Abstract
The high societal and individual cost of schizophrenia necessitates finding better, more effective treatment, diagnosis, and prevention strategies. One of the obstacles in this endeavor is the diverse set of etiologies that comprises schizophrenia. A substantial body of evidence has grown over the last few decades to suggest that schizophrenia is a heterogeneous syndrome with overlapping symptoms and etiologies. At the same time, an increasing number of clinical, epidemiological, and experimental studies have shown links between schizophrenia and inflammatory conditions. In this review, we analyze the literature on inflammation and schizophrenia, with a particular focus on comorbidity, biomarkers, and environmental insults. We then identify several mechanisms by which inflammation could influence the development of schizophrenia via the two-hit hypothesis. Lastly, we note the relevance of these findings to clinical applications in the diagnosis, prevention, and treatment of schizophrenia.
Keywords: Schizophrenia, Inflammation, Infection, Biomarkers, Comorbidity, Animal Models
1. Introduction
Schizophrenia is a serious mental illness characterized by psychotic symptoms, cognitive impairment, functional decline, and, in most cases, a chronic course. It occurs, on average, in ~1% of the population worldwide, with variability around this mean in different countries and cultures. Despite 100 years of research, much about schizophrenia remains unknown, including its etiology and the extent of its heterogeneity. An emerging consensus is that schizophrenia is best conceptualized as a category, like dementia, epilepsy, cancer, or anemia, with multiple causes and types. It is now generally accepted that schizophrenia is not a single disease state.
While there have been many attempts to characterize schizophrenia’s heterogeneity (e.g., based on level of premorbid functioning, types of symptoms, etc.), few have led to advances in treatment, and improvements in this endeavor are still needed (Corvin et al., 2013). At the same time, the validity of the classic subtypes has been challenged (Braff et al., 2013). Recent data on inflammatory processes in schizophrenia, and the role of maternal and/or patient infection as etiological factors, may aid in the effort to characterize heterogeneity by identifying specific subtypes of patients with one or more infectious/inflammatory processes, and perhaps by identifying subgroups with an absence of inflammation (i.e., other etiological factors) (Allan et al., 2009). It has recently been proposed to include a distinct sub-group of schizophrenia to be classified according to the presence of mild encephalitis (Bechter, 2013). Moreover, it is now clear that data on inflammatory and infectious processes can be integrated with evidence on neurotransmitter (e.g., glutamate) and receptor (e.g., N-Methyl-D-aspartic acid receptor, or NMDA-R) abnormalities (Muller and Schwarz, 2006), developmental history (e.g., links between child abuse and inflammatory processes) (Dennison et al., 2012), and the biological bases of specific symptoms and cognitive impairments (Meyer et al., 2011).
Therefore, the purpose of this review is to examine current data on infectious and inflammatory processes, with a particular focus on different developmental stages and their relevance for schizophrenia. We then try to fit the pieces together regarding the implications for understanding heterogeneity in research and clinical domains, including diagnosis, prevention, and treatment. In discussing developmental stages, we also emphasize the “two-hit” theory of schizophrenia, suggesting that inflammatory processes may represent the sequelae of the first hit, and therefore serve to increase overall risk in the event of a second adverse event.
2. Background
2.1 Two-Hit Hypothesis
The two-hit hypothesis of schizophrenia suggests that a combination of genetic susceptibility coupled with a distinct developmental insult can prime an individual for a later event that ultimately leads to onset of the full clinical syndrome (Bayer et al., 1999). In the case of schizophrenia, multiple genetic liabilities have been suggested to interact with environmental factors, ultimately affecting the development of the central nervous system in a way that creates an abnormal signaling network (van Os et al., 2008). This network, however, may not become abnormal until later in life: schizophrenia tends to first occur in the early and late 20s in males and females, respectively, suggesting that an event in adolescence or post-adolescence may be highly influential in the development of the disorder (this is explored below). A number of structural abnormalities in schizophrenia are associated with symptom development: Gray and white matter alterations, cerebral asymmetries, and ventricular enlargement, accompanied by abnormal neuronal density, size, shape, and migration (for review, see Hoistad et al., 2009; Walterfang et al., 2011; Rapoport et al., 2012; Shepherd et al., 2012) A multifactorial conceptualization of psychiatric disease is emerging in which multiple biologically significant events (or “hits”) are temporally distributed across early periods of the overall lifespan, and result in the development of schizophrenia-like diseases (Giovanoli et al., 2013). Until now, genetic abnormalities were viewed as the likely ‘first hit,’ however, as we suggest below, infection and inflammatory processes may also serve this function.
2.2 Inflammation/Immune System
There is ample evidence that cytokines and the immune system can influence and shape the development of the CNS and behavior (Bauer et al., 2007; Deverman and Patterson, 2009; Yirmiya and Goshen, 2011; Bilbo and Schwarz, 2012). The importance of this information as a factor in the etiology of schizophrenia has been strongly emphasized by different laboratories investigating the effects of prenatal immune activation, thereby resurrecting the immunologic theory of schizophrenia. Indeed, this notion has experienced a checkered history, owing to the complexity of the disease and the paucity of well-controlled studies. Early studies noted dysregulation of immune function and the presence of antibodies against CNS tissue in schizophrenic patients (Ganguli et al., 1994). This was consistent with an autoimmune theory of schizophrenia and related disorders, which has received renewed attention in recent years (Eaton et al., 2006; Strous and Shoenfeld, 2006; Eaton et al., 2010). For detailed review of this subject, see Fineberg & Ellman, 2013, and Meyer et al., 2011. Additionally, dementia or dementia-like symptoms have been associated with viral and bacterial infections (Itzhaki et al., 2004; Spudich and Ances, 2012), neurosyphilis can cause psychotic symptoms (Zheng et al., 2011), and streptococcal infections in children have been linked to the development of obsessive-compulsive disorders (Pavone et al., 2006). Other schizophrenia studies have shown increased inflammation via imaging (Potvin et al., 2008; van Berckel et al., 2008; Doorduin et al., 2009; Meyer et al., 2011).
To describe the immune system in its entirety is beyond the scope of this article, especially as this has already been done in relation to schizophrenia (Strous and Shoenfeld, 2006; Muller et al., 2009; Richard and Brahm, 2012). However, some comment is perhaps necessary regarding the terms ‘inflammation’ and ‘neuroinflammation.’ The term ‘inflammatory’ has been liberally used in the neuroscientific, psychiatric, and behavioral literature of late, with the general intent being to suggest the presence of physiological events that are driven by the immune system. As such, descriptions of an inflammatory event can incorporate components of the immune system, including cells, soluble mediators, and products (e.g., cytokines and antibodies), but at the same time can include products of other tissue cells, including the so-called acute phase proteins, such as c-reactive protein (CRP). Indeed, in some cases, such as neuroinflammation, the discussion is largely confined to the activities of glial cells, such as microglia, which have a myeloid cell origin and are considered the resident immune cells (tissue macrophages) of the brain (see reviews by Bilbo & Schwartz, 2009, 2012). Moreover, neuroinflammation can include infiltration of circulating immune cells into the brain or spinal cord, wherein there is further elaboration of cytokines and antibodies. Therefore, context and specificity are important factors in a closer examination of what is meant when an “inflammatory process” is credited with affecting or being associated with some set of behavioral or neural outcomes.
It is sometimes possible to discern the cause of increased inflammation (i.e. the immunostimulatory condition, such as infection), but in many clinical studies this is seldom known. As discussed further below, there are inferences of causation through antibody measures, such as prior exposure to Toxoplasma Gondii (T. Gondii). However, for the most part measures of increased cytokines are made with little knowledge of how this came about. In some cases, certain forms of reasoning have been applied by observing shifts in the balance of immune elements. For example, the helper T cell type-1 (Th-1) response is a component of the adaptive immune system, which is predominantly pro-inflammatory. The helper T cell type-2 (Th-2) response, in contrast, activates the humoral and antibody producing immune system, and tends to be largely anti-inflammatory. After cytokines (immunomodulating signals) were noted to be abnormal in schizophrenia, it was thought that the disorder was associated with a shift away from a Th-1 response towards a Th-2 response (Schwarz et al., 2001). More recent biomarker studies have demonstrated altered cytokine activity evident of a mixed response in schizophrenia, both of a type-1 responses, including cytokines Interleukin-2 (IL-2), Interferon-gamma (IFN-γ), Tumor Necrosis Factor alpha (TNF-α), and of a type-2 response, including IL-4 and IL-13 (Drexhage et al., 2011; Richard and Brahm, 2012; Jones and Thomsen, 2013). However, these findings can vary depending on disease chronicity, age of onset, duration, and medication. This remains a promising area for understanding and treating schizophrenia.
It is important to note that inflammatory processes have been implicated in multiple psychiatric disorders, including depression, bipolar disorder, dementia, degenerative disorders, and anxiety, among others (de Vries et al., 2006; Miller et al., 2009; Goldstein et al., 2009; Khansari and Sperlagh, 2012; Hou and Baldwin, 2012). The relevance of these concurrent findings is not entirely clear, but could relate to a number of factors: inflammatory responses could indicate a general psychiatric vulnerability factor, they could result from the increased burden of stress on individuals with psychiatric illnesses, or there may be specific inflammatory profiles that predispose individuals towards specific disorders.
In short, it is not uncommon to observe neuropsychiatric symptoms during or as a result of major infection or immune dysregulation. However, where this information may be of critical importance is with regard to sensitive periods of neural development. This is particularly pertinent to epidemiological reports of the increased incidence of schizophrenia among those individuals born to women who were pregnant during major influenza epidemics (Brown and Derkits, 2010). Indeed, a comprehensive review of the epidemiological prenatal infection literature has provided reasonable grounds for suspecting immunological involvement in schizophrenia-like conditions (Brown and Derkits, 2010). This has led to the hypothesis that prenatal infections, by virtue of activating the maternal immune system, disrupt normal development of the CNS, which then results in maladaptive and abnormal behavior.
2.3 Summary
In a notable number of cases, schizophrenia has several characteristics that suggest its etiology may involve infectious or autoimmune processes. As we will describe, a number of studies have demonstrated an association between psychosis and maternal (during pregnancy) and early childhood immunological insults. Similarly, in adults, schizophrenia is associated with increased levels of various inflammatory markers. We suggest that under this framework, it may be possible to account for some of the heterogeneous etiology of schizophrenia, and that some symptomatology and altered behavioral functioning in schizophrenia can be attributed to immune system function. Major emphasis is currently focused on increasing the specificity of diagnosis in schizophrenia spectrum disorders, and it is possible that immune system related biomarkers will be valuable in this endeavor. Furthermore, if inflammation is a factor in schizophrenia, then it presents a target for potential treatment.
3. Prenatal/Maternal Infection
3.1 Animal Studies
3.1.1 Relevance
Immunobiological and psychosocial manipulations in the young animal have long demonstrated a significant impact on later-life endocrine, immune and behavioral functions (Coe, 1993; Walker et al., 2009; Sominsky et al., 2013). With regard to the etiology of schizophrenia-like symptoms, the strongest argument has emerged from studies that examined the postnatal development of mice, rats and, in some cases, non-human primates (Short et al., 2010; Willette et al., 2011) that received prenatal immunologic and/or infectious exposure. This has come to be referred to as maternal immune activation (MIA), and capitalizes on the notion that the immune response influences the developing nervous system of the embryo and/or fetus. The number of studies documenting significant postnatal effects has grown dramatically, and this area has been thoroughly reviewed (Meyer et al., 2005; Boksa, 2010; Meyer and Feldon, 2012). A variety of outcomes have been assessed, including dopaminergic, GABA-ergic and glutamatergic changes, as well as several of tests of altered behavior. It is understood that the full spectrum of schizophrenia symptoms are difficult to replicate in animals, and to some extent the behaviors that are assessed may apply to other conditions, such as autism and depression. For example, changes in social exploration, hedonic capacity, anxiety, learning and memory and prepulse inhibition (PPI) can be found in a range of psychiatric conditions. Even with PPI, a measure of sensorimotor gating, caution has been urged in adopting this as a gold standard in the diagnosis of schizophrenia (Swerdlow et al., 2008), since PPI deficits are also evident in individuals with high anxiety sensitivity (McMillan et al., 2012) and obsessive-compulsive disorder (Ahmari et al., 2012). Therefore, while the effects of maternal (or prenatal) infection or immune activation are relevant to schizophrenia, it is probably more helpful to view these findings as indicators of the contribution that prenatal infection may make towards specific endophenotypes (Gottesman and Gould, 2003; Gould and Gottesman, 2006) that cluster with other symptoms relevant to a diagnosis of schizophrenia. An important perspective here is that an animal model is useful for understanding schizophrenia if it can reproduce one or more aspects of the condition, even if that aspect is not unique to the disorder, which can be viewed as an open construct with fluid boundaries with other conditions (Mitchell et al., 2013b). No animal model should be expected to reproduce all aspects of schizophrenia and it is recognized that this is an impossible goal given some of its distinctly human aspects (e.g., formal thought disorder). Furthermore, given that significant postnatal and early adolescent environmental events are likely to be superimposed on intrauterine neurodevelopmental dysregulation due to prenatal infection, it is likely that additive and even synergistic interactions may trigger truly abnormal behavioral changes that warrant psychiatric intervention. To this end, recent studies have tested the two-hit hypothesis, in which postnatal exposure to psychogenic stressors or cannabinoid receptor agonists among offspring from infected mothers show more dramatic behavioral deficits than those seen due to maternal infection alone (Dalton et al., 2012; Giovanoli et al., 2013).
3.1.2 Immunologic Promotion of Altered Neurobehavioral Development: Preclinical Studies
The form of immune stimulation that has been used in animal MIA studies has relied largely on the use of molecular agents that activate the innate immune system, which includes cells such as the polymorphonuclear phagocytic cells (e.g., macrophages) and monocytes. Activation of these cells with pathogenic stimuli can stimulate phagocytic function and the production of proinflammatory cytokines (e.g., the classical proinflammatory cytokines IL-1β, IL-6, and TNF-α). Many studies that have examined the role of neural-immune interactions in vivo have used lipopolysaccharide (LPS; also referred to as ‘endotoxin’) as an immune stimulus. This molecule is found in the cell membrane of gram negative bacteria, such as E. coli, and as such mimics the immunological effects of gram negative infections. Also in prominent use, although to a lesser degree than LPS, is polyribosinic: polyribocytidylic acid (poly I:C), a synthetic double-stranded RNA that mimics viral infections. Both LPS and Poly I:C are known to stimulate innate immune cells via Toll-like receptors (TLRs) expressed on the cell surface. The discovery of TLRs was based on the ability of innate immune cells to recognize pathogen-associated membrane patterns on infectious microbial agents (Broz and Monack, 2013). With regard to LPS, it was found that this endotoxin binds TLR4, whereas Poly I:C selectively stimulates TLR3 (Engel et al., 2011). Therefore, while both stimuli target the innate immune compartment, each does so via a different surface receptor.
It is well established that LPS stimulates monocytes and macrophages to produce a range of proinflammatory cytokines (e.g., IL-1, IL-6, TNF-α) that can have a variety of neurobehavioral effects (Dantzer et al., 2008). Similarly, Poly I:C induces proinflammatory cytokine production and exerts neural and behavioral effects, including increased turnover of monoamine neurotransmitter in the brain and the induction of sickness behavior (Gibb et al., 2011; Meyer and Feldon, 2012). It should be noted, however, that in normal adult mice, differences have been observed in the profile of neurochemical changes after challenge with either LPS or Poly I:C. For example, LPS can have pronounced effects on brain monoamine neurotransmitter alterations, although dopaminergic changes are minimal in the prefrontal cortex (PFC) (Lacosta et al., 1999). Similarly, the effects of poly I:C appear to be more modest or absent after Poly I:C challenge in the prefrontal and limbic brain regions (Gandhi et al., 2007). Whether these differences are important with regard to impact on the developing embryo and fetus is not known. Moreover, it should be kept in mind that there have been few attempts to correlate the magnitude of the mother’s immune response to immune challenge, and resulting neurochemical and endocrine changes, with the postnatal development of her pups. This is technically difficult, but nonetheless, should be an important consideration for future studies.
Postnatal behaviors assessed in animals from mothers that have been subjected to MIA have included exploratory behavior, social interaction, cognitive function, and sensorimotor gating. To a large extent, many of these behaviors have been altered by MIA (Meyer and Feldon, 2012), and this has been demonstrated in rats, mice, and non-human primates. In studies that have used LPS or Poly I:C, normally only a single injection has been used, given mostly during mid-to-late gestation. While injection timing has not been extensively investigated, one study observed that Poly I:C injections of pregnant mice at gestational day 9 (GD9), but not GD17, resulted in impaired sensorimotor gating, as measured by the acoustic PPI procedure (Meyer et al., 2008). However, injection of Poly I:C on GD17 impaired working memory (Meyer et al., 2008), demonstrating that immune activation can cause differential phenotypic changes in the nervous system depending on the developmental stage of the embryo.
Perhaps of most relevance to any preclinical animal studies purporting to identify antecedents to schizophrenia-like abnormalities is evidence of neuroanatomical and neurotransmitter and receptor alterations. For many years, a dominant hypothesis in the etiology of schizophrenia has been dysregulation of the dopaminergic system (Heinz and Schlagenhauf, 2010). However, in recent years, it has become clear that impaired function of other forms of neurotransmitter communication, such as the inhibitory GABAergic and excitatory glutamatergic systems may be present in schizophrenia (Wallace et al., 2011; Yin et al., 2012). Therefore, it is noteworthy that in rat offspring from mothers challenged with LPS on GD15/16 that dopamine receptor 2 (D2R) expression in the medial PFC, and numbers of D2R expressing cells, were reduced on postnatal days 35 and 60 (Baharnoori et al., 2013). This was consistent with murine postnatal dopaminergic changes in the PFC and hippocampus pursuant to maternal Poly I:C challenge (Bitanihirwe et al., 2010). Changes in the brain GABAergic system have also been reported (Richetto et al., 2013), including a reduction in the concentration of hippocampal GABA (Bitanihirwe et al., 2010). Furthermore, cortical expression of both variants (65 and 67) of glutamic acid decarboxylase (GAD), which is necessary for synthesis of GABA, is reduced in the offspring of mice challenged with Poly I:C (Soumiya et al., 2011; Richetto et al., 2013). Indeed, these changes, as well as alterations in subunit expression for the GABA-A receptor, were noted in the prefrontal cortex and found to correlate with impaired working memory performance (Richetto et al., 2013). As such, these studies reveal altered development of dopaminergic and GABAergic neurotransmitter systems in the offspring of pregnant mice and rats exposed to immunological activation during pregnancy.
Complementing these neurochemical analyses, electrophysiological approaches have been used to determine neurodevelopmental alterations in synaptic activity in offspring from rats prenatally challenged with LPS. A common method for studying synaptic plasticity involves high frequency stimulation of presynaptic neurons to generate enhanced electrophysiological effects in postsynaptic neurons, a phenomenon known as long-term potentiation (LTP). This typically involves glutamate signaling, and likely reflects the physical basis of memory formation. However, alterations in glutamatergic signaling, presumably through downregulation of the postsynaptic glutamate receptor, can result in attenuating effects on postsynaptic electrophysiological responses after high rates of presynaptic stimulation, an effect referred to as long-term depression (LTD). Interestingly, it has been noted that glutamatergic signaling is altered in offspring from rats challenged with LPS, in that electrophysiological long-term depression in the CA1 region of the hippocampus was impaired from reduced synaptic function of the glutamate NMDA receptor (Burt et al., 2013). In principle, this study is in agreement with other evidence that prenatal immune activation alters the glutamate system (for review, see Meyer and Feldon, 2012). Moreover, it suggests that communication at glutamatergic synapses may be deficient, affecting the strength of synaptic activity necessary for cognitive functions.
Finally, the normal operation of the brain is dependent on efficient communication between different regions that specialize in cognitive, emotional, and motoric functions. This is mediated through well-defined neuroanatomical circuits that integrate cortical activity with subcortical information processing (e.g., sensory events). Addressing this aspect of brain function, electrophysiological studies of rats showed that prenatal challenge with Poly I:C produced asynchronous EEG activity in the medial prefrontal cortex (PFC) and hippocampus (Dickerson et al., 2010). Given that synchronous activity (i.e. activity that is temporally coincidental) in these two areas underlies successful working memory performance (Sigurdsson et al., 2010), the asynchronous activity may reflect altered cognitive capacity. Therefore, recent evidence supports the hypothesis that following prenatal immune challenge there is impaired communication between important areas of the brain that subserve reasoning and memory skills, potentially arising from glutamatergic hypofunction.
3.1.3 Animal Studies Involving Infection
Given that epidemiological infection data has energized the immunological hypothesis for developmental origins of schizophrenia, it is relevant to determine whether actual infection of the mother alters the neurobehavioral development of the offspring. Aronsson et al. (2002) showed that intranasal infection of pregnant mice with a specific strain of influenza A virus resulted in deposition of the virus in the fetal brain. The viral RNA was shown to persist in the brains of offspring up to the age of 90 days (Aronsson et al., 2002). This suggests that aside from known immunologic changes (e.g., cytokine expression) that can occur in the fetal brain as a result of maternal immune challenge (Shi et al., 2003; Elovitz et al., 2011; Oskvig et al., 2012), the infectious agent in and of itself can traverse the placenta and gain access to the developing organism. However, this interpretation is not altogether conclusive, as others have found no evidence for cross-placental migration of influenza virus into the fetal brain (Shi et al., 2005). Although this issue is largely unresolved, and may depend on a variety of different factors, it is likely that significant changes result in neurobiological function following maternal viral infection as a result of the maternal immune response. Indeed, as with the non-infectious models (e.g., LPS, Poly I:C) prenatal infection with human influenza has been shown to produce deficits in PPI of the acoustic startle response, as well as reduced exploration of novel environments and objects (Shi et al., 2003). Moreover, this can result in reduced numbers of Purkinje cells in specific layers of the cerebellum (Shi et al., 2009). Finally, maternal infection of rhesus monkeys with influenza virus was shown to reduce the amount of white matter in the cerebellum and the gray matter density in the prefrontal cortex, frontal cortex (extending throughout the precentral and secondary motor areas), the cingulate, insula, the parietal cortex and the superior region of the temporal cortex (Short et al., 2010). No major behavioral deficits were observed in the infants, although accelerated autonomy from the mother and reduced orienting or attentional behavior were noted in primates from infected mothers. Interestingly, the use of low nanogram amounts of LPS as a prenatal immune challenge in pregnant rhesus monkeys at 6 weeks prior to term produced notable behavioral effects, including a deficit in PPI, as well as reduced frequency of hostile and/or fearful reactions to a human intruder test (Willette et al., 2011). These changes were associated with a measured increase in white and gray matter in prefrontal, frontal and parietal regions of the cortex. While the source of the discordance in findings between viral infection and endotoxin exposure studies has yet to be determined, it is apparent that disruption of normal brain development takes place in non-human primates born to mothers that generate immune responses to pathogenic stimuli during pregnancy.
Overall, there is sufficient evidence to suggest that maternal immune activation interferes with the normal development of the fetal and neonatal brain. The particular mechanisms involved in exerting this effect are presently unknown, although the role of proinflammatory microglial cells in the CNS are beginning to received increased attention (Bilbo and Schwarz, 2009; Juckel et al., 2011; Williamson et al., 2011; Beumer et al., 2012). Moreover, the possibility exists that prenatal immunologic influences are more profound in genetically vulnerable individuals (Abazyan et al., 2010; Lipina et al., 2013). Finally, while this section has focused on maternal immune challenges, there is also evidence for altered sensorimotor gating and cognitive deficits in animals that were infected or immunologically challenged as neonates (Rothschild et al., 1999; Williamson et al., 2011). Combined, the immune response and/or inflammation can now be considered valid variables in the multifactorial conceptualization of the two-hit hypothesis and schizophrenia development.
3.2 Human studies
Environmental factors have been associated with schizophrenia since the disorder was first classified. Increased risk of developing schizophrenia occurs for offspring whose second trimester occurred during cold weather months or during periods of famine, such as during the Dutch famine towards the end of World War II (Hoek et al., 1998; Susser et al., 1998). It is now fairly well understood that a number of environmental factors can occur during pregnancy that affect the developing nervous system, including stress and illness (for review, see Brown and Derkits, 2010; Brown and Patterson, 2011; Clarke et al., 2012; Morgan et al., 2013). Influenza is a very well studied example: offspring of mothers who developed influenza during the first half of gestation had triple the risk of later developing schizophrenia, and it rose to seven fold when occurring during the first trimester (Brown et al., 2004). Birth cohort studies have found associations between schizophrenia and maternal infection with Toxoplasma Gondii (T. Gondii) and Herpes Simplex Virus Type 2 (HSV-2) during pregnancy (Buka et al., 2001; Brown et al., 2005; Mortensen et al., 2007a; Buka et al., 2008). Other associations have been documented with rubella, mumps, respiratory infection, measles, and polio (Brown and Patterson, 2011).
The important issue is the mechanism by which maternal diseases are associated with later onset of schizophrenia. It is less likely a result of the specific pathogen itself, as often the antigens themselves are unable to affect the fetus during pregnancy, but rather may be a result of the maternal immune response (Bilbo and Schwarz, 2009). For instance, elevated levels of inflammatory cytokines have been noted in mothers whose offspring later develop schizophrenia (Brown and Patterson, 2011), and a recent study found that high levels of antibody to dietary antigens increased risk for psychosis in offspring (Karlsson et al., 2012). These observations support the notion that an immune insult during gestation can be a vulnerability factor for developing schizophrenia, and is supported by the animal studies previously described.
4. Early life
Schizophrenia is typically considered an adult disease, although children sometimes will be diagnosed. However, neural development is still widely active during childhood and into adolescence, with changes in white and grey matter not fully developed until well into adulthood (Gogtay et al., 2004; Fields, 2008). There is significant evidence, furthermore, that stressful events in childhood are associated with the later development of mental health problems, including psychosis, and complementary evidence that stress can alter cortical development (Bale et al., 2010; Sideli et al., 2012; Morgan et al., 2013). Other events that have been linked to future development of schizophrenia include cannabis use and severe head trauma, indicating neurochemical alterations can predispose individuals for mental illness (van Os et al., 2010; Molloy et al., 2011; Brown, 2011).
A recent meta-analysis of childhood infection has shown there appears to be a significant link between viral infections of the CNS during childhood and future development of schizophrenia (Khandaker et al., 2012). Among studies showing a positive correlation, Dalman et al. (2008) found infections most strongly correlated with later development of schizophrenia were cytomegalovirus and mumps. Furthermore, infection with viruses such as HSV-1 can exacerbate schizophrenia symptoms, with decreased cognitive performance and neurological changes associated with HSV-1 affected compared to unaffected patients (Dickerson et al., 2003; Prasad et al., 2007; Schretlen et al., 2010; Prasad et al., 2011; Yolken et al., 2011).
Poor premorbid functioning, including cognitive impairment, social deficit, and movement irregularities, is found in the histories of many people with schizophrenia (Schenkel and Silverstein, 2004). Whether infection plays a role in this is unclear, but this issue should be examined in future studies. It is possible for a compound effect to exist between the factors associated with abnormal premorbid functioning and the stressor-effect of infection, such that a vulnerable CNS is primed early, and results in schizophrenia following exposure to relevant environment interactions.
5. Adulthood, biomarkers, and comorbidity
5.1 Biomarkers
5.1.1 Cytokine profiles
The study of biomarkers in adult schizophrenia is currently a key avenue for research into diagnosis and treatment. Several recent studies have shown that serum metabolite and chemical signatures can identify differences between individuals with schizophrenia and healthy controls, although not yet at the level of adequate diagnostic specificity (Kaddurah-Daouk et al., 2007; Schwarz et al., 2012; He et al., 2012; Yang et al., 2013). In a study by Schwarz and colleagues (2012), among the identifiers were several inflammatory markers. Other studies have shown differences between both oxidative stress markers and inflammatory cytokines between controls and schizophrenia patients (Kunz et al., 2011; Francesconi et al., 2011; Pedrini et al., 2012), and more recent studies have observed that increased immune response or inflammatory markers may be associated with more severe psychopathology (Fan et al., 2007; Fan et al., 2010; Hope et al., 2013). Inflammatory cytokines have also been shown to be increased in first degree relatives of schizophrenia patients (Gaughran et al., 2002; Nunes et al., 2006; Martinez-Gras et al., 2012). Two recent meta-analyses have revealed significant changes in cytokine profiles among schizophrenia patients relative to controls. The first, by Potvin and colleagues (2008), examined 62 studies and showed increases in IL-1RA, sIL-2R, and IL-6, and a decrease in IL-2 levels in schizophrenia patients relative to controls. The other, by Miller and colleagues (2011), examined 40 studies, and found that differing profiles depended on the nature of the hospitalization (first episode or acute relapse) with IL-1β, IL-6, and transforming growth factor-β (TGF-β) increased in both groups, but high IL-12, IFN-γ, TNF-α and sIL-2R levels observed in the relapsing patient group. IL-1β was decreased for both groups. Other more recent studies have looked at the protein or mRNA levels of post mortem cortex and found similar upregulation of inflammatory factors, including IL-6, IL-8, IL-1β, SERPINA3 (Fillman et al., 2013), and arachidonic acid (AA) cascade markers (Rao et al., 2013). An important implication of these studies is that cytokine profiles may represent endophenotypes of certain schizophrenia populations, but that the levels may be dependent on a number of factors, including genetics, phase/duration of the disorder, and medication. Indeed, in the study by Fillman et al., when the population was divided into clusters, those with the shortest illness duration had the larger inflammatory responses.
5.1.2 Imaging
There have been several MRI studies of schizophrenia and inflammation with conflicting results. One recent review, however, showed that in a variety of different disorders (aging, Alzheimer’s disease, major depression disorder, and schizophrenia), imaging-detected brain irregularities were coupled with inflammatory processes (Frodl and Amico, 2013). A different study also showed that initial inflammatory processes at first episode presentation may be categorically different from that which is observed when the disorder reaches a chronic state, implying there may be a critical window during which inflammation plays a significant role in disorder progression (Pasternak et al., 2012).
5.1.3 Infection
The causal nature of inflammation is still a major object of inquiry, as importantly, increased inflammation could result from the stress of a major disorder, such as schizophrenia. Infections have also been documented with increased frequency in individuals with schizophrenia, specifically in that relapse is associated with recent infection (Benros et al., 2011; Benros et al., 2012) and with increased frequency of concurrent urinary tract infection (Miller et al., 2013a). It is likely that infections may play a role in triggering the observed inflammation associated with schizophrenia, but also possible that the decrease in hygiene associated with individuals with schizophrenia, especially during a psychotic episode or the emergence of negative symptoms, leaves them more prone to infection.
5.1.4 T. Gondii
Toxoplasma Gondii (T. Gondii) is a coccidian protozoan whose preferential host is the cat, but the parasite is also carried by many types of warm blooded animals serving as intermediate hosts. Oocytes are most commonly transmitted to humans by means of ingesting undercooked meat or inhaling particles in areas frequented by cats. While most people exposed to T. Gondii are asymptomatic, it occasionally can cause toxoplasmosis, especially in immunocompromised individuals. Acute infection is often associated with fever, after which the parasite rarely causes further symptoms, but severe toxoplasmosis can lead to neurologic conditions including seizure and encephalitis. Whereas the presence of antibody to T. Gondii is believed to be fairly common (estimates range from 10% to 75% of all individuals in various populations), and reflects exposure to the protozoan, recent studies have indicated that this is associated with schizophrenia (Tenter et al., 2000). Serological investigations have confirmed a high level of antibody to T. Gondii is linked to increased risk for schizophrenia (Yolken et al., 2001; Leweke et al., 2004; Emelia et al., 2012). Moreover, animal and human studies have also shown that genetics can heavily impact the response to infection (Suzuki et al., 1996; Luder et al., 1998; Jamieson et al., 2008; Hermes et al., 2008; Lees et al., 2010).
Two recent meta-analyses by Torrey et al., consisted of 38 studies that examined the relationship of serological levels of T. Gondii antibodies and schizophrenia (23 were examined in the first meta-analysis, and an additional 15 were analyzed in the second). The studies found that the combined odds-ratio for patients was ~ 2.7 (Torrey et al., 2007; Torrey et al., 2012). All of the studies reviewed in the analyses measured levels of T. Gondii antibody in schizophrenia patients (both first episode and at all levels of clinical manifestation) relative to controls. Individuals with schizophrenia were significantly more likely to have higher levels of T. Gondii antibody, which conferred risk levels comparable to using cannabis and having minor physical anomalies. Similarly, in register-based studies in Denmark and the US, higher serological levels of antibodies from pregnant mothers and infants were predictive of future development of schizophrenia in both mothers and their offspring (Brown et al., 2005; Mortensen et al., 2007a; Mortensen et al., 2007b; Pedersen et al., 2011).
The T. Gondii relationship with human behavior is not limited solely to the association with schizophrenia, but also with cognitive traits, both independent of and related to schizophrenia. Higher T. Gondii levels are correlated with automobile and work accidents, lower intelligence, decreased novelty seeking, decreased reaction time, personality change, suicide attempts, and poor memory (Flegr et al., 2000; Flegr et al., 2003; Yolken et al., 2009; Flegr et al., 2009; Okusaga et al., 2011; Ling et al., 2011; Alvarado-Esquivel et al., 2012). In individuals with schizophrenia, the presence of T. Gondii antibodies is associated with more severe psychopathology, specifically positive symptoms (Holub et al., 2013), decreased education and a family history of mental illness (Park et al., 2012), and reduced grey matter density (Horacek et al., 2012). The cognitive changes caused by T. Gondii are well established in the animal literature, and include diminished memory, learning, fear aversion, and increased motor activity (Webster, 2007; Kannan and Pletnikov, 2012).
How these relative cognitive changes may occur is unknown, but there are several potential mechanisms. One is that acute T. Gondii infection can have a direct impact on neurotransmitter signaling, altering processes such as dopamine metabolism, kynurenic acid (KYNA) activity, and glutamate signaling (Schwarcz and Hunter, 2007; Prandovszky et al., 2011; Haroon et al., 2012). Further, T. Gondii can effectively colonize both glial and neuronal cells (Fischer et al., 1997a). Specifically, glial cells, among them the microglial cells, which perform immune-like functions in the brain (e.g., cytokine production and phagocytosis), are modified by T. Gondii insult, which can involve altered glial cell migration, brain cytokine concentrations, and neurotransmitter activity. After an immune insult, such as T. Gondii infection, reactive astrocytes and microglia initiate a series of complex signaling cascades, involving the production of prostaglandins and members of the TGF, interleukin, and interferon cytokine families (Fischer et al., 1997b).
The effect of T. Gondii on the peripheral immune response may play a role in the development of mental illness (for a comprehensive review of T. Gondii and the immune system, see Lang et al., 2007). Asymptomatic hosts infected with T. Gondii must have elevated immune responses to counter the infection, and this creates a dynamic environment in which parasite and host alter their environment to their respective advantages. To avoid immune detection, T. Gondii decreases pro-inflammatory cytokines and increases down-regulatory cytokines in infected cells and limits the production of nitric oxide (NO), allowing the parasite to propagate in the host (Luder et al., 1998; Luder et al., 2003). This leads to alterations in cytokine levels, notably Interferon-γ and TGF-β, which are upregulated upon T. Gondii infection, and combat microglia stimulated NO overproduction, a degenerative process of particular importance in immunocompromised individuals (Rozenfeld et al., 2005). T. Gondii infection also reduces the number of cytotoxic CD8+ T cells, which play a role in combating infection, and could augment cognitive disturbances in schizophrenia (Bhadra et al., 2013). In addition, genetic differences could affect the balance of the compensating immune responses induced by T. Gondii and natural immune responses, predisposing some individuals to increased inflammatory cytokine responses.
Higher levels of serological T. Gondii antibodies in pregnant mothers are associated with future development of schizophrenia in offspring (Blomstrom et al., 2012): as is the case with influenza, a more reactive maternal immune system may translate to altered neural biochemistry and development in offspring. Along those lines, early life exposure to T. Gondii could cause an immune response, and consequent neural development changes, in young children. The relationship between T. Gondii infection and schizophrenia could thereby result from change during both development and adulthood. In keeping with the two-hit hypothesis, the immune response caused by T. Gondii infection could represent the first hit, and the neurochemical alterations it causes later in life could further lower the threshold for developing schizophrenia.
5.2 Comorbidity
5.2.1 Overview
One avenue of research for understanding inflammation in schizophrenia is to examine comorbidity with other disorders, events, and syndromes. For example, there is now significant evidence that the metabolic syndrome observed in many individuals with schizophrenia may be associated with inflammatory and immune processes (Fan et al., 2010; Leonard et al., 2012; Miller et al., 2013b). Many studies have examined the relationships between schizophrenia and specific medical conditions, and it is beyond the scope of this review to thoroughly explore each of these relationships (Cournos et al., 2005; Leucht et al., 2007; Beary et al., 2012; Fan et al., 2013; Mitchell et al., 2013a). What is perhaps most interesting about available data is the suggestion that schizophrenia is genetically protective against a variety of other diseases, including cancer and rheumatoid arthritis. This hypothesis would be supported by reduced rates of comorbidity of these disorders, especially in families with multiple cases of schizophrenia.
5.2.2 Cancer
The relationship between cancer and schizophrenia has a long and ambiguous history (for review, see Bushe et al., 2009; Hodgson et al., 2010), but could nevertheless prove useful for understanding the etiology of both conditions. Studies have provided heterogeneous results. While an increase in risk and incidence for certain cancers in schizophrenia patients and their first degree relatives has been observed (Mortensen, 1989; Gal et al., 2012), there is alternative evidence for a decreased cancer risk among certain schizophrenia patient and first degree relative demographics (Lichtermann et al., 2001; Cohen et al., 2002; Barak et al., 2005; Levav et al., 2007; Catts et al., 2008; Gal et al., 2012; Ji et al., 2013). Other studies have shown mixed or null results, with the risk for specific types of cancer increased or decreased, and still others showing no difference relative to control groups (Goldacre et al., 2005; Grinshpoon et al., 2005; Hippisley-Cox et al., 2007; Gal et al., 2012; Lin et al., 2013b; Chen et al., 2013). Further complicating these findings are gender, age, and race interactions concerning schizophrenia and cancer rates (Ji et al., 2013; Chen et al., 2013). Two recent registry studies have offered some interesting suppositions: That schizophrenia patients may be underdiagnosed with cancer (Crump et al., 2013), and that there is an inverse relationship between cancer mortality and age among schizophrenia patients (Lin et al., 2013a). These findings suggest that younger schizophrenia patients may be more likely to die of cancer resulting from improper treatment and diagnosis, but older schizophrenia patients may have increased resistance to developing cancer.
The difficulty in determining these relationships is evident by the wide array of genetic and environmental factors involved in both schizophrenia and cancer, and further complicated by the distinction between spontaneous and familial schizophrenia. Another compounding factor is that psychiatric presentation can be a first manifestation of certain types of cancer, usually of the brain, and this may be underrepresented in large registry samples (Benros et al., 2009). More research is needed to explain the relationships between specific cancers and both spontaneous and familial schizophrenia before there can be conclusive evidence of a link. Other compounding factors include the increased incidence of smoking among schizophrenia patients, the age and gender of the patients, the difficulty in confirming diagnosis of schizophrenia in population registry studies, competing comorbidities, the heterogeneity of the samples used for analysis, and the identification of genetic and epigenetic factors involved in both schizophrenia and cancer.
There are many detailed reviews covering the interaction of cancer and the immune system (Dranoff, 2004; Dunn et al., 2004; de la Cruz-Merino et al., 2008; see Bronte and Mocellin, 2009; Schreiber et al., 2011; Finn, 2012; Murphy et al., 2012). In brief, immunoediting is the term coined to describe the interactions between cancer and the immune system; a process that can be inductive or suppressive of tumor formation (Dunn et al., 2004). It consists of several stages: elimination, equilibrium, and escape. The elimination phase is when the host immune system (both the innate and adaptive components) is able to successfully detect and destroy nascent tumor cells. Aggressive tumors, however, may develop mutations to either a) avoid detection by the host immune system or b) manipulate the microenvironment against the production of regulatory cytokines. Equilibrium is when a tumor has grown to the point where the immune system can inhibit metastatic spread of the tumor cells. The tumor may be considered dormant at this stage, as it might not be metastasizing or expressing proteins identifiable to the immune system. Finally, in the escape stage, a tumor previously in the equilibrium phase is able to evade the host immune system and metastasize. This can happen through mutations that prevent previously recognized tumor antigens from being identified by the host, and adaptations that prevent the tumor cells from being destroyed. Furthermore, the tumor may manipulate the cytokines in its microenvironment, shifting the balance to favor its own growth and immune evasion.
Although this is a brief summary of an incredibly complex and dynamic system that varies greatly between different types of cancers and different individuals, it offers some clues to how individuals prone to schizophrenia may be more resistant to cancers. The molecule p53 is a well characterized tumor suppressor protein with prominent roles in apoptosis. It is a typical target for tumor immunosuppression, and is often mutated in cancers. Several studies have shown an association between TP53 (the gene encoding p53) function and schizophrenia (Catts and Catts, 2000; Ni et al., 2005; Lung et al., 2009). Further, some TP53 polymorphisms found in schizophrenia may confer reduced vulnerability to lung cancer (Park et al., 2004), suggesting that in some instances of schizophrenia, resistance to cancer may be mediated by tumor-suppressing TP53 variants.
Another signaling factor, vascular endothelial growth factor (VEGF), is often upregulated by tumors in the microenvironment to increase angiogenesis (Kryczek et al., 2005; Roskoski, 2007), but has been shown to have decreased expression in schizophrenia (Fulzele and Pillai, 2009; Harris et al., 2012). Despite these examples, it has also been proposed that chronic inflammation, as has now been observed in individuals with schizophrenia, can have differential effects on tumor growth, depending on tumor type and stage (Schreiber et al., 2011).
If future experiments confirm the findings that incidences of specific cancer are decreased in schizophrenia patients and their relatives, and reveal more polymorphisms that are distinctive in cancer and schizophrenia, it would support the hypothesis that an overactive immune system is a vulnerability factor for developing schizophrenia. Genetic abnormalities that lead to increased apoptosis or limit cerebral blood flow, for example, can represent liabilities to abnormal neural development or altered immune response. Furthermore, a genetic predisposition to an overactive immune system, while potentially more effective at identifying and attacking cancer cells, could increase the likelihood of immune episodes in pregnant mothers, or increase the likelihood for inflammatory events later in life.
5.2.3 Autoimmune diseases
A number of recent papers have investigated the relationship between autoimmune diseases and schizophrenia using large population cohorts from national registries. Relative to control populations, studies have found links to a variety of autoimmune diseases, including Grave’s disease, celiac disease, thyrotoxicosis, interstitial cystitis, psoriasis, pernicious anaemia, systemic lupus erythematosus, and hypersensitive vasculitis (Eaton et al., 2006; Benros et al., 2011; Chen et al., 2012b). The individual microbiome, or the complete aggregation of microbes, including their genetic makeup and environmental interactions, may also contribute to autoimmunity in psychiatric disorders (Hornig, 2013). Consistent with the idea that there may be a genetic interaction between the development of schizophrenia and autoimmune disease, other studies found an increase in the incidence of autoimmune disorders in the families of schizophrenia patients (Gilvarry et al., 1996; Wright et al., 1996; Eaton et al., 2006). Risk for developing schizophrenia has also been found to increase synergistically with autoimmune disease and infections (Benros et al., 2011; Benros et al., 2012). This may result from increased immune response load, or because infections may increase permeability of the blood brain barrier and allow circulating auto-antibodies to interact with the central nervous system. The observation of blood brain barrier dysfunction in schizophrenia supports this suggestion (Bechter et al., 2010).
Two other disorders commonly believed to result from autoimmune processes, multiple sclerosis (MS) (Feinstein et al., 1992; Bartzokis et al., 2007), and Systemic Lupus Erythematosus (SLE) (Meszaros et al., 2012), sometimes present with psychosis. Their comorbidity with schizophrenia per se, however, is not necessarily higher than that of the general population; in fact, there is some evidence that schizophrenia is less well represented among individuals with MS (Marrie et al., 2009). Nevertheless, schizophrenia does share similar etiological aspects with both of these disorders. White matter disruption is the hallmark of multiple sclerosis, but is also present in schizophrenia (Walterfang et al., 2011). SLE has been associated with glutamate receptor antibodies (Lauvsnes and Omdal, 2012), and as will be discussed in section 6.5, NMDA-R antibodies have been implicated in schizophrenia. Going forward, it may be possible to learn more about the shared symptomatology between these disorders by investigating their common biological processes.
5.2.4 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a recurring chronic inflammatory disease of unknown etiology, but is believed to be autoimmune related and associated with a significant increase in mortality and joint degradation (for review, see Carmona et al., 2010; Boissier et al., 2012). Autoantibodies, including rheumatoid factor and anti-citrullinated peptide antibody, contribute to the degradation of autoantigens within and outside joints. The disorder involves numerous interactions between adaptive and innate immune processes, and many cytokines implicated in schizophrenia are similarly active during RA, such as TNF-α and multiple members of the interleukin signaling family.
Similarly to schizophrenia, RA occurs in roughly 1% of the population, involves complex genetic and environmental interactions, and has high (15–30%) heritability. Like schizophrenia, the genetic components are largely unknown, but most likely involve a heterogeneous assortment of genes with interacting effects. Several studies have now replicated the finding that schizophrenia appears to have an inverse relationship with RA, with some estimates putting the risk for RA among schizophrenia patients at 30–50% that of control counterparts (Eaton et al., 1992; Oken and Schulzer, 1999; Mors et al., 1999; Chen et al., 2012a). Conversely, there may also be a reduced risk for developing schizophrenia among individuals with RA (Gorwood et al., 2004). Furthermore, RA has distinct demographic patterns from schizophrenia: females are more than three times as likely to develop RA and there is no relationship between season of birth and RA diagnosis; however, both disorders are associated with infections (e.g. T. Gondii) and genes involved in the HLA complex (Torrey and Yolken, 2001).
What are the reasons for the mutually protective effects? One possibility is the complexities involved in the genetic and environmental interaction with the immune system. Individuals with genetic susceptibility and exposure to infectious agents could develop one disorder or the other depending on the temporal occurrence of the insult during development. This hypothesis would be supported if first degree relatives of individuals with schizophrenia had an increased risk of developing RA (and vice versa), but to our knowledge this has not been studied. Several studies have investigated the association of RA susceptibility genes in schizophrenia without finding positive associations (Morar et al., 2007; Shirts et al., 2007; Watanabe et al., 2009). Other factors could include medication, environmental effects, or the interaction of immune and genetic factors with different components in the central and peripheral nervous system.
One area that may help account for the mutual protection of RA and schizophrenia lies in the domain of lipid membranes (Elaborated upon in section 6.4). Both schizophrenia and RA are associated with membrane phospholipid abnormalities involving the production of prostaglandins from arachidonic acid. RA is associated with overall increase in membrane phospholipids, such as prostaglandin E2 (PGE2) levels (McCoy et al., 2002; Westman et al., 2004; Korotkova et al., 2011). Prostaglandin levels are also abnormally regulated in schizophrenia, and PGE2 levels also elevated (Elaborated in section 6.4). However, as prostaglandin and membrane protein alterations can produce heterogeneous activities depending on temporal and geographical context, the reason for their mutually protective effects could lie in the membrane composition, the subsequent relative activity of the prostaglandin signaling receptors, or in a separate or indirect system – for example, both disorders have associations with class II human leukocyte antigen system antigens (Torrey and Yolken, 2001). However, the immune responses of both disorders are incredibly complex and overlap across a number of signaling pathways, rendering any postulated direct connections tenuous. The reduction in comorbidity most likely results from a combination of factors (e.g. differences in immune response, lipid membrane composition) that may contribute to their respective pathologies, all of which should be studied in more depth.
6. Mechanisms
6.1 Human endogenous retrovirus type-W (HERV-W)
A potential bridge between environmental stressors, such as the influenza virus, and the later development of schizophrenia is the human endogenous retrovirus type-W. Approximately 8% of the human genome is composed of retroviruses; HERVs are a family of retroviruses that are heritable, can code for proteins, are found in the CNS (Kim et al., 2008), and are associated with disorders such as MS and schizophrenia (for review, see Brodziak et al., 2012; Leboyer et al., 2013). HERV-W expression, however, is variable and typically inactive, but can be activated by specific environmental triggers, which can induce an immune response (Nellaker et al., 2006). HERV-W associated retroviral envelope proteins can produce a pro-inflammatory response, beginning a cascade with the activation of CD14 and TLR, in turn leading to increased production of schizophrenia associated cytokines such as IL-1, IL-6, TNF-α (Perron et al., 2001; Rolland et al., 2006).
Increased HERV-W element expression has been found in individuals with schizophrenia through serum, CSF, and tissue analyses, and is more prominent during first episode psychosis (Deb-Rinker et al., 1999; Karlsson et al., 2001; Perron et al., 2008; Yao et al., 2008; Dickerson et al., 2012; Perron et al., 2013). During embryonic development, there is a reduction of DNA-methylation, creating an inductive environment for activating usually inactive HERV-W elements (Lees-Murdock and Walsh, 2008; Leboyer et al., 2013). The potentially elevated expression in response to environmental stressors fits within the two-hit hypothesis of schizophrenia: Responses to HERV-W can have both developmental effects and acute inflammatory effects after development is complete. For example, during pregnancy, influenza, in addition to inducing the immune response, may also help activate a second pro-inflammatory cascade in the embryo through induced expression of HERV-W elements, and subsequently alter neuronal development. This may also sensitize individuals to respond to immune stressors at later time points, creating a cyclical reaction: future stressors and infections may initiate additional HERV-W expression and subsequent inflammatory events. TNF-α, BDNF, and dopamine receptor D3 may be elevated during immune response to HERV-W, all of which may play roles in altering neuronal communication during the later phases of schizophrenia (Rolland et al., 2006; Huang et al., 2011).
However, it is important to note that the increased incidence of HERV activity in various autoimmune disorders could be triggered by certain pro-inflammatory agents (Brodziak et al., 2012). A more thorough understanding of the relationship between retroviruses and disease will be necessary to determine their specific role in disorders such as schizophrenia.
6.2 Sex Differences in schizophrenia
Sex differences in schizophrenia have long been recognized. There are slightly higher rates and earlier onset in males (typically ages 18–25), compared to females (25–30), while higher incidence rates occur for females compared to males after age 50 (Goldstein and Walder, 2006). Also, higher rates of schizophrenia transmission occur from fathers to daughters and from mothers to sons (Goldstein et al., 2011). In addition, there are substantial gender differences in syndrome presentation, with women tending to present with more affective and paranoid psychotic symptoms, men tending to present with more negative symptoms, and women overall presenting with less severe clinical course (for review, see Leung and Chue, 2000; Abel et al., 2010). Other biological differences include increased lipid peroxidation (Ramos-Loyo et al., 2013) and decreased fatty acid levels (McNamara et al., 2007; Kale et al., 2010) in males compared to females with schizophrenia, which could differentially alter cell membrane integrity (this is discussed in section 6.4). While the reasons for these sex differences remain unknown, the later onset and post-menopausal increase in incidence among females suggest that sex hormones may have some effect.
The estrogen theory posits a protective effect of estrogen on schizophrenia, with periods of protection occurring when estrogen levels are high, such as during puberty and pregnancy (Leung and Chue, 2000). Treatment studies using estrogen or related hormones have shown some benefit in treating schizophrenia and cognitive impairments (Begemann et al., 2012; Hayes et al., 2012; Kulkarni et al., 2012). Further, there is evidence in multiple systems that estrogens exhibit neuroprotective effects and can interact with immune processes to reduce CNS inflammation and activate a Th-2 response (Tenenbaum et al., 2007; Benedusi et al., 2012; Oertelt-Prigione, 2012), although there is some evidence that they may have pro-inflammatory effects as well (for review, see Strom et al., 2011). Estrogens have a variety of other neuroprotective effects, including reducing free radicals, facilitating synaptic plasticity, and facilitating NMDA signaling (Brann et al., 2007). Estrogen signaling has also been shown in animal models to mitigate reactive microglial activity, another potential factor in schizophrenia, discussed below (Bruce-Keller et al., 2000; Arevalo et al., 2012).
In summary, there is compelling evidence that gender differences in schizophrenia may be due to differences in hormonal activity. Clinical trials are beginning to show the benefits of estrogen treatment in schizophrenia, and this remains a promising area of study. Investigating the causes of sex differences in schizophrenia may continue to yield important insights into its etiology.
6.3 Microglial Activity and Neuron Pruning
The role of microglia during psychiatric disease is beginning to be better understood, particularly in schizophrenia, but they are also relevant to other affective and degenerative psychiatric disorders (Beumer et al., 2012; Prokop et al., 2013). Microglia produce, and respond to, many different cytokines, and a number of anti-psychotic drugs directly act by limiting microglial activity and inflammatory cytokine production (Monji et al., 2009), all of which implicate microglia in the etiology of schizophrenia. In adults, post-mortem, serological, and imaging studies have shown increased microglia activity in individuals with schizophrenia (Wierzba-Bobrowicz et al., 2005; Steiner et al., 2006; Steiner et al., 2008; van Berckel et al., 2008; Doorduin et al., 2009). A recent review by Monji and colleagues highlights the relevant cellular processes affected by altered microglia in schizophrenia, including neurogenesis, apoptosis, and white matter development (Monji et al., 2013). There is also increasing evidence from animal studies that both prenatal and early-life infections or stress can lead to primed microglia (Bland et al., 2010; Juckel et al., 2011; Frank et al., 2012), that is, microglia that are more easily activated later in life in response to insult. Once activated, the composition of glutamate receptors on microglial cells themselves may become abnormal, resulting in increased pro-inflammatory cytokine release (Beppu et al., 2013). This sequence could represent both hits in the two-hit hypothesis: Early activation of microglia (through immune insult in the CNS) can sensitize them for later activation (Hickie et al., 2009).
Microglia can also extensively regulate the glutamatergic signaling pathway. They express and respond to glutamate, have dynamic interactions with NMDA receptors and glutamate signaling (Hayashi et al., 2006; Takaki et al., 2012; Berger et al., 2012; Domercq et al., 2013), and prenatal activation of microglia has been shown to alter glutamatergic synaptic function later in life (Roumier et al., 2008). Furthermore, microglia regulate synapses throughout adulthood (Wake et al., 2009). Altered membrane fatty acid levels in microglia can lead to altered microglial function, thus interfering with and exacerbating the aforementioned processes (Hjorth and Freund-Levi, 2012) and contributing to glutamate hypofunction.
Another mechanism incorporating inflammation and microglia with the two-hit and the glutamate hypofunction hypotheses is synaptic pruning (for review, see Hua and Smith, 2004; Schafer and Stevens, 2010; Paolicelli et al., 2011; Chung and Barres, 2012; Boksa, 2012). Pruning is the process by which synapses are culled in an activity dependent manner during late adolescence, essentially preserving high load synaptic connections at the expense of low use, potentially extraneous connections. This primarily occurs during early developmental stages and later, during adolescence. Its activity in humans, however, has been postulated from post-mortem and imaging studies, and not explicitly observed. Many experiments, however, have indicated that individuals with schizophrenia have reduced gray and white matter (van Haren et al., 2008; Chan et al., 2009), in addition to a disproportionate decrease in the amount of dendritic spines beginning near adolescence (Glantz and Lewis, 2000; Bennett, 2011; Glausier and Lewis, 2012). This may represent aberrant synaptic pruning, which could in turn lead to disruptions in a number of neurotransmitter systems. The overlap of critical periods of synaptic pruning and first onset of schizophrenia symptoms implicate pruning as a potentially altered process in schizophrenia.
Glial signaling actively regulates synaptic pruning. Microglia are well known to regulate synaptic plasticity and pruning during development throughout the cortex (Tremblay et al., 2011; Paolicelli et al., 2011; Blank and Prinz, 2013) and both synaptic pruning and microglial activity are altered by inflammatory processes (Schafer and Stevens, 2010). Consistent with the two-hit hypothesis, the combination of genetic and environmental insult early in life could alter neurodevelopmental processes. An end result of fewer synapses could subsequently result from overactivation of the pruning response, possibly by means of exaggerated microglial activity, but could also result from starting with fewer synapses or failing to reach a normal level of synapses before pruning initiates (Hoffman and McGlashan, 1993).
6.4 Membrane Phospholipids
6.4.1 Polyunsaturated fatty acids (PUFAs)
A common finding among schizophrenia studies is abnormal membrane phospholipid composition, including abnormalities in PUFAs and their metabolites, such as prostaglandins. This literature has been well reviewed (Horrobin, 1998; Skosnik and Yao, 2003; du Bois et al., 2005), and two recent meta-reviews showed that blood cells of schizophrenia patients have decreased PUFA levels, which include docosahexaenoic acid (DHA, an ω-3 fatty acid), docosapentaenoic acid (DPA, an ω-3 fatty acid), and arachidonic acid (AA, an ω-6 fatty acid) (van der Kemp et al., 2012; Hoen et al., 2013). Further, erythrocyte PUFA levels may accurately represent brain PUFA levels (Carver et al., 2001; Yao et al., 2002). How and why PUFA levels are abnormal in schizophrenia is incompletely understood, but could result from increased breakdown and reduced incorporation into neural membranes, as assayed via animal studies, post mortem and platelet analyses, niacin skin flush observations, and various imaging studies.
AA and its metabolites, termed eicosanoids, are involved in numerous processes, including neurotransmitter function and release, immune function, inflammation, and pain regulation (Skosnik and Yao, 2003; Smith, 2006; Harizi et al., 2008). Alterations in PUFA levels, therefore, could have wide-ranging effects both on neural functioning, inflammation, and any connections between the two. Abnormal pain reporting in schizophrenia has long been noted (Bonnot et al., 2009; Levesque et al., 2012), and eicosanoids could also be relevant in that domain. Interestingly, low levels of PUFAs in schizophrenia patients have been associated with negative symptoms (Bentsen et al., 2011; Bentsen et al., 2012), positive symptoms (Sumiyoshi et al., 2008), and poor cognition (Condray et al., 2008; Condray and Yao, 2011), indicating that they may be associated with pathology.
6.4.2 Prostaglandins
Prostaglandins are fatty acids derived from AA via a signaling cascade involving cyclooxygenase enzyme 1or 2 (i.e. Cox-1 and Cox-2). They have long been recognized as abnormally regulated in schizophrenia, although because of the many different types and receptors, their effects may be quite heterogeneous (Horrobin, 1996, 1998; Smesny, 2004). Specifically, while PUFAs in general seem to be decreased in schizophrenia, including anti-inflammatory prostaglandins E1 (PGE1) and 15d-PGJ2 (Smesny, 2004; Martinez-Gras et al., 2011), pro-inflammatory PGE2 levels may be increased, as a result of inflammation or compensation from decreased anti-inflammatory PUFAs (Lokesh et al., 1986; Laye, 2010; Ricciotti and FitzGerald, 2011). Phospholipase A2, which regulates conversion of AA to prostaglandins, may have increased activity in schizophrenia (Smesny et al., 2010), resulting in possible short term overproduction of various prostaglandins but long term depletion of the AA substrate. The relevance of prostaglandins in schizophrenia treatment has been shown with a number of studies involving Cox-2 inhibitors, which reduce the amount of prostaglandins, notably PGE2, produced from precursors, and can have therapeutic effects (reviewed in Muller et al., 2013). The treatment benefits of Cox-2 inhibition, however, are not observed in chronic schizophrenia (Muller et al., 2004). One recent study showed that components of prostaglandin production decrease with illness duration, possibly accounting for the more beneficial effect of anti-inflammatory Cox-2 inhibitors applied earlier in illness relative to later (Tang et al., 2012).
Prostaglandin signaling is regulated by complex mechanisms: members of the signaling family can have synergistic or antagonistic responses to the same signal, depending on temporal and cellular context, creating both pro- and anti-inflammatory responses (for review, see Hata and Breyer, 2004; Ricciotti and FitzGerald, 2011). Additionally, they affect glutamate stimulation, neuroprotection, and cell death (Akaike et al., 1994; Cazevieille et al., 1994; Hata and Breyer, 2004; McCullough et al., 2004; Ahmad et al., 2005; Carlson et al., 2009). While there is much more yet to be understood, it is clear that prostaglandin dysregulation interacts with inflammatory components of the immune system and contributes to membrane composition abnormalities in schizophrenia.
6.4.3 Treatment of schizophrenia with PUFAs
The essential fatty acids (EFAs) comprise α-linolenic acid, an ω-3 fatty acid (n-3) that is metabolized to longer chained forms EPA and DHA, and linolenic acid, an ω-6 fatty acid (n-6) that is metabolized to AA, and are required for proper membrane function but must be obtained through diet. There is evidence that a modern Western diet has a vastly higher ratio of n-6 to n-3 fatty (15 or 20:1) acids relative to that of human ancestors (closer to 1:1), and that this imbalance is associated with a variety of health effects (Simopoulos, 2008). Improper accumulation of n-3 fatty acids into membranes, or an imbalance in the relative amount of n-3 compared to n-6 fatty acids, can lead to problems in many domains: for example, PUFA incorporation into membranes is critically tied to neurotransmitter signaling and receptor activity (du Bois et al., 2005). Membrane phospholipid integrity is also critically integrated into the immune response and n-3 fatty acids have been shown to reduce and inhibit inflammatory cytokines (for review, see Laye, 2010; McMurray et al., 2011; Shaikh et al., 2012; Calder, 2013). There are also many studies showing n-3 fatty acid levels are involved in neuron development and synaptogenesis (Moriguchi et al., 2000; Lim et al., 2005; Cao et al., 2009; Kim et al., 2011). Furthermore, anti-inflammatory n-3 fatty acids tend to counter and compete with the more pro-inflammatory n-6 derivatives (Schmitz and Ecker, 2008), and an imbalance in this ratio could create a more pro-inflammatory profile
To determine if lack or imbalance of PUFAs could serve as a potential treatment target, several groups have performed clinical trials by supplementing diets of schizophrenia patients with n-3 fatty acids. The results have been mixed: two recent literature reviews of clinical trials did not find statistical differences compared to placebo (Fusar-Poli and Berger, 2012; Akter et al., 2012); however, overall sample size was low, methodology was heterogeneous, and there were some positive effects. One other recent study showed beneficial effects of n-3 fatty acids on treatment and attenuating conversion to psychosis among high risk groups (Amminger et al., 2010), and several literature reviews have shown dose-responsive positive effects on cognition and vision with DHA and AA supplementation in the general population (SanGiovanni et al., 2000; Hoffman et al., 2009; Ryan et al., 2010). Since n-3 fatty acids have also been shown to limit the inflammatory response in fetal membranes (Frew et al., 2013), abnormal EFA levels could represent a prenatal vulnerability to inflammatory insults and potentially a target for early (prodromal or first episode) intervention. More research, however, is clearly needed on the clinical efficacy of PUFA supplementation in schizophrenia.
6.4.4 Potential for membrane alterations to affect Schizophrenia
Based on the extended literature on the roles of membrane phospholipids, there are several different ways they could contribute to the pathogenesis of schizophrenia and do so in a manner consistent with the two-hit hypothesis. Dysregulation of PUFAs (including AA, prostaglandins, and n-3 fatty acids) could alter early neural development, exaggerate inflammatory responses, or lead to aberrant neurotransmitter functioning, all of which could prime the nervous system for insult and dysfunction during early or later developmental windows.
6.5 Glutamate
6.5.1 Glutamate hypofunction
The glutamate hypofunction hypothesis of schizophrenia states that low levels of glutamate signaling can explain the symptomatology associated with schizophrenia, and could be the result of developmental abnormalities. A wide variety of in vitro and in vivo studies have shown dysregulation of the glutamate signaling systems in humans and in animal models of schizophrenia, and many genes implicated in schizophrenia are involved in glutamate signaling (Phillips and Silverstein, 2003; Lisman et al., 2008; Adell et al., 2012; Lin et al., 2012). Under this model, aberrant glutamate signaling affects multiple pathways. One is afferent glutamate signaling to GABA-ergic interneurons in the descending cortico-brainstem pathway; in normal signaling, these signals limit mesolimbic dopamine activity through interneurons in the ventral tegmental area (VTA) by means of tonic inactivation. A decrease in glutamate signaling, via NMDA-Rs, reverses the tonic inhibition of the interneurons and leads to overexcitation of the dopaminergic receptors in the limbic region, resulting in the positive symptoms associated with schizophrenia. Concurrently, the lack of afferent glutamatergic signaling can directly downregulate activity in the cortex, resulting in the negative and disorganization symptoms. The glutamate hypothesis fits within the context of the two-hit hypothesis. Developmental events can affect the eventual circuitry of the CNS through alterations in synapse formation, dendritic spine density and number, receptor localization, and neurotransmitter production, such that glutamate neurotransmitter function is abnormal.
6.5.2 Glutamate and Inflammation
Glutamate dysregulation is also a consequence of inflammation, potentially resulting from cytokine and glial activity. One of the more well studied signaling cascades that regulates this interaction is the kynurenine system (for review, see Muller et al., 2009; Mandi and Vecsei, 2012; Anderson and Maes, 2013). In this pathway, kynurenine (KYN) is converted to tryptophan (TRY) with the help of indoleamine 2,3-dioxygenase (IDO), expressed predominantly by CNS astrocytes, or tryptophan 2,3-dioxygenase (TDO), predominantly expressed by microglia. Through a signaling cascade, resulting metabolites can be kynurenic acid (KYNA), the only known naturally occurring NMDA antagonist, or quinolinic acid (QUIN), an NMDA agonist. Tellingly, KYNA levels in the CSF or tissue of schizophrenia patients have been found to be higher than in those of controls (Schwarcz et al., 2001; Erhardt et al., 2001; Nilsson et al., 2005; Sathyasaikumar et al., 2011; Linderholm et al., 2012), supporting the glutamate hypofunction hypothesis.
The kynurenine signaling system is inherently tied to the immune system. As mentioned previously, its two major catalysts, TDO and IDO, are both produced by CNS glial cells, which become upregulated in response to inflammation and stress. IDO and KYNA levels are also increased in response to TNF-α and IFN-γ, two inflammatory cytokines upregulated in schizophrenia (Robinson et al., 2005; Guillemin et al., 2007; Asp et al., 2011). However, KYNA has multiple interactions with immune processes and may serve as a feedback mechanism for reducing TNF-α activity (Mandi and Vecsei, 2012). KYNA can alter glutamatergic signaling through non-competitive antagonism, and also via downstream signaling through α7 nicotinic acetylcholine receptors and GABA-ergic interneurons (Banerjee et al., 2012a; Banerjee et al., 2012b). This can result in extensive neurotransmitter dysregulation, altering levels of glutamate, dopamine, acetylcholine, and GABA, that could be relevant in causing or exacerbating symptoms in schizophrenia.
A number of animal studies have shown an important developmental role of this pathway. KYNA levels are increased after exposure to Influenza, both in vitro and during development (Holtze et al., 2008; Asp et al., 2010), and by T. Gondii infection, mediated through inflammatory cytokines (Silva et al., 2002; Fujigaki et al., 2002; Schwarcz and Hunter, 2007). Animals exposed to KYNA during early development have higher levels as adults, lower glutamate levels, and show abnormal increased behaviors associated with animal models of schizophrenia (Alexander et al., 2012; Pocivavsek et al., 2012). These studies illustrate a pathway through which glutamatergic hypofunctioning can be facilitated through inflammatory regulation of KYNA activity.
6.5.3 Glutamate and membrane composition
Membrane phospholipids are essential for normal glutamatergic signaling. The prostaglandin signaling system is significantly and complexly associated not only with the immune system, but with glutamate and glial signaling. PGE2 can modulate calcium currents involved in glial and neuronal NMDA signaling (Bezzi et al., 1998; Sanzgiri et al., 1999; Katafuchi et al., 2005; Dave et al., 2011), therefore an alteration in PGE2 abundance could alter glutamate signaling strength. Prostaglandins also mediate the effect of Cox-2 inhibitors in decreasing KYNA (Schwieler et al., 2005)
There are other interactions between membrane signaling components and glutamate activity. DHA and AA potentiate intracellular calcium signaling initiated by glutamate and potentiate synaptic glutamate signaling (Miller et al., 1992; Nishikawa et al., 1994; Casado and Ascher, 1998). Reduction in AA and DHA, as observed in schizophrenia, could therefore have significant effects on NMDA mediated glutamate signaling and long term potentiation. N-3 fatty acid deficiencies have been modeled in animals, showing age related decreases in glutamate signaling (Latour et al., 2013). Taken together, there is significant evidence that membrane abnormalities could be involved in glutamate hypofunction observed in schizophrenia.
6.5.4 NMDA-R Antibodies
The last decade has seen a substantial number of studies published on autoantibody related encephalitis disorders, most prominently anti-NMDA-R encephalitis (for review, see Dalmau et al., 2011; Wandinger et al., 2011). This has been determined to be a progressive illness that first manifests with psychotic features, memory deficits and seizures, subsequently followed by dyskinesias, hypoventilation, loss of consciousness, and death. The mechanism of action is a reduction in NMDA receptors caused by excessive antibodies to the NR1 subunit, where patients’ antibodies are directly responsible for decreasing NMDA-R density and localization (Dalmau et al., 2008; Hughes et al., 2010). The disorder is much more common in females by a ratio of 4:1, and is often associated with ovarian teratomas. Since this discovery, other encephalopathies have been identified as being autoimmune responses to other synaptic antigens, including components of GABAB receptors, components of AMPA receptors, and glioma-inactivated 1 (Lai et al., 2009; Lai et al., 2010; Lancaster et al., 2010).
Many patients eventually diagnosed with anti-NMDA-R encephalitis exhibit positive, disorganized, and negative symptoms associated with schizophrenia. Where the symptoms differ from those of schizophrenia patients is after the initial course, wherein symptoms become increasingly associated with brainstem activity and autonomic function regulation (e.g. lethargy, seizures, hypoventilation, and comas). If given a correct diagnosis, many patients respond with complete or partial recovery to immunosuppressive therapy and tumor removal (if a tumor is identified), with better prognosis associated with early diagnosis (Dalmau et al., 2008; Iizuka et al., 2008; Irani et al., 2010; Dalmau et al., 2011). Interestingly, retrospective analysis of previously unidentified encephalitis patients were shown to have high levels of anti-NMDA-R antibodies, and some individuals with disorders such as first episode, postpartum, and drug induced psychosis have been rediagnosed with anti NMDA-R-encephalitis, indicating the disorder may be more prevalent than previously thought, owing to prior misdiagnosis (Gable et al., 2009; Dale et al., 2009; Pruss et al., 2010; Mesquita and Siva, 2011; Mann et al., 2012). Recently, NMDA-R antibodies have been found in individuals with schizophrenia and cognitive impairment without encephalitis (Zandi et al., 2011; Pruss et al., 2012). The largest study to date (121 schizophrenia patients) found a 10% prevalence of multiple types of NMDA-R antibodies (Steiner et al., 2013).
As recent studies have shown, glutamate receptor antibodies can play significant roles in psychosis and potentially in schizophrenia. However, much more research needs to be done in this area. NMDA-R antibodies play roles in many different illnesses, including epilepsy (Takahashi et al., 2003; Ganor et al., 2004; Ganor et al., 2005), ischemia (Dambinova et al., 2002; Dambinova et al., 2003), lupus, and encephalitis. Furthermore, their effects can be wide ranging depending on a number of factors, including receptor subtype, location, circuit involvement, temporal activity, and access to the central nervous system. Causality is similarly complicated: while there have been several studies showing the excitotoxic effect of certain glutamate receptor autoantibodies (reviewed in Pleasure, 2008), NMDA-R autoantibodies can be associated with neuroprotective effects to adverse events (During et al., 2000). Activity on some receptor subtypes increases Ca+2 into the neuron, leading to increased neurotoxicity, whereas activity on others can decrease signaling activity, leading to hypofunction. The type of antibody may make a large difference in presentation: IgG antibodies to NMDA-R are often responsible for encephalopathies, but IgA antibodies may more easily cross the blood brain barrier and cause more subtle, progressive effects (Pruss et al., 2012).
There are a number of implications of these collective studies. First, certain receptors and channels involved in glutamate signaling are prone to degradation during specific auto-immune related encephalopathies, and the similarity between behaviors and symptoms associated with these conditions and schizophrenia can be considered strong evidence in support of the glutamate hypofunction hypothesis of schizophrenia. Second, patients admitted to hospitals with psychosis-like symptoms but no history of mental health disorders should be screened for tumors and CSF antibodies. Third, a subset of schizophrenia patients may be identified by biomarkers related to NMDA-R, AMPA-R, and potassium channel antibodies. Fourth, there may be specific avenues of treatment for these subsets of patients, including immunosuppressive therapy in conjunction with antipsychotic medication.
6.6 Integrating mechanisms
What is emerging is a picture of schizophrenia as a dynamic biological disorder. The two-hit hypothesis emphasizes that an insult during the prenatal or early life stages may prime the nervous system to develop either: 1) abnormally, such that functioning is noticeably disordered even before individuals are diagnosed with a schizophrenia-spectrum disorder, or 2) normally, but is primed such that a second event later in life (such puberty or later life immune attack or a significant life stressor) disrupts neurological processes. What is also emerging is that abnormalities in glutamate signaling and microglial activity may be inherently intertwined in this process.
Under the framework of the two-hit hypothesis, it is clear that distinct but connected systems can interact to disrupt normal functioning (Fig 1). Immune insults can occur at early developmental stages, leading to immediate or delayed abnormalities in neural activity. This can occur through disruption of neurotransmitter signaling, most notably hypofunction of glutamate activity, via processes such as excessive pruning, microglial activity, or an abnormal immune response, each potentially affecting components of the signaling pathway (e.g. dendrites, synapses, receptors, or membranes). Such effects can be facilitated or inhibited by a variety of vulnerability factors, including genetic predisposition, membrane phospholipid composition, and hormonal regulation. Approaching schizophrenia within this framework will help to understand more about its heterogeneity and treatment options.
Figure 1.
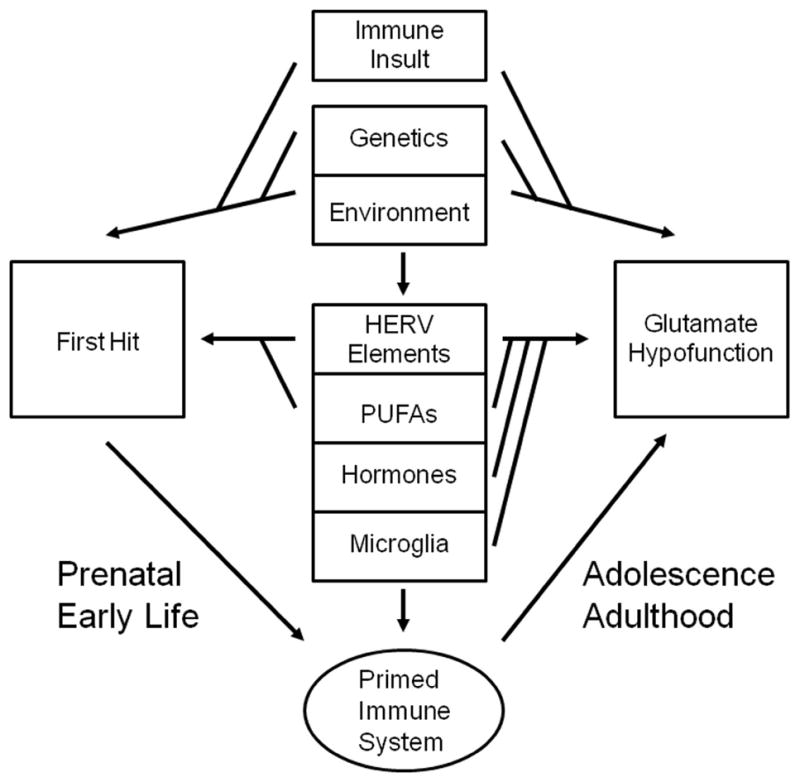
The two-hit hypothesis of schizophrenia relates to glutamate hypofunction. This figure summarizes how glutamate hypofunction can occur as the outcome of a number of different pathways. An inflammatory event at the early stages of development, either in utero or as an infant or young child, can be considered the first ‘hit.’ This can occur because of environmental stressors, often in the form of an immune event, and is modulated by genetic makeup. Retroviral elements and fatty acids/membrane composition can also influence this stage. After the initial insult, development can occur abnormally, such that neural development and glutamate signaling is altered permanently, or normally. In either case, the immune system may become ‘primed’ for a second insult. This can involve enhanced microglial activity, may be protected by hormonal balance, and can be heavily influenced by genetics, fatty acids/membrane composition, and environmental stressors. After adolescence, glutamate hypofunction can then result from directly from altered development, or from a second ‘hit’ by the primed immune system, possibly via activated microglia. This stage can also be regulated by additional immune insults, environmental stressors, or genetics.
7. Conclusions
7.1 Diagnosis
Certain cytokines, inflammatory markers, and anti-inflammatory markers are consistently altered in individuals with schizophrenia, as are antibodies to certain pathogens. Many research groups are currently working to identify biomarkers for schizophrenia. Future research should examine if certain subtypes of schizophrenia are more associated with specific markers, and to make serum analysis a viable tool to aid in diagnosis (CSF fluid analysis may be more thorough currently, but it is highly invasive). If certain subtypes of schizophrenia are characterized by certain immune/inflammatory profiles, the next step would be to identify the causal relationships therein. As of yet, this has not been done: while the meta-analysis by Miller et al. (2011) showed evidence that cytokine profiles are related to illness state, studies showing relationships between cytokine profile and symptom ratings were not reliably replicated. However, because of the heterogeneous nature of the studies (i.e. treatment groups, medication, cytokines assayed), additional studies need to be conducted to determine a more standard set of biomarkers. Positive prediction of conversion to psychosis among high risk individuals is improving, but still lacks sensitivity, with commonly used predictors including family history, neurocognitive markers, electrophysiological measures, environmental factors, imaging, and clinical assessment (Fusar-Poli et al., 2013; Morgan et al., 2013). Identifying inflammatory indicators in addition to these other measures could add another tool for effectively identifying and treating individuals who will transition to schizophrenia. More research is needed for imaging studies to determine if inflammation and microglial activity are useful indicators of specific subtypes of illness, and to what extent regional location and temporal context may contribute to specific symptoms. Similarly, certain antibody levels have been linked to behavioral changes; further understanding of these interactions could help determine the cognitive changes in individuals with schizophrenia.
7.2 Prevention
There have now been an abundance of studies showing that increasing prenatal stressors increases the risk for the offspring to develop schizophrenia (Brown and Derkits, 2010; Brown and Patterson, 2011; Clarke et al., 2012). It is therefore of upmost importance to increase pregnancy health and awareness, especially among vulnerable populations. Better and cheaper influenza vaccinations show sound promise. Additional population cohort studies would add significant understanding to this issue. More research into the relationship between inflammation and acute/chronic diseases is needed to identify potential anti-inflammatory interventions for at risk individuals, including people with prodromal symptoms, environmentally at risk individuals, and previously diagnosed people in remission. Early identification of CNS infection and encephalitis in children and adolescents would go a long way to treat not only the primary infection, but future mental health problems as well. If reducing inflammation becomes a viable method to prevent or treat schizophrenia, early intervention may be far more effective, and more studies explicitly combining anti-inflammatory treatments to existing protocols for treating prodromal and high-risk patients should be tried. Family history of autoimmune disease should be considered a small risk for developing schizophrenia.
7.3 Treatment
The inflammation hypothesis also highlights the importance of novel therapeutic approaches, including targeting the immune system, the blood brain barrier, stress pathways, and glia. More clinical trials are needed to identify anti-inflammatory medications that may be used in conjunction with anti-psychotics. Müller and colleagues (2013) reviewed treatment studies to date, including anti-inflammatory agents, such as n-3 fatty acids, erythropoietin, tetracycline, and cox-2 inhibitors. While inconsistent, there is evidence that these treatments may be effective in various domains of function and recovery, as suggested in the study by Amminger et al. (2010) showing a reduced rate of conversion to psychosis among high risk individuals treated with n-3 fatty acids. While early studies of glucocorticoids have provided mixed results (Knight et al., 2007), a more recent one found positive results (Laan et al., 2009) and a recent meta-analysis of 5 studies involving NSAID treatment found they were effective at reducing symptom severity (Sommer et al., 2012). However, there are many challenges in this approach. Since inflammatory profiles may change depending on disease state, therapy may need to be flexible in this respect. The heterogeneity of schizophrenia is a further impediment: it is highly probable that different subtypes of schizophrenia may be characterized by distinct inflammatory profiles, akin to the genetic disparities observed in the disorder. Nevertheless, these are prime areas to target for research, and may yield large payoffs in discovering better treatment options, understanding critical periods in schizophrenia development, and characterizing the illness.
7.4 Summary
As we and others have illustrated, there is now a large amount of literature exploring the associations between schizophrenia and the inflammatory response. The important point going forward is how to use the information to determine the best areas on which to focus research, treatment, and therapy. What is emerging is a picture of schizophrenia as a dynamic biological disorder. The two-hit hypothesis emphasizes that an insult at the prenatal or early life stage may prime the nervous system to develop one of two ways. The first is abnormally, such that functioning is noticeably disordered even before individuals are diagnosed with a schizophrenia-spectrum disorder. This may result from alterations to neurons, membranes, dendrites, or neurotransmitter integrity and functioning. The second is normally, but such that the CNS is primed so a second event later in life (e.g. microglial activity, synaptic pruning, immune insult) disrupts neurological processes.
Given the enormous body of research relating infectious and inflammatory factors to neural development and behavior, the immune system represents a highly relevant variable in the two-hit hypothesis for development of schizophrenia. This may contribute to schizophrenia risk in several ways. In particular, abnormal glutamate signaling is now a well established feature of schizophrenia, and as we have reviewed, there are multiple ways in which immune system activity can lead to its disruption. Overall, given the preponderance of evidence, there is significant promise in further exploring these relationships.
Highlights.
We review the literature on schizophrenia and inflammation
The literature is consistent with the two-hit hypothesis of schizophrenia
Mechanisms include microglial activity, synaptic pruning, and glutamate signaling
Further research could improve schizophrenia diagnosis, prevention, and treatment
Acknowledgments
Dr. Feigenson’s work was supported by the Biomedical Science Education Postdoctoral Training Program – NIH Grant No: 5K12GM093854. Dr. Kusnecov’s work was supported by NIEHS Grant P30 ES005022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alex W. Kusnecov, Email: Kusnecov@rci.rutgers.edu.
Steven M. Silverstein, Email: Silvers1@ubhc.rutgers.edu.
References
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biological Psychiatry. 2010;68:1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Adell A, Jimenez-Sanchez L, Lopez-Gil X, Romon T. Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr Bull. 2012;38:9–14. doi: 10.1093/schbul/sbr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad AS, Ahmad M, de Brum-Fernandes AJ, Dore S. Prostaglandin EP4 receptor agonist protects against acute neurotoxicity. Brain Research. 2005;1066:71–77. doi: 10.1016/j.brainres.2005.10.068. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology. 2012;37:1216–1223. doi: 10.1038/npp.2011.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S. Prostaglandin E2 protects cultured cortical neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Research. 1994;663:237–243. doi: 10.1016/0006-8993(94)91268-8. [DOI] [PubMed] [Google Scholar]
- Akter K, Gallo DA, Martin SA, Myronyuk N, Roberts RT, Stercula K, Raffa RB. A review of the possible role of the essential fatty acids and fish oils in the aetiology, prevention or pharmacotherapy of schizophrenia. Journal of Clinical Pharmacy and Therapeutics. 2012;37:132–139. doi: 10.1111/j.1365-2710.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology. 2012;220:627–637. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan CL, Cardno AG, Rijsdijk FV, Kalidindi S, Farmer A, Murray RM, McGuffin P. Twin study of illness history variables in psychosis. Schizophr Res. 2009;115:237–244. doi: 10.1016/j.schres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Torres-Castorena A, Liesenfeld O, Estrada-Martinez S, Urbina-Alvarez JD. High seroprevalence of Toxoplasma gondii infection in a subset of Mexican patients with work accidents and low socioeconomic status. Parasit Vectors. 2012;5:13. doi: 10.1186/1756-3305-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of General Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M. Schizophrenia: Linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;42:5–19. doi: 10.1016/j.pnpbp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. Journal of Neuroendocrinology. 2012;24:183–190. doi: 10.1111/j.1365-2826.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Aronsson F, Lannebo C, Paucar M, Brask J, Kristensson K, Karlsson H. Persistence of viral RNA in the brain of offspring to mice infected with influenza A/WSN/33 virus during pregnancy. J Neurovirol. 2002;8:353–357. doi: 10.1080/13550280290100480. [DOI] [PubMed] [Google Scholar]
- Asp L, Holtze M, Powell SB, Karlsson H, Erhardt S. Neonatal infection with neurotropic influenza A virus induces the kynurenine pathway in early life and disrupts sensorimotor gating in adult Tap1−/− mice. Int J Neuropsychopharmacol. 2010;13:475–485. doi: 10.1017/S1461145709990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp L, Johansson AS, Mann A, Owe-Larsson B, Urbanska EM, Kocki T, Kegel M, Engberg G, Lundkvist GB, Karlsson H. Effects of pro-inflammatory cytokines on expression of kynurenine pathway enzymes in human dermal fibroblasts. J Inflamm (Lond) 2011;8:25. doi: 10.1186/1476-9255-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharnoori M, Bhardwaj SK, Srivastava LK. Effect of maternal lipopolysaccharide administration on the development of dopaminergic receptors and transporter in the rat offspring. PLoS One. 2013;8:e54439. doi: 10.1371/journal.pone.0054439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biological Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J, Alkondon M, Albuquerque EX. Kynurenic acid inhibits glutamatergic transmission to CA1 pyramidal neurons via alpha7 nAChR-dependent and -independent mechanisms. Biochemical Pharmacology. 2012a;84:1078–1087. doi: 10.1016/j.bcp.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Banerjee J, Alkondon M, Pereira EF, Albuquerque EX. Regulation of GABAergic inputs to CA1 pyramidal neurons by nicotinic receptors and kynurenic acid. Journal of Pharmacology and Experimental Therapeutics. 2012b;341:500–509. doi: 10.1124/jpet.111.189860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D. Reduced cancer incidence among patients with schizophrenia. Cancer. 2005;104:2817–2821. doi: 10.1002/cncr.21574. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Nuechterlein KH, Gitlin M, Doi C, Edwards N, Lieu C, Altshuler LL, Mintz J. Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophrenia Research. 2007;93:13–22. doi: 10.1016/j.schres.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Beary M, Hodgson R, Wildgust HJ. A critical review of major mortality risk factors for all-cause mortality in first-episode schizophrenia: clinical and research implications. J Psychopharmacol. 2012;26:52–61. doi: 10.1177/0269881112440512. [DOI] [PubMed] [Google Scholar]
- Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;42:71–91. doi: 10.1016/j.pnpbp.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. Journal of Psychiatric Research. 2010;44:321–330. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophr Res. 2012;141:179–184. doi: 10.1016/j.schres.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology. 2012;153:2777–2788. doi: 10.1210/en.2011-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR. Schizophrenia: susceptibility genes, dendritic-spine pathology and gray matter loss. Progress in Neurobiology. 2011;95:275–300. doi: 10.1016/j.pneurobio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Benros ME, Laursen TM, Dalton SO, Mortensen PB. Psychiatric disorder as a first manifestation of cancer: a 10-year population-based study. International Journal of Cancer. 2009;124:2917–2922. doi: 10.1002/ijc.24274. [DOI] [PubMed] [Google Scholar]
- Benros ME, Mortensen PB, Eaton WW. Autoimmune diseases and infections as risk factors for schizophrenia. Annals of the New York Academy of Sciences. 2012;1262:56–66. doi: 10.1111/j.1749-6632.2012.06638.x. [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. American Journal of Psychiatry. 2011;168:1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- Bentsen H, Solberg DK, Refsum H, Bohmer T. Clinical and biochemical validation of two endophenotypes of schizophrenia defined by levels of polyunsaturated fatty acids in red blood cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2012;87:35–41. doi: 10.1016/j.plefa.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Bentsen H, Solberg DK, Refsum H, Gran JM, Bohmer T, Torjesen PA, Halvorsen O, Lingjaerde O. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105. doi: 10.1016/j.biopsych.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Beppu K, Kosai Y, Kido MA, Akimoto N, Mori Y, Kojima Y, Fujita K, Okuno Y, Yamakawa Y, Ifuku M, Shinagawa R, Nabekura J, Sprengel R, Noda M. Expression, subunit composition, and function of AMPA-type glutamate receptors are changed in activated microglia; possible contribution of GluA2 (GluR-B)-deficiency under pathological conditions. Glia. 2013;61:881–891. doi: 10.1002/glia.22481. [DOI] [PubMed] [Google Scholar]
- Berger JV, Dumont AO, Focant MC, Vergouts M, Sternotte A, Calas AG, Goursaud S, Hermans E. Opposite regulation of metabotropic glutamate receptor 3 and metabotropic glutamate receptor 5 by inflammatory stimuli in cultured microglia and astrocytes. Neuroscience. 2012;205:29–38. doi: 10.1016/j.neuroscience.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, Steiner J, Connor TJ, Harkin A, Versnel MA, Drexhage HA. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. Journal of Leukocyte Biology. 2012;92:959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bhadra R, Cobb DA, Weiss LM, Khan IA. Psychiatric disorders in toxoplasma seropositive patients--the CD8 connection. Schizophr Bull. 2013;39:485–489. doi: 10.1093/schbul/sbt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain, Behavior, and Immunity. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia. 2013;61:62–70. doi: 10.1002/glia.22372. [DOI] [PubMed] [Google Scholar]
- Blomstrom A, Karlsson H, Wicks S, Yang S, Yolken RH, Dalman C. Maternal antibodies to infectious agents and risk for non-affective psychoses in the offspring--a matched case-control study. Schizophrenia Research. 2012;140:25–30. doi: 10.1016/j.schres.2012.06.035. [DOI] [PubMed] [Google Scholar]
- Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac’h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun. 2012;39:222–228. doi: 10.1016/j.jaut.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Boksa P. Abnormal synaptic pruning in schizophrenia: Urban myth or reality? Journal of Psychiatry and Neuroscience. 2012;37:75–77. doi: 10.1503/jpn.120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnot O, Anderson GM, Cohen D, Willer JC, Tordjman S. Are patients with schizophrenia insensitive to pain? A reconsideration of the question. Clin J Pain. 2009;25:244–252. doi: 10.1097/AJP.0b013e318192be97. [DOI] [PubMed] [Google Scholar]
- Braff DL, Ryan J, Rissling AJ, Carpenter WT. Lack of Use in the Literature From the Last 20 Years Supports Dropping Traditional Schizophrenia Subtypes From DSM-5 and ICD-11. Schizophrenia Bulletin. 2013;39:751–753. doi: 10.1093/schbul/sbt068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodziak A, Ziolko E, Muc-Wierzgon M, Nowakowska-Zajdel E, Kokot T, Klakla K. The role of human endogenous retroviruses in the pathogenesis of autoimmune diseases. Med Sci Monit. 2012;18:RA80–88. doi: 10.12659/MSM.882892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Mocellin S. Suppressive influences in the immune response to cancer. Journal of Immunotherapy. 2009;32:1–11. doi: 10.1097/CJI.0b013e3181837276. [DOI] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Progress in Neurobiology. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Archives of General Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. American Journal of Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophrenia Bulletin. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013;13:551–565. doi: 10.1038/nri3479. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Buka SL, Cannon TD, Torrey EF, Yolken RH. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biological Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Archives of General Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Burt MA, Tse YC, Boksa P, Wong TP. Prenatal immune activation interacts with stress and corticosterone exposure later in life to modulate N-methyl-d-aspartate receptor synaptic function and plasticity. Int J Neuropsychopharmacol. 2013:1–14. doi: 10.1017/S1461145713000229. [DOI] [PubMed] [Google Scholar]
- Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE. Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophrenia Research. 2009;114:6–16. doi: 10.1016/j.schres.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proceedings of the Nutrition Society. 2013;72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson NG, Rojas MA, Black JD, Redd JW, Hille J, Hill KE, Rose JW. Microglial inhibition of neuroprotection by antagonists of the EP1 prostaglandin E2 receptor. J Neuroinflammation. 2009;6:5. doi: 10.1186/1742-2094-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona L, Cross M, Williams B, Lassere M, March L. Rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2010;24:733–745. doi: 10.1016/j.berh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Research Bulletin. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J Physiol. 1998;513 ( Pt 2):317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Catts SV. Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res. 2000;41:405–415. doi: 10.1016/s0920-9964(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Catts VS, Catts SV, O’Toole BI, Frost AD. Cancer incidence in patients with schizophrenia and their first-degree relatives - a meta-analysis. Acta Psychiatrica Scandinavica. 2008;117:323–336. doi: 10.1111/j.1600-0447.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- Cazevieille C, Muller A, Meynier F, Dutrait N, Bonne C. Protection by prostaglandins from glutamate toxicity in cortical neurons. Neurochemistry International. 1994;24:395–398. doi: 10.1016/0197-0186(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Chan WY, Chia MY, Yang GL, Woon PS, Sitoh YY, Collinson SL, Nowinski WL, Sim K. Duration of illness, regional brain morphology and neurocognitive correlates in schizophrenia. Annals of the Academy of Medicine, Singapore. 2009;38:388–388. [PubMed] [Google Scholar]
- Chen SJ, Chao YL, Chen CY, Chang CM, Wu EC, Wu CS, Yeh HH, Chen CH, Tsai HJ. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. British Journal of Psychiatry. 2012a;200:374–380. doi: 10.1192/bjp.bp.111.092098. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Chao YL, Chen CY, Chang CM, Wu EC, Wu CS, Yeh HH, Chen CH, Tsai HJ. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. Br J Psychiatry. 2012b;200:374–380. doi: 10.1192/bjp.bp.111.092098. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Lin GM, Li YH. Cancer risk before schizophrenia diagnosis in taiwan, 1995–2009. Schizophrenia Bulletin. 2013;39:729–731. doi: 10.1093/schbul/sbt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Barres BA. The role of glial cells in synapse elimination. Current Opinion in Neurobiology. 2012;22:438–445. doi: 10.1016/j.conb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MC, Kelleher I, Clancy M, Cannon M. Predicting risk and the emergence of schizophrenia. Psychiatr Clin North Am. 2012;35:585–612. doi: 10.1016/j.psc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Coe CL. Psychosocial factors and immunity in nonhuman primates: a review. Psychosom Med. 1993;55:298–308. doi: 10.1097/00006842-199305000-00007. [DOI] [PubMed] [Google Scholar]
- Cohen M, Dembling B, Schorling J. The association between schizophrenia and cancer: a population-based mortality study. Schizophrenia Research. 2002;57:139–146. doi: 10.1016/s0920-9964(01)00308-5. [DOI] [PubMed] [Google Scholar]
- Condray R, Yao JK. Cognition, dopamine and bioactive lipids in schizophrenia. Front Biosci (Schol Ed) 2011;3:298–330. doi: 10.2741/s153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condray R, Yao JK, Steinhauer SR, van Kammen DP, Reddy RD, Morrow LA. Semantic memory in schizophrenia: association with cell membrane essential fatty acids. Schizophr Res. 2008;106:13–28. doi: 10.1016/j.schres.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvin A, Buchanan RW, Carpenter WT, Kennedy JL, Keshavan MS, MacDonald A, III, Sass L, Wessa M. Which Aspects of Heterogeneity Are Useful to Translational Success? In: Silverstein SM, Moghaddam B, Wykes T, editors. Schizophrenia: Evolution and Synthesis. Cambridge: MIT Press; 2013. [PubMed] [Google Scholar]
- Cournos F, McKinnon K, Sullivan G. Schizophrenia and comorbid human immunodeficiency virus or hepatitis C virus. Journal of Clinical Psychiatry. 2005;66(Suppl 6):27–33. [PubMed] [Google Scholar]
- Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. American Journal of Psychiatry. 2013;170:324–333. doi: 10.1176/appi.ajp.2012.12050599. [DOI] [PubMed] [Google Scholar]
- Dale RC, Irani SR, Brilot F, Pillai S, Webster R, Gill D, Lang B, Vincent A. N-methyl-D-aspartate receptor antibodies in pediatric dyskinetic encephalitis lethargica. Annals of Neurology. 2009;66:704–709. doi: 10.1002/ana.21807. [DOI] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Gunnell D, Harrison G, Kristensson K, Lewis G, Lofving S, Rasmussen F, Wicks S, Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. American Journal of Psychiatry. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton VS, Verdurand M, Walker A, Hodgson DM, Zavitsanou K. Synergistic Effect between Maternal Infection and Adolescent Cannabinoid Exposure on Serotonin 5HT1A Receptor Binding in the Hippocampus: Testing the “Two Hit” Hypothesis for the Development of Schizophrenia. ISRN Psychiatry. 2012;2012:451865. doi: 10.5402/2012/451865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clinical Chemistry. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Skoromets AA. Multiple panel of biomarkers for TIA/stroke evaluation. Stroke. 2002;33:1181–1182. doi: 10.1161/01.str.0000014922.83673.86. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KA, Platel JC, Huang F, Tian D, Stamboulian-Platel S, Bordey A. Prostaglandin E2 induces glutamate release from subventricular zone astrocytes. Neuron Glia Biol. 2011:1–7. doi: 10.1017/S1740925X10000244. [DOI] [PubMed] [Google Scholar]
- de la Cruz-Merino L, Grande-Pulido E, Albero-Tamarit A, Codes-Manuel de Villena ME. Cancer and immune response: old and new evidence for future challenges. The Oncologist. 2008;13:1246–1254. doi: 10.1634/theoncologist.2008-0166. [DOI] [PubMed] [Google Scholar]
- de Vries EF, Dierckx RA, Klein HC. Nuclear imaging of inflammation in neurologic and psychiatric disorders. Curr Clin Pharmacol. 2006;1:229–242. doi: 10.2174/157488406778249334. [DOI] [PubMed] [Google Scholar]
- Deb-Rinker P, Klempan TA, O’Reilly RL, Torrey EF, Singh SM. Molecular characterization of a MSRV-like sequence identified by RDA from monozygotic twin pairs discordant for schizophrenia. Genomics. 1999;61:133–144. doi: 10.1006/geno.1999.5946. [DOI] [PubMed] [Google Scholar]
- Dennison U, McKernan D, Cryan J, Dinan T. Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychological Medicine. 2012;42:1865–1871. doi: 10.1017/S0033291712000074. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Lillehoj E, Stallings C, Wiley M, Origoni A, Vaughan C, Khushalani S, Sabunciyan S, Yolken R. Antibodies to retroviruses in recent onset psychosis and multi-episode schizophrenia. Schizophr Res. 2012;138:198–205. doi: 10.1016/j.schres.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Archives of General Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Domercq M, Vazquez-Villoldo N, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci. 2013;7:49. doi: 10.3389/fncel.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of Nuclear Medicine. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TA, Cohen D, Versnel MA, Nolen WA, van Beveren NJ, Drexhage HA. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int J Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- du Bois TM, Deng C, Huang XF. Membrane phospholipid composition, alterations in neurotransmitter systems and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:878–888. doi: 10.1016/j.pnpbp.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annual Review of Immunology. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- During MJ, Symes CW, Lawlor PA, Lin J, Dunning J, Fitzsimons HL, Poulsen D, Leone P, Xu R, Dicker BL, Lipski J, Young D. An oral vaccine against NMDAR1 with efficacy in experimental stroke and epilepsy. Science. 2000;287:1453–1460. doi: 10.1126/science.287.5457.1453. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, Mortensen PB. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. American Journal of Psychiatry. 2006;163:521–528. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Hayward C, Ram R. Schizophrenia and rheumatoid arthritis: a review. Schizophrenia Research. 1992;6:181–192. doi: 10.1016/0920-9964(92)90001-l. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disord. 2010;12:638–646. doi: 10.1111/j.1399-5618.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29:663–671. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emelia O, Amal RN, Ruzanna ZZ, Shahida H, Azzubair Z, Tan KS, Noor Aadila S, Siti NA, Aisah MY. Seroprevalence of anti-Toxoplasma gondii IgG antibody in patients with schizophrenia. Trop Biomed. 2012;29:151–159. [PubMed] [Google Scholar]
- Engel AL, Holt GE, Lu H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert Rev Clin Pharmacol. 2011;4:275–289. doi: 10.1586/ecp.11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- Fan X, Liu EY, Freudenreich O, Park JH, Liu D, Wang J, Yi Z, Goff D, Henderson DC. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophrenia Research. 2010;118:211–217. doi: 10.1016/j.schres.2010.02.1028. [DOI] [PubMed] [Google Scholar]
- Fan X, Pristach C, Liu EY, Freudenreich O, Henderson DC, Goff DC. Elevated serum levels of C-reactive protein are associated with more severe psychopathology in a subgroup of patients with schizophrenia. Psychiatry Research. 2007;149:267–271. doi: 10.1016/j.psychres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and the risk of cardiovascular diseases: A meta-analysis of thirteen cohort studies. J Psychiatr Res. 2013;47:1549–1556. doi: 10.1016/j.jpsychires.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Feinstein A, du Boulay G, Ron MA. Psychotic illness in multiple sclerosis. A clinical and magnetic resonance imaging study. British Journal of Psychiatry. 1992;161:680–685. doi: 10.1192/bjp.161.5.680. [DOI] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends in Neurosciences. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol Psychiatry. 2013;73:951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Annals of Oncology. 2012;23(Suppl 8):viii6–9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer HG, Nitzgen B, Reichmann G, Gross U, Hadding U. Host cells of Toxoplasma gondii encystation in infected primary culture from mouse brain. Parasitology Research. 1997a;83:637–641. doi: 10.1007/s004360050311. [DOI] [PubMed] [Google Scholar]
- Fischer HG, Nitzgen B, Reichmann G, Hadding U. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. European Journal of Immunology. 1997b;27:1539–1548. doi: 10.1002/eji.1830270633. [DOI] [PubMed] [Google Scholar]
- Flegr J, Klose J, Novotna M, Berenreitterova M, Havlicek J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J, Kodym P, Tolarova V. Correlation of duration of latent Toxoplasma gondii infection with personality changes in women. Biological Psychology. 2000;53:57–68. doi: 10.1016/s0301-0511(00)00034-x. [DOI] [PubMed] [Google Scholar]
- Flegr J, Preiss M, Klose J, Havlicek J, Vitakova M, Kodym P. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii Dopamine, a missing link between schizophrenia and toxoplasmosis? Biological Psychology. 2003;63:253–268. doi: 10.1016/s0301-0511(03)00075-9. [DOI] [PubMed] [Google Scholar]
- Francesconi LP, Cereser KM, Mascarenhas R, Stertz L, Gama CS, Belmonte-de-Abreu P. Increased annexin-V and decreased TNF-alpha serum levels in chronic-medicated patients with schizophrenia. Neuroscience Letters. 2011;502:143–146. doi: 10.1016/j.neulet.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew L, Sugiarto NU, Rajagopal SP, He J, Leask R, Norman JE, Riley SC, Stock SJ. The effect of omega-3 polyunsaturated fatty acids on the inflammatory response of the amnion. Prostaglandins Leukotrienes and Essential Fatty Acids. 2013;89:221–225. doi: 10.1016/j.plefa.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013 doi: 10.1016/j.pnpbp.2012.12.013. (in press) [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, Seishima M. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002;70:779–786. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele S, Pillai A. Decreased VEGF mRNA expression in the dorsolateral prefrontal cortex of schizophrenia subjects. Schizophr Res. 2009;115:372–373. doi: 10.1016/j.schres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Berger G. Eicosapentaenoic acid interventions in schizophrenia: meta-analysis of randomized, placebo-controlled studies. J Clin Psychopharmacol. 2012;32:179–185. doi: 10.1097/JCP.0b013e318248b7bb. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, Keshavan M, Wood S, Ruhrmann S, Seidman LJ, Valmaggia L, Cannon T, Velthorst E, De Haan L, Cornblatt B, Bonoldi I, Birchwood M, McGlashan T, Carpenter W, McGorry P, Klosterkotter J, McGuire P, Yung A. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable MS, Gavali S, Radner A, Tilley DH, Lee B, Dyner L, Collins A, Dengel A, Dalmau J, Glaser CA. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. European Journal of Clinical Microbiology and Infectious Diseases. 2009;28:1421–1429. doi: 10.1007/s10096-009-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal G, Goral A, Murad H, Gross R, Pugachova I, Barchana M, Kohn R, Levav I. Cancer in parents of persons with schizophrenia: is there a genetic protection? Schizophrenia Research. 2012;139:189–193. doi: 10.1016/j.schres.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Brar JS, Rabin BS. Immune abnormalities in schizophrenia: evidence for the autoimmune hypothesis. Harv Rev Psychiatry. 1994;2:70–83. doi: 10.3109/10673229409017120. [DOI] [PubMed] [Google Scholar]
- Ganor Y, Goldberg-Stern H, Amrom D, Lerman-Sagie T, Teichberg VI, Pelled D, Futerman AH, Zeev BB, Freilinger M, Verheulpen D, Van Bogaert P, Levite M. Autoimmune epilepsy: some epilepsy patients harbor autoantibodies to glutamate receptors and dsDNA on both sides of the blood-brain barrier, which may kill neurons and decrease in brain fluids after hemispherotomy. Clin Dev Immunol. 2004;11:241–252. doi: 10.1080/17402520400001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganor Y, Goldberg-Stern H, Lerman-Sagie T, Teichberg VI, Levite M. Autoimmune epilepsy: distinct subpopulations of epilepsy patients harbor serum autoantibodies to either glutamate/AMPA receptor GluR3, glutamate/NMDA receptor subunit NR2A or double-stranded DNA. Epilepsy Research. 2005;65:11–22. doi: 10.1016/j.eplepsyres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Gaughran F, O’Neill E, Sham P, Daly RJ, Shanahan F. Soluble interleukin 2 receptor levels in families of people with schizophrenia. Schizophrenia Research. 2002;56:235–239. doi: 10.1016/s0920-9964(01)00275-4. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Poulter MO, Anisman H. Effects of stressors and immune activating agents on peripheral and central cytokines in mouse strains that differ in stressor responsivity. Brain Behav Immun. 2011;25:468–482. doi: 10.1016/j.bbi.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Gilvarry CM, Sham PC, Jones PB, Cannon M, Wright P, Lewis SW, Bebbington P, Toone BK, Murray RM. Family history of autoimmune diseases in psychosis. Schizophrenia Research. 1996;19:33–40. doi: 10.1016/0920-9964(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. Schizophrenia and cancer: an epidemiological study. British Journal of Psychiatry. 2005;187:334–338. doi: 10.1192/bjp.187.4.334. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. Journal of Clinical Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Seidman LJ, Petryshen TL, Fitzmaurice G, Tsuang MT, Buka SL. Sex-specific rates of transmission of psychosis in the New England high-risk family study. Schizophr Res. 2011;128:150–155. doi: 10.1016/j.schres.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Walder DJ. Sex differences in schizophrenia: the case for developmental origins and etiological implications. In: Sharma T, Harvey PD, editors. The Early Course of Schizophrenia. Oxford, United Kingdom: Oxford University Press; 2006. [Google Scholar]
- Gorwood P, Pouchot J, Vinceneux P, Puechal X, Flipo RM, De Bandt M, Ades J. Rheumatoid arthritis and schizophrenia: a negative association at a dimensional level. Schizophrenia Research. 2004;66:21–29. doi: 10.1016/s0920-9964(03)00017-3. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Grinshpoon A, Barchana M, Ponizovsky A, Lipshitz I, Nahon D, Tal O, Weizman A, Levav I. Cancer in schizophrenia: is the risk higher or lower? Schizophrenia Research. 2005;73:333–341. doi: 10.1016/j.schres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. Journal of Neuroscience. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–469. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Haroon F, Handel U, Angenstein F, Goldschmidt J, Kreutzmann P, Lison H, Fischer KD, Scheich H, Wetzel W, Schluter D, Budinger E. Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS One. 2012;7:e35516. doi: 10.1371/journal.pone.0035516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One. 2012;7:e46368. doi: 10.1371/journal.pone.0046368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacology and Therapeutics. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia. 2006;53:660–668. doi: 10.1002/glia.20322. [DOI] [PubMed] [Google Scholar]
- Hayes E, Gavrilidis E, Kulkarni J. The role of oestrogen and other hormones in the pathophysiology and treatment of schizophrenia. Schizophr Res Treatment. 2012;2012:540273. doi: 10.1155/2012/540273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yu Z, Giegling I, Xie L, Hartmann AM, Prehn C, Adamski J, Kahn R, Li Y, Illig T, Wang-Sattler R, Rujescu D. Schizophrenia shows a unique metabolomics signature in plasma. Transl Psychiatry. 2012;2:e149. doi: 10.1038/tp.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, Mayr T, Kirisits MJ, Wollmann R, Ferguson DJ, Roberts CW, Hwang JH, Trendler T, Kennan RP, Suzuki Y, Reardon C, Hickey WF, Chen L, McLeod R. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie IB, Banati R, Stewart CH, Lloyd AR. Are common childhood or adolescent infections risk factors for schizophrenia and other psychotic disorders? Medical Journal of Australia. 2009;190:S17–21. doi: 10.5694/j.1326-5377.2009.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Archives of General Psychiatry. 2007;64:1368–1376. doi: 10.1001/archpsyc.64.12.1368. [DOI] [PubMed] [Google Scholar]
- Hjorth E, Freund-Levi Y. Immunomodulation of microglia by docosahexaenoic acid and eicosapentaenoic acid. Curr Opin Clin Nutr Metab Care. 2012;15:134–143. doi: 10.1097/MCO.0b013e32835017cc. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J Psychopharmacol. 2010;24:51–60. doi: 10.1177/1359786810385489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW, Brown AS, Susser E. The Dutch famine and schizophrenia spectrum disorders. Social Psychiatry and Psychiatric Epidemiology. 1998;33:373–379. doi: 10.1007/s001270050068. [DOI] [PubMed] [Google Scholar]
- Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukotrienes and Essential Fatty Acids. 2009;81:151–158. doi: 10.1016/j.plefa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Parallel distributed processing and the emergence of schizophrenic symptoms. Schizophrenia Bulletin. 1993;19:119–140. doi: 10.1093/schbul/19.1.119. [DOI] [PubMed] [Google Scholar]
- Hoistad M, Segal D, Takahashi N, Sakurai T, Buxbaum JD, Hof PR. Linking white and grey matter in schizophrenia: oligodendrocyte and neuron pathology in the prefrontal cortex. Front Neuroanat. 2009;3:9. doi: 10.3389/neuro.05.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtze M, Asp L, Schwieler L, Engberg G, Karlsson H. Induction of the kynurenine pathway by neurotropic influenza A virus infection. Journal of Neuroscience Research. 2008;86:3674–3683. doi: 10.1002/jnr.21799. [DOI] [PubMed] [Google Scholar]
- Holub D, Flegr J, Dragomirecka E, Rodriguez M, Preiss M, Novak T, Cermak J, Horacek J, Kodym P, Libiger J, Hoschl C, Motlova LB. Differences in onset of disease and severity of psychopathology between toxoplasmosis-related and toxoplasmosis-unrelated schizophrenia. Acta Psychiatrica Scandinavica. 2013;127:227–238. doi: 10.1111/acps.12031. [DOI] [PubMed] [Google Scholar]
- Hope S, Ueland T, Steen NE, Dieset I, Lorentzen S, Berg AO, Agartz I, Aukrust P, Andreassen OA. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophrenia Research. 2013;145:36–42. doi: 10.1016/j.schres.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Horacek J, Flegr J, Tintera J, Verebova K, Spaniel F, Novak T, Brunovsky M, Bubenikova-Valesova V, Holub D, Palenicek T, Hoschl C. Latent toxoplasmosis reduces gray matter density in schizophrenia but not in controls: voxel-based-morphometry (VBM) study. World J Biol Psychiatry. 2012;13:501–509. doi: 10.3109/15622975.2011.573809. [DOI] [PubMed] [Google Scholar]
- Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Current Opinion in Rheumatology. 2013;25:488–795. doi: 10.1097/BOR.0b013e32836208de. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. Schizophrenia as a membrane lipid disorder which is expressed throughout the body. Prostaglandins Leukotrienes and Essential Fatty Acids. 1996;55:3–7. doi: 10.1016/s0952-3278(96)90138-6. [DOI] [PubMed] [Google Scholar]
- Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophrenia Research. 1998;30:193–208. doi: 10.1016/s0920-9964(97)00151-5. [DOI] [PubMed] [Google Scholar]
- Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol. 2012;27:6–14. doi: 10.1002/hup.1259. [DOI] [PubMed] [Google Scholar]
- Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nature Neuroscience. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- Huang W, Li S, Hu Y, Yu H, Luo F, Zhang Q, Zhu F. Implication of the env gene of the human endogenous retrovirus W family in the expression of BDNF and DRD3 and development of recent-onset schizophrenia. Schizophrenia Bulletin. 2011;37:988–1000. doi: 10.1093/schbul/sbp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. Journal of Neuroscience. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hata T, Dalmau J. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging. 2004;25:619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Jamieson SE, de Roubaix LA, Cortina-Borja M, Tan HK, Mui EJ, Cordell HJ, Kirisits MJ, Miller EN, Peacock CS, Hargrave AC, Coyne JJ, Boyer K, Bessieres MH, Buffolano W, Ferret N, Franck J, Kieffer F, Meier P, Nowakowska DE, Paul M, Peyron F, Stray-Pedersen B, Prusa AR, Thulliez P, Wallon M, Petersen E, McLeod R, Gilbert RE, Blackwell JM. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS One. 2008;3:e2285. doi: 10.1371/journal.pone.0002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Sundquist K, Ning Y, Kendler KS, Sundquist J, Chen X. Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophr Bull. 2013;39:527–536. doi: 10.1093/schbul/sbs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Molecular and Cellular Neurosciences. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Juckel G, Manitz MP, Brune M, Friebe A, Heneka MT, Wolf RJ. Microglial activation in a neuroinflammational animal model of schizophrenia--a pilot study. Schizophrenia Research. 2011;131:96–100. doi: 10.1016/j.schres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Molecular Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, Mahadik S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Kannan G, Pletnikov MV. Toxoplasma gondii and cognitive deficits in schizophrenia: an animal model perspective. Schizophrenia Bulletin. 2012;38:1155–1161. doi: 10.1093/schbul/sbs079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Bachmann S, Schroder J, McArthur J, Torrey EF, Yolken RH. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4634–4639. doi: 10.1073/pnas.061021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Blomstrom A, Wicks S, Yang S, Yolken RH, Dalman C. Maternal antibodies to dietary antigens and risk for nonaffective psychosis in offspring. American Journal of Psychiatry. 2012;169:625–632. doi: 10.1176/appi.ajp.2012.11081197. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Duan S, Take S, Yoshimura M. Modulation of glutamate-induced outward current by prostaglandin E(2) in rat dissociated preoptic neurons. Brain Research. 2005;1037:180–186. doi: 10.1016/j.brainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: A meta-analysis of population-based studies. Schizophrenia Research. 2012;139:161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari PS, Sperlagh B. Inflammation in neurological and psychiatric diseases. Inflammopharmacology. 2012;20:103–107. doi: 10.1007/s10787-012-0124-x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ahn K, Kim DS. Quantitative expression of the HERV-W env gene in human tissues. Archives of Virology. 2008;153:1587–1591. doi: 10.1007/s00705-008-0159-x. [DOI] [PubMed] [Google Scholar]
- Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochemical Journal. 2011;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JG, Menkes DB, Highton J, Adams DD. Rationale for a trial of immunosuppressive therapy in acute schizophrenia. Molecular Psychiatry. 2007;12:424–431. doi: 10.1038/sj.mp.4001959. [DOI] [PubMed] [Google Scholar]
- Korotkova M, Daha NA, Seddighzadeh M, Ding B, Catrina AI, Lindblad S, Huizinga TW, Toes RE, Alfredsson L, Klareskog L, Jakobsson PJ, Padyukov L. Variants of gene for microsomal prostaglandin E2 synthase show association with disease and severe inflammation in rheumatoid arthritis. European Journal of Human Genetics. 2011;19:908–914. doi: 10.1038/ejhg.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Research. 2005;65:465–472. [PubMed] [Google Scholar]
- Kulkarni J, Gavrilidis E, Worsley R, Hayes E. Role of estrogen treatment in the management of schizophrenia. CNS Drugs. 2012;26:549–557. doi: 10.2165/11630660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kunz M, Cereser KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS, Belmonte-de-Abreu PS, Kauer-Sant’Anna M, Kapczinski F, Gama CS. Serum levels of IL-6, IL-10 and TNF-alpha in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr. 2011;33:268–274. doi: 10.1590/s1516-44462011000300010. [DOI] [PubMed] [Google Scholar]
- Laan W, Smeets H, de Wit NJ, Kahn RS, Grobbee DE, Burger H. Glucocorticosteroids associated with a decreased risk of psychosis. Journal of Clinical Psychopharmacology. 2009;29:288–290. doi: 10.1097/JCP.0b013e3181a44575. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818:291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, Mata S, Kremens D, Vitaliani R, Geschwind MD, Bataller L, Kalb RG, Davis R, Graus F, Lynch DR, Balice-Gordon R, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Annals of Neurology. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Gross U, Luder CG. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitology Research. 2007;100:191–203. doi: 10.1007/s00436-006-0306-9. [DOI] [PubMed] [Google Scholar]
- Latour A, Grintal B, Champeil-Potokar G, Hennebelle M, Lavialle M, Dutar P, Potier B, Billard JM, Vancassel S, Denis I. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell. 2013;12:76–84. doi: 10.1111/acel.12026. [DOI] [PubMed] [Google Scholar]
- Lauvsnes MB, Omdal R. Systemic lupus erythematosus, the brain, and anti-NR2 antibodies. Journal of Neurology. 2012;259:622–629. doi: 10.1007/s00415-011-6232-5. [DOI] [PubMed] [Google Scholar]
- Laye S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;82:295–303. doi: 10.1016/j.plefa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Tamouza R, Charron D, Faucard R, Perron H. Human endogenous retrovirus type W (HERV-W) in schizophrenia: a new avenue of research at the gene-environment interface. World J Biol Psychiatry. 2013;14:80–90. doi: 10.3109/15622975.2010.601760. [DOI] [PubMed] [Google Scholar]
- Lees-Murdock DJ, Walsh CP. DNA methylation reprogramming in the germ line. Epigenetics. 2008;3:5–13. doi: 10.4161/epi.3.1.5553. [DOI] [PubMed] [Google Scholar]
- Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. Journal of Immunology. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, Schwarz M, Myint AM. The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol. 2012;26:33–41. doi: 10.1177/0269881111431622. [DOI] [PubMed] [Google Scholar]
- Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116:317–333. doi: 10.1111/j.1600-0447.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatrica Scandinavica Supplementum. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Levav I, Lipshitz I, Novikov I, Pugachova I, Kohn R, Barchana M, Ponizovsky A, Werner H. Cancer risk among parents and siblings of patients with schizophrenia. British Journal of Psychiatry. 2007;190:156–161. doi: 10.1192/bjp.bp.106.024943. [DOI] [PubMed] [Google Scholar]
- Levesque M, Potvin S, Marchand S, Stip E, Grignon S, Pierre L, Lipp O, Goffaux P. Pain perception in schizophrenia: evidence of a specific pain response profile. Pain Med. 2012;13:1571–1579. doi: 10.1111/j.1526-4637.2012.01505.x. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Gerth CW, Koethe D, Klosterkotter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken RH. Antibodies to infectious agents in individuals with recent onset schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lonnqvist J. Incidence of cancer among persons with schizophrenia and their relatives. Archives of General Psychiatry. 2001;58:573–578. doi: 10.1001/archpsyc.58.6.573. [DOI] [PubMed] [Google Scholar]
- Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr Res. 2005;58:741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacology, Biochemistry and Behavior. 2012;100:665–677. doi: 10.1016/j.pbb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Lin CY, Lane HY, Chen TT, Wu YH, Wu CY, Wu VY. Inverse association between cancer risks and age in schizophrenic patients: a 12-year nationwide cohort study. Cancer Sci. 2013a;104:383–390. doi: 10.1111/cas.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GM, Chen YJ, Kuo DJ, Jaiteh LE, Wu YC, Lo TS, Li YH. Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997–2009. Schizophr Bull. 2013b;39:407–416. doi: 10.1093/schbul/sbr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–432. doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling VJ, Lester D, Mortensen PB, Langenberg PW, Postolache TT. Toxoplasma gondii seropositivity and suicide rates in women. Journal of Nervous and Mental Disease. 2011;199:440–444. doi: 10.1097/NMD.0b013e318221416e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH. Maternal Immune Activation during Gestation Interacts with Disc1 Point Mutation to Exacerbate Schizophrenia-Related Behaviors in Mice. J Neurosci. 2013;33:7654–7666. doi: 10.1523/JNEUROSCI.0091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosciences. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokesh BR, Hsieh HL, Kinsella JE. Peritoneal macrophages from mice fed dietary (n-3) polyunsaturated fatty acids secrete low levels of prostaglandins. Journal of Nutrition. 1986;116:2547–2552. doi: 10.1093/jn/116.12.2547. [DOI] [PubMed] [Google Scholar]
- Luder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. International Journal for Parasitology. 2003;33:833–844. doi: 10.1016/s0020-7519(03)00092-4. [DOI] [PubMed] [Google Scholar]
- Luder CG, Lang T, Beuerle B, Gross U. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clinical and Experimental Immunology. 1998;112:308–316. doi: 10.1046/j.1365-2249.1998.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Shu BC, Kao WT, Chen CN, Ku YC, Tzeng DS. Association of DRD4 uVNTR and TP53 codon 72 polymorphisms with schizophrenia: a case-control study. BMC Med Genet. 2009;10:147. doi: 10.1186/1471-2350-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandi Y, Vecsei L. The kynurenine system and immunoregulation. Journal of Neural Transmission. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- Mann A, Machado NM, Liu N, Mazin AH, Silver K, Afzal KI. A Multidisciplinary Approach to the Treatment of Anti-NMDA-Receptor Antibody Encephalitis: A Case and Review of the Literature. Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:247–254. doi: 10.1176/appi.neuropsych.11070151. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. The burden of mental comorbidity in multiple sclerosis: frequent, underdiagnosed, and undertreated. Multiple Sclerosis. 2009;15:385–392. doi: 10.1177/1352458508099477. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Garcia-Sanchez F, Guaza C, Rodriguez-Jimenez R, Andres-Esteban E, Palomo T, Rubio G, Borrell J. Altered immune function in unaffected first-degree biological relatives of schizophrenia patients. Psychiatry Res. 2012;200:1022–1025. doi: 10.1016/j.psychres.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Perez-Nievas BG, Garcia-Bueno B, Madrigal JL, Andres-Esteban E, Rodriguez-Jimenez R, Hoenicka J, Palomo T, Rubio G, Leza JC. The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res. 2011;128:15–22. doi: 10.1016/j.schres.2011.01.018. [DOI] [PubMed] [Google Scholar]
- McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. Journal of Clinical Investigation. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. Journal of Neuroscience. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan KA, Asmundson GJ, Zvolensky MJ, Carleton RN. Startle response and anxiety sensitivity: subcortical indices of physiologic arousal and fear responding. Emotion. 2012;12:1264–1272. doi: 10.1037/a0029108. [DOI] [PubMed] [Google Scholar]
- McMurray DN, Bonilla DL, Chapkin RS. n-3 Fatty acids uniquely affect antimicrobial resistance and immune cell plasma membrane organization. Chemistry and Physics of Lipids. 2011;164:626–635. doi: 10.1016/j.chemphyslip.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Hahn CG, Richtand NM, Stanford KE. Abnormalities in the fatty acid composition of the postmortem orbitofrontal cortex of schizophrenic patients: gender differences and partial normalization with antipsychotic medications. Schizophr Res. 2007;91:37–50. doi: 10.1016/j.schres.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita J, Siva L. Anti-NMDA receptor encephalitis suspected as cause of drug-induced psychosis. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:E2. doi: 10.1176/jnp.23.4.jnpe2. [DOI] [PubMed] [Google Scholar]
- Meszaros ZS, Perl A, Faraone SV. Psychiatric symptoms in systemic lupus erythematosus: a systematic review. Journal of Clinical Psychiatry. 2012;73:993–1001. doi: 10.4088/JCP.11r07425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J. To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62:1308–1321. doi: 10.1016/j.neuropharm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain, Behavior, and Immunity. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacology and Therapeutics. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Graham KL, Bodenheimer CM, Culpepper NH, Waller JL, Buckley PF. A prevalence study of urinary tract infections in acute relapse of schizophrenia. Journal of Clinical Psychiatry. 2013a;74:271–277. doi: 10.4088/JCP.12m08050. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Mellor A, Buckley P. Total and differential white blood cell counts, high-sensitivity C-reactive protein, and the metabolic syndrome in non-affective psychoses. Brain, Behavior, and Immunity. 2013b;31:82–89. doi: 10.1016/j.bbi.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013a;39:306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Durstewitz PO, Fenton AA, Gingrich JA, Gordon JA, Kelsh W, Moghaddam B, Phillips WA, Sawa A. How can models be better utlized to enhance outcome? In: Silverstein SM, Moghaddam B, Wykes T, editors. Schizophrenia: Evolution and Synthesis. Cambridge, MA: MIT Press; 2013b. pp. 13–34. [Google Scholar]
- Molloy C, Conroy RM, Cotter DR, Cannon M. Is traumatic brain injury a risk factor for schizophrenia? A meta-analysis of case-controlled population-based studies. Schizophrenia Bulletin. 2011;37:1104–1110. doi: 10.1093/schbul/sbr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry and Clinical Neurosciences. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M, Yamauchi Y, Yamada S, Kanba S. Neuroinflammation in schizophrenia especially focused on the role of microglia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;42:115–121. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Morar B, Schwab SG, Albus M, Maier W, Lerer B, Wildenauer DB. Evaluation of association of SNPs in the TNF alpha gene region with schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:318–324. doi: 10.1002/ajmg.b.30451. [DOI] [PubMed] [Google Scholar]
- Morgan C, O’Donovan M, Bittner RA, Cadenhead KS, Jones PB, McGrath J, Silverstein SM, Tost H, Uhlhaas P, Voineskos A. How can risk and resilience factors be leveraged to optimize discovery pathways? In: Silverstein SM, Moghaddam B, Wykes T, editors. Schizophrenia: Evolution and Synthesis. Vol. 13. Cambridge: MIT Press; 2013. pp. 137–164. [PubMed] [Google Scholar]
- Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- Mors O, Mortensen PB, Ewald H. A population-based register study of the association between schizophrenia and rheumatoid arthritis. Schizophrenia Research. 1999;40:67–74. doi: 10.1016/s0920-9964(99)00030-4. [DOI] [PubMed] [Google Scholar]
- Mortensen PB. The incidence of cancer in schizophrenic patients. Journal of Epidemiology and Community Health. 1989;43:43–47. doi: 10.1136/jech.43.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Torrey EF, Yolken RH. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biological Psychiatry. 2007a;61:688–693. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophrenia Bulletin. 2007b;33:741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Myint AM, Krause D, Weidinger E, Schwarz MJ. Anti-inflammatory treatment in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;42:146–153. doi: 10.1016/j.pnpbp.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Muller N, Myint AM, Schwarz MJ. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders--relation to drug treatment. Dialogues Clin Neurosci. 2009;11:319–332. doi: 10.31887/DCNS.2009.11.3/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–148. doi: 10.1007/BF03033242. [DOI] [PubMed] [Google Scholar]
- Muller N, Ulmschneider M, Scheppach C, Schwarz MJ, Ackenheil M, Moller HJ, Gruber R, Riedel M. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- Murphy MA, O’Leary JJ, Cahill DJ. Assessment of the humoral immune response to cancer. J Proteomics. 2012;75:4573–4579. doi: 10.1016/j.jprot.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Nellaker C, Yao Y, Jones-Brando L, Mallet F, Yolken RH, Karlsson H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology. 2006;3:44. doi: 10.1186/1742-4690-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Trakalo J, Valente J, Azevedo MH, Pato MT, Pato CN, Kennedy JL. Human p53 tumor suppressor gene (TP53) and schizophrenia: case-control and family studies. Neurosci Lett. 2005;388:173–178. doi: 10.1016/j.neulet.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH, Nordin C, Karanti A, Persson P, Erhardt S. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–322. doi: 10.1016/j.schres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes SO, Matsuo T, Kaminami MS, Watanabe MA, Reiche EM, Itano EN. An autoimmune or an inflammatory process in patients with schizophrenia, schizoaffective disorder, and in their biological relatives. Schizophrenia Research. 2006;84:180–182. doi: 10.1016/j.schres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Oertelt-Prigione S. Immunology and the menstrual cycle. Autoimmun Rev. 2012;11:A486–492. doi: 10.1016/j.autrev.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Oken RJ, Schulzer M. At issue: schizophrenia and rheumatoid arthritis: the negative association revisited. Schizophrenia Bulletin. 1999;25:625–638. doi: 10.1093/oxfordjournals.schbul.a033407. [DOI] [PubMed] [Google Scholar]
- Okusaga O, Langenberg P, Sleemi A, Vaswani D, Giegling I, Hartmann AM, Konte B, Friedl M, Groer MW, Yolken RH, Rujescu D, Postolache TT. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophrenia Research. 2011;133:150–155. doi: 10.1016/j.schres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Park JK, Lee HJ, Kim JW, Park YH, Lee SS, Chang HI, Song JY, Yoon DJ, Bahn GH, Shin YH, Kim YJ, Kim SA, Choe BK, Kim CJ, Chung JH. Differences in p53 gene polymorphisms between Korean schizophrenia and lung cancer patients. Schizophr Res. 2004;67:71–74. doi: 10.1016/s0920-9964(03)00155-5. [DOI] [PubMed] [Google Scholar]
- Park MH, Kwon YJ, Jeong HY, Lee HY, Hwangbo Y, Yoon HJ, Shim SH. Association between Intracellular Infectious Agents and Schizophrenia. Clin Psychopharmacol Neurosci. 2012;10:117–123. doi: 10.9758/cpn.2012.10.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. Journal of Neuroscience. 2012;32:17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavone P, Parano E, Rizzo R, Trifiletti RR. Autoimmune neuropsychiatric disorders associated with streptococcal infection: Sydenham chorea, PANDAS, and PANDAS variants. J Child Neurol. 2006;21:727–736. doi: 10.1177/08830738060210091401. [DOI] [PubMed] [Google Scholar]
- Pedersen MG, Stevens H, Pedersen CB, Norgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. American Journal of Psychiatry. 2011;168:814–821. doi: 10.1176/appi.ajp.2011.10091351. [DOI] [PubMed] [Google Scholar]
- Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC, Teixeira AL, Lobato MI, Walz JC, Belmonte-de-Abreu PS, Kauer-Sant’anna M, Kapczinski F, Gama CS. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. Journal of Psychiatric Research. 2012;46:819–824. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C, Sarrazin S, Leguen E, Houenou J, Delavest M, Moins-Teiserenc H, Bengoufa D, Yolken R, Madeira A, Garcia-Montojo M, Gehin N, Burgelin I, Ollagnier G, Bernard C, Dumaine A, Henrion A, Gombert A, Le Dudal K, Charron D, Krishnamoorthy R, Tamouza R, Leboyer M. Molecular characteristics of Human Endogenous Retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2013;3:e226. doi: 10.1038/tp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron H, Jouvin-Marche E, Michel M, Ounanian-Paraz A, Camelo S, Dumon A, Jolivet-Reynaud C, Marcel F, Souillet Y, Borel E, Gebuhrer L, Santoro L, Marcel S, Seigneurin JM, Marche PN, Lafon M. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287:321–332. doi: 10.1006/viro.2001.1045. [DOI] [PubMed] [Google Scholar]
- Perron H, Mekaoui L, Bernard C, Veas F, Stefas I, Leboyer M. Endogenous retrovirus type W GAG and envelope protein antigenemia in serum of schizophrenic patients. Biological Psychiatry. 2008;64:1019–1023. doi: 10.1016/j.biopsych.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behavioral and Brain Sciences. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- Pleasure D. Diagnostic and pathogenic significance of glutamate receptor autoantibodies. Archives of Neurology. 2008;65:589–592. doi: 10.1001/archneur.65.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. European Journal of Neuroscience. 2012;35:1605–1612. doi: 10.1111/j.1460-9568.2012.08064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biological Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. 2011;6:e23866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Eack SM, Goradia D, Pancholi KM, Keshavan MS, Yolken RH, Nimgaonkar VL. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: a longitudinal study. American Journal of Psychiatry. 2011;168:822–830. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Molecular Psychiatry. 2007;12:105–113. 101. doi: 10.1038/sj.mp.4001915. [DOI] [PubMed] [Google Scholar]
- Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013 doi: 10.1007/s00401-013-1182-x. [DOI] [PubMed] [Google Scholar]
- Pruss H, Dalmau J, Harms L, Holtje M, Ahnert-Hilger G, Borowski K, Stoecker W, Wandinger KP. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology. 2010;75:1735–1739. doi: 10.1212/WNL.0b013e3181fc2a06. [DOI] [PubMed] [Google Scholar]
- Pruss H, Holtje M, Maier N, Gomez A, Buchert R, Harms L, Ahnert-Hilger G, Schmitz D, Terborg C, Kopp U, Klingbeil C, Probst C, Kohler S, Schwab JM, Stoecker W, Dalmau J, Wandinger KP. IgA NMDA receptor antibodies are markers of synaptic immunity in slow cognitive impairment. Neurology. 2012;78:1743–1753. doi: 10.1212/WNL.0b013e318258300d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Loyo J, Medina-Hernandez V, Estarron-Espinosa M, Canales-Aguirre A, Gomez-Pinedo U, Cerdan-Sanchez LF. Sex differences in lipid peroxidation and fatty acid levels in recent onset schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;44:154–161. doi: 10.1016/j.pnpbp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophrenia Research. 2013 doi: 10.1016/j.schres.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard MD, Brahm NC. Schizophrenia and the immune system: pathophysiology, prevention, and treatment. American Journal of Health-System Pharmacy. 2012;69:757–766. doi: 10.2146/ajhp110271. [DOI] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal Immune Activation Induces Maturation-Dependent Alterations in the Prefrontal GABAergic Transcriptome. Schizophr Bull. 2013 doi: 10.1093/schbul/sbs195. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. Journal of Interferon and Cytokine Research. 2005;25:20–30. doi: 10.1089/jir.2005.25.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. Journal of Immunology. 2006;176:7636–7644. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Vascular endothelial growth factor (VEGF) signaling in tumor progression. Critical Reviews in Oncology/Hematology. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Rothschild DM, O’Grady M, Wecker L. Neonatal cytomegalovirus exposure decreases prepulse inhibition in adult rats: implications for schizophrenia. Journal of Neuroscience Research. 1999;57:429–434. [PubMed] [Google Scholar]
- Roumier A, Pascual O, Bechade C, Wakselman S, Poncer JC, Real E, Triller A, Bessis A. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One. 2008;3:e2595. doi: 10.1371/journal.pone.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld C, Martinez R, Seabra S, Sant’anna C, Goncalves JG, Bozza M, Moura-Neto V, De Souza W. Toxoplasma gondii prevents neuron degeneration by interferon-gamma-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-beta1 production by infected microglia. American Journal of Pathology. 2005;167:1021–1031. doi: 10.1016/s0002-9440(10)61191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N., Jr Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;82:305–314. doi: 10.1016/j.plefa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Parra-Cabrera S, Colditz GA, Berkey CS, Dwyer JT. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000;105:1292–1298. doi: 10.1542/peds.105.6.1292. [DOI] [PubMed] [Google Scholar]
- Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. Journal of Neurobiology. 1999;41:221–229. [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochemical Society Transactions. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- Schenkel LS, Silverstein SM. Dimensions of premorbid functioning in schizophrenia: a review of neuromotor, cognitive, social, and behavioral domains. Genetic, Social, and General Psychology Monographs. 2004;130:241–270. doi: 10.3200/MONO.130.3.241-272. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Progress in Lipid Research. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Vannorsdall TD, Winicki JM, Mushtaq Y, Hikida T, Sawa A, Yolken RH, Dickerson FB, Cascella NG. Neuroanatomic and cognitive abnormalities related to herpes simplex virus type 1 in schizophrenia. Schizophrenia Research. 2010;118:224–231. doi: 10.1016/j.schres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophrenia Bulletin. 2007;33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biological Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Guest PC, Rahmoune H, Harris LW, Wang L, Leweke FM, Rothermundt M, Bogerts B, Koethe D, Kranaster L, Ohrmann P, Suslow T, McAllister G, Spain M, Barnes A, van Beveren NJ, Baron-Cohen S, Steiner J, Torrey FE, Yolken RH, Bahn S. Identification of a biological signature for schizophrenia in serum. Molecular Psychiatry. 2012;17:494–502. doi: 10.1038/mp.2011.42. [DOI] [PubMed] [Google Scholar]
- Schwarz MJ, Muller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56:483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, Erhardt C, Engberg G. Prostaglandin-mediated control of rat brain kynurenic acid synthesis--opposite actions by COX-1 and COX-2 isoforms. Journal of Neural Transmission. 2005;112:863–872. doi: 10.1007/s00702-004-0231-y. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Jolly CA, Chapkin RS. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Molecular Aspects of Medicine. 2012;33:46–54. doi: 10.1016/j.mam.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neuroscience and Biobehavioral Reviews. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. Journal of Neuroscience. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain, Behavior, and Immunity. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Shirts BH, Kim JJ, Reich S, Dickerson FB, Yolken RH, Devlin B, Nimgaonkar VL. Polymorphisms in MICB are associated with human herpes virus seropositivity and schizophrenia risk. Schizophrenia Research. 2007;94:342–353. doi: 10.1016/j.schres.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH, Coe CL. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideli L, Mule A, La Barbera D, Murray RM. Do child abuse and maltreatment increase risk of schizophrenia? Psychiatry Investig. 2012;9:87–99. doi: 10.4306/pi.2012.9.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii: induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–868. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Yao JK. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;69:367–384. doi: 10.1016/j.plefa.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Smesny S. Prostaglandin-mediated signaling in schizophrenia. Int Rev Neurobiol. 2004;59:255–271. doi: 10.1016/S0074-7742(04)59010-4. [DOI] [PubMed] [Google Scholar]
- Smesny S, Milleit B, Nenadic I, Preul C, Kinder D, Lasch J, Willhardt I, Sauer H, Gaser C. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52:1314–1327. doi: 10.1016/j.neuroimage.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Smith HS. Arachidonic acid pathways in nociception. J Support Oncol. 2006;4:277–287. [PubMed] [Google Scholar]
- Sominsky L, Fuller EA, Bondarenko E, Ong LK, Averell L, Nalivaiko E, Dunkley PR, Dickson PW, Hodgson DM. Functional programming of the autonomic nervous system by early life immune exposure: implications for anxiety. PLoS One. 2013;8:e57700. doi: 10.1371/journal.pone.0057700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? a meta-analysis. Journal of Clinical Psychiatry. 2012;73:414–419. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- Soumiya H, Fukumitsu H, Furukawa S. Prenatal immune challenge compromises development of upper-layer but not deeper-layer neurons of the mouse cerebral cortex. J Neurosci Res. 2011;89:1342–1350. doi: 10.1002/jnr.22636. [DOI] [PubMed] [Google Scholar]
- Spudich SS, Ances BM. Neurologic complications of HIV infection. Top Antivir Med. 2012;20:41–47. [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of Psychiatric Research. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG, Bogerts B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Glanz W, Sarnyai Z, Bernstein HG, Vielhaber S, Kastner A, Skalej M, Jordan W, Schiltz K, Klingbeil C, Wandinger KP, Bogerts B, Stoecker W. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson A, Theodorsson E. Mechanisms of estrogens’ dose-dependent neuroprotective and neurodamaging effects in experimental models of cerebral ischemia. Int J Mol Sci. 2011;12:1533–1562. doi: 10.3390/ijms12031533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. Journal of Autoimmunity. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Itoh H, Higuchi Y, Arai H, Takamiya C, Kurachi M. Essential polyunsaturated fatty acids and social cognition in schizophrenia. Psychiatry Res. 2008;157:87–93. doi: 10.1016/j.psychres.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: The story of the Dutch Famine Study. American Journal of Epidemiology. 1998;147:213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Wong SY, Grumet FC, Fessel J, Montoya JG, Zolopa AR, Portmore A, Schumacher-Perdreau F, Schrappe M, Koppen S, Ruf B, Brown BW, Remington JS. Evidence for genetic regulation of susceptibility to toxoplasmic encephalitis in AIDS patients. Journal of Infectious Diseases. 1996;173:265–268. doi: 10.1093/infdis/173.1.265. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Mori H, Mishina M, Watanabe M, Fujiwara T, Shimomura J, Aiba H, Miyajima T, Saito Y, Nezu A, Nishida H, Imai K, Sakaguchi N, Kondo N. Autoantibodies to NMDA receptor in patients with chronic forms of epilepsia partialis continua. Neurology. 2003;61:891–896. doi: 10.1212/01.wnl.0000086819.53078.70. [DOI] [PubMed] [Google Scholar]
- Takaki J, Fujimori K, Miura M, Suzuki T, Sekino Y, Sato K. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: the ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflammation. 2012;9:275. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B, Capitao C, Dean B, Thomas EA. Differential age- and disease-related effects on the expression of genes related to the arachidonic acid signaling pathway in schizophrenia. Psychiatry Res. 2012;196:201–206. doi: 10.1016/j.psychres.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum M, Azab AN, Kaplanski J. Effects of estrogen against LPS-induced inflammation and toxicity in primary rat glial and neuronal cultures. J Endotoxin Res. 2007;13:158–166. doi: 10.1177/0968051907080428. [DOI] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. International Journal for Parasitology. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophrenia Bulletin. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophrenia Bulletin. 2012;38:642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. The schizophrenia-rheumatoid arthritis connection: infectious, immune, or both? Brain, Behavior, and Immunity. 2001;15:401–410. doi: 10.1006/brbi.2001.0649. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. Journal of Neuroscience. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van der Kemp WJ, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophr Res. 2012;141:153–161. doi: 10.1016/j.schres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biological Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophrenia Bulletin. 2008;34:1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. Journal of Neuroscience. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34:1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Velakoulis D, Whitford TJ, Pantelis C. Understanding aberrant white matter development in schizophrenia: an avenue for therapy? Expert Rev Neurother. 2011;11:971–987. doi: 10.1586/ern.11.76. [DOI] [PubMed] [Google Scholar]
- Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. Journal of Neuroimmunology. 2011;231:86–91. doi: 10.1016/j.jneuroim.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nunokawa A, Kaneko N, Muratake T, Arinami T, Ujike H, Inada T, Iwata N, Kunugi H, Itokawa M, Otowa T, Ozaki N, Someya T. Two-stage case-control association study of polymorphisms in rheumatoid arthritis susceptibility genes with schizophrenia. Journal of Human Genetics. 2009;54:62–65. doi: 10.1038/jhg.2008.4. [DOI] [PubMed] [Google Scholar]
- Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophrenia Bulletin. 2007;33:752–756. doi: 10.1093/schbul/sbl073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman M, Korotkova M, af Klint E, Stark A, Audoly LP, Klareskog L, Ulfgren AK, Jakobsson PJ. Expression of microsomal prostaglandin E synthase 1 in rheumatoid arthritis synovium. Arthritis and Rheumatism. 2004;50:1774–1780. doi: 10.1002/art.20286. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathologica. 2005;43:81–89. [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Knickmeyer RC, Short SJ, Styner M, Gilmore JH, Coe CL. Brain enlargement and increased behavioral and cytokine reactivity in infant monkeys following acute prenatal endotoxemia. Behav Brain Res. 2011;219:108–115. doi: 10.1016/j.bbr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Sham PC, Gilvarry CM, Jones PB, Cannon M, Sharma T, Murray RM. Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophrenia Research. 1996;20:261–267. doi: 10.1016/0920-9964(96)82950-1. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen T, Sun L, Zhao Z, Qi X, Zhou K, Cao Y, Wang X, Qiu Y, Su M, Zhao A, Wang P, Yang P, Wu J, Feng G, He L, Jia W, Wan C. Potential metabolite markers of schizophrenia. Molecular Psychiatry. 2013;18:67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Stanley JA, Reddy RD, Keshavan MS, Pettegrew JW. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol Psychiatry. 2002;52:823–830. doi: 10.1016/s0006-3223(02)01397-5. [DOI] [PubMed] [Google Scholar]
- Yao Y, Schroder J, Nellaker C, Bottmer C, Bachmann S, Yolken RH, Karlsson H. Elevated levels of human endogenous retrovirus-W transcripts in blood cells from patients with first episode schizophrenia. Genes Brain Behav. 2008;7:103–112. doi: 10.1111/j.1601-183X.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- Yin DM, Chen YJ, Sathyamurthy A, Xiong WC, Mei L. Synaptic dysfunction in schizophrenia. Adv Exp Med Biol. 2012;970:493–516. doi: 10.1007/978-3-7091-0932-8_22. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Bachmann S, Ruslanova I, Lillehoj E, Ford G, Torrey EF, Schroeder J. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clinical Infectious Diseases. 2001;32:842–844. doi: 10.1086/319221. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunology. 2009;31:706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophrenia Research. 2011;128:61–65. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Zandi MS, Irani SR, Lang B, Waters P, Jones PB, McKenna P, Coles AJ, Vincent A, Lennox BR. Disease-relevant autoantibodies in first episode schizophrenia. Journal of Neurology. 2011;258:686–688. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Zhou D, Zhao Z, Liu Z, Xiao S, Xing Y, Suo WZ, Liu J. The clinical presentation and imaging manifestation of psychosis and dementia in general paresis: a retrospective study of 116 cases. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:300–307. doi: 10.1176/jnp.23.3.jnp300. [DOI] [PubMed] [Google Scholar]


