Abstract
Purpose
Pulmonary hypertension (pHTN), a main determinant of survival in congenital diaphragmatic hernia (CDH), results from in utero vascular remodeling. Phosphodiesterase type 5 (PDE5) inhibitors have never been used antenatally to treat pHTN. The purpose of this study is to determine if antenatal PDE5 inhibitors can prevent pHTN in the fetal lamb model of CDH.
Methods
CDH were created in pregnant ewes. Postoperatively, pregnant ewes received oral placebo or tadalafil, a PDE5 inhibitor, until delivery. Near term gestation, lambs underwent resuscitations, and lung tissue was snap frozen for protein analysis.
Results
Mean cGMP levels were 0.53±0.11 in placebo-treated fetal lambs and 1.73±0.21 in tadalafil-treated fetal lambs (p=0.002). Normalized expression of eNOS was 82±12% in Normal-Placebo, 61±5% in CDH-Placebo, 116±6% in Normal-Tadalafil, and 86±8% in CDH-Tadalafil lambs. Normalized expression of β-sGC was 105±15% in Normal-Placebo, 82±3% in CDH-Placebo, 158±16% in Normal-Tadalafil, and 86±8% in CDH-Tadalafil lambs. Endothelial NOS and β-sGC were significantly decreased in CDH (p = 0.0007 and 0.01 for eNOS and β-sGC, respectively), and tadalafil significantly increased eNOS expression (p = 0.0002).
Conclusions
PDE5 inhibitors can cross the placental barrier. β-sGC and eNOS are downregulated in fetal lambs with CDH. Antenatal PDE5 inhibitors normalize eNOS and may prevent in utero vascular remodeling in CDH.
Keywords: congenital diaphragmatic hernia, DH, pulmonary hypertension, phosphodiesterase type 5 inhibitors, PDE5 inhibitors
Introduction
Congenital diaphragmatic hernia is a birth malformation that affects approximately 1:2000 newborns.1 Despite advancements in postnatal care, a recent multicenter worldwide study indicates that the overall survival is still only 68%.2 The current mainstay of treatment includes respiratory support and stabilization prior to repair of the diaphragmatic defect. During this period, some infants experience progressive acidosis and hypoxemia resistant to inhaled nitric oxide due to pulmonary hypoplasia, pulmonary hypertension and persistent fetal circulation. The severity of pulmonary hypoplasia and pulmonary hypertension contributes significant morbidity and mortality to infants with CDH and remains the main determinant of survival.2–4
The molecular mechanisms involved in pulmonary hypertension and pulmonary vascular remodeling in CDH are complex and multifactorial.5 Both abnormal muscularization of distal arterioles6–8 and an innate pulmonary vascular hyperreactivity3, 9 contribute to pulmonary hypertension, and these effects occur in both lungs, regardless of the laterality of the diaphragmatic hernia. Treatment includes inhaled nitric oxide, extracorporeal membrane oxygenation, endothelin receptor antagonists and phosphodiesterase type 5 (PDE5) inhibitors. PDE5 inhibitors are currently used to treat adults10 and children11, 12 with pulmonary hypertension. Sildenafil, a PDE5 inhibitor, has also been used in pregnant women with no adverse side-effects for mother or fetus.13 However, PDE5 inhibitors have never been used in humans antenatally to prevent pulmonary hypertension from developing.
A prenatal diagnosis of CDH offers a unique opportunity to treat fetuses antenatally with PDE5 inhibitors to potentially circumvent the pulmonary vascular remodeling that occurs during pulmonary development in CDH. PDE5 inhibitors prevent the breakdown of cyclic GMP (cGMP), which acutely acts on the vascular smooth muscle cells to prolong pulmonary vasodilation. However, chronically, they may be able to alter gene expression to prevent the vascular remodeling that contributes to pulmonary hypertension in utero. The purpose of this study is to determine if the cGMP pathway is altered in the fetal lamb model of CDH. We hypothesize that antenatal maternally-administered PDE5 inhibitors can decrease the severity of pulmonary hypertension in fetal lambs with surgical diaphragmatic hernias by altering expression of proteins in the cGMP pathway.
Methods
Animals
A total of 21 ewes and 26 lambs were used for this study. Specific “n” numbers for each experiment are delineated in the relevant figure.
Plasma tadalafil concentration
Maternal ewes underwent laparotomy and hysterotomy, and jugular venous catheters were placed in normal fetuses at gestational day 85–90 (this was the earliest gestational age at which the fetal tissue integrity could allow for securing of the catheters). Catheters were exteriorized onto the maternal ewe so that blood samples could be obtained from the fetus in utero. Postoperatively, maternal ewes received four days of tadalafil orally (dosed at either 1mg/kg/day or 2mg/kg/day) before serum concentrations were determined in order to achieve a steady state. After four days, blood was drawn at administration (0 hours) of the fifth dose, and at 2, 4, 6, 8, and 24 hours afterwards from maternal ewes. In fetal lambs, blood was drawn at administration (0 hours) and at 4, 8, 12, 16, 20, and 24 hours afterwards. Serum tadalafil levels were determined by liquid chromatography and mass spectrometry. Sample size was limited to one maternal ewe and one fetus for each dosage (total n = 2 maternal ewes and n = 2 fetuses) due to budgetary constraints and loss of additional fetuses to preterm labor, likely related to entanglement with the fetal catheters. Lambs were euthanized after the last blood draw.
Lung cGMP Levels
Unoperated ewes at mid-gestation were given either placebo or tadalafil at 2mg/kg/day orally for seven days before lungs were procured from fetal lambs (n=3 lambs/group) to assess levels of cyclic GMP (cGMP), measured with a commercially available competitive enzyme-linked immunoassay from Cayman Chemical (Ann Arbor, MI) according to the manufacturer’s protocol.
Lamb resuscitations
Diaphragmatic hernias were created in time-dated pregnant ewes at 75 days gestation. Postoperatively, pregnant ewes were randomized to receive either placebo (n = 7 ewes) or tadalafil at 2mg/kg/day (n = 8 ewes) orally. Unoperated twins, if present, served as controls. At 135 days gestation, ewes were anesthetized with ketamine (15mg/kg) and isoflurane, and intubated prior to laparotomy and hysterotomy. Polyvinyl catheters were placed into an artery and vein in one hind leg and advanced into the descending aorta and inferior vena cava, respectively, in fetal lambs. A left thoracotomy was performed in the third intercostal space in the lambs. An ultrasonic flow probe (Transonics Systems, Ithaca, NY) was placed on the main pulmonary artery to measure pulmonary blood flow. Polyvinyl catheters were placed in the main pulmonary artery to measure pulmonary artery pressures. The ductus arteriosus was isolated with a vessel loop, and the ribs were approximated. Lambs were intubated on placental circulation with a 4.5mm cuffed endotracheal tube before the umbilical cord was cut. Lambs were mechanically ventilated and anesthetized with ketamine (0.3 mg/kg/min) and diazepam (0.002 mg/kg/min). Normothermia was maintained (38°C) using a heated table, heating lamps and a makeshift incubator. Blood pressure and heart rate were measured continuously throughout the resuscitation. Arterial blood gases were drawn every 10 minutes throughout the 30 minute resuscitation. Baseline hemodynamic measurements were obtained, and then again after the ductus arteriosus was ligated, inhaled nitric oxide (iNO) was turned on to 40 ppm, and then iNO was turned off. The lambs were euthanized with a lethal injection of sodium pentobarbital followed by bilateral thoracotomy as described in the NIH Guidelines for the Care and Use of Laboratory Animals. Fetal lung tissue was isolated for protein analysis. All protocols and procedures were approved by the Committee of Animal Research of the University of California, Davis and University of California, San Francisco. Lung tissue was collected for analysis.
Western Blot Analysis
Protein extracts from peripheral lung tissue were prepared using lysis buffer (50mM Tris–HCl, pH 7.6, 0.5% Triton X-100, 20% glycerol) containing Halt™ protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). Protein concentration was determined using Pierce® BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Protein extracts (50μg) were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen, Carlsbad, CA) and transferred to Immuno-Blot™ PVDF membranes (Biorad, Hercules, CA) using electrophoresis. PVDF membranes were blocked in 5% milk/TBST for 1 hour, before overnight incubation with primary antibodies in 1% milk/TBST at 4°C overnight. Blots were washed three times with TBST for ten minutes each time and then incubated with secondary antibody at room temperature in 1% milk/TBST for one hour. Membranes were washed three times with TBST before detection of HRP activity with Pierce® ECL Western Blotting Substrate (Thermo Scientific, Rockford, IL). Protein densitometry was determined using Image J (Bethesda, MD), with β-actin as a loading control (equivalent expression defined as 100%).
Western antibodies
Mouse anti-eNOS(1:1000) and mouse anti-PDE5 (1:1000) primary antibodies were purchased from BD Biosciences (San Jose, CA). Rabbit anti-β-sGC (1:1000) was purchased from Cayman Chemical (Ann Arbor, MI). Mouse anti-β-actin (1:70,000) was purchased from Sigma-Aldrich (St. Louis, MO). Secondary antibodies horse anti-mouse antibody conjugated with HRP (1:5000 for eNOS and PDE5, 1:50,000 for β-actin) and goat anti-rabbit antibody conjugated with HRP (1:15,000 for β-sGC) were purchased from Vector Laboratory (Burlingame, CA).
Statistics
Lung cGMP levels were compared using Student t-test. Lamb resuscitation data and protein expression were compared using a Two-way ANOVA to account for the “CDH vs Normal” and “Tadalafil vs Placebo” variables. Subgroup analysis was performed using Student t-test when Two-way ANOVA was significant. Repeated-measures ANOVA and posthoc analysis with Bonferroni Multiple Comparison Test was used to analyze pulmonary artery pressure and total pulmonary blood flow data. P < 0.05 was considered significant.
Results
Maternally-Administered Tadalafil Crosses the Placental Barrier and Affects the Fetal Lamb Lung
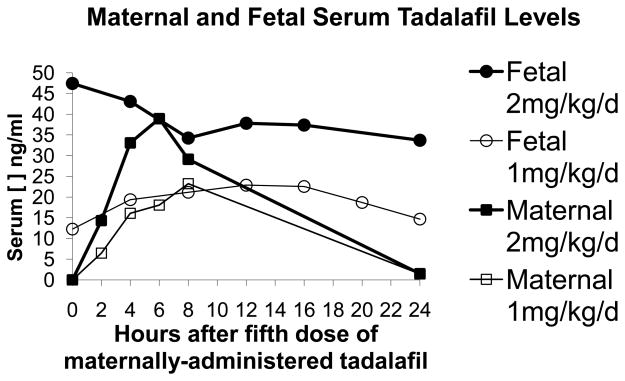
We performed an initial pilot study to determine if tadalafil would cross the placental barrier. Tadalafil, dosed at 1 or 2 mg/kg/day, was orally administered to pregnant ewes (n = 1 per dosage group) for five days before blood was drawn at the fifth treatment and at 2, 4, 6, 8, and 24 hours afterwards in maternal ewes. In fetal lambs, blood was drawn at the fifth treatment and at 4, 8, 12, 16, 20, and 24 hours afterwards. Time points for blood draws differed between maternal ewes and fetal lambs because at certain time points, catheters would not draw back. Ewes in this experiment were euthanized after blood draws were completed because the fetal lambs died after their umbilical cords became entangled with the catheters used for blood draws. Tadalafil concentrations in maternal ewes peaked between 6 and 8 hours after administration (Figure 1). In the fetal lambs, there was a steady state of tadalafil, with concentrations comparable to peak levels seen in the maternal ewe (Figure 1). There was a proportional increase in serum tadalafil concentration with increases in tadalafil dosage. Since serum concentrations were higher at 2mg/kg/day, the remaining experiments were performed with that dosage.
Figure 1.
Pharmacokinetics of antenatal maternally-administered tadalafil after 5 days of oral administration. Serum tadalafil concentrations were quantified at the fifth treatment (0 hours) and at 2, 4, 6, 8, and 24 hours afterwards in maternal ewes. In fetal lambs, blood was drawn at the fifth treatment (0 hours) and at 4, 8, 12, 16, 20, and 24 hours afterwards. In the maternal ewe, peak concentrations occur between 6–8 hours after administration. In the fetal lamb, there is a steady state of tadalafil, with concentrations comparable to peak levels seen in the maternal ewe. There is a proportional increase in serum tadalafil concentration with increases in tadalafil dosage.
Cyclic GMP levels were measured in the fetal lamb lungs after maternal ewes were administered either placebo or tadalafilat 2mg/kg/day for seven days to confirm that maternally-administered PDE5 inhibitors would have an appropriate end biochemical effect. Average cGMP levels in fetal lamb lungs from pregnant ewes treated with tadalafil at 2mg/kg/day (n = 3) were 1.73 ±0.21 pmol/ml. Average cGMP levels in fetal lamb lungs from pregnant ewes treated with placebo (n = 3) were 0.53±0.11 pmol/ml. Average cGMP levels were significantly elevated in fetal lungs from pregnant ewes treated with tadalafil at 2mg/kg/day in comparison to placebo, p = 0.002.
Antenatal Tadalafil Normalizes Expression of Proteins in the cGMP Pathway
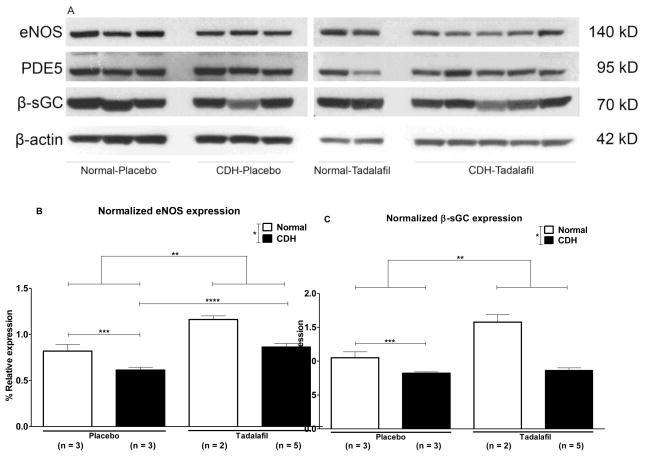
To quantify expression of proteins in the cGMP pathway, we performed Western blotting on protein isolated from fetal lamb lungs (Figure 2A). Expression of proteins in the cGMP pathway was normalized to β-actin expression. Normalized expression of eNOS was 82±12% in Normal-Placebo, 61±5% in CDH-Placebo, 116±6% in Normal-Tadalafil, and 86±8% in CDH-Tadalafil lambs (Figure 2B, n=3 for Normal-Placebo and CDH-Placebo, n = 2 for Normal-Tadalafil, and n = 5 for CDH-Tadalafil). Normalized eNOS expression was significantly decreased in CDH lambs compared to normal lambs (p = 0.0007), and treatment with tadalafil significantly increased eNOS expression (p = 0.0002). In a subgroup analysis, eNOS expression was significantly decreased in CDH-Placebo in comparison to Normal-Placebo, p = 0.05. Normalized eNOS expression was significantly increased in CDH-Tadalafil in comparison to CDH-Placebo (p = 0.003), and there was no significant difference in normalized eNOS expression between the Normal-Placebo and CDH-Tadalafil groups, (p = ns).
Figure 2.
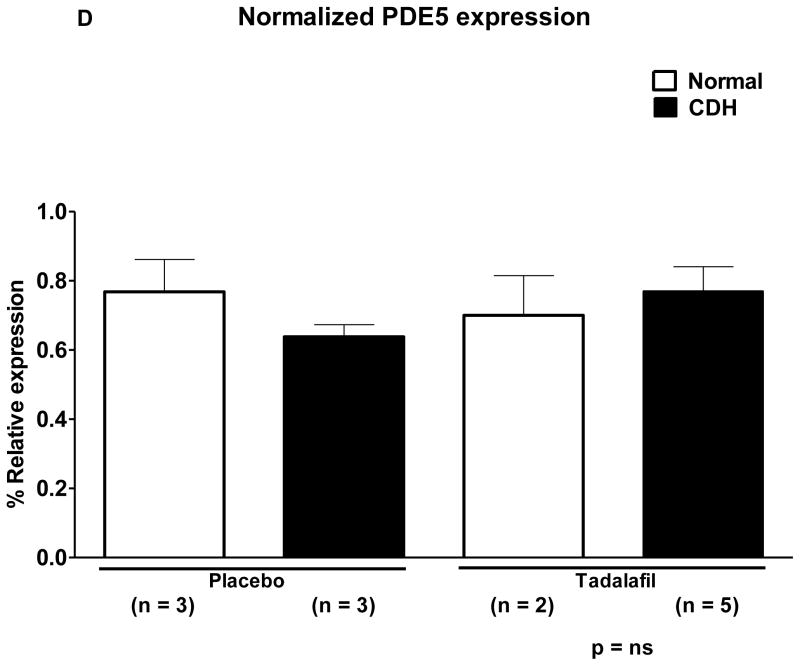
A. Western blot of protein isolated from fetal lamb lung. B. *Normalized expression of eNOS is significantly decreased in CDH lambs compared to normal lambs, p = 0.0007. **Tadalafil significantly increases eNOS expression in comparison to placebo, p = 0.0002. ***Endothelial NOS expression is significantly lower in CDH-Placebo lambs in comparison to Normal-Placebo lambs, p = 0.05, and ****eNOS expression is significantly increased in CDH-Tadalafil in comparison to CDH-Placebo, p = 0.003. There was no difference in eNOS expression between the Normal-Placebo and CDH-Tadalafil groups. C. *Normalized β-sGC expression is significantly lower in CDH lambs in comparison to normal lambs p = 0.01, and **tadalafil significantly increases β-sGC expression p = 0.0002. *** There was a trend towards decreased β-sGC expression in CDH-Placebo in comparison to Normal-Placebo, p = 0.06, but there was no significant difference in β-sGC between CDH-Placebo and CDH-Tadalafil lambs. D. There was no significant difference in normalized expression of PDE5 between groups, p = ns.
Normalized expression of β-sGC was 105±15% in Normal-Placebo, 82±3% in CDH-Placebo, 158±16% in Normal-Tadalafil, and 86±8% in CDH-Tadalafil lambs (Figure 2C). Normalized expression of β-sGC was significantly decreased in CDH lambs in comparison to normal (p = 0.01), and treatment with tadalafil significantly increased β-sGC expression (p = 0.0002). There was a trend towards decreased β-sGC expression in CDH-Placebo in comparison to Normal-Placebo in an isolated t-test (p = 0.06), but no significant difference between the CDH-Placebo and CDH-Tadalafil groups (p = ns).
Normalized expression of PDE5 was 77±16% in Normal-Placebo, 64±6% in CDH-Placebo, 70±16% in Normal-Tadalafil, and 77±16% in CDH-Tadalafil lambs (Figure 2D). Both CDH and tadalafil did not have a significant effect on normalized PDE5 expression, p = ns.
Effects of Antenatal Tadalafil on Body Weight and Lung Weight
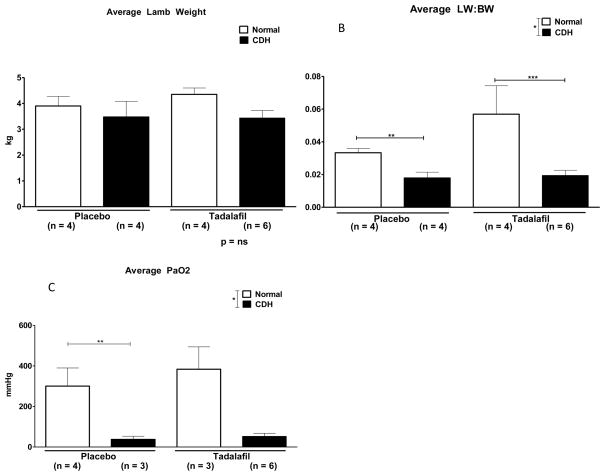
There was no significant difference in fetal lamb weight between groups (p = ns, Figure 3A). Average lung weight to body weight ratio (LW:BW) is an indicator of lung hypoplasia, and was significantly lower in CDH lambs compared to normal (p = 0.007, Figure 3B), but tadalafil did not have a significant effect on LW:BW (p = ns). LW:BW was significantly lower in CDH-Placebo compared to Normal-Placebo lambs (p = 0.01) and in CDH-Tadalafil compared to Normal-Tadalafil (p = 0.03). There was no significant difference in LW:BW between the CDH-Placebo and CDH-Tadalafil groups (p = ns).
Figure 3.
Tadalafil does not affect intrauterine growth and we were able to produce physiologically significant lung hypoplasia in our fetal lamb model of CDH. A. There was no significant difference in lamb weight between the groups. B. *Lung weight-to-body weight ratio (LW:BW) was significantly lower in CDH lambs in comparison to normal lambs, p = 0.007, and tadalafil did not affect LW:BW, p = ns. **LW:BW was significantly lower in CDH-Placebo in comparison to Normal-Placebo, p = 0.01, and also significantly lower in ***CDH-Tadalafil in comparison to Normal-Tadalafil, p = 0.03. There was no difference in LW:BW ratio between CDH-Placebo and CDH-Tadalafil. C. *Average PaO2 was significantly lower in CDH in comparison to Normal lambs, p = 0.0005, but tadalafil did not significantly increase average PaO2.**Average PaO2 was significantly lower in CDH-Placebo in comparison to Normal-Placebo, p = 0.05, but there was no significant difference in average PaO2 between CDH-Placebo and CDH-Tadalafil.
Both Maximum and Average PaO2 are Lower in CDH Lambs
Both maximum and average PaO2 were significantly lower in CDH lambs compared to normal (p = 0.0001 and 0.0005, respectively), but tadalafil did not have a significant effect on PaO2 (p = ns) (Figure 3C).
Pulmonary Hypertension in CDH Lambs
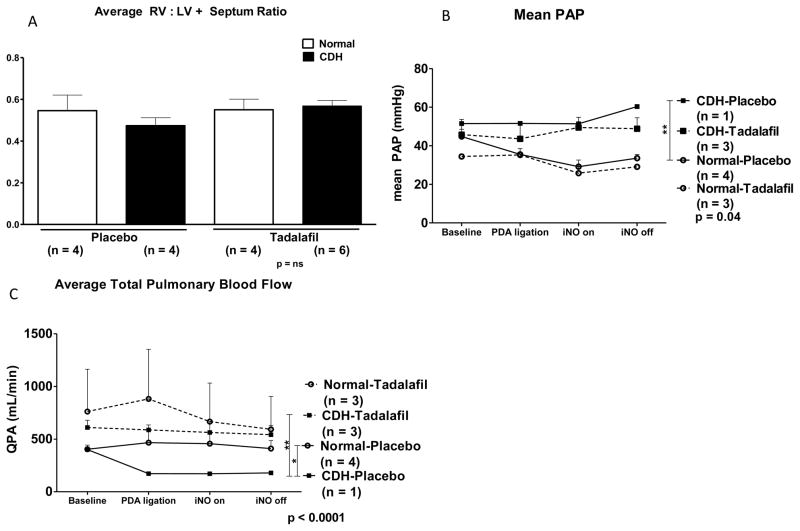
As a measure of right ventricular hypertrophy, we divided the right ventricular weight (RV) by the left ventricular and septum weights (LV+Septum) to get the RV:LV+Septum ratio. There was no significant difference in RV:LV+Septum ratio between groups (p = ns, Figure 4A).
Figure 4.
Assessment of pulmonary hypertension in fetal lambs. A. There was no significant difference in RV:LV+Septum ratio between groups. B. PAP is significantly different by Repeated Measures ANOVA, p = 0.04. **PAP is significantly higher in the CDH-Placebo group in comparison to the Normal-Placebo group by Bonferroni Multiple Comparisons Test, but there was no significant difference in PAP between the CDH-Placebo and CDH-Tadalafil group. C. QPA is significantly different between groups by Repeated Measures ANOVA, p < 0.0001. *QPA was significantly lower in the CDH-Placebo group in comparison to the Normal-Placebo Group by Bonferroni Multiple Comparisons Test. **QPA was also significantly higher in the CDH-Tadalafil group in comparison to the CDH-Placebo group in post-hoc as well. There was no significant difference in QPA between the CDH-Tadalafil and Normal-Placebo groups.
Pulmonary artery pressure (PAP) was measured in fetal lambs during resuscitations. PAP was significantly different between groups by Repeated Measures ANOVA, p = 0.04 (Figure 4B). PAP was significantly higher in the CDH-Placebo group compared to the Normal-Placebo group. However, there was no significant difference in PAP between the CDH-Placebo group and the CDH-Tadalafil group.
A flow probe was placed on the main pulmonary to quantify total pulmonary blood flow (QPA). QPA was significantly different between groups by Repeated-measures ANOVA, p < 0.0001 (Figure 4C). Total pulmonary blood flow was significantly lower in the CDH-Placebo group compared to the Normal-Placebo Group. QPA was higher in both normal and CDH lambs that were treated with tadalafil compared to normal and CDH lambs treated with placebo. In addition, QPA was significantly higher in the CDH-Tadalafil group in comparison to the CDH-Placebo group in post-hoc analysis, and there was no significant difference in QPA between CDH-Tadalafil and Normal-Placebo.
Discussion
Congenital diaphragmatic hernia (CDH) is a common cause of persistent pulmonary hypertension in the newborn, resulting in right-to-left shunting, a significant preductal-postductal saturation difference, and acute right heart failure.14, 15 Even infants with CDH who do not manifest clinically significant pulmonary hypertension have evidence of increased pulmonary vascular resistance on echocardiogram.14 Pulmonary hypertension in CDH is due to a combination of increased pulmonary vascular hyperreactivity,3, 9 increased muscularization of the pulmonary arterioles,5, 6 and decreased size of the pulmonary vascular bed, 4, 6, 11, 13 and the severity of pulmonary hypertension remains a main determinant of survival for neonates with CDH.
Initially, pulmonary hypertension is managed with inhaled nitric oxide15 and minimizing stimuli such as acidosis, hypoxemia, and hypercarbia. Patients with refractory pulmonary hypertension are resistant to inhaled nitric oxide and treated with extracorporeal membrane oxygenation, as well as endothelin antagonists, PDE5 inhibitors, prostacyclin, and prostaglandin E1.15, 16 However, the vascular remodeling that contributes to pulmonary hypertension postnatally actually occurs antenatally, in utero.17 Antenatal therapies designed to prevent vascular remodeling may be the key to improving outcomes for neonates with CDH.
PDE5 inhibitors are currently used to treat postnatal pulmonary hypertension in infants, but they have never been used antenatally in humans to decrease the severity of pulmonary hypertension in CDH. Luong et al18 published a study using antenatal sildenafil, a PDE5 inhibitor, in the teratogenic nitrofen rat model of CDH. Sildenafil was administered to pregnant rats through subcutaneous injection, which improved lung architecture and pulmonary vascular density, and also attenuated right ventricular hypertrophy in rat fetuses with nitrofen-induced CDH.18 However, they were not able to assess the physiologic effects of antenatal maternally-administered PDE5 inhibitors on fetuses with CDH in vivo. In our study, we analyzed the effects of antenatal maternally-administered PDE5 inhibitors in the well-established surgical fetal lamb model of CDH using the next generation of PDE5 inhibitors, tadalafil.
The half-life of tadalafil is approximately 17 hours, whereas the half-life of sildenafil is between 3–5 hours.19 Tadalafil has a longer half-life than sildenafil and can be administered once daily, which will be more convenient for patients. Tadalafil is also more specific for PDE5, and theoretically, will have less potential for adverse effects in the fetus because it has less cross-reactivity with PDE6, which is expressed in the retina.19
The pharmacokinetics of tadalafil in pregnancy is poorly understood. Before proceeding with our study, we confirmed that tadalafil, when administered orally to pregnant ewes, can cross the placental barrier. However, serum tadalafil levels were significantly lower in sheep in comparison to humans. We achieved serum tadalafil concentrations of 45 ng/mL only after pregnant ewes were administered a dose of 2mg/kg/day (approximately 150–200 mg/day). In contrast, serum tadalafil levels are approximately 600–800ng/mL at a dosage of 40mg/day in humans. We were initially concerned that serum tadalafil levels were significantly lower in sheep in comparison to humans at five times the dosage. However, Luong et al18 were able to demonstrate that sildenafil has a beneficial effect, despite the fact that metabolism of sildenafil in rats was 100 times that of humans. In our studies, cGMP levels were significantly elevated in fetal lamb lungs from pregnant ewes treated with 2mg/kg/day of tadalafil in comparison to those treated with placebo. Though we tried to dose tadalafil at 4mg/kg/day (data not shown), cGMP levels in fetal lamb lungs were comparable to those dosed at 2mg/kg/day. Therefore, tadalafil dosed at 2mg/kg/day had the appropriate and intended end biochemical effect.
Successful transition to extrauterine life depends on the ability of the pulmonary vasculature to decrease resistance within the first few minutes of life,6 and the cGMP pathway is only one of many biochemical pathways that mediate pulmonary vascular vasodilation. Nitric oxide can be delivered exogenously (inhaled) or produced endogenously in the vascular endothelium by endothelial nitric oxide synthase (eNOS). Nitric oxide can then diffuse into the smooth muscle cells to activate soluble guanylate cyclase to produce cGMP, which activates proteins downstream to cause smooth muscle relaxation.20 Nitric oxide synthase expression is developmentally regulated and maximally expressed at late gestation in the fetal rat lung to optimize nitric oxide-mediated pulmonary vasodilatation at birth.21 Unfortunately, many infants with CDH have pulmonary hypertension that is refractory to inhaled nitric oxide, which suggests that proteins in the cGMP pathway may have impaired expression or activity.
A study on human fetuses with lung hypoplasia reports that fetuses with CDH have a 75% decrease in eNOS expression by Western blotting,6, 22 and eNOS expression was decreased in the nitrofen rat model of CDH.23 Transgenic mice with knockout of the eNOS gene had increased pulmonary vascular resistance and increased muscularization of distal arterioles.24 Other studies have shown that both PDE5 25 and guanylate cyclase25, 26 activity are impaired in the fetal lamb model of CDH. In our study, we show that both eNOS and β-sGC expression are decreased in CDH lambs in comparison to normal lambs, and that tadalafil significantly increased eNOS expression. There was a significant difference in eNOS expression between CDH-Placebo and CDH-Tadalafil groups, and no significant difference in expression between the Normal-Placebo and CDH-Tadalafil groups. Though tadalafil normalized eNOS expression in CDH lambs, it did not normalize β-sGC expression in CDH lambs. These results suggest that decreased nitric oxide production contributes to impaired pulmonary vascular reactivity in fetal lambs with CDH, and treatment with PDE5 inhibitors can normalize expression of eNOS.
Though maternally-administered tadalafil normalizes eNOS protein expression, it did not adversely affect fetal weight. There was no significant difference in average weight groups. Lung weight-to-body weight ratio (LW:BW) was significantly lower CDH lambs in comparison to normal lambs, but tadalafil did not have a significant effect on LW:BW in CDH lambs. In addition, CDH lambs had significantly lower maximum and average PaO2 in comparison to normal lambs. Though we did not see a significant improvement in PaO2 in the CDH-Tadalafil in comparison to the CDH-Placebo group, we were able to show significant differences in total pulmonary blood flow (QPA). Patients with CDH have significantly elevated PAP,14, 27 but pulmonary blood flow is significantly lower in CDH in comparison to age-matched controls,27 which we were able to recapitulate in our fetal lamb model of diaphragmatic hernia. Tadalafil significantly improved QPA in fetal lambs with diaphragmatic hernia after maternal ewes were administered tadalafil orally.
Previous studies in both sheep and rats have shown that increased pulmonary blood flow is associated with increased eNOS expression.28, 29 Though both of these studies were performed in animal shunt models created to induce pulmonary hypertension, the pathway to development of pulmonary hypertension is different in shunt physiology in comparison to CDH. Patients with significant shunt physiology can eventually develop pulmonary hypertension, whereas patients with CDH are already born with intrinsic pulmonary hypertension. Those with shunt physiology have elevated total pulmonary blood flow, whereas patients with CDH have decreased pulmonary blood flow. Therefore, we postulate that tadalafil, a PDE5 inhibitor, prevents the breakdown of cGMP, which leads to smooth muscle relaxation and hence, increased pulmonary blood flow. Increased eNOS expression in tadalafil-treated lambs may be an adaptive response of the pulmonary circulation in order to accommodate increase in pulmonary blood flow.
In summary, we have shown that antenatal maternally-administered tadalafil can cross the placental barrier in sheep and has an appropriate end biochemical effect in the fetal lamb lung. Fetal lambs with surgical diaphragmatic hernias have decreased expression of eNOS and soluble guanylate cyclase, and maternally-administered tadalafil significantly increases eNOS expression. Tadalafil does not have deleterious effects on intrauterine growth in fetal lambs, and significantly improves total pulmonary blood flow. Currently, maternally-administered medical therapies for fetal diseases are limited to short courses of steroids to promote fetal lung maturity and antiarrhythmics to treat fetal cardiac arrhythmias. While pharmacokinetics in pregnancy and safety must be clearly demonstrated, “preventative medicine” may take on a whole new meaning as we explore possibilities for prenatal medical therapies.
Acknowledgments
United Therapeutics donated tadalafil and limited funding for this project (DM). National Institutes of Health K08 HL092062 (DM), T32 2T32HD049303-06A1 (ES), and T32 GM008258 (SCC), as well as the UCSF Academic Senate Individual Investigator Grant (DM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langham MR, Jr, Kays DW, Ledbetter DJ, et al. Congenital diaphragmatic hernia. Epidemiology and outcome. Clin Perinatol. 1996;23(4):671–88. [PubMed] [Google Scholar]
- 2.Cortes RA, Keller RL, Townsend T, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg. 2005;40(1):36–45. doi: 10.1016/j.jpedsurg.2004.09.037. discussion 45–6. [DOI] [PubMed] [Google Scholar]
- 3.Thebaud B, Mercier JC, Dinh-Xuan AT. Congenital diaphragmatic hernia. A cause of persistent pulmonary hypertension of the newborn which lacks an effective therapy. Biol Neonate. 1998;74(5):323–36. doi: 10.1159/000014050. [DOI] [PubMed] [Google Scholar]
- 4.Bohn D. Congenital diaphragmatic hernia. Am J Respir Crit Care Med. 2002;166(7):911–5. doi: 10.1164/rccm.200204-304CC. [DOI] [PubMed] [Google Scholar]
- 5.Miniati D. Pulmonary vascular remodeling. Semin Pediatr Surg. 2007;16(2):80–7. doi: 10.1053/j.sempedsurg.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Boucherat O, Franco-Montoya ML, Delacourt C, et al. Defective angiogenesis in hypoplastic human fetal lungs correlates with nitric oxide synthase deficiency that occurs despite enhanced angiopoietin-2 and VEGF. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L849–56. doi: 10.1152/ajplung.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamataka T, Puri P. Pulmonary artery structural changes in pulmonary hypertension complicating congenital diaphragmatic hernia. J Pediatr Surg. 1997;32(3):387–90. doi: 10.1016/s0022-3468(97)90587-x. [DOI] [PubMed] [Google Scholar]
- 8.Geggel RL, Murphy JD, Langleben D, et al. Congenital diaphragmatic hernia: arterial structural changes and persistent pulmonary hypertension after surgical repair. J Pediatr. 1985;107(3):457–64. doi: 10.1016/s0022-3476(85)80534-5. [DOI] [PubMed] [Google Scholar]
- 9.de Lagausie P, de Buys-Roessingh A, Ferkdadji L, et al. Endothelin receptor expression in human lungs of newborns with congenital diaphragmatic hernia. J Pathol. 2005;205(1):112–8. doi: 10.1002/path.1677. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 11.Mourani PM, Sontag MK, Ivy DD, et al. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr. 2009;154(3):379–84. 384 e1–2. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stultz JS, Puthoff T, Backes C, Jr, et al. Intermittent intravenous sildenafil for pulmonary hypertension management in neonates and infants. Am J Health Syst Pharm. 2013;70(5):407–13. doi: 10.2146/ajhp120364. [DOI] [PubMed] [Google Scholar]
- 13.Samangaya RA, Mires G, Shennan A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. 2009;28(4):369–82. doi: 10.3109/10641950802601278. [DOI] [PubMed] [Google Scholar]
- 14.Mohseni-Bod H, Bohn D. Pulmonary hypertension in congenital diaphragmatic hernia. Semin Pediatr Surg. 2007;16(2):126–33. doi: 10.1053/j.sempedsurg.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Thebaud B, Tibboel D. Pulmonary hypertension associated with congenital diaphragmatic hernia. Cardiol Young. 2009;19(Suppl 1):49–53. doi: 10.1017/S1047951109003965. [DOI] [PubMed] [Google Scholar]
- 16.Dhillon R. The management of neonatal pulmonary hypertension. Arch Dis Child Fetal Neonatal Ed. 2012;97(3):F223–8. doi: 10.1136/adc.2009.180091. [DOI] [PubMed] [Google Scholar]
- 17.Taira Y, Yamataka T, Miyazaki E, et al. Comparison of the pulmonary vasculature in newborns and stillborns with congenital diaphragmatic hernia. Pediatr Surg Int. 1998;14(1–2):30–5. doi: 10.1007/s003830050429. [DOI] [PubMed] [Google Scholar]
- 18.Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123(19):2120–31. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 19.Montani D, Chaumais MC, Savale L, et al. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther. 2009;26(9):813–25. doi: 10.1007/s12325-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 20.Celermajer DS, Dollery C, Burch M, et al. Role of endothelium in the maintenance of low pulmonary vascular tone in normal children. Circulation. 1994;89(5):2041–4. doi: 10.1161/01.cir.89.5.2041. [DOI] [PubMed] [Google Scholar]
- 21.North AJ, Star RA, Brannon TS, et al. Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung. Am J Physiol. 1994;266(6 Pt 1):L635–41. doi: 10.1152/ajplung.1994.266.6.L635. [DOI] [PubMed] [Google Scholar]
- 22.Solari V, Piotrowska AP, Puri P. Expression of heme oxygenase-1 and endothelial nitric oxide synthase in the lung of newborns with congenital diaphragmatic hernia and persistent pulmonary hypertension. J Pediatr Surg. 2003;38(5):808–13. doi: 10.1016/jpsu.2003.50172. [DOI] [PubMed] [Google Scholar]
- 23.North AJ, Moya FR, Mysore MR, et al. Pulmonary endothelial nitric oxide synthase gene expression is decreased in a rat model of congenital diaphragmatic hernia. Am J Respir Cell Mol Biol. 1995;13(6):676–82. doi: 10.1165/ajrcmb.13.6.7576705. [DOI] [PubMed] [Google Scholar]
- 24.Fagan KA, Fouty BW, Tyler RC, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest. 1999;103(2):291–9. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Buys Roessingh A, Fouquet V, Aigrain Y, et al. Nitric oxide activity through guanylate cyclase and phosphodiesterase modulation is impaired in fetal lambs with congenital diaphragmatic hernia. J Pediatr Surg. 2011;46(8):1516–22. doi: 10.1016/j.jpedsurg.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Thebaud B, Petit T, De Lagausie P, et al. Altered guanylyl-cyclase activity in vitro of pulmonary arteries from fetal lambs with congenital diaphragmatic hernia. Am J Respir Cell Mol Biol. 2002;27(1):42–7. doi: 10.1165/ajrcmb.27.1.4712. [DOI] [PubMed] [Google Scholar]
- 27.Zussman ME, Bagby M, Benson DW, et al. Pulmonary vascular resistance in repaired congenital diaphragmatic hernia vs. age-matched controls. Pediatr Res. 2012;71(6):697–700. doi: 10.1038/pr.2012.16. [DOI] [PubMed] [Google Scholar]
- 28.Black SM, Fineman JR, Steinhorn RH, et al. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol. 1998;275(5 Pt 2):H1643–51. doi: 10.1152/ajpheart.1998.275.5.H1643. [DOI] [PubMed] [Google Scholar]
- 29.Dai ZK, Tan MS, Chai CY, et al. Effects of increased pulmonary flow on the expression of endothelial nitric oxide synthase and endothelin-1 in the rat. Clin Sci (Lond) 2002;103(Suppl 48):289S–293S. doi: 10.1042/CS103S289S. [DOI] [PubMed] [Google Scholar]