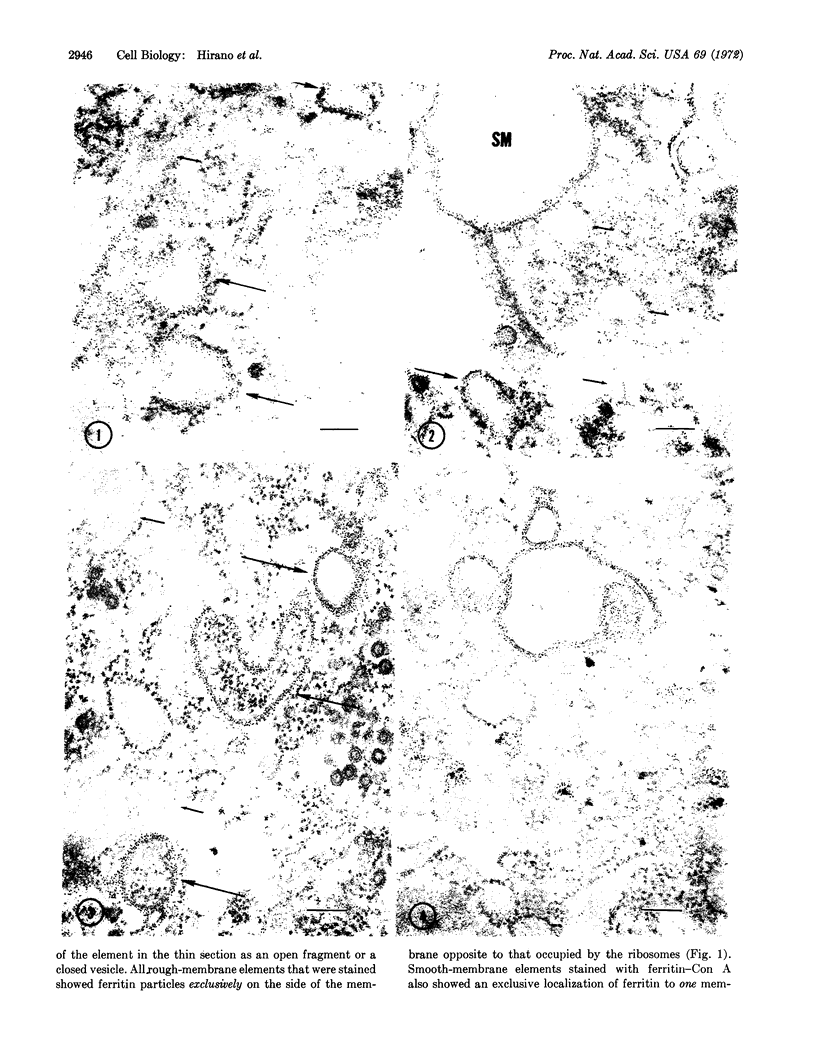
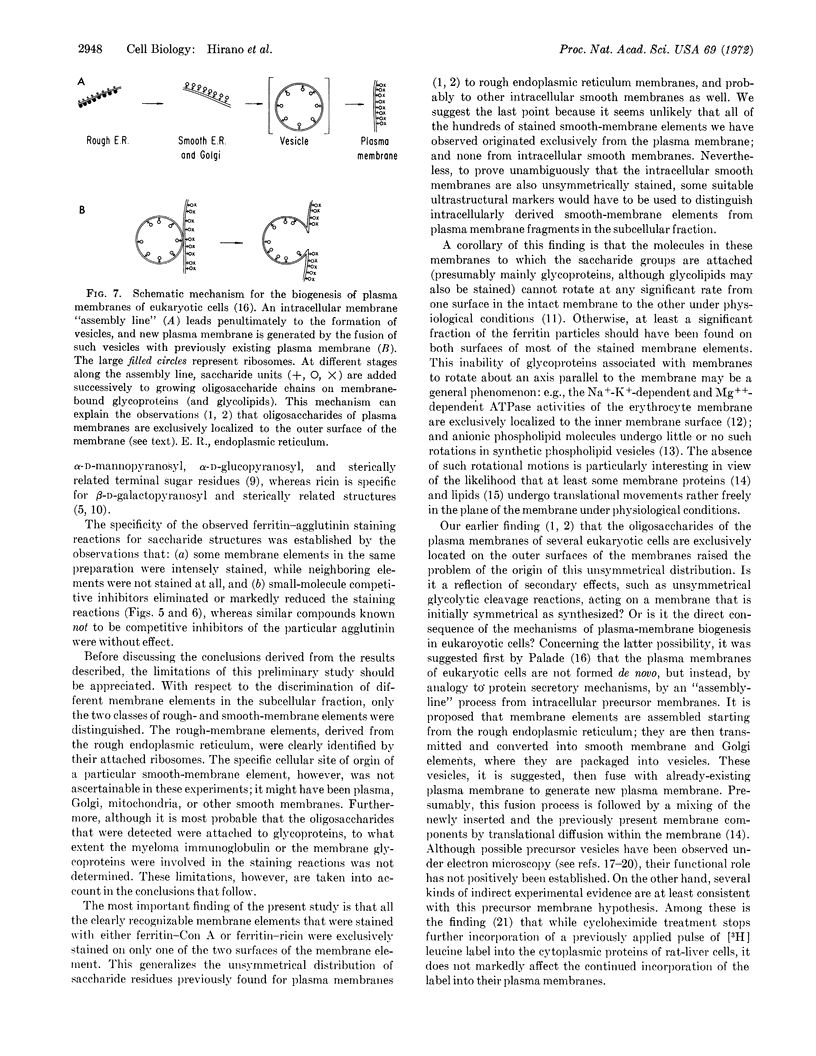
Abstract
Ferritin conjugates of two plant agglutinins, concanavalin A and ricin, have been used as specific electron microscopic stains for covalently-bound saccharide residues on membrane fragments from a myeloma-cell homogenate. The results indicate that different saccharide residues are uniformly localized to a single surface of each membrane fragment. In particular, the ferritin-concanavalin A conjugate binds exclusively to the cisternal side of membrane fragments of the rough endoplasmic reticulum. If it is postulated that the biogenesis of eukaryotic plasma membranes involves an assembly-line process from precursor intracellular membranes, these observed asymmetric distributions of saccharides on cell membranes can be explained.
Keywords: ferritin conjugates, plant agglutinins, smooth- and rough-membrane elements
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bennett G., Leblond C. P. Formation of cell coat material for the whole surface of columnar cells in the rat small intestine, as visualized by radioautography with L-fucose-3H. J Cell Biol. 1970 Aug;46(2):409–416. doi: 10.1083/jcb.46.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Knopf P. M., Lennox E. S. Intracellular transport and secretion of an immunoglobulin light chain. Biochemistry. 1971 Feb 16;10(4):668–679. doi: 10.1021/bi00780a019. [DOI] [PubMed] [Google Scholar]
- Choi Y. S., Knopf P. M., Lennox E. S. Subcellular fractionation of mouse myeloma cells. Biochemistry. 1971 Feb 16;10(4):659–667. doi: 10.1021/bi00780a018. [DOI] [PubMed] [Google Scholar]
- Drysdale R. G., Herrick P. R., Franks D. The specificity of the haemagglutinin of the Castor bean, Ricinus communis. Vox Sang. 1968;15(3):194–202. doi: 10.1111/j.1423-0410.1968.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol. 1967 Nov;35(2):385–404. doi: 10.1083/jcb.35.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar J., Sy D. Attachment of glucosamine to protein at the ribosomal site of rat liver. Biochemistry. 1967 Jul;6(7):1941–1947. doi: 10.1021/bi00859a009. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Marchesi V. T., Singer S. J. The localization of spectrin on the inner surface of human red blood cell membranes by ferritin-conjugated antibodies. J Cell Biol. 1971 Oct;51(1):265–272. doi: 10.1083/jcb.51.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretz R. D., Goldstein I. J. An examination of the topography of the saccharide binding sites of concanavalin A and of the forces involved in complexation. Biochemistry. 1970 Jul 7;9(14):2890–2896. doi: 10.1021/bi00816a021. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Hernandez W., Leblond C. P. Detection of complex carbohydrates in the Golgi apparatus of rat cells. J Cell Biol. 1969 Feb;40(2):395–414. doi: 10.1083/jcb.40.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A. Morphological and histochemical aspects of glycoproteins at the surface of animal cells. Int Rev Cytol. 1971;31:57–114. doi: 10.1016/s0074-7696(08)60057-1. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Lieberman I., Lansing A. I. Synthesis of the plasma membrane of the liver cell. Biochem Biophys Res Commun. 1968 Apr 5;31(1):54–58. doi: 10.1016/0006-291x(68)90030-2. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Stockem W. Pinocytose und Bewegung von Amöben. 3. Die Funktsion des Golgiapparates von Amoeba proteus und Chaos chaos. Histochemie. 1969;18(3):217–240. doi: 10.1007/BF00306169. [DOI] [PubMed] [Google Scholar]
- Whur P., Herscovics A., Leblond C. P. Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol. 1969 Nov;43(2):289–311. doi: 10.1083/jcb.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise G. E., Flickinger C. J. Cytochemical staining of the Golgi apparatus in amoeba proteus. J Cell Biol. 1970 Sep;46(3):620–626. doi: 10.1083/jcb.46.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise G. E., Flickinger C. J. Relation of the Golgi apparatus to the cell coat in amoebae. Exp Cell Res. 1970 Jul;61(1):13–23. doi: 10.1016/0014-4827(70)90252-1. [DOI] [PubMed] [Google Scholar]
- Zagury D., Uhr J. W., Jamieson J. D., Palade G. E. Immunoglobulin synthesis and secretion. II. Radioautographic studies of sites of addition of carbohydrate moieties and intracellular transport. J Cell Biol. 1970 Jul;46(1):52–63. doi: 10.1083/jcb.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]