Abstract
There is a great deal of variability in mother-infant interactions and infant behavior across the first year of life in rhesus monkeys. The current paper has two specific aims: 1) to determine if birth timing predicts variability in the mother-infant relationship and infant behavior during weaning and maternal breeding, and 2) to identify predictors of infant behavior during a period of acute challenge, maternal breeding. Forty-one mother-infant pairs were observed during weaning when infants were 4.5 months old, and 33 were followed through maternal breeding. Subjective ratings of 16 adjectives reflecting qualities of maternal attitude, mother-infant interactions, and infant attitude were factor analyzed to construct factors relating to the mother-infant relationship (Relaxed and Aggressive), and infant behavior (Positive Engagement and Distress). During weaning, late born infants were more Positively Engaged than peak born infants (ANOVA, P < 0.05); however, birth timing did not affect the mother-infant relationship factors Relaxed and Aggressive or the infant attitude factor Distress. During maternal breeding early born infants had less Relaxed relationships with their mothers than peak or late born infants, higher Positive Engagement scores than peak or late born infants, and tended to have higher Distress scores than peak born infants (Repeated-measures ANOVA, P < 0.05). In addition, Distress scores were higher during maternal breeding than during the pre- and post-breeding phases. Finally, multiple regression (P < 0.05) indicated that while infant behavioral responsiveness predicted infant Positive Engagement during the acute challenge of maternal breeding, qualities of the mother-infant relationship predicted infant Distress. These data suggest that birth timing influences the patterns of mother-infant interactions during weaning and maternal breeding. Additionally, infant behavioral responsiveness and mother-infant relationship quality impact infant social engagement and affect expression, respectively.
Keywords: Birth timing, rhesus monkey, mother-infant relationship, infant behavior, temperament
INTRODUCTION
Over the first year of a rhesus monkey’s life, the mother-infant relationship changes drastically, and variation in how this relationship changes could have implications for later development and social functioning. Whereas the first few months of life are characterized by high levels of maternal care, by the end of the first year infants are spending the majority of their time away from their mother [Hinde and Spencer-Booth 1967; Kaufman 1974]. Although there is a general decrease in the amount of time mothers and infants spend together, Hinde and Spencer-Booth [1967] reported a peak in maternal rejection and greater fluctuations in mother-infant contact between 4.0–7.5 months of age in rhesus monkeys than at any other time in the first year of life. What might cause this variation, and what might be the consequences of variation in the mother-infant relationship during this period?
Weaning and maternal resumption of breeding are two challenges that infants face when they are between 4 and 7.5 months of age, and variability in how mothers and infants negotiate these challenges may have important consequences for both the fitness of the mother and her offspring. Weaning in primates is a gradual process where the mother decreases infant suckling over a period of weeks to months, and can be defined as ending when the mother resumes reproductive cycling [Lee 1996]. It is also a period in which there is the potential for high levels of parent-offspring conflict over the degree of investment by the mother in the current offspring [Maestripieri 2002; Trivers 1974]. For the mother to maximize her fitness, she must ensure the survival of her current offspring yet must reduce infant suckling in order to resume estrus and conceive the next season’s offspring. This tradeoff is especially salient in rhesus monkeys as studies of reproductive physiology have shown that infant suckling is a potent inhibitor of ovulation, and sustained high rates of infant suckling have been associated with reduced ability to conceive, especially during the first half of the breeding season [Johnson et al. 1993]. The fitness of the infant, on the other hand, would be maximized by delaying maternal resumption of estrus and thus potentially securing more maternal investment.
Once infant suckling is sufficiently reduced, the mother begins reproductive cycling and the mother-infant relationship goes through a period of concentrated change as the mother shifts her attention away from infant care and toward breeding activities. When breeding, male and female rhesus monkeys form consortships, which are temporary associations that can last hours to days [Wolfe 1984]. During a consortship many male and female dyads will move to the periphery of the social group and isolate themselves from other group members, including the female’s own offspring [Berman et al. 1994; Stern and Smith 1984]. Berman and colleagues [1994] examined the mother-infant relationship during this period and have shown that there are marked decreases in mother-infant contact and proximity, and increased maternal rejection. These results led to the suggestion that the mother’s resumption of breeding may act as a naturalistic mother-infant separation. They also reported that this naturalistic separation led to infant distress and even a depressive response in some infants. After a consort relationship ends the mother will often resume a higher level of infant care than she exhibited while mating, although it is common for a female to cycle and enter into consortships more than once. As both weaning and maternal breeding occur at a time where a large degree of variation in mother-infant contact has been reported [Hinde and Spencer-Booth 1967], we sought to examine the factors that influence variability in the mother-infant relationship during these two periods.
Predictors of variability in the mother-infant relationship
One source of variability for mother-infant interactions is maternal style, which has been characterized as comprising two independent dimensions: protective [approaches, grooms, and restrains infant] and rejecting [rejects, breaks contact, and leaves infant; Fairbanks 1996]. These styles have been shown to be both constant over time with the same infant, and consistent for individual mothers across infants [Berman 1990; Fairbanks 1989; Fairbanks and McGuire 1987]. In addition to maternal style, prior maternal experience has been shown to influence maternal behavior such that primiparous mothers tend to be to be more excitable, less confident, and more protective or restrictive of their infants, and to allow their infants to suckle more frequently than do multiparous mothers [Gomendio 1991; Hooley and Simpson 1981; Kaufmann 1966; Maestripieri 1998; Schino et al. 1995]. The social environment can also affect maternal behavior; infants born into larger matrilines or that had older siblings were rejected more and spent less time on their mother [Berman 1992; Hooley and Simpson 1981; Schino et al. 1995]. In addition, under conditions of high risk for social aggression or low maternal rank mothers tend to be more protective of their infants [Maestripieri 1998; White and Hinde 1975]. Finally, environmental conditions like the availability or predictability of food resources can also influence maternal behavior [Hauser and Fairbanks 1988; Rosenblum and Paully 1984]. All of these findings indicate that the social and environmental context in which a mother rears her offspring, as well as her own individual style, will influence her behavior towards her infant.
A variety of infant factors have also been suggested as influencing the ways mothers and infants interact. Findings on the effects of infant sex on mother-infant interactions have been mixed; while Hooley and Simpson [1981] reported that infant sex and parity interacted such that primiparous mothers were more protective of daughters and multiparous mothers were more protective of sons, other studies have found no relationship [e.g. Fairbanks 1996]. Examinations of infant age indicated that younger infants delayed their mother’s breeding onset and were able to maintain higher rates of contact when their mothers did resume breeding [Berman et al. 1994]. While the impact of birth timing on maternal behavior has yet to be examined, birth timing may have an effect similar to infant age. Fürtbauer and colleagues [2010] reported that mothers that gave birth early in the birth season were more likely to have a 1-year interbirth interval (IBI) whereas mothers that gave birth late in the birth season were more likely to have a 2-year IBI. In a seasonally breeding species, mothers that give birth late in the birth season are likely to have infants that are younger and not fully weaned at the start of the breeding season. This may lead the mother to engage in one of two different strategies; to maximize her reproductive output the mother may limit her investment in her current infant by weaning it at an earlier age in order to begin reproductive cycling and produce another infant. On the other hand, she could maintain a higher level of investment in the current offspring and delay reproduction altogether as was suggested by the larger IBI for mothers of late born infants [Fürtbauer et al. 2010]. If this tradeoff exists, we would expect that mothers that give birth early in the birth season would be under different constraints as mothers that give birth late in the birth season. Therefore we might predict that the patterns of mother-infant interactions between early born infants and their mothers versus late born infants and their mothers would differ.
The degree of tension or conflict within the mother-infant relationship may be related to how well the behavior of the mother and infant “mesh” [Hinde 1977]. A greater degree of concordance in mother and infant behavior would likely lead to a more relaxed mother-infant relationship, whereas divergence in the behavior and goals of the mother and infant would lead to greater conflict. The factors discussed above may influence how well the behavior of mothers and infants mesh, and therefore the levels of conflict each dyad exhibits. For example, we might predict that due to the reproductive constraints on mothers of late born infants there may be a greater divergence of goals of the mother and infant during weaning, and therefore higher levels of conflict in the mother-infant relationship might be predicted to occur.
Predictors of Infant Behavior
Variation in maternal behavior may have long lasting effects on both infant physiological and behavioral development. For example, in rats, low amounts of maternal care have been associated with an increased fear of novelty and altered regulation of the hypothalamic-pituitary-adrenal axis [Cameron et al. 2005; Francis and Meaney 1999]. In primates, variation in maternal style has been associated with infant independence, environmental exploration, and infant distress. Protective mothers tend to have infants that show less interest in the external environment and longer latencies to enter a novel environment [Bardi and Huffman 2002; Fairbanks and McGuire 1988], and maternal rejection has been associated with greater social interaction and infant distress vocalizations [Bardi and Huffman 2002; Bardi and Huffman 2006].
Although maternal behavior is a strong organizing factor in infant development, infant temperament may also influence mother-infant interactions and infant behavior outside the context of the mother-infant relationship. One temperament trait, reactivity, is characterized by frequent expressions of fear and anxiety [Suomi 1999]. Highly reactive rhesus monkey infants were less likely to venture away from their mothers to explore their surroundings and could alter patterns of contact within the mother-infant relationship by soliciting more maternal care.
The current study aimed to investigate how infant sex, maternal rank, and birth timing influence the mother infant-relationship and infant behavior during weaning and maternal resumption of breeding. In particular, we examined variation in general themes or styles of mother-infant interactions and infant behavior or attitude. We predicted that infants born late in the birth season would have more conflictual relationships with their mothers during weaning than would late born infants. In addition, we explored whether maternal behavior and infant temperament predicted infant behavior during maternal breeding, a period of acute challenge for the infant.
METHODS
Subjects and Housing
Subjects were 41 rhesus monkeys (21 Male; Macaca mulatta) housed at the California National Primate Research Center (CNPRC). All animals were born to multiparous mothers between the ages of 5 and 15 years, and all mothers had reared at least one infant past nine months of age. Infants were observed in two cohorts, 20 (10 male) were born in 2007 and 21 (11 male) were born in 2008. Each mother contributed only one infant to the sample. Infants were born and reared in one of four large naturalistic social groups which were housed outdoors in 0.2 ha field cages consisting of 125–175 animals with age and sex compositions similar to that in the wild. Cages contained multiple perches, climbing structures, and small shelters to provide protection from the rain and wind. Food and water were available ad libitum. All experimental procedures were approved by the University of California-Davis Institutional Animal Care and Use Committee (IACUC) and adhered to the American Society of Primatologists principles for the ethical treatment of primates.
Birth Timing
At the CNPRC, the outdoor housed animals breed seasonally during the fall (September–December) and give birth in the spring (February–May). The start of the birth season was determined for each of the 4 field cages separately and was considered to start when the 1st infant was born in each cage. The birth timing measure was then calculated by determining the number of days each subsequent infant was born after the start of the birth season in that cage. Subjects were born an average of 50.7 days after the start of the birth season (range: 3–91 days). To allow for the use of ANOVA for analysis, the earliest 25%, the middle 50%, and the latest 25% of animals were grouped together to form early, peak, and late born groups.
Biobehavioral Infant Assessment
All subjects were participants in an infant BioBehavioral Assessment (BBA) program at the CNPRC when they were approximately 3.6 months of age (Range: 3.2 – 4.0 months). The methods of this assessment are described in detail elsewhere [Capitanio et al. 2006]. Briefly, infants were removed from their home cages, separated from their mothers, and relocated to an unfamiliar indoor testing environment where they were housed individually in temporary holding cages for a period of 25 h. During this period, infants were given a battery of tests to assess behavioral and physiological reactivity. All behavioral data were collected by trained observers who had established reliability greater than 85% agreement. At the end of the 25 h testing period infants’ temperament was assessed, and infants were reunited with their mothers for an hour, after which the mother-infant pairs were returned to their home cages.
Data from an assessment of behavioral responses to separation and relocation were used in the current analysis. This assessment consisted of a 5 minute focal observation at the start of the 25-hour testing period, and recorded both states (e.g. lie, crouch, locomote) and events (e.g. vocalizations, facial expressions). Exploratory and confirmatory factor analysis (using promax rotation) of the behaviors during these observations yielded a two factor structure [see details in Golub et al. 2009], Activity and Emotionality, that characterized the infants’ initial responsiveness to the separation and relocation. Factors scores were calculated by summing the z-scores of the items that loaded on the factor and reliability statistics indicated good reliability (Cronbach’s alphas ranged from 0.6–0.8). Factor scores were then expressed as z-scores calculated within each yearly cohort of animals.
Observation Schedule
Behavioral observations in the field cages occurred between 9 am–1 pm, 5–7 days per week. Observations began when infants were 4.5 months of age and continued until 2 weeks after the mother ended the first consortship of the breeding season. Observations consisted of twice weekly 10 minute focal observations unless the mother was engaged in consortship activities. During maternal breeding, observation density increased to daily 15 minute observations to ensure a total of at least 30 minutes of data was available. To determine the start of maternal breeding, consortship scans were conducted at least twice daily to assess the breeding status of all mothers in the sample. Consortship activity was evidenced by mate-guarding, contact maintenance through mutual following or grooming, mounting, sex presents, or other evidence of sexual activity [i.e. coagulated semen in the perineal region; Stern and Smith 1984; Wolfe 1984]. Mounting behavior or coagulated semen was sufficient evidence to identify a consortship; for all other indicators at least 2 different behaviors had to be seen to indicate a consortship was occurring.
The study period was divided into four phases. Phase 1 provided a snapshot of mother-infant interactions and infant behavior during weaning (which was at least 3 weeks prior to maternal resumption of breeding) and consisted of the observations conducted when infants were 4.5–5.0 months of age. Because maternal breeding occurred at variable infant ages, a baseline period just prior to maternal breeding was also identified (Phase 2), which consisted of the observations from the 2–3 weeks before the mother was recorded as engaging in consortship activities. (The week immediately preceding consortship activity was not used due to the difficulty in defining a precise date for the start of breeding activity for all mothers.) Once the mother was seen to engage in a consortship, Phase 3 began and continued until the breeding activity records indicated the mother had not engaged in consortship behavior for 4 consecutive days [Berman et al. 1994]. Because the mother may not have been seen to engage in consortship activities every day during Phase 3, we only included data collected on days in which consortship activities were clearly evident. After the completion of Phase 3, the post-breeding phase began (Phase 4) and consisted of 2 weeks of twice weekly 10 minute observations (see Table 1 for a summary). Phases 1, 2, and 4 each consisted of 3–4 observations over a two week period. Because consortship durations were variable, Phase 3 had a mean of 6 observations (Range: 2–16) per dyad. For each phase, behavioral data and adjective data (see below) were expressed as the mean rating (for the adjective ratings), mean rate (for events), or mean percent of observation (for states).
Table 1.
Observation Schedule
| Phase | Purpose | When | Duration |
|---|---|---|---|
| 1 | To assess behavior during weaning but prior to maternal resumption of breeding | Infant age: 4.5–5.0 months | Twice weekly 10 minute observations |
| 2 | To assess behavior immediately prior to maternal resumption of breeding | 2–3 weeks prior to maternal resumption of breeding activity Mean infant age: 5.9 mos; Range: 5.0 – 7.1 mos |
Twice weekly 10 minute observations |
| 3 | To assess behavior during maternal breeding | Days when the mother was recorded as engaging in consortship activities. Mean infant age: 6.6 mos; Range 5.7 – 7.8 mos |
Daily 15 minute observations |
| 4 | To assess behavior after maternal breeding has ended | 2 weeks immediately after the cessation of maternal breeding Mean infant age:7.1 mos; Range 6.2 – 8.2 mos |
Twice weekly 10 minute observations |
Behavioral Observations
Focal animal observations were conducted on each infant. Interactions with the mother and infant behaviors relating to anxiety or distress were recorded using a modified version of the transactional data collection scheme described by Mason and colleagues [1993]. For all mother-infant interactions, the state of association between the mother and infant was recorded and included: suckling, ventral contact, non-ventral contact, proximity (1–60 cm away from mother), or non-social (more than 60 cm away from the mother). In addition, information regarding who initiated the state (mother or infant), and the response of the receiver (accommodate, neutral, resist, or aggression) was recorded. All instances of grooming or aggression between the mother and infant were also recorded in this format but were treated as events and not states [Altmann 1974]. In addition, any occurrences of infant vocalizations (coo, gecker, scream) or potential indicators of infant distress or anxiety (scratch, yawn, self-groom) were also recorded [Martin and Bateson 1993]. Data were recorded using the observer handheld (Noldus Information Technology, Wageningen, The Netherlands). Inter-observer reliability was 88.7%.
Adjective Ratings
Adjective ratings were made at the end of each observation session to get an overall picture of the dynamic qualities and themes of the mother-infant interactions, and infant behavior outside the mother-infant relationship. Ratings were conducted in a manner similar to the way personality assessments are conducted [e.g. Capitanio 1999] but instead of assessing stable traits of the individual based on long acquaintance with the animals, ratings were designed to assess the attitudes of individuals using the observers’ impressions of the animals during an individual focal observation period [Capitanio et al. 1997]. This type of assessment allows for the incorporation of behaviors that are otherwise difficult to reliably capture during live data collection on animals housed in large social groups [ex. posture or gaze; Emery et al. 2001]. The adjective ratings consisted of 4 maternal attitude adjectives (nurturing, restrictive, indifferent, and aggressive), 4 mother-infant interaction theme adjectives (conflictual, tense, calm or relaxed, aggressive) and 8 infant attitude adjectives (fearful, relaxed, depressed, nervous, active, confident, affiliative, or playful; see Table 2 for adjective definitions). Adjectives were grouped into two sets for the purposes of analysis; adjectives relating to maternal attitude and the mother-infant interaction themes were combined and used to assess the dynamic qualities of the mother-infant relationship. Infant attitude adjectives were analyzed separately and were used to provide information on infant responses and attitude outside the mother-infant relationship. All ratings used a 7-point Likert scale with 1 indicating a total absence of the attribute and a 7 indicating an extremely large amount of the attribute was present. Inter-rater agreement was 92.2% when ratings were allowed to vary by 1 point.
Table 2.
Adjective Rating Definitions
| Maternal Attitude Adjectives
| |
| Nurture | Mother seems interested and motivated to interact with her infant and exhibits high levels of maternal behavior (grooming, ventral carrying). Mother also accommodates infant initiated interactions frequently. |
| Indifferent | Mother does not respond either negatively or positively to infant. |
| Abuse or aggression | The mother threatens, hits, or pushes infant often. |
| Restrictive | The mother restrains infant or resists infant attempts to leave mother. |
|
| |
| Mother-Infant Interaction Adjectives
| |
| Calm and Relaxed | Dyad interact in a calm, easy, and relaxed manner. |
| Conflictual | Interactions between the mother and infant frequently result in conflict, often evidenced by one of the pair resisting overtures by the other. |
| Tense | Mother and infant interact but the overtures appear cautious or timid. Infant exhibits a great degree of vigilance towards the mother but appears to be reluctant or cautious about initiating an interaction. Shows restraint in posture and movement during interactions. |
| Aggressive | Transactions between the mother and infant contain aggression and threats or overtures by one member of the dyad are met with aggression from the other. |
|
| |
| Infant Attitude Adjectives
| |
| Active | Moves about a lot, distance traveled by walking, running, climbing, or jumping. Not lethargic. |
| Confident | Behaves in a positive, assured manner, not restrained or tentative. |
| Affiliative | Actively seeks companionship and interaction with others. |
| Playful | Initiates play and joins in play when initiated by others. |
| Fearful | Fear grins; retreats readily from others. |
| Nervous | Anxious, jittery, not calm. Frequently exhibits anxiety related behaviors (e.g. scratch, yawn). |
| Depressed | Infant exhibits depressed posture in sitting position with the head hanging lower than the shoulders. Not sleeping. |
| Relaxed | Infant moves or interacts with others in a calm, easy and relaxed manner. Exhibits a loose posture. Not tense. |
Data analysis
Principal axis factoring using oblique rotation was conducted on the attitude ratings from the Phase 1 observations using the data from all 41 subjects in order to assess themes in 1) the mother-infant relationship (including 3 maternal attitude and 4 interaction theme adjectives) and 2) infant attitude (7 adjectives). One adjective was excluded from each factor analysis (maternal restrictiveness and infant depression, respectively) due to low variability in the ratings. Factor scores were constructed by adding together the scores from the adjectives that had loadings greater than 0.30, and were then expressed as a proportion of the maximal possible score for that factor resulting in each factor having a minimum of zero and maximum of one. Reliability of the resulting scales was determined using Cronbach’s alpha; if low reliability was indicated, that factor was adjusted by removing the adjective that showed the lowest reliability relative to the others and reliability was re-examined. Validity was established by examining the patterns of correlations with the behavioral data collected during the observations that preceded the adjective ratings. Of the 41 infants observed during weaning (Phase 1), complete data (for Phases 2–4) were available for 33 infants (17 male). Notably, two of the late born infants were excluded from further analyses because their mothers were not observed to engage in mating behavior. The missing data comprised 1 early, 2 peak, and 5 late born infants.
The effect of birth timing was examined during two different time periods, weaning (Phase 1) and breeding (Phases 2–4). For all further analyses the reduced, but complete, sample (N = 33) was used to allow for direct comparisons of effects between weaning and maternal breeding. The analysis of weaning data (Phase 1) consisted of a series of ANOVAs in which the effects of birth-timing (early, N = 9; peak, N = 18; and late, N = 6), infant sex, and maternal rank (high, N = 12; middle, N = 10; and low, N = 11) on the mother-infant relationship and infant attitude factors were examined. The breeding analysis (Phases 2–4) used a series of repeated measures ANOVAs to examine how qualities of the mother-infant relationship and infant attitude changed in the time surrounding maternal breeding. Birth timing, infant sex, and maternal rank were entered as between subjects variables and time (Phases 2–4) was the within-subjects variable. In addition, due to the fact that infants were of variable ages when maternal breeding resumed, infant age at maternal resumption of breeding was included in the analysis as a covariate. For all tests, if the assumption of sphericity was violated, the Huynh-Feldt adjustment was used; post hoc tests compared the estimated marginal means using the Sidak correction for multiple comparisons.
Finally, to examine whether the qualities of the mother-infant relationship and infant behavioral responses from the BioBehavioral Assessment were related to infant attitude during maternal breeding (Phase 3), hierarchical multiple regressions were conducted with each of the infant attitude factors as dependent variables. The first step of the regressions included birth timing, infant sex and maternal rank as covariates. The second step included the mother-infant relationship factors from maternal breeding (Phase 3), and the third step included the behavioral responsiveness factors, Activity and Emotionality, to determine if infant temperament would predict infant responses above and beyond what was predicted by maternal behavior. For each model, steps 2 and 3 were only retained if they resulted in a significant improvement in the predictive power (R2) of the model.
RESULTS
Mother-Infant Relationship and Infant Attitude Factors
The factor analysis of the maternal attitude and mother-infant interaction adjectives from the Phase 1 observations resulted in two factors that explained 52.97% of the common variance. The first factor, “Relaxed,” reflected an interaction style between the mother and infant that was calm and relaxed, not conflictual or tense, and a mother that was nurturing and not indifferent to her infant (see Table 3 for factor loadings). The second maternal attitude factor, “Aggressive,” was characterized by high levels of aggression or abuse by the mother and aggression within the mother-infant relationship. The mother-infant relationship factors were significantly negatively correlated (r = −0.470, P = 0.002).
Table 3.
Relationship and Infant Attitude Factor Loadings
| Mother-Infant Relationship
|
Infant Attitude
|
|||
|---|---|---|---|---|
| Relaxed | Aggressive | Positive Engagement | Distress | |
| Maternal and Dyad Adjectives | ||||
|
| ||||
| Nurture | 0.571 | −0.069 | ||
| Abusive or Aggressive | −0.046 | 0.903 | ||
| Indifferent | −0.404 | −0.008 | ||
| Conflict | −0.738 | 0.013 | ||
| Tense | −0.745 | 0.102 | ||
| Calm and Relaxed | 0.764 | 0.092 | ||
| Aggressive | 0.014 | 0.776 | ||
|
| ||||
| Infant Adjectives | ||||
|
| ||||
| Fearful | −0.020 | 0.551 | ||
| Nervous | −0.211 | 0.792 | ||
| Active | 0.885 | 0.222 | ||
| Confident | 0.868 | −0.101 | ||
| Affiliative | 0.794 | −0.126 | ||
| Playful | 0.785 | 0.001 | ||
| Relaxed | −0.231 | −0.402a | ||
|
| ||||
| Cronbach’s alpha | 0.710 | 0.743 | 0.893 | 0.618 |
Adjectives with factor loadings greater than 0.3 (indicated in bold) were used in the construction of that scale.
This adjective was removed to improve scale reliability.
Factor analysis of the infant attitude adjectives resulted in two factors that explained 57.72% of the common variance in infant attitude recorded outside the mother-infant relationship. These factors were named “Positive Engagement” and “Distress,” and were independent of each other (r = −0.105). Table 3 lists the adjectives and the reliability statistics for all the factors. The relationship factor Relaxed was significantly negatively associated with infant Positive Engagement (r = −0.416, P = 0.007) and Distress (r = −0.504, P = 0.001). The relationship factor Aggressive did not correlate significantly with either of the infant attitude factors (Positive Engagement: r = 0.197; Distress: r = .277).
Factor Scores and Behaviors
Factor scores correlated with the behaviors recorded during the preceding focal observations in predictable ways suggesting that these are valid reflections of characteristics of the mother-infant relationship and infant attitude. Within dyads scoring high on the relationship factor Relaxed, mothers spent more time nursing their infants and had a higher rate of nursing bouts, spent less time away from their infants, groomed their infants more, and resisted infant overtures less (See Table 4 for correlation coefficients and significance levels). Dyads scoring high on the Aggressive factor had a significantly greater rate of aggressive interactions. Infants that scored high on the Positive Engagement factor were less likely to interact with their mothers; they spent more time away from their mothers, less time nursing and exhibited lower rates of nursing, contact, mother initiated ventral contact, and mother initiated leaves. Finally, infants scoring high on the Distress infant attitude factor were more likely to scream, gecker, and had mothers that left them more frequently.
Table 4.
Correlations between Factors and Behaviora
| Mother-Infant Relationship
|
Infant Attitude
|
|||
|---|---|---|---|---|
| Relaxed | Aggressive | Positive Engagement | Distress | |
| Durations | ||||
|
| ||||
| Infant suckling | 0.465** | −0.137 | −0.635** | −0.059 |
| Away from mother (> 60 cm) | −0.404** | 0.082 | 0.670** | −0.075 |
|
| ||||
| Rates | ||||
|
| ||||
| Aggression (between mother an infant) | −0.151 | 0.686** | −0.216 | 0.214 |
| Contact | 0.159 | −0.011 | −0.435** | 0.242 |
| Infant Suckling | 0.319* | −0.069 | −0.381* | 0.069 |
| Mother leaving infant | −0.194 | 0.122 | −0.313* | 0.501** |
| Mother resisting infant overtures | −0.408** | 0.124 | 0.043 | 0.231 |
| Mother initiates ventral contact | 0.168 | 0.113 | −0.408** | 0.234 |
| Groom | 0.404** | 0.222 | −0.129 | −0.130 |
| Scream | −0.146 | 0.041 | −0.230 | 0.407** |
| Gecker | −0.129 | 0.211 | −0.159 | 0.315* |
p<0.05,
p < 0.01
Behavioral and adjective data collected during Phase 1.
The mother-infant relationship and infant attitude during weaning
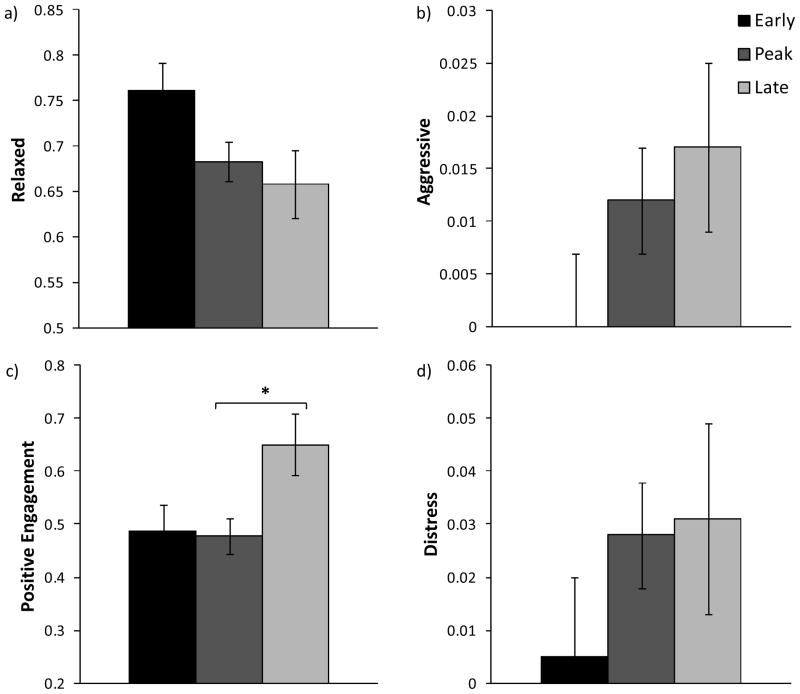
Birth timing was significantly related to Positive Engagement (F (2, 27) = 3.467, P = 0.046), but not to either of the mother-infant relationship factors or the infant attitude factor Distress. Post-hoc analyses indicate that late born infants had higher Positive Engagement scores than peak born infants (P = 0.049; see Figure 1c).
Figure 1.
Mean (± standard error) factor scores by birth timing group for the weaning phase (Phase 1; N=33). There was no significant effect of birth timing for the mother-infant relationship factor Relaxed (a) and Aggressive (b), or the infant attitude factor Distress (d). Late born infants had significantly higher Positive Engagement scores than peak born infants (c). * P < 0.05
The mother-infant relationship and infant attitude during maternal breeding
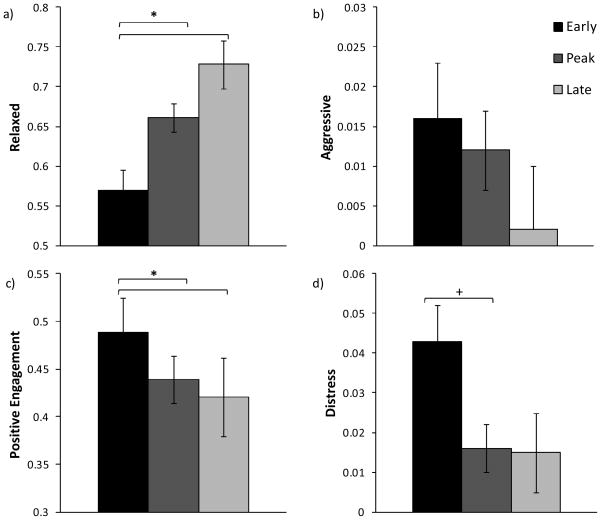
Birth timing was significantly related to the relationship factor Relaxed (F (2, 26) = 7.390, P = 0.003; See Figure 2a), but not to the Aggressive factor. Post hoc analysis indicated that early born infants and their mothers had, on average, significantly lower Relaxed factor scores than did peak (P = 0.041) or late born (P = 0.002) infants. There were no main effects of maternal rank, infant sex, or significant changes in the relationship factor Relaxed over the breeding period. There were no significant effects for the relationship factor Aggressive.
Figure 2.
Mean (± standard error) factor scores by birth timing group for the breeding period (Phases 2–4; N = 33). Early born infants had less Relaxed relationships with their mothers than peak or late born infants (a), higher Positive Engagement scores than peak or late born infants (c), and tended to have higher Distress scores than peak born infants (d). There was no significant main effect of birth timing for the mother-infant relationship factor Aggressive (b). * P < 0.05, + P < 0.1
Birth timing was related to Positive Engagement and Distress scores during maternal breeding and there were significant changes in infant Distress across the breeding period. Examination of the Positive Engagement attitude factor indicated a main effect for birth timing (F (2, 26) = 5.594, P = 0.010; See Figure 2c), but no significant effects for infant sex or maternal rank. Post hoc analysis indicated that early born infants were, on average, more Positively Engaged during maternal breeding than peak born (P = 0.015) or late born (P = 0.023) infants.
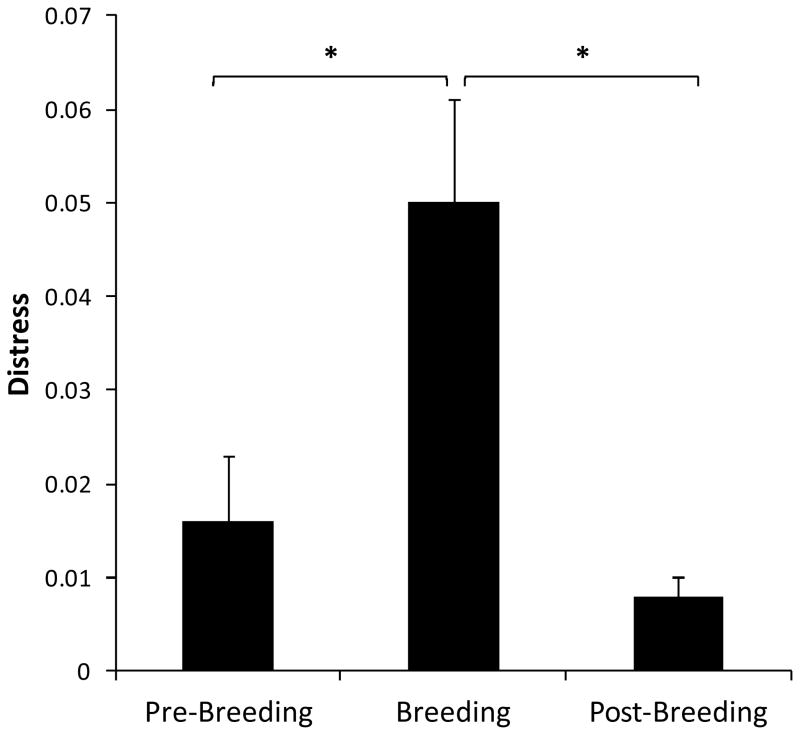
Birth timing was also related to infant Distress (F (2, 26) = 3.422, P = 0.048; See Figure 2d); early born infants tended to have higher Distress scores than peak born infants (P = 0.058). Finally, there was a significant within-subject effect for infant Distress (F (2, 52) = 3.547, P = 0.037; See Figure 3). Post hoc analyses indicated that infant Distress was higher during maternal breeding than during the two weeks before (P = 0.034) or after (P = 0.002) maternal breeding.
Figure 3.
Average Distress factor scores (means ± standard error) across the breeding period. Distress was higher during maternal breeding than during the pre- and post-breeding phases. * P < 0.05
Predictors of Infant Attitude during Maternal Resumption of Breeding
During breeding (Phase 3), the Positive Engagement factor was predicted by infant behavioral responsivity (from the BioBehavioral Assessment) while the Distress factor was predicted by qualities of the mother-infant relationship. For the Positive Engagement factor, entry of the behavioral responsivity factors (step 3), but not the mother-infant relationship factors (step 2), significantly added to the predictive power of the model (Step: F (2, 27) = 4.254, P = 0.025; adjusted R2 = 0.129): animals that responded with greater Activity during the separation and relocation at 3–4 months of age were more Positively Engaged (β = 0.501, P = 0.007) during maternal resumption of breeding. In contrast, the Distress factor scores during maternal resumption of breeding were predicted by the mother-infant relationship factors (step 2; F (2, 27) = 7.374, P = 0.003, adjusted R2 = 0.294), but not behavioral responsivity (step 3). Specifically, higher scores on the relationship factor Aggressive were associated with higher scores on the infant Distress factor (β = 0.447, P = 0.031).
DISCUSSION
The current study aimed to better understand individual differences in the mother-infant relationship during weaning and maternal breeding as well as what factors predict infant responses to maternal breeding. We identified two factors, Relaxed and Aggressive, that described the mother-infant relationship and two factors, Positive Engagement and Distress, that described infant attitude. During weaning, birth timing predicted Positive Engagement, with late born infants exhibiting higher Engagement scores than peak born infants. During maternal breeding, birth timing had significant effects on the mother-infant relationship factor Relaxed as well as on both infant attitude factors: on average, early born infants had less Relaxed relationships with their mothers than did peak or late born infants, higher Positive Engagement scores than peak or late born infants, and tended to have higher Distress scores than peak born infants. We also found that Distress scores were higher during maternal breeding (Phase 3) than in the pre- or post-breeding phases (Phases 2 and 4, respectively). Finally, we found that Activity, a measure of behavioral responsiveness during an earlier separation from mother, predicted infant Positive Engagement, whereas infant Distress was predicted by high Aggressive scores within the mother-infant relationship. We discuss these results in more detail below.
Quantifying the Mother-Infant Relationship and Infant Attitude
The current study used a subjective rating methodology to measure the qualities of mother-infant interactions and infant attitude. While some researchers question the use of subjective rating methods [e.g. Vanderwolf 1998], many studies have shown that rating methods have a strong empirical basis, high levels of inter-rater agreement, and predict future behavior [Capitanio 1999; Gosling 2001; Meagher 2009; Vazire et al. 2007]. In general, it has been suggested that ratings may be a more practical method for assessing global or broad traits of individuals, whereas behavior codings may be more appropriate for assessing how context (e.g. presence of absence of conspecifics) affects behavior [Vazire et al. 2007; Weinstein et al. 2008]. Like the transactional analysis of mother-infant relationships [Mason et al. 1993], a rating methodology provides a way to assess the affective tone of interactions within a relationship.
In the current study, two factors relating to the mother-infant relationship were identified that reflect the dynamic ways in which mothers and infants interact with each other. Examination of the correlations presented in the validity analysis (Table 4) suggests these factors correlate with the behavior codings in predictable ways. For example, the relationship factor Relaxed was associated with the mother and infant interacting in a positive way (positively correlated with infant suckling and maternal grooming and negatively correlated with the mother resisting infant overtures), but was not correlated with the rate of aggressive interactions. The Aggressive factor was correlated with the rate of aggressive interactions, but not the non-aggressive instances of the mother resisting infant overtures. These patterns of correlation provide evidence of both convergent and discriminate validity for these factors, and we propose the current methodology as a complementary approach to the more traditional methods of behavior recording that allows for quantification of affective or motivational meshing within a relationship.
Further examination of the correlations presented in Table 4 suggests that the current measures of the mother-infant relationship are influenced by, but are not synonymous with, maternal style. For example, the relationship factor Relaxed was positively correlated with maternal grooming of the infant (a component of maternal protectiveness) and was negatively correlated with maternal rejection [Fairbanks 1996], but was unrelated to other indicators of maternal style (ex. lack of correlation with mother-initiated ventral contact). The relationship factor Aggressive was strongly related to the occurrence of aggressive encounters between the mother and the infant. While it was strongly negatively correlated with the Relaxed factor (r = −0.470), evidence suggests that these are two distinct factors. Specifically, in the current analysis the aggression related adjectives did not load on the Relaxed factor, there were significant effects of birth timing on the Relaxed factor but not the Aggressive factor, and infant Distress was predicted by the Aggressive factor but not by the Relaxed factor. Although there is support for the two factor solution in the factor analysis, further data are needed to confirm this distinction.
The infant attitude factors, Positive Engagement and Distress, described two independent patterns of infant response. Infants scoring high on Positive Engagement had lower rates of interaction with their mothers, and interacted with their environment and social group in an active, confident, affiliative and playful manner. This factor may be related to the characteristic of “enterprise” discussed by Simpson, which was suggested to be one that motivates an infant to engage in activities and interactions away from the mother [Simpson and Datta 1991]. The Distress attitude factor, on the other hand, is one that likely reflects the experience of negative affect by the infant, as infants scoring high on Distress were more likely to exhibit distress vocalizations, and were rated more nervous and fearful.
The Role of Birth Timing
Our data indicate that birth timing influences the mother-infant relationship during maternal breeding but not during weaning. Based on the maternal constraints of lactational suppression of estrus, we predicted that mothers of late born infants would have more conflictual relationships with their offspring as they accelerate the weaning process in preparation for the breeding season. This hypothesis was not supported; however we did find that early born infants had less Relaxed relationships with their mothers than peak or late born infants during the breeding period. Although this result was unexpected we believe that this effect may be partially due to the higher levels of social aggression that are present during the breeding season [Wilson and Boelkins 1970]. Late born infants may be more vulnerable to aggression because they are likely to be younger, and potentially smaller, than other infants born that year. Therefore, mothers of late born infants may devote more time and resources to infant care during the breeding season, as suggested by the high Relaxed scores in dyads with late born infants, as a way to protect their infants [Berman et al. 1993]. Overall, these differences in qualities of the mother-infant relationship may indicate that weaning and maternal breeding may pose different levels of challenge for infants depending on whether they were born early or late in the birth season.
Birth timing also predicted both infant attitude factors during maternal breeding. Infants born early in the birth season were more Positively Engaged than peak and late born infants and more Distressed than peak born infants. These infant attitude effects are consistent with the findings for the mother-infant relationship factor Relaxed. If early born infants have less Relaxed relationships with their mothers then they may be less likely to spend time near their mother and instead engage in distress behaviors or social engagement. This would be consistent with effects reported by Bardi and colleagues [2006] where low maternal responsiveness was associated with greater infant anxiety and independence and high maternal rejection was associated with greater infant enterprise. Consistent with what has been previously reported [Berman et al. 1994], there was a significant increase in infant Distress during maternal breeding.
Predictors of infant attitude during maternal breeding
Examination of the predictors of infant attitude during the acute challenge of maternal breeding indicated that qualities of the mother-infant relationship predicted infant Distress, but infant behavioral responsiveness predicted infant Positive Engagement. The correlational analysis showed that both Positive Engagement and Distress were higher when the mother-infant relationship was not Relaxed suggesting that infants were less likely to display negative affect or interact with the social group when they were interacting with their mother in a calm and relaxed fashion. While Positive Engagement tended to be higher when the mother-infant relationship was not Relaxed, these data indicate that under conditions of challenge the infant’s Positive Engagement with the broader social environment was influenced by the temperament characteristics of the infant and not the way the infant interacted with the mother. These data also suggest a consistency in infant responses to the laboratory challenge of maternal separation and relocation, and the natural challenge of maternal breeding. Infants that responded to an experimental challenge at 3–4 months of age with greater activity were more likely to respond to a naturalistic challenge approximately 3 months later with activity and affiliation. In contrast, infant Distress was not predicted by infant temperament but instead by characteristics of the mother-infant relationship, specifically the relationship factor Aggressive. This suggests that infants having Aggressive relationships with their mothers may be more likely to display negative affect when faced with challenge.
Altogether, these results suggest that birth timing may be an important factor in understanding individual differences in the mother-infant relationship. Due to differences in developmental stage, late and early born infants may require different levels of support from their mothers when seasonal events, like maternal breeding, occur. These data suggest that mothers may respond to infant needs and alter the allocation of maternal resources. This variation in mother-infant interactions could have important consequences for maternal fitness and have organizational effects on infant behavioral and physiological development.
Acknowledgments
All procedures were approved by the Institutional Animal Care and Use Committee of the University of California at Davis, comply with all relevant laws of the United States, and adhere to the American Society of Primatologists principles for the ethical treatment of nonhuman primates. We would like to thank L. DelRosso and L. Calonder for their work on the BBA program. We also thank the American Society of Primatologists for the Best Poster Award, given to J. Vandeleest for her presentation of pilot data for the current study. This research was supported by NIH grants #RR000169 (to California National Primate Research Center) and RR019970 (to J.P.C.).
References
- Altmann J. Observational study of behavior: Sampling methods. Behaviour. 1974;49(3–4):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Bardi M, Huffman MA. Effects of maternal style on infant behavior in Japanese macaques (Macaca fuscata) Developmental Psychobiology. 2002;41(4):364–372. doi: 10.1002/dev.10065. [DOI] [PubMed] [Google Scholar]
- Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Dev Psychobiol. 2006;48(1):1–9. doi: 10.1002/dev.20111. [DOI] [PubMed] [Google Scholar]
- Berman CM. Consistency in maternal behavior within families of free-ranging rhesus monkeys: An extension of the concept of maternal style. American Journal of Primatology. 1990;22(3):159–169. doi: 10.1002/ajp.1350220303. [DOI] [PubMed] [Google Scholar]
- Berman CM. Immature siblings and mother-infant relationships among free-ranging rhesus monkeys on Cayo Santiago. Animal Behaviour. 1992;44(2):247–258. [Google Scholar]
- Berman CM, Rasmussen KL, Suomi SJ. Responses of free-ranging rhesus monkeys to a natural form of social separation. I. Parallels with mother-infant separation in captivity. Child Development. 1994;65(4):1028–41. [PubMed] [Google Scholar]
- Berman CM, Rasmussen KLR, Suomi SJ. Reproductive consequences of maternal care patterns during estrus among free-ranging rhesus monkeys. Behavioral Ecology and Sociobiology. 1993;32(6):391–399. [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29(4–5):843–65. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: Prediction of behaviors across time and situation. American Journal of Primatology. 1999;47(4):299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Bond JC, Mason WA. “A state of mind or feeling”: Assessment of the attitudinal domain of social interaction. American Journal of Primatology. 1997;42(2):98–99. [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery Rearing of Nonhuman Primates in the 21st Century. New York: Springer; 2006. pp. 191–214. [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Fairbanks LA. Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Developmental Psychobiology. 1989;22(7):669–681. doi: 10.1002/dev.420220703. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA. Individual differences in maternal style: Causes and consequences for mothers and offspring. Advances in the Study of Behavior. 1996;25:579–611. [Google Scholar]
- Fairbanks LA, McGuire MT. Mother-infant relationships in vervet monkeys: Response to new adult males. International Journal of Primatology. 1987;8(4):351–366. [Google Scholar]
- Fairbanks LA, McGuire MT. Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Developmental Psychobiology. 1988;21(7):711–724. doi: 10.1002/dev.420210708. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9(1):128–34. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Fürtbauer I, Schülke O, Heistermann M, Ostner J. Reproductive and life history parameters of wild female Macaca assamensis. International Journal of Primatology. 2010;31(4):501–517. doi: 10.1007/s10764-010-9409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Developmental Psychobiology. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomendio M. Parent/offspring conflict and maternal investment in rhesus macaques. Animal Behaviour. 1991;42(6):993–1005. [Google Scholar]
- Gosling SD. From mice to men: what can we learn about personality from animal research? Psychological Bulletin. 2001;127(1):45–86. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Fairbanks LA. Mother-offspring conflict in vervet monkeys: variation in response to ecological conditions. Animal Behaviour. 1988;36(3):802–813. [Google Scholar]
- Hinde RA. Mother infant separation and the nature of inter individual relationships: Experiments with rhesus monkeys. Proceedings of the Royal Society of London Series B Biological Sciences. 1977;196(1122):29–50. doi: 10.1098/rspb.1977.0027. [DOI] [PubMed] [Google Scholar]
- Hinde RA, Spencer-Booth Y. The behaviour of socially living rhesus monkeys in their first two and a half years. Animal Behaviour. 1967;15(1):169–96. doi: 10.1016/s0003-3472(67)80029-0. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Simpson MJA. A comparison of primiparous and multiparous mother-infant dyads in Macaca mulatta. Primates. 1981;22(3):379–392. [Google Scholar]
- Johnson RL, Berman CM, Malik I. An integrative model of the lactational and environmental control of mating in female rhesus monkeys. Animal Behaviour. 1993;46(1):63–78. [Google Scholar]
- Kaufman IC. Mother/infant relations in monkeys and humans. In: White NF, editor. Ethology and Psychiatry. Toronto: University of Toronto Press; 1974. pp. 47–68. [Google Scholar]
- Kaufmann JH. Behavior of infant rhesus monkeys and their mothers in a free-ranging band. Zoologica. 1966;51:17–28. [Google Scholar]
- Lee PC. The meanings of weaning: Growth, lactation, and life history. Evolutionary Anthropology. 1996;5(3):87–96. [Google Scholar]
- Maestripieri D. Social and demographic influences on mothering style in pigtail macaques. Ethology. 1998;104(5):379–385. [Google Scholar]
- Maestripieri D. Parent-offspring conflict in primates. International Journal of Primatology. 2002;23(4):923–951. [Google Scholar]
- Martin P, Bateson P. Measuring Behavior: An introductory guide. 2. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Mason W, Long D, Mendoza S. Temperament and mother-infant conflict in macaques: A transactional analysis. In: Mason WA, Mendoza SP, editors. Primate Social Conflict. Albany: SUNY Press; 1993. pp. 205–227. [Google Scholar]
- Meagher RK. Observer ratings: Validity and value as a tool for animal welfare research. Applied Animal Behaviour Science. 2009;119:1–14. [Google Scholar]
- Rosenblum LA, Paully GS. The effects of varying environmental demands on maternal and infant behavior. Child Development. 1984;55(1):305–314. [PubMed] [Google Scholar]
- Schino G, d’Amato FR, Troisi A. Mother-infant relationships in Japanese macaques: Sources of inter-individual variation. Animal Behaviour. 1995;49(1):151–158. [Google Scholar]
- Simpson MJA, Datta SB. Predicting infant enterprise from early relationships in rhesus macaques. Behaviour. 1991;116(1/2):42–63. [Google Scholar]
- Stern BR, Smith DG. Sexual behavior and paternity in 3 captive groups of rhesus monkeys Macaca mulatta. Animal Behaviour. 1984;32(1):23–32. [Google Scholar]
- Suomi SJ. Attachment in rhesus monkeys. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. New York, NY, US: Guilford Press; 1999. pp. 181–197. [Google Scholar]
- Trivers RL. Parent Offspring Conflict. American Zoologist. 1974;14(1):249–264. [Google Scholar]
- Vanderwolf CH. Brain, behavior, and mind: what do we know and what can we know? Neuroscience and Biobehavioral Reviews. 1998;22(2):125–42. doi: 10.1016/s0149-7634(97)00009-2. [DOI] [PubMed] [Google Scholar]
- Vazire S, Gosling SD, Dickey AS, Schapiro SJ. Measuring personality in nonhuman animals. In: Robins RW, Fraley RC, Krueger RF, editors. Handbook of research methods in personality psychology. New York: Guilford Press; 2007. pp. 190–206. [Google Scholar]
- Weinstein TAR, Capitanio JP, Gosling SD. Personality in animals. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality. 3. New York: Guilford Press; 2008. pp. 328–348. [Google Scholar]
- White LF, Hinde RA. Some factors affecting mother infant relations in rhesus monkeys. Animal Behaviour. 1975;23(3):527–542. doi: 10.1016/0003-3472(75)90130-x. [DOI] [PubMed] [Google Scholar]
- Wilson AP, Boelkins RC. Evidence for seasonal variation in aggressive behaviour by Macaca mulatta. Animal Behaviour. 1970;18(4):719–24. doi: 10.1016/0003-3472(70)90017-5. [DOI] [PubMed] [Google Scholar]
- Wolfe LA. Japanese macaque female sexual behavior: A comparison of Arishiyama east and west. In: Small MF, editor. Female Primates: Studies by Women Primatologists. New York: AR Liss; 1984. pp. 141–158. [Google Scholar]