Abstract
Introduction
Cryptic epitopes (CE) can be encoded by any of the 5 alternate reading frames (ARF, 2 sense and 3 antisense) of a known gene. While CE responses are commonly detected during HIV-1 infection, it is not known whether these responses are induced following vaccination.
Methods
Using a bioinformatic approach, we determined that vaccines with codon-optimized HIV inserts significantly skewed CE sequences and are not likely to induce cross-reactive responses to natural HIV CE. We then evaluated the CE- and protein-specific T-cell responses using Gag, Pol and ARF peptide pools among participants immunized with a noncodon-optimized vaccine regimen of two pGA2/JS7 DNA primes followed by two MVA/HIV62 Gag-Pol-Env vector boosts or four saline injections.
Results
Vaccinees had significantly more IFN-γ ELISpot responses toward Gag (p= 0.003) but not Pol protein than placebo recipients. However, CE-specific T-cell responses were low in magnitude and their frequencies did not differ significantly between vaccine and placebo recipients. Additionally, most positive CE responses could not be mapped to individual peptides. After expanding responses in a cultured assay, however, the frequency and median magnitude of responses to ARF peptides was significantly greater in vaccinees (p<0.0001), indicating CE-specific T cell responses are present but below an ex vivo assay’s limit of detection.
Conclusions
Our data demonstrates that HIV-1 vaccines currently in clinical trials are poorly immunogenic with regards to CE-specific T cell responses. Therefore, the context of HIV-1 immunogens may need to be modified as a comprehensive strategy to broaden vaccine-induced T cell responses.
Keywords: HIV-1, cryptic epitopes, alternative reading frames, CD8+ T cells, HVTN 205 vaccine trial, codon-optimized
INTRODUCTION
The extensive sequence diversity of human immunodeficiency virus type 1 (HIV-1) poses a formidable challenge in vaccine development [1]. The search for a universal HIV-1 vaccine has focused on induction of adaptive immune responses through multiclade, polyvalent viral vectors like the recombinant canarypox vector used in RV-144, which offered some efficacy in protection against infection [2,3]. While modest protection against HIV-1 acquisition during the RV-144 trial in Thailand mainly correlated with binding antibodies, preclinical studies in non-human primates and observational studies in HIV-infected adults have established that epitope-specific T cell responses play a critical role in controlling viral replication following infection [4–10]. Furthermore, simian immunodeficiency virus (SIV) vaccines that induce a greater breadth of virus-specific T cell responses at high frequencies correlated with better viral control [11–13]. A broader CD4 and CD8 T cell response has also correlated with better markers of HIV disease progression [14–17]. Thus, mitigating cell-mediated immunity through T cell responses may be imperative for protection against disease progression in those where antibodies failed to protect against infection. Such optimization will likely require identification of new targets to induce a more broadly reactive effector population.
Cryptic epitopes (CE) are a unique set of major histocompatibility class I (MHC-I)-restricted T cell epitopes recognized during infection with SIV, HIV, murine leukemia virus (LP-BM5), and influenza virus and provide a novel source of HIV-1 antigens for vaccine development [18–22]. In contrast with traditional, protein-derived epitopes, CE are encoded by forward (F2, F3) and reverse (R1, R2, R3) alternative reading frames (ARFs) of existing genes, corresponding to translation of sense and antisense transcripts of messenger RNA [23–26]. Thus, a double-stranded DNA vector can express traditional epitopes from the primary reading frame (F1) of the sense transcript while expressing CE in the remaining two frames (F2–3) of the same transcript or the three reverse frames (R1–3) of the antisense transcript. Therefore, the prevalence of epitopes could be 5 times greater in ARFs, making CE ideal for expanding the breadth of vaccine-induced responses without expanding the current size of the vector inserts. Despite this advantage, all vector inserts to date have been designed to preserve targets encoded by the primary reading frame (F1) without considering the effects on the ARFs. While insert sequences still contain ARFs (F2–3, R1–3), neither the sequence conservation of CE from these frames or the potential of current vaccine vectors to induce CE-specific T cells responses has been directly evaluated.
Because of the importance of broadening T cell response for HIV vaccine design and the potential implications of broadening these responses through CE targeting, we evaluated whether select HIV vaccines in clinical development are able to induce these types of responses. We found that recombinant vaccines with codon-optimized inserts skewed CE to such an extent that they poorly represented naturally occurring HIV strains. Analysis of samples from recipients of a vaccine regimen encoding noncodon-optimized HIV inserts revealed vaccination could induce CE-specific T cell populations but at frequencies that were low in comparison to protein-derived epitopes. These findings suggest that current HIV vaccines have the capacity to express CE and, if the ARFs that encode them are prioritized, vaccination could further increase the breadth of vector-induced T cell responses.
MATERIALS AND METHODS
Bioinformatic Analyses
The gag and RT region nucleotide sequences for each full length HIV-1 clade B transmitted founder virus (TFV) genome that was available from 12 acutely-infected patients in our cohort [53] were aligned before determining a single consensus sequence for each reading frame (LANL’s Consensus Maker tool, http://www.hiv.lanl.gov/content/sequence/CONSENSUS/SimpCon.html). This reference set was then translated (ExPASy’s Translate tool, http://web.expasy.org/translate/), along with 1 noncodon-optimized vaccine insert (MVA/HIV62, Acc. AY528646), 2 codon-optimized vaccine inserts (MRKAd5, obtained through personal communication with M. Betts; VRCrAd5, Acc.VRC-4306), and the 2 wild type parent sequences that were optimized (wtMRKAd5 gag, CAM-1 Acc. D10112, pol LAV-1 Acc. K02013 and wtVRCrAd5, NL4.3 Acc. M19921). Pairwise alignment scores were computed by Geneious (BLOSUM62 cost matrix with a gap open penalty of 12.0 and gap extend penalty of 3.0) for each frame of the TFV consensus sequences and all 5 test sequences [27]. Homology was plotted (GraphPad Prism6 v6.0b) as the proportion of TFV consensus residues that were conserved in the sequence. Significance was determined using two-tailed Fisher’s exact tests calculated by GraphPad QuickCalcs (http://www.graphpad.com/quickcalcs/ConfInterval1.cfm).
In a separate analysis, vaccine inserts and their wild type parent amino acid sequences were compared to individual TFV consensus sequences. Alignment scores for all amino acids translated from all six reading frame of gag and RT were computed by Geneious using the matrix described above and the Jukes-Cantor correction model. Unrooted, neighbor-joining phylogenetic trees were plotted to infer amino acid sequence divergence among the sequences without assuming any ancestral relationships.
Study Cohort
Previously cryopreserved peripheral blood mononuclear cells (PBMCs) were obtained from 126 participants based on their chronological enrollment in a Phase 2a trial (HVTN 205). Five individuals were excluded after their response rate for negative controls repeatedly exceeded the statistical threshold for positivity (described below) and one was excluded because the individual seroconverted three months post final vaccination. Statistical analyses were performed for 120 participants who received intramuscular injections of either a 0.9% sodium chloride placebo (40) or vaccine treatment (80) in a testing schema with two 3 mg DNA primes followed by two 1 mL of 1×10≥7.5TCID50 MVA boosts at 0, 2, 4, and 6 months, respectively. Vaccine doses of the pGA/JS7 DNA prime contained JS7DNA plasmid encoding clade B HIV-1 HXB2 gag, HXB2/ADA env-tat-rev-vpu, and BH10 PR-RT with an inactivating point mutation in PR; the MVA boost, HIV/MVA62, is a modified vaccinia Ankara vector encoding the same gag, PR-RT, and env sequences [40]. PBMC samples were obtained 2 weeks post last vaccination. Stratification of participants into responders and non-responders was finalized prior to reporting results to the Statistical Center for HIV/AIDS Research & Prevention (SCHARP) for unblinding.
Peptide Design
Consensus clade B 15mer peptides overlapping by 11 amino acids (aa) for HIV-1 Gag and Pol proteins were obtained through the NIH AIDS Reagent Program (catalog #8117, #6208, respectively). Full length Gag, Protease (PR), and Reverse Transcriptase (RT) proviral DNA regions of the MVA/HIV62 vector were used to design overlapping ARF peptides (OLPs; 8 to 18mers overlapping by 10 residues) for potential CE using PeptGen (http://www.hiv.lanl.gov/content/sequence/PEPTGEN/peptgen.html) and synthesized in a 96 well array format by New England Biolabs [28–30]. Individual OLPs were combined by reading frame into subpools (approximately 10 peptides/pool) and large pools of no more than 248 peptides for traditional proteins and 98 peptides for potential CE (range of 33 – 98 with means of 42 and 86 for gag and PR-RT regions, respectively; Supplemental Table 1). Individual peptide, subpool, and large pool stocks were reconstituted at 40mM in DMSO, diluted to 100uM in deionized water, and stored at −80C until use.
Ex vivo IFNγ ELISpot Assay
Previously cryopreserved PBMCs were thawed and rested overnight at 37°, 5% CO2 to improve recovery rates, which retained approximately 85% of the cells per vial with average viabilities of 90% by trypan blue staining. ELISpot assays were performed as previously described using protein (NIH AIDS Reagents Program) and ARF peptide pools (New England Biolabs) at final concentrations of 5µM per peptide per subpool and 2µM per peptide per large pool. Antigens were tested in duplicate with 100,000 PBMCs/well in Millipore nitrocellulose 96-well plates while unstimulated cells were plated in quadruplicate as a negative control and phytohemagglutinin (PHA) in duplicate as a positive control. Spots that developed on the nitrocellulose membrane were counted by an ELISpot reader (ImmunoSpot; CTL) as a measure of interferon gamma (IFNγ) cytokine production. T cell responses to HIV-1 Gag, Pol, or their respective ARF peptide pools were reported as positive in the IFNγ ELISpot if 1) the negative control response averaged less than 55 SFU/106 PMBCs, 2) the average positive control response was greater than 500 SFU/106 PBMCs, and 3) the duplicate average of the wells was greater than the mean negative control response of all samples plus 3 standard deviations and 4) greater than or equal to 3 times the average response of the sample’s negative control. Responses between both groups were analyzed for differences in magnitude using two-tailed non-parametric Mann-Whitney U tests calculated by GraphPad Prism v6.0b and frequency using two-tailed Fisher’s exact tests calculated by GraphPad QuickCalcs (www.graphpad.com/quickcalcs/ConfInterval1.cf).
Cultured IFNγ ELISpot Assay
In a subset of PBMC samples, a 12 day in vitro cultured assay was performed concurrently with ex vivo ELISpot assays. Cryopreserved PBMCs were prepared for overnight resting and stimulated with the same antigens as described above. In brief, cells were suspended after resting in 10% HyClone FBS culture medium with 25 ng/mL IL-7 (R&D Systems, #207-IL) before being plated in 24 or 48-well culture plates at 1 mil PBMCs/mL. Cells were stimulated with subpools (5µM final concentration per peptide) and/or large pools (2µM final concentration per peptide) corresponding to the previous positive large pool responses in the participant. As a control, each sample was stimulated with a previously negative ARF large pool in addition to using unstimulated cells and PHA as negative and positive controls, respectively. On days 2 and 7, IL-2 (BD, #354043) was added to each well at a final concentration of 40–45 BRMP units/mL. Cultures were incubated an additional 2 days before washing cells three times with PBS and resting in IL-2-free R10 media for 30–36 h. After resting, cultured cells were tested in an IFNγ ELISpot assay as described above. Based on previous studies using cultured cells, samples with media background values greater than the media mean for all samples plus 2 standard deviations (210 SFU/1 mil PBMCs) were excluded [32–36]. Positive responders were identified when the magnitude of a subpool response exceeded the 3x the sample’s background and media mean for all samples plus two standard deviations. Groups were compared with the Mann-Whitney statistical U test using background-subtracted responses.
RESULTS
Codon-optimized vector inserts significantly skew all ARF sequences
Because CE responses are dominantly due to CD8 T cells, our strategy was to analyze HIV recombinant adenoviral serotype 5 (Ad5) vector vaccines as these can be potent inducers of CD8 T cells. In order to enhance protein expression without changing the natural amino acid sequence, some HIV-1 vaccines (including all recombinant Ad5 vectors) are codon-optimized [37,38]. However, CE are encoded in ARFs, and their conservation is not prioritized during codon optimization of traditional genes.
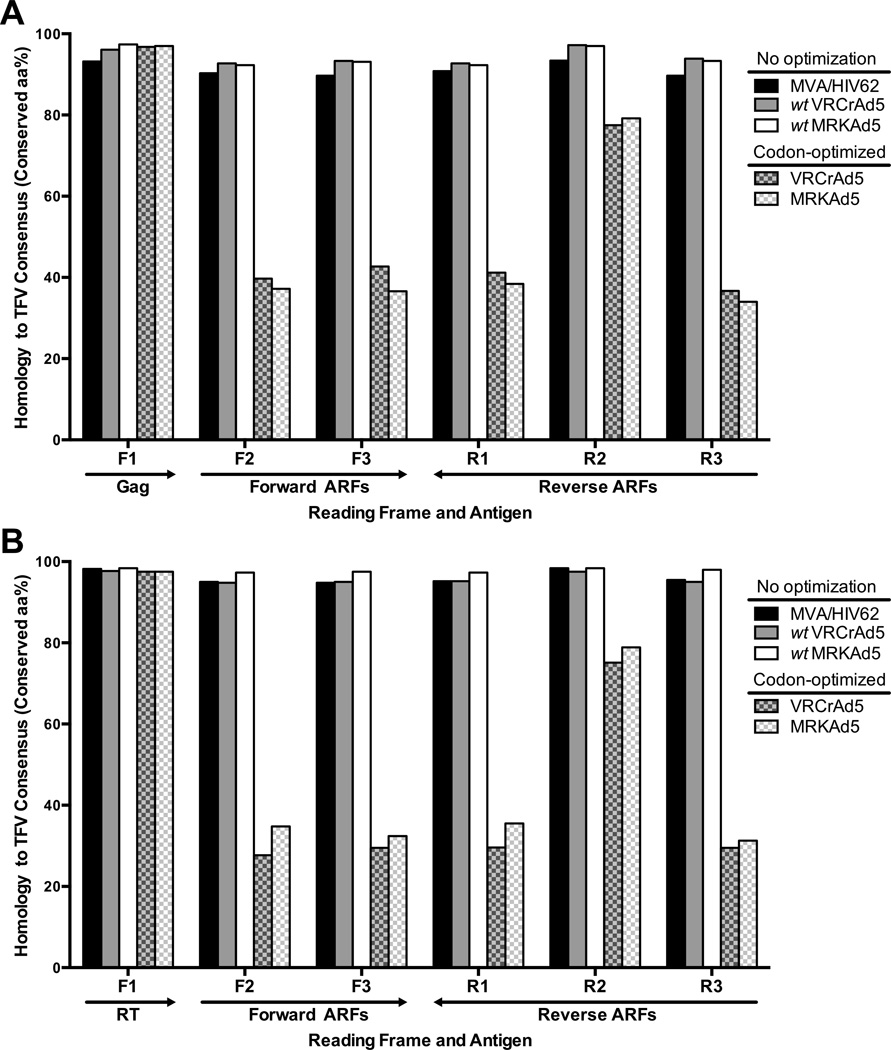
To quantify the impact on ARF sequences following codon-optimization, we compared the amino acid identities of synthetic constructs to ARFs of naturally-occurring transmitted founder virus sequences (TFV). Since TFV sequences are derived from sequencing at the earliest disease stage, each TFV sequence corresponds to the transmitted virus’s genome after it established infection and prior to little or any experience of immune pressure in the new host [18]. Thus, our use of TFV sequences ensured epitopes encoded by vector inserts would be scored against a reference sequences that accurately represents vaccine targets [39]. Using 12 full length HIV-1 clade B TFV sequences that were available from our acutely infected cohort, we compiled a reference set containing 1 amino acid consensus sequence per reading frame and gene region. In a preliminary analysis of a previously recognized CE encoded by gag (Gag-CE), we found that both the Gag and CE residues encoded by a noncodon-optimized vector insert were homologous to the TFV sequence (Supplementary Figure 1). In contrast, a codon-optimized vector encoded the same Gag sequence but the CE incurred an 11% loss in homology (1 of 9 residues) when compared to the TFV sequence and the wild type, noncodon-optimized sequence from which its insert was designed. Given these results at the epitope level, pairwise alignments for the full length of the inserts were then performed to measure amino acid conservation with respect to the TFV reference set for gene regions that were common (gag and reverse transcriptase, RT) to 1 noncodon-optimized vector insert (MVA/HIV62), 2 codon-optimized vector inserts (MRKAd5, VRCAd5), and both wild type sequences from which the codon-optimized inserts were derived. We found that codon optimizing the protein-coding frame (F1) amplified the effect seen at the epitope level, leading to a significant decrease (Fisher’s exact, p<0.0001) in amino acid identity for all ARFs of gag (mean, 46.3%, range of 34.0% – 79.2%; Fig. 1A) and RT (mean, 40.4%; range of 27.7% – 78.9%, Fig. 1B) that could encode CE (ARFs F2–3, R1–3; [27]).
Figure 1. Codon optimization significantly alters natural HIV-1 ARFs.
The homology of amino acid sequences is plotted as the percent of residues that were conserved with respect to a reference, the translated TFV consensus sequence, for 1 noncodon-optimized insert (MVA/HIV62; black), 2 codon-optimized inserts (VRCrAd5, MRKAd5; grey and white pattern, respectively), and the 2 wild type sequences from which the codon-optimized insert were designed (wtVRCrAd5, wtMRKAd5; grey and white). Amino acid identities were compared for all reading frames of the gag (A) and RT (B) regions.
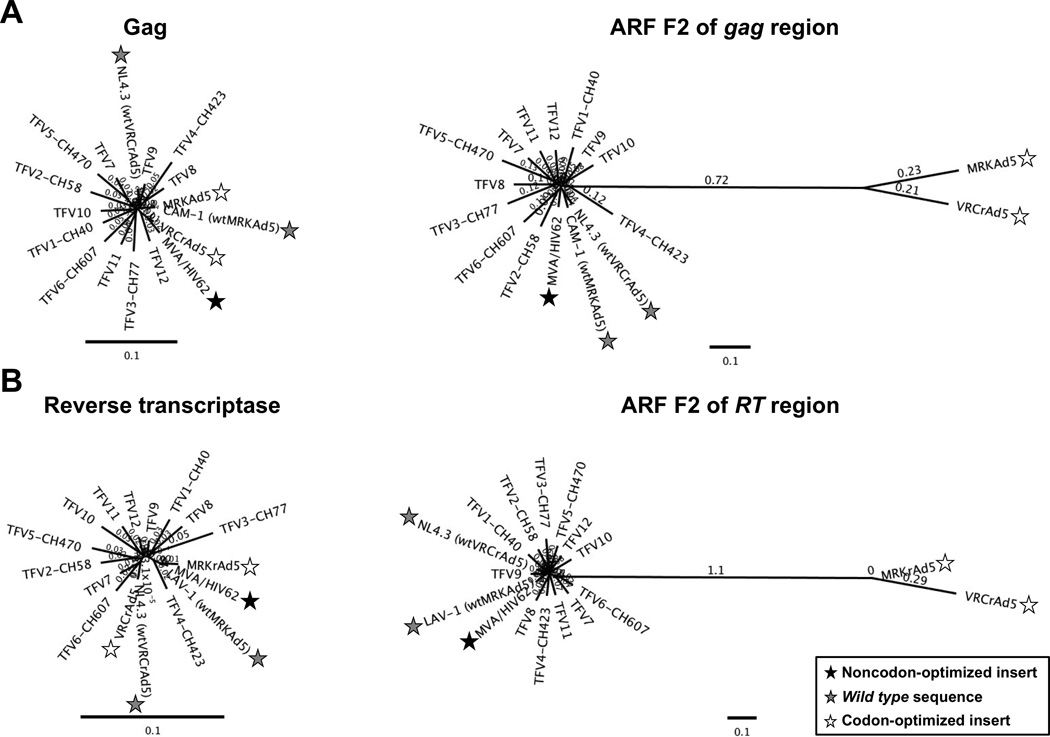
To further evaluate the cummulatlve effect of codon optimization on vector inserts, we used phylogenetic analyses to compare individual TFV and insert sequences from two vaccine vectors (MVA/HIV62 and VRCrAd5) for which we had access to clinical trial samples. Genetic distances between the TFV reference set, all vector inserts, and wild type sequences were calculated for both proteins (F1) from the gag and reverse transcriptase (RT) regions and all ARF amino acid sequences (F2, F3; R1–3), which would include any potential CE (Fig. 2). Tight clustering of tree branches indicates amino acid sequences for Gag and RT proteins (F1) are genetically similar (Figs. 2A and 2B, left panels). Noncodon-optimized sequences demonstrated tight clustering for both proteins (F1) and potential CE (F2–3, R1–3; Fig. 2, all panels). Similarly, codon-optimized Gag and RT (F1) did not significantly diverge from the TFV reference set. However, codon-optimized ARFs had distances 2 to 5 times greater than the TFV reference set and the wild type noncodon-optimized sequences (Figs. 2A and 2B, right panels). The genetic disparity shown for F2 of gag and pol was representative of the codon-optimized CE encoded by the remaining ARFs (F3, R1–3; data not shown). These results confirmed that regardless of the method used to encode the insert, there was little difference between residues of proteins encoded by the noncodon-optimized, codon-optimized, wild type, or TFV consensus sequences. Conversely, analysis of the sequences from each of the 5 ARFs in the gag and RT regions of the genome demonstrated that codon optimization of inserts can lead to a significant decrease in amino acid conservation for ARFs and a greater than 2-fold decrease in homology between targets encoded by TFVs and vaccine-derived CE.
Figure 2. Genetic distances of ARF sequences are greatly increased for codon-optimized vector inserts.
Genetic distances were determined for amino acid sequences translated from 12 individual clade B TFV sequences, 1 noncodon-optimized vector insert (MVA/HIV62; black stars), 2 codon-optimized vector inserts (VRCrAd5, MRKAd5; white stars), and 2 wild type, noncodon-optimized sequences (wtVRCrAd5, wtMRKAd5; grey stars). Distances are plotted for Gag and RT proteins (A and B, respectively; left panels) and forward frame 2 (F2), a representative example of distances for an alternative reading frame (ARF; A and B, right panels). Branch lengths reflect sequence divergence. Scale bars indicate 10% genetic distance.
Lack of CE-specific T cell responses in recipients of a noncodon-optimized vaccine
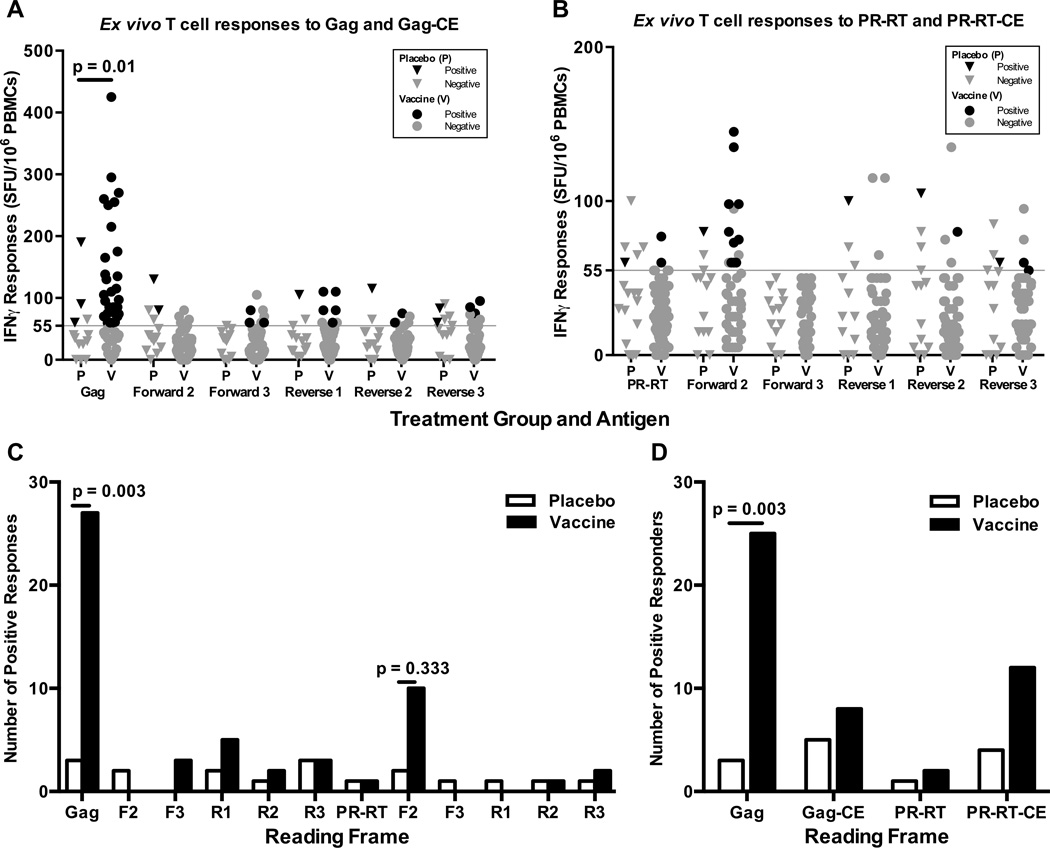
Based on the results above, we exclusively evaluated CE-specific T cell responses to a noncodon-optimized vaccine vector, MVA/HIV62. Responses to proteins and CE encoded by the gag and PR-RT inserts were measured with an interferon-gamma (IFN-γ) ELISpot assay using peripheral blood mononuclear cells (PBMC) collected from participants enrolled in HVTN 205 who received two pGA2/JS7 DNA injections at 0 and 2 months followed by two vector boosts at 4 and 6 months or placebo. As expected from prior studies with this product, Gag but not Pol-specific T cell responses were present only in vaccine recipients (Fig. 3, [40]). CE-specific responses were occasionally detected in vaccinees, especially in PR-RT ARF F2. However, these were not significantly greater than the responses seen in the placebo controls for any of the gag or PR-RT ARFs. Furthermore, subsequent mapping of the PR-RT ARF F2 responses failed to identify any peptide, indicating that the detected peptide pool responses were either weak or not present at all.
Figure 3. Lack of detectable CE-specific cytotoxic T cell responses to ARF peptide pools.
The magnitude of responses to large, single frame overlapping peptide pools from the gag (A) and PR-RT (B) regions was measured by IFNγ ELISpot. All responses for are shown for positive responders who received vaccine (N= 32; V, circles) or placebo (N=8; P, triangles) treatment during HVTN 205. Positive values (black) exceeded both the positivity threshold (grey line) and 3 times the sample background. (C) The total number of positive responses to 12 large, single frame peptide pools are shown for positive responders according to treatment group. (D) The proportion of participants with a positive response to either protein or any CE encoded by the gag or PR-RT regions is shown as a percentage according to group.
In vitro cultured assay detected a higher frequency of responders in vaccinees
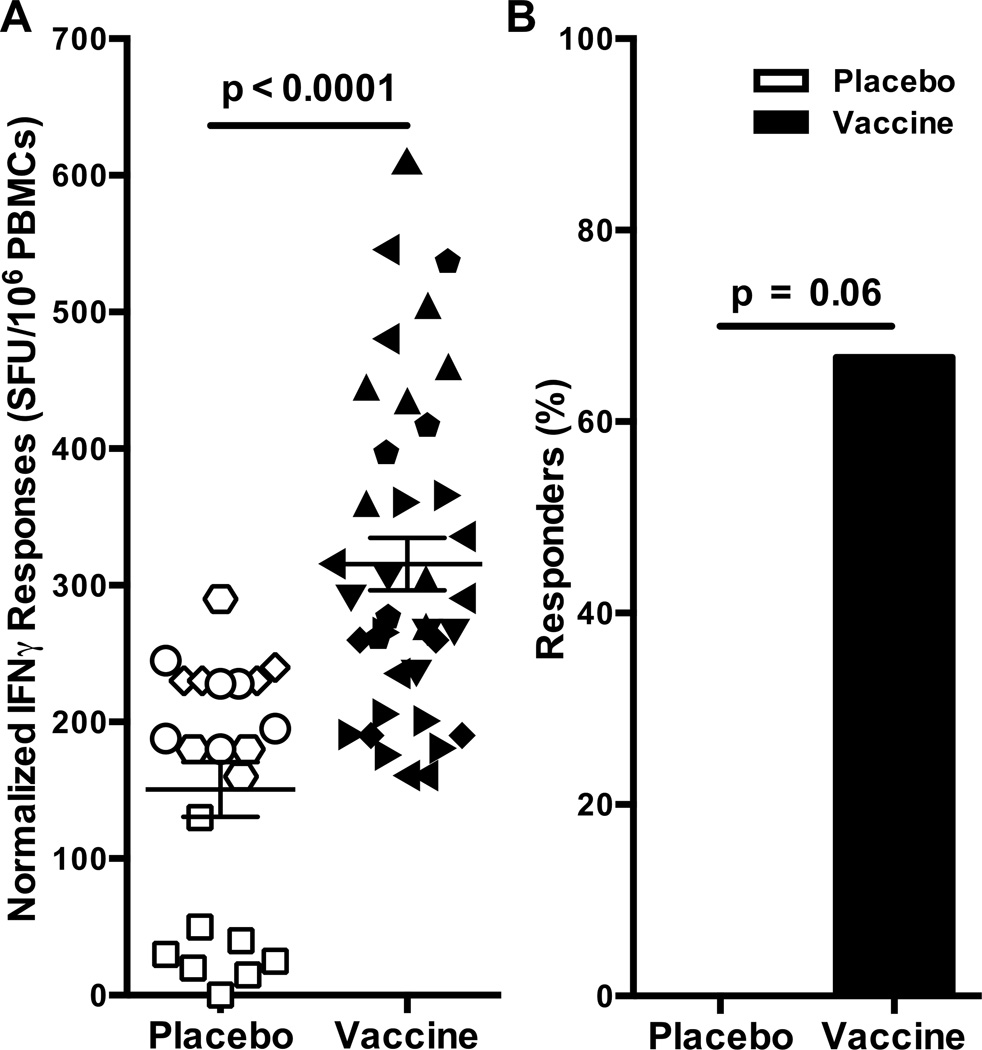
A cultured assay was performed on a select set of positive responders to determine if low frequency CE specific responses were present and could be expanded after a 12 day in vitro expansion. This assay’s extended period of antigenic stimulation allows for proliferation of low frequency HIV-1-specific T cells and therefore achieves greater sensitivity than an ex vivo ELISpot assay [32–34]. Eighteen participant samples from both treatment groups were cultured with peptides from gag and PRT-RT ARFs but high background seen in samples from 7 participants excluded them from further analysis. In the 11 remaining samples with acceptable background, the magnitude of CE-specific T cells was significantly greater in vaccinees (p<0.0001; Fig. 4A). Furthermore, CE-specific responses were only detected in the vaccinees and none of the placebo controls (Fisher’ exact test, p= 0.06; Fig. 4B). In addition, none of the irrelevant ARF pools (those that were negative at the initial testing) were positive upon culturing. Taken together, these data suggests that CE responses may be induced by this DNA/MVA vaccine regimen, albeit at low frequencies.
Figure 4. Increased detection of proliferating HIV-1 CE-specific T cells in a cultured IFNγ ELISpot assay.
T cells were evaluated following 10 days in culture with subpools of peptides encoded by ARFs. (A) Data are presented as the number of responses normalized to the lowest magnitude subpool response after subtracting background. Each symbol represents all positive and negative responses for an individual vaccinee. Bars represent the standard error of the mean. (B) The proportion of participants with a positive response to any ARF subpool is shown as a percentage according to group.
DISCUSSION
Translation of cryptic epitopes from alternative reading frames highlights the immense coding capacity of HIV-1’s genome and increases the demand for immunologic coverage through vaccination [7,9,10,12]. These immunogens present a new source of HIV-1 antigen diversity that further challenges the development of a broadly protective HIV-1 vaccine [1,4]. In retrospect, vaccine candidates in ongoing clinical trials were designed prior to research that established the immunological relevance of CE in HIV-1-infected patients [18–20]. Consequently, current vaccines prioritize preservation of primary reading frames rather than ARFs, which may affect the generation of CE [37,38,41]. We therefore assessed the CE coding capacity of current vector designs, which supported investigation of CE-specific T cell responses in recipients of a noncodon-optimized HIV-1 vaccine regimen.
Our comparison of vector inserts demonstrated that codon optimization of HIV-1 genes preserves amino acid sequences encoded by the primary reading frame (F1) but decreases amino acid conservation of potential CE from ARFs (F2–3, R1–3). Because of the high magnitude of responses to PR-RT-CE for ARF F2, we tested the same large pool of peptides in participants vaccinated with the VRC DNA prime rAd5 vaccine as part of HVTN 084 [59]. However, no T cell responses specific to ARF F2 were detected when evaluated ex vivo (unpublished results). Although not investigated by our group, it is also possible that codon optimization skews the normal CE production by skewing the regulatory sequences governing the synthesis of transcriptional or translational products [54,55]. SImilarly, recognition of optimized CE may be skewed because previous immunogenicity studies have shown that at least two-thirds of a peptide’s residues must be conserved for cross-recognition by epitope-specific T cells [42]. The average percent (46.3% and 40.4%, gag and PR-RT regions, respectively) of amino acid conservation for codon-optimized CE fell well below this threshold with exception of frame R2. The higher homology in R2 results from shared positioning of optimized codons in the primary reading frame (F1). Since codon optimization of a gene (F1) frequently changes the nucleotide in the third or wobble position of codons, its complementary base in the second reverse reading frame (R2) is also changed at the wobble position, which minimizes the decrease in homology for this reading frame [43]. In comparison, all frames of noncodon-optimized inserts were nearly identical to TFV sequences from acutely-infected patients, indicating a higher probability of inducing cross-reactive, CE-specific T cells during a transmission event. Moreover, these observations suggest that the native context of HIV-1’s genomic architecture is more rigid than was previously suspected, where manipulation of gene sequences may result in decreased antigen production, presentation, and/or recognition [44,45].
In participants of HVTN 205 receiving a vector encoding a noncodon-optimized insert, we were unable to detect significant CE responses ex vivo. Such a finding could be due to a number of factors including the use of cryopreserved specimens and the method of resting cells overnight both of which may have contributed to enhanced cell death. We find these possibilities unlikely since both systems are used extensively by our group and the HVTN and do not significantly impact responses seen to epitopes in the protein reading frame [56–58]. It seems more likely that the poor responses observed to CE lies in the fact that even with HIV infection responses to CE are low in magnitude and relatively infrequent [18,19]. Furthermore, the average OLP length (15mer, with a range of 8 – 18mer) exceeded the length of peptides commonly recognized by MHC-I (8 – 11mer), which potentially reduced sensitivity to CD8+ T cell responses in our assays. In addition, the higher frequency of stop codons in ARFs further restricted the size and number of OLPs that could be artificially synthesized. Accordingly, CE generated through non-conventional translation methods, including stop codon readthrough and doublet decoding, may have been undetectable with the described set of OLPs [30,46]. Furthermore, the low immunogenicity of Gag- and PR-RT-CE suggests MVA/HIV62 may not have been processed efficiently or effectively presented to the immune system [47,48]. During infection, translation of PR-RT from the gag-pol transcript relies on a native −1 ribosomal frameshift, which reduces PR-RT expression to one-tenth the level of Gag protein. Since the vector insert maintained the same out-of-frame nucleotide code for the gag and PR-RT regions, vaccine-derived PR-RT levels may have been insufficient for priming central memory T cell populations and limited the number of effector responses directed against Pol protein in this study. Alternatively, 90% of gag-pol reads do not result in frameshifting, such that translation could occur downstream of gag and generate CE from ARF F2 of PR-RT. Expression from this region is consistent with the trend seen for PR-RT-CE F2 responses (Fig. 3B) and our previous data, which suggested that epitopes in the pol region are predominantly cryptic [18]. Nevertheless, a cultured assay was performed with the same OLPs and detected CE-specific T cell responses exclusively in vaccinees.
Our inability to detect vaccine-induced CE responses ex vivo contrast with what has been reported in rhesus macaques immunized with SIV vaccines [26,49]. In fact, CE responses have been reported in up to one-quarter of these vaccinated macaques [50]. However, these macaques were vaccinated with SIV rAd5 vectors that potently induce CD8 T cell responses unlike MVA recombinant vectors, used in our study, that induce relatively weak CD8 responses and are more skewed towards CD4. Additionally, the breadth of vaccine-induced responses is significantly greater in macaques compared to humans likely due to the fact that the former primates are able to encode many more MHC type I alleles than the six available to humans [51]. As such it seems reasonable to expect that that this greater vaccine-induced breadth would extend to CE as well.
Together, our in silico and in vitro analyses suggest that codon optimization negatively impacts the breadth of CE-specific T cell responses when compared with the repertoire generated through infection or vaccination with a noncodon-optimized vector. Vaccination with the noncodon-optimized MVA/HIV62 vector induced cellular responses that were low in magnitude and frequency, indicating HIV-1 CE are presented but at levels that may not be adequate for recognition by peripheral T cells. Still, the immunity afforded to animals vaccinated with naturally encoded constructs supports delivery of noncodon-optimized vectors to expand the pool of vaccine-induced epitopes [52]. As future studies determine the importance of CE responses in viral control, our research may provide direction for developing vaccine designs that increase the breadth of T cell responses by either preserving as many natural HIV-1 targets as possible or specifically engineering vaccines to express CE as immune targets.
Supplementary Material
Conservation of a previously recognized CE (AF9; arrow) encoded by reverse frame 2 of the pol region was evaluated using a consensus sequence (top, left quadrant) of 12 acutely-infected individuals’ TFV sequences. Differences in nucleotides and amino acids are highlighted with respect to the consensus sequence for a noncodon-optimized vector insert (MVA/HIV62; bottom, left quadrant), a codon-optimized vector insert (MRKAd5; bottom, right quadrant) and the wild type sequence (LAV-1, i.e. noncodon-optimized MRKAd5) from which it was derived. Reading frames (forward, F; reverse, R) refer to translation of codons beginning at base 1, 2, or 3 of the 5’ terminus in the sense or antisense transcript, respectively.
Table 1.
Vaccine dosage and schema for participants of HVTN205.
| Participants | Dose | Vaccination Schedule (months) | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Number | DNA (mg) | MVA (TCID50) | 0 | 2 | 4 | 6 | 6.5 |
| Vaccine | 80 | 3 | 1×108 | DNA | DNA | MVA | MVA | Endpoint |
| Placebo | 40 | 0 | 0 | Placebo | Placebo | Placebo | Placebo | Endpoint |
Analyses were performed for HIV-1 uninfected, vaccinia-naïve adults who were vaccinated per protocol. Participants received either a prime-boost regimen with HIV-1 pGA2/JS7 Gag-PR-RT-Env-Tat-Rev-Vpu DNA and a clade B Gag-PR-RT-Env MVA/HIV62 vector or 0.9% sodium chloride via intramuscular injection. Immunogenicity was tested at 6.5 months (2 weeks post last boost).
ACKNOWLEDGEMENTS
The authors thank all of the volunteers who participated in the HVTN 205 study. We also thank the HIV Vaccine Trials Network and GeoVax, Inc. for their collaboration in supporting this work.
Support: NIAID: RO1 A1 084772 (Goepfert)
Abbreviations used
- CE
cryptic epitopes
- ARF
alternative reading frames
- TFV
transmitted founder virus
- OLP
overlapping peptides
- HLA-I
human leukocyte antigen class I
- MHC-I/II
major histocompatibility class I or II
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings: Interim results from our study were presented at The Global HIV Vaccine Enterprise’s 2010 AIDS Vaccine meeting in Atlanta (oral #OA01.02) and AAI’s 100th Annual Meeting: IMMUNOLOGY 2013 in Honolulu (oral #179.10, poster #P4495).
Conflicts of Interest: The authors report no scientific or financial conflicts of interest.
REFERENCES
- 1.Taylor BS, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;359:1965–1966. doi: 10.1056/NEJMc086373. [DOI] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 3.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009;83:6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, et al. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brumme ZL, Brumme CJ, Carlson J, Streeck H, John M, et al. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J Virol. 2008;82:9216–9227. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal A, Carlson J, Yan J, Akinsiku OT, Schaefer M, et al. CD8 T cell response and evolutionary pressure to HIV-1 cryptic epitopes derived from antisense transcription. J Exp Med. 2010;207:51–59. doi: 10.1084/jem.20092060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger CT, Carlson JM, Brumme CJ, Hartman KL, Brumme ZL, et al. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J Exp Med. 2010;207:61–75. doi: 10.1084/jem.20091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Champiat S, Raposo RA, Maness NJ, Lehman JL, Purtell SE, et al. Influence of HAART on Alternative Reading Frame Immune Responses over the Course of HIV-1 Infection. Plos One. 2012;7:e39311. doi: 10.1371/journal.pone.0039311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaur A, Green WR. Role of a cytotoxic-T-lymphocyte epitope-defined, alternative gag open reading frame in the pathogenesis of a murine retrovirus-induced immunodeficiency syndrome. J Virol. 2005;79:4308–4315. doi: 10.1128/JVI.79.7.4308-4315.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolan BP, Li L, Takeda K, Bennink JR, Yewdell JW. Defective ribosomal products are the major source of antigenic peptides endogenously generated from influenza A virus neuraminidase. J Immunol. 2010;184:1419–1424. doi: 10.4049/jimmunol.0901907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michael NL, Vahey MT, d'Arcy L, Ehrenberg PK, Mosca JD, et al. Negative-strand RNA transcripts are produced in human immunodeficiency virus type 1-infected cells and patients by a novel promoter downregulated by Tat. J Virol. 1994;68:979–987. doi: 10.1128/jvi.68.2.979-987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yewdell JW, Anton LC, Bennink JR. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules. J Immunol. 1996;157:1823–1826. [PubMed] [Google Scholar]
- 25.Cardinaud S, Moris A, Fevrier M, Rohrlich PS, Weiss L, et al. Identification of cryptic MHC I-restricted epitopes encoded by HIV-1 alternative reading frames. Journal of Experimental Medicine. 2004;199:1053–1063. doi: 10.1084/jem.20031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maness NJ, Valentine LE, May GE, Reed J, Piaskowski SM, et al. AIDS virus-specific CD8(+) T lymphocytes against an immunodominant cryptic epitope select for viral escape. Journal of Experimental Medicine. 2007;204:2505–2512. doi: 10.1084/jem.20071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drummond AJAB, Buxton S, Cheung M, Heled J, Kearse M, Moir R, Stones-Havas S, Thierer TWA. Geneious. 2012 doi: 10.1093/bioinformatics/bts199. 5.6 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draenert R, Brander C, Yu XG, Altfeld M, Verrill CL, et al. Impact of intrapeptide epitope location on CD8 T cell recognition: implications for design of overlapping peptide panels. AIDS. 2004;18:871–876. doi: 10.1097/00002030-200404090-00004. [DOI] [PubMed] [Google Scholar]
- 29.Yewdell JW, Hickman HD. New lane in the information highway: alternative reading frame peptides elicit T cells with potent antiretrovirus activity. J Exp Med. 2007;204:2501–2504. doi: 10.1084/jem.20071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullock TN, Patterson AE, Franlin LL, Notidis E, Eisenlohr LC. Initiation codon scanthrough versus termination codon readthrough demonstrates strong potential for major histocompatibility complex class I-restricted cryptic epitope expression. J Exp Med. 1997;186:1051–1058. doi: 10.1084/jem.186.7.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal A, Yue L, Conway J, Yusim K, Tang J, et al. Immunological control of chronic HIV-1 infection: HLA-mediated immune function and viral evolution in adolescents. AIDS. 2007;21:2387–2397. doi: 10.1097/QAD.0b013e3282f13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muturi MGC, Mutua G, Anzala O. Cultured enzyme-linked immunospot assay (ELISPOT) enhances detection of low HIV-specific immune responses in exposed seronegative individuals in Kenya. Baltimore, USA: Retrovirology; 2006. p. 44. [Google Scholar]
- 33.Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006;80:4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winstone N, Guimaraes-Walker A, Roberts J, Brown D, Loach V, et al. Increased detection of proliferating, polyfunctional, HIV-1-specific T cells in DNA-modified vaccinia virus Ankara-vaccinated human volunteers by cultured IFN-gamma ELISPOT assay. Eur J Immunol. 2009;39:975–985. doi: 10.1002/eji.200839167. [DOI] [PubMed] [Google Scholar]
- 35.Campion S, Cohen MS, McMichael AJ, Galvin S, Goonetilleke N. Improved detection of latent Mycobacterium tuberculosis infection in HIV-1 seropositive individuals using cultured cellular assays. Eur J Immunol. 2011;41:255–257. doi: 10.1002/eji.201040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritchie AJ, Campion SL, Kopycinski J, Moodie Z, Wang ZM, et al. Differences in HIV-specific T cell responses between HIV-exposed and -unexposed HIV-seronegative individuals. J Virol. 2011;85:3507–3516. doi: 10.1128/JVI.02444-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagata T, Uchijima M, Yoshida A, Kawashima M, Koide Y. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem Biophys Res Commun. 1999;261:445–451. doi: 10.1006/bbrc.1999.1050. [DOI] [PubMed] [Google Scholar]
- 38.Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol Res. 2008;42:219–232. doi: 10.1007/s12026-008-8076-3. [DOI] [PubMed] [Google Scholar]
- 39.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 203:610–619. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra I, Wamachi AN, Mungai PL, Mzungu E, Koech D, et al. Fine specificity of neonatal lymphocytes to an abundant malaria blood-stage antigen: epitope mapping of Plasmodium falciparum MSP1(33) J Immunol. 2008;180:3383–3390. doi: 10.4049/jimmunol.180.5.3383. [DOI] [PubMed] [Google Scholar]
- 43.Kotlar D, Lavner Y. The action of selection on codon bias in the human genome is related to frequency, complexity, and chronology of amino acids. BMC Genomics. 2006;7:67. doi: 10.1186/1471-2164-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW, Jr., et al. Architecture and secondary structure of an entire HIV-1 RNA genome. Nature. 2009;460:711–716. doi: 10.1038/nature08237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephenson KE, SanMiguel A, Simmons NL, Smith K, Lewis MG, et al. Full-length HIV-1 immunogens induce greater magnitude and comparable breadth of T lymphocyte responses to conserved HIV-1 regions compared with conserved-region-only HIV-1 immunogens in rhesus monkeys. J Virol. 2012;86:11434–11440. doi: 10.1128/JVI.01779-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruce AG, Atkins JF, Gesteland RF. tRNA anticodon replacement experiments show that ribosomal frameshifting can be caused by doublet decoding. Proc Natl Acad Sci U S A. 1986;83:5062–5066. doi: 10.1073/pnas.83.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal A, Gough E, Sabbaj S, Ritter D, Yusim K, et al. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS. 2005;19:241–250. [PubMed] [Google Scholar]
- 48.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maness NJ, Wilson NA, Reed JS, Piaskowski SM, Sacha JB, et al. Robust, vaccine-induced CD8(+) T lymphocyte response against an out-of-frame epitope. J Immunol. 2010;184:67–72. doi: 10.4049/jimmunol.0903118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maness NJ, Walsh AD, Piaskowski SM, Furlott J, Kolar HL, et al. CD8+ T cell recognition of cryptic epitopes is a ubiquitous feature of AIDS virus infection. J Virol. 2010;84:11569–11574. doi: 10.1128/JVI.01419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otting N, Heijmans CM, Noort RC, de Groot NG, Doxiadis GG, et al. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci U S A. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schirmbeck R, Riedl P, Fissolo N, Lemonnier FA, Bertoletti A, et al. Translation from cryptic reading frames of DNA vaccines generates an extended repertoire of immunogenic, MHC class I-restricted epitopes. J Immunol. 2005;174:4647–4656. doi: 10.4049/jimmunol.174.8.4647. [DOI] [PubMed] [Google Scholar]
- 53.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cannarozzi G, Schraudolph NN, Faty M, von Rohr P, Friberg MT, Roth AC, et al. A role for codon order in translation dynamics. Cell. 2010;141:355–367. doi: 10.1016/j.cell.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 55.Smith JM, Amara RR, Campbell D, Xu Y, Patel M, Sharma S, et al. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res Hum Retroviruses. 2004;20:1335–1347. doi: 10.1089/aid.2004.20.1335. [DOI] [PubMed] [Google Scholar]
- 56.Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filbert H, Attig S, Bidmon N, Renard BY, Janetzki S, Sahin U, et al. Serum-free freezing media support high cell quality and excellent ELISPOT assay performance across a wide variety of different assay protocols. Cancer Immunol Immunother. 2013;62:615–627. doi: 10.1007/s00262-012-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janetzki S, Price L, Britten CM, van der Burg SH, Caterini J, Currier JR, et al. Performance of serum-supplemented and serum-free media in IFNgamma Elispot Assays for human T cells. Cancer Immunol Immunother. 2010;59:609–618. doi: 10.1007/s00262-009-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graham BS, Enama ME, Nason MC, Gordon IJ, Peel SA, Ledgerwood JE, et al. DNA vaccine delivered by a needle-free injection device improves potency of priming for antibody and CD8+ T-cell responses after rAd5 boost in a randomized clinical trial. PLoS One. 2013;8:e59340. doi: 10.1371/journal.pone.0059340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Conservation of a previously recognized CE (AF9; arrow) encoded by reverse frame 2 of the pol region was evaluated using a consensus sequence (top, left quadrant) of 12 acutely-infected individuals’ TFV sequences. Differences in nucleotides and amino acids are highlighted with respect to the consensus sequence for a noncodon-optimized vector insert (MVA/HIV62; bottom, left quadrant), a codon-optimized vector insert (MRKAd5; bottom, right quadrant) and the wild type sequence (LAV-1, i.e. noncodon-optimized MRKAd5) from which it was derived. Reading frames (forward, F; reverse, R) refer to translation of codons beginning at base 1, 2, or 3 of the 5’ terminus in the sense or antisense transcript, respectively.