Kaplan J. C., Kushner S. R., Mahler I. Enzymes involved in the early stages of repair of ultraviolet-irradiated DNA. Cold Spring Harb Symp Quant Biol. 1968;33:229–234. doi: 10.1101/sqb.1968.033.01.026. [DOI] [PubMed] [Google Scholar]
- Hariharan P. P., Cerutti P. A. Repair of gamma-ray-induced thymine damage in Micrococcus radiodurans. Nat New Biol. 1971 Feb 24;229(8):247–249. doi: 10.1038/newbio229247a0. [DOI] [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation and repair of gamma-ray induced thymine damage in Micrococcus radiodurans. J Mol Biol. 1972 Apr 28;66(1):65–81. doi: 10.1016/s0022-2836(72)80006-8. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen I., Gurvin I., Rupp W. D. The formation of single-strand breaks in intracellular DNA by x-rays. Radiat Res. 1971 Dec;48(3):599–612. [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA, 1. Purification of two enzymes involved in the excision of thymine dimers from ultraviolet-irradiated DNA. Proc Natl Acad Sci U S A. 1969 May;63(1):144–151. doi: 10.1073/pnas.63.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Repair of radiation-induced damage in Escherichia coli. II. Effect of rec and uvr mutations on radiosensitivity, and repair of x-ray-induced single-strand breaks in deoxyribonucleic acid. J Bacteriol. 1970 Jul;103(1):49–54. doi: 10.1128/jb.103.1.49-54.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Kaiser A. D. Lambda dv: an autonomously replicating DNA fragment. Cold Spring Harb Symp Quant Biol. 1968;33:769–775. doi: 10.1101/sqb.1968.033.01.088. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Boyle J. M., Setlow R. B. Ultraviolet- and X-ray-induced responses of a deoxyribonucleic acid polymerase-deficient mutant of Escherichia coli. J Bacteriol. 1971 Jul;107(1):61–67. doi: 10.1128/jb.107.1.61-67.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson M. C., Roozen K. J. Photoreactivatiion, excision, and strand-rejoining repair in R factor-containing minicells of Escherichia coli K-12. J Bacteriol. 1972 Apr;110(1):71–80. doi: 10.1128/jb.110.1.71-80.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. C., Meun D. H. Repair of radiation-induced damage in Escherichia coli. I. Effect of rec mutations on post-replication repair of damage due to ultraviolet radiation. J Mol Biol. 1970 Aug;51(3):459–472. doi: 10.1016/0022-2836(70)90001-x. [DOI] [PubMed] [Google Scholar]
- Strauss B. S. DNA repair mechanisms and their relation to mutation and recombination. Curr Top Microbiol Immunol. 1968;44:1–85. [PubMed] [Google Scholar]
- Takagi Y., Sekiguchi M., Okubo S., Nakayama H., Shimada K., Yasuda S., Nishimoto T., Yoshihara H. Nucleases specific for ultraviolet light-irradiated DNA and their possible role in dark repair. Cold Spring Harb Symp Quant Biol. 1968;33:219–227. doi: 10.1101/sqb.1968.033.01.025. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]



