Abstract
A delicate balance exists between the central dopaminergic and cholinergic neurotransmitter systems with respect to motor function. An imbalance can result in motor dysfunction as observed in Parkinson’s disease patients and in patients treated with antipsychotic compounds. Cholinergic receptor antagonists can alleviate extrapyramidal symptoms in Parkinson’s disease and motor side effects induced by antipsychotics. The effects of anticholinergics are mediated by muscarinic receptors of which five subtypes (M1-M5) exist. Muscarinic M4 receptors are found in high concentrations in motor parts of the striatum, suggesting a role for muscarinic M4 receptors in the motor side effects of antipsychotics, and in the alleviation of these side effects by anticholinergics. To this end, we investigated the potential role of the muscarinic M4 receptor in catalepsy induced by antipsychotics (haloperidol and risperidone) as well as the anti-cataleptic effects of the non-selective anticholinergic drug scopolamine in fully backcrossed muscarinic M4 receptor knockout mice. The drug-induced catalepsy was strongly attenuated, but not abolished, in M4 knockout mice as compared to wild-type controls. Scopolamine further attenuated the cataleptic response in M4 knockout mice, suggesting that non-M4 muscarinic receptors also participate in the anti-cataleptic effects. In conclusion, these data indicate an important role for M4 receptors in antipsychotic-induced motor side effects and suggest that M4 receptors could be a target for future pharmacological treatment of antipsychotic-induced as well as idiopathic parkinsonism.
Keywords: Muscarinic M4 receptor, catalepsy, haloperidol, risperidone, dopamine, scopolamine, motoric side effects
1. Introduction
Muscarinic acetylcholine receptors are highly expressed in the central nervous system (Levey 1993; Vilaro 1993) where they are involved in numerous functions, e.g. learning, memory, reward, and motor performance (Bymaster et al., 2003; Wess, 2004). It is well known that a delicate balance exists between the central dopaminergic and cholinergic transmitter system with respect to motor function. Imbalance between the two systems can result in motor dysfunction as observed in Parkinson’s disease patients and in patients with parkinsonian symptoms following treatment with antipsychotic compounds (Di Chiara et al., 1994), i.e. dopamine D2 receptor antagonists (Seeman et al., 1976). Cholinergic receptor antagonists can alleviate extrapyramidal symptoms in Parkinson’s disease and motor side effects induced by antipsychotics. The effects of anticholinergics are mediated by muscarinic receptors of which five subtypes exist (M1-M5). Muscarinic M4 receptors are highly expressed in striatal areas (Hersch et al., 1994), and involvement of M4 receptors in motor activities has been suggested (Gomeza et al., 1999). However, many antipsychotic compounds, e.g. haloperidol and risperidone at high doses, frequently induce extrapyramidal side effects in patients (Gao et al., 2008) an effect most likely caused by dopamine D2 receptor blockade (Arnt, 1998). It is well-known that muscarinic M4 receptors are co-localized with dopamine D1 receptors on striatal medium spiny out-put neurons (Ince et al., 1997) with opposite effects on cAMP formation (Ince et al., 1997; Hulme et al., 1990). However, many antipsychotic compounds have no substantial receptor binding affinity for dopamine D1 receptors (Schotte et al., 1996) and consequently, the effect of muscarinic receptor antagonism on extrapyramidal side effects cannot simply be explained by opposite effects of dopamine D1 receptors and muscarinic M4 receptors on medium spiny striatal output neurons. A more likely explanation is the following: Striatal cholinergic interneurons express dopamine D2 receptors (Le Moine, 1990) and these interneurons innervate medium spiny output neurons projecting to the substantia nigra or to the globus pallidus (Robertson et al., 1992). Blockade of dopamine D2 receptors in the striatum enhances striatal release of acetylcholine (Damsma et al., 1990; DeBoer and Abercrombie, 1996) and striatal Fos expression (Dragunow et al., 1990; Fink-Jensen and Kristensen, 1994). The cataleptic effects of dopamine D2 receptor antagonists may be caused by increased release of striatal acetylcholine that subsequently binds to muscarinic receptors located on medium spiny striatopallidal or striatonigral neurons.
To what extent muscarinic M4 receptors play a role in this response has not been elucidated. Catalepsy is a behavioural phenomenon where the rodent is not able to change an externally imposed posture, and antipsychotic-induced catalepsy is a rodent model for the motoric side effects often seen when using antipsychotic medication (Hoffman and Donovan, 1995). One previous study byKarasawa et al. (2003) showed no difference in the cataleptic response to haloperidol in M4 receptor knockout mice (M4−/−) as compared to wildtype mice (M4+/+), suggesting that the M4 receptor was not involved in mediating the cataleptic effect of haloperidol. However, in the study by Karasawa et al. 2003 catalepsy was only measured 30 min following haloperidol administration and the mice were maintained on a mixed genetic background, which complicate interpretation of transgenic studies (Gerlai, 1996). Consequently, the full effect of the absence of M4 receptors might not have been fully elucidated.
Both antipsychotic-induced motor side effects in patients and antipsychotic-induced catalepsy in rodents can be alleviated by non-selective muscarinic antagonists (Katzenschlager et al., 2003; Klemm, 1985; McEvoy, 1983) and somewhat surprisingly Karasawa and collaborators (2003) found that the anti-cataleptic effect of the muscarinic antagonist scopolamine was attenuated in M4−/− mice, suggesting a role for M4 in the anti-cataleptic effect of scopolamine. To further investigate the involvement of the muscarinic M4 receptor in antipsychotic-induced catalepsy, we studied fully backcrossed (10 generations) muscarinic M4−/− mice and M4+/+ littermates and their cataleptic responses to the first generation antipsychotic haloperidol and the second generation antipsychotic risperidone. In the same M4−/− mice and M4+/+ littermates, we also tested the ability of scopolamine to modify haloperidol- and risperidone-induced catalepsy. Basal locomotor activity was also tested in order to insure that changes in catalepsy were not related to a general decline in motoric capabilities.
2. Methods
2.1. Animals
M4 receptor knockout mice were generated, as previously described (Gomeza et al., 1999), by disrupting the muscarinic M4 receptor gene in TC1 (129ScEv) embryonic stem cells. Founder mice of mixed genetic background (129SvEv/CF1) were then backcrossed to the C57BL/6Ntac strain for 10 generations to produce congenic mice. M4−/−mice, heterozygotes (M4+/−) and their M4+/+ littermates were bred at the animal facilities at the Panum Institute, University of Copenhagen. Genotyping was performed on mouse-tail DNA using the Polymerase Chain Reaction (PCR).
The mice were acclimatized to the animal facilities at Rigshospitalet where experiments were conducted for at least one week prior to experiments. Animals were housed in standard cages (macrolon type III) on wood-chip bedding with food and water available ad libitum and kept on a 12 h light/dark cycle in a temperature (22–24°C) and humidity (55%) controlled room. Cardboard pipes and nesting material were provided for enrichment. All experiments were carried out with experimentally naïve adult male mice, weighing 22–35 g. All testing was conducted during the light-phase of the circadian cycle (9.00 AM – 5.00 PM). The experiments were performed in accordance with guidelines from the Animal Experimentation Inspectorate, Denmark.
2.2. Locomotor Activity
Basal locomotor activity as well as locomotor activity following saline injection was measured in monitoring frames equipped with seven horizontal infrared light beams along the long axis of the frame placed 4.3 cm apart and 3.3 cm above the surface. Standard cages (macrolon type III) with a scant lining of fresh wood-chip bedding were placed in the monitoring frames and covered with plexiglas tops with ventilation holes. The set-up was situated in a ventilated soundproof room with dimmed light settings. A computer program (YMOT16) recorded interruptions of the light beams as counts of photo beam breaks in minimum intervals of one minute. A constant interruption of a photo beam would only be recorded once, with the intention that an animal remaining in a small area was recorded as being static.
Mice were transported to the experimental room on the test day and allowed at least one hour of habituation before the experiment was started. Mice used for baseline activity evaluation did not receive any pre-treatment, whereas saline injections (i.p.) were administered to M4−/− and M4+/+ mice immediately before evaluation of saline-induced locomotion. The mice were placed individually in cages in the monitoring frames, the experiment was started, and the experimenter left the room. For the assessment of both basal and saline-induced locomotor activity beam breaks were measured for 120 min. In both locomotion experiments, the experimenter was blind to the genotype. The mice were only tested once.
2.3. Catalepsy
M4−/− and M4+/+ mice were weighed and injected i.p. with 0.9 % saline or test-drug (haloperidol 0.3 and 1.0 mg/kg (Serenase, 5 mg/ml, Janssen-Cilag, DK) or risperidone 1.0 and 3.0 mg/kg (Sigma-Aldrich, DK)). After injection the mice were returned to their home cage where they remained until testing. The mice were tested for cataleptic responses 30, 60, and 90 min after the initial injection. This was done by lifting the mouse by the tail and placing it with its front paws on a steel bar and the hind legs on the plane surface. The steel bar was 15 cm long, had a diameter of 0.5 mm and was maintained horizontal 5.5 cm above the surface level. The centre of the steel bar was marked to assure identical placement of the mice during the tests. If the mouse did not stay in the position, the procedure was repeated two times more, and if the mouse still did not remain in the position, the cataleptic response time was registered as zero seconds. Time spent in the unnatural position was measured with a cut-off at 60 s. If the mice removed the front paws from the steel bar or the hind legs from the plane surface during the 60 s, the testing ended, and the time maintained in the position was registered. After each testing, the animal was returned to its home cage until next testing. Reversal of the cataleptic effect was investigated by randomly assigning the mice to treatment-groups according to their genotype and administering either saline or scopolamine (5.0 mg/kg, i.p., Scopolamine hydrobromide, Sigma-Aldrich, DK) immediately after the last test, i.e., 90 min after the test-drug injection. These mice were then tested for residual cataleptic response 120 min after their initial test-drug injection.
3. Data Analysis and Statistics
Basal locomotor activity and saline-induced locomotion, expressed as number of beam breaks in intervals of 30 min, were analysed by a two-way analysis of variance (ANOVA) with genotype as between-subjects factor and time interval as within-subjects factor followed by a post hoc t-test.
Antipsychotic-induced catalepsy was analysed by a two-way ANOVA with genotype as between-subjects factor and time point as within-subjects factor, followed by a Bonferroni corrected pair-wise comparisons. Reversal of the cataleptic effect with scopolamine was investigated with a two-way ANOVA with genotype and treatment as factors followed by a Bonferroni corrected pair-wise comparisons.
4. Results
4.1. Locomotor Activity
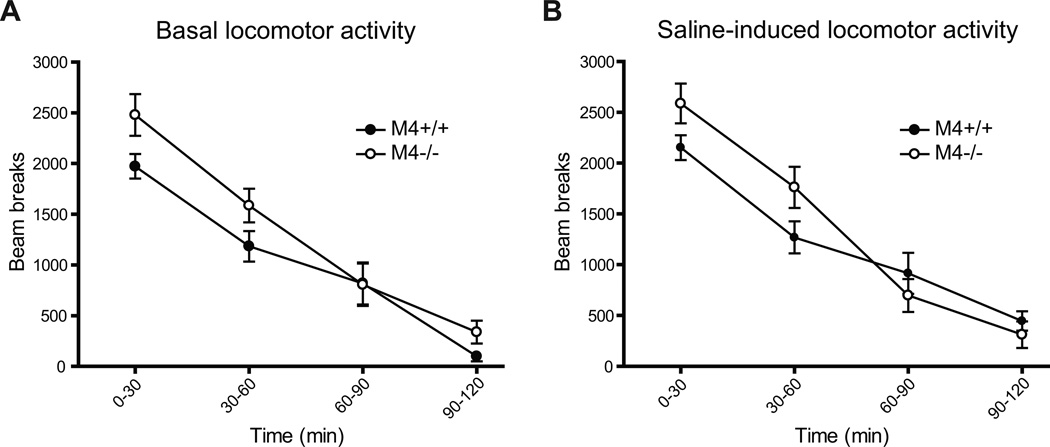
Basal and saline-induced locomotor activity was measured for 120 min and is presented in 30 min. intervals in Fig. 1, A and B, respectively. Both M4−/− mice and their M4+/+ controls displayed a gradual decrease in locomotor activity, expressed as number of beam breaks during the observation period. Although the M4−/− mice showed slightly higher levels of locomotor activity during the first hour of measurement (Fig. 1A), a two-way ANOVA of the basal locomotor activity data revealed a significant effect of time (F75,3=121.02, p < 0.0001) but no effect of genotype and no interaction over the 120 min period. Similarly, a two-way ANOVA of saline-induced activity data showed significant effect of time (F57,3=117.03, p < 0.0001), with no effect of genotype but a significant interaction (F57,3=5.32, p < 0.01; Fig. 1B). Post-hoc t-test on the individual intervals also showed no differences between the genotypes.
Fig. 1.
No significant differences were observed between M4−/− and M4+/+ mice with regard to (A) basal and (B) saline-induced locomotion.. Abscissas; time, the 120 minute measuring period divided into 30 min. intervals. Ordinates; locomotor activity measured in beam breaks. Data are group means ± S.E.M. Group sizes: N=10–14.
4.2. Catalepsy
The cataleptic response in M4−/− and M4+/+ mice was measured 30, 60, and 90 min after administration of test-drug. Subsequently, saline or scopolamine was administered to investigate reversal of drug-induced catalepsy and catalepsy was measured 120 min following administration of the antipsychotic test drug.
Initial control experiment showed that saline injections and repeated testing (after 30, 60, 90 and 120 min) did not induce notable signs of catalepsy (mean time in position on steel bar ± S.E.M. in M4−/− and M4+/+ mice was 0.438 sec ± 0.157 sec and 0.188 sec ± 0.063 sec, respectively) and a two-way ANOVA revealed no significant effect of genotype (F1,24=1.33, p = 0.26), test time (F3,24=0.556, p = 0.65) or interaction between test time and genotype (F3,24=0.667, p = 0.58).
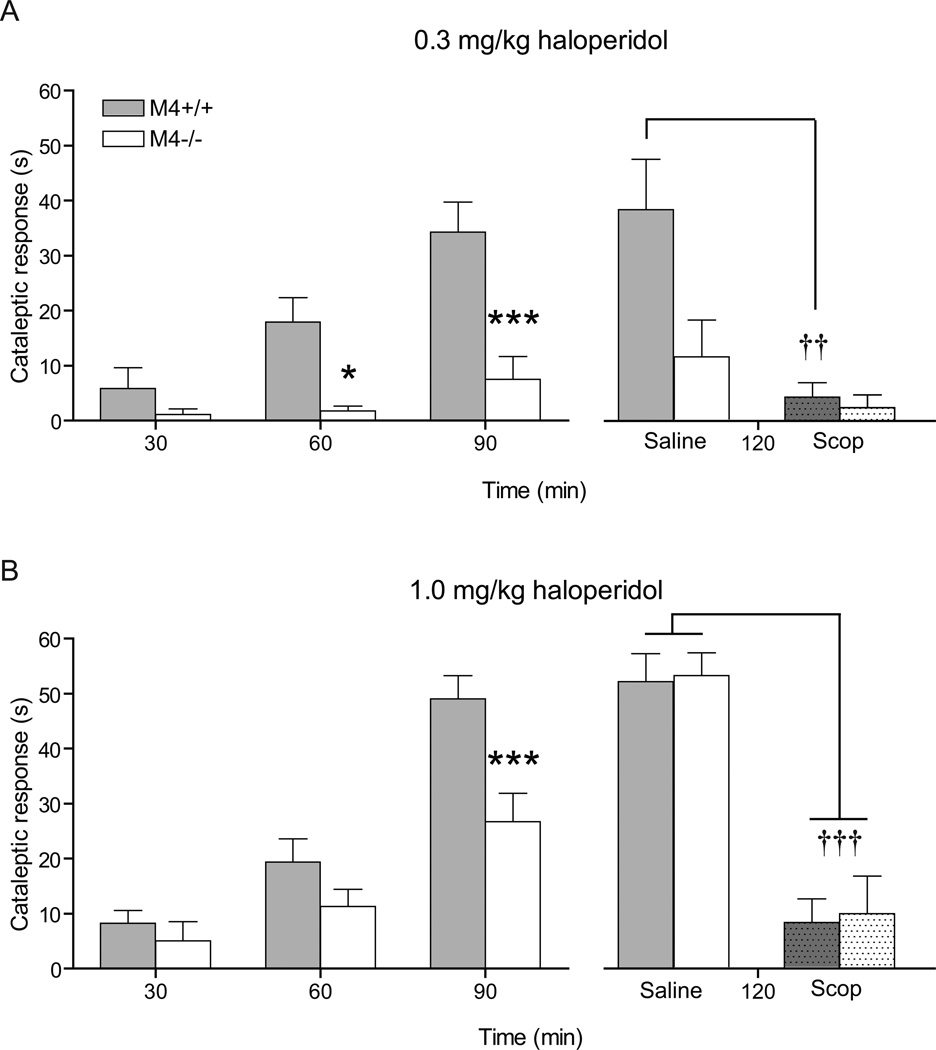
At haloperidol 0.3 mg/kg, two-way ANOVA revealed a significant effect of genotype (F1,60=14.98, p < 0.001) and test time (F2,60=15.77, p < 0.0001) as well as a significant interaction between test time and genotype (F2,60=6.15, p < 0.01). Post-hoc analysis showed that the genotypes differed significantly after 60 and 90 min (p < 0.01 and p < 0.001, respectively) (Fig. 2A left panel). At 120 min after haloperidol administration, scopolamine (5.0 mg/kg) reduced the cataleptic response. Two-way ANOVA showed a significant effect of genotype (F1,28=6.26, p<0.01), treatment (F1,28=13.86, p<0.001) and interaction (F1,28=4.76, p<0.05). Post-hoc analysis showed that the effect was only significant in the M4+/+ mice (p<0.01) (Fig. 2A right panel).
Fig. 2.
Haloperidol-induced catalepsy was measured 30, 60, and 90 min after drug-injection. Haloperidol 0.3 mg/kg (A left panel) and 1.0 mg/kg (B left panel) caused a strongly attenuated cataleptic response in M4−/− mice as compared to M4+/+ mice. N=13–17. * indicates p < 0.05 and *** indicates p < 0.001 vs. M4+/+ mice in Bonferroni-corrected post-hoc t-tests. Reversal of haloperidol-induced catalepsy was examined by administration of 5.0 mg/kg scopolamine 90 min after the mice were injected with 0.3 mg/kg haloperidol (A right panel) or 1.0 mg/kg haloperidol (B right panel). The effect of scopolamine (Scop) as compared to saline was examined 120 min after the initial haloperidol injection. Scopolamine significantly reduced 0.3 mg/kg haloperidol-induced catalepsy in M4+/+ mice (A), whereas a significant reduction in the cataleptic response of M4−/− mice was only detectable after 1.0 mg/kg haloperidol (B). N=7–9. †† indicates p < 0.01 and ††† indicates p < 0.001 vs. saline control. * indicates p < 0.01 vs. M4+/+ saline group in Bonferroni-corrected post-hoc t-tests. Abscissas; time, measured in min subsequent to haloperidol injection. Ordinates; cataleptic response measured in seconds. Cut-off = 60 sec. Data are group means ± S.E.M.
Haloperidol 1.0 mg/kg also caused a significant effect of genotype (F1,66=12.40, p < 0.01), test time (F2,66=40.01, p < 0.0001), and interaction between test time and genotype (F2,66=3.54, p < 0.05). Post-hoc analysis revealed that the genotypes differed significantly after 90 min (p < 0.001) (Fig. 2B left panel). Treatment with scopolamine resulted in an overall significant effect of treatment (F1,30=72.29, p < 0.0001) but no significant effect on genotype (F1,30=0.067, p =0.80) or interaction between treatment and genotype (F1,30=0.012, p = 0.913) (Fig. 2B right panel).
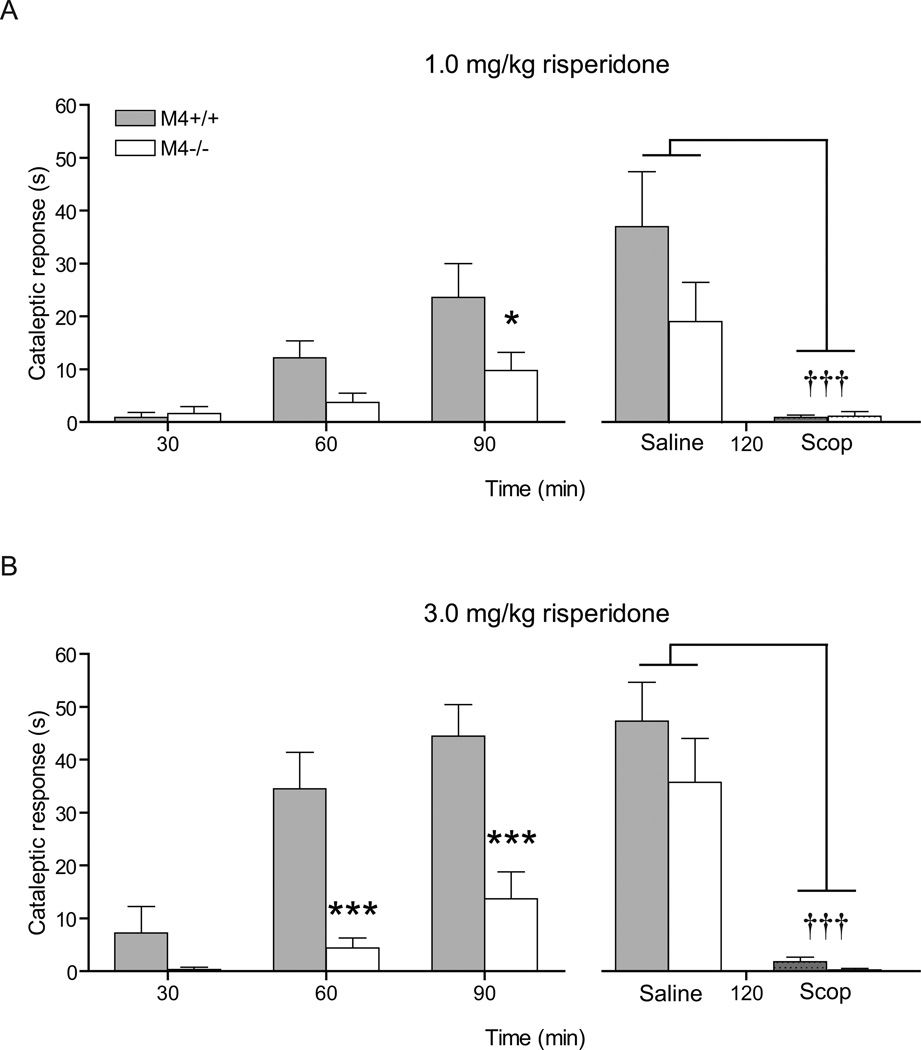
At risperidone 1.0 mg/kg, two-way ANOVA showed a significant effect of genotype (F1,46=4.37, p < 0.05) and test time (F2,46=15.25, p < 0.0001) as well as a significant interaction between test time and genotype (F2,46=3.49, p < 0.05). Post-hoc analysis revealed that the genotypes differed significantly after 90 min (p < 0.05) (Fig. 3A left panel). Two-way ANOVA revealed an overall significant effect of treatment with scopolamine (F1,19=70.44, p < 0.0001) but no significant effect on genotype (F1,21=1.77, p =0.20) or interaction between treatment and genotype (F1,21=1.97, p = 0.17) (Fig 3A right panel).
Fig. 3.
Risperidone 1.0 mg/kg (A) and 3.0 mg/kg (B) resulted in an attenuated cataleptic response in M4−/− compared to M4+/+ mice. N=11–13. * indicates p < 0.05 and *** indicates p < 0.001 vs. M4+/+ mice in Bonferroni-corrected post-hoc t-tests. Scopolamine (Scop) at 5.0 mg/kg as compared to saline significantly reduced risperidone-induced catalepsy in M4+/+ mice at both doses (A and B) . N=4–7. ††† indicates p < 0.001 versus saline control in Bonferroni-corrected post-hoc t-tests. Abscissas; time, measured in min subsequent to risperidone injection. Ordinates; cataleptic response measured in seconds. Cut-off = 60 sec. Data are group means ± S.E.M.
At risperidone 3.0 mg/kg, a two-way ANOVA also revealed a significant effect of genotype (F1,42=21.18, p < 0.001) and test time (F42,2=17.72, p < 0.0001) as well as a significant interaction between test time and genotype (F2,42=5.03, p < 0.05). Post-hoc analysis revealed that the genotypes differed significantly after both 60 and 90 min (p < 0.001) (Fig. 3B left panel). Two-way ANOVA revealed an overall significant effect of treatment with scopolamine (F1,19=70.44, p < 0.0001) but no significant effect of genotype (F1,19=1.80, p =0.20) or interaction between treatment and genotype (F1,19=1.11, p = 0.30) (Fig. 3B right panel).
5. Discussion
It is well known that muscarinic receptor agonists worsen haloperidol-induced catalepsy whereas muscarinic receptor antagonists exhibit the opposite effect (Klemm, 1985). The precise mechanism of this action is unknown. The muscarinic M4 receptor would be an obvious target, due to its pronounced localization in the striatum (Hersch et al., 1994). It has been difficult to elucidate the physiological role of the various muscarinic receptor subtypes due to the lack of subtype selective ligands (Wess et al., 2007). The development of muscarinic M4−/− mice has made it possible to investigate the functional role of the muscarinic M4 receptor (Gomeza et al., 1999). In these mice an enhanced locomotor response to dopamine receptor stimulation was observed (Gomeza et al., 1999) which is in accordance with the suggested involvement of the M4 receptor in motor activity.
In the present study, we found that male M4−/− mice backcrossed for ten generations showed slightly higher levels of locomotor activity during the first hour of the two hours of observation, but there was no general significant effect of genotype. Interestingly, in the initial study the hyperactivity was only observed in the M4−/− mice during the first 40 min of the one hour observation period (Gomeza et al., 1999). Therefore, reduced cataleptic responses in M4−/− mice could not be explained by a lack of overall motoric capability. The most important result of the present sets of experiments was the pronounced reduction in cataleptic responses to the first generation antipsychotic haloperidol and to the second generation antipsychotic risperidone compared to controls. The difference in cataleptic response between M4−/− and M4+/+ mice at the highest doses tested decreased over time which is most likely caused by a ceiling effect. In accordance with this explanation, 50% and 75% and 50% and 50% of the M4+/+ mice in the two haloperidol-groups and the two risperidone groups, respectively reached the 60 sec cut-off limit at 120 min, as opposed to 0% and 56% (two haloperidol groups) and 13% and 25% (two risperidone groups) of the M4−/− mice. The observed reduction in the cataleptic response does not appear to be related to changes in dopamine D1 or D2 receptor expression in CPu and NAc since no significant change in dopamine D1 and D2 receptor density was observed in these areas (Schmidt et al., submitted for publication). Our data indicate that the muscarinic M4 receptor plays a central role in mediating the motor side effect profile of the tested antipsychotics. The responses in M4−/− mice are consistent with one of our recent studies using transgenic mice that lack the M4 muscarinic receptor selectively on D1 dopamine receptor-expressing cells, where reduced antipsychotic-induced catalepsy was observed (Jeon et al., 2010). However, non-selective muscarinic receptor antagonists can completely abolish the cataleptic response to antipsychotics (Karasawa et al., 2003; Arnt and Christensen, 1981), and since the response was not totally abolished in muscarinic M4−/− mice in the present study, other muscarinic receptor subtypes must be involved.
Karasawa et al. (2003) did not observe a difference in haloperidol-induced cataleptic response between mice lacking the M4 receptor and wild type controls which is in discordance with the present data. The reason for this discrepancy could well be that catalepsy was only assessed 30 min after administration of haloperidol in the former study (Karasawa et al., 2003) whereas we monitored the cataleptic response 30, 60, and 90 min after haloperidol injection. We found no difference 30 min after injection of 0.3 mg/kg of haloperidol (in accordance with (Karasawa et al., 2003)) while marked differences were observed after 60 and 90 min. Furthermore, the M4 mutant mice of Karasawa and associates (2003) were not fully backcrossed. Polymorphisms in knockout mice on a mixed genetic background complicate the interpretation of phenotyping studies (Gerlai, 1996). Backcrossing to a specific strain for several generations can reduce the influence of genetic background (Gerlai, 1996).
The neuroanatomical basis for the influence of muscarinic M4 receptors on the dopaminergic system remains to be determined. The regulation of dopaminergic homeostasis by muscarinic M4 receptors could involve numerous brain regions due to the wide distribution of the M4 receptor subtype. For instance, M4 muscarinic receptors are located on striatal cholinergic interneurons and on striatal medium spiny GABAergic output neurons (Bernard et al., 1992; Weiner et al., 1990; Yan et al., 2001), as well as on cholinergic neurons projecting from the pedunculopontine and laterodorsal tegmental nuclei to dopaminergic neurons in the midbrain (Sugaya et al., 1997).
In the striatum, muscarinic M4 receptors are stimulated by acetylcholine released from large cholinergic interneurons that are under tonic inhibition through dopaminergic input from the substantia nigra (Damsma et al., 1990). Release of striatal acetylcholine is increased when dopamine D2 receptors are blocked by haloperidol (Damsma et al., 1990). Consequently, stimulation of muscarinic M4 receptors localized on medium spiny output neurons is increased, and this may influence the balance between the so-called direct and indirect pathways from the striatum to substantia nigra and globus pallidus, respectively, thereby mediating the observed cataleptic response. Interestingly, M4 receptors are coexpressed with D1 dopamine receptors in a specific subset of striatal medium spiny neurons which contain GABA as the major neurotransmitter and give rise to the so-called striatonigral pathway (Di Chiara et al., 1994; Ince et al., 1997; Bernard et al., 1992). However, both haloperidol and risperidone exhibit more than 100 fold higher affinity for dopamine D2 than D1 receptors (Arnt and Skarsfeldt, 1998), making it less likely that the cataleptic effects are mediated by a direct effect on dopamine D1 receptors localized on medium spiny output neurons.
Cholinergic neurons, projecting from the pedunculopontine and laterodorsal tegmental nuclei to midbrain dopaminergic neurons that again project to the forebrain have been suggested to function as autoreceptors, regulating acetylcholine release in the midbrain (Tzavara et al., 2004). In support of this suggestion, basal extracellular levels of acetylcholine were elevated in the midbrain and basal extracellular levels of dopamine were elevated in the striatum of M4−/− mice (Tzavara et al., 2004). The elevated dopamine tonus in striatum may reduce the dopamine D2 receptor antagonist-induced elevation of striatal acetylcholine and thereby restoring part of the imbalance between the direct and indirect pathways from striatum to substantia nigra and globus pallidus, respectively, thereby reducing the cataleptic response. The non-selective muscarinic receptor antagonist scopolamine inhibited antipsychoticinduced catalepsy in the present study, in agreement with earlier studies (Karasawa et al., 2003; Arnt and Christensen, 1981). The scopolamine-induced inhibition of catalepsy was also observed in mice lacking the M4 receptor, but the difference was only significant at the highest doses of haloperidol (1.0 mg/kg) or risperidone (3.0 mg/kg) tested. This may be due to the very attenuated cataleptic response observed in muscarinic M4−/− mice at lower doses of haloperidol and risperidone, making it more difficult to establish a further significant reduction. Risperidone is a second generation antipsychotic which at lower doses only causes few motor adverse events in patients but at higher doses converge to the side effect profile of haloperidol (Gao et al., 2008). A similar profile is observed in rodents, where risperidone at high doses causes Fos-induction in the motor-related dorsolateral striatum in a manner similar to the effect of haloperidol (Fink-Jensen and Kristensen, 1994).
The anti-cataleptic effect of scopolamine observed in muscarinic M4−/− mice in the present experiment is in discordance with the data from (Karasawa et al. 2003) who found no significant effect of scopolamine in muscarinic M4−/− mice (Karasawa et al. 2003). The reason for this discrepancy remains unclear.
In conclusion, the present study indicates that muscarinic M4 receptors play a pivotal role in catalepsy induced by antipsychotics and suggests that the muscarinic M4 receptor may be a new therapeutic target in the medical treatment of Parkinson’s disease and antipsychoticinduced parkinsonian symptoms. The muscarinic M4 receptor seems to play a less pronounced role in the reversal of catalepsy by anticholinergic drugs.
Acknowledgements
This work was supported by grants from the Ivan Nielsen Foundation and the Lundbeck Foundation.
References
- Arnt J. Pharmacological differentiation of classical and novel antipsychotics. Int Clin Psychopharmacol. 1998;13:S7–S14. doi: 10.1097/00004850-199803003-00002. [DOI] [PubMed] [Google Scholar]
- Arnt J, Christensen AV. Differential reversal by scopolamine and THIP of the antistereotypic and cataleptic effects of neuroleptics. Eur J Pharmacol. 1981;69:107–111. doi: 10.1016/0014-2999(81)90608-7. [DOI] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, McKinzie DL, Felder CC, Wess J. Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res. 2003;28:437–442. doi: 10.1023/a:1022844517200. [DOI] [PubMed] [Google Scholar]
- Damsma G, de Boer P, Westerink BH, Fibiger HC. Dopaminergic regulation of striatal cholinergic interneurons: an in vivo microdialysis study. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:523–527. doi: 10.1007/BF00169040. [DOI] [PubMed] [Google Scholar]
- DeBoer P, Abercrombie EM. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996;277:775–783. [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson GS, Faull RL, Robertson HA, Jansen K. D2 dopamine receptor antagonists induce fos and related proteins in rat striatal neurons. 1990;37:287–294. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Kristensen P. Effects of typical and atypical neuroleptics on Fos protein expression in the rat forebrain. Neurosci Lett. 1994;182:115–118. doi: 10.1016/0304-3940(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Gao KM, Kemp DE, Ganocy SJ, Gajwani P, Xia GH, Calabrese JR. Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia - a systematic review. J Clin Psychopharmacol. 2008;28:203–209. doi: 10.1097/JCP.0b013e318166c4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M-4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of M1-M4 muscarinic receptor proteins in the rat striatum - light and electron-microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychoticdrugs with extrapyramidal side-effect liability. Psychopharmacology (Berl) 1995;120:128–133. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- Hulme EC, Birdsall NJM, Buckley N. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wortwein G, Woldbye DPD, Cui Y, Davis AA, Levey AI, Schutz G, Sager TN, Mork A, Li C, Deng CX, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa H, Taketo MM, Matsui M. Loss of anti-cataleptic effect of scopolamine in mice lacking muscarinic acetylcholine receptor subtype 4. Eur J Pharmacol. 2003;468:15–19. doi: 10.1016/s0014-2999(03)01642-x. [DOI] [PubMed] [Google Scholar]
- Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev. 2003;2:CD003735. doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm WR. Evidence for a cholinergic role in haloperidol-induced catalepsy. Psychopharmacology (Berl) 1985;85:139–142. doi: 10.1007/BF00428402. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Tison F, Bloch B. D2 dopamine receptor gene expression by cholinergic neurons in the rat striatum. Neurosci Lett. 1990;117:248–252. doi: 10.1016/0304-3940(90)90671-u. [DOI] [PubMed] [Google Scholar]
- Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. The clinical use of anticholinergic drugs as treatment for extrapyramidal side-effects of neuroleptic drugs. J Clin Psychopharmacol. 1983;3:288–302. [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DPD, Wörtwein G, Fink-Jensen A. Increased self-administration in M4 muscarinic acetylcholine receptor knockout mice. doi: 10.1007/s00213-011-2225-4. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte A, Janssen PFM, Gommeren W, Luyten WHML, Gompel P, Lesage AS, Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124(1–2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Clamp C, Bryan D, McKinney M. mRNA for the m4 muscarinic receptor subtype is expressed in adult rat brain cholinergic neurons. Brain Res Mol Brain Res. 1997;50:305–313. doi: 10.1016/s0169-328x(97)00199-x. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Vilaro MT, Mengod G, Palacios JM. Advances and limitations of the molecular neuroanatomy of cholinergic receptors: the example of multiple muscarinic receptors. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor messenger-RNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]