Abstract
The extracellular signal-regulated kinases (ERKs) 1, 2, and 5 have been shown to play distinct roles in proliferation, differentiation, and neuronal viability. In this study, we examined ERK1, 2, and 5 expression and activation in the substantia nigra (SN), striatum (STR), and ventral tegmental area (VTA) during aging. An age-related decrease in phosphorylated ERK5 was observed in the SN and STR, whereas an increase in total ERK1 was observed in all three regions. In primary cultures of the SN and VTA, inhibition of ERK5 but not ERK1 and 2 significantly decreased DA neuronal viability. These data suggest that ERK5 is essential for the basal survival of SN and VTA dopaminergic neurons. These are the first studies to examine ERK1, 2, and 5 expression and activation in the SN, STR, and VTA during aging and the relative roles of ERK1, 2, and 5 in basal survival of SN and VTA dopaminergic neurons. These data raise the possibility that a decline ERK5 signaling may play a role in age-related impairments in dopaminergic function.
Keywords: Brain, aging, ERK5, ERK1/2, Striatum, Substantia nigra, Ventral tegmental area, Fischer-344xBrown Norway F1 hybrids (F344xBN F1), MAP kinases
1. Introduction
Extracellular signal-regulated kinase (ERK) 1, 2, and 5 members of the mitogen-activated protein kinase (MAPK) family regulate many physiological processes during neurodevelopment, including apoptotic cell death and the maintenance of neuronal viability following oxidative insults (Cavanaugh, 2004; Liu et al., 2003; 2006; Satoh et al., 2011; Watson et al., 2001; Xia et al., 1995). Age-associated changes in ERK1 and 2 expression and activation have been observed, although the nature of those changes is controversial. For example, Song and colleagues (2007) reported an increase in p-ERK1 and 2 levels in the hippocampus, frontal cortex, and striatum in 24-month old animals. However, Mo and colleagues (2005) demonstrated a decrease in p-ERK1 and 2 levels in these same brain regions in 22-month old rats. Although, ERK5 expression has not been studied in the aging brain, Liu and colleagues (2003; 2006) observed high levels of ERK5 in early embryonic stages that decreased in the whole brain and cortex of postnatal and adult Sprague-Dawley rats.
To date, no studies have been performed to examine the relative expression and activation of ERK1, 2 and 5 in dopaminergic regions of the brain with aging or their roles in the survival of dopamine (DA) neurons. Although ERK5 has been shown to be crucial for the basal survival of MN9D dopaminergic cells (Cavanaugh et al., 2006), its role in the survival of primary DA neurons has not been explored. In this study, we examined the expression and activation of ERK1, 2, and 5 in the SN, STR, and VTA during aging. Further, we sought to examine the relative roles of these ERK isoforms in DA neuronal survival. Due to the involvement of dopaminergic neuronal systems in Parkinson’s disease and other mental disorders, elucidation of cellular signaling alterations that responsible for the decrease in dopaminergic function with age may advance our understanding of neurological disorders and lead to the identification of novel drug targets for these conditions.
2. Materials and Methods
Animals
To determine ERK expression in the SN, STR, and VTA with age, young (3 mo.), middle-aged (13 mo.), and old (23 mo.) male Fischer-344xBrown Norway F1 hybrids (F344xBN F1) rats were obtained from the National Institute on Aging colonies (Harlan Sprague-Dawley, Indianapolis, IN). For primary neuronal cultures, timed pregnant Sprague-Dawley rats (Hilltop Laboratory Inc., Scottdale, PA) were used. Animals were single-housed in a 12:12 light: dark cycle and provided with water and rat chow ad libitum. All procedures were conducted in accordance with the guidelines for the NIH Care and Use of Laboratory Animals and approved by the Duquesne University or the University of Pittsburgh Institutional Animal Care and Use Committees.
Western blot analysis
Tissues were isolated, frozen on dry ice and stored at −80 °C. All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Tissue samples were sonicated in ice-cold 1:20 (w/v) lysis buffer as previously described (Allen et al., 2011). Total homogenate was centrifuged at 11,000 rpm for 30 minutes at 4 °C and the supernatant was collected and stored at −80 °C. Total protein content was assessed by Bradford protein assay (Bio-Rad, Hercules, CA) and 60 µg of protein was loaded on an 8% SDS-PAGE gel and transferred to a nitrocellulose membrane (Licor Biosciences, Lincoln, NE). After transfer, membranes were washed for 5 minutes with 1X PBS and blocked for 1 h in a caseinblocking buffer (Licor Biosciences) at room temperature. Membranes were incubated overnight at 4°C in primary antibody in the casein blocking buffer with 0.1% Tween-20. Antibodies included rabbit anti-phospho-ERK1/2 (Cell Signaling Cat. No. 9101), mouse anti-total ERK1/2 (Cell Signaling Cat. No. 9107), rabbit anti-phospho-ERK5 (Cell Signaling Cat. No. 3371), and rabbit anti-total ERK5 (Sigma-Aldrich Cat. No. E1523). Mouse anti-α-Tubulin (Sigma-Aldrich Cat. No. T5168) was used as a loading control. After incubation with primary antibody, blots were washed in 1X PBS solution with 0.1% Tween-20 (1X PBS-T) and incubated with goat antirabbit (Licor Biosciences, Cat. No 926-68021) and goat anti-mouse (Licor Biosciences, Cat. No 926-32210) secondary antibodies for 1 h at room temperature. After washing the membranes with 1X PBS-T, the protein bands were visualized on an Odyssey Infrared Imager and quantified with Odyssey software (Licor Biosciences).
Primary dissociated dopaminergic SN and VTA neuronal culture and treatment
Cultures were prepared as previously described in Ding et al, 2004 with minor modifications. Postnatal day 0 rat brains were isolated under sterile condition into cold Gey’s Balanced Salt Solution (1.55 mM CaCl2, 5 mM KCl, 0.22 mM KH2PO4, 1.05 mM MgCl2, 137 mM NaCl, 2.7 mM NaHCO3, 0.84 mM NaH2PO4, and 5.5 mM glucose pH 7.25). Coronal sections of the SN and VTA were isolated under a dissecting microscope. The tissue was then incubated in a solution containing 20 units/ml of papain and dissolved in a disassociation media (DM) containing 90 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2, 0.25 mM CaCl2, 10 mM 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (Hepes), 20 mM glucose, 0.5% phenol red, 1 mM kynurenic acid and 10 mM MgCl2 for 1 h in a roller drum incubator (Schuett-biotec Gmbh, Göttingen, Germany) at 35 °C. After incubation in the papain, the tissue was washed several times in DM, followed by washes in trituration media (TM; 1 mg/ml Bovine Serum Albumin (BSA), 10 mM Hepes, 1mg/ml trypsin inhibitor, 1 mM kynurenic acid, 10 mM MgCl2, 5% fetal bovine serum), then finally two washes with feeding media (FM; 2% rat serum, 2% fetal bovine serum, B27 Supplement Supplement (Life Technologies, Grand Island, NY), 0.225% glucose, 1 mM L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, 10 mM Hepes, and 0.9 mM sodium pyruvate in BME). After washes the tissue was mechanically dissociated with a fire-polished pipet in TM. The slurry of cells was put through a concentration gradient 10mg/ml BSA, 10mg/ml trypsin inhibitor, 1 mM kynurenic acid, 10 mM MgCl2, 5% fetal bovine serum in BME to concentrate neurons and remove debris. The pellet was resuspended in FM. Cells were plated on 16 well Nunc chambered slides (Thermofischer Scientific, Pittsburgh, PA), coated with 200 µg/ml poly-d-lysine and 5 µg/ml laminin (Life Technologies) at a concentration of 30,000 live cells/well determined by trypan blue cell exclusion with a hemocytometer. Cultures were maintained at 37 °C in 5% CO2. As soon as the cultured primary cells are attached to the plate, at least one hour following plating, the treatments with DMSO and inhibitors BIX02189 (Selleck Chemicals, Houston, TX), or U0126 (Sigma-Aldrich) (all 10 µM) were done.
Immunocytochemical staining
At 2, 4, 6, and 8 days in vitro (DIV) slides were fixed in 4% paraformaldehyde and 4% sucrose in PBS for 30 minutes. The slides were washed 3 times in wash buffer (0.1% Tween 20, sodium azide in PBS). Following washes, the slides were incubated in blocking solution of 5% BSA (Sigma-Aldrich), 0.1% glycine, 5% goat serum (Jackson Immuno, West Grove, PA), and 0.3% Triton X-100 (Bio-Rad) in PBS with sodium azide for 1 h. After blocking, cultures were incubated in rabbit anti-TH antibody (PelFreez, Rogers, AR ) overnight at a concentration of 1:5000 in blocking buffer. Following three washes, the cultures were incubated with Alexa Fluoro 546 goat anti-mouse (1:1000, Molecular Probes, Life Technologies) in blocking buffer 2 h and Hoechst 33342 (10 µg/ml) for 15 minutes. The slides were washed and cover slipped with Fluoromount (Southern Biotech Birmingham, AL).
Data Collection and Image Analysis
Images were captured with MetaMorph Imaging Software (7.1, Universal Imaging, Downingtown, PA) using a Retiga 1300R digital CCD camera (QImaging, Burnaby, British Columbia, Canada). TH+ cells and total cell number were obtained using the cell count macro within the MetaMorph software after verifying the accuracy with manual counting of the cells.
SH-SY5Y cells culture and treatment
Human dopaminergic neuroblastoma SH-SY5Y cells were cultured in DMEM (Gibco Life Technologies) supplemented with 10% (v/v) fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 0.05 U/ml penicillin and 0.05 mg/ml streptomycin and maintained at 37 °C in a humidified 5% CO2 atmosphere. For treatment with inhibitors, SHSY5Y cells were seeded in 96-well plates at a density of 15,000 cells/well. Cell viability was assessed after 24 and 48 h of U0126 (10 µM) or BIX 02189 (10 µM) exposure.
Cell Titer-Glo assay
To determine SH-SY5Y cell viability, ATP levels were assessed using luciferase-based Cell Titer Glo assay with a modification (25µl reagent in 50µl media; Promega Inc., Madison, WI). Luminescence was measured on a microplate reader (Victor3 1420 multilabel counter, PerkinElmer, MA, USA).
Statistical Analysis
GraphPad Prism 5 Software (San Diego, CA) was used for statistical analysis. Data are expressed as mean ± SEM. For the aging study, statistical comparison was performed using a one-way analysis of variance (ANOVA) followed by the appropriate post hoc test as noted for each analysis. A two-tailed Student's t-test for unpaired data was also used for statistical comparisons. For viability studies, statistical significance was determined by two-way ANOVA followed by the Bonferroni post hoc test. Statistical significance was defined at p < 0.05.
3. Results
Age-related changes in ERK5 phosphorylation
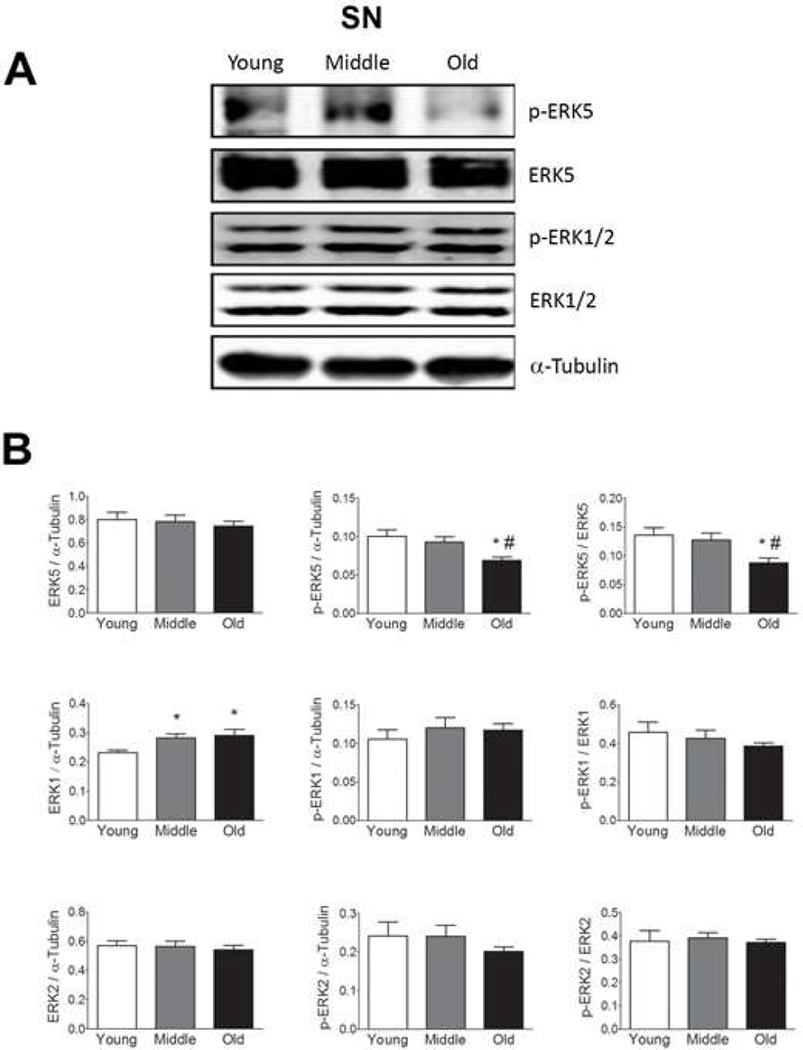
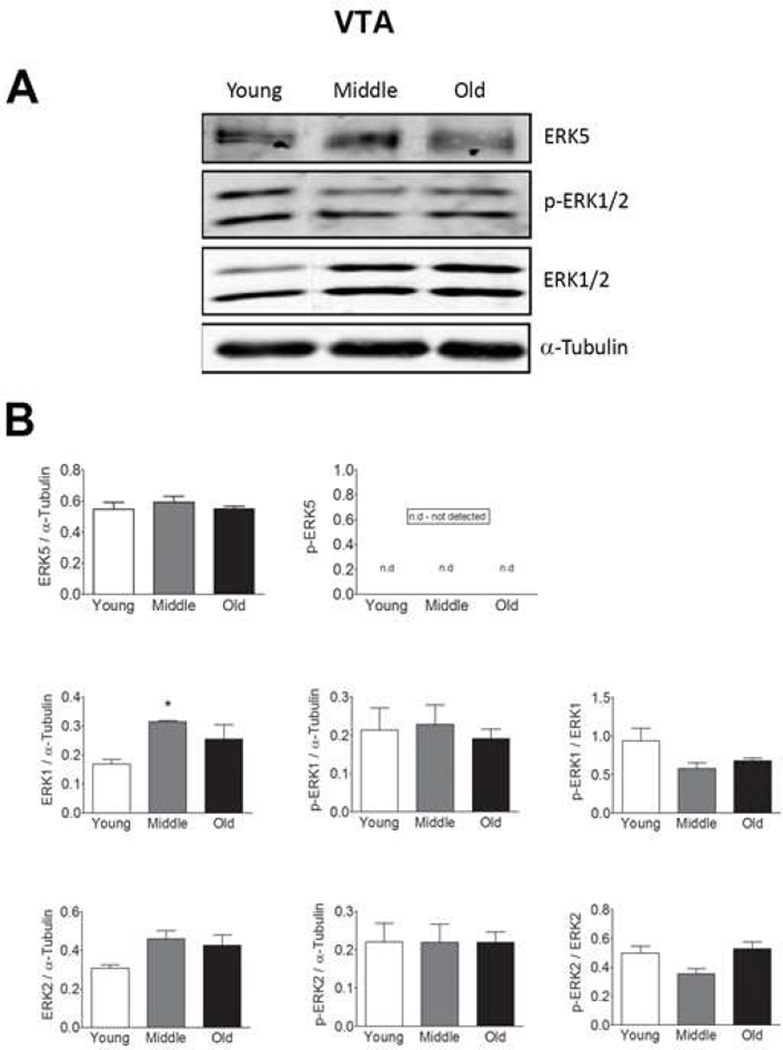
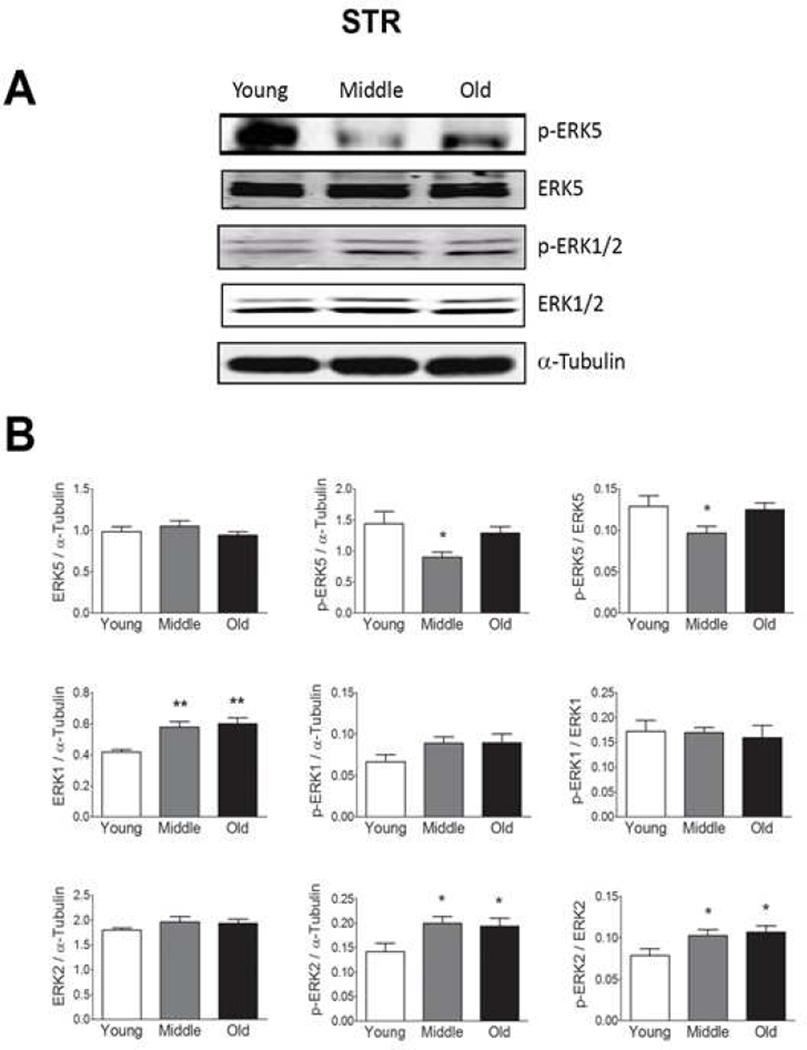
We observed no age-related changes in total ERK5 expression in the SN, STR, or VTA (Fig 1–3), but found a decrease in p-ERK5 in old and middle aged animals as compared to young animals in the SN and STR (Figs. 1 and 2, respectively). The decline in p-ERK5 with aging was observed when the ratio of p-ERK5 was taken with either α-tubulin or total ERK5. We were not able to detect p-ERK5 in the VTA (Fig. 3), which could be due to either low ERK5 expression or low phosphorylation in this region.
Figure 1. Total and phosphorylated ERK1, 2, and 5 expressions in the substantia nigra (SN) from young, middle and old male F344xBN F1 hybrid rats.
(A) Immunoblot analysis of total and phosphorylated ERK1, 2, and 5 expression in the SN. (B) Quantification of the immunoblots using Licor Odyssey software. The values are calculated using the integrated intensity. Protein expression was standardized to α-Tubulin and basal total protein. n=10 for each age. Data are expressed as mean ± SEM. * p < 0.05 indicates a difference compared to the young age group. # p < 0.05 indicates a difference compared to the middle age group. p values were determined by analysis of variance followed by post hoc Student-Newman-Keuls.
Figure 3. Total and phosphorylated ERK1, 2, and 5 expressions in the ventral tegmental area (VTA) from young, middle and old male F344xBN F1 hybrid rats.
(A) Immunoblot analysis of total and phosphorylated ERK1, 2, and 5 expression in the VTA. (B) Quantification of the immunoblots using Licor Odyssey software. The values are calculated using the integrated intensity. Protein expression was standardized to α-Tubulin and basal total protein. n=5 for each age. Data are expressed as mean ± SEM. * p < 0.05 indicates a difference compared to the young age group by analysis of variance followed by post hoc Student-Newman-Keuls.
Figure 2. Total and phosphorylated ERK1, 2, and 5 expressions in the striatum (STR) from young, middle and old male F344xBN F1 hybrid rats.
(A) Immunoblot analysis of total and phosphorylated ERK1, 2, and 5 expression in the STR. (B) Quantification of the immunoblots using Licor Odyssey software. The values are calculated using the integrated intensity. Protein expression was standardized to α-Tubulin and basal total protein. n=10 for each age. Data are expressed as mean ± SEM. ** p < 0.01 and * p < 0.05 compared to the young age group by analysis of variance followed by post hoc Student-Newman-Keuls.
Age-related changes in ERK1 and 2
In contrast to ERK5, age-related increases in total ERK1 expression were observed in each of these regions (Figs. 1, 2, and 3). In the SN and VTA, we did not observe any changes in ERK2 expression but in the VTA we observe a trending increase in ERK2 expression starting at middle age (Fig. 1–3). This trend is significant when the young and middle age groups were compared using student’s t-test (p = 0.023, Fig. 3). We did not observe any significant changes in p-ERK1 and p-ERK2 with age in SN (Fig. 1) or VTA (Fig. 3) regions. However, an increase in p-ERK2 was observed in the STR starting at middle age (Fig. 2) when the ration of p-ERK2 was taken with either α-tubulin or total ERK2. In the VTA, trending decreases in the amount of p-ERK1 and p-ERK2 were observed with age when the ratio of p- ERK1 and p-ERK2 were taken with total ERK1 and total ERK2, respectively (Fig. 3). However, this trend was not significant.
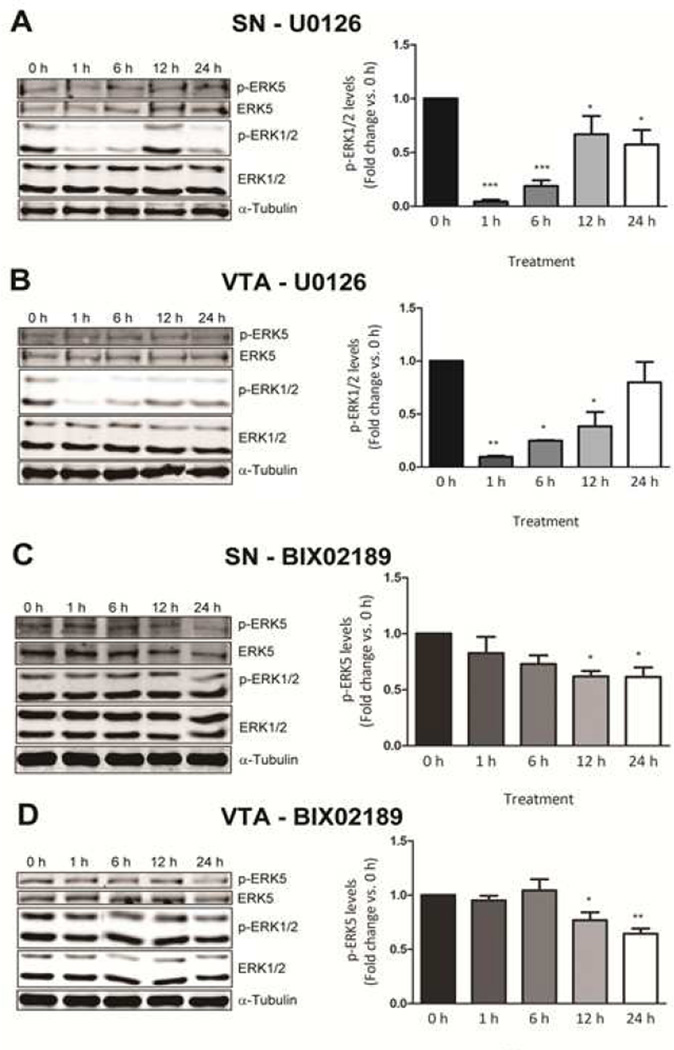
Inhibition of ERK1/2 or ERK5 activation by U0126 or BIX02189
In order to determine the distinct roles of the forms of ERK in the basal survival of DA neurons, we examined the impact of MEK inhibitors that specifically block either ERK1 and 2 or ERK5 phosphorylation by western analysis in primary cultures from the SN and VTA. It has been reported that the MEK1/2 inhibitor, U0126, also blocks MEK5 phosphorylation, thereby inhibiting ERK1, 2, and 5 activities (Cavanaugh et al., 2006; Kamakura et al., 1999). However, others have reported that U0126 does not inhibit ERK5 signaling (Mody et al., 2001). This suggests that U0126 could be specific depending on the type of cells, stimulation, and concentration used. Hence, it is important to test whether or not U0126 blocks ERK1, 2, and/or 5 phosphorylation in our system. Our results indicate that U0126 significantly blocked ERK1 and 2, but not ERK5, phosphorylation in primary SN and VTA cultures at any time point or dose tested in this study (Fig. 4A and 4B).
Figure 4. Inhibition of ERK1/2 and ERK5 phosphorylation by U0126 and BIX02189, respectively.
Immunoblot analysis indicating selective inhibition by U0126 (10 µM) of ERK1/2 phosphorylation in the primary dissociated neuronal culture prepared from (A) substantia nigra and (B) ventral tegmental area. Immunoblot analysis indicating selective inhibition by BIX02189 (10 µM) of ERK5 phosphorylation in the primary dissociated neuronal culture prepared from (C) substantia nigra and (D) ventral tegmental area. Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01, and *** p < 0.001 indicates indicate a difference compared to the no treatment by analysis of variance followed by post hoc Student-Newman-Keuls.
Recently, BIX02189 was developed as a selective pharmacological inhibitor of the MEK5/ERK5 pathway (Obara et al., 2011; Tatake et al., 2008). Our results confirm these reports as BIX02189 (10 µM) blocked ERK5, but not ERK1 or 2, phosphorylation at 12 and 24 hours in primary SN and VTA cultures (Fig. 4C and 4D).
Role of ERK5 in the basal survival of primary cells in SN and VTA cultures
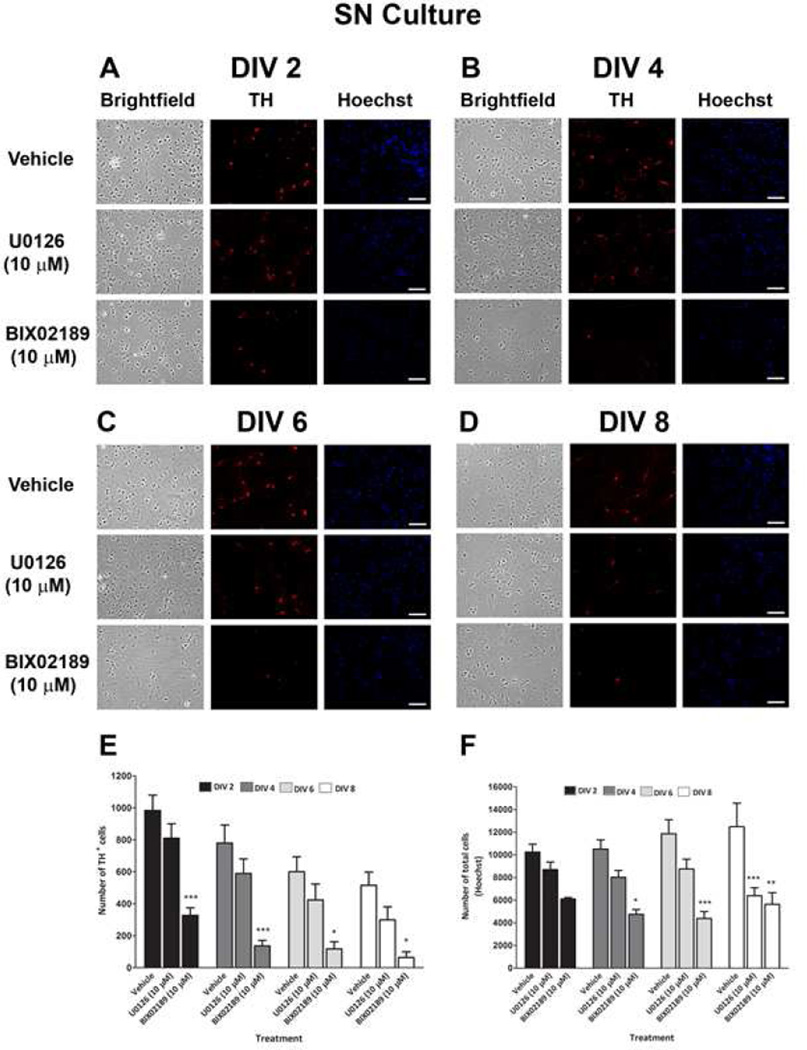
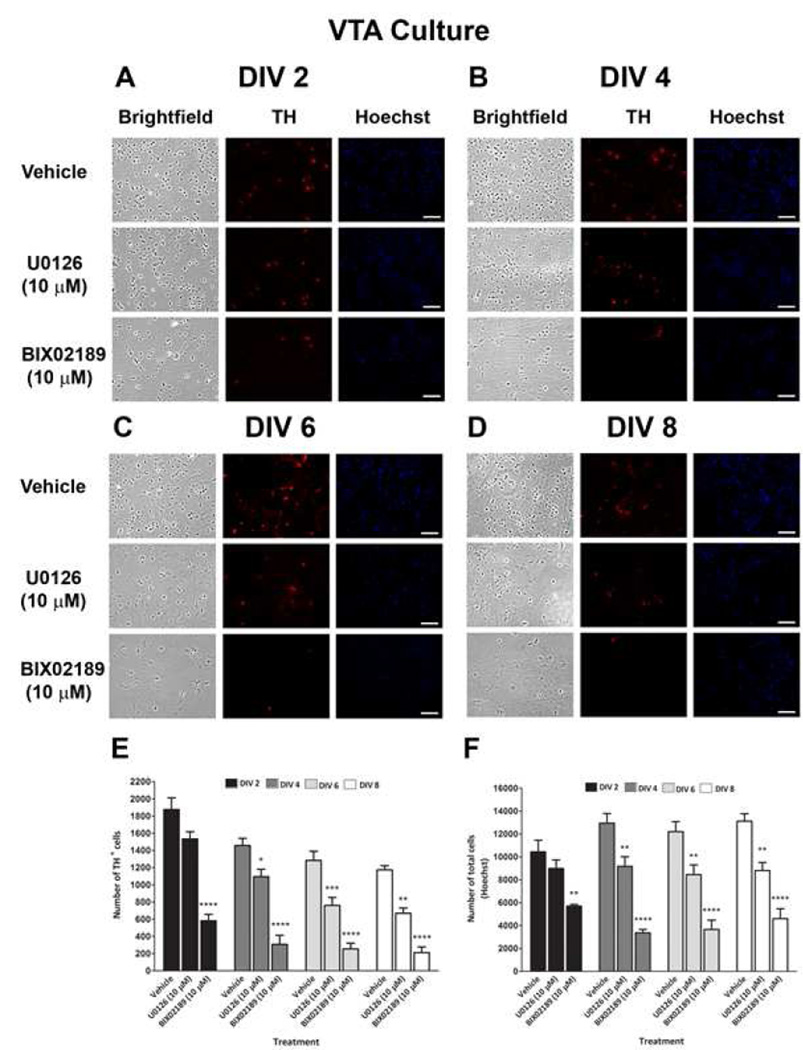
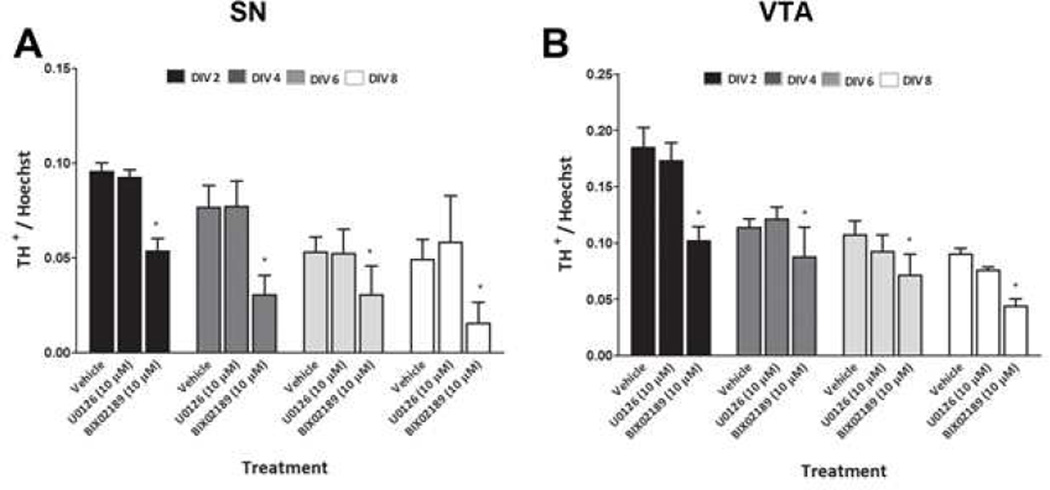
To examine the role of the ERK pathways in the basal survival of DA neurons, we treated SN and VTA cultures on DIV 0 with U0126 (10 µM) or BIX02189 (10 µM). The DA neuronal and total cellular viability was then determined at DIV 2–8 by counting TH+ neurons and Hoechst-stained nuclei. As is usual with these primary DA cultures, we observed a decline in the number of TH+ neurons and an increase in the number of Hoechst-stained nuclei in both the SN and VTA with increasing DIV in the vehicle group. The TH+ neurons are only 5–10% of the total cells in culture. Therefore even as the number of DA neurons decreases, the percent of total cells may not decrease. In addition, the glia in the culture continue to divide with time. By DIV 2, treatment of SN and VTA primary cultures with BIX02189 decreased the number of cell in both the SN (45%; Fig. 5F) and VTA cultures (47%; Fig. 6F). This effect was even more profound when DA neurons were counted separately: DA neurons were reduced by 67% in SN and 69% in VTA (Fig. 5E and 6E) culture. Similarly, at DIV 4, 6, and 8, significant losses of DA neurons and total cells were observed in the SN and VTA cultures treated with BIX02189 as compared to the vehicle treated cells. In contrast, U0126 treatment did not cause a significant decrease in DA neurons or total cells in cultures of the SN (Fig. 5E and 5F) or VTA (Fig. 6E and 6F) by DIV 2. A significant loss of DA neurons was observed at DIV 4, 6, and 8 with U0126 in the VTA (Fig. 6E), but not SN (Fig. 5E). Similarly, a significant loss of total cells was observed at DIV 4, 6, and 8 with U0126 in the VTA (Fig. 6F). In contrast, a significant decrease in the total number of cells was only observed at DIV 8 in the SN following U0126 treatment (Fig. 5F). At every time point examined the percentage loss of both DA neurons and total cells in the SN and VTA cultures was greater following BIX02189 treatment as compared to U0126 treatment. When the ratio of the number of DA neurons to that of total cells was determined for SN and VTA cultures, there was a significant loss of DA neurons following BIX02189, but not U0126, treatment as compared to the vehicle controls for all the DIV (Fig.7A and 7B).
Figure 5. Effect of pharmacological inhibition of ERK1/2 and ERK5 phosphorylation on the basal survival of DA neurons in primary dissociated substantia nigra cultures.
At DIV 0, cultures were treated with U0126 (10 µM) and BIX02189 (10 µM) and on A) DIV 2, B) DIV 4, C) DIV 6 and D) DIV 8 cultures were fixed and immunostained for TH (red fluorescence) to label DA neurons and bisbenzimide H33258 (Hoechst – blue fluorescence) to label all cell nuclei. Data were quantified for E) number of TH+ cells and F) number of total cells (Hoechst). Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001 indicates a difference as compared to respective vehicle treatment by two-way ANOVA followed by the Bonferroni post hoc test.
Figure 6. Effect of pharmacological inhibition of ERK1/2 and ERK5 phosphorylation on the basal survival of DA neurons in primary dissociated ventral tegmental area cultures.
At DIV 0, cultures were treated with U0126 (10 µM) and BIX02189 (10 µM) and on A) DIV 2, B) DIV 4, C) DIV 6 and D) DIV 8 cultures were fixed and immunostained for TH (red fluorescence) to label DA neurons and bisbenzimide H33258 (Hoechst – blue fluorescence) to label all cell nuclei. Data were quantified for E) number of TH+ cells and F) number of total cells (Hoechst). Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 indicates a difference as compared to respective vehicle treatment by two-way ANOVA followed by the Bonferroni post hoc test.
Figure 7.
Loss of TH+ cells with respect to total cell loss in primary dissociated neuronal cultures prepared from A) substantia nigra and B) ventral tegmental area for all the DIV. Data are expressed as mean ± SEM. Data are expressed as mean ± SEM. * p < 0.05 indicate a difference as compared to respective vehicle treatment by two-way ANOVA followed by the Bonferroni post hoc test.
ERK5 is necessary for basal survival of SH-SY5Y cells
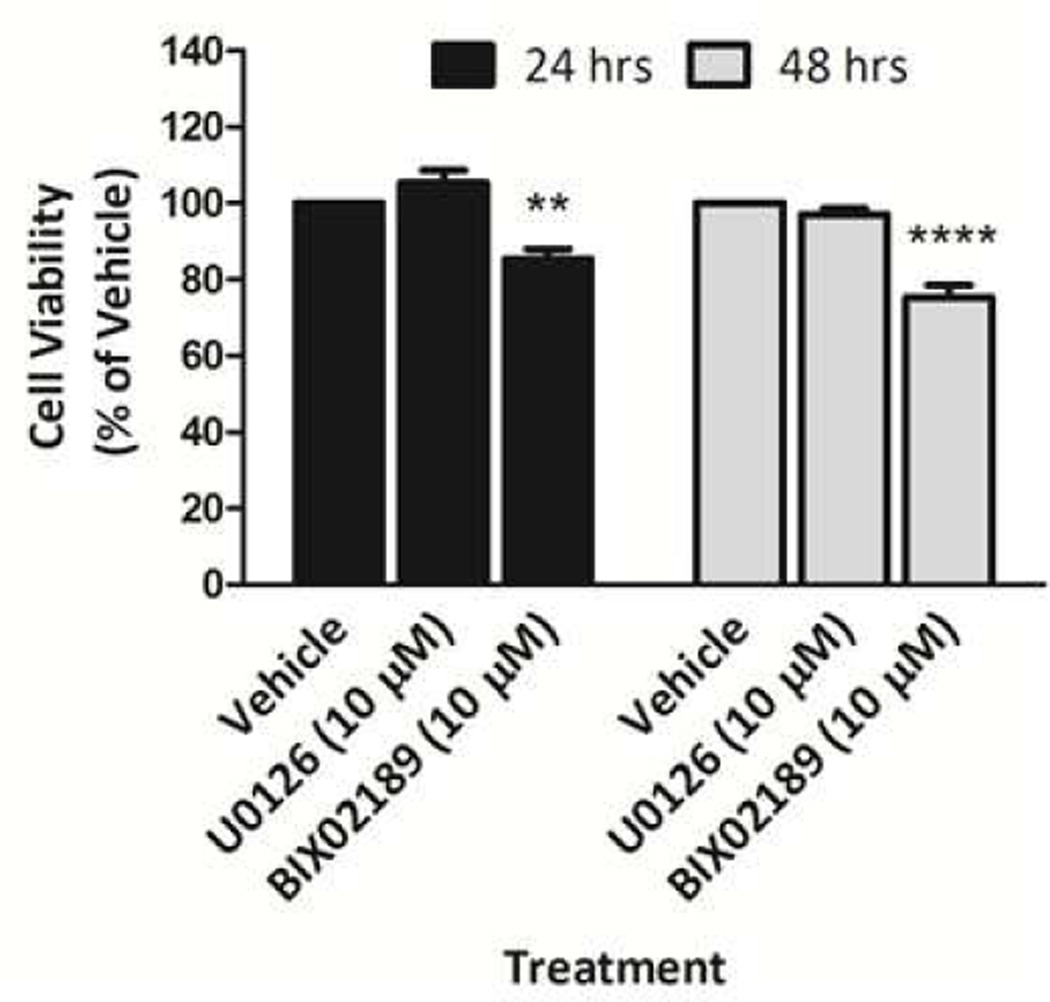
Primary dissociated SN and VTA cultures contain other neuronal cell types (e.g., GABA neurons) and glia that have been shown to protect DA neurons against oxidative stress (Hou et al., 1997). Thus, it is possible that the MEK inhibitors were influencing DA neurons indirectly via the inhibition of ERK signaling in other cells. In an attempt to examine this possibility, we tested the effect of MEK inhibitors on the human neuroblastoma SH-SY5Y cell line, which has been shown to possess many characteristics of dopaminergic neurons, including the presence of TH and dopamine β-hydroxylase and the capacity to synthesize DA (Biedler et al., 1973; Cheung et al., 2009; Oyarce and Fleming, 1991; Xie et al., 2010). SH-SY5Y cells were treated with U0126 (10 µM) or BIX02189 (10 µM) for 24 and 48 hrs. Our results show a loss of SH-SY5Y cell viability with BIX02189, but not U0126, treatment at 24 and 48 hrs (Fig. 8).
Figure 8. Effect of pharmacological inhibition of ERK1/2 and ERK5 phosphorylation on the basal survival of human dopaminergic neuroblastoma SH-SY5Y cells.
SH-SY5Y cells were treated with U0126 (10 µM) and BIX02189 (10 µM) for 24 and 48 h. After 24 and 48 h, cell viability was determined by measurement of ATP levels using a Cell Titer-Glo Luminescent Cell Viability Assay. Data are expressed as mean ± SEM. ** p < 0.01 and *** p < 0.001 indicates a difference as compared to respective vehicle treatment by two-way ANOVA followed by the Bonferroni post hoc test.
4. Discussion
We examined the levels of total and phosphorylated ERK 1, 2, and 5 in several dopaminergic brain regions brain regions as a function of age. We then sought to determine the importance of ERK phosphorylation in the survival of DA neurons emanating from the SN and VTA. In contrast to previous studies in which no ERK5 mRNA or protein expression was detected in the SN, STR and VTA of the adult mouse brain and generally thought to be absent in most areas of the adult brain (Di Benedetto et al., 2007; Pan et al., 2012), we were able to detect both total and phosphorylated ERK5 protein in each adult brain region examined with the exception of p-ERK5 in the VTA. Unpublished data from our laboratory show that we are able to detect ERK5 expression using immunohistochemistry and western blot in the same strain of mice (C57Bl/6) used by Pan et al., 2012 and Di Benedetto et al., 2007. Therefore, we think this difference could be associated with the primary ERK5 antibody and/or the technique used for detection. For example, Pan and colleagues used an ERK5 antibody generated in their laboratory. Moreover, Pan and colleagues (2012) used immunohistochemistry to detect ERK5 protein and Di Benedetto and colleagues (2007) used in situ hybridization to detect mRNA. Neither group performed western blot analysis to confirm the presence of ERK5 protein in adult brain. Using western blot analysis, several studies have reported the presence of ERK5 in several adult brain regions such as the hippocampus and cortex/prefrontal/frontal cortex in humans and rodents (Yoon et al., 2005, Liu et al., 2006, Dwivedi et al., 2007). Our findings suggest that whereas total ERK 1, 2, and 5 and phosphorylated ERK 1 and 2 either remain unchanged or increase with age in the SN, STR, and VTA, ERK5 phosphorylation decreases with age in the SN and STR. Moreover, inhibition of ERK5 phosphorylation by BIX02189 reduced the survival of cultured DA neurons with little effect of inhibition of ERK 1 and 2. The function and viability of dopaminergic neurons depend on the availability of neurotrophic factors and subsequent activation of survival signaling (Lindgren et al., 2008; Kramer et al., 2007; Pascual et al, 2008; Kim et al., 2011; Hidalgo-Figueroa et al., 2012). This study highlights the importance of ERK5 signaling in supporting the viability of dopamine neurons that may play a crucial role in maintaining mature dopaminergic neurons during adulthood. Age-related declines in this signaling could lead to the degeneration and death of dopaminergic neurons.
ERK 1, 2, and 5 as a function of age
In the present study, we observed an age-related decrease in ERK5 phosphorylation in the SN and STR (Fig. 1 and 2). In addition, we observed an increase in ERK2 phosphorylation in the STR with age (Fig. 2). These data suggest the possibility that cross-talk between these signaling pathways occurs in STR such that an increase p-ERK2 expression occurs to compensate for the decrease in p-ERK5. Evidence for cross-talk between the ERK1, 2, and 5 pathways has been previously suggested (Barros and Marshall, 2005; Mody et al., 2001). For example, pharmacological inhibition of ERK1 and 2 phosphorylation leads to a sustained activation of MEK5 and ERK5 following growth factor stimulation in HeLa cells (Mody et al., 2001).
Role of ERK5 in the survival of DA neurons
Previously, we have reported that ERK1, 2, and 5 are important for the basal survival of MN9D cells (Cavanaugh et al., 2006). However, this phenomenon had not previously been tested in primary DA neuronal cultures due to the lack of specific pharmacological inhibitors of the MEK5/ERK5 pathway. The development of these specific inhibitors, such as BIX02189, has allowed comparison of the roles of ERK1/2 and ERK5 isoforms in the basal survival of primary dissociated SN and VTA cultures. Using BIX02189 as a specific inhibitor of the upstream ERK5 kinase, MEK5, we observed a significant loss of DA neurons, total cells, and the number of DA neurons as a percentage of the total cells starting at DIV 2 in the SN and VTA cultures (Fig. 5–7). In contrast, U0126, an inhibitor of ERK1/2 phosphorylation had no such effect at DIV 2 in the SN and VTA cultures (Fig. 5–6). Similarly, in SH-SY5Y cells, cell viability significantly decreased following BIX02189, but not U0126, treatment (Fig. 8). However, following U0126 treatment there is a significant decline in the DA neurons and total cells at later time points (DIV 4, 6, and 8) in the VTA culture (Fig. 6). Although not significant, the percentage loss of DA neurons and total cells in the SN culture were similar to the VTA culture following U0126 treatment at DIV 4, 6 and 8. These data suggest that a loss of ERK1/2 signaling during early DIV results in an increased vulnerability of SN and VTA DA neurons and other cells at later time points. However, the number of DA neurons as a ratio of total cells is not decreased in these same cultures at any time point following U0126 treatment (Fig. 7). Together, these data suggest that ERK5 is necessary for the basal survival of DA neurons in the SN and VTA at early DIV. These results are in accord with a previous study in which targeted deletion of ERK5 in the developing nervous system reduced the density of dopaminergic neurons in the olfactory bulb (Zou et al., 2012).
Possible relation to neurotrophic factors
Loss of motor functions and PD are associated with an age-related loss of nigral DA neurons (Eriksen et al., 2009; Fearnley and Lees, 1991; Fox et al., 2001; Morgan et al., 1987) and/or dysfunction in the nigrostriatal dopaminergic pathways (Carlsson and Winblad 1976; Gerhardt et al., 1995; Irwin et al. 1994; McGeer et al., 1977; Yue et al., 2012; Yurek et al., 1998). These DA neuronal impairments may be associated with the age-related decreases in ERK5 activation observed in the present study. Neurotrophic factors (NTFs) essential for DA neuron survival, including glial cell line-derived neurotrophic factor (GDNF), brain derived neurotrophic factor (BDNF), nerve growth factor (NGF) and neuregulin (NRG), have been shown to activate the ERK5 pathway (Carlsson et al., 2011; Cavanaugh et al., 2001; Dickerson, 2010; Esparis-Ogando et al., 2002; Fox et al., 2001; Hayashi et al., 2001; Obara et al., 2009). Moreover, a critical role for ERK5 in NTF-induced survival of immature cortical, cerebellar, dorsal root ganglia, and superior cervical ganglion neurons has been reported (Finegan et al., 2009; Liu et al., 2003; Shalizi et al., 2003; Watson et al., 2001). Age-related declines in GDNF protein and GDNF and NRG receptors (GFR α-1 and ErbB4, respectively) have been noted in dopaminergic brain regions (Dickerson et al., 2009; Pruett and Salvatore, 2010; Yurek and Fletcher-Turner, 2001) and several NTFs are particularly low in the SN of patients with Parkinson’s disease (Jenner and Olanow, 1998; Mogi et al., 1999; Siegel and Chauhan, 2000).
These observations, along with our current data, suggest that age-associated declines in nigral DA neurons (Eriksen et al., 2009; Fearnley and Lees, 1991; Fox et al., 2001; Morgan et al., 1987) and motor functions (Allen et al., 2011; Bennett et al., 1996; Boger et al., 2006; Irwin et al., 1994; Yue et al., 2012) could be a consequence of decreased ERK5 activation resulting from reduced NTF signaling. It may also be noteworthy that inhibition of the ERK5 pathway has been shown to reduce GDNF mRNA and protein levels in some cells (Obara et al., 2011), suggesting a feedback loop in which MEK5-ERK5 signaling is further decreased.
In conclusion, we present evidence for age-related changes in total and phosphorylated ERK1, 2, and 5 in DA-rich brain regions during normal aging. Further, we have elucidated distinct roles of ERK1/2, and 5 in the basal survival of DA neurons and total cells in SN and VTA primary cultures. As our data suggest that ERK5 activation is essential for the survival of dopaminergic neurons, the use of NTFs that activate ERK5 could be a viable therapeutic option to decrease DA neuronal vulnerability and, thereby, reduce age-related motor deficits and the risk of parkinsonism.
Acknowledgements
These studies were funded by the National Institute of Aging (AG25848) and the National Institute of Neurological Disorders and Stroke (NS070825), Duquesne University, and the University of Pittsburgh. S.L.W. was supported in part by summer student fellowship (PDFSFW- 1215) from the Parkinson's Disease Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statements
a) The authors have no actual or potential conflicts of interest.
b) All animals were maintained in temperature-controlled rooms in the barrier facility maintained by Duquesne University or the University of Pittsburgh. Animals were housed in accordance with Guidelines for the Care and Use of Animals, at 23 °C with lights on between 1900 and 0700 hr. Animals were fed and watered ad libitum, and the woodchips in their cages changed every other day. Full-time trained animal care personnel performed all animal handling and maintenance. The directors of the Duquesne University and the University of Pittsburgh animal facilities and veterinarians supervised animal care. All animal care was overseen by the Institutional Animal Care and Use Committee (IACUC) within each university.
References
- Allen E, Carlson KM, Zigmond MJ, Cavanaugh JE. L-DOPA reverses motor deficits associated with normal aging in mice. Neurosci.Lett. 2011;489:1–4. doi: 10.1016/j.neulet.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros JC, Marshall CJ. Activation of either ERK1/2 or ERK5 MAP kinase pathways can lead to disruption of the actin cytoskeleton. J.Cell.Sci. 2005;118:1663–1671. doi: 10.1242/jcs.02308. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N.Engl.J.Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]
- Boger H, Middaugh L, Huang P, Zaman V, Smith A, Hoffer B, Tomac A, Granholm A. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp.Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Winblad B. Influence of age and time interval between death and autopsy on dopamine and 3-methoxytyramine levels in human basal ganglia. J.Neural Transm. 1976;38:271–276. doi: 10.1007/BF01249444. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Schindler FR, Höllerhage M, Depboylu C, Arias-Carrión O, Schnurrbusch S, Rösler TW, Wozny W, Schwall GP, Groebe K. Systemic administration of neuregulin-1β1 protects dopaminergic neurons in a mouse model of Parkinson’s disease. J.Neurochem. 2011;117:1066–1074. doi: 10.1111/j.1471-4159.2011.07284.x. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JE. Role of extracellular signal regulated kinase 5 in neuronal survival. Eur.J.Biochem. 2004;271:2056–2059. doi: 10.1111/j.1432-1033.2004.04131.x. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JE, Ham J, Hetman M, Poser S, Yan C, Xia Z. Differential regulation of mitogen-activated protein kinases ERK1/2 and ERK5 by neurotrophins, neuronal activity, and cAMP in neurons. J.Neurosci. 2001;21:434–443. doi: 10.1523/JNEUROSCI.21-02-00434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JE, Jaumotte JD, Lakoski JM, Zigmond MJ. Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response to oxidative stress. J.Neurosci.Res. 2006;84:1367–1375. doi: 10.1002/jnr.21024. [DOI] [PubMed] [Google Scholar]
- Cheung Y, Lau WK, Yu M, Lai CS, Yeung S, So K, Chang RC. Effects of all trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Di Benedetto B, Hitz C, Holter SM, Kuhn R, Vogt Weisenhorn DM, Wurst W. Differential mRNA distribution of components of the ERK/MAPK signalling cascade in the adult mouse brain. J.Comp.Neurol. 2007;500:542–556. doi: 10.1002/cne.21186. [DOI] [PubMed] [Google Scholar]
- Dickerson JW, Hemmerle AM, Numan S, Lundgren KH, Seroogy KB. Decreased expression of ErbB4 and tyrosine hydroxylase mRNA and protein in the ventral midbrain of aged rats. Neuroscience. 2009;163:482–489. doi: 10.1016/j.neuroscience.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson JW. Neuroprotective and neurorestorative effects of neuregulins in the injured and aged dopaminergic nigrostriatal system. 2010 [Google Scholar]
- Ding YM, Jaumotte JD, Signore AP, Zigmond MJ. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J.Neurochem. 2004;89:776–787. doi: 10.1111/j.1471-4159.2004.02415.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Teppen T, Sasaki N, Chen H, Zhang H, Roberts RC, Conley RR, Pandey GN. Aberrant extracellular signal-regulated kinase (ERK) 5 signaling in hippocampus of suicide subjects. Neuropsychopharmacology. 2007;32:2338–2350. doi: 10.1038/sj.npp.1301372. [DOI] [PubMed] [Google Scholar]
- Eriksen N, Stark AK, Pakkenberg B. Age and Parkinson's disease-related neuronal death in the substantia nigra pars compacta. J.Neural Transm.Suppl. 2009;(73):203–213. doi: 10.1007/978-3-211-92660-4_16. [DOI] [PubMed] [Google Scholar]
- Esparis-Ogando A, Diaz-Rodriguez E, Montero JC, Yuste L, Crespo P, Pandiella A. Erk5 participates in neuregulin signal transduction and is constitutively active in breast cancer cells overexpressing ErbB2. Mol.Cell.Biol. 2002;22:270–285. doi: 10.1128/MCB.22.1.270-285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Finegan KG, Wang X, Lee EJ, Robinson AC, Tournier C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ. 2009;16:674–683. doi: 10.1038/cdd.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CM, Gash DM, Smoot MK, Cass WA. Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res. 2001;896:56–63. doi: 10.1016/s0006-8993(00)03270-4. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Henson M, Zhang Z, Ovadia A, Hoffer BJ, Gash DM. Age-related changes in potassium-evoked overflow of dopamine in the striatum of the rhesus monkey. Neurobiol.Aging. 1995;16:939–946. doi: 10.1016/0197-4580(95)02013-6. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Iwashita T, Murakamai H, Kato Y, Kawai K, Kurokawa K, Tohnai I, Ueda M, Takahashi M. Activation of BMK1 via tyrosine 1062 in RET by GDNF and MEN2A mutation. Biochem.Biophys.Res.Commun. 2001;281:682–689. doi: 10.1006/bbrc.2001.4338. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Figueroa M, Bonilla S, Gutiérrez F, Pascual A, López-Barneo J. GDNF is predominantly expressed in the PV neostriatal interneuronal ensemble in normal mouse and after injury of the nigrostriatal pathway. 2012;32:864–872. doi: 10.1523/JNEUROSCI.2693-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JG, Cohen G, Mytilineou C. Basic fibroblast growth factor stimulation of glial cells protects dopamine neurons from 6-hydroxydopamine toxicity: involvement of the glutathione system. J.Neurochem. 1997;69:76–83. doi: 10.1046/j.1471-4159.1997.69010076.x. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeill T, Chan P, Forno LS, Murphy GM, Jr, Di Monte DA, Sandy MS, Langston JW. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann.Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases Identification and characterization of a signaling pathway to the nucleus. J.Biol.Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C, During M, Kholodilov N, Burke RE. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann.Neurol. 2011;70:110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Leak RK, Carlson KM, Smith AD, Zigmond MJ. Activation of the extracellular signal-regulated kinases 1 and 2 by glial cell line-derived neurotrophic factor and its relation to neuroprotection in a mouse model of Parkinson's disease. J.Neurosci.Res. 2008;86:2039–2049. doi: 10.1002/jnr.21641. [DOI] [PubMed] [Google Scholar]
- Liu L, Cavanaugh JE, Wang Y, Sakagami H, Mao Z, Xia Z. ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc.Natl.Acad.Sci.U.S.A. 2003;100:8532–8537. doi: 10.1073/pnas.1332804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cundiff P, Abel G, Wang Y, Faigle R, Sakagami H, Xu M, Xia Z. Extracellular signal-regulated kinase (ERK) 5 is necessary and sufficient to specify cortical neuronal fate. 2006;103:9697–9702. doi: 10.1073/pnas.0603373103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki JS. Aging and extrapyramidal function. Arch.Neurol. 1977;34:33. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- Mo L, Ren Q, Duchemin AM, Neff NH, Hadjiconstantinou M. GM1 and ERK signaling in the aged brain. Brain Res. 2005;1054:125–134. doi: 10.1016/j.brainres.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Mody N, Leitch J, Armstrong C, Dixon J, Cohen P. Effects of MAP kinase cascade inhibitors on the MKK5/ERK5 pathway. FEBS Lett. 2001;502:21–24. doi: 10.1016/s0014-5793(01)02651-5. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson's disease. Neurosci.Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- Morgan DG, May PC, Finch CE. Dopamine and serotonin systems in human and rodent brain: effects of age and neurodegenerative disease. J.Am.Geriatr.Soc. 1987;35:334–345. doi: 10.1111/j.1532-5415.1987.tb04641.x. [DOI] [PubMed] [Google Scholar]
- Obara Y, Yamauchi A, Takehara S, Nemoto W, Takahashi M, Stork PJ, Nakahata N. ERK5 activity is required for nerve growth factor-induced neurite outgrowth and stabilization of tyrosine hydroxylase in PC12 cells. J.Biol.Chem. 2009;284:23564–23573. doi: 10.1074/jbc.M109.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara Y, Nemoto W, Kohno S, Murata T, Kaneda N, Nakahata N. Basic fibroblast growth factor promotes glial cell-derived neurotrophic factor gene expression mediated by activation of ERK5 in rat C6 glioma cells. Cell.Signal. 2011;23:666–672. doi: 10.1016/j.cellsig.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Oyarce AM, Fleming PJ. Multiple forms of human dopamine β-hydroxylase in SHSY5Y neuroblastoma cells. Arch.Biochem.Biophys. 1991;290:503–510. doi: 10.1016/0003-9861(91)90573-2. [DOI] [PubMed] [Google Scholar]
- Pan YW, Zou J, Wang W, Sakagami H, Garelick MG, Abel G, Kuo CT, Storm DR, Xia Z. Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis. J.Biol.Chem. 2012 doi: 10.1074/jbc.M112.344762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R, López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat.Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pruett BS, Salvatore MF. GFR alpha-1 receptor expression in the aging nigrostriatal and mesoaccumbens pathways. J.Neurochem. 2010;115:707–715. doi: 10.1111/j.1471-4159.2010.06963.x. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Kobayashi Y, Takeuchi A, Pagès G, Pouysségur J, Kazama T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. 2011;31:1149–1155. doi: 10.1523/JNEUROSCI.2243-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res.Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Shalizi A, Lehtinen M, Gaudilliere B, Donovan N, Han J, Konishi Y, Bonni A. Characterization of a neurotrophin signaling mechanism that mediates neuron survival in a temporally specific pattern. J.Neurosci. 2003;23:7326–7336. doi: 10.1523/JNEUROSCI.23-19-07326.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GY, Kang JS, Lee SY, Myung CS. Region-specific reduction of Gbeta4 expression and induction of the phosphorylation of PKB/Akt and ERK1/2 by aging in rat brain. Pharmacol.Res. 2007;56:295–302. doi: 10.1016/j.phrs.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Tatake RJ, O’Neill MM, Kennedy CA, Wayne AL, Jakes S, Wu D, Kugler SZ, Kashem MA, Kaplita P, Snow RJ. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem.Biophys.Res.Commun. 2008;377:120–125. doi: 10.1016/j.bbrc.2008.09.087. [DOI] [PubMed] [Google Scholar]
- Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat.Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xie H, Hu L, Li G. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin.Med.J. 2010;123:1086–1092. [PubMed] [Google Scholar]
- Yoon SC, Ahn YM, Jun SJ, Kim Y, Kang UG, Park J, Kim YS. Regionspecific phosphorylation of ERK5–MEF2C in the rat frontal cortex and hippocampus after electroconvulsive shock. Prog.Neuro-Psychopharmacol.Biol.Psychiatry. 2005;29:749–753. doi: 10.1016/j.pnpbp.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Yue F, Zeng S, Wu D, Yi D, Zhang YA, Chan P. Age-related decline in motor behavior and striatal dopamine transporter in cynomolgus monkeys. J.Neural Transm. 2012;119:943–952. doi: 10.1007/s00702-012-0770-6. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Zou J, Pan YW, Wang Z, Chang SY, Wang W, Wang X, Tournier C, Storm DR, Xia Z. Targeted deletion of ERK5 MAP kinase in the developing nervous system impairs development of GABAergic interneurons in the main olfactory bulb and behavioral discrimination between structurally similar odorants. J.Neurosci. 2012;32:4118–4132. doi: 10.1523/JNEUROSCI.6260-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]