Abstract
Purpose
To describe the minimum inhibitory concentration (MIC) of fungal isolates to natamycin and voriconazole, and to compare these MICs to previous ocular susceptibility studies.
Design
Experimental laboratory study using isolates from a randomized clinical trial.
Methods
The Mycotic Ulcer Treatment Trial I was a randomized, double-masked, multicenter trial comparing topical natamycin and voriconazole for fungal keratitis treatment. Susceptibility testing to natamycin and voriconazole were performed according to Clinical and Laboratory Standards Institute methods. The relationship between organism and MIC was assessed. A literature review was performed to compare results to previous ocular susceptibility studies.
Results
Of the 323 patients enrolled in the trial, MICs were available for 221 (68%). Fusarium (N=126) and Aspergillus species (N=52) were the most commonly isolated organisms. MICs to natamycin and voriconazole were significantly different across all genera (P<0.001). The MIC median (MIC50) and 90th percentile (MIC90) for natamycin were equal to or higher than voriconazole for all organisms, except Curvularia species. Compared to other organisms, Fusarium species isolates had the highest MICs to voriconazole and A. flavus isolates had the highest MICs to natamycin. Our results were similar to previous reports except the voriconazole MIC90 against Aspergillus species was 2-fold higher and the natamycin MIC90 against A. fumigatus was 4-fold higher in our study.
Conclusion
In this large susceptibility study, Fusarium isolates were least susceptible to voriconazole and A. flavus isolates were least susceptible to natamycin when compared to other filamentous fungi. In the future, susceptibility testing may help guide therapy if performed in a timely manner.
INTRODUCTION
Fungal keratitis is a leading cause of visual impairment worldwide. It is endemic in tropical areas, such as South India, where up to half of all infectious keratitis cases are caused by fungus.1-3 Filamentous fungi, especially Fusarium species, are the predominant cause of fungal ulcers in tropical regions and are thought to be particularly virulent.4,5 Currently, fungal keratitis treatment is largely empirical, with no consensus on the role of susceptibility testing in guiding treatment decisions. Natamycin has long been considered the standard of care for filamentous fungal keratitis and is the only topical ophthalmic antifungal approved by the US Food and Drug Administration. However, newer azoles, including voriconazole, are reported to have good in vitro activity against most isolates from fungal ulcers, though there is mixed evidence regarding activity against Fusarium species.5,6
Antifungal susceptibility studies frequently use systemic isolates or focus on yeast. There are limited reports on filamentous fungi, likely due to the absence of established minimum inhibitory concentration (MIC) clinical breakpoints, which classify isolates as susceptible, intermediate, or resistant to an antimicrobial agent. Susceptibility studies investigating natamycin are also limited, as natamycin is used primarily for treating fungal keratitis.6-10 The ocular studies that are present often have small sample sizes5,11-13 or focus on one particular genus or species.8-10 Here, we report the in vitro activity of natamycin and voriconazole against filamentous fungal isolates collected as part of a large, randomized comparative trial on fungal keratitis treatment,14 and investigate the association between organism and MIC. Our relatively large sample size of isolates provides more precision in the estimation of the MIC median (MIC50) and 90th percentile (MIC90) than previously available. For comparison purposes, we also performed a literature review to identify ocular susceptibility studies on filamentous fungi using similar antifungals.
METHODS
The Mycotic Ulcer Treatment Trial I (MUTT I) was a randomized, double-masked trial comparing clinical outcomes of filamentous fungal keratitis in patients receiving 5% topical natamycin (Natacyn, Alcon, Fort Worth, TX) versus 1% topical voriconazole (VFEND IV, Pfizer, New York, NY).14 Detailed methods for MUTT I have been reported previously.14 In brief, we enrolled 323 patients with fungal keratitis who had presenting visual acuity of 0.3 logMAR (20/40) to 1.3 logMAR (20/400) at the Aravind Eye Care System (Madurai, Pondicherry, and Coimbatore) in India. The dosing schedules were identical in both treatment arms and consisted of 1 drop to the affected eye every 1 hour while awake for 1 week, then every 2 hours while awake until 3 weeks from enrollment.14 Continuation of the masked treatment was then at the discretion of the physician. For ethical reasons, physicians were allowed to add or change medications if deemed medically necessary. The MUTT I trial obtained informed consent from all patients, adhered to the Declaration of Helsinki, and received prospective Institutional Review Board (IRB) approval at Aravind, Dartmouth, and the University of California San Francisco (UCSF). MUTT is registered at Clinicaltrials.gov (NCT00996736).
Microbiology
Detailed microbiological methods have been described previously.6,7 In brief, corneal scrapings were obtained from all patients who were eligible for the trial, and Gram stains and potassium hydroxide (KOH) wet mounts were performed. Eligible patients were required to have a KOH wet mount positive for fungus and a Gram stain negative for bacteria at enrollment. Fungal cultures were determined to be positive if there was growth on two or more media, or if there was moderate to heavy growth on one medium. Fungal identification was performed using gross and microscopic characteristics, as described previously.7
All samples with a positive fungal culture had susceptibility testing to natamycin and voriconazole performed according to standardized methods outlined in the Clinical and Laboratory Standards Institute (CLSI) document M38-A2.15 Briefly, broth microdilutions were performed for susceptibility testing using Dimethyl sulfoxide (DMSO) as the drug diluent for voriconazole and natamycin.15 Aspergillus flavus ATCC20430 was included as a quality control isolate. MIC was defined as the lowest concentration that exhibited a 100% visual reduction in turbidity when compared with the control tube for natamycin at 48 hours, and an 80% reduction in turbidity for voriconazole.15 Only natamycin and voriconazole were tested since these were the treatments used in the clinical trial.
Statistical Analyses
Differences in clinical characteristics between isolates with MIC values and those without were analyzed using Student’s t-test for continuous variables, chi-square test or Fisher’s exact test for categorical variables, and log-rank test for time to reepithelialization. Multiple comparisons were corrected for using the Holm’s method in R 3.0.0 (R Foundation for Statistical Computing, www.R-project.org, Vienna, Austria). The lowest antibiotic concentration that inhibits bacterial growth is termed the MIC and concentrations that inhibit 50% (MIC50) or 90% (MIC90) The MIC50 and MIC90 were estimated using the PERCENTILE function in Microsoft Excel (Microsoft Inc, Redmond, Washington) and verified by hand to ensure accuracy. The MIC90 was estimated for organisms with at least 9 observations, the smallest number where extrapolation would not be necessary. The 95% confidence intervals (CI) for the MIC50 and MIC90 were estimated as bootstrap percentile confidence intervals in Mathematica 8 (Wolfram, Champaign, IL) for genus and species with at least 9 observations. Differences in MIC across groups of organisms were analyzed with one-way analysis of variance (ANOVA). For each genus and species, the MIC to natamycin was compared with the MIC to voriconazole using Wilcoxon signed-rank test. All statistical analyses were conducted using Stata 10.0 (StataCorp, College Station, Texas) unless otherwise specified.
Literature Search for Prior Susceptibility Studies
To identify studies reporting MIC data for ocular isolates tested against natamycin and voriconazole, searches were conducted in Web of Science (all dates up to 9/27/13) and PubMed (all dates up to 9/28/13) using the topic search terms (corneal ulcer or keratitis) and (fungal or fungus or fungi) and (susceptibility or susceptibilities). Titles and abstracts were screened to exclude ineligible studies. Eligible studies included those that used ocular fungal isolates and reported MICs, MIC50, MIC90 or MIC range for Aspergillus genus, A. flavus, A. fumigatus, Fusarium genus or F. solani against natamycin, voriconazole, or amphotericin B using CLSI protocols (M38-P, M38-A, or M38-A2). Excluded studies included review articles; studies using only systemic isolates, bacterial or parasitic isolates, or animal samples; non-English language studies; and articles without an accessible web link. In addition, the bibliographies of included studies were searched to identify additional studies.
RESULTS
Of the 323 patients enrolled in the trial, 256 samples (79%) had a positive fungal culture, and 221 (68%) had MIC results available and were included in the analysis. The 35 isolates that were fungal culture positive but missing MIC values had difficult growth during susceptibility testing. Identification of these 35 isolates revealed 13 (37%) unidentified hyaline organisms, 12 (34%) unidentified dematiaceous organisms, 3 (9%) Curvularia species, 2 (6%) Fusarium species, 2 (6%) Aspergillus species, 1 (3%) Alternaria species, 1 (3%) Exserophilum species, and 1 (3%) Lasodiplopia species. Table 1 shows the clinical characteristics of isolates with MIC values compared to those without. No statistically significant differences were seen in the baseline characteristics between the two groups. Among isolates with MIC values present, the most common genus was Fusarium (N=126, 57%) followed by Aspergillus (N=52, 24%).
Table 1.
Clinical Characteristics of Fungal Keratitis Isolates With and Without Minimum Inhibitory Concentration Values
| Characteristics | Isolates with MIC values (N=221) |
Isolates without MIC values (N=102) |
P-valuea |
|---|---|---|---|
| Treatment arm, N (%) | 0.50 | ||
| Natamycin | 108 (49) | 54 (53) | |
| Voriconazole | 113 (51) | 48 (47) | |
|
| |||
| At Presentation: | |||
|
| |||
| Age (years), median (IQR) | 46 (38-55) | 48 (40-60) | 0.21 |
| Gender, N (%) | 0.06 | ||
| Male | 133 (60) | 50 (49) | |
| Female | 88 (40) | 52 (51) | |
| Duration of symptoms (days), median (IQR) | 5 (3-9) | 7 (3-10) | 0.29 |
| Trauma, N (%) | 111 (50) | 50 (50) | 0.87 |
| Ocular surface disease, N (%) | 3 (1) | 0 (0) | 0.55 |
| Systemic inflammatory disease, N (%) | 14 (6) | 8 (8) | 0.64 |
| Fungal culture positive, N (%) | 221 (100) | 35 (34) | ND |
| Visual acuity (logMAR), mean (SD) | 0.71 (0.39) | 0.66 (0.38) | 0.29 |
| Infiltrate/Scar size (mm), mean (SD) | 3.35 (1.15) | 3.26 (1.26) | 0.56 |
|
| |||
| Clinical Outcomes: | |||
|
| |||
| 3-month visual acuity (logMAR), mean (SD) | 0.53 (0.64) | 0.37 (0.52) | 0.03 |
| 3-month scar size (mm), mean (SD) | 3.71 (1.66) | 3.33 (1.80) | 0.08 |
| Corneal perforations, N (%) | 41 (19) | 11 (11) | 0.08 |
| Epithelial defect healed, N (%)b | 136 (62) | 71 (70) | 0.16 |
| Time to reepithelialization (days), median (IQR)b | 15.5 (7-21) | 9.5 (2.5-21) | 0.15 |
Abbreviations: N, number; ND, not determined; MIC, minimum inhibitory concentration; SD, standard deviation; IQR, interquartile range; logMAR, logarithm of the minimum angle of resolution.
Used Holm method to correct for multiple comparisons. The adjusted significance level threshold started at P≤0.004.
Right censored at 21 days
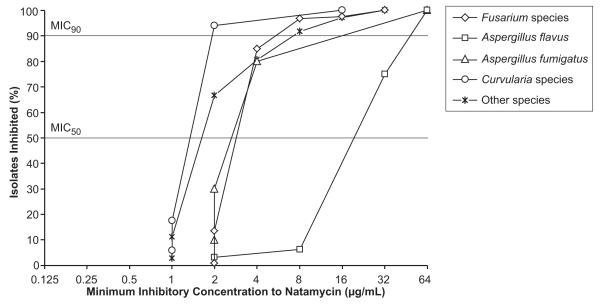
The MIC50 and MIC90 for natamycin were equal to or higher than those for voriconazole for all organisms except Curvularia species, which had a higher MIC90 for voriconazole (Table 2). For Aspergillus isolates, the MICs for natamycin were significantly higher than those for voriconazole (P<0.001). In particular, A. flavus isolates had the highest MICs to natamycin relative to other organisms (Figure, top panel). Fusarium isolates had the highest MICs to voriconazole compared to other organisms (Figure, bottom panel).
Table 2.
Natamycin and Voriconazole Minimum Inhibitory Concentration Values From a Fungal Keratitis Clinical Trial
| Organism | N | Natamycin (μg/mL) | Voriconazole (μg/mL) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MIC50 (95% CI)b |
MIC90a (95% CI)b |
MIC Range | MIC50 (95% CI)b |
MIC90a (95% CI)b |
MIC Range | ||
| Identified hyaline | 178 | 4 | 32 | 1 to 64 | 2 | 8 | 0.25 to 16 |
| Fusarium species | 126 | 4 (4 to 4) | 8 (4 to 8) | 2 to 32 | 4 (4 to 4) | 8 (8 to 16) | 0.25 to 16 |
| Aspergillus species | 52 | 32 (16 to 32) | 64 (32 to 64) | 1 to 64 | 0.5 (0.5 to 0.5) | 2 (1-4) | 0.25 to 8 |
| A. flavus | 32 | 32 (32 to 32) | 64 (32 to 64) | 2 to 64 | 0.5 (0.5 to 0.5) | 2 (1-4) | 0.25 to 8 |
| A. fumigatus | 10 | 4 (2 to 4) | 64 (4 to 64) | 2 to 64 | 0.5 (0.25 to 0.5) | 1.3 (0.5 to 4) | 0.25 to 4 |
| A. niger | 2 | 5 | ND | 2 to 8 | 3 | ND | 2 to 4 |
| A. terreus | 3 | 16 | ND | 8 to 16 | 0.5 | ND | 0.5 to 0.5 |
| Other | 5 | 4 | ND | 1 to 32 | 0.5 | ND | 0.5 to 2 |
|
Unidentified
hyaline |
4 | 4 | ND | 2 to 8 | 0.75 | ND | 0.03 to 8 |
|
| |||||||
|
Identified
dematiaceous |
34 | 2 | 2 | 1 to 16 | 0.5 | 2 | 0.25 to 8 |
| Curvularia species | 17 | 2 (2 to 2) | 2 (2 to 16) | 1 to 16 | 0.25 (0.25 to 0.5) | 4 (0.5 to 8) | 0.25 to 8 |
|
Exserohilum species |
8 | 2 | ND | 1 to 2 | 1 | ND | 0.5 to 2 |
| Alternaria species | 2 | 2 | ND | 2 to 2 | 1 | ND | 1 to 1 |
| Bipolaris species | 4 | 2 | ND | 2 to 2 | 0.25 | ND | 0.25 to 1 |
|
Lasiodiplodia species |
3 | 2 | ND | 2 to 2 | 0.5 | ND | 0.5 to 2 |
|
Unidentified
dematiaceous |
3 | 4 | ND | 2 to 4 | 1 | ND | 0.25 to 2 |
| Other species | 2 | 2 | ND | 2 to 2 | 8.25 | ND | 0.5 to 16 |
|
| |||||||
| Total | 221 | 4 | 32 | 1 to 64 | 2 | 8 | 0.03 to 16 |
| P-Value | <0.001c | <0.001c | |||||
Abbreviations: N, number; ND, not determined; MIC, minimum inhibitory concentration; CI, confidence interval; MIC50, minimum inhibitory concentration median; MIC90, minimum inhibitory concentration 90th percentile.
MIC90 estimated for genus or species with at least 9 isolates, the smallest number where extrapolation would not be necessary.
Estimation of 95% CI only performed for genus or species with at least 9 isolates
Analysis of variance test (ANOVA) comparing MIC values among genera
Based upon our search criteria, 21 studies were found to have explored in vitro antifungal susceptibility patterns to natamycin, voriconazole, or amphotericin B using ocular Fusarium or Aspergillus isolates (Table 3).6-13,16-28 These studies used different methods to report MIC (MIC50, MIC90, and range) and demonstrated variable MICs. The natamycin and voriconazole MIC50 and MIC90 found in our study for Fusarium isolates were within the range of published values for F. solani and for all Fusarium species. Among Aspergillus species, our natamycin MIC values for A. flavus were consistent with previous study results, but the MIC90 for A. fumigatus was 4-fold higher than previous reports. Our voriconazole MIC90 against Aspergillus species exceeded values reported in the ophthalmic literature by 2-fold.
Table 3.
Prior Ocular Susceptibility Studies With Minimum Inhibitory Concentrations to Natamycin, Voriconazole or Amphotericin B against Fusarium and Aspergillus species
| Studya | Species (Number of Isolates) |
Natamycin (μg/mL) | Voriconazole (μg/mL) | Amphotericin B (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | ||
| Debourgogne et al., 201222 |
F. solani (48) | – | – | – | – | 8 | 1 to 16 | – | 2 | 0.13 to 2 |
|
| ||||||||||
| Edelstein et al., 201223 | F. solani (1) | – | – | 16 | – | – | 8 | – | – | 4 |
|
| ||||||||||
| Giaconi et al., 200616 | Fusarium spp. (1) | – | – | – | – | – | 8 | – | – | – |
|
| ||||||||||
| Homa et al., 201317 | Fusarium spp. (60) | 8c | >64c | 2 to >64 | 64c | >64c | 0.13 to >64 | 16c | >64c | 0.13 to >64 |
| F. solani (43) | 8c | >64c | 2 to >64 | 64c | >64c | 0.13 to >64 | 8c | 64c | 0.13 to >64 | |
| F. non-solani (17) | 8c | >64c | 2 to >64 | 64c | >64c | 16 to >64 | 16c | >64c | 4 to >64 | |
|
| ||||||||||
| Iqbal et al., 20089 | Fusarium spp. (85) | – | – | 4 to 16 | – | – | 0.5 to >8 | – | – | 0.5 to >8 |
| F. solani (57) | 8 | – | 4 to 16 | >8 | – | 8 to >8 | 2 | – | 0.5 to >8 | |
| F. non-solani (35) | – | – | 4 to 8 | – | – | 0.5 to >8 | – | – | 1 to 4 | |
|
| ||||||||||
| Kondori et al., 201118 | Fusarium spp. (5) | – | – | – | – | – | 0.25 to >8 | – | – | – |
| F. solani (1) | – | – | – | – | – | −>8 | – | – | – | |
| F. non-solani (4) | – | – | – | – | – | 0.25 to 1 | – | – | – | |
|
| ||||||||||
| Lalitha et al., 20076 | Fusarium spp. (38) | 8 | 16 | – | 2 | 4 | – | 4 | 4 | – |
|
| ||||||||||
| Lalitha et al., 200819 | Fusarium spp. (41) | 4 | 4 | 2 to 8 | – | – | – | – | – | – |
|
| ||||||||||
| Lalitha et al., 20127 | Fusarium spp. (44) | 4 | 8 | 2 to 16 | 4 | 16 | 2 to 16 | – | – | – |
|
| ||||||||||
| Li et al., 200820 | Fusarium spp. (38) | 4 | 16 | 2 to 16 | – | – | – | 4 | 4 | 2 to 16 |
|
| ||||||||||
| Oechsler et al., 20138 | F. solani (NAT 44, VRC 15, AMB 43) |
4.8 | 4.8 | 2.4 to 9.6 | 16 | 16 | 4 to ≥16 | 2 | 2 | 1 to ≥16 |
| F. non-solani (NAT 14, VRC 12, AMB 14) |
2.4 | 4.8 | 2.4 to 4.8 | 4 | 4 | 2 to ≥16 | 2 | 2 | 1 to 2 | |
|
| ||||||||||
| Ozdemir et al., 201213 | Fusarium spp. (9) | – | – | – | 4c | 8c | 1 to 8 | 1c | 2c | 0.5 to 2 |
| F. solani (2) | – | – | – | 4c | – | 4 to 8 | 1c | – | 1 to 2 | |
| F. non-solani (7) | – | – | – | 4c | – | 1 to 4 | 0.5c | – | 0.5 to 2 | |
|
| ||||||||||
| Pradhan et al., 201112, d | Fusarium spp. (15) | – | – | 4 to 8 | – | – | – | – | – | – |
|
| ||||||||||
| Shapiro et al., 201011 | Fusarium spp. (23) | 8 | 16 | 4 to 16 | – | – | – | – | – | – |
|
| ||||||||||
| Sponsel et al., 200224, b | F. solani (1) | – | – | 32 | – | – | – | – | – | 2 |
|
| ||||||||||
| Taylan et al., 201225 | F. solani (1) | – | – | – | – | – | 8 | – | – | 0.5 |
|
| ||||||||||
| Tu et al., 200726 | F. solani (1) | – | – | – | – | – | 8 | – | – | 4 |
|
| ||||||||||
| Wang et al., 200921 | Fusarium spp. (71) | – | – | – | – | – | – | 0.5 | 1 | 0.06 to 1 |
|
| ||||||||||
| Xie et al., 200827 | F. solani (34) | – | – | – | – | – | – | 1 | 2 | – |
|
| ||||||||||
| Total Range | Fusarium spp. | 4 to 8 |
4 to
>64 |
2 to >64 |
2 to
64 |
4 to
>64 |
0.13 to >64 |
0.5 to
16 |
1 to
>64 |
0.06 to
>64 |
|
| ||||||||||
| F. solani |
4.8 to
8 |
4.8 to
>64 |
2 to >64 |
4 to
64 |
8 to
>64 |
0.13 to >64 | 1 to 8 |
2 to
64 |
0.13 to
>64 |
|
|
| ||||||||||
| F. non-solani |
2.4 to
8 |
4.8 to
>64 |
2 to >64 |
4 to
64 |
4 to
>64 |
0.25 to >64 |
0.5 to
16 |
2 to
>64 |
0.5 to >64 | |
|
| ||||||||||
| Kondori et al., 201118 | Aspergillus spp. (29) | – | – | – | – | – | 0.13 to 8 | – | – | – |
| A. fumigatus (23) | – | – | – | – | – | 0.13 to 8 | – | – | – | |
|
| ||||||||||
| Lalitha et al., 20076 | Aspergillus spp. (41) | 32 | >32 | – | 0.25 | 0.5 | – | 2 | 4 | – |
|
| ||||||||||
| Lalitha et al., 200819 | Aspergillus spp. (59) | – | – | 1 to 64 | – | – | – | – | – | – |
| A. flavus (32) | 32 | 64 | 8 to 64 | – | – | – | – | – | – | |
| A. fumigatus (18) | 4 | 4 | 1 to 4 | – | – | – | – | – | – | |
|
| ||||||||||
| Lalitha et al., 20127 | Aspergillus spp. (17) | 32 | 32 | 2 to 32 | 1 | 1 | 0.13 to 2 | – | – | – |
| A. flavus (11) | 32 | 32 | – | 1 | 1 | – | – | – | – | |
| A. fumigatus (5) | 4 | 8 | – | 0.5 | 0.5 | – | – | – | – | |
|
| ||||||||||
| Manikandan et al., 201310 |
A. flavus (74) | 128 | 128 | 4 to 128 | 0.5 | 1 | 0.25 to 1 | 2 | 8 | 0.5 to 16 |
| A. fumigatus (14) | 4 | 16 | 4 to 64 | 0.5 | 0.5 | 0.1 to 1 | 0.5 | 1 | 0.25 to 1 | |
|
| ||||||||||
| Nayak et al., 201128 | A. flavus (64) | – | – | – | – | – | – | 3.12 | 12.5 | 0.03 to 25 |
| A. fumigatus (43) | – | – | – | – | – | – | 3.12 | 3.12 | 0.03 to 12.5 |
|
|
| ||||||||||
| Pradhan et al., 201112, d | Aspergillus spp. (24) | – | – | 2 to 32 | – | – | – | – | – | – |
|
| ||||||||||
| A. flavus (13) | – | – | 8 to 32 | – | – | – | – | – | – | |
| A. fumigatus (9) | – | – | 2 to 8 | – | – | – | – | – | – | |
|
| ||||||||||
| Shapiro et al., 201011 | Aspergillus spp. (24) | 32 | 64 | 8 to 64 | – | – | – | – | – | – |
| A. flavus (18) | 32 | 64 | 16 to 64 | – | – | – | – | – | – | |
| A. fumigatus (1) | – | – | 8 | – | – | – | – | – | – | |
|
| ||||||||||
| Wang et al., 200921 | Aspergillus spp. (15) | – | – | – | – | – | – | 1 | 1 | 0.25 to 1 |
|
| ||||||||||
| Xie et al., 200827 | A. flavus (9) | – | – | – | – | – | – | 2 | 4 | – |
| A. fumigatus (9) | – | – | – | – | – | – | 1 | 2 | – | |
|
| ||||||||||
| Total Range | Aspergillus spp. | 32 |
32 to
64 |
1 to 64 |
0.25
to 1 |
0.5 to
1 |
0.13 to 8 | 1 to 2 | 1 to 4 | 0.25 to 1 |
|
| ||||||||||
| A. flavus |
32 to
128 |
32 to
128 |
4 to 128 |
0.5 to
1 |
1 | 0.25 to 1 |
2 to
3.12 |
4 to
12.5 |
0.03 to 25 | |
|
| ||||||||||
| A. fumigatus | 4 |
4 to
16 |
1 to 64 | 0.5 | 0.5 | 0.1 to 8 |
0.5 to
3.12 |
1 to
3.12 |
0.03 to
12.5 |
|
Abbreviations: N, number; MIC, minimum inhibitory concentration; MIC50, minimum inhibitory concentration median; MIC90, minimum inhibitory concentration 90th percentile; spp., species; NAT, natamycin; VRC, voriconazole; AMB, amphotericin B.
Susceptibility testing was performed using Clinical and Laboratory Standards Institute (CLSI) documents M38-P, M38-A, or M38-A2, except as noted.
Susceptibility testing method was not specified.
Estimated MIC50 or MIC90 using raw data provided in the article.
Used natamycin eye drop (Sun Pharmaceutical Ind., Ltd, Mumbai, India) for susceptibility testing instead of standard natamycin pharmaceutical-grade powder.
DISCUSSION
In this study, we investigated the in vitro activity of natamycin and voriconazole against 221 patient isolates from a fungal keratitis clinical trial and compared our findings to prior ocular susceptibility studies. Overall, organisms in our study had lower MICs to voriconazole than natamycin, though MICs were significantly different across all genera. Relative to other organisms, A. flavus isolates appeared least susceptible (highest MICs) to natamycin, while Fusarium isolates were least susceptible to voriconazole. The current literature has few studies on susceptibility testing of filamentous fungi against natamycin and voriconazole. The relatively large sample size of this study allows precision in the estimation of the MIC50 and MIC90 for Fusarium and Aspergillus species in particular, crucial for determining if antifungal susceptibility testing can actually optimize treatment decisions.
We found that Fusarium isolates had the same MIC50 and MIC90 for natamycin and voriconazole, though the estimated 95% CI for the MIC90 was higher for voriconazole than natamycin, respectively (8 to 16 vs 4 to 8, Table 2). Relative to other genera, Fusarium isolates had lower MICs to natamycin and higher MICs to voriconazole. These observations suggest that Fusarium isolates may have increased susceptibility to natamycin and decreased susceptibility to voriconazole, compared with other filamentous fungi. Two previous studies found similar results for Fusarium cases.6,7 These results may be clinically relevant since Fusarium ulcers have been shown to have poor clinical outcome with voriconazole compared to natamycin.14,29 In MUTT I, natamycin-treated cases performed significantly better than voriconazole-treated cases, largely due to improvement in Fusarium ulcers.14 Subgroup analysis of Fusarium cases showed 4-line improvement in 3-month visual acuity with natamycin treatment compared to voriconazole treatment.14
Reports suggest F. solani isolates are more resistant to antifungals and have worse outcomes than other Fusarium species isolates.8,9,18,30 In the Mycotic Ulcer Therapeutic Exploratory Trial (N=120) performed in preparation for MUTT, Fusarium speciation was performed using DNA sequencing (Lalitha P, written communication, 9/2013).31 The vast majority of Fusarium isolates were F. solani (N=39/43, 91%), followed by Giberrela fujikuroi (N=3/43, 7%) and F. dimerum (N=1/43, 2%) (Lalitha P, written communication, 9/2013). The MIC50 to voriconazole for F. solani was 3 μg/mL compared to 1 μg/mL for G. fujikuroi and F. dimerum (Lalitha P, written communication, 9/2013).8 The MIC50 to natamcyin was 2 μg/mL for F. solani, 3 μg/mL for G. fujikuroi, and 1 μg/mL for F. dimerum (Lalitha P, written communication, 9/2013). Since the majority of ulcers were due to F. solani, it was difficult to make substantial comparisons among Fusarium species regarding susceptibility. While reports have shown that F. solani isolates have higher levels of resistance to voriconazole than F. non-solani isolates (Table 3),8, 9, 18 our exploratory trial results found minimal difference between the strains. Since MUTT participants came from the same population as the exploratory trial, we expected the breakdown of Fusarium species to be similar with F. solani in the majority. As such, we did not speciate Fusarium isolates in MUTT.
For A. flavus and A. fumigatus, natamycin MICs were significantly higher than voriconazole MICs. Interestingly, A. flavus had the highest MIC to natamycin compared to other genera and species, including A. fumigatus. Four studies have reported similar findings regarding A. flavus.7,10,12,32 From our results, it is likely that A. flavus isolates have decreased susceptibility to natamycin. It is not known if this finding of decreased susceptibility is inherent to the species or an emerging resistance. To the best of our knowledge, no report of A. flavus resistance to natamycin has been described to date. In MUTT I, Aspergillus cases had better clinical outcomes with voriconazole treatment than natamycin treatment, though this was not significant (Sun CQ, written communication, 4/2013). Other studies have also shown that voriconazole treatment is efficacious against Aspergillus ulcers, while natamycin treatment has poor efficacy.12,33, 34 Prior in vitro susceptibility studies have demonstrated that A. flavus and A. fumigatus have MICs that are highest for natamycin, followed by amphotericin B, and then voriconazole, confirming that voriconazole may be most effective against Aspergillus species (Table 3).6,7,10-12,18,19,21,27,28
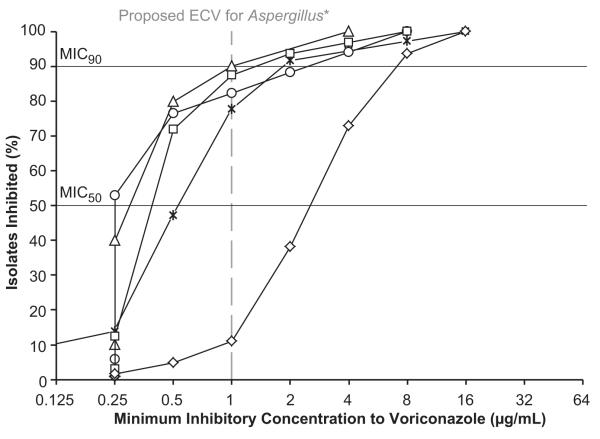
The absence of established MIC clinical breakpoints for antifungals against all filamentous fungi makes the interpretation of MIC data challenging.35 Breakpoints are the MIC values used to classify isolates as susceptible, intermediate, or resistant to antimicrobials. The CLSI has not established breakpoints for filamentous fungi, but the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has for voriconazole against A. fumigatus.36,37 In the absence of breakpoints, systemic epidemiological cut-off values (ECVs) have been proposed for voriconazole and Aspergillus species using CLSI methodology.38-40 The upper limit of the MIC distribution for the wild-type population (strains that exhibit no acquired resistance to the drug in question) is defined as the ECV.41 The ECV should encompass at least 95% of isolates in the wild-type distribution.41 Non-wild-type isolates have MICs greater than the ECV and may have acquired resistance mechanisms.41 Using the proposed CLSI ECV for voriconazole against A. flavus (1μg/ml) and A. fumigatus (1μg/ml),38,39 we found that the percentage of non-wild-type isolates in our study were 12.5% (N=4/32) and 10% (N=1/10), respectively, more than had been noted in previous systemic reports (Figure 1B).38,41,42 Since the proposed ECVs were determined and tested using systemic isolates, it is not known if they are different for ocular isolates. Further studies using ocular isolates are necessary to confirm these findings.
FIGURE. Percentage of Fungal Isolates Inhibited at Different Drug Concentrations in a Fungal Keratitis Clinical Trial.
Percentage of fungal isolates inhibited at increasing concentrations of natamycin (Top) or voriconazole (Bottom). Organisms include Fusarium species (N=126), Aspergillus flavus (N=32), Aspergillus fumigatus (N=10), Curvularia species (N=17), and all other fungal species (N=36). Horizontal black lines represent the threshold for the minimum inhibitory concentration median (MIC50) and 90th percentile (MIC90). *Vertical grey dashed line (Bottom) represents the proposed epidemiological cut-off value (ECV) of 1μg/mL using Clinical and Laboratory Standards Institute methodology for A. flavus and A. fumigatus against voriconazole.38, 39 The ECV distinguishes wild-type strains (exhibit no acquired resistance to the drug in question) from non-wild-type strains.41 Non-wild-type strains have MICs greater than the ECV and may have acquired resistance mechanisms.41
Conclusions about treatments for fungal keratitis using in vitro susceptibility results have certain limitations. As discussed, standardized systemic or topical breakpoints using CLSI methodology are not yet available for antifungals against filamentous fungi. This limitation makes it challenging to correlate studies with clinical outcome, as can be done in bacterial infections.43 In addition, while organisms overall had lower MICs to voriconazole than natamycin, the concentration of natamycin used clinically (5%) is typically 5-fold higher than that of voriconazole (1%). More information about the actual therapeutic corneal concentration and bioavailability of the antifungals would aid in the interpretation of MIC results.
This is a large filamentous fungal susceptibility study using ocular isolates from keratitis cases in South India. We describe the in vitro activity of natamycin and voriconazole against filamentous fungal isolates and compare our findings to prior ocular susceptibility studies. Overall, organisms had lower MICs to voriconazole than natamycin, though MICs were significantly different across genera. Specifically, Fusarium isolates were less susceptible to voriconazole, relative to other organisms. A. flavus isolates appeared to have lower susceptibility to natamycin compared to other organisms. In vitro susceptibility testing may help guide treatment decisions when performed in a timely manner. The establishment of MIC clinical breakpoints for filamentous fungi will likely help facilitate this practice.
Acknowledgements
A) Funding/Support: This work was supported by the following grants: National Eye Institute U10EY018573 (TML) and K23 EY017897 (NRA); That Man May See (NRA, SDM, TML); the Harper/Inglis Trust (TML); The South Asia Research Foundation (TML); Research to Prevent Blindness (NRA, TML); and the UCSF Academic Senate Committee on Research (Sun). Natamycin and voriconazole were donated by Alcon and Pfizer, respectively. The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
D) Other Acknowledgements: none
Appendix. Mycotic Ulcer Treatment Trial Group
Clinical Centers
Aravind Eye Hospital, Madurai, Tamil Nadu, India: N. Venkatesh Prajna, MD (principal investigator), Prajna Lalitha, MD, Jeena Mascarenhas, MD, Muthiah Srinivasan, MD, Thirukkonda Subramanian Chandravathi, MA, R. Somu Saravanan, MA, Rajarathinam Karpagam, Malaiyandi Rajkumar, Rajendran Mahalakshmi, MSc, S.R. Sumithra, and Charles Sundar; Aravind Eye Hospital, Coimbatore, Tamil Nadu, India: Revathi Rajaraman,MD (site director), Anita Raghavan, MD, and P. Manikandan, MPhil; Aravind Eye Hospital, Pondicherry, Tamil Nadu, India: Tiruvengada Krishnan, MD (site director), and N. Shivananada, MD; Francis I. Proctor Foundation, University of California, San Francisco: Thomas M. Lietman, MD (principal investigator), Nisha R. Acharya, MD, MS (principal investigator), Stephen D. McLeod, MD, John P. Whitcher, MD, MPH, Salena Lee, OD, Vicky Cevallos, MT (ASCP), Catherine E. Oldenburg, MPH, Kieran S. O’Brien, MPH, and Kevin C. Hong, BA.
Data and Safety Monitoring Committee
Marian Fisher, PhD (chair), Anthony Aldave, MD, Donald F. Everett, MA, Jacqueline Glover, PhD, K. Ananda Kannan, MD, Steven Kymes, PhD, and Ivan Schwab, MD.
Resource Centers
Coordinating Center, Francis I. Proctor Foundation, University of California, San Francisco: Thomas M. Lietman, MD (principal investigator), Nisha R. Acharya, MD, MS (principal investigator), David Glidden, PhD, Stephen D. McLeod, MD, John P. Whitcher, MD, MPH, Salena Lee, OD, Kathryn J. Ray, MA, Vicky Cevallos, MT (ASCP), Catherine E.Oldenburg, MPH, Kevin C. Hong, BA, Kieran S. O’Brien, MPH; Project Office, National Eye Institute, Rockville, Maryland: Donald F. Everett, MA; Photography Reading Center, Dartmouth Medical School, Lebanon, New Hampshire: Michael E. Zegans, MD, and Christine M. Kidd, PhD.
Footnotes
B) Financial Disclosures: Dr. Nisha Acharya served as a consultant to Xoma and Santan.
C) Contributions to Authors in each of these areas: Design and conception of the study (PL, NVP, SDM, NRA, TML); Analysis and interpretation of data (PL, CQS, KSO, VC, SDM, TML); Writing the manuscript (PL, CQS); Critical revision of the article (All authors); Final approval of the article (All authors); Data collection (PL, RK, GM); Provision of materials, patients or resources (PL, NVP); Statistical expertise (TML); Obtaining funding (NVP, SDM, NRA, TML); Literature search (CQS).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81(11):965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15(4):321–327. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 4.O’Day DM. Selection of appropriate antifungal therapy. Cornea. 1987;6(4):238–245. doi: 10.1097/00003226-198706040-00002. [DOI] [PubMed] [Google Scholar]
- 5.Marangon FB, Miller D, Giaconi JA, Alfonso EC. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol. 2004;137(5):820–825. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 6.Lalitha P, Shapiro BL, Srinivasan M, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol. 2007;125(6):789–793. doi: 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]
- 7.Lalitha P, Prajna NV, Oldenburg CE, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31(6):662–667. doi: 10.1097/ICO.0b013e31823f8ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oechsler RA, Feilmeier MR, Miller D, Shi W, Hofling-Lima AL, Alfonso EC. Fusarium Keratitis: Genotyping, In Vitro Susceptibility and Clinical Outcomes. Cornea. 2013;32(5):667–673. doi: 10.1097/ICO.0b013e318277ac74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal NJ, Boey A, Park BJ, Brandt ME. Determination of in vitro susceptibility of ocular Fusarium spp. isolates from keratitis cases and comparison of Clinical and Laboratory Standards Institute M38-A2 and E test methods. Diagn Microbiol Infect Dis. 2008;62(3):348–350. doi: 10.1016/j.diagmicrobio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Manikandan P, Varga J, Kocsube S, et al. Epidemiology of Aspergillus keratitis at a tertiary care eye hospital in South India and antifungal susceptibilities of the causative agents. Mycoses. 2013;56(1):26–33. doi: 10.1111/j.1439-0507.2012.02194.x. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro BL, Lalitha P, Loh AR, et al. Susceptibility testing and clinical outcome in fungal keratitis. Br J Ophthalmol. 2010;94(3):384–385. doi: 10.1136/bjo.2009.158675. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan L, Sharma S, Nalamada S, Sahu SK, Das S, Garg P. Natamycin in the treatment of keratomycosis: correlation of treatment outcome and in vitro susceptibility of fungal isolates. Indian J Ophthalmol. 2011;59(6):512–514. doi: 10.4103/0301-4738.86328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozdemir HG, Oz Y, Ilkit M, Kiraz N. Antifungal susceptibility of ocular fungal pathogens recovered from around the world against itraconazole, voriconazole, amphotericin B, and caspofungin. Med Mycol. 2012;50(2):130–135. doi: 10.3109/13693786.2011.584328. [DOI] [PubMed] [Google Scholar]
- 14.Prajna NV, Krishnan T, Mascarenhas J, et al. The Mycotic Ulcer Treatment Trial: A Randomized Trial Comparing Natamycin vs Voriconazole. JAMA Ophthalmol. 2013;131(4):422–429. doi: 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 2nd ed. CLSI; Wayne, PA: 2008. Approved standard M38-A2. [Google Scholar]
- 16.Giaconi JA, Marangon FB, Miller D, Alfonso EC. Voriconazole and fungal keratitis: a report of two treatment failures. J Ocul Pharmacol Ther. 2006;22(6):437–439. doi: 10.1089/jop.2006.22.437. [DOI] [PubMed] [Google Scholar]
- 17.Homa M, Shobana CS, Singh YR, et al. Fusarium keratitis in South India: causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses. 2013;56(5):501–511. doi: 10.1111/myc.12062. [DOI] [PubMed] [Google Scholar]
- 18.Kondori N, Svensson E, Mattsby-Baltzer I. In vitro susceptibility of filamentous fungi to itraconazole, voriconazole and posaconazole by Clinical and Laboratory Standards Institute reference method and E-test. Mycoses. 2011;54(5):E318–E322. doi: 10.1111/j.1439-0507.2010.01913.x. [DOI] [PubMed] [Google Scholar]
- 19.Lalitha P, Vijaykumar R, Prajna NV, Fothergill AW. In vitro natamycin susceptibility of ocular isolates of Fusarium and Aspergillus species: comparison of commercially formulated natamycin eye drops to pharmaceutical-grade powder. J Clin Microbiol. 2008;46(10):3477–3478. doi: 10.1128/JCM.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Wang Z, Li R, Luo S, Sun X. In vitro evaluation of combination antifungal activity against Fusarium species isolated from ocular tissues of keratomycosis patients. Am J Ophthalmol. 2008;146(5):724–728. doi: 10.1016/j.ajo.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol. 2009;37(8):763–771. doi: 10.1111/j.1442-9071.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 22.Debourgogne A, de Hoog S, Lozniewski A, Machouart M. Amphotericin B and voriconazole susceptibility profiles for the Fusarium solani species complex: comparison between the E-test and CLSI M38-A2 microdilution methodology. Eur J Clin Microbiol Infect Dis. 2012;31(4):615–618. doi: 10.1007/s10096-011-1323-x. [DOI] [PubMed] [Google Scholar]
- 23.Edelstein SL, Akduman L, Durham BH, Fothergill AW, Hsu HY. Resistant Fusarium keratitis progressing to endophthalmitis. Eye Contact Lens. 2012;38(5):331–335. doi: 10.1097/ICL.0b013e318235c5af. [DOI] [PubMed] [Google Scholar]
- 24.Sponsel WE, Graybill JR, Nevarez HL, Dang D. Ocular and systemic posaconazole(SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br J Ophthalmol. 2002;86(7):829–830. doi: 10.1136/bjo.86.7.829-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylan Sekeroglu H, Erdem E, Yagmur M, et al. Successful medical management of recalcitrant Fusarium solani keratitis: molecular identification and susceptibility patterns. Mycopathologia. 2012;174(3):233–237. doi: 10.1007/s11046-012-9542-y. [DOI] [PubMed] [Google Scholar]
- 26.Tu EY, McCartney DL, Beatty RF, Springer KL, Levy J, Edward D. Successful treatment of resistant ocular fusariosis with posaconazole (SCH-56592) Am J Ophthalmol. 2007;143(2):222–227. doi: 10.1016/j.ajo.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 27.Xie L, Zhai H, Zhao J, Sun S, Shi W, Dong X. Antifungal susceptibility for common pathogens of fungal keratitis in Shandong Province, China. Am J Ophthalmol. 2008;146(2):260–265. doi: 10.1016/j.ajo.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Nayak N, Satpathy G, Prasad S, Titiyal JS, Pandey RM, Vajpayee RB. Molecular characterization of drug-resistant and drug-sensitive Aspergillus isolates causing infectious keratitis. Indian J Ophthalmol. 2011;59(5):373–377. doi: 10.4103/0301-4738.83614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prajna VN, Lalitha PS, Mascarenhas J, et al. Natamycin and voriconazole in Fusarium and Aspergillus keratitis: subgroup analysis of a randomised controlled trial. Br J Ophthalmol. 2012;96(11):1440–1441. doi: 10.1136/bjophthalmol-2012-301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones BR. Principles in the management of oculomycosis. XXXI Edward Jackson memorial lecture. Am J Ophthalmol. 1975;79(5):719–751. doi: 10.1016/0002-9394(75)90730-8. [DOI] [PubMed] [Google Scholar]
- 31.Prajna NV, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128(6):672–678. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalitha P, Prajna NV, Kabra A, Mahadevan K, Srinivasan M. Risk factors for treatment outcome in fungal keratitis. Ophthalmology. 2006;113(4):526–530. doi: 10.1016/j.ophtha.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 33.Rahman MR, Johnson GJ, Husain R, Howlader SA, Minassian DC. Randomised trial of 0.2% chlorhexidine gluconate and 2.5% natamycin for fungal keratitis in Bangladesh. Br J Ophthalmol. 1998;82(8):919–925. doi: 10.1136/bjo.82.8.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson EM, Szekely A, Warnock DW. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J Antimicrob Chemother. 1998;42(6):741–745. doi: 10.1093/jac/42.6.741. [DOI] [PubMed] [Google Scholar]
- 35.Espinel-Ingroff A, Canton E, Peman J. Updates in antifungal susceptibility testing of filamentous fungi. Curr Fungal Infect Rep. 2009;3(3):133–141. [Google Scholar]
- 36.European Committee on Antimicrobial Susceptibility Testing [Accessed May 4, 2013];Voriconazole: Rationale for the clinical breakpoints. (version 1.0). 2012 http://www.eucast.org.
- 37.Hope WW, Cuenca-Estrella M, Lass-Florl C, Arendrup MC. EUCAST Technical Note on Voriconazole and Aspergillus spp. Clin Microbiol Infect. 2013;19(6):E278–280. doi: 10.1111/1469-0691.12148. [DOI] [PubMed] [Google Scholar]
- 38.Espinel-Ingroff A, Diekema DJ, Fothergill A, et al. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document) J Clin Microbiol. 2010;48(9):3251–3257. doi: 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaller MA, Diekema DJ, Ghannoum MA, et al. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J Clin Microbiol. 2009;47(10):3142–3146. doi: 10.1128/JCM.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Tudela JL, Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Monzon A, Cuenca-Estrella M. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52(7):2468–2472. doi: 10.1128/AAC.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfaller M, Boyken L, Hollis R, et al. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J Clin Microbiol. 2011;49(2):586–590. doi: 10.1128/JCM.02136-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller MA, Messer SA, Boyken L, et al. In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J Clin Microbiol. 2008;46(8):2568–2572. doi: 10.1128/JCM.00535-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis. 2002;35(8):982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]