Abstract
Background:
Antiretroviral regimens (ART) changes occur frequently among HIV-infected persons. Duration and type of initial highly active antiretroviral therapy (HAART) and factors associated with regimen switching were evaluated in the Multicenter AIDS cohort Study.
Methods:
Participants were classified according to the calendar period of HAART initiation: T1 (1996-2001), T2 (2002-2005) and T3 (2006-2009). Kaplan Meier curves depicted time from HAART initiation to first regimen changes within 5.5 years. Cox proportional hazards regression models were used to examine factors associated with time to switching.
Results:
Of 1009 participants, 796 changed regimen within 5.5 years after HAART initiation. The percentage of participants who switched declined from 85% during T1 to 49 % in T3., The likelihood of switching in T3 decreased by 50% (p<0.01) compared to T1 after adjustment for pre-HAART ART use, age, race and CD4 count. Incomplete HIV suppression decreased over time (p<0.01) but predicted switching across all time periods. Lower HAART adherence (≤ 95% of prescribed doses) was predictive of switching only in T1. In T2, central nervous system symptoms predicted switching (RH = 1.7, p= 0.012). Older age at HAART initiation was associated with increased switching in T1 (RH=1.03 per year increase) and decreased switching in T2 (RH = 0.97 per year increase).
Conclusions:
During the first 15 years of the HAART era, initial HAART regimen duration lengthened and regimen discontinuation rates diminished. Both HIV RNA non-suppression and poor adherence predicted switching prior to 2001 while side effects that were possibly ART-related were more prominent during T2.
Introduction
Highly active antiretroviral therapy (HAART) use has resulted in a dramatic and sustained decrease in AIDS-related morbidity and mortality1-5. HAART’s effectiveness is commonly measured by its ability to durably suppress HIV replication and to affect immune system reconstitution as measured by increases in circulating CD4+ T lymphocyte (CD4) cell counts 6-8, which in turn result in decreased rates of HIV clinical progression, AIDS-related opportunistic diseases and death 4,5,9-12.
Newer antiretroviral agents, including fixed-dose combinations and single tablet daily regimen preparations, have become available over the past decade; these newer therapies have been associated with greater efficacy, tolerability, and convenience13,14. Early in the HAART era, HIV non-suppression and treatment-related adverse effects were reported as common causes for changing regimens15-18
We hypothesized that use of more modern antiretroviral therapies that are more potent, convenient, and associated with fewer adverse effects are more likely to facilitate improved adherence and result in increases in the durability of initial HAART regimens. We also postulated that factors associated with switching from initial HAART have changed over time.
Methods
Study population
Study subject were participants in the Multicenter AIDS Cohort Study (MACS), an ongoing prospective study of HIV-1 infection and treatment among men who have sex with men (MSM) in the United States begun in 1984 at four sites; Chicago, Baltimore, Pittsburgh and Los Angeles 19,20. In 1984-1985 4,954 HIV-infected and uninfected MSM were enrolled. In 1987 recruitment was reopened to increase the number of African American participants; 668 men were enrolled. During 2001-2003 another 1350 younger men, predominantly from minority populations, were enrolled. Another MACS expansion commenced at the beginning of 2010; 71 men had been recruited through September 2012. Every 6 month, study visits include interviews focused upon symptoms and treatment. In addition, quality of life assessments, focused physical examinations and collection of biological specimen were obtained. The institutional review boards of each center approved the protocols and informed consent was obtained from each participant. A detailed description of the MACS study has been published and only methods for the present analyses are presented here 19.
Combination antiretroviral regimens were considered highly active antiretroviral therapies (HAART) if they met the United States Department of Health and Human services guidelines 21,22. Information regarding therapies received was obtained by participant self-report and updated at each semi-annual MACS visit. MACS participants who initiated HAART (n=1009) after enrolling in the MACS were included and classified as either pre-HAART antiretroviral treatment (ART) experienced (TE) or pre-HAART ART naïve (TN). Individuals were further classified according to the calendar period during which they initiated HAART: T1 (1996-2001), T2 (2002-2005) and T3 (2006-2009). Participants were censored at death, withdrawal from the MACS; if HAART was initiated before entering the MACS study and end of study visit 57 (9/30/2012). All participants who initiated HAART were followed for the first five and one half year of their therapy for this analysis.
Outcome Variable
Time to change of the first HAART regimen was the primary outcome of interest. Switching HAART was defined as the changing of at least one antiretroviral drug contained in a HAART regimen. The change from two individual agents (e.g., zidovudine + lamivudine) to a fixed-dose combination preparation drug (e.g., Combivir) was not considered a regimen switch. Entry time was the date of HAART initiation; the duration of the first regimen was calculated from the initiation date until switching if it occurred within 5.5 years after HAART initiation, or at the last visit occurred if it occurred within 5.5 years after HAART initiation for persons who did not switch HAART. We used 5.5 years after HAART initiation as the cutoff so that the person-time contributed to the analysis was comparable during all the three time periods.
Exposure Variables
The primary exposure of interest was calendar period of HAART initiation (T1, T2, and T3). The fixed characteristics of ART-experience pre-HAART (TE: treatment-experienced and TN: treatment-naive), and type of first HAART regimen received were examined as predictors of switching. We classified first HAART regimens into four categories: (1) protease inhibitor (PI)-based (reference group), (2) non-nucleoside reverse transcriptase inhibitor (NNRTI)-based, (3) only nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs)-based, and (4) entry inhibitor (including fusion inhibitors) (EI) or integrase inhibitor (II)-based. Time-varying characteristics, including possible adverse effects, HIV RNA ≥50 copies/ml, convenience, estimated pill/tablets of antiretroviral agents taken daily, drug holidays and regimen adherence, using information collected at each visit up to the time of HAART switch or until 5.5 years after HAART initiation. Possible drug effects were defined by evaluating participant-reported symptom(s) that occurred while receiving the initial HAART regimen. Hepatitis B virus (HBV) co-infection was defined based on positive HBV surface antigen; hepatitis C virus (HCV) co-infection as a positive serum antibody or positive HCV RNA. Symptoms and signs included were gastrointestinal (GI, diarrhea, nausea, vomiting, abdominal pain or bloating), central nervous system (CNS, nightmares, vivid dreams or insomnia) hyperglycemia (fasting glucose>110 mg/dL or if the participant reported high blood sugar/diabetes), hyperlipidemia (fasting total cholesterol >210 mg/dL or a self report of high cholesterol/ high triglycerides), or a self-report of change in body fat distribution (lipodystrophy). Self-report of having stopped all prescribed ART for at least sequential 2 days since the last visit was considered a drug holiday. Self-reported level of adherence to HAART at each visit prior to switching was dichotomized as ≥95% (reference) vs. < 95% of prescribed doses. Age at HAART initiation, history of intravenous drug use, self-reported race (White (reference), non-White, Hispanic) and education (college degree or above vs. below college (reference) at baseline, nadir CD4 cell count and pre-HAART CD4 cell count (cells/mm3) were also included to adjust for possible confounding23.
Statistical Analysis
The Fisher exact test or Kruskal-Wallis nonparametric test was used to test unadjusted differences in proportions and distributions of study population characteristics among the three time periods, as appropriate. Modified Poisson regression with robust variance 24 was used to test for trends in reasons for HAART switching. Median regression was used to test whether the number of pills used in the 1st HAART regimen changed over time. Cox proportional hazards models were used to determine the relative hazards of switching by time period and treatment experience prior to HAART initiation. Associations between exposure variables and time to ART regimen change from 1st HAART were estimated using Cox proportional hazards models with time-dependent covariates. Separate models were run for different time periods.
Results
Between 1996 and 2009, 1009 MACS participants initiated HAART after study enrollment. Participant demographics at the time of HAART initiation for each of the 3 time periods are presented in Table 1. Men who initiated HAART in T3 were older (median 47 years versus 43 in T1 and T2, p< 0.05). The proportion of HAART initiators who were non-white increased during the later two time periods (12%-T1, 34%-T2, 28 %-T3; p<0.05), though this was anticipated as a consequence of the targeted enhanced recruitment of MSM from minority populations in 2001-2003. The level of education was lower in the later two time periods: 37 % and 44% of men in T3, and T2 versus 53 % in T1, respectively, had completed college, (p<0.05). The nadir CD4 cell count increased over time (median: 211 (T1), 278 (T2) and 310 cells/ul, (T3), respectively p<0.05) as well as the pre-HAART CD4 cell counts (median 302 (T1), 329 (T2), to 380 cells/ul (T3), respectively p<0.05). The percentages of participants with HBV co-infection in the three time periods were similar (8%, 8% and 7 % in T1, T2, T3, respectively, p=0.81), as were the percentages of men with HCV co-infection (6%, 9 % and 8% in T1, T2 and T3, respectively, p=0.32). Likewise, the prevalence of participants with a history of intravenous drug use was stable over time (14%, 14%, 18% in T1, T2 and T3 respectively, p=0.39).
Table 1.
Characteristics of HAART initiators in the Multicenter AIDS Cohort Study by Calendar Period at HAART Initiation
| Participant characteristics at HAART initiation | 1996-2001 | 2002-2005 | 2006/2009 | P value |
|---|---|---|---|---|
| (n=690) | (n=189) | (n=130) | ||
| Race/Ethnicity | <0.001 | |||
| Hispanic (%) | 6 | 13 | 16 | |
| Non-white (%) | 12 | 34 | 28 | |
| Baseline education >= College (%) |
53 | 44 | 37 | <0.001 |
| History of IDU(%) | 14 | 14 | 18 | 0.591 |
| Chronic HBV (%) | 8 | 8 | 8 | 1.0 |
| Chronic HCV (%) | 6 | 9 | 8 | 0.321 |
| Age (years): Med (IQR) |
43 (39-48) |
43 (37-49) |
47 (39-53) |
0.005 |
| Hyperglycemia/DM* (% of prevalence) | 8.3 | 13.2 | 18.7 | <0.001 |
| Hyperlipidemia *(% of prevalence) | 58.9 | 28.1 | 35.2 | <0.001 |
| CD4 count (cells/μl) Nadir: Med (IQR) |
211 (109-321) |
278 (154-373) |
310 (214-419) |
<0.001 |
| CD4 count (cells/μl) Pre-HAART: Med (IQR) |
302 (164-461) |
329 (193-432) |
380 (237-527) |
<0.001 |
| Pre-HAART RNA (log10 cp/mL): Med (IQR) |
4.4 (3.5-4.9) | 4.6 (3.9-5.1) | 4.5 (4.0-5.0) | 0.046 |
P values were obtained from the Fisher exact test or the Kruskal-Wallis non-parametric test, as appropriate.
Med = median, IQR = interquartile range
among pre-HAART HIV-infected person-visits by calendar periods.
Hyperglycemia/diabetes mellitus was defined as FG<110mg/dL or self-report of high blood sugar or on anti-diabetic drug Hyperlipidemia was defined as fasting total cholesterol > 210 mg/dL or self report of high total cholesterol or triglycerides or on lower-lipids medication.
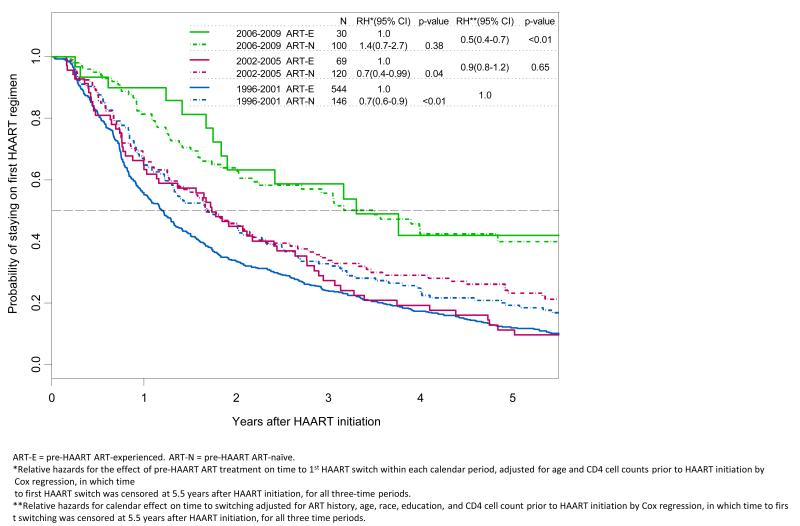
Among the 1009 men who initiated HAART, 796 (78.9%) switched therapy within 5.5 years of initiation; 85% (n=585), 78% (n=147) and 49 % (n=64) of men, respectively, switched HAART during T1, T2 and T3. Data available through 2012 were used to characterize HAART use through 2009. Among men who initiated HAART before 2006 and switched within 5.5 years, the median and 75th percentile of time to switch was 1.1 years and 2.2 years respectively. Among men who did not switch within 5.5 years, the percentage of men with follow up time beyond 2.2 years (the 75th percentile of time to switch) were 74%, 69% and 76% respectively in T1, T2 and T3 respectively (p=0.729) showing that men who started HAART in T3 had the same opportunity to observe switching as men in the earlier periods. The prevalence of HAART discontinuation was 10%, 16.4% and 13.5% for the three- calendar period studied, respectively. Compared to T1, the likelihood of switching in T2 decreased by 10% (Figure 1, p=0.65) and by 50 % (p<0.01) during T3 after adjusting for pre-HAART ART use, age, race, education and CD4 cell count prior to HAART initiation. The proportion of treatment-experienced (TE) participants decreased over time as expected: 79 % during T1, 37% during T2, and 23 % during T3 (p<0.01 for trend). Treatment-naïve (TN) participants were significantly less likely to switch from their first HAART regimen than TE patients in T1 and T2 (figure 1, relative hazard (RH) = 0.7, p<0.01 and RH 0.7, p= 0.04 respectively after adjusting for age and pre-HAART CD4 cell counts), but this difference by pre-HAART ART experience was not observed during T3. Among the TN participants, 77% switched from their first HAART regimen within 5.5 years after HAART initiation in T1 (113/146) and in T2 73% (88/120), while only 51% (51/100) switched in T3. The univariate relative hazard of switching among TN during T1 or T2 compared to T3 was 1.9 and 1.7 respectively (p<0.01) for each comparison. Among TE patients, 87% (472/544) switched therapy during T1, 86% (59/69) during T2 and 43% (13/30) during T3. The univariate relative hazard of switching for T2 versus T3 was 2.3 (p<0.01) and for T1 versus T3, HR= 2.6 (p<0.01)
Figure 1.
Kaplan-Meier probability curves depicting time from HAART initiation to first HAART switches that occurred within 5.5 years after HAART initiation.
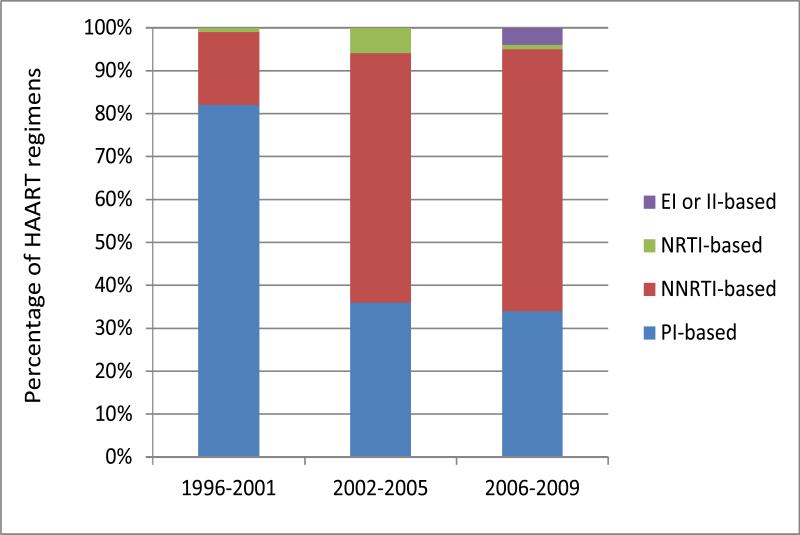
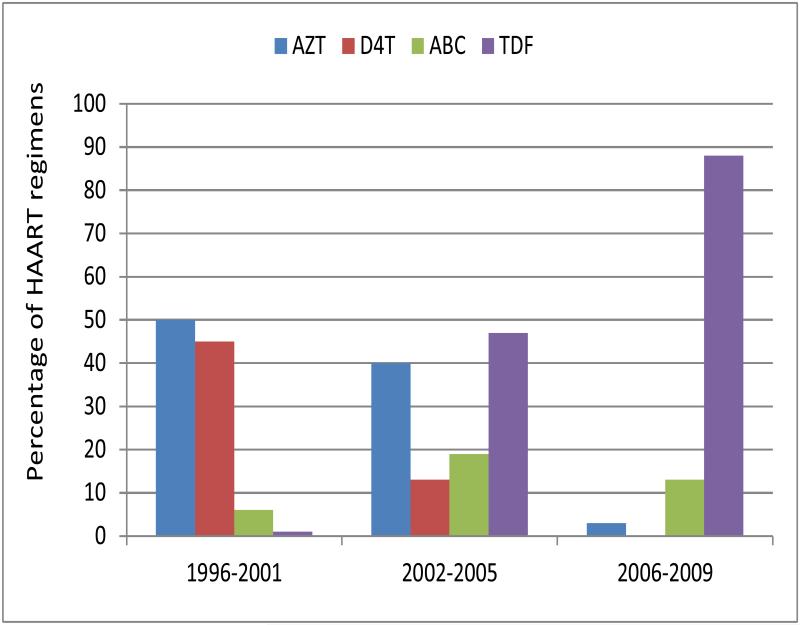
Initial HAART regimens (Figures 2a,) were less likely to be protease inhibitor (PI)-based over time (82% in T1 vs. 34 % in T3, p<0.05) and changed over time (figure SDC). Use of non-nucleoside reverse transcriptase inhibitor (NNRTI) based regimens increased from T1 to T3 (17% to 61 %, p<0.05). The principal nucleoside reverse transcriptase inhibitors (NRTIs) used in HAART regimens also changed over time (Figure 2b); zidovudine and stavudine (d4T) were used in 50% and 45% of initial HAART regimens respectively in T1, but in only 3% of regimens initiated during T2 and d4T not at all in T3. Among initial HAART regimens prescribed in T3, 88% included tenofovir.
Figure 2a.
First HAART regimen types during the 3 times periods. PI = protease inhibitor NNRTIs = non-nucleoside reverse transcriptase inhibitors, NRTI= nucleoside/nucleotide reverse transcriptase inhibitors, EI = entry inhibitors (including fusion inhibitors), II = integrase inhibitors.
Figure 2b.
Prevalence of select NRTI use in initial HAART regimen by Calendar Period
At the visit prior to switching the percentage of men with ≥ 50 copies/ml of HIV RNA among switchers decreased over time (64%, 41% and 35% for T1, T2, and T3 respectively, p<0.01) The percentage who switched and reported gastrointestinal symptoms at the prior visit was not significantly different in the three time periods (29% in T1, 34% in T2 and 25% in T3, p= 0.27). In contrast, more participants who switched in T3 had fasting hyperglycemia or diabetes (1% in T1, 7% in T2 and 15% in T3, p<0.01 for trend) and/or hyperlipidemia at the visit prior to HAART switch (5% in T1, 23% in T2 and 21% in T3, p<0.01 for trend) (data not shown). Fewer HAART users reported CNS symptoms in T3 compared to T2 (46% versus 58%, p=0.13). Information regarding CNS symptoms was not collected during T1. In the univariate Cox proportional hazard models (data not shown), participants who stopped HAART for at least 2 days in a row during the six months prior to switching were subsequently more likely to switch in all three time periods (RH=1.59 with p= 0.008 in T1, RH= 1.93 with p= 0.007 in T2 and RH= 1.80 with p= 0=082 in T3. Participants who received NNRTI based regimens versus PI based regimens were less likely to change regimens initiated in T1 (RH=0.69 p= 0.015 and T3 (RH= 0.50, p=0.020). Non-whites were more likely to switch in T2 (RH= 1.66 with p=0.013). Higher pre HAART CD4 cell counts were associated with switching in T3 (RH= 1.09 per 100 cells increase, p= 0.037).
CNS symptoms were recorded during T2 and T3, report of having such symptoms was significantly associated with switching in T2 (RH= 1.67, p=0.012). Self reported levels of adherence of less than 95% to the first HAART regimen were significantly associated with switching in all three time periods in the univariate model (RH=1.91 with p<0.001 for T1, RH= 1.96 with p=0.007 for T2 and RH= 3.38 with p= 0.001 for T3) but a significant association was seen only in T1 (RH=1.87 with p=0.001) after controlling for other factors in the multivariate analysis.
In the multivariate Cox proportional hazard models (Table 2) that examined time-varying exposures, men with detectable plasma HIV RNA levels were significantly more likely to switch in all 3-time periods (relative hazards (RH) = 1.9, 1.9 and 3.0, respectively, in T1, T2, and T3, p<0.05 in comparison to men with undetectable HIV RNA levels). Older age at HAART initiation was associated with increased switching in T1 and T3 (RH=1.03 and 1.04 per year increase respectively), but with decreased switching in T2 (RH = 0.97 per year increase).
Table 2.
Multivariate relative hazard (RH) associated with the first HAART switching that occurred within 5.5 years after HAART initiation using Cox proportional hazards models with time dependent covariates stratified by the calendar time of HAART initiation
| 1996-2001 | 2002-2005 | 2006-2009 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RH | 95% CI | p- value |
RH | 95% CI | p- value |
RH | 95% CI | p-value | |
| Detectable HIV RNA* | 1.87 | 1.44-2.44 | <0.001 | 1.94 | 1.26-2.99 | 0.003 | 3.03 | 1.46-6.30 | 0.003 |
| CNS symptoms* | NA | 1.66 | 1.12-2.45 | 0.012 | 1.00 | 0.51-1.94 | 0.997 | ||
| NNRTI-basedvs. PI based HAART regimen |
0.75 | 0.54-1.04 | 0.081 | 0.96 | 0.63-1.47 | 0.849 | 0.59 | 0.30-1.16 | 0.126 |
| Adherence* <95% | 1.87 | 1.30-2.68 | 0.001 | 1.06 | 0.59-1.93 | 0.840 | 2.16 | 0.83-5.65 | 0.116 |
| Age at HAART initiation, per 1 year |
1.03 | 1.01-1.05 | 0.001 | 0.97 | 0.95-0.99 | 0.011 | 1.04 | 1.00-1.08 | 0.055 |
Measured at each visit prior to switching for persons who switched from their 1st HAART regimen within 5.5 years after HAART initiation, or prior to the last visit seen within 5.5 years after initiation for persons who did not switch HAART regimens within 5.5 years.
Other factors examined in the multivariate model but not show in table 2 due to having p>0.1 in all three time periods included ART-naïve before HAART, GI symptoms, hyperglycemia, hyperlipidemia, lipodystrophy, stopped HAART for at least 2 days in a row in the past 6 months, race, education and history of intravenous drug use.
Overall, the median number and interquartile range (IQR=25%-75%) of ART pills/tablets taken per day for initial HAART regimen for men who switched was 4 (3-7), 3 (3-5) and 3(1-3) during T1, T2 and T3 respectively and 5 (3-7), 4 (3-6) and 3 (2-4) after switching. Over time, decreases in the daily number of pills/tablets taken during the first HAART regimen were significant (p< 0.05 for trend). For TN participants, the median number of pills taken in first regimens went from 4 (3-5), 3 (2-4) and 2 (1-3) for T1, T2 and T3 respectively and for TE patients, they were 5 (3-7), 4 (3-5) and 3 (2-4) in these time periods. Among TN patients initial HAART regimens contained significantly fewer pills /tablets than for TE patients during T2 (p<0.05) (data not shown).
Discussion
In the MACS, the duration of first HAART regimens increased significantly from 1996-2009, the calendar years represented in this analysis. Among men who initiated HAART in each successive time period and who were subsequently followed 5.5 years, switching became less common. Observed changes in first HAART regimen length occurred simultaneously with the availability of newer, more potent and better-tolerated antiretroviral therapies. While side effects that were possibly ART-related were associated with an increased likelihood of ART regimen switching during T1, ART-related toxicities were less often clearly related to switching during later time periods.
Some of the observed increases in first HAART duration over time were partially, but not completely, accounted for by progressive increases in the proportion of HAART initiators who were pre-HAART ART naïve. Furthermore, the average CD4 cell count prior to HAART initiation increased with time, consonant with simultaneous changes in the guidelines for treatment initiation 21,22. Guidelines published early in the HAART era recommended that therapy could be initiated at CD4 cell counts as low as 200-250 cells/ul while more recent recommendations have advised starting HAART at progressively higher CD4 cell counts and, most recently, at any CD4 count if the patient is willing to initiate therapy 8. Our data reflect these trends 25,26. In the MACS, HAART initiators during T1 were more likely to have been diagnosed with AIDS, and both nadir CD4+ cells (median 211cells/ml) and CD4 at the visit prior to HAART initiation (median 302 cells/ml) were lower than levels seen in later time periods. This phenomenon was likely a consequence of several factors: 1) patients starting HAART during T1 were more likely to have received less effective mono or combination antiretroviral regimens prior to the first HAART initiation; 2) HAART in T1 was less potent, tolerable and convenient than regimens that became available later and 3) changing treatment standards in the timing of HAART initiation, as discussed above.27,28.
There were clearly changes over time in the prevalence of symptoms reported before switching that were feasibly ART-related. CNS symptoms had diminished in frequency by the most recent time period studied, likely at least in part due to changes in the composition of HAART use. While the overall number of switchers diminished over time, the proportion of switchers for whom adverse metabolic sequelae (including hyperglycemia and hyperlipidemia) existed increased over time, however neither hyperglycemia nor hyperlipidemia were significantly associated with switching in multivariable models in period 3 (data not shown). It is possible that the aging of this cohort may have, influenced the prevalence of these metabolic conditions.
The extent to which regimen switches were made pre-emptively in later time periods, by the desire to receive more “metabolic-friendly” agents is unclear. This phenomenon may have been operative in this cohort of aging men with extended survival who were increasingly diagnosed with or at least wary of age-related chronic co-morbiditie compared with HIV infected men in the pre HAART era,
Changes in the composition of initial HAART regimens received by MACS participants over time reflect the availability of newer, more potent, better tolerated and easier to administer individual drugs and fixed dose combinations 29-32. These changes were associated with a progressive increase in the duration of initial HAART regimens. For example, PI-based regimens were overwhelmingly used during T1 (1996-2001) and often included older PI’s such as indinavir and nelfinavir that were commonly associated with treatment-limiting side effects. Inclusion of PI’s in initial HAART regimens gradually decreased over time following the introduction of NNRTI’s, particularly efavirenz, and more recently (during T3) integrase inhibitors (ISTI).33-35 The increased convenience represented by the availability of fixed-dose ART combinations and daily single tablet regimens is reflected in our data and is likely associated with the longer duration of the first HAART regimen during the most recent time period. During T1 (1996-2001), more than 80% of prescribed HAART regimens included the thymidine-analogue NRTI’s zidovudine or stavudine, and use of either is associated with diverse short- and long-term treatment limiting adverse side effects. During T3 (2006-2009), the most recent time period analyzed, neither of these agents was received by more than 3 % of our cohort. While we cannot directly associate specific ART use and individual reasons for HAART switching, we believe that we can plausibly link changes in reported rates of some side effects with evolving use of ART over time. 36,37.38. Tenofovir replaced zidovudine and stavudine as the NRTI of choice for inclusion in initial HAART during T2 and T3, and its use probably was associated with fewer overall HAART switches undertaken for reasons of efficacy or tolerability39. Data from randomized prospective studies have demonstrated comparatively lower rates of treatment-limiting side effects associated with tenofovir compared to zidovudine or stavudine use, including the likelihood of lipoatrophy, pro-atherogenic hyperlipidemia, glucose intolerance, GI intolerance and bone marrow suppression (in the case of zidovudine)40,41. Further, it is likely that some patients (and their clinicians) may have pre-emptively switched from zidovudine or stavudine-containing HAART in order to avoid or ameliorate potential adverse effects42.
A major limitation of this analysis is the dependence upon data collected at semiannual MACS visits, which were not oriented specifically at capturing reasons for changing of ART regimens. The questionnaire used was designed to provide health information as reported by participants in the six months prior to the visit. Similarly, we have only laboratory data obtained at each semi-annual visit, not necessarily reflecting values present immediately preceding a switch in therapy, values which may or may not have been determinants in the changing of regimens. In addition, switching from agents taken separately to a fixed-dose combination of the same drugs was not considered a change in therapy for this analysis. Thus, we cannot evaluate specifically the issue of increased treatment convenience as a motivation for undertaking regimen switching. The impact upon our findings of the deliberate enrichment of the MACS from 1999-2001 with younger men of color is difficult to ascertain. Finally, the MACS participants are volunteers with a high degree of education and motivation, which limits the generalizability of these findings.
In summary, the frequency of switching from first HAART regimen during the first 5.5 years of therapy diminished progressively over time in the MACS during the first 15 years of the HAART era, while the duration of first HAART regimens increased. While HIV non-suppression appears to remain as a central reason for modifying first HAART therapy, rates of viral non-suppression have decreased over time in the MACS. Potential ART-related treatment-limiting adverse effects have evolved over time. There were fewer reports CNS side symptoms, more changes of regimen potentially linked to occurrence or avoidance of adverse metabolic outcomes in recent years and suggestive of overall increases in HAART regimen tolerability. This fact, along with the improved HIV suppressive efficacy and increased convenience of newer regimens as evidenced by progressive reductions over time in the mean the number of pills received in initial HAART regimens, indicate that current HIV treatment optimize the likelihood of extended first HAART durability and improved patient quality of life.
Supplementary Material
Acknowledgements
The MACS is funded by the National Institute of Allergy and Infectious Diseases. Dr Laurence Slama was supported by: an unrestricted grant for the study from Gilead, a grant from the International AIDS Education Project Inc. and by a grant from AMEVIH (not- for profit foundations), a grant from Jansen, Roche and Merck to support her visiting scholar position at Northwestern University.
Footnotes
conflict of interest disclosure:
Xiuhong Li: no conflict of interest
Dr Todd Brown is on a board membership for EMD-Serono and consults for the following commercial entities: ViiV Healthcare, Merck, Gilead, BMS, Abbott
Lisa Jacobson: no conflict of interest
Dr Bernard Macatangay: no conflict of interest
Dr Robert Bolan: no conflict of interest.
Dr. John Phair serves on a Data Safety and Monitoring Board for Pfizer.
Dr. Frank Palella is on the speakers’ bureau and consults for the following commercial entities: Gilead Sciences, Bristol Myers Squibb, Janssen Pharmaceuticals, and Merck.
Dr Gilles Pialoux serves a board from: Abbott, BMS, Gilead, MSD, Tibotec-Janssen, ViiV Healthcare and participated on symposium organized by
Abbott, Boehringer-Ingelheim, BMS, GSK, Gilead, MSD, Pfizer, Roche, Nephrotec, Tibotec-Janssen, ViiV Healthcare. He was also invited to national and international meeting by Abbott, BMS, Gilead, ViiV Healthcare.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. The New England journal of medicine. 1997 Sep 11;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. The New England journal of medicine. 1997 Sep 11;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA : the journal of the American Medical Association. 1998 Nov 4;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 5.Hessol NA, Kalinowski A, Benning L, et al. Mortality among participants in the Multicenter AIDS Cohort Study and the Women's Interagency HIV Study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007 Jan 15;44(2):287–294. doi: 10.1086/510488. [DOI] [PubMed] [Google Scholar]
- 6.Casado JL, Perez-Elias MJ, Antela A, et al. Predictors of long-term response to protease inhibitor therapy in a cohort of HIV-infected patients. Aids. 1998 Jul 30;12(11):F131–135. doi: 10.1097/00002030-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Grabar S, Le Moing V, Goujard C, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000 Sep 19;133(6):401–410. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson LP, Li R, Phair J, et al. Evaluation of the effectiveness of highly active antiretroviral therapy in persons with human immunodeficiency virus using biomarker-based equivalence of disease progression. American journal of epidemiology. 2002 Apr 15;155(8):760–770. doi: 10.1093/aje/155.8.760. [DOI] [PubMed] [Google Scholar]
- 9.Phair J, Jacobson L, Detels R, et al. Acquired immune deficiency syndrome occurring within 5 years of infection with human immunodeficiency virus type-1: the Multicenter AIDS Cohort Study. Journal of acquired immune deficiency syndromes. 1992;5(5):490–496. [PubMed] [Google Scholar]
- 10.Graham NM, Zeger SL, Park LP, et al. The effects on survival of early treatment of human immunodeficiency virus infection. The New England journal of medicine. 1992 Apr 16;326(16):1037–1042. doi: 10.1056/NEJM199204163261601. [DOI] [PubMed] [Google Scholar]
- 11.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998 Nov 28;352(9142):1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 12.Seaberg EC, Wiley D, Martinez-Maza O, et al. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer. 2010 Dec 1;116(23):5507–5516. doi: 10.1002/cncr.25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willig JH, Abroms S, Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. Aids. 2008 Oct 1;22(15):1951–1960. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smit M, Smit C, Cremin I, Garnett GP, Hallett T, de Wolf F. Could better tolerated HIV drug regimens improve patient outcome? Aids. 2012 Sep 24;26(15):1953–1959. doi: 10.1097/QAD.0b013e32835722bd. [DOI] [PubMed] [Google Scholar]
- 15.Mocroft A, Youle M, Moore A, et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. Aids. 2001 Jan 26;15(2):185–194. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- 16.Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004 Jan 20;170(2):229–238. [PMC free article] [PubMed] [Google Scholar]
- 17.Llibre JM, Domingo P, Palacios R, et al. Sustained improvement of dyslipidaemia in HAART-treated patients replacing stavudine with tenofovir. Aids. 2006 Jun 26;20(10):1407–1414. doi: 10.1097/01.aids.0000233574.49220.de. [DOI] [PubMed] [Google Scholar]
- 18.Curran A, Ribera E. From old to new nucleoside reverse transcriptase inhibitors: changes in body fat composition, metabolic parameters and mitochondrial toxicity after the switch from thymidine analogs to tenofovir or abacavir. Expert opinion on drug safety. 2011 May;10(3):389–406. doi: 10.1517/14740338.2011.542145. [DOI] [PubMed] [Google Scholar]
- 19.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. American journal of epidemiology. 1987 Aug;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 20.Multicenter AIDS Cohort Study (MACS) Public Data Set: Release PO4. National Technical Information Service; Springfield, VA: Sep 14, 1995. 2009. http://www.statepi.jhsph.edu/macs/pdt.html. [Google Scholar]
- 21.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA : the journal of the American Medical Association. 2000 Jan 19;283(3):381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 22.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; Washington, DC: Dec 30, 2011. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 23.Oh DL, Sarafian F, Silvestre A, et al. Evaluation of adherence and factors affecting adherence to combination antiretroviral therapy among White, Hispanic, and Black men in the MACS Cohort. Journal of acquired immune deficiency syndromes. 2009 Oct 1;52(2):290–293. doi: 10.1097/QAI.0b013e3181ab6d48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Kimmel AD, Goldie SJ, Walensky RP, et al. Optimal frequency of CD4 cell count and HIV RNA monitoring prior to initiation of antiretroviral therapy in HIV-infected patients. Antiviral therapy. 2005;10(1):41–52. [PubMed] [Google Scholar]
- 26.Wolbers M, Babiker A, Sabin C, et al. Pretreatment CD4 cell slope and progression to AIDS or death in HIV-infected patients initiating antiretroviral therapy--the CASCADE collaboration: a collaboration of 23 cohort studies. PLoS Med. 2010 Feb;7(2):e1000239. doi: 10.1371/journal.pmed.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita TE, Phair JP, Munoz A, et al. Immunologic and virologic response to highly active antiretroviral therapy in the Multicenter AIDS Cohort Study. Aids. 2001 Apr 13;15(6):735–746. doi: 10.1097/00002030-200104130-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011 Oct;15(7):1381–1396. doi: 10.1007/s10461-011-9942-x. [DOI] [PubMed] [Google Scholar]
- 29.Kleeberger CA, Buechner J, Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. Aids. 2004 Mar 5;18(4):683–688. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 30.DeJesus E, Rockstroh JK, Henry K, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012 Jun 30;379(9835):2429–2438. doi: 10.1016/S0140-6736(12)60918-0. [DOI] [PubMed] [Google Scholar]
- 31.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012 Jun 30;379(9835):2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 32.Cicconi P, Cozzi-Lepri A, Castagna A, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naive patients. HIV medicine. 2010 Feb;11(2):104–113. doi: 10.1111/j.1468-1293.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 33.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. The New England journal of medicine. 2008 May 15;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockstroh JK, Lennox JL, Dejesus E, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Oct;53(8):807–816. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 35.Molina JM, Lamarca A, Andrade-Villanueva J, et al. Efficacy and safety of once daily elvitegravir versus twice daily raltegravir in treatment-experienced patients with HIV-1 receiving a ritonavir-boosted protease inhibitor: randomised, double-blind, phase 3, non-inferiority study. The Lancet infectious diseases. 2012 Jan;12(1):27–35. doi: 10.1016/S1473-3099(11)70249-3. [DOI] [PubMed] [Google Scholar]
- 36.Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007 Jul 7;370(9581):49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 37.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. Journal of acquired immune deficiency syndromes. 2010 Mar;53(3):323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 38.Arnaiz JA, Mallolas J, Podzamczer D, et al. Continued indinavir versus switching to indinavir/ritonavir in HIV-infected patients with suppressed viral load. Aids. 2003 Apr 11;17(6):831–840. doi: 10.1097/00002030-200304110-00008. [DOI] [PubMed] [Google Scholar]
- 39.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA : the journal of the American Medical Association. 2004 Jul 14;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 40.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. The New England journal of medicine. 2006 Jan 19;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 41.Madruga JR, Cassetti I, Suleiman JM, et al. The safety and efficacy of switching stavudine to tenofovir df in combination with lamivudine and efavirenz in hiv-1-infected patients: three-year follow-up after switching therapy. HIV clinical trials. 2007 Nov-Dec;8(6):381–390. doi: 10.1310/hct0806-381. [DOI] [PubMed] [Google Scholar]
- 42.Moyle GJ, Sabin CA, Cartledge J, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. Aids. 2006 Oct 24;20(16):2043–2050. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.