Abstract
Biochar can influence native soil organic carbon (SOC) mineralisation through “priming effects”. However, the long-term direction, persistence and extent of SOC priming by biochar remain uncertain. Using natural 13C abundance and under controlled laboratory conditions, we show that biochar-stimulated SOC mineralisation (“positive priming”) caused a loss of 4 to 44 mg C g−1 SOC over 2.3 years in a clayey, unplanted soil (0.42% OC). Positive priming was greater for manure-based or 400°C biochars, cf. plant-based or 550°C biochars, but was trivial relative to recalcitrant C in biochar. From 2.3 to 5.0 years, the amount of positively-primed soil CO2-C in the biochar treatments decreased by 4 to 7 mg C g−1 SOC. We conclude that biochar stimulates native SOC mineralisation in the low-C clayey soil but that this effect decreases with time, possibly due to depletion of labile SOC from initial positive priming, and/or stabilisation of SOC caused by biochar-induced organo-mineral interactions.
Biochar production for use as a soil amendment is proposed as a novel strategy to mitigate climate change, with potential to offset 1.8 to 9.5 Pg (1015 g) carbon dioxide (CO2)-C equivalent emissions annually1,2. The estimated climate change mitigation potential of biochar systems arises largely from the combined impact of long-term C sequestration due to biochar's high stability2,3,4,5,6, co-generation of bioenergy during pyrolysis1,7, and avoidance of nitrous oxide and methane emissions from biomass decomposition1,7. Enhancement of crop yield8 and reduction in nitrous oxide emissions from soil9,10 can also contribute to the mitigation potential of biochar systems. Uncertainty remains about the influence of biochar on native SOC mineralisation in terrestrial ecosystems11,12. Biochar application is reported to stimulate11,13 or conversely, to suppress14,15 the mineralisation of native SOC; these effects are termed positive and negative priming, respectively13,14,15. Wardle et al.11 reported strong stimulation of humus decomposition over a 10-year period in boreal forests in the presence of 450°C charcoal, however, these findings may not necessarily be applicable to mineral soil16. Luo et al.13 observed positive priming of native SOC mineralisation in the presence of 350 or 700°C Miscanthus biochar, which decreased in intensity over the 87 days of incubation. In contrast, Kuzyakov et al.6 observed sustained suppression of native SOC mineralisation (over 3.2 years) following the application of 400°C ryegrass biochar in a nutrient-limited loess soil, an effect attributed to reduced microbial activity caused by sorption of nutrients onto biochar. Other studies have also reported positive, negative or no priming effects, however the results are based on limited observations over shorter incubation periods15,17 The divergent results observed in previous studies may have resulted from variations in the proportion of labile C in biochars13, presence or absence of plant-derived labile organic matter input in soil14, and the extent of biochar ageing in soil15,17,18. Spokas & Reicosky19 found no correlation of the CO2 production in biochar-amended versus the control soil with feedstock type, pyrolysis temperature, or biochar properties (elemental composition and surface area). Clearly, a thorough understanding and quantification of the priming effects across a range of biochars over a longer period is important to assessing the true climate change mitigation value of biochar application to agricultural soils1,2,7.
Stimulation of SOC mineralisation in the presence of biochar is thought to occur through (i) the positive co-metabolic effect of labile components on microbial growth6,13,20,21 and (ii) provision of habitat for microorganisms22 that protects microorganisms from predation, and concurrently supports microbial growth through co-locating labile organic matter on biochar surfaces23. Biochar can also influence microbial activity through other mechanisms, for example by altering soil pH, nutrient availability and/or water holding capacity, which may contribute to positive priming13,15. On the other hand, suppression of SOC mineralisation in response to biochar addition is hypothesised to occur due to (i) direct sorption of labile organic matter onto and within the pore network of biochar23,24 and/or (ii) biochar-induced stabilisation or protection of relatively labile organic matter in soil within organo-mineral fractions4,14,18,25,26. Furthermore, a transient shift of microbial communities to utilise relatively more labile C in biochar (cf. native SOC), a phenomenon known as “preferential substrate utilisation”27,28, may also contribute to negative priming, and this phenomenon may be prominent in low-C soil receiving nutrient application29.
To address the issue of uncertainty about the direction, persistence and extent of priming effects of biochar on native SOC, we conducted laboratory incubations of contrasting biochars, produced from four feedstocks at 400 or 550°C, in a clayey soil under controlled conditions over a 5 year period. Our hypotheses were that (i) biochar will positively prime native SOC mineralisation initially, in proportion to its lability, and (ii) biochar-induced positive priming will decrease gradually, possibly due to depletion of labile native SOC from initial positive priming, along with depletion of labile biochar C and interaction of “aged” biochar with soil components in a reactive, smectitic-rich, clayey soil.
The application of biochar with distinctly more negative δ13C signature than the soil allowed us to separately quantify the C mineralisation of biochar and native soil organic matter using an isotopic mixing model (see Table 1; Supplementary Information). The incubations were performed under controlled conditions of soil temperature (22°C) and moisture (70% of maximum water holding capacity) and nutrient supply, to provide ideal C mineralisation conditions and to minimise potential priming effects resulting from changes in these abiotic factors following biochar application to the soil.
Table 1. Mean (1 standard error in parenthesis, n = 3) for total carbon (C), δ13C of biochars and soil, and the fraction of total biochar C or soil C that is labile or mineralised in the first 2 months or over 5 years. The estimated mean residence times (MRT) of biochars, and the amount of native soil organic carbon (SOC) lost via positive priming over 2.3 years, are also shown.
| Treatmentsa | Total Cb (g kg−1) | δ13Cc (‰) | Labile Cc (g kg−1 C) | Cmin2-mo (g kg−1 C) | Cmin5-yr (g kg−1 C) | MRTc (years) | Primed C2.3-yr (mg g−1 SOC) | |
|---|---|---|---|---|---|---|---|---|
| 400°C biochars | ||||||||
| Wood400 | 697.4 (4.3) | −28.36 (0.07) | 2.6 (0.5) | 2.5 (0.2) | 19.7 (0.3) | 294 (5) | 12.8 (2.2) | |
| Leaf400A | 662.8 (2.9) | −28.13 (0.07) | 6.2 (0.4) | 5.5 (0.3) | 24.8 (0.8) | 270 (10) | 18.5 (0.5) | |
| PL400 | 431.1 (6.8) | −25.18 (0.02) | 32.9 (0.8) | 32.0 (0.8) | 69.4 (1.3) | 129 (7) | 43.8 (3.1) | |
| CM400 | 175.0 (1.5) | −27.45 (0.05) | 17.3 (1.5) | 18.3 (1.3) | 72.5 (3.0) | 90 (5) | 14.7 (0.2) | |
| 550°C biochars | ||||||||
| Wood550 | 836.1 (7.6) | −28.74 (0.08) | 1.3 (0.1) | 1.4 (0.2) | 4.8 (0.6) | 1616 (252) | 5.9 (1.6) | |
| Leaf550A | 719.8 (5.3) | −28.21 (0.07) | 3.0 (0.2) | 3.3 (1.1) | 12.4 (0.9) | 572 (32) | 16.3 (4.0) | |
| PL550A | 413.2 (1.8) | −25.22 (0.12) | 6.7 (0.2) | 7.5 (0.2) | 20.5 (1.5) | 396 (57) | 14.1 (4.2) | |
| CM550A | 165.3 (2.5) | −27.71 (0.16) | 4.9 (1.1) | 4.8 (0.7) | 22.4 (1.4) | 313 (38) | 4.4 (3.3) | |
| Smectitic soil (Vertisol) | ||||||||
| Control | 4.2 (0.06) | −14.20 (0.03) | 10.6 (0.6) | 13.4 (0.4) | 96.9 (2.1) | 58 (2) | NA | |
aTreatment details are given in Methods.
bAcid washing of biochars38 did not significantly change their δ13C value (see Table S1), thus suggesting negligible presence of inorganic carbon in biochars. Similarly, inorganic C was also absent in soil3.
cLabile C content and MRT of biochar or native SOC were determined by fitting a two-pool exponential model to the pattern of cumulative % of added biochar C or native OC mineralised over 5 years3.
NA = Not applicable.
Results
Priming effects and lability of biochar in soil
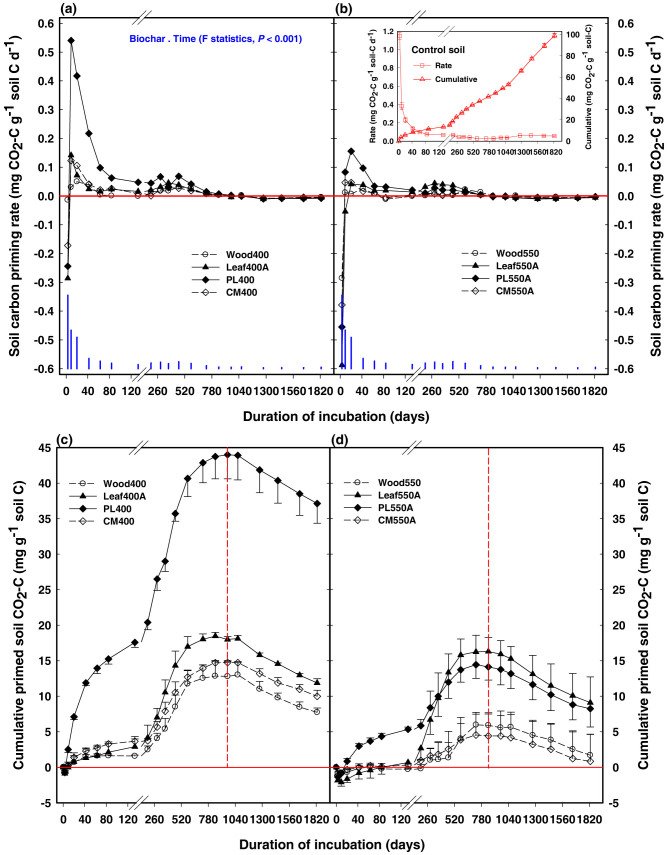
The native SOC mineralisation rate in the control soil decreased in an exponential manner with time. On a cumulative basis, 99.1 mg of CO2-C per gram of native SOC was released over 5 years (Inset in Fig. 1b). The positive or negative priming effects represent the additional native SOC released or stabilised, respectively, in the presence of biochar, relative to the control (Fig. 1).
Figure 1. Soil carbon priming rate (upper panel) and cumulative amount of positively-primed carbon (lower panel) per gram of soil carbon over 5 years in the presence of 400°C (Fig. 1a,c) and 550°C (Fig. 1b,d) biochars in Vertisol.
The main graphs (Fig. 1a–d) are plotted relative to control. Blue bars in Fig. 1a,b show least significant differences (at 5% level, LSD0.05). Error bars for the cumulative data represent 1 standard error (SE) of the means (n = 3). Treatment details are given in Methods. The inset in Fig. 1b shows the mean values (±SE) of both rates and cumulative amounts of carbon mineralised in the control soil (The x-axis title of the inset is the same as for the main graphs). Note the change of scale after the break on the x-axis (i.e. duration of incubation). The dashed vertical line in Fig. 1c,d represents the initiation of a period in which the amount by which mineralisation of native SOC exceeded that of control (positive priming) gradually diminished.
Biochar application resulted in an immediate (on day 1) suppression of native SOC mineralisation (P<0.05), especially for the 550°C biochars (Fig. 1a,b). Subsequently, the rates of native SOC mineralisation were stimulated by biochars and peaked within 2–3 weeks, although the positive priming was significant (P<0.05) for the poultry litter biochars only (Fig. 1a,b). The positive priming effect continued for at least 2.3 years, causing an additional loss of 4 to 44 mg C g−1 SOC across all biochars in the period to 2.3 years. On a cumulative basis, native SOC mineralisation due to the positive priming was greater for the manure- than the plant-based biochars (Fig. 1c,d). Furthermore, the 400°C plant- and manure-based biochars produced a larger positive priming effect (12–44 mg C g−1 soil C) than the corresponding 550°C biochars (4–18 mg C g−1 soil C) (Fig. 1c,d; Table 1). The initial dominating positive priming phase was followed by a period in which the amount by which mineralisation of native SOC exceeded that of control gradually diminished, because the rate of mineralisation of native SOC after about 3 years became lower in the biochar treatments than the control (Fig. 1a,b). This caused a decrease in the initial positively-primed soil CO2-C by 4–7 mg C g−1 soil C between 2.3 to 5.0 years, with little difference in the rate of decrease of the primed SOC mineralisation across the feedstock type or pyrolysis temperature. Overall, there was a net loss of 1–37 mg C g−1 SOC over 5 years (Fig. 1c,d).
The labile C pool (model-derived) in biochars in the 5-year study ranged between 1.3 and 32.9 g C kg−1 biochar C across biochars (Table 1); correspondingly, of the total biochar C mineralised (4.8–72.5 g C kg−1 biochar C across biochars) over 5 years, between 1.4 and 32.0 g C kg−1 biochar C (13–46% of the total) was mineralised in the first 2 months (Table 1). Based on the extent of biochar C mineralised, its labile C content and the estimated mean residence time in the soil (Table 1), the long-term stability of biochars can be ranked in the order of manure biochar < leaf biochar < wood biochar. Furthermore, the low-temperature biochars mineralised faster, and to a greater degree, than the high-temperature biochars from the same feedstock (Table 1).
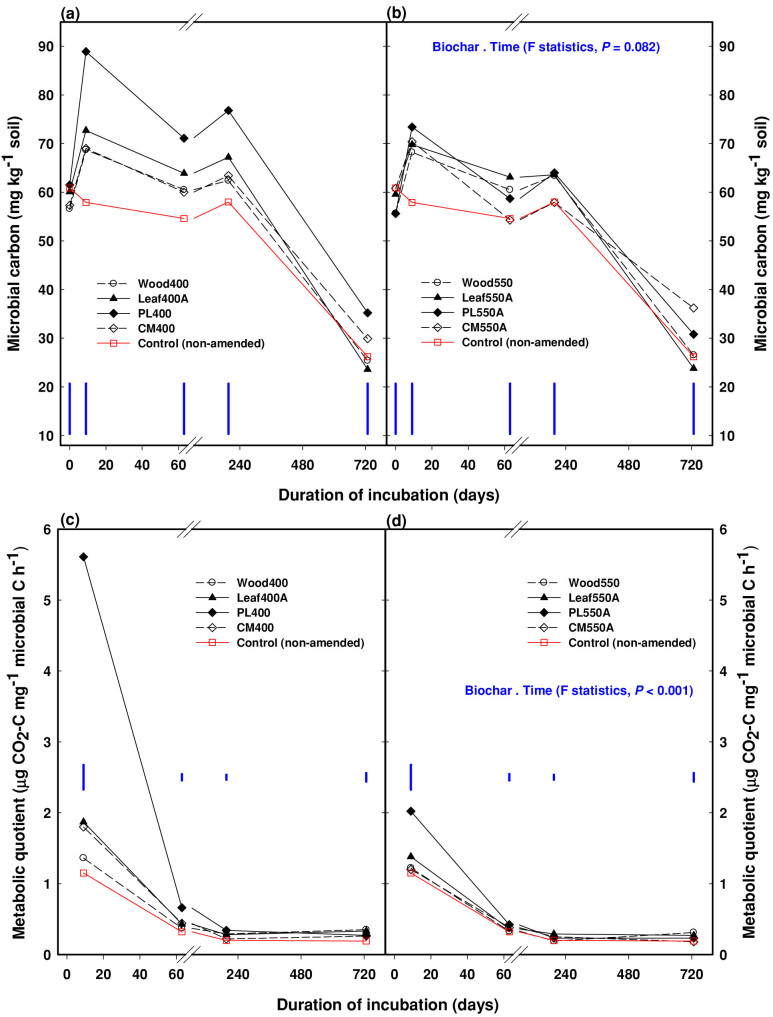
Biochar influence on microbial carbon, metabolic quotient and populations
Biochar application did not change the initial (day zero) microbial biomass C in soil (Fig. 2a,b). Corresponding to the initial pattern of positive soil C priming (Fig. 1) and the relative proportion of labile C in biochars (Table 1), the soil microbial C and associated metabolic quotient (an indicator of microbial C use efficiency) were significantly (P<0.05) higher for the manure- and leaf-based biochars than the control on day 9 (Fig. 2a–d). Averaged over 2 years, microbial C was sustained at higher levels in the biochar-amended Vertisol relative to the control, although microbial C was significantly (P<0.05) higher for the leaf- and manure-based biochars only (Fig. 2a,b). Bacterial and fungal populations (but not actinomycetes) were also larger or similar in the biochar-amended Vertisol (cf. control) 6.5 months and 2 years after incubation (Table 2). Microbial C in both the amended and non-amended treatments decreased significantly at 2 years after incubation (Fig. 2a,b), whereas microbial populations were similar at 6.5 months and 2 years across the treatments (Table 2).
Figure 2. Soil microbial carbon (upper panel) and metabolic quotient (lower panel) in plant- and manure-based biochar, 400°C (Fig. 2a,c) and 550°C (Fig.2b,d), amended and non-amended (control) soil at different times for up to 2 years.
Note the change of scale after the break on the x-axis. The least significant differences (at 5% level, LSD0.05) are shown as blue bars. Treatment details are given in Methods.
Table 2. Colony forming units (CFU ± standard error, n = 3) of microbial populations in biochar-amended and control treatments, determined by a pour plate viable count method, 6.5 months and 2 years after incubation. The least significant differences (LSD0.05) among treatment means are also shown at each time-point when P<0.05.
| Treatments | Bacteria CFU (104) g−1 soil | Actinomycetes CFU (103) g−1 soil | Fungi CFU (102) g−1 soil | |||
|---|---|---|---|---|---|---|
| 6.5 mo | 2 yr | 6.5 mo | 2 yr | 6.5 mo | 2 yr | |
| 400°C biochars | ||||||
| Wood400 | 81±3 | 153±38 | 44±2 | 68±7 | 75±5 | 58±13 |
| Leaf400A | 155±16 | 195±65 | 95±4 | 87±6 | 56±1 | 78±10 |
| PL400 | 268±12 | 290±47 | 101±3 | 120±10 | 131±18 | 94±16 |
| CM400 | 90±11 | 158±48 | 84±3 | 129±5 | 50±5 | 68±5 |
| 550°C biochars | ||||||
| Wood550 | 93±8 | 108±31 | 46±5 | 81±4 | 100±5 | 75±21 |
| Leaf550A | 45±3 | 168±56 | 42±1 | 55±8 | 48±7 | 67±28 |
| PL550A | 102±20 | 153±24 | 59±1 | 91±4 | 60±10 | 56±25 |
| CM550A | 58±2 | 140±50 | 91±9 | 93±7 | 74±3 | 71±8 |
| Control (Vertisol) | ||||||
| Control | 51±5 | 170±42 | 82±5 | 143±10 | 32±3 | 33±6 |
| Treatment (P value) | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | NS |
| LSD0.05 | 31 | 63 | 11 | 19 | 20 | – |
Discussion
Our study shows that biochar application can stimulate mineralisation of native SOC due to positive priming in the short term i.e. within 2 to 3 weeks, which may continue with decreased intensity over the medium term (for at least 2.3 years). Furthermore, we found that the observed positive priming of biochar is related to biochar lability (cf. Fig. 1; Table 1). For example, the manure-based biochars (e.g. poultry litter biochars), which possessed higher proportions of labile C, mineralised to a greater extent, and correspondingly showed higher positive priming effects than the plant-based biochars. Similarly, the relatively labile 400°C biochars showed higher positive priming effects than the corresponding 550°C biochars.
The results also suggest that biochar may transiently suppress native SOC mineralisation immediately after application (Fig. 1a,b). This could result from: (i) sorption of labile soil organic matter onto biochar particles, thus decreasing its mineralisation23; (ii) a very short-term inhibitory effect on microbial activity of biochar-associated volatile organic compounds30,31; and/or (iii) a transient shift to more labile substrates in biochar, relative to those in the low-C clayey soil29. Since the transient negative priming effect (on day 1) was more pronounced for the 550°C biochars, which would have a lower proportion of labile C (Table 1) and also volatile organic compounds31, but higher surface area and porosity14, this effect is more likely attributed to the sorption mechanism suggested above.
Following the transient suppression phase, biochar treatments increased mineralisation of native SOC by supporting the activity and growth of microorganisms (Fig. 2) possibly by (i) providing intrinsic labile organic components for microbial utilisation21,32, thus inducing the co-metabolic effect13,20,21; and (ii) serving as a habitat for microorganisms22. The higher-temperature or activated biochars would possess high specific surface area and porosity14,33, and hence may provide greater microbial habitat in soil than the lower-temperature or non-activated biochars15,22. Nevertheless, the positive priming effect was clearly greater for the lower-temperature biochars than the higher-temperature biochars (Fig. 1). Furthermore, the positive priming effect was similar for the activated and non-activated biochars (data not shown). These data suggest that it is the intrinsic lability (rather than the habitat characteristic) of biochar that plays an important role in determining the extent of shorter-term positive priming (Fig. 1). Some studies have demonstrated that even trace amounts of low molecular weight organic substrates (e.g. glucose, amino acids, root exudates) may induce a trigger response to stimulate microbial activity and biomass34,35 and consequently induce ‘apparent’ or ‘real’ positive priming effects36. Biochar may contain small quantities of water-soluble low molecular organic compounds37 among predominantly complex C substrates3, which could trigger priming effects. Further research is required to determine forms of low molecular weight organic molecules in biochar and their relevance to the positive or negative priming effects on native SOC mineralisation.
The non-C fraction (e.g. nutrients in biochar) may also influence SOC priming if present in disproportionate amounts in different biochar treatments. Indeed there was a variation in the content of nutrients (N, P, K, S, Ca, Mg and a suite of micronutrients) among biochars38. In the present study, however, both major and micro nutrients were added in excess (see Methods), thereby minimising the influence of contrasting nutrient addition through biochar on SOC priming across the treatments. Furthermore, any stimulation of native SOC mineralisation by the addition of alkalinity through the biochars is expected to be minimal in the alkaline Vertisol (pH = 8.1) used in the present study. The pH of biochars used ranged from 6.9 to 10.3, and the CaCO3 equivalence ranged from −9.2 to 86.3 mg g−1 biochar, with the 550°C biochars being more alkaline, and having higher CaCO3 equivalence, than the 400°C counterparts38.The 550°C biochars used in this study generally had higher or similar content of mineral nutrients compared with the corresponding 400°C biochars (see Singh et al.38). Nevertheless, the positive priming effect was larger for the 400°C biochars than the corresponding 550°C biochars (Fig. 1), thus suggesting that any further additions of alkalinity or nutrients through biochar, which could increase native SOC mineralisation27,39, did not contribute to the observed positive priming effect.
Our positive priming results over the short- to medium-term are consistent with the findings of Luo et al.13, who observed greater positive priming effects for the 350°C than the 700°C biochar; the positive priming effect peaked within 1–13 days and was sustained over the 87-d incubation period. Similarly, Farrell et al.21 observed a short-lived (up to 9 days) pulse of positively-primed soil CO2 in the presence of biochar followed by a decrease in the magnitude of positive priming, which persisted throughout the 74-d incubation period. Luo et al.13 hypothesised rapid stimulation of r-strategist microorganisms by labile biochar C components, causing C substitution in microbial pool and enhancing inherent microbial C turnover, thus leading to the ‘apparent’ positive priming effect36. Consistent with these hypotheses, we observed high microbial metabolic quotients in the biochar-amended Vertisol initially (Fig. 2c,d). These results suggest the occurrence of uncoupled growth and turnover (i.e. greater turnover than growth) of microbial biomass40 using the labile organic components of biochar and are consistent with ‘apparent’ positive priming36,41. However, because the positive priming effect was sustained for at least 2.3 years, this suggests co-occurrence of both ‘apparent’ and ‘real’ positive priming effects during incubation41. Positive priming may have been induced by the increased activity of both r-strategist and K-strategist micro-organisms (activated in the early and mid- to later-stages of incubation, respectively) and hence maintaining increased mineralisation of native SOC, albeit at a lower-rate with time than observed in the shorter-term (Fig. 1). Furthermore, since microbial biomass and metabolic quotient decreased over time, possibly due to depletion of labile C and shifts in microbial communities toward efficient C users21; this would have contributed to lower rates of positive priming with time. Nevertheless, microbial population counts were sustained over the 2 years of observations in the biochar-amended soil, relative to the control (Table 2), thus ensuring equivalent presence of microbial communities for potential positive priming effects.
Importantly, through measurements over the longer term (from 2.3 to 5 years), our study observed a partial decrease in the cumulative amount of positively-primed soil CO2-C across all biochar treatments (Fig. 1). We suggest that this reduction in SOC mineralisation at later stages may have resulted from (i) depletion of labile SOC due to the previous positive priming (SOC depletion hypothesis) and/or (ii) stabilisation of SOC due to biochar-induced organo-mineral interactions (SOC stabilisation hypothesis)14,15, which may become prominent as labile biochar components are depleted and as biochar ages over time3. We note that the extent and timing of reduction in positively-primed SOC mineralisation were similar across the biochar treatments despite differences in the levels of positive priming between the biochar treatments (Fig. 1). Hence, the SOC depletion hypothesis, described above, does not seem to provide complete explanation for the observed reduction in the primed SOC mineralisation at later stages. Alternatively, as biochar “ageing” results in the formation of negatively-charged organic functional groups on biochar surfaces26,42, this may enhance interactions of biochar with native organic matter and clay minerals, e.g. through ligand exchange or cation bridging mechanisms43. These interactions would then cause stabilisation of native SOC and biochar in mineral soil4,14,26,44 and may result in negative priming, which would enhance the C sequestration potential of biochar in the soil. However, stabilisation through organo-mineral interaction, if occurring, would hasten the timing of reduction in positively-primed SOC mineralisation, for example, by removing SOC from labile pools to relatively stable pools, but this is not obvious from the primed SOC mineralisation data (Fig. 1). Another possible explanation for why the reduction in the primed SOC mineralisation did not occur sooner in relatively labile biochar treatments is because of their persistently higher positive priming effect over the initial 2.3 years, compared with the less labile biochars (Fig. 1) and this could have counteracted the impact of SOC depletion on the timing of reduced mineralisation rates. While we hypothesise that our data in the later stages of 5-year incubation could indicate SOC stabilisation through organo-mineral interactions, further work is required to test this suggestion. Future work should employ physical fractionation to evaluate evidence for increased mineral-associations of native or added organic C in biochar-amended soil18,44. The findings presented here are specific to the smectitic-rich soil type, and to the range of biochars and the controlled laboratory conditions employed, where the SOC depletion hypothesis is likely to predominate to explain reductions in positive priming of native SOC with time. Future work investigating the importance of organo-mineral interactions in the organic C stabilisation process should assess biochar-amended soils of different clay mineralogy and organic C contents, preferably under the constant supply of labile organic matter from plants.
Stabilisation of native SOC via biochar-induced organo-mineral interactions has been hypothesised to occur in Anthrosols containing aged biochar and kaolinite-dominated clay minerals, where the newly added C in sugarcane residues was rapidly stabilised in organo-mineral fractions18. Using the same Vertisol and similar wood biochars as in the present study, Keith et al.14 found that sugarcane residue-C applied in the presence of biochar was stabilised rapidly and this negative priming effect increased with increasing input of residue-C. These results from Keith et al.14 also suggest that high labile organic matter content in a clayey soil may further assist the C stabilisation process, possibly through intensifying microbial activity and biochar organo-mineral interactions14,17,26. The formation of organo-mineral associations during ageing over longer terms may reduce the accessibility of labile organic C to microbial attack18,25. In Slavich et al.45, the observation of increased soil C stocks over and above the addition of C through biochar in a reactive clayey soil after several years of biochar ageing in the field can be attributed to biochar-induced stabilisation of plant-derived C in the field, supporting the hypothesis of SOC stabilisation through organo-mineral interaction14,18.
Due to its high surface area and porosity, biochar may also stabilise native SOC through direct sorption of dissolved organic matter and microbial enzymes on surfaces and within pore spaces of biochar14,15,23. However, biochar particles alone would possess limited sorption ability, which may decrease further due to clogging of pore network of biochar and attenuation of biochar's surface activity by high molecular weight organic substances in soil24. It should also be noted that SOC stabilisation mechanisms such as protection of labile organic matter within biochar pores or in stable organo-mineral fractions would occur close to biochar particles and their relevance at the whole soil scale would be a function of biochar mass per unit soil volume.
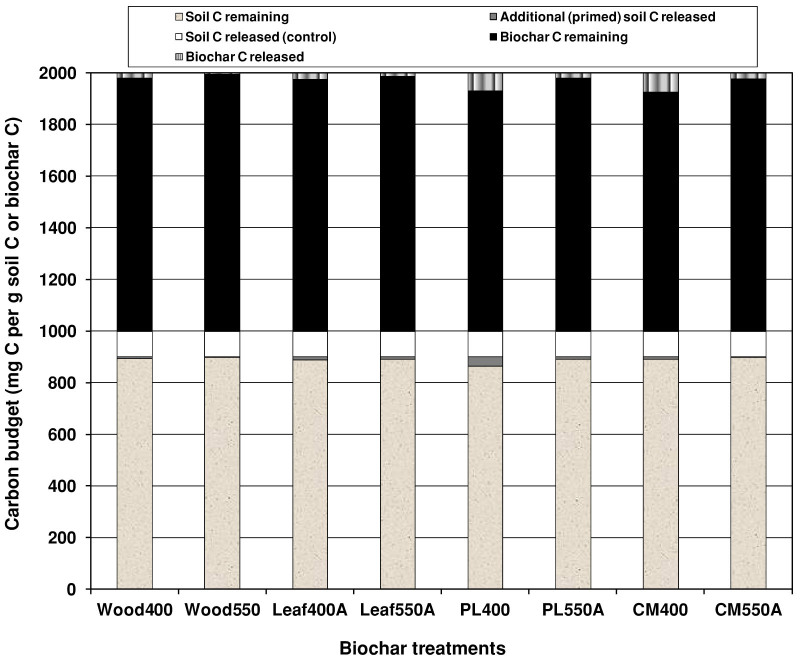
Based on the proportions of added (biochar) and native C released during the 5-year incubation, we have shown that the additional loss of native SOC due to positive priming by biochars was very small (0.4–4.4% of initial native SOC) relative to recalcitrant C in biochar added to the soil (Fig. 3; Table 1). Thus, the native SOC loss due to positive priming is likely to only partially offset the direct C sequestration of biochar based on its stability in the soil. On the positive side, short- to medium-term positive priming events caused by biochar may enhance nutrient cycling in soil27. Furthermore, our data show that the initial dominating positive priming phase was followed by the phase of a gradual reduction in the amount of positively-primed soil CO2-C in the biochar treatments (Fig. 1). This later decrease in the positively-primed SOC mineralisation could be a result of biochar-induced microbial proliferation initially depleting labile native SOC (positive priming), simultaneously generating relatively stable SOC, and possibly further stabilising in situ microbial-derived C through organo-mineral interactions in the clayey soil. This later decrease in relative rate of native SOC mineralisation then partially reduced the cumulative amount of positively-primed C, by 0.4–0.7% over the period from 2.3 to 5.0 years. These findings suggest that stimulation of native SOC loss due to biochar application may not continue over a longer term in an unplanted low-C clayey soil. Thus we assert that observations of positive priming of native SOC by biochar in incubation studies without labile organic inputs from plants should be interpreted with caution, and may not represent the long term outcomes of biochar application in the field.
Figure 3. Budget of the proportions of added biochar carbon and native soil carbon released and remaining in soil, including primed carbon per gram of native soil carbon over 5 years in Vertisol amended with 400°C and 550°C plant- and manure-based biochars.
Treatment details are given in Methods.
Methods
Soil and biochars
The Vertisol soil was collected from a treeless grassland dominated by C4 tussock Mitchell grass (Astrebla spp.) over 100 years, at Toorak Research Station near Julia Creek (Lat 21°02′49″S, Long 141°78′55″E), Queensland, Australia3,14. The relevant properties of the soil are: pH1:5 water 8.1; organic C 4.2 g kg−1; δ13C −14.2‰; clay content 530 g kg−1; >80% smectite. The analytical procedures used for determining these soil properties are described in Keith et al.14.
Ten biochars were produced using slow pyrolysis (“Daisy” Reactor, Batch Pyrolyser; Pacific Pyrolysis, Somersby NSW, Australia; http://pacificpyrolysis.com) from four C3-biomass feedstocks3,38: Eucalyptus saligna wood, (C 47.9%, N ~0.03%, δ13C -27.6‰); Eucalyptus saligna leaves (C 50.1%, N 1.33%, δ13C −28.2‰); poultry litter, which was made up of poultry manure on rice hulls (39.3% C, 6.09% N, δ13C −24.9‰); and cow manure (20% C, 1.96% N, δ13C −27.4‰). Biomass was heated in the Daisy Reactor at 5–10°C/min and then retained at the highest heating temperature (HHT), 400° or 550°C, for 40 min. Nitrogen (N2) gas was then added into the reactor during the cooling-off period to maintain the inert environment. Some biochars were activated by introducing steam into the reactor when HHT was reached, for the entire 40 minute residence time, to increase porosity of biochars33.
Biochars were produced at 400 and 550°C from the E. saligna wood without steam activation (Wood400 and Wood550, respectively) and with steam activation (Wood400A and Wood550A, respectively). The E. saligna leaf biochars were produced at 400°C (Leaf400A) and 550°C (Leaf500A) with steam activation only. Biochars from poultry litter and cow manure were produced each at 400°C without steam activation (PL400 and CM400, respectively) and at 550°C with steam activation (PL550A and CM550A, respectively). Before mixing with the soil, biochar samples were ground and sieved to < 2-mm. Chemical and nutrient properties of biochars and associated analytical methods are reported elsewhere3,38.
We used a Micromass IsoPrime isotope ratio mass spectrometer (with analytical precision (1σ) < 0.10–0.15‰ determined from acetanilide standard)3 or a Delta V Thermo Finnigan isotope ratio mass spectrometer (with analytical precision (1σ) < 0.10‰ determined from beet sucrose standards)14 to determine δ13C values of finely ground (<53 μm) soil, feedstock and biochar samples.
Repeated acid-washing of biochar samples was performed38 to determine whether biochars contained 13C-enriched carbonates. The δ13C values of biochars did not change after repeated acid washing, indicating that carbonates were essentially absent in the biochars (cf. Table 1 and Table S1).
Furthermore, inorganic C was also absent in this soil and hence total C equates to total organic C in the soil.
Incubation experiment
The air-dried soil [Vertisol, equivalent to 612 g oven-dry basis, 2 mm sieved], adjusted to ~67% of water holding capacity using a nutrient solution of NH4NO3 (at 50 mg N kg−1 dry soil), and inoculated with a soil microbial inoculum3,14, was placed in 600 ml plastic jars. The soil containers were then placed in sealed 5-L buckets and pre-incubated for 10 days at 22±1°C. After pre-incubation, a nutrient solution (6 ml) that contained (kg−1 soil) ca. 500 mg N, 80 mg P, 200 mg K, 30 mg S, and trace elements was uniformly mixed with the soil to ensure adequate supply of essential nutrients for microbial growth46. Each of the biochars was mixed with the soil at 8.17 g kg−1 soil (oven-dry basis). A control comprising soil without biochar that received identical moisture adjustment, nutrient solution addition and mixing to that in the biochar treatments was also prepared. The soil-biochar mixtures and the control soil were repacked in 600 ml plastic jars (bulk density: 1.2 g cm−3). The jars were then placed in separate 5-L sealed buckets containing (i) 100 mL of water to maintain humidity, and (ii) 30 mL of 2 M NaOH to absorb CO2 evolved. The sealed buckets were placed in a dark room at 22±1°C. The incubation experiment comprised three replicates for each of the 11 treatments (10 biochars plus one control). The CO2 trap was removed and replaced 20 times over 5 years and was analysed for total CO2-C and δ13C. See Supplementary Information online for further details on the incubation experiment.
Biochar carbon mineralisation, priming effect, and mean residence time
The CO2 trapped in 1 ml of 2 M NaOH was precipitated with 10 ml of 0.20 M BaCl2 and the residual NaOH was titrated with 0.1 M HCl using phenolphthalein as the indicator3. To another 10 ml aliquot of 2 M NaOH, 10 ml of 1 M SrCl2.6H2O was added to precipitate the trapped CO2 as SrCO3. The SrCO3 precipitate was repeatedly washed (~8 times) with deionised water to obtain a neutral pH in the supernatant3. The supernatant was discarded and the precipitate was dried at 60°C and finely-ground for δ13C analysis by IRMS (1.5 mg SrCO3 + 3 mg V2O5 using a Delta V Thermo Finnigan IRMS or 4 mg SrCO3 + 6 mg V2O5 using a Micromass IsoPrime IRMS)47.
The amount (mg kg−1 soil) of biochar C mineralised from biochar-amended soil, CB, was calculated as:
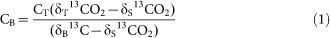 |
where CT is the total C mineralised from biochar-amended soil, δT13CO2 is the δ13C value of the CO2-C evolved from biochar-amended soils, δS is the δ13C value of CO2-C evolved from the control (non-amended) soil, and δB13C is the initial δ13C value of biochar3,14 (see Supplementary Information online for further details on the use of δ13C of biochar and respired CO2 from the control as end members in Equation 1). The δ13C values of the CO2-C evolved from biochar-amended and non-amended (control) soils during the incubation are given in the Supporting Information of Singh et al.3 (see Fig. S6c,d online in Singh et al.3). Information about why we did not look into the results for the papermill sludge biochar in this paper in Scientific Reports is given in the Supplementary Information online.
The amount of soil-C mineralised from biochar-amended soil was determined by subtracting CB from CT. The amounts of biochar C and soil C mineralised were then normalised to per gram of biochar C and soil C, respectively.
The changes in native SOC mineralisation induced by biochar application (i.e. the positive or negative priming effect (PE)) were calculated as:
 |
where, 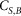 is the amount (mg kg−1 soil) of soil-derived CO2-C evolved from biochar-amended soil, isolated using Equation 1.
is the amount (mg kg−1 soil) of soil-derived CO2-C evolved from biochar-amended soil, isolated using Equation 1. 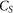 is the amount (mg kg−1 soil) of CO2-C evolved from the control soil14 The priming effect data were expressed per gram of native soil C (see Fig. 1).
is the amount (mg kg−1 soil) of CO2-C evolved from the control soil14 The priming effect data were expressed per gram of native soil C (see Fig. 1).
Recognising the difference in total C content between different biochars (ranging from 17 to 84%; Table 1), the priming effect data were also normalised to 1% biochar C addition rate (10-g biochar C kg−1 soil) to evaluate comparison of the priming effect at an equivalent loading rate of biochar C in the soil (see Supplementary Fig. S1 online). However, care should be taken in interpreting the priming results on an equivalent biochar C basis, because it is not known whether the priming effect is a linear function of biochar C addition rate; other factors such as biochar pH, nutrient content, labile C content, and surface area may also influence native SOC priming. Furthermore, although there were differences in the extent of positive priming across the biochar treatments, we did not find differences in the extent of reduction in native SOC mineralisation per unit of biochar C observed in the later phase, except for the greater reduction in the cow-manure biochar, which contained the lowest C content (see Supplementary Fig. S1 online).
The effect of steam activation on biochar C mineralisation3, soil microbial C and soil C priming was tested for wood biochar only (i.e. comparing activated and non-activated wood biochars at each pyrolysis temperature). These properties were not significantly different between the activated and non-activated biochar treatments; hence results for only the non-activated wood biochar are presented here. The activation treatment was also found to have no significant effect on chemical properties of wood biochar38, including the chemical structure as determined by cross and direct polarisation 13C NMR techniques3.
A two-pool exponential model was fitted to the pattern of cumulative % C mineralised over 5 year incubations3, to estimate the MRT of biochar C and native SOC (see further details in Supplementary Information online).
Microbial analysis
The fumigation-extraction procedure was used to determine soil microbial C48: the soils were fumigated for 10 days with chloroform (stabilised by 0.006% v/v amylene). The fumigated and non-fumigated soils were extracted for 1 h with 0.5 M K2SO4 solution and soil microbial biomass C was determined from the difference between the amounts of organic C extracted from the fumigated and non-fumigated samples, divided by 0.67, to adjust for greater release of microbial cytoplasm during 10 days of fumigation49, instead of 0.45 for 1-day fumigation48. Soil microbial metabolic quotient, i.e. soil C mineralization rate per unit of microbial C in the biochar-amended and control soils was determined using the corresponding values of SOC mineralisation rate and microbial C for each replicate.
The counts of viable microbial populations (bacteria, actinomycetes, fungi) were determined in biochar-amended and control (non-amended) soils at day 196 and 2 year after incubation using a pour plate method50. Briefly, the counts of viable bacteria, actinomcyetes and fungi were determined by pour plating on peptone-yeast agar, glycerol-casein agar and rose Bengal-streptomycin agar, respectively, using 10-fold serial dilutions50.
Statistical analysis
Repeated measures analysis was performed for soil C mineralisation rate, microbial biomass C and microbial metabolic quotient in biochar-amended and control (non-amended) soils using a linear mixed model framework in the Genstat® statistical package (14th Edition)51. Each analysis consisted of fixed effects of biochar treatments, time, and all associated interactions, and random effects of replicate and replicate by time. To allow for correlation between repeated measures on the same units, a first order autoregressive model was fitted for soil C mineralisation. For microbial biomass C and metabolic quotient, random effects of units were fitted (implying a uniform correlation or split plot in time model) since there was no evidence of auto-correlation. Heterogeneity in the residual variances between time-points was included for soil C mineralisation and metabolic quotient as it was found to be significant. Univariate analyses of microbial populations (Table 2) were conducted in biochar-amended and control soils at each time-point separately (6.5 months and 2 years) using a linear mixed model framework in the Genstat® statistical package (14th Edition)51, with each analysis consisted of fixed effects of biochar and random effects of replicate. Wald-type F statistics (with Kenward-Roger adjustments) were calculated for all fixed terms (treatment, time or treatment by time): where the F-statistic was significant, the predicted means were compared using the LSD test at 5% level.
Author Contributions
B.P.S. and A.L.C. conceived and designed the experiment. B.P.S. performed the experiment and interpreted the data with contributions from A.L.C. B.P.S. drafted the manuscript and B.P.S. and A.L.C. reviewed the manuscript.
Supplementary Material
Supplementary document
Acknowledgments
This study was supported by funding from the NSW Office of Environment and Heritage (formerly Department of Environment, Climate Change and Water). We thank Evelyn Krull, Yunying Fang and Balwant Singh for providing comments on an earlier version of the manuscript and Adriana Downie of Pacific Pyrolysis (Australia) for supplying biochars. We are grateful to David Giles, Kamaljeet Kaur and Satvinder Bawa for excellent technical support. Damian Collins' assistance with statistical analysis is greatly appreciated. We thank Claudia Keitel of the University of Sydney and Brett Kuskopf of the University of Melbourne for the δ13C analysis. We also thank David Phelps and Peggy Olsson for supplying the soil.
References
- Woolf D., Amonette J. E., Street-Perrott F. A., Lehmann J. & Joseph S. Sustainable biochar to mitigate global climate change. Nat. Commun. 1, 56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Gaunt J. & Rondon M. Bio-char sequestration in terrestrial ecosystems–a review. Mitig. Adapt. Strat. Glob. Change 11, 403–427 (2006). [Google Scholar]
- Singh B. P., Cowie A. L. & Smernik R. J. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 46, 11770–11778 (2012). [DOI] [PubMed] [Google Scholar]
- Fang Y., Singh B., Singh B. P. & Krull E. Biochar carbon stability in four contrasting soils. Eur. J. Soil Sci. 10.1111/ejss.12094 (2013). [Google Scholar]
- Zimmerman A. R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 44, 1295–1301 (2010). [DOI] [PubMed] [Google Scholar]
- Kuzyakov Y., Subbotina I., Chen H., Bogomolova I. & Xu X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 41, 210–219 (2009). [Google Scholar]
- Roberts K. G., Gloy B. A., Joseph S., Scott N. R. & Lehmann J. Life cycle assessment of biochar systems; estimating the energetic, economic and climate change potential. Environ. Sci. Technol. 44, 827–833 (2010). [DOI] [PubMed] [Google Scholar]
- Waters D. et al. [Biochar in soil for climate change mitigation and adaptation]. Soil Health and Climate Change [Singh, B. P., Cowie, A. L. & Chan, K. Y. (eds.)] [345–368] (Springer-Verlag, Berlin, 2011). [Google Scholar]
- Singh B. P., Hatton B. J., Singh B., Cowie A. L. & Kathuria A. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 39, 1224–1235 (2010). [DOI] [PubMed] [Google Scholar]
- Cayuela M. L. et al. Biochar's role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosys. Environ. 10.1016/j.agee.2013.10.009 (2013). [Google Scholar]
- Wardle D. A., Nilsson M. C. & Zackrisson O. Fire-derived charcoal causes loss of forest humus. Science 320, 629–629 (2008). [DOI] [PubMed] [Google Scholar]
- Woolf D. & Lehmann J. Modelling the long-term response to positive and negative priming of soil organic carbon by black carbon. Biogeochem. 111, 83–95 (2012). [Google Scholar]
- Luo Y., Durenkamp M., De Nobili M., Lin Q. & Brookes P. C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 43, 2304–2314 (2011). [Google Scholar]
- Keith A., Singh B. & Singh B. P. Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ. Sci. Technol. 45, 9611–9618 (2011). [DOI] [PubMed] [Google Scholar]
- Zimmerman A. R., Gao B. & Ahn M. -Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 43, 1169–1179 (2011). [Google Scholar]
- Lehmann J. & Sohi S. Comment on "Fire-Derived Charcoal Causes Loss of Forest Humus". Science 321, 1295 (2008). [DOI] [PubMed] [Google Scholar]
- Cross A. & Sohi S. P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 43, 2127–2134 (2011). [Google Scholar]
- Liang B. Q. et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 41, 206–213 (2010). [Google Scholar]
- Spokas K. A. & Reicosky D. C. Impacts of sixteen different biochars on soil greenhouse gas production. Ann. Environ. Sci. 3, 179–193 (2009). [Google Scholar]
- Hamer U., Marschner B., Brodowski S. & Amelung W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem. 35, 823–830 (2004). [Google Scholar]
- Farrell M. et al. Microbial utilisation of biochar-derived carbon., Sci. Total Environ. 465, 288–297 (2013). [DOI] [PubMed] [Google Scholar]
- Pietikäinen J., Kiikkilä O. & Fritze H. Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 89, 231–242 (2000). [Google Scholar]
- Lehmann J. et al. Biochar effects on soil biota–A review. Soil Biol. Biochem. 43, 1812–1836 (2011). [Google Scholar]
- Pignatello J. J., Kwon S. & Lu Y. Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids. Environ. Sci. Technol. 40, 7757–7763 (2006). [DOI] [PubMed] [Google Scholar]
- Brodowski S., John B., Flessa H. & Amelung W. Aggregate-occluded black carbon in soil. Euro. J. Soil Sci. 57, 539–546 (2006). [Google Scholar]
- Lin Y., Munroe P., Joseph S., Kimber S. & Van Zwieten L. Nanoscale organo-mineral reactions of biochars in ferrosol: an investigation using microscopy. Plant Soil 357, 369–380 (2012). [Google Scholar]
- Kuzyakov Y., Friedel J. K. & Stahr K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498 (2000). [Google Scholar]
- Gontikaki E., Thornton B., Huvenne V. A. I. & Witte U. Negative priming effect on organic matter mineralisation in NE Atlantic slope sediments. PLoS One 8(6), e67722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenet B., Leloup J., Raynaud X., Bardoux G. & Abbadie L. Negative priming effect on mineralization in a soil free of vegetation for 80 years. Eur. J. Soil Sci. 61, 384–391 (2010). [Google Scholar]
- Spokas K. A., Baker J. M. & Reicosky D. C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 333, 443–452 (2010). [Google Scholar]
- Spokas K. A. et al. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 85, 869–882 (2011). [DOI] [PubMed] [Google Scholar]
- Luo Y. et al. Microbial biomass growth, following incorporation of biochars produced at 350°C or 700°C, in a silty-clay loam soil of high and low pH. Soil Biol. Biochem. 57, 513–523 (2013). [Google Scholar]
- Downie A., Crosky A. & Munroe P. [Physical properties of biochar]. Biochar for Environmental Management: Science and Technology [Lehmann, J. & Joseph, S. (eds.)] [13–32] (Earthscan, London, 2009). [Google Scholar]
- Jenkins S. N. et al. Taxon-specific responses of soil bacteria to the addition of low level C inputs. Soil Biol. Biochem. 42, 1624–1631 (2010). [Google Scholar]
- De Nobili M., Contin M., Mondini C. & Brookes P. C. Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol. Biochem. 33, 1163–1170 (2001). [Google Scholar]
- Blagodatskaya E. & Kuzyakov Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils 45, 115–131 (2008). [Google Scholar]
- Lin Y., Munroe P., Joseph S., Henderson R. & Ziolkowski A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 87, 151–157 (2012). [DOI] [PubMed] [Google Scholar]
- Singh B., Singh B. P. & Cowie A. L. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 48, 516–525 (2010). [Google Scholar]
- Li X.-G., Rengel Z., Mapfumo E. & Bhupinderpal-Singh. Increase in pH stimulates mineralization of ‘native’ organic carbon and nitrogen in naturally salt-affected sandy soils. Plant Soil 290, 269–282 (2007). [Google Scholar]
- Bhupinderpal-Singh., Rengel Z. & Bowden J. W. Carbon, nitrogen and sulphur cycling following incorporation of canola residue of different sizes into a nutrient-poor sandy soil. Soil Biol. Biochem. 38, 32–42 (2006). [Google Scholar]
- Kuzyakov Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 42, 1363–1371 (2010). [Google Scholar]
- Cheng C. H., Lehmann J., Thies J. E., Burton S. D. & Engelhard M. H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 37, 1477–1488 (2006). [Google Scholar]
- Lützow M. V. et al. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review. Euro. J. Soil Sci. 57, 426–445 (2006). [Google Scholar]
- Singh N. et al. Transformation and stabilization of pyrogenic organic matter in a temperate forest field experiment. Glob. Change Biol. 10.1111/gcb.12459 (2013). [DOI] [PubMed] [Google Scholar]
- Slavich P. G. et al. Contrasting effects of manure and green waste biochars on the properties of an acidic ferralsol and productivity of a subtropical pasture. Plant Soil 366, 213–227 (2013). [Google Scholar]
- Chapman S. J. Carbon substrate mineralization and sulphur limitation. Soil Biol. Biochem. 29, 115–122 (1997). [Google Scholar]
- Harris D., Porter L. K. & Paul E. A. Continuous flow isotope ratio mass spectrometry of carbon dioxide trapped as strontium carbonate. Commun. Soil Sci. Plan. Anal. 28, 747–757 (1997). [Google Scholar]
- Vance E. D., Brookes P. C. & Jenkinson D. S. An extraction method for measuring microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- Sparling G. P., Gupta V. V. S. R. & Zhu C. Release of ninhydrin-reactive compounds during fumigation of soil to estimate microbial C and N. Soil Biol. Biochem. 25, 1803–1805 (1993). [Google Scholar]
- Brendecke J. W., Axelson R. D. & Pepper I. L. Soil microbial activity as an indicator of soil fertility: long-term effects of municipal sewage sludge on an arid soil. Soil Biol. Biochem. 25, 751–758 (1993). [Google Scholar]
- Payne R. W., Murray D. A., Harding S. A., Baird D. B. & Soutar D. M. An Introduction to GenStat for Windows (14th Edition) (VSN International, Hemel Hempstead, UK, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary document