Abstract
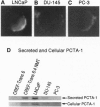
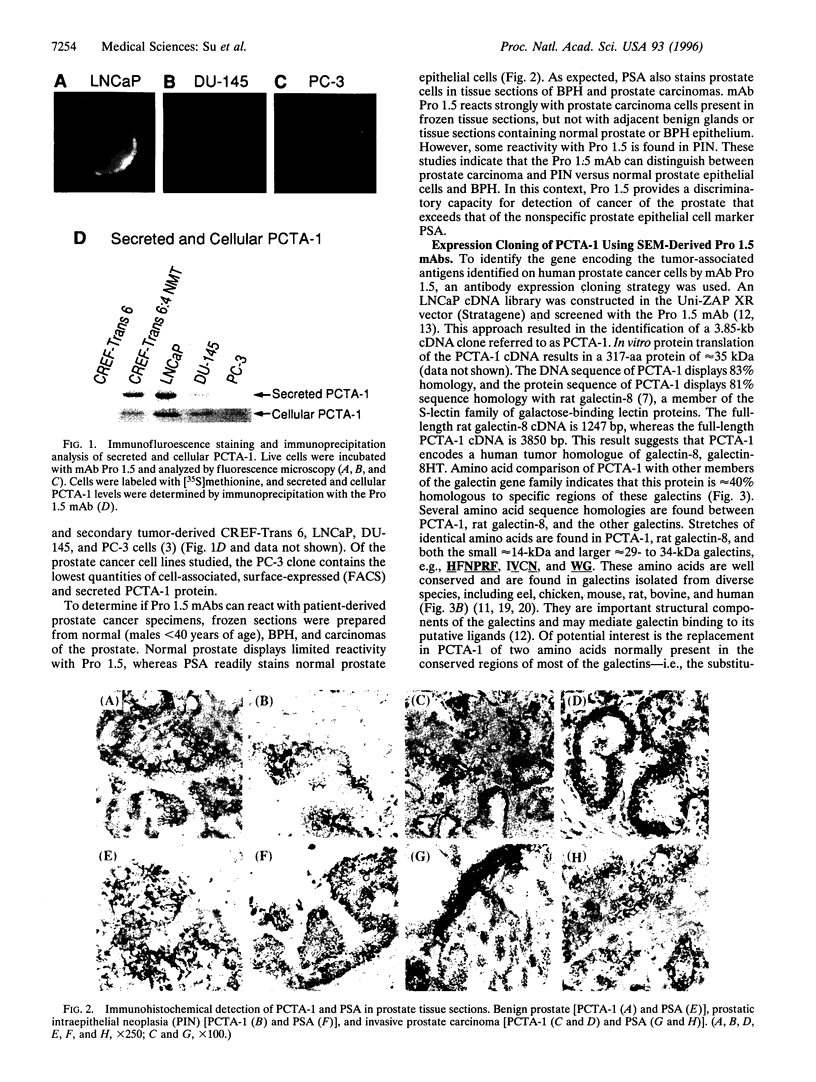
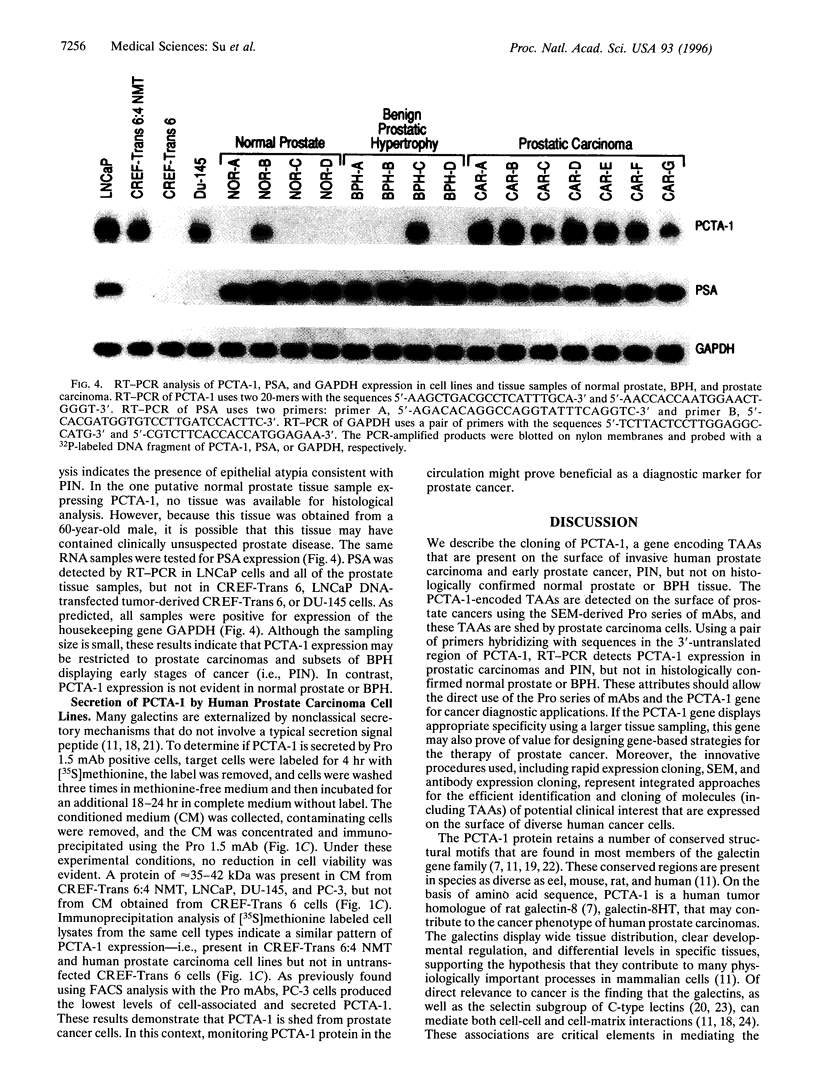
The selective production of monoclonal antibodies (mAbs) reacting with defined cell surface-expressed molecules is now readily accomplished with an immunological subtraction approach, surface-epitope masking (SEM). Using SEM, prostate carcinoma (Pro 1.5) mAbs have been developed that react with tumor-associated antigens expressed on human prostate cancer cell lines and patient-derived carcinomas. Screening a human LNCaP prostate cancer cDNA expression library with the Pro 1.5 mAb identifies a gene, prostate carcinoma tumor antigen-1 (PCTA-1). PCTA-1 encodes a secreted protein of approximately 35 kDa that shares approximately 40% sequence homology with the N-amino terminal region of members of the S-type galactose-binding lectin (galectin) gene family. Specific galectins are found on the surface of human and marine neoplastic cells and have been implicated in tumorigenesis and metastasis. Primer pairs within the 3' untranslated region of PCTA-1 and reverse transcription-PCR demonstrate selective expression of PCTA-1 by prostate carcinomas versus normal prostate and benign prostatic hypertrophy. These findings document the use of the SEM procedure for generating mAbs reacting with tumor-associated antigens expressed on human prostate cancers. The SEM-derived mAbs have been used for expression cloning the gene encoding this human tumor antigen. The approaches described in this paper, SEM combined with expression cloning, should prove of wide utility for developing immunological reagents specific for and identifying genes relevant to human cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Dubnick M., Kerlavage A. R., Moreno R., Kelley J. M., Utterback T. R., Nagle J. W., Fields C., Venter J. C. Sequence identification of 2,375 human brain genes. Nature. 1992 Feb 13;355(6361):632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994 Aug 19;269(33):20807–20810. [PubMed] [Google Scholar]
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Drickamer K., Taylor M. E. Biology of animal lectins. Annu Rev Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- Hadari Y. R., Paz K., Dekel R., Mestrovic T., Accili D., Zick Y. Galectin-8. A new rat lectin, related to galectin-4. J Biol Chem. 1995 Feb 17;270(7):3447–3453. doi: 10.1074/jbc.270.7.3447. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J., Kasai K. The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993 Aug;3(4):297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Kawinski E., Karr J. P., Rosenthal H., Chu T. M., Mirand E. A., Murphy G. P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983 Apr;43(4):1809–1818. [PubMed] [Google Scholar]
- Irimura T., Matsushita Y., Sutton R. C., Carralero D., Ohannesian D. W., Cleary K. R., Ota D. M., Nicolson G. L., Lotan R. Increased content of an endogenous lactose-binding lectin in human colorectal carcinoma progressed to metastatic stages. Cancer Res. 1991 Jan 1;51(1):387–393. [PubMed] [Google Scholar]
- Jiang H., Lin J., Su Z. Z., Herlyn M., Kerbel R. S., Weissman B. E., Welch D. R., Fisher P. B. The melanoma differentiation-associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene. 1995 May 4;10(9):1855–1864. [PubMed] [Google Scholar]
- Leon J. A., Goldstein N. I., Fisher P. B. New approaches for the development and application of monoclonal antibodies for the diagnosis and therapy of human cancer. Pharmacol Ther. 1994;61(1-2):237–278. doi: 10.1016/0163-7258(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Apodaca G., Barondes S. H., Mostov K. E., Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993 Jun 5;268(16):11750–11757. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Lotan R., Ito H., Yasui W., Yokozaki H., Lotan D., Tahara E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int J Cancer. 1994 Feb 15;56(4):474–480. doi: 10.1002/ijc.2910560404. [DOI] [PubMed] [Google Scholar]
- Lotan R., Lotan D., Raz A. Inhibition of tumor cell colony formation in culture by a monoclonal antibody to endogenous lectins. Cancer Res. 1985 Sep;45(9):4349–4353. [PubMed] [Google Scholar]
- Meromsky L., Lotan R., Raz A. Implications of endogenous tumor cell surface lectins as mediators of cellular interactions and lung colonization. Cancer Res. 1986 Oct;46(10):5270–5275. [PubMed] [Google Scholar]
- Miranda A. F., Duigou G. J., Hernandez E., Fisher P. B. Characterization of mutant human fibroblast cultures transformed with simian virus 40. J Cell Sci. 1988 Apr;89(Pt 4):481–493. doi: 10.1242/jcs.89.4.481. [DOI] [PubMed] [Google Scholar]
- Ohannesian D. W., Lotan D., Thomas P., Jessup J. M., Fukuda M., Gabius H. J., Lotan R. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells. Cancer Res. 1995 May 15;55(10):2191–2199. [PubMed] [Google Scholar]
- Raz A., Carmi P., Pazerini G. Expression of two different endogenous galactoside-binding lectins sharing sequence homology. Cancer Res. 1988 Feb 1;48(3):645–649. [PubMed] [Google Scholar]
- Raz A., Carmi P., Raz T., Hogan V., Mohamed A., Wolman S. R. Molecular cloning and chromosomal mapping of a human galactoside-binding protein. Cancer Res. 1991 Apr 15;51(8):2173–2178. [PubMed] [Google Scholar]
- Raz A., Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 1987;6(3):433–452. doi: 10.1007/BF00144274. [DOI] [PubMed] [Google Scholar]
- Raz A., Meromsky L., Lotan R. Differential expression of endogenous lectins on the surface of nontumorigenic, tumorigenic, and metastatic cells. Cancer Res. 1986 Jul;46(7):3667–3672. [PubMed] [Google Scholar]
- Raz A., Zhu D. G., Hogan V., Shah N., Raz T., Karkash R., Pazerini G., Carmi P. Evidence for the role of 34-kDa galactoside-binding lectin in transformation and metastasis. Int J Cancer. 1990 Nov 15;46(5):871–877. doi: 10.1002/ijc.2910460520. [DOI] [PubMed] [Google Scholar]
- Rosen S. D. Cell surface lectins in the immune system. Semin Immunol. 1993 Aug;5(4):237–247. doi: 10.1006/smim.1993.1028. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeppner H. L., Raz A., Ho S. B., Bresalier R. S. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995 Jun 15;75(12):2818–2826. doi: 10.1002/1097-0142(19950615)75:12<2818::aid-cncr2820751206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Shen R., Su Z. Z., Olsson C. A., Fisher P. B. Identification of the human prostatic carcinoma oncogene PTI-1 by rapid expression cloning and differential RNA display. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6778–6782. doi: 10.1073/pnas.92.15.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R., Su Z. Z., Olsson C. A., Goldstein N. I., Fisher P. B. Surface-epitope masking: a strategy for the development of monoclonal antibodies specific for molecules expressed on the cell surface. J Natl Cancer Inst. 1994 Jan 19;86(2):91–98. doi: 10.1093/jnci/86.2.91. [DOI] [PubMed] [Google Scholar]
- Su Z. Z., Olsson C. A., Zimmer S. G., Fisher P. B. Transfer of a dominant-acting tumor-inducing oncogene from human prostatic carcinoma cells to cloned rat embryo fibroblast cells by DNA-transfection. Anticancer Res. 1992 Mar-Apr;12(2):297–304. [PubMed] [Google Scholar]
- Waldmann T. A. Monoclonal antibodies in diagnosis and therapy. Science. 1991 Jun 21;252(5013):1657–1662. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]