Abstract
Background
Although epidemiologic studies have shown associations between sedentary behavior and mortality, few have focused on older women with adequate minority representation and few have controlled for both physical activity and functional status.
Purpose
The objective of this study was to determine the relationship between sedentary time and total; cardiovascular disease (CVD); coronary heart disease (CHD); and cancer mortality in a prospective, multiethnic cohort of postmenopausal women.
Methods
The study population included 92,234 women aged 50–79 years at baseline (1993–1998) who participated in the Women’s Health Initiative Observational Study through September 2010. Self-reported sedentary time was assessed by questionnaire and examined in 4 categories (≤4, >4–8, ≥8–11, >11 hours). Mortality risks were examined using Cox proportional hazard models adjusting for confounders. Models were also stratified by age, race/ethnicity, body mass index, physical activity, physical function, and chronic disease to examine possible effect modification. Analyses were conducted in 2012–2013.
Results
The mean follow-up period was 12 years. Compared with women who reported the least sedentary time, women reporting the highest sedentary time had increased risk of all-cause mortality in the multivariate model (HR=1.12, 95% CI=1.05, 1.21). Results comparing the highest versus lowest categories for CVD, CHD, and cancer mortality were as follows: HR=1.13, 95% CI=0.99, 1.29; HR=1.27, 95% CI=1.04, 1.55; and HR=1.21, 95% CI=1.07, 1.37, respectively. For all mortality outcomes, there were significant linear tests for trend.
Conclusions
There was a linear relationship between greater amounts of sedentary time and mortality risk after controlling for multiple potential confounders.
Introduction
There is strong evidence that 10-minute or more bouts of moderate to vigorous physical activity (MVPA) provide substantial health benefits, with higher doses producing greater benefits.1 There is also increasing evidence that less time spent in sedentary activities provides health benefits. Epidemiologic studies suggest that less sedentary time may protect against premature death, independent of MVPA.2–7 Other studies have reported that more sitting time was associated with increased risk for coronary heart disease (CHD)8; cardiovascular disease (CVD)9; and adverse metabolic effects such as obesity and insulin resistance.10–15 In addition, it was estimated that life expectancy in the U.S. would increase by 2 years if people reduced sitting time to fewer than 3 hours/day.16
There appear to be plausible physiologic mechanisms by which sedentary time affects health. One possible explanation is that more sedentary time decreases metabolic activity of muscle and diminishes energy utilization, leading to insulin resistance and metabolic derangements. It has been demonstrated in animal models that lipoprotein lipase is deactivated in the muscle tissue during prolonged physical inactivity.10 Another study showed that prolonged sitting reduced blood clearance of glucose and reduced insulin secretion.17
Analyses of NHANES accelerometer data show that the most sedentary age group is adults aged ≥60 years, with adults aged 60–69 years engaging in 8.4 hours/day of sedentary behaviors and adults aged 70–85 years engaging in 9.3 hours/day.18 However, long-term data on associations of sedentary time and mortality focusing specifically on women aged ≥50 years are limited; in fact, only one prior study of this age group has included a follow-up period of 10 years or longer.6
The goal of the present study was to determine the association between sedentary time and mortality—all-cause mortality and mortality due to CVD, CHD, and cancer. Given the large and diverse sample of this study, possible differences by age, race/ethnicity, BMI, physical activity, physical function, and multiple morbidity were also examined, as these variables are related to mortality and may also influence sedentary time.
Methods
Study Population
The cohort for this study was participants in the Women’s Health Initiative (WHI) Observational Study (OS) and Extension Study (ES). Postmenopausal women aged between 50 and 79 years were recruited from 1993 to 1998 at 40 clinical centers across the U.S. through several recruitment activities (e.g., mass mailings, community presentations) and included 93,676 racially and ethnically diverse postmenopausal women aged 50–79 years at baseline. Data were collected using in-person interviews, physical measurements, blood samples, and self-report questionnaires. When the main WHI study ended in 2005, participants were invited to enroll in the ES. Those who consented were followed up annually by mail from 2005 through 2010. The study data analysis included events through September 30, 2010 (collected as of March 31, 2011). Subjects provided written, informed consent and each study site IRB approved the study. Additional details about WHI have been previously described.19–22
Study Variables
Data on sedentary time were collected at baseline using self-report questionnaires.23 Respondents estimated the total hours spent sitting in response to the question: During a usual day and night, about how many hours do you spend sitting? Be sure to include the time you spend sitting at work, sitting at the table eating, driving or riding in a car or bus, and sitting up watching TV or talking. They also estimated total hours spent lying down: During a usual day and night, about how many hours do you spend sleeping or lying down with your feet up? Be sure to include the time you spend sleeping or trying to sleep at night, resting or napping, and lying down watching TV. A third question asked participants to estimate the number of hours typically spent sleeping each night. To calculate total sedentary time, sitting time plus lying time were summed, and sleeping time was subtracted. In a diverse sample of 1902 women aged 50–79 years, this questionnaire demonstrated moderate to substantial test–retest reliability.23 Mortality was documented through hospital records, autopsy reports, death certificates, and records from the National Center for Health Statistic’s National Death Index.24
The covariates included age; race/ethnicity; education; marital status; BMI (kg/m2); smoking; alcohol use; number of chronic diseases (CHD, congestive heart failure [CHF], stroke, diabetes, cancer, arthritis, hypertensive [on medications or high blood pressure], number of falls in the past year, chronic obstructive pulmonary disease [COPD] and hip fractures before age of 55); hormone use; depressed mood; living alone; number of falls in past 12 months; activities of daily living disability; hypertension; treated diabetes; self-reported health; and history of the following: stroke, CHD, CHF, COPD, falls, cancer, arthritis, and hip fracture over age 55.
Physical activity (as MVPA) level and physical function score were key covariates of interest. For physical activity, respondents were asked to classify the duration, frequency, and intensity of walking and other recreational activities. MVPA was measured using the WHI physical activity questionnaire, which has acceptable validity and reliability.23,25,26 Physical function was measured by self-report using ten items from the Rand-36.27,28 Items asked whether current health limits physical function in four domains (moderate/vigorous activities [two items], strength [four], walking abilities [three], and self-care [one]). SF-36 scores ranged from 0 to 100, with higher scores indicating better physical function.
Outcome data were collected annually by mail. For women with unknown cause of death, lost to follow-up, or no longer followed, additional outcome data were sought by linkage with the National Death Index (NDI). The most recent NDI search was conducted in 2010 and included outcomes through December 2008.
Statistical Analysis
All women for whom sedentary time data were available were included in these analyses, yielding an analytic sample of 92,234 (of 93,676 women). Women who dropped out of the study or were lost to follow-up were censored at their last known contact or end of the NDI search, whichever was later.
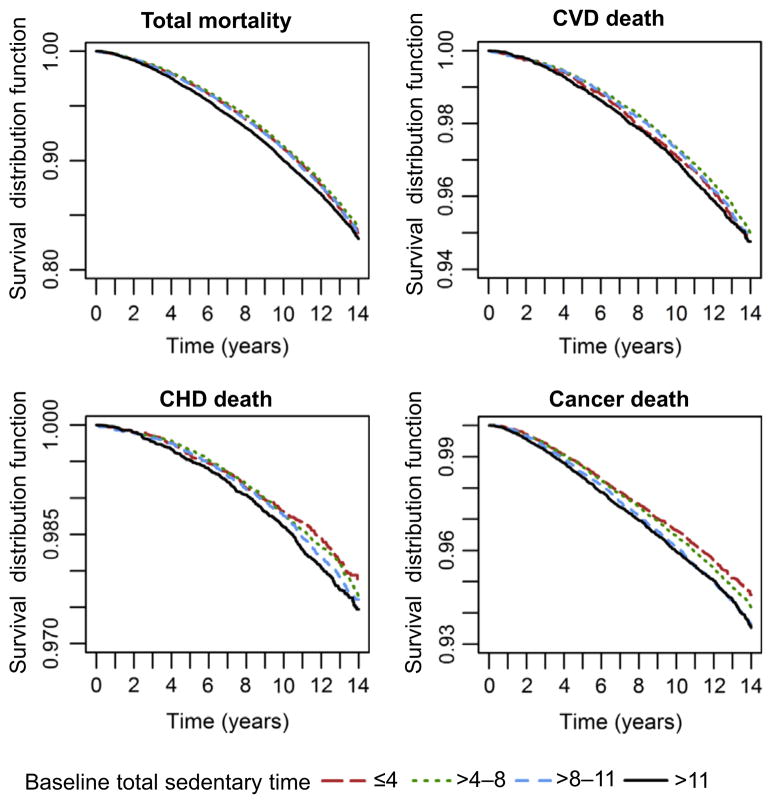
Baseline descriptive characteristics were examined in 4 categories of baseline total sedentary time (in hours/day) using cut-points commonly found in previous studies5,29: ≤4, >4–8, ≥8–11, >11 hours. Frequencies and percentages were presented for categorical variables, whereas mean and SD were shown for continuous variables (Table 1). Two-sided p-values comparing baseline characteristics across categories were based on the chi-square test for categorical variables, or ANOVA for continuous variables. Differences in survival probabilities of all-cause, cancer, CHD, and CVD death across categories of sedentary time were examined using Kaplan–Meier survival curves (Figure 1).
Table 1.
Baseline characteristics of participants by total sedentary time (N=92,234)a
| Variable | All | ≤4 hours | >4–8 hours | >8–11 hours | ≥11 hours | p-valueb |
|---|---|---|---|---|---|---|
| Follow-up time (years) | 92,234; 12.2 (2.4) | 13,134; 12.1 (2.3) | 35,668; 12.2 (2.3) | 23,245; 12.2 (2.4) | 20,187; 12.2 (2.5) | <0.0001 |
| Age (years) | 92,234; 63.6 (7.4) | 13,134; 64.1 (7.3) | 35,668; 64.3 (7.2) | 23,245; 63.5 (7.4) | 20,187; 62.2 (7.5) | <0.0001 |
| Age (years) | <0.0001 | |||||
| 50–59 | 29,280 (31.7) | 3,845 (29.3) | 9,766 (27.4) | 7,532 (32.4) | 8,137 (40.3) | |
| 60–69 | 40,610 (44.0) | 5,884 (44.8) | 16,531 (46.3) | 10,079 (43.4) | 8,116 (40.2) | |
| 70–79 | 22,344 (24.2) | 3,405 (25.9) | 9,371 (26.3) | 5,634 (24.2) | 3,934 (19.5) | |
| Race/ethnicity | <0.0001 | |||||
| White | 77,082 (83.6) | 9,315 (70.9) | 30,481 (85.5) | 20,050 (86.3) | 17,236 (85.4) | |
| Black | 7,415 (8.0) | 1,870 (14.2) | 2,494 (7.0) | 1,529 (6.6) | 1,522 (7.5) | |
| Hispanic | 3,402 (3.7) | 1,119 (8.5) | 1,167 (3.3) | 596 (2.6) | 520 (2.6) | |
| American Indian | 403 (0.4) | 98 (0.7) | 145 (0.4) | 96 (0.4) | 64 (0.3) | |
| Asian/Pacific Islander | 2,645 (2.9) | 475 (3.6) | 907 (2.5) | 661 (2.8) | 602 (3.0) | |
| Unknown | 1,287 (1.4) | 257 (2.0) | 474 (1.3) | 313 (1.3) | 243 (1.2) | |
| Education | <0.0001 | |||||
| None–high school diploma | 19,553 (21.4) | 3,869 (29.8) | 7,773 (21.9) | 4,296 (18.6) | 3,615 (18.0) | |
| School after HS | 33,459 (36.6) | 4,864 (37.4) | 13,016 (36.7) | 8,408 (36.5) | 7,171 (35.8) | |
| College degree | 38,494 (42.1) | 4,265 (32.8) | 14,630 (41.3) | 10,352 (44.9) | 9,247 (46.2) | |
| Physical activity, METs/week | <0.0001 | |||||
| Inactive (0 MET) | 12,422 (13.6) | 1,717 (13.2) | 3,972 (11.2) | 3,010 (13.1) | 3,723 (18.6) | |
| <5 | 17,521 (19.2) | 2,348 (18.1) | 6,314 (17.9) | 4,490 (19.5) | 4,369 (21.8) | |
| 5–<12 | 21,580 (23.6) | 2,882 (22.2) | 8,174 (23.1) | 5,654 (24.5) | 4,870 (24.4) | |
| ≥12 | 39,881 (43.6) | 6,058 (46.6) | 16,892 (47.8) | 9,895 (42.9) | 7,036 (35.2) | |
| Physical function score (0–100) | 90,792; 81.1 (20.4) | 12,807; 81.6 (20.5) | 35,092; 82.9 (18.7) | 22,966; 81.1 (20.1) | 19,927; 77.5 (23.1) | <0.0001 |
| Self-rated health status | <0.0001 | |||||
| Excellent | 16,412 (17.9) | 2,337 (17.9) | 6,659 (18.7) | 4,162 (17.9) | 3,254 (16.2) | |
| Very good | 37,302 (40.6) | 4,891 (37.4) | 15,030 (42.3) | 9,523 (41.1) | 7,858 (39.0) | |
| Good | 29,302 (31.9) | 4,285 (32.8) | 11,021 (31.0) | 7,423 (32.0) | 6,573 (32.7) | |
| Fair/poor | 8,915 (9.7) | 1,566 (12.0) | 2,819 (7.9) | 2,084 (9.0) | 2,446 (12.2) | |
| BMI | <0.0001 | |||||
| <18.5 | 1,091 (1.2) | 206 (1.6) | 428 (1.2) | 258 (1.1) | 199 (1.0) | |
| 18.5–24.9 | 36,193 (39.7) | 5,280 (40.6) | 15,015 (42.6) | 8,934 (38.9) | 6,964 (35.0) | |
| 25–29.9 | 30,968 (34.0) | 4,466 (34.4) | 12,165 (34.5) | 7,898 (34.4) | 6,439 (32.3) | |
| >30.0 | 22,904 (25.1) | 3,048 (23.4) | 7,646 (21.7) | 5,893 (25.6) | 6,317 (31.7) | |
| Smoking status | <0.0001 | |||||
| Never | 46,334 (50.9) | 7,103 (55.0) | 18,484 (52.5) | 11,439 (49.8) | 9,308 (46.6) | |
| Past | 39,030 (42.9) | 4,933 (38.2) | 14,761 (41.9) | 10,155 (44.2) | 9,181 (46.0) | |
| Current | 5,708 (6.3) | 890 (6.9) | 1,965 (5.6) | 1,385 (6.0) | 1,468 (7.4) | |
| Number of falls in previous 12 months | <0.0001 | |||||
| None | 61,760 (67.7) | 9,111 (70.3) | 24,224 (68.7) | 15,335 (66.7) | 13,090 (65.6) | |
| 1 time | 18,136 (19.9) | 2,367 (18.3) | 6,958 (19.7) | 4,712 (20.5) | 4,099 (20.5) | |
| 2 times | 7,470 (8.2) | 968 (7.5) | 2,755 (7.8) | 1,963 (8.5) | 1,784 (8.9) | |
| 3 or more times | 3,837 (4.2) | 507 (3.9) | 1,348 (3.8) | 991 (4.3) | 991 (5.0) | |
| Activity of daily living disability | 1,624 (1.8) | 227 (1.8) | 492 (1.4) | 361 (1.6) | 544 (2.8) | <0.0001 |
N, M, and SD are shown for continuous variable, n and percentage are shown for categorical variable. All cells n (%) unless noted otherwise
p-value for comparing difference in the baseline variable across four groups of total sedentary time was based on χ2 test for categorical variable and ANOVA test for continuous variable
Figure 1.
Unadjusted Kaplan–Meier survival curves for all-cause; cardiovascular disease (CVD); coronary heart disease (CHD); and cancer mortality by sedentary time
To examine the multivariate relationship between sedentary time and mortality, hazard ratios (HRs) and 95% CIs were estimated with Cox proportional hazards models, controlling for age and all potentially confounding covariates as described above. For each outcome, the time-to-event was defined as the number of days from WHI enrollment to death; for women still alive at the last known contact, censoring time was defined as number of days from WHI enrollment to last known contact or end of NDI search. Three models for the four mortality categories were examined: (1) age-adjusted; (2) partially adjusted: age, race, physical activity, and physical function; and (3) fully adjusted multivariate model. To assess a dose–response relationship between sedentary time and mortality, trend tests were performed in a Cox model by including the total sedentary time as a continuous variable in the model (Table 2). All reported p-values are two-sided. Because 12 p-values are reported for the main analysis, the critical p-value was set at 0.004 (0.05/12) using the Bonferroni correction to adjust for Type-I error in the context of multiple comparisons. The proportional hazard model assumption was checked and was deemed acceptable (p>0.05).
Table 2.
Cox proportional hazards models: relationship between sedentary time and all-cause, CVD, CHD, and cancer mortality
| Baseline sedentary time | p-value for trenda | ||||
|---|---|---|---|---|---|
| ≤4 hours | >4–8 hours | >8–11 hours | ≥11 hours | ||
| N | 13,134 | 35,668 | 23,245 | 20,187 | |
| Person-year of follow-up | 158,703.06 | 436,043.51 | 284,539.86 | 246,139.09 | |
| All-cause mortality | |||||
| Number of deaths | 1,855 | 4,993 | 3,395 | 3,073 | |
| Age-adjusted hazard ratio | 1.00 | 0.95 (0.90, 1.01) | 1.06 (1.00, 1.12) | 1.26 (1.19, 1.34) | <0.0001 |
| Age, race, physical activities, and physical function score adjusted | 1.00 | 0.99 (0.94, 1.05) | 1.05 (0.99, 1.11) | 1.12 (1.06, 1.19) | <0.0001 |
| Multivariate hazard ratiob | 1.00 | 1.03 (0.97, 1.10) | 1.07 (0.99, 1.14) | 1.12 (1.05, 1.21) | 0.0006 |
| CVD death | |||||
| Number of deaths | 551 | 1,445 | 987 | 895 | |
| Age-adjusted hazard ratio | 1.00 | 0.93 (0.84, 1.03) | 1.05 (0.94, 1.16) | 1.30 (1.17, 1.44) | <0.0001 |
| Age, race, physical activities, and physical function score adjusted | 1.00 | 1.01 (0.92, 1.12) | 1.06 (0.95, 1.18) | 1.14 (1.02, 1.28) | 0.0049 |
| Multivariate hazard ratiob | 1.00 | 1.04 (0.92, 1.17) | 1.06 (0.93, 1.20) | 1.13 (0.99, 1.29) | 0.0446 |
| CHD death | |||||
| Number of deaths | 222 | 657 | 459 | 418 | |
| Age-adjusted hazard ratio | 1.00 | 1.05 (0.90, 1.22) | 1.21 (1.03, 1.42) | 1.50 (1.27, 1.77) | <0.0001 |
| Age, race, physical activities, and physical function score adjusted | 1.00 | 1.17 (1.00, 1.37) | 1.24 (1.05, 1.46) | 1.32 (1.11, 1.57) | 0.0019 |
| Multivariate hazard ratiob | 1.00 | 1.18 (0.98, 1.42) | 1.19 (0.98, 1.45) | 1.27 (1.04, 1.55) | 0.0421 |
| Cancer death | |||||
| Number of deaths | 605 | 1,771 | 1,280 | 1,103 | |
| Age-adjusted hazard ratio | 1.00 | 1.05 (0.95, 1.15) | 1.21 (1.10, 1.33) | 1.31 (1.19, 1.45) | <0.0001 |
| Age, race, physical activities, and physical function score adjusted | 1.00 | 1.04 (0.94, 1.14) | 1.18 (1.07, 1.31) | 1.22 (1.10, 1.36) | <0.0001 |
| Multivariate hazard ratiob | 1.00 | 1.09 (0.98, 1.22) | 1.21 (1.07, 1.35) | 1.21 (1.07, 1.37) | 0.0002 |
Note: Values within parentheses are 95% CIs.
p-value for linear trend was obtained by using the computed value of total sedentary time as continuous variable in the model
Model adjusted for age, race/ethnicity, education, marital status, BMI, self-rated health status, smoking, alcohol consumption, number of chronic diseases, hormone use, depressed mood, living alone, number of falls in the past 12 months, activity of daily living disability, history of CHD and/or CHF, physical functioning score, physical activity level, history of stroke, treated diabetes, hypertensive, arthritis, cancer, chronic obstructive pulmonary disease, and history of hip fracture over age 55; CHD, coronary heart disease
CHF, congestive heart failure; CVD, cardiovascular disease
Sensitivity analyses were conducted to examine (1) effect of only sitting time (rather than combined, total sedentary time) and (2) effect of eliminating first 2 years of mortality data (to account for possible reverse causality with sedentary time at baseline). An additional sensitivity analysis was conducted to adjust for potential measurement error in sedentary time. For a subsample of 536 WHI OS participants (513 included in the analytic sample), the measurements of sitting time, sleeping time, and total sleeping plus lying time were again ascertained approximately 10 weeks after the first administration of the questionnaire. The reliability ratio of total sedentary time in this subsample was 0.77.23 Hazard ratios were estimated adjusting for measurement error using a modification of the regression calibration approach (Appendix A and B, available at www.ajpmonline.org). Standard errors for the HRs were obtained through bootstrapping.
To explore the possibility that associations might differ by key baseline characteristics, the Cox regression models were stratified on age, BMI, race/ethnicity, physical activity level, physical function, and history of chronic diseases. Interaction tests were performed by including the product term of the baseline characteristic and categories of total sedentary time into the fully adjusted multivariate model. Because 24 interactions were reported, chance alone could produce a significant interaction at the 0.05 level for approximately one factor in the analysis. All statistical analyses were performed during 2012–2013 using SAS statistical software (version 9.2).
Results
Descriptive statistics, displayed by categories of sedentary time, are shown in Table 1. The mean (SD) age of participants was 63.6 (7.4), and mean (SD) sedentary time was 8.5 (4.0) hours/day. In general, women who reported ≤4 hours/day of sedentary time were less likely to be white and had lower education compared with women in other categories of sedentary time: 70.9% of women with ≤4 hours/day sedentary time were white, compared with 85.5%, 86.3%, and 85.4% in women who reported >4–8 hours, >8–11 hours, and >11 hours/day sedentary time, respectively; 32.5% of women with ≤4 hours/day sedentary time had a college degree, compared with 41.3%, 44.9%, and 46.2% in women who reported >4–8 hours, >8–11 hours, and >11 hours/day sedentary time. Women reporting >11 hours/day sedentary time had higher BMIs and lower levels of physical activity and physical function. Sedentary women were more likely to be smokers, report fair/poor health, and more falls in the past 12 months. The p-values from chi-square tests indicated difference across groups among all the variables presented in Table 1.
During 1,125,426 years of follow-up, 13,316 women died. Overall, it appeared that unadjusted survival time declined with increasing level of sedentary time, especially cancer deaths, as shown in the Kaplan–Meier survival curves for all-cause, CVD, CHD, and cancer mortality (Figure 1).
Table 2 presents the results of the Cox proportional hazards models for total mortality and death from CVD, CHD, or cancer. There was a linear, dose–response relationship between sedentary time and all-cause mortality (multivariate HR of highest versus lowest category: HR=1.12, 95% CI=1.05, 1. 21); CVD death (HR=1.13, 95% CI=0.99, 1.29); CHD death (HR=1.27, 95% CI=1.04, 1.55); and cancer death (HR=1.21, 95% CI=1.07, 1.37) in the fully adjusted models.
Tests for trend were all significant. The HRs from these multivariate models changed slightly from the minimally adjusted model. In the sensitivity analysis examining sitting time only (rather than total sedentary time) as well as the sensitivity analysis in which the first 2 years of mortality data were removed, HRs were similar to those in Table 2.
In the sensitivity analysis adjusting for measurement error, the HRs slightly strengthened: all-cause mortality (multivariate HR of highest versus lowest category: HR=1.19, 95% CI=1.07, 1.30); CVD death (HR=1.17, 95% CI=0.98, 1.37); CHD death (HR=1.38, 95% CI=1.02, 1.73); and cancer death (HR=1.42, 95% CI=1.19, 1.65).
Potential differences by age, race/ethnicity, BMI, physical activity level, physical function score, and chronic disease were examined for all-cause and cause-specific mortality using fully adjusted multivariate models (Table 3). Interaction tests indicated that race/ethnicity modified the relationship between sedentary time and mortality from all causes, with the stratum-specific results showing somewhat stronger associations in black women and women in the “other” race group, which included Asians, Native Americans, Pacifoc Islanders, and multi-racial women. Comparing the highest with lowest category of sedentary time, a 37% increase in the multivariate HR was noted for black women (HR=1.37, 95% CI=1.11, 1.70) and a 45% increase for women in the other race category (HR=1.45, 95% CI=0.95, 2.20), whereas white and Hispanic women had a 9% increase (HR=1.09, 95% CI=1.11, 1.18) and a 6% increase (HR=1.06, 95% CI=0.66, 1.70), respectively. For cancer mortality, stronger associations were seen in women aged <70 years comparing the highest to lowest reported sedentary time, the 50–59 age group and 60–69 age group had a 34% and 36% increase in HR sedentary time (HR=1.34, 95% CI=0.93, 1.91, and HR=1.36, 95% CI=1.14, 1.62), respectively. There was no suggestion that associations went in opposite directions across strata of any baseline characteristic examined.
Table 3.
Effect modification by age, race/ethnicity, BMI, physical activity level, physical function, and chronic disease
| Variable | No. of events | Total sedentary time (95% CI) | p-valuea for interaction | |||
|---|---|---|---|---|---|---|
| ≤4 hours | >4–8 hours | >8–11 hours | ≥11 hours | |||
| All-cause mortality | ||||||
| Age (years) | 0.3470 | |||||
| 50–59 | 1,623 | 1.00 (ref) | 1.31 (1.03, 1.67) | 1.30 (1.01, 1.67) | 1.32 (1.03, 1.69) | |
| 60–69 | 5,178 | 1.00 (ref) | 1.02 (0.93, 1.13) | 1.13 (1.01, 1.25) | 1.14 (1.02, 1.27) | |
| 70–79 | 6,515 | 1.00 (ref) | 0.99 (0.90, 1.08) | 0.98 (0.89, 1.08) | 1.08 (0.97, 1.19) | |
| Race/ethnicity | 0.0347 | |||||
| White | 11,269 | 1.00 (ref) | 1.01 (0.94, 1.08) | 1.03 (0.95, 1.11) | 1.09 (1.01, 1.18) | |
| Black | 1,168 | 1.00 (ref) | 0.98 (0.80, 1.19) | 1.35 (1.09, 1.66) | 1.37 (1.11, 1.70) | |
| Hispanic | 341 | 1.00 (ref) | 1.26 (0.90, 1.78) | 1.14 (0.76, 1.71) | 1.06 (0.66, 1.70) | |
| Otherb | 343 | 1.00 (ref) | 1.27 (0.87, 1.86) | 1.16 (0.76, 1.77) | 1.45 (0.95, 2.20) | |
| BMI | 0.8532 | |||||
| <25 | 5,076 | 1.00 (ref) | 1.02 (0.92, 1.12) | 1.05 (0.94, 1.16) | 1.12 (1.00, 1.26) | |
| 25–<30 | 4,277 | 1.00 (ref) | 1.05 (0.94, 1.18) | 1.09 (0.97, 1.23) | 1.10 (0.97, 1.25) | |
| ≥30 | 3,781 | 1.00 (ref) | 1.03 (0.91, 1.18) | 1.10 (0.96, 1.25) | 1.20 (1.05, 1.37) | |
| Physical activity level | 0.1609 | |||||
| Q1 (0–3 METs hours/ week) | 4,179 | 1.00 (ref) | 1.11 (0.99, 1.25) | 1.09 (0.96, 1.23) | 1.22 (1.08, 1.38) | |
| Q2 (>3–<10 METs hours/week) | 3,392 | 1.00 (ref) | 1.08 (0.95, 1.24) | 1.21 (1.05, 1.39) | 1.19 (1.03, 1.38) | |
| Q3 (10–19.75 METs hours/week) | 3,002 | 1.00 (ref) | 1.01 (0.88, 1.15) | 1.09 (0.94, 1.26) | 1.17 (1.01, 1.36) | |
| Q4 (>19.75 METs hours/week) | 2,586 | 1.00 (ref) | 0.92 (0.80, 1.04) | 0.90 (0.78, 1.04) | 0.94 (0.80, 1.11) | |
| Physical functioning score | 0.4659 | |||||
| T1 (0–75) | 6,542 | 1.00 (ref) | 1.01 (0.92, 1.11) | 1.06 (0.96, 1.18) | 1.17 (1.06, 1.29) | |
| T2 (>75–90) | 3,651 | 1.00 (ref) | 0.98 (0.87, 1.11) | 0.99 (0.87, 1.13) | 1.02 (0.89, 1.17) | |
| T3 (>90–100) | 2,831 | 1.00 (ref) | 1.09 (0.95, 1.24) | 1.16 (1.01, 1.34) | 1.15 (0.99, 1.35) | |
| History of chronic disease | 0.3623 | |||||
| No | 1,394 | 1.00 (ref) | 0.90 (0.75, 1.09) | 0.95 (0.78, 1.16) | 1.04 (0.84, 1.29) | |
| Yes | 11,922 | 1.00 (ref) | 1.05 (0.98, 1.13) | 1.09 (1.02, 1.18) | 1.16 (1.08, 1.25) | |
| CVD death | ||||||
| Age | 0.8795 | |||||
| 50–59 | 307 | 1.00 (ref) | 1.05 (0.61, 1.81) | 1.18 (0.68, 2.06) | 1.17 (0.68, 2.04) | |
| 60–69 | 1,298 | 1.00 (ref) | 1.02 (0.83, 1.26) | 1.13 (0.91, 1.40) | 1.12 (0.90, 1.40) | |
| 70–79 | 2,273 | 1.00 (ref) | 1.02 (0.88, 1.19) | 1.00 (0.84, 1.17) | 1.13 (0.95, 1.34) | |
| Race/ethnicity | 0.4207 | |||||
| White | 3,228 | 1.00 (ref) | 1.02 (0.89, 1.17) | 1.01 (0.87, 1.16) | 1.12 (0.97, 1.30) | |
| Black | 390 | 1.00 (ref) | 1.00 (0.71, 1.41) | 1.32 (0.91, 1.92) | 1.57 (1.09, 2.26) | |
| Hispanic | 94 | 1.00 (ref) | 0.97 (0.50, 1.91) | 1.11 (0.53, 2.30) | 0.64 (0.23, 1.82) | |
| Otherb | 105 | 1.00 (ref) | 1.31 (0.65, 2.64) | 1.42 (0.66, 3.05) | 0.85 (0.35, 2.05) | |
| BMI | 0.9656 | |||||
| <25 | 1,369 | 1.00 (ref) | 0.97 (0.80, 1.17) | 0.99 (0.81, 1.22) | 1.11 (0.90, 1.38) | |
| 25–<30 | 1,233 | 1.00 (ref) | 1.11 (0.90, 1.36) | 1.13 (0.90, 1.41) | 1.17 (0.92, 1.48) | |
| ≥30 | 1,222 | 1.00 (ref) | 1.05 (0.83, 1.32) | 1.09 (0.86, 1.38) | 1.19 (0.94, 1.51) | |
| Physical activity level | 0.1340 | |||||
| Q1 (0–3 METs hours/ week) | 1,250 | 1.00 (ref) | 1.17 (0.95, 1.46) | 1.07 (0.85, 1.34) | 1.20 (0.95, 1.51) | |
| Q2 (>3–<10 METs hours/week) | 1,024 | 1.00 (ref) | 1.28 (0.99, 1.64) | 1.44 (1.11, 1.87) | 1.37 (1.04, 1.80) | |
| Q3 (10–19.75 METs hours/week) | 847 | 1.00 (ref) | 0.88 (0.68, 1.12) | 0.93 (0.71, 1.22) | 1.22 (0.93, 1.61) | |
| Q4 (>19.75 METs hours/week) | 703 | 1.00 (ref) | 0.87 (0.67, 1.11) | 0.86 (0.65, 1.14) | 0.89 (0.66, 1.22) | |
| Physical functioning score | 0.5999 | |||||
| T1 (0–75) | 2,164 | 1.00 (ref) | 0.97 (0.82, 1.14) | 0.99 (0.83, 1.17) | 1.10 (0.93, 1.30) | |
| T2 (>75–90) | 980 | 1.00 (ref) | 1.02 (0.80, 1.28) | 1.02 (0.80, 1.31) | 1.13 (0.87, 1.48) | |
| T3 (>90–100) | 627 | 1.00 (ref) | 1.28 (0.96, 1.69) | 1.42 (1.05, 1.93) | 1.31 (0.93, 1.83) | |
| History of chronic disease | 0.1888 | |||||
| No | 291 | 1.00 (ref) | 0.89 (0.59, 1.34) | 1.14 (0.74, 1.75) | 0.86 (0.52, 1.42) | |
| Yes | 3,587 | 1.00 (ref) | 1.06 (0.94, 1.20) | 1.07 (0.94, 1.23) | 1.20 (1.05, 1.37) | |
| CHD death | ||||||
| Age | 0.9361 | |||||
| 50–59 | 138 | 1.00 (ref) | 1.89 (0.74, 4.85) | 2.06 (0.80, 5.33) | 1.89 (0.73, 4.89) | |
| 60–69 | 607 | 1.00 (ref) | 1.29 (0.94, 1.77) | 1.33 (0.95, 1.86) | 1.41 (1.00, 1.97) | |
| 70–79 | 1,011 | 1.00 (ref) | 1.08 (0.86, 1.37) | 1.08 (0.84, 1.38) | 1.16 (0.90, 1.50) | |
| Race/ethnicity | 0.8766 | |||||
| White | 1,440 | 1.00 (ref) | 1.11 (0.90, 1.37) | 1.11 (0.89, 1.38) | 1.26 (1.00, 1.57) | |
| Black | 200 | 1.00 (ref) | 1.37 (0.83, 2.27) | 1.58 (0.91, 2.74) | 1.74 (1.01, 3.00) | |
| Hispanic | 42 | 1.00 (ref) | 2.00 (0.71, 5.64) | 1.46 (0.44, 4.78) | 0.64 (0.13, 3.23) | |
| Otherb | 46 | 1.00 (ref) | 1.14 (0.39, 3.32) | 1.20 (0.35, 4.09) | 0.00 (0.00, inf) | |
| BMI | 0.8623 | |||||
| <25 | 583 | 1.00 (ref) | 1.09 (0.81, 1.46) | 1.16 (0.85, 1.60) | 1.10 (0.78, 1.55) | |
| 25–<30 | 552 | 1.00 (ref) | 1.22 (0.88, 1.69) | 1.23 (0.87, 1.74) | 1.32 (0.92, 1.88) | |
| ≥30 | 592 | 1.00 (ref) | 1.29 (0.91, 1.83) | 1.30 (0.91, 1.86) | 1.53 (1.07, 2.19) | |
| Physical activity level | 0.2532 | |||||
| Q1 (0–3 METs hours/ week) | 599 | 1.00 (ref) | 1.26 (0.91, 1.75) | 1.25 (0.89, 1.75) | 1.30 (0.93, 1.82) | |
| Q2 (>3 – <10 METs hours/week) | 442 | 1.00 (ref) | 1.62 (1.07, 2.46) | 1.85 (1.20, 2.85) | 1.86 (1.19, 2.90) | |
| Q3 (10 – 19.75 METs hours/week) | 411 | 1.00 (ref) | 0.90 (0.63, 1.30) | 1.05 (0.72, 1.55) | 1.32 (0.89, 1.96) | |
| Q4 (>19.75 METs hours/week) | 284 | 1.00 (ref) | 1.12 (0.76, 1.67) | 0.84 (0.53, 1.33) | 0.79 (0.47, 1.32) | |
| Physical functioning score | 0.8747 | |||||
| T1 (0–75) | 1,016 | 1.00 (ref) | 1.12 (0.88, 1.44) | 1.22 (0.94, 1.57) | 1.28 (0.99, 1.66) | |
| T2 (>75–90) | 414 | 1.00 (ref) | 1.27 (0.87, 1.84) | 1.14 (0.76, 1.72) | 1.43 (0.94, 2.18) | |
| T3 (>90–100) | 263 | 1.00 (ref) | 1.24 (0.82, 1.86) | 1.30 (0.83, 2.04) | 1.13 (0.68, 1.89) | |
| History of chronic disease | 0.7150 | |||||
| No | 111 | 1.00 (ref) | 1.77 (0.81, 3.86) | 2.10 (0.92, 4.75) | 1.83 (0.76, 4.39) | |
| Yes | 1,645 | 1.00 (ref) | 1.17 (0.97, 1.41) | 1.18 (0.97, 1.45) | 1.29 (1.05, 1.59) | |
| Cancer death | ||||||
| Age | 0.0031 | |||||
| 50–59 | 850 | 1.00 (ref) | 1.46 (1.03, 2.06) | 1.36 (0.95, 1.96) | 1.34 (0.93, 1.91) | |
| 60–69 | 2,140 | 1.00 (ref) | 1.13 (0.96, 1.33) | 1.45 (1.22, 1.72) | 1.36 (1.14, 1.62) | |
| 70–79 | 1,769 | 1.00 (ref) | 0.98 (0.83, 1.15) | 0.94 (0.78, 1.12) | 1.02 (0.84, 1.24) | |
| Race/ethnicity | 0.3205 | |||||
| White | 4,129 | 1.00 (ref) | 1.06 (0.94, 1.20) | 1.16 (1.02, 1.32) | 1.16 (1.01, 1.32) | |
| Black | 367 | 1.00 (ref) | 1.13 (0.80, 1.61) | 1.64 (1.13, 2.38) | 1.44 (0.98, 2.11) | |
| Hispanic | 97 | 1.00 (ref) | 1.86 (0.96, 3.62) | 1.18 (0.50, 2.79) | 3.05 (1.40, 6.64) | |
| Otherb | 106 | 1.00 (ref) | 1.13 (0.56, 2.29) | 1.16 (0.54, 2.46) | 1.40 (0.65, 3.04) | |
| BMI | 0.7601 | |||||
| <25 | 1,850 | 1.00 (ref) | 1.06 (0.90, 1.25) | 1.13 (0.95, 1.36) | 1.21 (1.00, 1.47) | |
| 25–<30 | 1,578 | 1.00 (ref) | 1.08 (0.89, 1.31) | 1.16 (0.95, 1.42) | 1.13 (0.92, 1.40) | |
| ≥30 | 1,283 | 1.00 (ref) | 1.20 (0.95, 1.52) | 1.46 (1.15, 1.85) | 1.40 (1.10, 1.79) | |
| Physical activity level | 0.5147 | |||||
| Q1 (0–3 METs hours/ week) | 1,370 | 1.00 (ref) | 1.08 (0.88, 1.34) | 1.13 (0.91, 1.40) | 1.21 (0.97, 1.50) | |
| Q2 (>3 – <10 METs hours/week) | 1,167 | 1.00 (ref) | 1.06 (0.84, 1.33) | 1.26 (0.99, 1.59) | 1.17 (0.92, 1.51) | |
| Q3 (10 – 19.75 METs hours/week) | 1,124 | 1.00 (ref) | 1.11 (0.88, 1.40) | 1.40 (1.10, 1.77) | 1.24 (0.96, 1.60) | |
| Q4 (>19.75 METs hours/week) | 1,053 | 1.00 (ref) | 1.13 (0.91, 1.40) | 1.08 (0.85, 1.37) | 1.30 (1.00, 1.68) | |
| Physical functioning score | 0.2447 | |||||
| T1 (0–75) | 1,841 | 1.00 (ref) | 1.02 (0.85, 1.23) | 1.12 (0.93, 1.35) | 1.22 (1.01, 1.47) | |
| T2 (>75–90) | 1,450 | 1.00 (ref) | 0.97 (0.80, 1.18) | 1.08 (0.88, 1.32) | 1.05 (0.84, 1.30) | |
| T3 (>90–100) | 1,387 | 1.00 (ref) | 1.29 (1.06, 1.58) | 1.48 (1.19, 1.83) | 1.36 (1.07, 1.71) | |
| History of chronic disease | 0.2651 | |||||
| No | 663 | 1.00 (ref) | 0.89 (0.68, 1.16) | 0.92 (0.69, 1.23) | 1.02 (0.75, 1.38) | |
| Yes | 4,096 | 1.00 (ref) | 1.14 (1.01, 1.29) | 1.28 (1.13, 1.45) | 1.27 (1.11, 1.45) | |
p-value for interaction was calculated by including a product term of total sedentary time and the effect modifier into the model. This model adjusted for the same set of covariates as the multivariate model in Table 2
Other included American Indian, and Asian/Pacific Islander. Unknown race/ethnicity was excluded from this analysis
CHD, coronary heart disease; CVD, cardiovascular disease
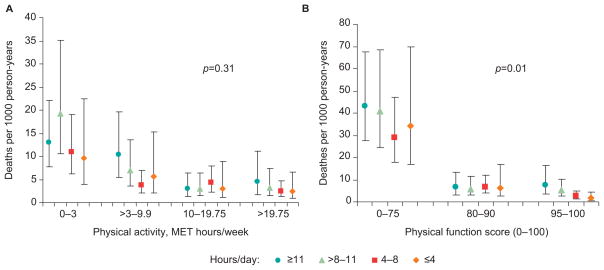
Figure 2 displays age-adjusted death per 1000 person-years for the four categories of sedentary time across physical activity and physical functioning categories, respectively. The overall interaction p-value was not significant (p=0.31) for physical activity, but was significant (p-value=0.01) for physical functioning; the relationship appeared to be strongest among women within the diminished physical function (score=0–75) category (Figure 2B).
Figure 2.
Age-adjusted death rates per 1000 person-years according to category of sedentary time, stratified by level of physical activity (A) and physical function (B). p-values indicate effect modification of relationship between sedentary time and mortality risk by (A) METs of moderate to vigorous physical activity (MVPA) and (B) SF-36 physical function score
Discussion
Postmenopausal women who reported greater amounts of sedentary time had an increased risk of all-cause mortality after controlling for physical activity, physical function, and other relevant covariates. Mortality risk was modestly elevated in a dose–response manner and consistent across the causes of death examined, although CVD and CHD were not significant in the multivariate analysis after adjustment for multiple comparisons. The magnitude of the association between sedentary time and mortality was greater than or similar to some studies2,30 but somewhat smaller than others.4–7
These differences may be due, in part, to the combination of unique characteristics of this sample, the methods for measuring sedentary time, the covariates examined, and the length of the follow-up period. For example, in a population-based sample of Canadian men and women aged 18–90 years, Katzmarzyk et al.3 reported a multivariate adjusted HR of 1.11 for adults who sat “half of the time” and an HR of 1.54 for adults who sat “almost all of the time” compared to “almost none of the time.” In the analyses here, sedentary time of >11 hours/day compared to <4 hours/day was associated with an adjusted HR of 1.12. This weaker association may be due to competing causes of mortality in older adults; the mean age of the population in the Katzmarzyk study was 42 years. The true deleterious effect of sedentary time on human physiological health indicators may not be necessarily weaker with age, but rather may be attenuated by the presence of competing risk factors for mortality with advancing age. Consistent with this, multivariate adjustment resulted in a reduction in strength of association (Table 2). Effect sizes may also differ because sedentary time questions varied across studies,3 including studies in which self-reported screen time (watching TV, computer use) were the measure of sedentary behavior.30–32
To our knowledge, there is only one other study examining sedentary time and mortality risk in which more than two race/ethnicity groups were included4 and the only one in which possible differences by race/ ethnicity are displayed.33 In that study, black women and women from the “other” race category had stronger associations than white and Hispanic women. This finding may be attributable to Type-I error. Differential reporting of sedentary time could have influenced these associations, which has been observed for reported energy intake among black women. If the interaction can be replicated in other studies, it is possible that black women may be more sensitive to adverse health effects of sedentary time, such as abdominal obesity and insulin resistance, and the higher prevalence of obesity and diabetes. Given the relatively small numbers of nonwhite women in this study, this interaction is possible but requires further investigation in future research.
Strengths of this current study include a follow-up period that was longer than most previous studies, outcome data collected using standardized protocols with rigorous quality control,24 and the adjustment for physical function and multiple morbidity. Most studies have not accounted for physical function or have only used a single-item question.5 Older adults commonly have chronic conditions that reduce the ability to do physical activity and lead to more sedentary behavior; however, diminished physical function is not necessarily due to chronic disease, nor does chronic disease necessarily affect function. Thus, both variables are important in examining the association between sedentary time and mortality among aging individuals, and likely contributed to the smaller—yet still significant—effect sizes.
A further unique strength of this study was the ability to adjust for measurement error. To our knowledge, no prior studies have done this. Indeed, the HRs were slightly strengthened after accounting for measurement error. An important area for future studies is the use of accelerometry data in large, diverse populations for extended follow-up periods. In the one accelerometry-based sedentary time and mortality analysis of NHANES data, Koster and colleagues4 found that individuals with the greatest sedentary time had a significantly increased risk of death, independent of MVPA. However, the follow-up period in the Koster study was relatively short (2.8 years); thus, accelerometry studies with longer follow-up are needed.
It is important to note that these findings may not be generalizable to men or to younger segments of the population. A limitation of this study is the use of self-report to obtain sedentary time, which may introduce measurement bias. This could lead to underestimation of associations between sedentary time and mortality risk, but several things provide reassurance. Similar to previous studies, validity of the exact sedentary time questions used was not examined; however, they are similar to those in the International Physical Activity Questionnaire, which has significant but modest correlation (r=0.33) with sedentary behavior as measured by accelerometer.34,35 It is also worth noting that the average hours of sitting time (9–10 hours/day) in the WHI women aged 50–79 years was similar to the average hours of sitting time in women aged 60–69 years (8.4 hours/day) estimated by the accelerometer data in NHANES.18 Finally, the analyses attempted to adjust for multiple testing issues by lowering the critical alpha value for statistical significance; however, 95% CIs are reported to conform to standard practice, and caution should thus be exercised with this in mind when interpreting results.
In summary, this study demonstrates strong evidence for a modest dose–response relationship between sedentary time and mortality among an ethnically diverse sample of older women after controlling for MVPA, physical function, chronic disease status, and other relevant factors. Future research should assess MVPA and sedentary time using accelerometers and questionnaires to determine the extent to which the method of assessment affects the strength of association. As older adults have high levels of sedentary time, they should be included in future intervention trials designed to decrease sedentary time to determine whether this modifiable risk factor can extend active life. Furthermore, with consideration to prior studies with similar populations of focus, recommendations from health care providers as well as public health messaging that specifically discourage extended daily sedentary behaviors by aging individuals appear well supported.
Supplementary Material
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, U.S. Department of Health and Human Services through Contracts N01WH22110, 24152, 32100-32102, 32105-32106, 32108-32109, 32111-32113, 32115, 32118-32119, 32122, 42107-42126, 42129-42132, and 44221 This work was also supported by PO1 CA53996, R01AG025441-03, and T32 AG027677.
Dr. Seguin was supported by a grant from the National Heart, Lung, and Blood Institute (K01 HL108807). Dr. Buchner is supported in part as a Shahid and Ann Carlson Khan Professor of Applied Health Sciences, and by grants from the National Heart, Lung, and Blood Institute (5 R01 H205065-02) and from USDA NIFA (2011-27001-30101). Ms. Liu was supported by N01WH22110 sponsored by the National Heart, Lung, and Blood Institute. Dr. Manini is supported by the National Institute on Aging (R21AG031974) and by University of Florida Claude D. Pepper Center awarded by the National Institute on Aging (P30AG028740).
Dr. Wang was supported by the National Cancer Institute (P01 CA53996) and the National Institute of Environmental Health Sciences (R01 ES017030). Dr. LaCroix was supported by 5R01AG025441-03 sponsored by the National Institute of Aging.
Appendix Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.amepre.2013.10.021.
Footnotes
Program Office: National Heart, Lung, and Blood Institute, Bethesda, Maryland: Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, Washington: Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan. Medical Research Labs, Highland Heights, Kentucky: Evan Stein. University of California at San Francisco, San Francisco, California: Steven Cummings.
Clinical Centers: Albert Einstein College of Medicine, Bronx, New York: Sylvia Wassertheil-Smoller. Baylor College of Medicine, Houston, Texas: Aleksandar Rajkovic. Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts: JoAnn E. Manson. Brown University, Providence, Rhode Island: Charles B. Eaton. Emory University, Atlanta, Georgia: Lawrence Phillips. Fred Hutchinson Cancer Research Center, Seattle, WA: Shirley Beresford. George Washington University Medical Center, Washington, D.C.: Lisa Martin. Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center, Torrance, California: Rowan Chlebowski. Kaiser Permanente Center for Health Research, Portland, Oregon: Yvonne Michael. Kaiser Permanente Division of Research, Oakland, California: Bette Caan. Medical College of Wisconsin, Milwaukee, WI: Jane Morley Kotchen. MedStar Research Institute/Howard University,Washington, D.C.: Barbara V. Howard. Northwestern University, Chicago/ Evanston, Illinois: Linda Van Horn. Rush Medical Center, Chicago, Illinois: Henry Black. Stanford Prevention Research Center, Stanford, California: Marcia L. Stefanick. State University of New York at Stony Brook, Stony Brook, New York: Dorothy Lane. The Ohio State University, Columbus, Ohio: Rebecca Jackson. University of Alabama at Birmingham, Birmingham, Alabama: Cora E. Lewis. University of Arizona, Tucson/Phoenix, Arizona: Cynthia A. Thomson. University at Buffalo, Buffalo, New York: Jean Wactawski-Wende. University of California at Davis, Sacramento, California: John Robbins. University of California at Irvine, California: F. Allan Hubbell. University of California at Los Angeles, Los Angeles, California: Lauren Nathan. University of California at San Diego, La Jolla/Chula Vista, California: Robert D. Langer. University of Cincinnati, Cincinnati, Ohio: Margery Gass. University of Florida, Gainesville/Jacksonville, Florida: Marian Limacher. University of Hawaii, Honolulu, Hawaii: J. David Curb. University of Iowa, Iowa City/Davenport, Iowa: Robert Wallace. University of Massachusetts/Fallon Clinic, Worcester, Massachusetts: Judith Ockene. University of Medicine and Dentistry of New Jersey, Newark, New Jersey: Norman Lasser. University of Miami, Miami, Florida: Mary Jo O’Sullivan. University of Minnesota, Minneapolis, Minnesota: Karen Margolis. University of Nevada, Reno, Nevada: Robert Brunner. University of North Carolina, Chapel Hill, North Carolina: Gerardo Heiss. University of Pittsburgh, Pittsburgh, Pennsylvania: Lewis Kuller. University of Tennessee Health Science Center, Memphis, Tennessee: Karen C. Johnson. University of Texas Health Science Center, San Antonio, Texas: Robert Brzyski. University of Wisconsin, Madison, Wisconsin: Gloria E. Sarto. Wake Forest University School of Medicine, Winston-Salem, North Carolina: Mara Vitolins. Wayne State University School of Medicine/Hutzel Hospital, Detroit, Michigan: Michael Simon.
No conflicts of interest were reported by the authors of this paper.
References
- 1.USDHHS. Physical Activity Guidelines Advisory Committee Report. Washington DC: USDHHS; 2008. [Google Scholar]
- 2.Matthews CE, George SM, Moore SC, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in U.S. adults. Am J Clin Nutr. 2012;95:437–45. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 4.Koster A, Caserotti P, Patel KV, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7:e37696. doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 6.Patel AV, Bernstein L, Deka A, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of U.S. adults. Am J Epidemiol. 2010;172:419–29. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leon-Munoz LM, Martinez-Gomez D, Balboa-Castillo T, Lopez-Garcia E, Guallar-Castillon P, Rodriguez-Artalejo F. Continued sedentariness, change in sitting time, and mortality in older adults. Med Sci Sports Exerc. 2013;45(8):1501–7. doi: 10.1249/MSS.0b013e3182897e87. [DOI] [PubMed] [Google Scholar]
- 8.Chomistek AK, Manson JE, Stefanick ML, et al. The relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol. 2013;23:2346–54. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 10.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551:673–82. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31:661–6. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 12.Healy GN, Dunstan DW, Shaw JE, Zimmet PZ, Owen N. Beneficial associations of physical activity with 2-h but not fasting blood glucose in Australian adults: the AusDiab study. Diabetes Care. 2006;29:2598–604. doi: 10.2337/dc06-0313. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–67. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int J Sport Nutr Exerc Metab. 2001;11(S):S97–S104. doi: 10.1123/ijsnem.11.s1.s97. [DOI] [PubMed] [Google Scholar]
- 15.Dunstan DW, Daly RM, Owen N, et al. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005;28:3–9. doi: 10.2337/diacare.28.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Katzmarzyk PT, Lee IM. Sedentary behaviour and life expectancy in the USA: a cause-deleted life table analysis. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunstan DW, Salmon J, Owen N, et al. Physical activity and television viewing in relation to risk of undiagnosed abnormal glucose metabolism in adults. Diabetes Care. 2004;27:2603–9. doi: 10.2337/diacare.27.11.2603. [DOI] [PubMed] [Google Scholar]
- 18.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the U.S. 2003–2004. Am J Epidemiol. 2008;167:875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9S):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 20.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9S):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 21.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Seguin R, Lamonte M, Tinker L, et al. Sedentary behavior and physical function decline in older women: findings from the Women’s Health Initiative. J Aging Res. 2012;2012:271589. doi: 10.1155/2012/271589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test–retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–8. doi: 10.1249/MSS.0b013e31818ace55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9S):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth B, Haskell W, Whitt M, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9S):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Pettee Gabriel K, McClain JJ, Lee CD, et al. Evaluation of physical activity measures used in middle-aged women. Med Sci Sports Exerc. 2009;41:1403–12. doi: 10.1249/MSS.0b013e31819b2482. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 28.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 29.Pavey TG, Peeters GG, Brown WJ. Sitting-time and 9-year all-cause mortality in older women. Br J Sports Med. 2012 doi: 10.1136/bjsports-2012-091676. [DOI] [PubMed] [Google Scholar]
- 30.Wijndaele K, Brage S, Besson H, et al. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk study. Int J Epidemiol. 2011;40:150–9. doi: 10.1093/ije/dyq105. [DOI] [PubMed] [Google Scholar]
- 31.Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121:384–91. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 32.Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011;57:292–9. doi: 10.1016/j.jacc.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 33.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women’s Health Initiative. Am J Epidemiol. 2008;167:1247–59. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg DE, Bull FC, Marshall AL, Sallis JF, Bauman AE. Assessment of sedentary behavior with the International Physical Activity Questionnaire. J Phys Act Health. 2008;5(S1):S30–S44. doi: 10.1123/jpah.5.s1.s30. [DOI] [PubMed] [Google Scholar]
- 35.Craig CL, Marshall AL, Sjostrom M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.