Abstract
Basal phosphorylation of sarcoplasmic reticulum (SR) Ca2+ proteins is high in sinoatrial nodal cells (SANC), which generate partially synchronized, spontaneous, rhythmic, diastolic local Ca2+ releases (LCRs), but low in ventricular myocytes (VM), which exhibit rare diastolic, stochastic SR-generated Ca2+ sparks. We tested the hypothesis that in a physiologic Ca2+ milieu, and independent of increased Ca2+ influx, an increase in basal phosphorylation of SR Ca2+ cycling proteins will convert stochastic Ca2+ sparks into periodic, high-power Ca2+ signals of the type that drives SANC normal automaticity. We measured phosphorylation of SR-associated proteins, phospholamban (PLB) and ryanodine receptors (RyR), and spontaneous local Ca2+ release characteristics (LCR) in permeabilized single, rabbit VM in physiologic [Ca2+], prior to and during inhibition of protein phosphatase (PP) and phosphodiesterase (PDE), or addition of exogenous cAMP, or in the presence of an antibody (2D12), that specifically inhibits binding of the PLB to SERCA-2. In the absence of the aforementioned perturbations, VM could only generate stochastic local Ca2+ releases of low power and low amplitude, as assessed by confocal Ca2+ imaging and spectral analysis. When the kinetics of Ca2+ pumping into the SR were increased by an increase in PLB phosphorylation (via PDE and PP inhibition or addition of cAMP) or by 2D12, self-organized, “clock-like” local Ca2+ releases, partially synchronized in space and time (Ca2+ wavelets), emerged, and the ensemble of these rhythmic local Ca2+ wavelets generated a periodic high-amplitude Ca2+ signal. Thus, a Ca2+ clock is not specific to pacemaker cells, but can also be unleashed in VM when SR Ca2+ cycling increases and spontaneous local Ca2+ release becomes partially synchronized. This unleashed Ca2+ clock that emerges in a physiological Ca2+ milieu in VM has two faces, however: it can provoke ventricular arrhythmias; or if harnessed, can be an important feature of novel bio-pacemaker designs.
Keywords: cardiac ventricular myocytes, calcium clock, calcium cycling, protein phosphorylation, spontaneous local calcium releases
1. Introduction
Spontaneous, rare, stochastic local diastolic Ca2+ releases (“Ca2+ sparks”) [1] that occur in basal-state cardiac ventricular myocytes (VM) provide an important SR Ca2+ leak pathway [2]. β-adrenergic receptor stimulation (β-ARS) of VM organizes those local diastolic Ca2+ releases into partially synchronized spontaneous, periodic diastolic Ca2+ signals (Ca2+ waves) that, unlike “Ca2+ sparks”, can be of sufficient amplitude to generate abnormal spontaneous diastolic after-depolarizations that can initiate spontaneous abnormal action potentials (APS) [3]. During β-ARS, two distinct, but related, phosphorylation-dependent events occur: (i) an increase in Ca2+ influx into the cell, and (ii) increased Ca2+ pumping rate into and release from SR. An increase in intracellular Ca2+, due to an increase in Ca2+ influx effected by a β-ARS-induced increase in phosphorylation of L-type Ca2+ channel subunits is thought to be the major mechanism involved in organization of local, stochastic Ca2+ signals into spontaneous, roughly periodic Ca2+ waves [4]. One viewpoint, however, is that, although β-ARS initially increases Ca2+ influx, the steady-state cell Ca2+ load during β-ARS does not increase (vs. that in the basal state), because Ca2+ efflux from the cell increases to match influx [5]. Sarcoplasmic reticulum (SR) Ca2+ cycling proteins, e.g. phospholamban (PLB) and ryanodine receptors (RyRs) also become phosphorylated during β-ARS, and an increase in the phosphorylation state is associated with enhanced Ca2+ pumping into SR, and to changes in spontaneous activation of RyRs. A role for enhanced SR Ca2+ cycling in the organization of partially synchronized, roughly periodic spontaneous diastolic SR Ca2+ releases in VM, in the absence of Ca2+ overload, however, has not been directly demonstrated.
A clue that increased SR Ca2+ cycling in the absence of Ca2+ overload can indeed generate roughly periodic spontaneous local Ca2+ releases (referred as “LCRs”), however, has emerged from recent studies in sinoatrial nodal pacemaker cells (SANC), in which basal levels of phosphorylation of Ca2+ cycling proteins are well above those in basal VM in a physiologic Ca2+ milieu [6]. These studies in SANC, in which the surface membrane had been permeabilized, clearly demonstrated that an enhanced rate of SR Ca2+ cycling effected by increased basal phosphorylation of SR Ca2+ cycling proteins enables inherently stochastic, sub-sarcolemmal LCRs via RyRs to become organized into roughly periodic Ca2+ signals (Ca2+ wavelets), even when the ambient steady [Ca2+] is buffered constantly at physiologic levels [6, 7]. LCRs are Ca2+ wavelets, i.e. larger and more organized than Ca2+ sparks, but, unlike Ca2+ waves, propagate only locally for relatively short distances (3 to 7 μm). Since SR generated LCRs are roughly periodic, the SR in SANC has been dubbed a “Ca2+ clock” [8].
In SANC with intact sarcolemma, spontaneous, periodic Ca2+ wavelets during diastole generated by the SR Ca2+ clock are of sufficient amplitude to effect local membrane depolarization (via activation of Na+/Ca2+ exchanger) that are critically linked to the occurrence of spontaneous, rhythmic APs, i.e. normal automaticity of the cardiac impulse [8]. Since high basal levels of Ca2+ cycling protein phosphorylation in SANC organize stochastic Ca2+ releases into local wavelet-like rhythmic LCRs, i.e. Ca2+ clock, we hypothesized that suppression of basal SR Ca2+ cycling in VM linked to a suppression of basal phosphorylation of SR Ca2+ cycling, prevents the emergence of periodic, organized LCRs (i.e. prevents the emergence of a Ca2+ clock in VM); instead only stochastic, low-amplitude “Ca2+ sparks” occur. Specifically, we hypothesized that even in a physiologic Ca2+ milieu, when the basal SR Ca2+ cycling rate increases, e.g. either in response to an increase in SR Ca2+ protein phosphorylation in VM when protein phosphatase (PP) and phosphodiesterase (PDE) activities are inhibited, or when PLB- SERCA interaction is inhibited by a specific monoclonal antibody, spontaneous stochastic sparks will self-organize into synchronized, periodic LCRs, i.e., a “Ca2+ clock” will emerge in VM.
2. Methods
Spontaneous local Ca2+ release characteristics (LCR), the phosphorylation status of SR-associated proteins, PLB and RyRs in permeabilized rabbit VM bathed in 100 nM free [Ca2+], and cytosolic Ca2+ signal in electrically stimulated rabbit VM with intact sarcolemma were measured. Shortly, intact VM were permeabilized with 0.01% saponin. After washing out saponin, solution was exchanged to the recording solution that contained 0.03 mM fluo-4 pentapotassium salt, 0.114 mM CaCl2 (free [Ca2+] ~ 100 nM), 100 mM C4H6NO4K (DL-aspartic acid potassium salt), 25 mM KCl, 10 mM NaCl, 3 mM MgATP, 0.81 mM MgCl2, 20 mM Hepes, 0.5 mM EGTA, 10 mM phosphocreatine, and creatine phosphokinase (5 U/ml), pH 7.2 [6]. The cytosolic free Ca2+ at given total Ca2+, Mg2+, ATP, and EGTA concentrations was calculated using a computer program (WinMAXC 2.50, Stanford University). A detailed description of all methods is available in the Online Data Supplementary.
Data were reported as mean ± SEM. A Student’s t test, or, when appropriate, one-way ANOVA, was applied to determine statistical significance of the differences. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Phosphorylation of sarcoplasmic reticulum Ca2+ cycling proteins, PLB and RyRs increases in permeabilized VM when PP and PDE activities are inhibited
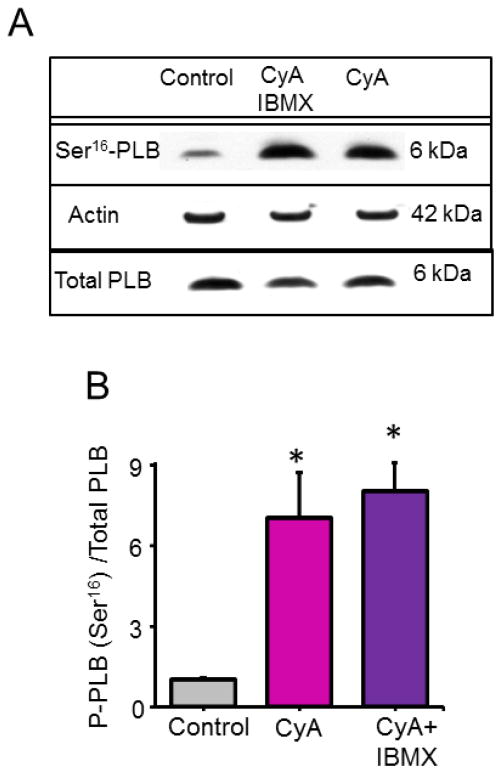
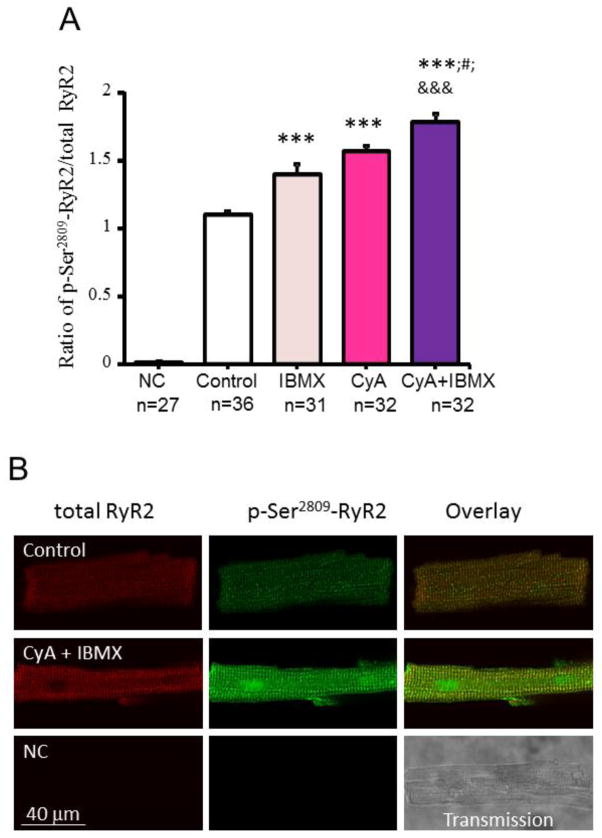
Inhibition of protein phosphatase (PP) by Calyculin A (CyA, 0.5 μM) or by CyA plus a broad spectrum PDE inhibitor IBMX (20 μM) markedly increased PLB phosphorylation at a protein kinase A (PKA)-specific Ser16 site, detected by Western blots (Fig. 1) and RyR phosphorylation at PKA-dependent Ser2809 site, detected by duo-immunolabeling (Fig. 2).
Fig. 1.
Enhancement of PLB phosphorylation at a protein kinase A (PKA)-specific Ser16 site detected by Western blots in response to PP and PP + PDE inhibition in permeabilized VM. (A) Representative Western blots. (B) Average data of phosphorylated PLB normalized to total PLB in response CyA (0.5 μM) or CyA + IBMX (20 μM) (n= 3 blots). *P < 0.05.
Fig. 2.
Enhancement of Ensemble RyR2 phosphorylation at Ser2809 detected by phospho-imaging of permeabilized VM in response to PDE or PP inhibition or to PP + PDE inhibition. (A) Average phosphorylation of RyR at Ser2809 by RyR duo-immunolabeling, in permeabilized VM in control (n=36) and in response to IBMX (20 μM, n=31), CyA (0.5 μM, n=32) or CyA + IBMX (20 μM, n=32). The primary antibody was omitted, and only the secondary antibodies were applied to the negative control (NC, n=27). The phosphorylation level was indexed by the average fluorescence density of phosphorylated RyR at Ser2809 normalized by the total RyR fluorescence density of a given cell; ***P < 0.001 vs. Control; #P< 0.05 vs. CyA; &&&P<0.001 vs. IBMX via one-way ANOVA. (B) Representative confocal images of permeabilized VM immunolabeled for both total RyR (red) and phosphorylated RyR at Ser2809 (green) in control, in response to 2 min incubation with CyA (0.5 μM) + IBMX (20 μM) and negative control.
3.2. Periodic, high-power Ca2+ signals emerge from stochastic Ca2+ sparks when phosphorylation of SR Ca2+ cycling proteins becomes increased in response to PP and PDE inhibition or exogenous cAMP
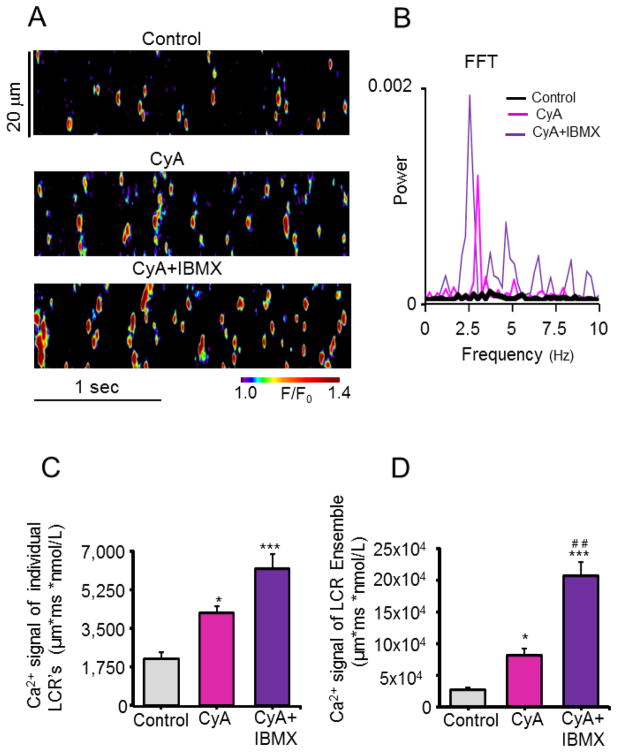
In a free [Ca2+] of 100 nM spontaneous Ca2+ sparks in VM are stochastic, non-periodic event of low power in the frequency domain, and of a low amplitude in the space-time domain (Control, Figs. 3A–D). When, in response to PP inhibition by CyA, PKA-dependent PLB phosphorylation is increased (Fig. 1) and the kinetics of SR Ca2+ cycling increase, multiple wavelet-like, rhythmic local Ca2+ oscillations, i.e. LCRs, emerge (CyA, Fig. 3A and B). When studied in the frequency domain by Fourier analysis, LCRs are synchronized at a dominant frequency of 2.5 Hz (Fig. 3B) and in the space-time domain of the confocal image resulted in high-amplitude individual LCRs Ca2+ signals (CyA, Fig. 3C) and summation of these individual Ca2+ signals produced a high-amplitude whole-cell (macroscopic) Ca2+ signal (ensemble of LCRs) (CyA, Fig. 3D). In other terms, a “Ca2+ clock” emerges in VM in a physiologic Ca2+ milieu. In the presence of CyA the addition of IBMX, a broad spectrum PDE inhibitor that increases cAMP, and leads to an increase in PKA-dependent phosphorylation [9] (Figs. 1 and 2), further increases the power of the partially synchronized Ca2+ signal in the frequency domain (CyA+IBMX, Fig. 3B) and this enhanced synchronization not only further amplified the space-time domain Ca2+ signal of individual LCR’s (CyA+IBMX, Fig. 3C), but also amplified the Ca2+ signal of the LCR ensemble by 8-fold over control (CyA+IBMX, Fig. 3D). On average, LCR periodicity in the frequency domain was observed in 77% and 86% of cells in response to inhibition of PP or PP plus PDE, respectively (Fig. S1A), and the dominant LCR period averaged 3.2 ±0.2 Hz (Fig. S1B).
Fig. 3.
Inhibition of PP and PP + PDE in permeabilized VM organizes stochastic sparks into synchronized, rhythmic, high power, spontaneous local Ca2+ releases (LCR’s/wavelets). (A) Representative confocal line-scan images of a permeabilized VM bathed in 100 nM free [Ca2+] in control and after incubation with CyA (0.5 μM) and CyA + IBMX (20 μM). (B) Respective fast Fourier transforms (FFT) of the rhythmic of Ca2+ oscillations recorded in A. (C, D) Average amplitudes of Ca2+ signals of individual LCR’s and of the LCR ensemble in control and in response to CyA and CyA + IBMX. *P < 0.05, ***P < 0.001 and ##P < 0.01 vs. CyA, n = 4 – 7 cells for each data point.
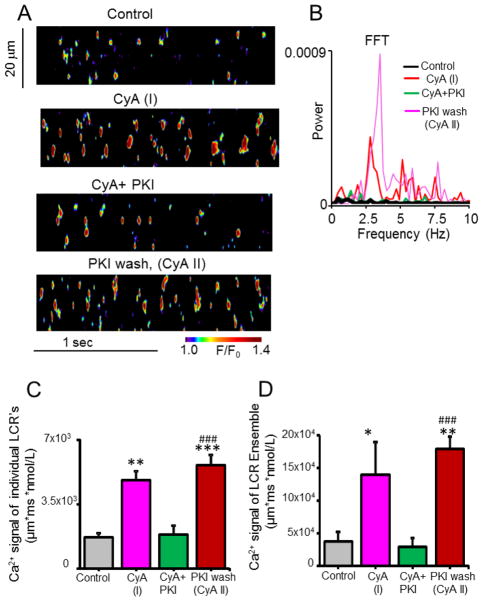
We employed PKI, a specific peptide inhibitor of PKA activity, to ascertain a specific role for PKA-dependent phosphorylation in the emergence of rhythmic spontaneous local Ca2+ releases in the presence of CyA+PKI reversibly abolished LCR periodicity in the frequency domain (CyA+PKI, Figs. 4A, B and Figs. S1A, B) and reduced Ca2+ signal amplitude in the space-time domain (CyA+PKI, Figs. 4A–D). Thus, when phosphatase activity was inhibited by CyA, PKA-dependent protein phosphorylation was required for synchronization of stochastic Ca2+ sparks into partially synchronized, high-amplitude LCRs.
Fig. 4.
PKI, a specific PKA inhibitor peptide, reversibly abolished LCR (wavelets) periodicity and reduced the amplitude of Ca2+ signals affected by PP inhibition with CyA. (A) Representative confocal line-scan images in control and during incubation with CyA (0.5 μM) or CyA + PKI (15 μM), and after washing out PKI, with CyA (0.5 μM) still present. (B) Respective Fast Fourier Transforms (FFT) of the LCR’s recorded in A. (C, D) Average amplitudes of Ca2+ signals of individual LCR’s and of the LCR ensemble in control, in response to CyA, to CyA + PKI, and following wash out of PKI with CyA. *P < 0.05, **P < 0.01, ***P < 0.001vs. Control and ###P < 0.001 vs. CyA+PKI, n = 4 – 6 cells for each data point.
Specific changes in the characteristics of individual local Ca2+ releases that become organized into periodic high-amplitude Ca2+ signals in the space-time domain are illustrated in Supplemental Figure S2. Note that the increase of space-time integral of each LCR Ca2+ signal results from increases in the average amplitude, width, duration and an increase in number of wavelet occurrences within the time window of observation (Fig. S2). This increase in amplitude of individual Ca2+ wavelets at a given locus may in part involve local recruitment of activation of neighboring RyRs which, in part may be attributed to Ca2+-induced Ca2+ release. That the number of sites at which Ca2+ wavelets occur triples compared to control (Fig. S2), however, indicates that in addition to Ca2+-induced Ca2+ release, RyR activation must become partially synchronized among remote sites (along a confocal line scan image), some of which were not visualized in control. To determine the cell-wide emergence of periodicity and synchronization of local Ca2+ signaling, we captured the local Ca2+ release in permeabilized VM in two-dimensional whole-cell images by Hamamatsu camera before and during PP inhibition with 0.1 μmol/L CyA (Fig. 5, Fig. S3, and movie 1-4). Supplemental Figure S3 illustrates how a phosphorylation-induced emergence of a powerful whole cell Ca2+ signal emerges from the ensemble of local Ca2+ oscillators following of CyA exposure. As exposure time in CyA increases, during which time a protein phosphorylation likely increases, LCRs evolve from rare small Ca2+ sparks in control (Fig. 5A and movie 1) into larger, powerful, and rhythmic Ca2+ oscillations over the entire cell (Figs. 5B–D and movie 2–4). In the frequency domain, partial synchronization of local spontaneous Ca2+ releases results in a Ca2+ signal as a sharp, high-amplitude peak in the power spectra (Fig. 5D).
Fig. 5.
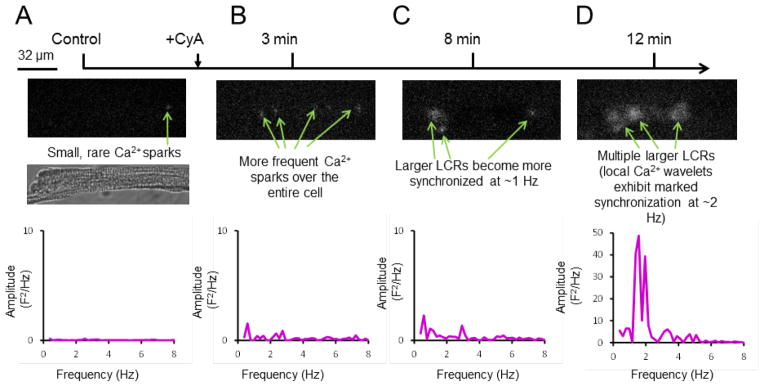
Time dependent emergence of the LCR (wavelets) synchronization during protein phosphatase (PP) inhibition in a representative saponin-permeabilized VM, recorded in two dimensions by a high speed camera. (A-D) Whole cell 2D images and their power spectra recorded in control and during 3 minutes, 8 minutes and 12 minutes of CyA (0.1 μmol/L) exposure. Shown is a representative example from 5 cells tested. Panel A also shows the transmission light image of the permeabilized cell (middle panel).
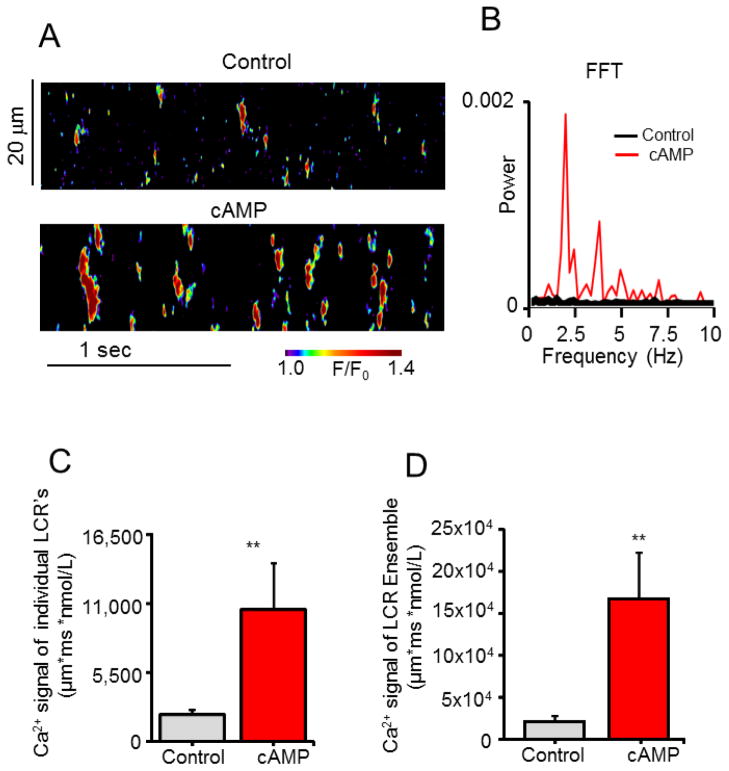
Figure 6 illustrates, that following the addition of exogenous cAMP (3 μM), similar to PP and PDE inhibition, rhythmic “clock-like” partially synchronized LCRs in the frequency and space-time domains emerge from stochastic Ca2+ sparks. This occurred in 78% of cells tested (cAMP, Fig. S1A), the average size of the local Ca2+ release increased 1.6 fold, and the average duration increased 1.5 fold, and the number of individual releases occurring per unit time increased by 1.9 fold (Fig. S2A). The space-time Ca2+ signal of individual LCRs increased by 4.9 fold (Fig. 6C), and that the LCR ensemble increased by 7.7 fold (Fig. 6D).
Fig. 6.
Addition of cAMP organizes stochastic sparks into synchronized, rhythmic, high power, spontaneous local Ca2+ wavelets (LCR’s) in permeabilized VM. (A) Representative confocal line-scan images in control and in response to 3 μM cAMP. (B) Respective Fast Fourier Transforms (FFT), of the local Ca2+ oscillations recorded in A. (C, D) Average amplitude of Ca2+ signals of individual LCR’s and of the LCR ensembles in control and in response to cAMP. **P < 0.01, n = 9 cells.
3.3. A specific monoclonal antibody that inhibits the PLB-SERCA interaction mimics the effects of PLB and RyR phosphorylation on synchronization of local Ca2+ releases in VM
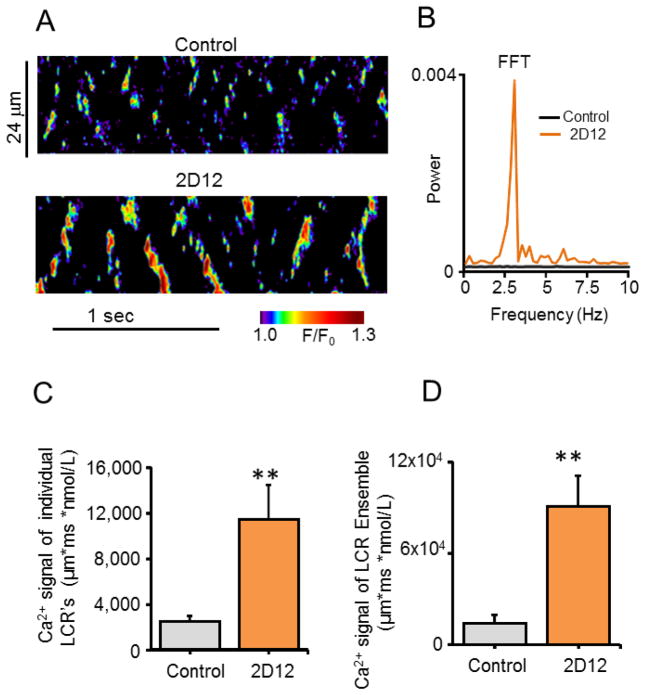
To establish a link between an increase in SR Ca2+ pumping and the emergence of rhythmic local Ca2+ wavelets when Ca2+ cycling protein phosphorylation is increased (Figs. 1, 2), we employed a specific anti-PLB monoclonal antibody, (2D12), that mimics the effect of PLB phosphorylation by inhibiting the PLB-SERCA-2 interaction, markedly increasing Ca2+ pumping into SR [10-12], without directly effecting the PLB phosphorylation. Indeed, similar to PDE and PP inhibition or cAMP application (Figs. 3-6), a 2-minute incubation with 2D12 (13.1μg/1ml) induced periodic, high-power rhythmic Ca2+ signals in the frequency domain (2D12, Figs. 7A, B) and high-amplitude ensemble local Ca2+ wavelets (2D12, Figs. 7C, D) in permeabilized VM. Periodicity emerged in 50% of cells tested (2D12, Fig. S1A), and the dominant frequency averaged at 2.6±0.17Hz, i.e. similar to interventions that increased SR Ca2+ cycling protein phosphorylation (Fig. S1B). In response to 2D12 and in response to any other perturbation employed in our study, the increase in the Ca2+ signal of LCR ensemble was highly correlated with the percent of cells that produced periodic LCRs (wavelets) (Fig. S5). The increased size, duration and number of local Ca2+ releases in response to 2D12 also resembled that of other perturbations that increase protein phosphorylation (Fig. S2). The Ca2+ signal of individual LCR’s in response to 2D12 increased by 4.6 fold (Fig. 7C) and that of the LCR ensemble increased by 6.4 fold over control (Fig. 7D), powerful, rhythmic Ca2+ signals that are strikingly similar to those induced by increased protein phosphorylation.
Fig. 7.
A monoclonal antibody against phospholamban (2D12) that inhibits SERCA2a-PLB interaction, like PLB phosphorylation, organizes stochastic sparks into synchronized, rhythmic, high power, spontaneous local Ca2+ wavelets (LCR’s) in permeabilized VM. (A) Representative confocal line-scan images in control and after 2 min application of 2D12 (13.1μg/1ml). (B) Respective Fast Fourier Transforms (FFT) of the local Ca2+ oscillations recorded in A. (C, D) Average amplitude of Ca2+ signals of individual LCR’s and of LCR ensembles in control and in response to 2D12. **P < 0.01, n = 6 cells.
3.4. How partially synchronized SR Ca2+ depletion in local microdomains in a physiologic Ca2+ milieu affects global SR Ca2+ load as assessed by caffeine in permeabilized VM
The Ca2+ release measured by a rapid caffeine application in permeabilized VM bathed in 100 nM [Ca2+] is the SR Ca2+ load determined by difference between the magnitudes of Ca2+ pumped into SR throughout the cell and Ca2+ lost from SR in microdomains by the ensemble of spontaneous local Ca2+ releases. When the magnitude of spontaneous local Ca2+ releases equals that of the Ca2+ influx into SR due to enhanced Ca2+ pumping throughout the cell, the amplitude of the caffeine-induced SR Ca2+ release will be the same as that in control, even though SR Ca2+ pumping had been markedly increased throughout of the cell. In this case it might appear as if perturbations that increase Ca2+ pumping into SR do not increase the SR Ca2+ load, but this would be an inaccurate conclusion, because the caffeine-induced Ca2+ release reports the sum SR Ca2+ load within local domains in which spontaneous Ca2+ releases had not just occurred prior to caffeine application, and in other domains in which partially synchronized spontaneous Ca2+ releases had just occurred.
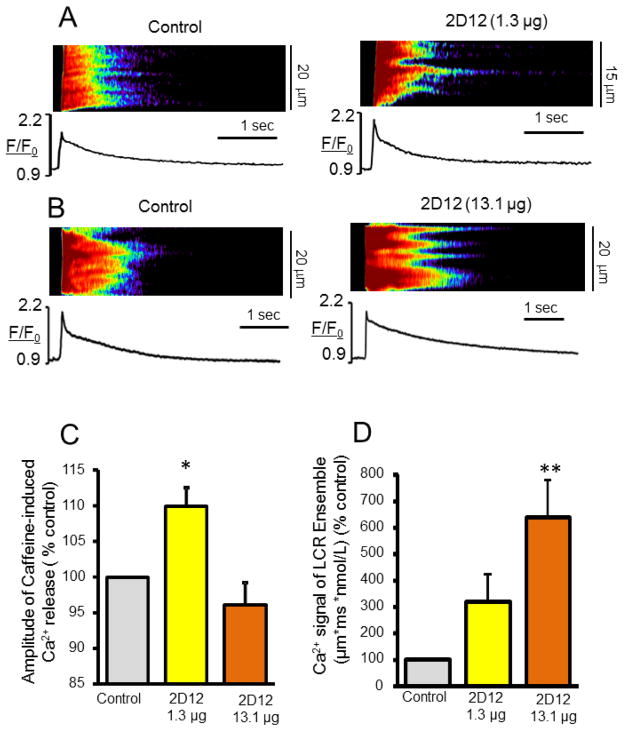
The effect of gradations in the amplitude of spontaneous, partially synchronized local SR Ca2+ releases in response to a perturbation that increases SR Ca2+ loading on caffeine-induced Ca2+ release is illustrated in Figure 8. In response to a low concentration of 2D12 (1.31μg/1ml) the amplitude of the caffeine-induced Ca2+ release increases indicating, therefore, that concomitant increase in the local SR Ca2+ depletion resulting from partially synchronized local SR Ca2+ releases, is less (2D12, 1.31μg/1ml, Fig. 8D) than the effect of 2D12 to increase cell wide Ca2+ pumping into SR (2D12, 1.31μg/1ml, Fig. 8C). A 10 fold increase in 2D12 concentration (2D12, 13.1μg/1ml, Fig. 8D), that maximally increases Ca2+ pumping into SR (Fig. 9D in [11]) also markedly further increases spontaneous Ca2+ releases within the local microdomains. In this case, the net SR Ca2+ load detected by caffeine does not increase even though Ca2+ pumping into SR is markedly enhanced, indicating this increased Ca2+ pumped into SR is balanced by an enhanced synchronization and magnitude of local Ca2+ release fluxes throughout the cell (Fig. 5 and Figs. S3, S5). It is noteworthy in this regard, that further synchronization of spontaneous local Ca2+ releases, per se, is a mechanism that increases the summated spontaneous RyR release flux. Enhanced local spontaneous Ca2+ flux likely increases local RyR phosphorylation by activating CaMKII. Both an increase in RyR phosphorylation and disengagement PLB from SERCA, which increases the kinetics of Ca2+ pumping into SR, are mechanisms that underlie the extent of this augmentation of synchronization of local spontaneous Ca2+ releases.
Fig. 8.
The relationship of the magnitude of the summated Ca2+ signal of spontaneous partially synchronized local Ca2+ releases (LCR’s/wavelets) to the amplitude of the cell-wide caffeine-induced SR Ca2+ release. (A, B) Representative confocal images and caffeine-induced Ca2+ transients elicited by 20 mM caffeine in permeabilized VM in control conditions bathed in 100 nM [Ca2+] (left) and after 2 min treatment with 2D12 (1.31μg/1ml and 13.1μg/1ml) (right). (C, D) Average changes in the amplitudes of the caffeine-induced SR Ca2+ release (n=6 to 18 cells for each data point) and Ca2+ signal of the LCR ensembles (n= 6 cells for each data point) in permeabilized VM in control and after application of a low (1.31μg/1ml) or a high (13.1μg/1ml) concentration of 2D12. *P < 0.05, **P < 0.01.
Similar to the case of a high 2D12 concentration, the amplitude of Ca2+ released by caffeine did not, on average, significantly increase from control in response to cAMP or PDE/PP inhibition, indicating that the magnitude of the partially synchronized ensemble of LCRs within microdomains in permeabilized VM bathed in 100nM [Ca2+] equals that of the magnitude of increased Ca2+ pumped into SR (Fig. S4).
The results presented thus far have reported how synchronization of stochastic local Ca2+ sparks to produce periodic Ca2+ wavelets (LCR’s) is affected by perturbations that increase Ca2+ cycling through the SR in the context of a physiologic free [Ca2+]. Numerous prior studies in VM have indicated that an increase in the bathing [Ca2+], which causes cell and SR [Ca2+] loading to increase also induces spontaneous Ca2+ waves [13, 14].
4. Discussion
The present study demonstrates, for the first time, that similar to pacemaker cells, self-organized, partial synchronization of spontaneous local Ca2+ releases operates in the absence of Ca2+ overload in the form of partially synchronized local rhythmic Ca2+ wavelets (LCRs) that generate a powerful ensemble signal, observed as a sharp, high-power peak in the frequency domain, and a high-amplitude ensemble Ca2+ signal in the space-time domain.
In our previous work by Lyashkov et al. Circ Res. 2007 [15], we have compared the distribution of RyR in rabbit SANC and VM. We showed that RyR’s within SANC are located both beneath sarcolemma and in the interior of the cell, with the highest density beneath the sarcolemma. The localization of RyR’s in transverse bands spaced ~2 mm apart within the SANC interior resemble the sarcomere spacing in VM, but does not depend on the presence of T-tubules, as those are not present in SANC [15, 16].
The ability of permeabilized SANC, but not permeabilized VM to generate periodic clock-like Ca2+ releases, demonstrated in our previous work, has been attributed to an experimentally detected in SANC versus VM increase in the abundance of SERCA, reduced abundance of the SERCA inhibitor protein, PLB, and to increased Ca2+-dependent basal phosphorylation of PLB and RyR [6].
4.1. Emergent partial synchronization and periodicity of spontaneous local Ca2+ releases is due, in part at least, to increased Ca2+ pumping into SR
In the present study we show that in contrast to pacemaker cells, the spontaneous Ca2+ clock in VM does not normally operate in the basal state, but must be unleashed by factors that accelerate SR Ca2+ cycling, e.g. by inhibiting the mechanisms that restrain Ca2+ and cAMP-dependent SR protein phosphorylation (such as phosphatases and phosphodiesterases) or during β-ARs. A phosphorylation-dependent facilitation of restitutions distributed across many SR functions mechanisms that govern SR Ca2+ cycling kinetics distributed across different processes is a general mechanism that underlies the self-organization of low-amplitude, stochastic oscillations (Ca2+ sparks) into cell-wide (line scan) Ca2+ signals of substantial amplitude when phosphorylation of SR Ca2+ cycling proteins increases. These functions include: 1) an increase in the rate of SR Ca2+ pumping into SR; 2) an increase in kinetics of mechanisms that remove RyR inactivation following a Ca2+ release; 3) a reduction in the threshold required for spontaneous RyR activation (see below).
Cyclic AMP-mediated, protein kinase A (PKA)-dependent phosphorylation of phospholamban (PLB), an accessory protein of the SR Ca2+ pump (SERCA-2) causes PLB to disengage from SERCA-2 [17], enhancing the rate of Ca2+ pumping into SR. Detailed studies of regulation of the kinetics of Ca2+ pumping by SERCA2 indicate that removal of PLB regulation converts SERCA2A to a functionally oligomeric state with increased intersubunit free energy exchange [18-20]. To demonstrate that effect of PLB phosphorylation induced by PP and PDE inhibition increases kinetics Ca2+ pumping into SR and that this effect is linked to partial synchronization of local Ca2+ releases from SR, we employed a non-phosphorylation-dependent mechanism, i.e. a specific antibody, 2D12, that inhibits PLB-SERCA interaction [11] and increases Ca2+ pumping into SR. Indeed, 2D12 reproduces the synchronizing effects of PP and PDE inhibition on spontaneous Ca2+ release. While this effect of selective inhibition of the association of PLB and SERCA2 by 2D12 initiates self-organized emergence of high amplitude, partially synchronized spontaneous local SR Ca2+ releases, it cannot be interpreted to indicate that an increased rate of Ca2+ pumping into SR is sufficient to fully account for this phenomenon. Indeed, the local high [Ca2+] in the vicinity of RyRs generated by the high-amplitude LCRs occurring in the contest of an increase in local SR Ca2+ load due to increase in Ca2+ pumping into SR can affect Ca2+-dependent RyR phosphorylation, via e.g. calmodulin kinase II (CAMKII) [21], which in VM also increases when PDE and PP are inhibited [22, 23]. Although some studies provide evidence that RyR phosphorylation by PKA in VM modulates SR luminal Ca2+ sensitivity [24], how RyR phosphorylation by CAMKII affects RyR gating or activation threshold, however, remains controversial [25-27].
4.2. Partial synchronization of phases of release and restitution of SR Ca2+ cycling within and among cell loci (microdomains) create a rhythmic clock in VM
The emergence of a macroscopic Ca2+ oscillator (i.e. whole-cell Ca2+ clock) in the present study involves the emergence of partial synchronization of local Ca2+ releases; i.e. Ca2+ oscillations having nearly the same period. This permits cooperative (and partially synchronized) operation of an ensemble of local Ca2+ oscillators in cell areas that are remote from each other. This type of synchronization mechanism is conceptually similar to that reported by Kort et al. in 1985 [28] and Stern et al. [29], who numerically modeled the emergence of powerful periodic macroscopic Ca2+ signal (whole cell signal in our case) on the basis of summation of local (microscopic) intracellular Ca2+ oscillators whose periods become synchronized around same value. This emergent macroscopic signal of VM in the present study is conceptually similar to the powerful Ca2+ signals in SANC when the phases of the intracellular Ca2+ clock synchronized by the last action potential prior to a voltage clamp at the maximum diastolic potential remain partially synchronized during the voltage clamps (e.g. Fig. 3 in [30]).
4.3. Relevance of synchronizing effects of phosphorylation-mediated SR Ca2+ cycling in permeabilized VM in the present study to electrically paced cells with intact sarcolemmal function during β-ARs
β-ARs during external pacing of intact VM engages the same mechanisms used in our experiments to augment Ca2+ cycling protein phosphorylation. β-ARS (1) suppresses protein phosphatase activity, via PKA-dependent phosphorylation of the phosphatase inhibitor I-1[31], as does PP inhibition in permeabilized VM in the present study (Figs 2, 3D and Figs. S2, S3, S4); (2) increases PLB phosphorylation via increased activation of protein kinase A, by activating adenylyl cyclase resulting in an increase in cAMP production [32], as does PDE plus PP inhibition (Fig. 1) or application of cAMP to permeabilized VM in the present study (Fig. 6 and Figs. S2, S4). The increase in cAMP-mediated, PKA-dependent phosphorylation of PLB during β-ARs in intact VM permits Ca2+ to be pumped into the SR at a greater rate [33], as does disengagement PLB from SERCA2 in permeabilized VM by the 2D12 antibody (Figs. 7, 8 and Fig. S2).
During βARs the robust Ca2+ “clock-like” behavior of SR Ca2+ cycling in VM demonstrated by the present results has two faces: when regulated normally, it not only prepares the myocardium to generate a strong organized Ca2+ release in response to APs occurring at a given frequency; but when it becomes dysregulated, it has the potential to trigger spontaneous abnormal APs that can initiate life-threatening arrhythmias.
4.3.1. The “upside” of β-ARs is to optimally synchronize local RyR activation in response to an AP
During β-ARs rhythmic APs occur at an increased rate, requiring that restitution of SR Ca2+ cycling processes becomes accelerated. The effect of phosphorylation of SR Ca2+ cycling proteins demonstrated in permeabilized cells in the present study would be expected to poise RyRs to activate at sooner times and with increased synchrony in response to subsequent single L-type channel activation, which is also enhanced by phosphorylation of its subunits during β-ARS [34]. The net result of phosphorylation-dependent synchronization of both single L-type Ca2+ channels and SR Ca2+ cycling within and among Ca2+ release units of a single ICaL-SR junction, and among ICaL-SR junctions cell-wide in response to an AP entrains the periodicity of VM SR Ca2+ cycling to ensure optimal synchronized RyR activation in response to that AP [33]. In other words, the SR Ca2+ clock and L-type channel gating clock mechanisms become more synchronized during β-ARS to generate an AP-induced cytosolic Ca2+ signal of increased amplitude that elicits a contraction of not increased amplitude, but of increased synchrony as well [33].
4.3.2. The “downside of β-ARS to synchronize local spontaneous diastolic Ca2+ releases
During β-ARS, the periods of spontaneous local Ca2+ releases of the SR Ca2+ clock are partially synchronized in part by phosphorylation of its proteins, and in part by the relatively cell-wide homogeneous SR Ca2+ depletion induced by the prior AP. During β-ARS regularly occurring APs arrive at the ventricular myocardium at shorter intervals and this faster heart rate entrains the SR Ca2+ clock to AP firing rate, i.e., the SR Ca2+ clock ticks faster. When the external stimulation rate during β-ARS is abruptly lowered, the entrained the SR clock generates partially synchronized Ca2+ releases spontaneously during diastole at or shortly following the time at which the next regular AP at the higher pacing rate was due. Supplemental Figure 6A illustrates the emergence of highly organized spontaneous diastolic Ca2+ releases when the Ca2+ clock ticks faster than the external pacing rate when the external pacing rate is abruptly lowered from 2Hz to 0.5 Hz. The periods of spontaneous Ca2+ release (measured as the time of their occurrence following the prior AP induced makes systolic Ca2+ transient ranged from 0.9 second up to 1.57 second (Fig. S6A). In the continued presence of β-ARs, increasing external pacing frequency to 3Hz, with a frequency less than Ca2+ clocks spontaneous Ca2+ release overdrives the spontaneous SR Ca2+ clock (Fig. S6A (iii)).
It is widely recognized that spontaneous diastolic Ca2+ releases can generate spontaneous diastolic depolarization, which, in single myocytes, are capable of generating spontaneous abnormal APs [3]. But it is also widely recognized that in ventricular myocardium spontaneous diastolic depolarization of the surface membrane of a single VM source in which a DAD arises would dissipate into the sink of surrounding VM, and if therefore, an AP occurred within the single VM, it would not likely excite adjacent cells, due to the well-known large source-sink current mismatch [35].
Based upon the phosphorylation induced emergence of synchronized spontaneous local SR Ca2+ releases as demonstrated in the present study, we propose that during β-ARS local Ca2+ releases not only become synchronized within a given VM, but also become partially synchronized among VM residing within local “neighborhoods” of ventricular tissue, and that partial synchronization of periods of spontaneous local diastolic Ca2+ releases among cells is a mechanism to explain how spontaneous diastolic Ca2+ oscillations within cells can generate spontaneous APs that trigger arrhythmias that arise in ventricular tissue. Supplemental Figure 6B demonstrates spontaneous Ca2+ oscillations occurring in the ten different VM residing together in a “neighborhood” of myocardium. Following a pause in the external stimulation during β-ARs spontaneous Ca2+ releases occur with roughly, i.e. not exactly, the same period. Note, the summated spontaneous Ca2+ release signal among the ten cells generates a high-amplitude spontaneous Ca2+ signal (red trace in Fig. S6B). This phosphorylation-dependent mechanism to synchronize emergent Ca2+ clocks among different myocytes during β-ARs can explain how spontaneous Ca2+ oscillations arising within individual VM can overcome the “source-sink” safety feature of ventricular myocardium (that protects the heart under normal conditions against an eventual afterpotential of single cell or a few cell) [35, 36]. This mechanism of abnormal automaticity originating from partially synchronized spontaneous diastolic Ca2+ releases among VM within ventricular tissue would be likely to occur during a bradycardic pause or between regular impulses emanating from the SA node at inadequately low rates that are unable to override the intrinsic “Ca2+ clock”. It is important to note that this clock-like synchronization mechanism to trigger a focal tissue excitation does not directly require or involve cell-to-cell interactions, and operates without dependence on cell density. In other words, the “source-sink” safety feature of ventricular myocardium (that protects the heart under normal conditions against an eventual after-depolarization of a single cell or a few cells) to generate an AP, can be overcome, because not a single VM, but a large number of VM within the “neighborhood” are the “source” in this instance.
4.4. Relevance of Present Results to Bio-pacemaker Design
Ca2+ clocks within SANC are persistently activated and driven by cAMP-activated PKA- dependent phosphorylation, due to presence and activation of Ca2+-activated adenylyl cyclases [37]. Novel designs of genetically engineered biological pacemakers, conceptually, could feature emergent Ca2+ clocks within VM and among other cardiac cells that are uncoupled from the impulses generated by SA node but are sufficiently coupled to activation of the membrane clock [38]. A recent study numerically tested hundreds of thousands of different bio-pacemaker designs, concluded that a Ca2+ clock is required for not only robust, but also flexible pacemaker function [39]. One specific biological pacemaker design (that mimics nature’s design) [37, 40] employs genetically controlled overexpression of Ca2+-activated adenylyl cyclases that drives the phosphorylation-driven automaticity. Overexpression of a Ca2+-activated adenylyl cyclase has been shown to be sufficient, in the absence of “funny-current” activation, to produce biological pacemaking for seven days in experimental dogs [41]. Another new and interesting approach to create natural coupled-clock functionality within a bio-pacemaker is to reactivate the genetic program of the SA node within VM. A recent study, successfully demonstrated a proof principle for this approach, using tbx18-infected VM that exhibit that “Ca2+ clock mechanisms of automaticity” pacing the heart for up to 8 weeks [42].
Supplementary Material
Highlights.
A Ca2+ clock emerges in permeabilized ventricular myocytes at normal [Ca2+]i
The Ca2+ clock has phosphorylation-dependent mechanism
The Ca2+ clock is manifested by rhythmic local Ca2+ releases as in pacemaker cells
The Ca2+ clock can be activated by PP and PDE inhibition
The Ca2+ clock can be arrhythmogenic and also a candidate for biopacemaker design
Acknowledgments
We thank Bruce Ziman and Ruth Sadler for excellent technical support.
Sources of Funding
This work is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland, and, in part, by National Institutes of Health Grant HL49428 (Larry Jones).
Abbreviations
- AP
Action potential
- β-ARs
β-adrenergic receptor stimulation
- Ca2+
Calcium
- IBMX
Isobutyl-1-methylxanthine
- LCR
Local Ca2+ releases
- PDE
Phosphodiesterase
- PKA
Protein kinase A
- PLB
Phospholamban
- PP
Protein phosphatases
- RyRs
Ryanodine receptors
- SANC
Sinoatrial node cells
- SERCA-2
SR Ca2+ pump
- SR
Sarcoplasmic reticulum
- VM
Ventricular myocytes
Footnotes
Disclosures
The authors are co-inventors in the patent application “Engineered Biological Pacemakers” [38].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–4. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 2.Zima AV, Bovo E, Bers DM, Blatter LA. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol. 2010;588:4743–57. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capogrossi MC, Houser SR, Bahinski A, Lakatta EG. Synchronous occurrence of spontaneous localized calcium release from the sarcoplasmic reticulum generates action potentials in rat cardiac ventricular myocytes at normal resting membrane potential. Circ Res. 1987;61:498–503. doi: 10.1161/01.res.61.4.498. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 5.Eisner D, Bode E, Venetucci L, Trafford A. Calcium flux balance in the heart. J Mol Cell Cardiol. 2013;58:110–7. doi: 10.1016/j.yjmcc.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Sirenko S, Yang D, Li Y, Lyashkov AE, Lukyanenko YO, Lakatta EG, et al. Ca2+-dependent phosphorylation of Ca2+ cycling proteins generates robust rhythmic local Ca2+ releases in cardiac pacemaker cells. Sci Signal. 2013;6(260):ra6. doi: 10.1126/scisignal.2003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circ Res. 2006;98:505–14. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 8.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010;106:659–73. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verde I, Vandecasteele G, Lezoualc’h F, Fischmeister R. Characterization of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br J Pharmacol. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akin BL, Chen Z, Jones LR. Superinhibitory phospholamban mutants compete with Ca2+ for binding to SERCA2a by stabilizing a unique nucleotide-dependent conformational state. J Biol Chem. 2010;285:28540–52. doi: 10.1074/jbc.M110.151779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akin BL, Jones LR. Characterizing phospholamban to sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a) protein binding interactions in human cardiac sarcoplasmic reticulum vesicles using chemical cross-linking. J Biol Chem. 2012;287:7582–93. doi: 10.1074/jbc.M111.334987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sham JS, Jones LR, Morad M. Phospholamban mediates the beta-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am J Physiol. 1991;261:H1344–9. doi: 10.1152/ajpheart.1991.261.4.H1344. [DOI] [PubMed] [Google Scholar]
- 13.Capogrossi MC, Stern MD, Spurgeon HA, Lakatta EG. Spontaneous Ca2+ release from the sarcoplasmic reticulum limits Ca2+-dependent twitch potentiation in individual cardiac myocytes. A mechanism for maximum inotropy in the myocardium. J Gen Physiol. 1988;91:133–55. doi: 10.1085/jgp.91.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–59. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 15.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, et al. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ Res. 2007;100:1723–31. doi: 10.1161/CIRCRESAHA.107.153676. [DOI] [PubMed] [Google Scholar]
- 16.Rigg L, Heath BM, Cui Y, Terrar DA. Localisation and functional significance of ryanodine receptors during beta-adrenoceptor stimulation in the guinea-pig sino-atrial node. Cardiovasc Res. 2000;48:254–64. doi: 10.1016/s0008-6363(00)00153-x. [DOI] [PubMed] [Google Scholar]
- 17.Brini M, Carafoli E. Calcium pumps in health and disease. Physiol Rev. 2009;89:1341–78. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 18.Mahaney JE, Albers RW, Kutchai H, Froehlich JP. Phospholamban inhibits Ca2+ pump oligomerization and intersubunit free energy exchange leading to activation of cardiac muscle SERCA2a. Ann N Y Acad Sci. 2003;986:338–40. doi: 10.1111/j.1749-6632.2003.tb07208.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahaney JE, Albers RW, Waggoner JR, Kutchai HC, Froehlich JP. Intermolecular conformational coupling and free energy exchange enhance the catalytic efficiency of cardiac muscle SERCA2a following the relief of phospholamban inhibition. Biochemistry. 2005;44:7713–24. doi: 10.1021/bi048011i. [DOI] [PubMed] [Google Scholar]
- 20.Waggoner JR, Huffman J, Froehlich JP, Mahaney JE. Phospholamban inhibits Ca-ATPase conformational changes involving the E2 intermediate. Biochemistry. 2007;46:1999–2009. doi: 10.1021/bi061365k. [DOI] [PubMed] [Google Scholar]
- 21.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishida A, Shigeri Y, Taniguchi T, Kameshita I. Protein phosphatases that regulate multifunctional Ca2+/calmodulin-dependent protein kinases: from biochemistry to pharmacology. Pharmacol Ther. 2003;100:291–305. doi: 10.1016/j.pharmthera.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Rao YJ, Xi L. Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts. Acta Pharmacol Sin. 2009;30:1–24. doi: 10.1038/aps.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullrich ND, Valdivia HH, Niggli E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol. 2012;53:33–42. doi: 10.1016/j.yjmcc.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12. 6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 27.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–52. [PubMed] [Google Scholar]
- 28.Kort AA, Lakatta EG, Marban E, Stern MD, Wier WG. Fluctuations in intracellular calcium concentration and their effect on tonic tension in canine cardiac Purkinje fibres. J Physiol. 1985;367:291–308. doi: 10.1113/jphysiol.1985.sp015825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stern MD, Kort AA, Bhatnagar GM, Lakatta EG. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J Gen Physiol. 1983;82:119–53. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ releases during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res. 2004;94:802–9. doi: 10.1161/01.RES.0000122045.55331.0F. [DOI] [PubMed] [Google Scholar]
- 31.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol. 2009;47:365–71. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–77. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 33.Lakatta EG. Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium. 2004;35:629–42. doi: 10.1016/j.ceca.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Zhou P, Zhao YT, Guo YB, Xu SM, Bai SH, Lakatta EG, et al. Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel. Proc Natl Acad Sci U S A. 2009;106:18028–33. doi: 10.1073/pnas.0906560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for afterdepolarizations to propagate in tissue. Biophys J. 2010;99:1408–15. doi: 10.1016/j.bpj.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capogrossi MC, Lakatta EG. Frequency modulation and synchronization of spontaneous oscillations in cardiac cells. Am J Physiol. 1985;248:H412–8. doi: 10.1152/ajpheart.1985.248.3.H412. [DOI] [PubMed] [Google Scholar]
- 37.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, et al. Ca2+ -stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. J Biol Chem. 2008;283:14461–8. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maltsev VA, Lakatta EG, Zahanich I, Sirenko SG, inventors. Engineered Biological Pacemakers. U.S. Provisional Patent Application No. 61/180,491. Federal Register. 2009;74(199):53268.
- 39.Maltsev VA, Lakatta EG. Numerical models based on a minimal set of sarcolemmal electrogenic proteins and an intracellular Ca2+ clock generate robust, flexible, and energy-efficient cardiac pacemaking. J Mol Cell Cardiol. 2013;59:181–95. doi: 10.1016/j.yjmcc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the If pacemaker current. J Physiol. 2007;582:1195–203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boink GJ, Nearing BD, Shlapakova IN, Duan L, Kryukova Y, Bobkov Y, et al. Ca2+-stimulated adenylyl cyclase AC1 generates efficient biological pacing as single gene therapy and in combination with HCN2. Circulation. 2012;126:528–36. doi: 10.1161/CIRCULATIONAHA.111.083584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31:54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.